Abstract
Background
The primary reported indication for the Associating Liver Partition with Portal vein Ligation for Staged hepatectomy (ALPPS) technique is in patients with very low future liver remnant volumes. Given the elevated incidence of major morbidity (40%) and liver-related mortality (12%) with ALPPS, we sought to determine the safety and efficacy of percutaneous portal vein embolization (PVE) in a similar patient population.
Patients and Methods
Tumor resectability and morbidity/mortality rates were reviewed for 144 consecutive liver tumor patients with future liver remnant to body weight ratios (LR/BW) less than 0.5%. All patients were referred for preoperative percutaneous right plus segment IV PVE using embolic microspheres, with planned reassessment of the LR/BW 30 days after PVE. Post-PVE outcomes were compared to reported outcomes for ALPPS.
Results
Percutaneous PVE was successfully performed in 141 of the 144 study patients (97.9%). Adequate regeneration was observed in 139 patients (98.5%) with median post-PVE LR/BW rising from 0.33% to 0.52% (p<0.0001), representing a per-patient median regeneration of 62% (range: 0.3 – 379%). In total, 104 patients underwent extended right hepatectomy (n=102) or right hepatectomy (n=2). The remaining 40 patients (27.8%) were not resectable due to short-interval disease progression (27 patients, 18.5%), insufficient liver regeneration (5 patients, 3.5%), and medical comorbidities (8 patients, 5.6%). After resection, the following outcomes were observed: major morbidity: 33.0% (34/104), liver insufficiency: 12.5% (13/104), and 90-day liver-related mortality: 5.8% (6/104). These oncologic and technical results compare favorably to those of ALPPS.
Conclusion
Based on its ability to select oncologically resectable patients and superior safety and efficacy profiles, percutaneous right+segment IV PVE and interval surgery remains the standard of care for patients with very low future liver remnant volumes.
INTRODUCTION
Patients with extensive intrahepatic tumor involvement from primary or metastatic liver malignancy present significant challenges to hepatobiliary surgeons. In these cases, the safety of resection is dependent on the volume and function of the future liver remnant (FLR). Patients with inadequate future liver remnant volume and/or function are more likely to experience postoperative liver insufficiency and elevated mortality risk. Extensive clinical and translational research has determined minimum volume thresholds for FLR volume that minimize these risks. However, adequate functional tests of the FLR are lacking, leading to variability in outcomes for any given FLR volume.
For patients who require extensive liver resection, the most impactful advance within the field of hepatobiliary surgery in the last 20 years has been development of techniques that manipulate portal blood flow to induce hypertrophy of the future liver remnant. Initially accomplished with extrahepatic portal vessel ligation,1–4 these techniques have evolved toward interventional percutaneous approaches with microparticle and coil embolization of small and large intrahepatic portal branches.5–7 Depending on technique, these approaches are able to induce adequate contralateral liver hypertrophy to convert a majority of patients from unresectable situations to safe major hepatectomy candidates. Furthermore, we have learned that the rate and overall ability to hypertrophy in response to portal vein embolization (PVE) is an indicator of FLR function that is associated with the avoidance of postoperative liver insufficiency.8,9
To address patients with extensive liver malignancy and inadequate FLR volume, a European group recently reported safety and efficacy data for a short-interval 2-stage liver surgery technique consisting of initial open right portal vein ligation with in situ splitting of the liver parenchyma followed by re-exploration for right trisectionectomy, named Associating Liver Partition and Portal vein ligation for Staged hepatectomy or “ALPPS”.10 Proponents of this technique indicate that it produces rapid FLR hypertrophy and that it is better able to induce the substantial hypertrophy required in patients with very small FLR volumes [defined as a liver remnant to body weight ratio (LR/BW) ≤0.5%], compared to traditional PVE techniques. However, their report is notable for an elevated incidence of major morbidity (40%) and inpatient mortality (12%) associated with the ALPPS procedure. As the technique has disseminated to other centers even higher mortality rates (13–22%) have been observed.11–13
Given the issues regarding capacity to induce adequate regeneration in patients with very small FLR volumes and the risks associated with extended hepatectomy in patients with very low LR/BW, this study was designed to assess the FLR hypertrophy rates and subsequent outcomes for patients treated with percutaneous right plus segment IV PVE (rPVE+IV) using embolic microspheres followed by interval re-evaluation of FLR volume and resection. The aim of this study was to compare these outcomes to the recently reported outcomes for ALPPS-treated patients.
