Abstract
Recent studies have revealed the presence of TMPRSS2-ERG gene fusion in both primary and metastatic prostatic cancers (PCAs). However, the relationship between primary and corresponding metastatic PCAs with respect to the status of this gene fusion remains unclear. Using fluorescence in situ hybridization, we evaluated the rearrangement of the ERG gene in the radical prostatectomy (RP) specimens and corresponding lymph node metastases from 19 patients with PCA. The mean age of the patients was 61 years and the median Gleason score in the RP specimens was 7 (4+3). PCA was unifocal in 6 cases and multifocal in 13 cases, including 10 with 2 foci and 3 with 3 foci. In the primary PCAs, rearrangement of the ERG gene was observed in 13 cases and associated with deletion of the 5’ ERG gene in 8 cases. In the metastases, the ERG rearrangement was present in 10 cases and associated with deletion of the 5’ ERG gene in 6 cases. In unifocal PCAs, the status of the ERG rearrangement was concordant between the primary PCA and metastasis in 5 of 6 cases. In multifocal PCA, despite a significant interfocal discordance, the status of the ERG rearrangement was concordant between the index (largest) primary tumor focus and metastasis in all 13 cases. Our study demonstrates a close relationship of the TMPRSS2-ERG gene fusion status between primary and metastatic PCA. The concordance of the ERG gene rearrangement status between the index primary tumor focus and metastasis suggests that metastasis most likely arises from the index tumor focus in multifocal PCA.
Keywords: TMPRSS2-ERG gene fusion, prostate cancer, metastasis, multifocality
Introduction
Recent studies have demonstrated a unique recurrent gene fusion in most prostate cancers (PCAs).1-3 This gene fusion is characterized by fusion of the 5’ region of the TMPRSS2 gene with the 3’ region of the ERG gene. TMPRSS2 is regulated by androgen in the prostate,4 and ERG, a member of the ETS family, is an oncogene.5 The TMPRSS2-ERG gene fusion leads to aberrant function of ERG in the prostate, which is believed to be involved in the oncogenesis of PCA.1 Other members of the ETS family, such as ETV1, ETV4 and ETV5, also fuse with the 5’ end of TMPRSS2, although at a much lower frequency.6,7 Likewise, other genes, such as SLC45A3 and NDRG1, may fuse with the 3’ region of ETS.8,9 Nonetheless, the TMPRSS2-ERG gene fusion accounts for the majority of recurrent gene fusions in PCA.10
The TMPRSS2-ERG gene fusion is also highly prevalent in metastatic PCA.11,12 Mehra et al found that all the TMPRSS2-ERG gene fusion in metastatic PCA was associated with deletion of the 5’ end of ERG gene.11 Furthermore, multiple metastases from an individual patient shared the same pattern of TMPRSS2-ERG gene fusion, suggesting that metastases developed as a result of clonal expansion of a single focus of primary PCA. However, the relationship of TMPRSS2-ERG gene fusion between primary and metastatic PCA remains unclear. In the current study, we compared the gene fusion status (presence or absence) between primary PCA in radical prostatectomy specimens and their corresponding metastases in lymph nodes.
Materials and methods
Case Selection and Pathologic Evaluation
With the approval of Institutional Review Board (IRB), we retrospectively searched our pathology file at The University of Texas MD Anderson Cancer Center and selected 19 patients who underwent radical prostatectomy (RP) and pelvic lymph nodes dissection from 2003 to 2009. All patients had primary PCA in the RP specimens and metastatic PCA in the pelvic lymph nodes. No patient had received treatment for PCA before RP. Pathologic features were collected from histologic examination of the specimens. In the RP specimens, each tumor focus was graded according to the Gleason grading system, and the tumor volume was calculated using a previously described formula.13 Patients’ demographic and clinical features were obtained from medical records.
