Abstract
Cilengitide (EMD121974) is a selective inhibitor of integrins αvβ3 and αvβ5. The αvβ3 promotes the proliferation of tumor-associated endothelial cells and potentially the survival of melanoma cells. We conducted a randomized phase II trial in patients with metastatic melanoma to evaluate the clinical efficacy of cilengitide. Patients with stage IV or unresectable stage III melanoma who were either chemo-naïve or who had previously received one systemic therapy were enrolled. Patients were randomly assigned to either 500 mg or 2,000 mg of cilengitide administered intravenously twice weekly. The primary objective of this study was to determine the progression-free survival rate at 8 weeks. Tumor samples and blood samples were collected for pharmacodynamic and pharmacokinetic studies. Twenty-nine patients were enrolled, of whom 26 were treated (14 at 500 mg and 12 at 2,000 mg). Among those treated, only three were progression-free at 8 weeks: two in the 500-mg arm and one in the 2,000-mg arm. One patient in the 2,000-mg arm had a prolonged partial response after an initial 28% enlargement of her target lesions. The treatment was well tolerated without clinically significant adverse events. The sole responder and one of two patients with stable disease had no αvβ3 expression at baseline. Overall, αvβ3 expression was decreased by day 8 of the treatment (P=0.05). Cilengitide was well tolerated by patients in both treatment arms but had minimal clinical efficacy as a single-agent therapy for metastatic melanoma, and the efficacy was not related to baseline αvβ3 expression.
Keywords: Phase II, Cilengitide, αvβ3 integrin, melanoma
INTRODUCTION
Angiogenesis is required for the invasion and metastasis of cancer cells, including melanoma. Integrins are a class of cell surface adhesion molecules essential in endothelial cell proliferation and tumor angiogenesis, and they are widely expressed in tumor-associated endothelial cells [1, 2]. Specifically, integrin αvβ3 binds to extracellular matrix components such as vitronectin, fibronectins, matrix degradation products, and thrombospondins, leading to a decrease in p21, inhibition of p53, and suppression of bax-mediated cell death [3-7]. Thus, integrin αvβ3 is crucial in the process of neovascularization or angiogenesis.
Consistent with melanoma cells inducing angiogenesis, tumor-associated endothelial cells in melanoma have upregulated αvβ3, [8] which is essential in endothelial cell proliferation, maturation and survival. In addition to being expressed in endothelial cells, αvβ3 is expressed in melanoma cells. αvβ3 is expressed in 86% of melanomas during the tumorigenic vertical growth phase and in 96% of metastatic lesions, and it has been suggested that this integrin plays an important role in the metastatic process of melanoma [9]. αvβ3 expression has also been found to be strongly associated with poor prognosis in patients with melanoma [10, 11]. Therefore, inhibition of integrin αvβ3 may provide a clinical benefit in patients with advanced melanoma through an antivascular effect as well as a possible direct antitumor effect.
Cilengitide (EMD 121974) is the inner salt of a cyclic pentapeptide and is a potent and selective integrin inhibitor [12]. It binds most specifically to integrin αvβ3 at a nanomolar range (~ 2 nM) and integrin αvβ5 to inhibit the binding of this integrin to vitronectin. Preclinical studies demonstrated that cilengitide blocked cytokine-induced basic fibroblast growth factor (bFGF)- and vascular endothelial growth factor (VEGF)-mediated angiogenesis in a chorioallantoic membrane model (Cilengitide, Investigators Brochure), and inhibited tumor growth in chick chorioallantoic membrane model, nude mice, and severe combined immunodeficient mice inoculated with human tumor cells, including melanoma cells (Cilengitide, Investigators Brochure).
Phase I trials of cilengitide demonstrated that doses up to 2,400 mg/m2 administered twice weekly were well tolerated, but the maximum-tolerated dose has not been established [13, 14]. In these trials, a maximum concentration equivalent to that noted to be active in preclinical studies (11-13 μg/mL) was achieved at a dose as low as 120 mg/m2, although this concentration was not sustained for a long time due to a relatively short half-life (~2.3-4.0 hours for doses of 120-1,200 mg/m2). Complete responses were reported at 360 mg/m2 and 2,400 mg/m2 in patients with recurrent malignant glioma [14] The responses observed at both low and high dose levels suggest that high doses or prolonged exposure of cilengitide may not be necessary for clinical activity.
In light of the previous clinical experience with cilengitide in solid and hematologic tumors, we conducted a randomized phase II trial to assess the clinical benefit of cilengitide at two different doses in patients with advanced melanoma and performed a pharmacokinetic analysis and correlative studies to examine the molecular effects of cilengitide on melanoma and endothelial cells. Because the estimated progression-free survival (PFS) rate at 8 weeks is approximately 40% for dacarbazine [15], the standard therapy for advanced melanoma at the time of study design, we assume that a PFS rate of at least 65% at 8 weeks would be considered efficacious.
METHODS
Patient Selection
The protocol for this study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. All patients gave written informed consent before enrollment.
Eligible patients were required to have histologically confirmed stage IV or unresectable stage III nonchoroidal melanoma with measurable metastases and to have undergone no more than one prior systemic treatment, not counting adjuvant interferon-alfa. Patients had to be at least 18 years of age with an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate bone marrow, kidney and liver function. Patients with brain metastases must have had their brain lesions controlled, asymptomatic for at least 3 months after an adequate local treatment. Patients with any history of wound-healing disorders or advanced coronary disease or a recent history (within 6 months) of peptic ulcer disease were excluded.