PATIENTS AND METHODS
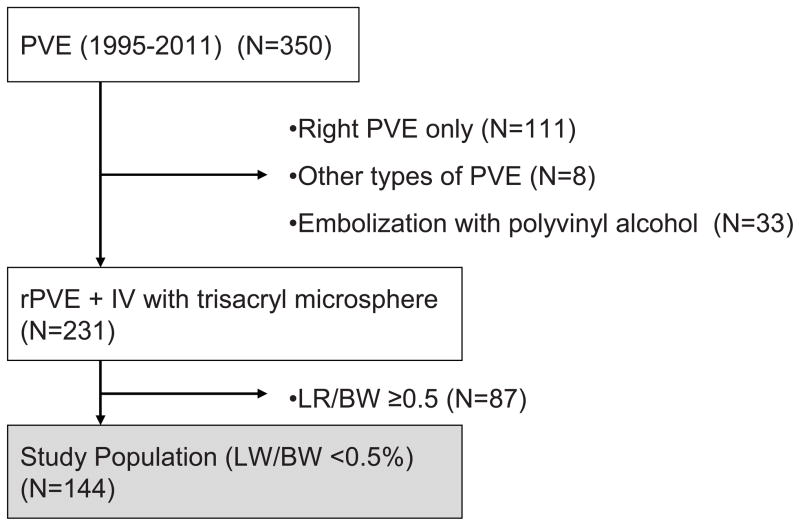
The Institutional Review Board of The University of Texas, MD Anderson Cancer Center, approved this study protocol (PA12-0575). From a prospective hepatobiliary database maintained by the Department of Surgical Oncology, 350 consecutive patients who underwent PVE between January 1993 and March 2013 were identified. Among these, 144 consecutive patients who underwent rPVE+IV for very low LR/BW were studied in detail (rPVE+IV group) (Figure 1). To evaluate the efficacy of rPVE+IV, these hypertrophy data along with short-term and mid-term postoperative outcomes were compared to those included in a recently published multicenter series of ALPPS-treated patients10 (ALPPS group).
Figure 1.
Study population
Pre-PVE liver volumetry, calculation of FLR volume, and PVE
All patients with potentially resectable disease underwent preoperative liver volumetry based on computed tomography (CT) imaging, and FLR volume was estimated according to the previously reported method.14 Standardized liver volume was calculated using the following formula: SLV = −794.41 + 1267.28 x body surface area (m2).15 PVE was considered when FLR was less than 20% in patients with normal liver, less than 30% in patients with evidence of fibrosis or liver injury, or less than 40% in patients with liver cirrhosis.16–18 All embolizations were performed by the ipsilateral percutaneous transhepatic approach using tris-acryl microspheres ranging in size from 100–700 microns and coils. Both right PVE and segment IV PVE were completed as previously described.5,14,19,20
Post-PVE liver volumetry and calculation of the contralateral hypertrophy
Planned post-PVE liver volume assessments were performed with 3-dimensional CT volumetry at 4 weeks after PVE. FLR hypertrophy was calculated using the following formula: [(Post-PVE FLR – Pre-PVE FLR) / (Pre-PVE FLR)] x 100. If hypertrophy at the first post-PVE volume assessment was insufficient, serial radiographic volumetric assessments were performed until the FLR volume was sufficient to permit resection. In these cases, systemic therapy was administered during this interval, if applicable.
Definitions of outcomes
Postoperative complications were classified using standard criteria, and major complications were defined as grade III or higher complications.21 Bile leaks were classified according to the definition of the International Study Group of Liver Surgery.22 Postoperative hepatic insufficiency was defined as a peak total bilirubin level greater than 7mg/dL and/or typical clinical manifestations of hepatic insufficiency, including massive ascites or encephalopathy.23 Postoperative mortality rates were calculated at 90 days after surgical resection.