Determination of the TMPRSS-ERG Gene Fusion
To evaluate the TMPRSS2-ERG gene fusion in the primary and metastatic PCA, we analyzed the rearrangement of the ERG gene with break-apart fluorescence in situ hybridization (FISH). The break-apart probes, a rhodamine-labeled 5′ ERG probe (BAC RP11-95I21) and a fluorescein isothiocyanate-labeled 3′ ERG probe (BAC RP11-476D17), were obtained from Children’s Hospital of Oakland Research Institute (Oakland, CA). Tissue pretreatment was performed using Paraffin Pretreatment Kit I (Vysis, Des Plaines, IL), and hybridization and washing were performed using Vysis hybridization reagents, following the manufacturer’s protocols. The specificity and quality of the probes were confirmed by hybridization to the metaphase spread of normal peripheral lymphocytes. A mean of 100 cells was evaluated per tumor focus.
Results
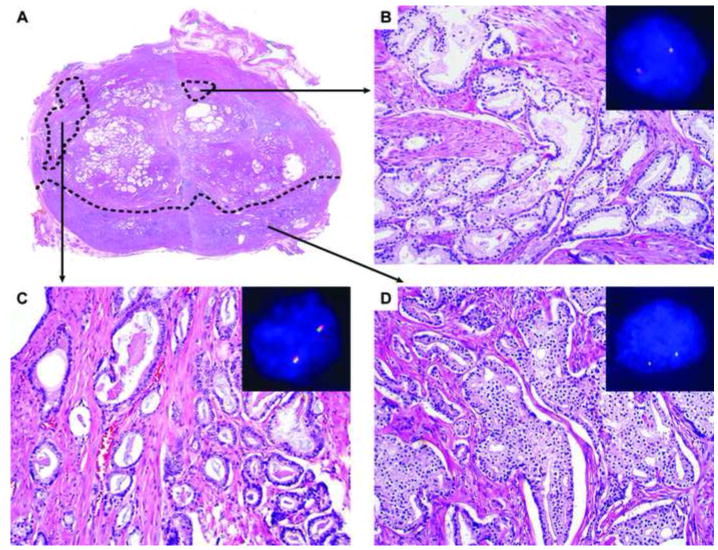
The average age of patients was 61 years (range 43-76 years). In the RP specimens, the PCA was unifocal in 6 cases and multifocal in 13 cases (Table 1). In the RP specimens, 23 tumor foci were in the peripheral zone and 8 foci were in the transition zone. Two tumor foci involved multiple zones and its zonal origin was considered undetermined. The RPs with unifocal PCA had a median Gleason score 9 (4+5) (range 7-9) and a mean tumor volume was 7.45 cm3 (range 2.80-12.16 cm3). The multifocal PCA included 2 foci in 10 cases and 3 foci in 3 cases (Figure 1A). In the RPs with multifocal PCA, the index (largest) tumor focus had a mean volume of 3.80 cm3 (range 1.44 10.08 cm3) with a median Gleason score of 7 (4+3; range 7-9), and the secondary tumor foci had a mean volume of 0.27 cm3 (range 0.01-2.16 cm3) with a median Gleason score of 6 (3+3) (range 6-7). While the secondary tumor foci (n=16) were located in either the transition zone (n=8; Figure 1B) or peripheral zone (n=8; Figure 1C), all the index tumor foci were located in the peripheral zone (Figure 1D). All patients received dissection of the pelvic lymph nodes, and a mean number of 14 (range 4-41) lymph nodes were removed. Metastatic PCA was present in all cases and a mean number of positive lymph nodes was 2 (range 1-6) (Figure 2).
Table 1.