Statistical analysis
Where the structure of the data permitted, comparison of hypertrophy and postoperative outcome data was made between the rPVE+IV group and the ALPPS group with standard statistical methods using IBM SPSS software (ver19.0. SPSS Inc., IL, USA). Although the FLR calculation algorithm used to make treatment decisions for our study patients was based on a formula that is standardized to body surface area, inorder to make comparisons with ALPPS reports, the LW/BW ratio was used to select patients for this study and to estimate FLR volume changes in this analysis. Variables with continuous values are reported as medians with ranges and were compared using the Mann-Whitney U test. Variables with categorical values were compared using Pearson’s chi-squared test or Fisher’s exact test, as appropriate. Overall survival was measured from the date of PVE until the date of death or the date of last clinical follow-up. Recurrence-free survival was measured from the date of hepatic resection until the date of radiographic detection of recurrence or last clinical follow-up. Survival curves were generated using the Kaplan-Meier method and compared using log-rank tests. All statistical tests were two-sided, and significance was set at P < .05.
RESULTS
Demographic and clinical characteristics of percutaneous right plus segment IV PVE patients
Demographic and clinical characteristics of the 144 percutaneous rPVE+IV patients are summarized in Table 1. Median age was 58 (range: 33–79 years) and 106 (74%) patients were male. Colorectal liver metastasis was the most common tumor type (91, 63%) and 94 (66%) patients received preoperative chemotherapy. Of the 144 percutaneous rPVE+IV patients, 32 (22%) were performed following a first stage partial left hepatectomy as part of a 2-stage hepatectomy approach as previously described24, and in the remaining 112 cases percutaneous PVE was performed prior to intended single-stage hepatic resection.
Table 1.
Background characteristics of 144 percutaneous plus segment IV PVE patients
| Age | 58 (33–79) |
| Gender, male (%) | 38 (26) |
| Diagnosis (%) | |
| Colorectal liver metastases | 91 (63) |
| Hepatocellular carcinoma | 14 (10) |
| Neuroendocrine tumor | 14 (10) |
| Hilar cholangiocarcinoma | 8 (6) |
| Intrahepatic cholangiocarcinoma | 4 (3) |
| Gallbladder cancer | 3 (2) |
| Others | 10 (7) |
| HBs-Ag positive (%) | 1 (0.7) |
| HCV-Ab positive (%) | 1 (0.7) |
| Diabetes | 20 (14) |
| Fibrosis* (%) | |
| F0–4 | 13 (11) |
| F5–6 | 1 (0.7) |
| Steatosis (%) | |
| None or minimal (<5%) | 95 (68) |
| Mild (5–33%) | 37 (27) |
| Moderate (33–66%) | 5 (4) |
| Severe (>66%) | 2 (1) |
| History of chemotherapy | |
| Yes (%) | 94 (66) |
| Duration, median (range) | 16 weeks (2–96 weeks) |
| PVE after 1st stage partial hepatectomy | 32 (22) |
Figures represent median (range) unless indicated.
Ishak score27.
Abbreviations. PVE, portal vein embolization; HBs-Ag, hepatitis B surface antigen; HCV-Ab, anti-hepatitis C antibody
Efficacy of PVE: FLR hypertrophy rates
Percutaneous rPVE+IV was attempted in 144 patients and completed in 141 patients (97.9%), with 3 patients unable to proceed due to decreases in left portal venous flow during the procedure. In addition, 2 patients developed postprocedure portal flow abnormalities that inhibited hypertrophy, yielding an overall technical success rate of PVE of 96.5% (139/144). In the remaining 139 patients, adequate increase in FLR was observed from a preprocedure mean LR/BW of 0.33% (range: 0.13–0.49%) to a postprocedure mean LR/BW of 0.52% (range: 0.18–1.03%) (P<0.0001). On a per-patient basis this represented a final median hypertrophy increase above pre-PVE values of 62% (0.3–379%) at 34 days (range: 12–385 days) (Figure 2).
Figure 2.