Summary of pathologic features and TMPRSS2-ERG gene fusion status in primary and metastatic prostate cancer
| Case No. | Metastasis gene fusion status | Primary tumor
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index tumor | Secondary tumor #1 | Secondary tumor #2 | |||||||||||
|
| |||||||||||||
| GS | Vol. (cm3) | Fusion status | Zone | GS | Vol. (cm3) | Fusion status | Zone | GS | Vol. (cm3) | Fusion status | Zone | ||
| 1 | Trans. | 5+4 | 3.36 | Trans. | PZ | ||||||||
| 2 | Neg. | 4+3 | 5.60 | Del. | PZ | ||||||||
| 3 | Trans. | 4+5 | 12.16 | Trans. | UZ | ||||||||
| 4 | Del. | 4+5 | 2.80 | Del. | PZ | ||||||||
| 5 | Trans. | 4+5 | 9.60 | Trans. | UZ | ||||||||
| 6 | Neg. | 4+3 | 11.20 | Neg. | PZ | ||||||||
| 7 | Neg. | 4+3 | 2.00 | Neg. | PZ | 3+3 | 0.17 | Trans. | PZ | ||||
| 8 | Neg. | 4+3 | 2.87 | Neg. | PZ | 3+3 | 0.08 | Neg. | PZ | ||||
| 9 | Del. | 4+5 | 4.20 | Del. | PZ | 3+3 | 0.01 | Neg. | PZ | ||||
| 10 | Trans. | 3+4 | 1.44 | Trans. | PZ | 3+4 | 0.35 | Neg. | PZ | ||||
| 11 | Del. | 4+3 | 1.92 | Del. | PZ | 3+4 | 0.50 | Del. | TZ | ||||
| 12 | Del. | 4+5 | 10.08 | Del. | PZ | 3+3 | 0.01 | Neg. | TZ | ||||
| 13 | Neg. | 4+3 | 1.92 | Neg. | PZ | 3+3 | 0.05 | Neg. | TZ | ||||
| 14 | Del. | 4+5 | 4.00 | Del. | PZ | 3+3 | 0.03 | Del. | PZ | ||||
| 15 | Neg. | 4+4 | 6.57 | Neg. | PZ | 3+4 | 2.16 | Neg. | TZ | ||||
| 16 | Neg. | 4+5 | 2.52 | Neg. | PZ | 4+3 | 0.11 | Neg. | PZ | ||||
| 17 | Del. | 4+5 | 2.04 | Del. | PZ | 3+3 | 0.13 | Neg. | PZ | 3+3 | 0.01 | Neg. | TZ |
| 18 | Neg. | 4+3 | 3.20 | Neg. | PZ | 3+3 | 0.12 | Neg. | TZ | 3+3 | 0.10 | Neg. | TZ |
| 19 | Neg. | 4+5 | 7.20 | Neg. | PZ | 3+3 | 0.44 | Del. | PZ | 3+3 | 0.03 | Neg. | TZ |
Del., ERG gene rearrangement associated with deletion; GS, Gleason score; Neg., negative for ERG gene rearrangement; PZ, peripheral zone; Trans., ERG gene rearrangement associated with translocation; TZ, transition zone; UZ, undetermined zone; Vol., volume.
Figure 1.
The TMPRSS2-ERG gene fusion in primary prostate cancer. Three separate tumor foci, two in the peripheral zone and one in the transition zone, are present in a cross-section of the prostate (A). The tumor focus in the transition zone had normal ERG arrangement (B). The smaller tumor focus in the peripheral zone has normal ERG arrangement (C). The larger (index) tumor focus in the peripheral zone shows ERG rearrangement with deletion of the 5’ ERG gene (D).
Figure 2.
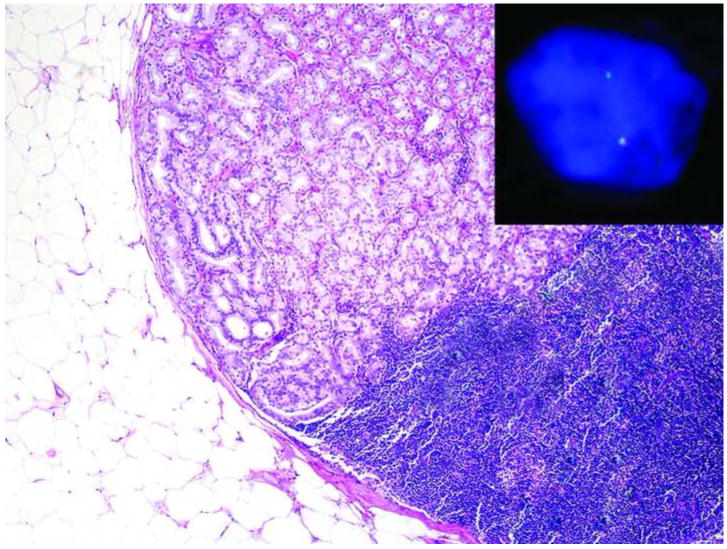
The TMPRSS2-ERG gene fusion in metastatic prostate cancer. Metastatic prostate cancer in a lymph node shows ERG rearrangement with deletion of the 5’ ERG gene.
The rearrangement of the ERG gene was evaluated in all tumor foci of each RP specimen using the break-apart probes (Table 1). In FISH analysis, the normal pattern of the ERG gene showed 2 pairs of colocalized green and red signals in the nuclei (Figure 1B and 1C), rearrangement of the ERG gene was characterized by only 1 pair of colocalized green and red signals in the nuclei (Figure 1 D). The other pair in the ERG rearrangement was broken apart, resulting in deletion or translocation of the red signal (the 5’ ERG gene). In 5 of the 6 patients with unifocal PCA, ERG rearrangement was observed. In 8 of the 13 patients with multifocal PCA, ERG rearrangement was present either in the index tumor focus only (n=4), a secondary tumor focus only (n=2) or both (n=2).
The rearrangement of the ERG gene was also evaluated in metastatic PCA in the lymph nodes. The ERG rearrangement was observed in 10 cases, and the rearrangement was associated with deletion of the 5’ ERG gene in 6 cases (Figure 2).
The status of the ERG gene rearrangement was compared between the primary tumor foci and metastasis in each patient. Among the 6 cases of unifocal PCA, there was a concordance of the ERG gene rearrangement status between the primary PCA and metastasis in 5 cases, including rearrangement in 4 cases and no rearrangement in 1 case. Among the 13 cases of multifocal PCA, although 6 cases showed an interfocal heterogeneity of the ERG gene rearrangement status, all cases showed a concordance of the ERG gene rearrangement status between the primary index tumor focus and the metastasis.
Discussion
Our study demonstrates a close relationship between primary and metastatic PCA with respect to the status of TMPRSS2-ERG gene fusion. Like primary PCA in our study, the majority of metastatic prostate cancers carried the TMPRSS2-ERG gene fusion, which was associated with either deletion or translocation of the 5’ ERG gene. Furthermore, we compared the gene fusion status between the metastasis and its corresponding primary PCA in the RP specimen. In patients with unifocal disease in the RP specimen, the TMPRSS2-ERG gene fusion status was concordant between the primary tumor and metastasis in most cases. In patients with multifocal disease in the RP specimen, although there was significant interfocal heterogeneity of TMPRSS2-ERG gene fusion status in the primary PCA, the TMPRSS2-ERG gene fusion status was concordant between the index tumor focus and the metastatic disease, suggesting that the metastatic disease likely originated from the index tumor focus.
Since the discovery of TMPRSS2-ERG gene fusion in PCA, there has been considerable interest in the clinical significance of this gene fusion. Although some studies have reported that the presence of TMPRSS2-ERG gene fusion in PCA is associated with an unfavorable prognosis,15,16 findings from other studies have not confirmed this association.17,18 Recently, Attard et al17 reported that duplication of TMPRSS2-ERG gene fusion is associated with a significantly worse prognosis, a finding that has been supported by a subsequent study 18; however, this duplication is not specific to the TMPRSS2-ERG locus and likely reflects a general increase in chromosome copy number due to genetic instability in aggressive PCA.18 Therefore, TMPRSS2-ERG gene fusion is likely an early event in PCA oncogenesis but may not be a major factor in determining the patient’s outcome.19,20
The majority of primary PCAs are multifocal.13 Separate tumor foci are likely to arise independently in the same prostate gland, as evidenced by discordant patterns of allelic loss among various tumor foci.21 Several groups have attempted to study TMPRSS2-ERG gene fusion in multifocal PCAs.22,23 Although the TMPRSS2-ERG gene fusion status usually demonstrates an intrafocal homogeneity, this status in multifocal PCA demonstrates a significant interfocal heterogeneity. Previous studies found that about 40-50% of RP specimens with multifocal PCA showed discordant TMPRSS2-ERG gene fusion status.22,23 In our study, 6 of 13 (46%) multifocal PCAs also showed an interfocal heterogeneity of the TMPRSS2-ERG gene fusion status, supporting that different tumor foci in the same prostate gland may arise independently from clonal expansion.