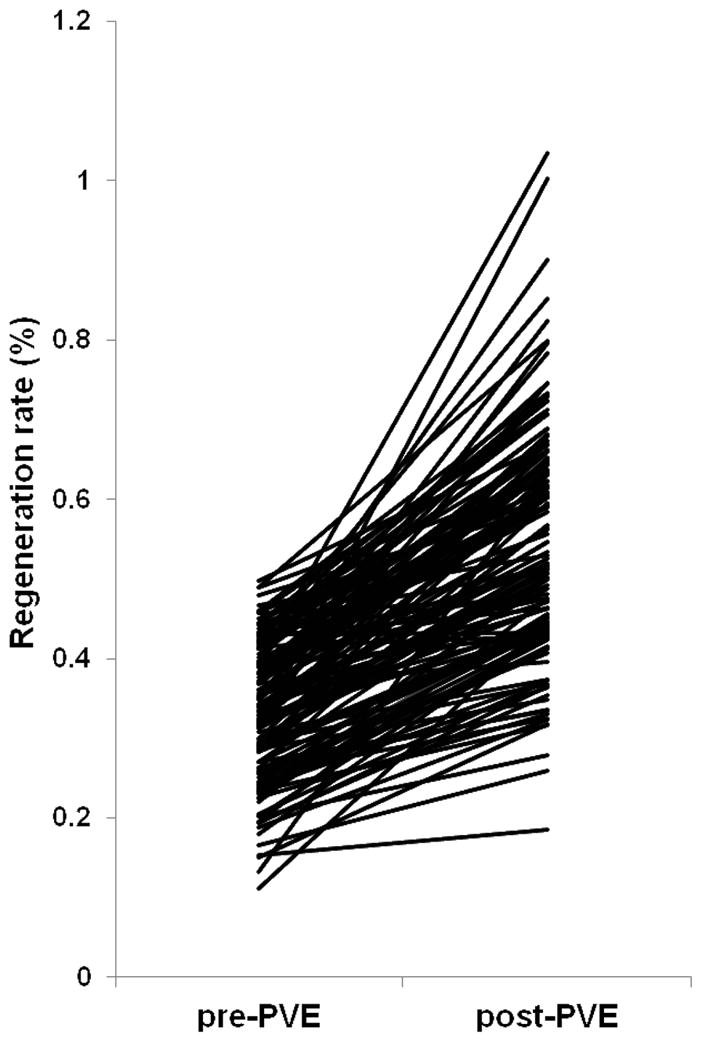
Increase in future liver remnant – body weight ratio (LR/BW) after right and segment 4 portal vein embolization for patients with LR/BW <0.5 (n = 139)
Resectability and surgical outcomes
Among the 144 patients with attempted percutaneous right plus segment IV PVE, 104 patients (72.2%) achieved curative extended right hepatectomy (n=102) or right hemihepatectomy (n=2) using a standardized technique.25 The remaining 40 patients (27.8%) were not resectable due to short-interval disease progression (27 patients, 18.5%) which was diagnosed before surgery (n=24) or at laparotomy (n=3), insufficient liver regeneration (5 patients, 3.5%), medical comorbidities or severe chemotherapy-associated injury to the underlying liver (8 patients, 5.6%) (Figure 3). Of the 27 patients who were not resected due to short-interval disease progression, 17 had developed extrahepatic disease and the remaining 10 patients had developed new lesions in the FLR. For the 102 resected patients, the overall morbidity rate was 57.7% (60/104) including 34 patients (33.0%) who experienced a major morbidity. Postoperative hepatic insufficiency occurred in 13 patients (12.5%) and the 90-day liver-related mortality rate was 5.8% (6/104). The overall 90-day mortality rate was 8.6% (9/104).
Figure 3.
Resectability after right and segment 4 portal vein embolization
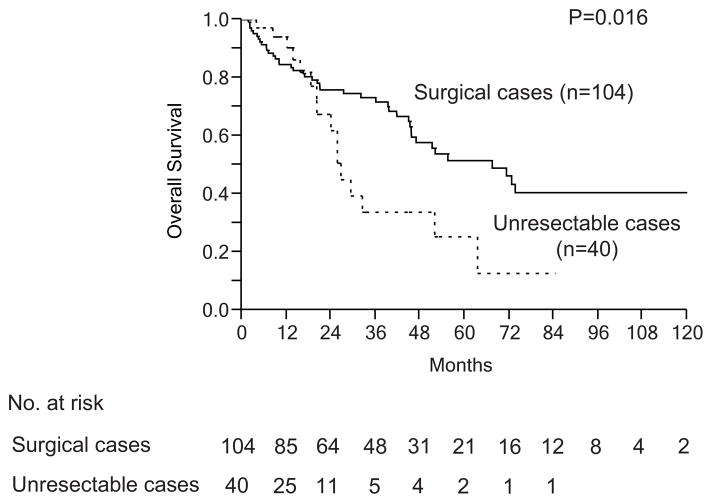
At a median clinical follow-up interval of 43.0 months (range: 1–127 months), the 1-year and 3-year overall survival rates and median overall survival time from the date of PVE was determined to be 86.0%, 72.8% and 67.4 months, respectively (Figure 4). In addition, patients who received PVE but did not proceed to surgery experienced relatively positive long-term outcomes with a median survival of 24 months. Although not reported in the Schnitzbauer paper, these survivals are likely to compare favorably to the survival of ALPPS treated patients who do not reach the second stage resection procedure.
Figure 4.
Long-term outcomes after right and segment 4 portal vein embolization
Overall survival from PVE (curative resection, n=104 vs. unresectable cases, n=40)
Efficacy and safety of percutaneous right plus segment IV PVE compared to the ALPPS approach
Clinical and demographic characteristics, including the percentage of patients that required a left liver resection, were equivalent between the two groups except for a clinically insignificant disproportion in patient gender (Table 2). With regard to the degree of hypertrophy achieved with each approach, the ALPPS FLR volume increase median (74%) and range (21% to 192%) were equivalent to the observed median and range FLR volume increase following rPVE+IV of 62% and (range: 0.3% to 379%). In contrast, there were clear differences in postoperative morbidity and mortality rates between the two groups. Specifically, the incidences of bile leak (grade B or C), sepsis, and relaparotomy for postoperative complication were considerably higher with the ALPPS approach group (24%, 20%, and 28%, respectively) compared to the rPVE+IV group (5.8%, 0%, and 2.9%, respectively). In addition, rates of overall morbidity, major morbidity, and liver-related mortality were higher in patients treated with the ALPPS approach.
Table 2.
Comparison of clinical results between percutaneous PVE and ALPPS
| rPVE+IV (n = 144) | ALPPS Approach (n = 25) | P | |
|---|---|---|---|
| Age (median) | 58 (33–79) | 63 (32–75) | - |
| Gender (male, %) | 106 (74) | 14 (56) | 0.02 |
| Diagnosis (%) | |||
| Colorectal liver metastases | 91 (63) | 14 (56) | 0.91 |
| Hepatocellular carcinoma | 14 (10) | 3 (12) | |
| Neuroendocrine tumor | 14 (10) | 0 (0) | |
| Hilar cholangiocarcinoma | 8 (6) | 2 (8) | |
| Intrahepatic cholangiocarcinoma | 4 (3) | 2 (8) | |
| Gallbladder cancer | 3 (2) | 1 (4) | |
| Others | 10 (7) | 3 (12) | |
| History of Chemotherapy (%) | 94 (66) | 12 (48) | 0.10 |
| Duration of chemotherapy (weeks) | 16 (2–96) | 19 (4–76) | 0.57 |
| FLR volume before PVE or ALPPS (mL) | 275 (135–541) | 310 (197–444) | - |
| FLR volume after PVE or ALPPS (mL) | 426 (218–1028) | 536 (273–881) | - |
| Pure volume increase (mL) | 162 (1–636) | 225 (54–490) | - |
| LR/BW before PVE or ALPPS | 0.33% (0.13–0.49%) | 0.38% (0.25–0.49%) | - |
| LR/BW after PVE or ALPPS | 0.52% (0.18–1.03%) | 0.61% (0.35–0.95%) | - |
| FLR regeneration rate | 62% (0.3–379%) | 74% (21–192%) | - |
| Time interval from PVE or ALPPS to final volume measurement (days) | 34 (12–385) | 9 (5–28) | - |
| Concomitant left partial hepatectomy | 16/104 (16) | 8/25 (32) | 0.10 |
| Morbidity | |||
| Any | 60/104 (57.7) | 16/25 (64.0) | 0.73 |
| Major | 34/104 (32.7) | 10/25 (40.0) | 0.49 |
| Bile leak (Grade B or C) | 6/104 (5.8) | 6/25 (24.0) | 0.01 |
| Postoperative hemorrhage | 2/104 (1.9) | 3/25 (7.5) | 0.08 |
| Sepsis | 0/104 (0) | 5/25 (20.0) | <0.0001 |
| Relaparotomy due to complication | 3/104 (2.9) | 7/25 (28) | 0.0001 |
| Liver related mortality* | 6/104 (5.8) | 3/25 (12.0) | 0.51 |
Figures represent median (range) unless indicate.
90-day mortality for MDACC Approach and in hospital death for ALPPS approach.