In a previous study, we found that TMPRSS2-ERG gene fusion is associated with the zonal origin of PCAs.24 While this gene fusion is prevalent in PCAs arising from the peripheral zone, it is uncommon in PCAs arising from the transition zone. In the current study, only 1 of 8 (12.5%) PCAs of the transition zone showed ERG rearrangement. In contrast, 12 of 25 (48%) PCAs of the peripheral zone showed ERG rearrangement. Therefore, the findings of the current study support our previous observation that the TMPRSS2-ERG gene fusion in the transition zone is significantly less common than in the peripheral zone, although it may not be completely absent.
Several studies have demonstrated the presence of TMPRSS2-ERG gene fusion in the majority of metastatic PCA.11,12,25 Using 30 metastatic tissue samples acquired by rapid autopsy tissue procurement, Mehra et al found several variants of TMPRSS2-EST gene fusion. The EST gene most commonly involved in gene fusion was ERG, followed by ETV1 and ETV4. Interestingly, they also found that TMPRSS2-ERG gene fusion in all metastases was associated with the deletion of the 5’ ERG gene. However, in the current study, although the majority of ERG rearrangement was associated with the deletion, the gene rearrangement in 5 cases was associated, instead, with translocation. Similarly, a study using circulating PCA cells from patients with castration-resistant metastatic PCA found that TMPRSS2-ERG gene fusion was associated with translocation of the 5’ ERG gene in 34% of the cases.25 Therefore, TMPRSS2-ERG gene fusion in metastatic PCA is not exclusively associated with the deletion of the 5’ ERG gene.
PCA metastases are often present in various anatomic locations, such as lymph nodes, bone, liver and lung. Several studies have attempted to investigate whether multifocal metastases arise from a single focus of primary PCA. 11,12,26 Using high-resolution genomewide analysis of single nucleotide polymorphisms and copy-number alterations, Liu et al found that most metastatic PCAs have monoclonal origins and maintain a unique signature copy-number pattern of the primary PCA.26 FISH analyses also demonstrated that various foci of metastatic PCA within an individual specimen harbored the same TMPRSS2-ERG gene fusion pattern. Despite a significant interfocal heterogeneity in multifocal primary PCA, multifocal metastatic PCA most likely arises from a single focus of primary PCA.11,12 These observations also imply that TMPRSS-ERG gene fusion is likely an early event and is not required for metastasis.
Perner et al12 also studied the relationship between the primary PCA and metastatic disease with respect to TMPRSS2-ERG gene fusion status. They found that there was generally concordance of the ERG gene rearrangement status between the index tumor focus in multifocal PCA and the metastasis, despite discordance in a small subset of cases; furthermore, when multifocal PCA contained a tumor focus with ERG rearrangement and another without the rearrangement, the metastatic disease showed the ERG gene rearrangement in all cases, suggesting that metastatic disease likely arose from the tumor focus with the ERG rearrangement. In the current study, we found concordance of the ERG rearrangement status between the index tumor focus and the metastasis in all cases of multifocal PCA, suggesting that the metastasis most likely arose from the index tumor focus. But it cannot be excluded that the metastasis may also arise from the secondary focus, particularly in the patients whose index and secondary tumor foci show similar pattern of the TMPRSS2-ERG gene fusion. Although metastatic PCA is not routinely given a Gleason score, we found that the metastatic PCA in the current study had a Gleason score of at least 8 (4+4). The high Gleason score in the metastatic PCA favors that it likely arises from the index tumor focus, as the index tumor focus had a higher Gleason score than the secondary tumor focus in all multifocal PCAs except for one case in our study. Six patients with multifocal PCA in our study contained one tumor focus with the ERG rearrangement and another without rearrangement. Four of the 6 patients showed rearrangement of the ERG gene in the metastases, and the other 2 patients did not have rearrangement in the metastases, despite that they had the ERG rearrangement in the primary tumor (both in the secondary focus), including one with deletion and the other with translocation. Thus the metastasis may also arise from the tumor focus without rearrangement of the ERG gene in multifocal PCA.