Abbreviations. PVE, portal vein embolization; FLR, future liver remnant; LR/BW, future liver remnant - body weight ratio
DISCUSSION
In this study, we analyzed the efficacy and clinical outcomes of percutaneous right plus segment IV PVE in a large cohort of patients with very low FLR volume (LR/BW<0.5). Systematic percutaneous embolization of right and segment IV portal vein branches using embolic microspheres and coils was accomplished in 97.9% of candidates and yielded sufficient FLR growth in 96.5% with a per-patient median hypertrophy rate of 62%. These rates are comparable to the FLR hypertrophy rates associated with the ALPPS approach, contradicting the claim that ALPPS is the only therapeutic option for patients with very small anticipated FLR volumes.
This analysis also identified significant differences in morbidity and mortality rates following each approach. These data are very important to hepatobiliary surgeons and patients with extensive bilateral liver tumor involvement. As there appears to be equivalent efficacy regarding the degree of FLR hypertrophy achievable with each approach, it is apparent that this group of patients can achieve curative resectability with techniques that avoid the elevated risks associated with the ALPPS approach.
We are aware that it is challenging to compare our mature experience with percutaneous right plus segment IV PVE to the initial report of the ALPPS experience. However, with specific regard to the comparison of degree of hypertrophy between the two approaches we feel this is a valid comparison. Our data are reproducible and equivalent to the ALPPS hypertrophy data. With regard to clinical outcomes, we recognize that the early experience with any radical technique may be associated with elevated morbidity and mortality rates. We know that already several modifications to patient selection (limiting the procedure to patients under age 70 [personal communication, Pierre Alain Clavien, MD]) and surgical technique (abandoning right bile duct ligation [personal communication, Rene Adam, MD]) have been made in response to center specific high morbidity and mortality rates. Some of these modifications, however, such as the elimination of right bile duct ligation due to excessive severe biliary complications, may lessen the effectiveness of the FLR hypertrophy achievable with ALPPS, further favoring the percutaneous PVE and interval surgery strategy.
With regard to FLR hypertrophy, the major existing difference between the two techniques is the timing of the major hepatectomy. With the ALPPS approach, reoperation is performed at a short-interval from the portal vein manipulation (median: 9 days, range: 5–28 days). This short interval is mandated by the in situ split portion of the procedure that leaves segment IV ischemic and at risk for liver abscess. This short interval has some advantages in that, barring complications, the patient will have a single hospitalization that may be preferable to a longer interval between procedures with additional admissions. On the other hand, from an oncological perspective, we find value in this waiting period when the patient can clear any existing infections, can improve their performance status prior to major hepatectomy and can be reassessed for the development of malignant disease progression.
Patients who are candidates for either approach have locally advanced malignancy, and are by definition at high risk for malignant disease progression at all points during the treatment strategy. One of the proposed benefits of ALPPS is the ability to remove the tumor(s) approximately 4–6 earlier than preoperative PVE followed by resection, theoretically preventing patient drop-out due to rapid disease progression of existing liver tumors. Although 40 patients (28%) in the rPVE+IV group were not resected, this was mainly due to short-interval progression of their malignant disease (27/144, 19%). The majority of short interval recurrences were observed at extrahepatic locations, with fewer recurrences noted in the liver. However, the overall patterns of recurrence indicate that no patients were lost to resectability due only to progression of existing intrahepatic disease. These data suggest that the theoretical ALPPS benefit of rapid tumor removal to prevent patient dropout due to progression of existing disease is unlikely to be clinically or oncologically relevant.
The oncologic value of the staged approach with percutaneous PVE is further emphasized by the recently published ALPPS series that identified a 20% recurrence rate at only 187 days of postoperative follow-up.26 Given the favorable long-term outcomes observed in the 104 percutaneous PVE and resection patients, with a 3-year survival rate of 72.8% and median survival of 67.4 months, we view the time interval between PVE and resection not as a negative, but as a positive feature that allows us to select oncologically appropriate candidates for major hepatectomy (Figure 4).
Similar to the oncologic concerns, the magnitude of hepatectomy in both approaches is extreme and carries a certain level of inherent risk. Indeed, the reported mortality using the preoperative PVE followed by surgery approach was not zero. Of the nine postoperative mortalities reported in this series, six were related to postoperative liver failure, and three were independent of liver causality. All six patients who experienced liver failure and death had marginal liver remnant hypertrophy after PVE with kinetic FLR growth rates (KGR) less than 2% per week.9 Since inclusion of the KGR into our multifactorial decision making process for selection of patients for hepatic resection (2010 to present), no posthepatectomy deaths have occurred in preoperative PVE-treated patients. These data underscore our current understanding that both the rate of hypertrophy and the degree of hypertrophy induced by PVE are important determinants of postoperative FLR function. Examination of the degree of hypertrophy curves from both approaches indicates that each has a number of ‘slow growers’ who do not respond to the initial intervention, emphasizing that another major benefit of the preoperative PVE followed by surgery strategy is a period of hypertrophy evaluation that allows for selection of those patients with the regenerative potential to safely support radical hepatectomy.
In conclusion, for patients with extensive intrahepatic malignancy and a very small FLR, percutaneous right plus segment IV PVE offers both sufficient FLR hypertrophy and acceptable surgical outcomes. Based on its ability to select oncologically resectable patients and its superior safety compared to the ALPPS approach, percutaneous right plus segment IV PVE followed by extended right hepatectomy remains the standard of care for patients with very low FLR volumes.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672
Footnotes
Disclosure information: Nothing to disclose.
Presented at the Western Surgical Association 120th Scientific Session, Colorado Springs, CO, November 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bax HR, Mansens BJ, Schalm L. Atrophy of the liver after occlusion of the bile ducts or portal vein and compensatory hypertrophy of the unoccluded portion and its clinical importance. Gastroenterology. 1956 Aug;31(2):131–155. [PubMed] [Google Scholar]
- 2.Honjo I, Suzuki T, Ozawa K, Takasan H, Kitamura O. Ligation of a branch of the portal vein for carcinoma of the liver. American journal of surgery. 1975 Sep;130(3):296–302. doi: 10.1016/0002-9610(75)90389-x. [DOI] [PubMed] [Google Scholar]
- 3.Rous P, Larimore LD. Relation of the Portal Blood to Liver Maintenance : A Demonstration of Liver Atrophy Conditional on Compensation. The Journal of experimental medicine. 1920 Apr 30;31(5):609–632. doi: 10.1084/jem.31.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayasu K, Muramatsu Y, Shima Y, Moriyama N, Yamada T, Makuuchi M. Hepatic lobar atrophy following obstruction of the ipsilateral portal vein from hilar cholangiocarcinoma. Radiology. 1986 Aug;160(2):389–393. doi: 10.1148/radiology.160.2.3014598. [DOI] [PubMed] [Google Scholar]
- 5.Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005 Feb;16(2 Pt 1):215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 6.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990 May;107(5):521–527. [PubMed] [Google Scholar]
- 7.Nagino M, Kamiya J, Kanai M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000 Feb;127(2):155–160. doi: 10.1067/msy.2000.101273. [DOI] [PubMed] [Google Scholar]
- 8.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007 Nov;94(11):1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 9.Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013 Feb;216(2):201–209. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012 Mar;255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 11.Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013 Feb;100(3):388–394. doi: 10.1002/bjs.8955. [DOI] [PubMed] [Google Scholar]
- 12.Dokmak S, Belghiti J. Which limits to the “ALPPS” approach? Ann Surg. 2012 Sep;256(3):e6. doi: 10.1097/SLA.0b013e318265fd64. author reply e16–17. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in Right Trisectionectomy: a Safe Procedure to Avoid Postoperative Liver Failure? J Gastrointest Surg. 2012 doi: 10.1007/s11605-012-2132-y. [DOI] [PubMed] [Google Scholar]
- 14.Truty MJ, Vauthey JN. Uses and limitations of portal vein embolization for improving perioperative outcomes in hepatocellular carcinoma. Semin Oncol. 2010 Apr;37(2):102–109. doi: 10.1053/j.seminoncol.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2002 Mar;8(3):233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 16.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002 Jun;137(6):675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 17.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004 May;239(5):722–730. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Annals of surgery. 2000 Nov;232(5):665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology. 2003 Apr;227(1):251–260. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 20.Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008 Nov;144(5):744–751. doi: 10.1016/j.surg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery. 2004 Aug;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011 May;149(5):680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007 May;204(5):854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862–854. [DOI] [PubMed] [Google Scholar]
- 24.Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: Response-based selection and complete resection define outcome. J Clin Oncol. 2011;29(8):1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005 Aug;242(2):172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sala S, Ardiles V, Ulla M, Alvarez F, Pekolj J, de Santibanes E. Our initial experience with ALPPS technique: encouraging results. Updates in surgery. 2012 Sep;64(3):167–172. doi: 10.1007/s13304-012-0175-y. [DOI] [PubMed] [Google Scholar]
- 27.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995 Jun;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]