The current study has some limitations. Although the definition of index tumor focus in multifocal PCa was originally based on the tumor size, other criteria, such as Gleason score and tumor stage, may also be used to determine the index tumor focus in multifocal PCa.27 In the current study, we used the original definition (tumor size) to determine the index tumor. Thus our findings remain to be verified in the index tumors based on different criteria. In 7 cases of multifocal PCA, the ERG rearrangement status in the secondary tumor focus was similar to that in the index tumor focus, which raises the possibility that the metastasis may have arisen from the secondary tumor focus in these cases. In 1 case, the primary PCA had the gene fusion while the metastasis lacked it, probably because the large tumor focus resulted from the fusion of several separate tumor foci. Finally, the number of cases in our study was limited by the availability of specimens. Further study in a large cohort of patients may help to further elucidate the relationship of TMRPSS2-ERG gene fusion between primary and metastatic PCA.
Acknowledgments
This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
We have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 2.Clark J, Merson S, Jhavar S, et al. Diversity of TMPRSS2-ERG fusion transcripts in the human prostate. Oncogene. 2007;26:2667–2673. doi: 10.1038/sj.onc.1210070. [DOI] [PubMed] [Google Scholar]
- 3.Mehra R, Tomlins SA, Shen R, et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod Pathol. 2007;20:538–544. doi: 10.1038/modpathol.3800769. [DOI] [PubMed] [Google Scholar]
- 4.Lin B, Ferguson C, White JT, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 5.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 6.Tomlins SA, Mehra R, Rhodes DR, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396–3400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- 7.Helgeson BE, Tomlins SA, Shah N, et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008;68:73–80. doi: 10.1158/0008-5472.CAN-07-5352. [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, Laxman B, Dhanasekaran SM, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 9.Pflueger D, Rickman DS, Sboner A, et al. N-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancer. Neoplasia. 2009;11:804–811. doi: 10.1593/neo.09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah RB, Chinnaiyan AM. The discovery of common recurrent transmembrane protease serine 2 (TMPRSS2)-erythroblastosis virus E26 transforming sequence (ETS) gene fusions in prostate cancer: significance and clinical implications. Adv Anat Pathol. 2009;16:145–153. doi: 10.1097/PAP.0b013e3181a12da7. [DOI] [PubMed] [Google Scholar]
- 11.Mehra R, Tomlins SA, Yu J, et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68:3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perner S, Svensson MA, Hossain RR, et al. ERG rearrangement metastasis patterns in locally advanced prostate cancer. Urology. 2010;75:762–767. doi: 10.1016/j.urology.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ME, Johnston DA, Tang K, et al. Detailed mapping of prostate carcinoma foci: biopsy strategy implications. Cancer. 2000;89:1800–1809. doi: 10.1002/1097-0142(20001015)89:8<1800::aid-cncr21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 15.Demichelis F, Fall K, Perner S, et al. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 16.Lapointe J, Kim YH, Miller MA, et al. A variant TMPRSS2 isoform and ERG fusion product in prostate cancer with implications for molecular diagnosis. Mod Pathol. 2007;20:467–473. doi: 10.1038/modpathol.3800759. [DOI] [PubMed] [Google Scholar]
- 17.Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–263. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 20.Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6:429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L, Song SY, Pretlow TG, et al. Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst. 1998;90:233–237. doi: 10.1093/jnci/90.3.233. [DOI] [PubMed] [Google Scholar]
- 22.Barry M, Perner S, Demichelis F, Rubin MA. TMPRSS2-ERG fusion heterogeneity in multifocal prostate cancer: clinical and biologic implications. Urology. 2007;70:630–633. doi: 10.1016/j.urology.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehra R, Han B, Tomlins SA, et al. Heterogeneity of TMPRSS2 gene rearrangements in multifocal prostate adenocarcinoma: molecular evidence for an independent group of diseases. Cancer Res. 2007;67:7991–7995. doi: 10.1158/0008-5472.CAN-07-2043. [DOI] [PubMed] [Google Scholar]
- 24.Guo CC, Zuo G, Cao D, et al. Prostate cancer of transition zone origin lacks TMPRSS2-ERG gene fusion. Mod Pathol. 2009;22:866–871. doi: 10.1038/modpathol.2009.57. [DOI] [PubMed] [Google Scholar]
- 25.Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Kwast TH, Amin MB, Billis A, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol. 2011;24:16–25. doi: 10.1038/modpathol.2010.156. [DOI] [PubMed] [Google Scholar]