Treatment Plan
This was a double-blind, randomized phase II clinical trial. Upon enrollment, all patients underwent mandatory core-needle or skin punch biopsy 5-7 days before the first treatment to assess baseline αvβ3 expression by immunohistochemical (IHC) staining. Patients were randomly assigned in a 1:1 ratio to receive either 500 mg or 2,000 mg of cilengitide by intravenous infusion over 1 hour twice weekly. Patients were stratified using the Pocock-Simon minimization method [16] by the following factors: prior systemic treatment (no/yes); visceral metastases (no/yes); serum lactate dehydrogenase level (normal/abnormal); and tumor αvβ3 overexpression (no/yes) where overexpression was defined as >25% of melanoma cells’ expressing this marker.
No intrapatient dose escalation was permitted. Each treatment cycle lasted 4 weeks, with a maximum of six treatment cycles administered.
On day 8 of the first cycle, an optional tumor biopsy was performed within 6 hours after the completion of treatment in patients who consented to correlative studies. Because of possible complications with wound healing from the biopsy, treatment was omitted on day 11 of the first cycle.
Response Evaluation
Radiologic assessment including computed tomography was performed after every two cycles. Clinical responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) [17]. Overall clinical response included both complete and partial responses. Progression-free survival (PFS; time to disease progression from the date of treatment initiation) and overall survival (OS; time to death of any cause from the date of treatment initiation) were measured from day 1 of treatment.
Toxicity Evaluation
Toxicities were evaluated and recorded according to the National Cancer Institute’s Common Toxicity Criteria version 3.0. Complete blood counts and serum chemistry panels were obtained every 2 weeks.
Population Pharmacokinetic Analysis
Blood samples were drawn into heparin-containing tubes at the following time points: predose and 2, 4 and 24 hours after the start of treatment infusion on day 1 of the first and second cycles and predose and 4 hours after the start of treatment on day 8 of the first and second cycles. Blood samples then immediately centrifuged at 1,500 rpm for 10 minutes at 4°C for separation. Plasma samples were aliquoted and stored in labeled cryovials at approximately -70°C until analysis. Cilengitide concentrations were determined by validated liquid chromatography-mass spectrometry protocol at Merck KGaA. The validated calibration range was 0.2-100 μg/ml.
Because of the limited number of sampling time points, no standard compartmental or noncompartmental data analyses were performed and only concentration data are presented.
Tumoral αvβ3 Expression Analyses
Tissue samples were analyzed using IHC staining for integrin αvβ3 (LM 609, Millipore), The stained tissue slides were examined by two pathologists, who reached a consensus for the grade of each sample on the basis of the percentage of cells expressing αvβ3. The IHC labeling grades were: 0, no positive cells; +1, 1% to 10% positive cells; +2, 11% to 25% positive cells; +3, 26% to 75% positive cells; and +4, greater than 75% positive cells. For stratification, we defined 0, +1 and +2 as negative expression and +3 and +4 as positive expression.
Pharmacodynamic Studies
To assess the downstream molecular changes within the tumor by cilengitide treatment, the expressions of p-ERK1/2 (E-4 [sc-7383], Santa Cruz Biotechnology, Inc.), p-AKT (Phospho-Akt [Ser473] Antibody [IHC Specific] [9277], Cell Signaling Technology) and p-FAK1 (FAK pY861 [44-626G], BioSource) were analyzed by IHC staining with methods identical to those used to determine αvβ3 expression, as described above. The IHC grades were 0, 5% or fewer positive cells; +1, 6% to 25% positive cells; +2, 26% to 75% positive cells; and +3, greater than 75% positive cells.
Statistical Analysis
This trial implemented Simon’s optimal two-stage design, [18] with a proposed sample size of 28 for each dose group. The primary endpoint was the PFS rate at 8 weeks. The assumed null rate was 40%, and the target rate was 65% for both dose groups. The design was set with a type I error rate of 10% and a power of 90%. If 5 or fewer of the first 13 patients in each group were free of disease progression after two treatment cycles, that group would be terminated early due to futility.
Pharmacokinetic data were summarized using mean, standard deviation and percent coefficient of variation by group. αvβ3 expression analyzed by IHC staining before and after treatment were compared using the paired t-test. Correlations of changes in αvβ3 expression with changes in other kinases were assessed using the Spearman’s correlation test. PFS and OS were estimated using the Kaplan-Meier method. Patients whose disease did not progress or who did not die were censored at the last follow-up date. Median PFS and OS and the PFS rate at 8 weeks were estimated and corresponding 95% confidence intervals (CIs) were provided for the entire group of patients and for each dose group. All analyses were performed on an intent-to-treat basis. Statistical tests were two-sided and P≤0.05 was considered statistically significant. Statistical analysis was carried out with SAS version 9 (SAS Institute).
RESULTS
Patient Characteristics
From August 2004 to March 2006, 29 patients were enrolled and 26 were treated with cilengitide. Two patients withdrew consent before the randomization, and one patient was not eligible to receive the study drug because the baseline tumor biopsy of the supraclavicular lymph node revealed a non-small cell carcinoma. Pretreatment characteristics of the patients are listed in Table 1. All 26 treated patients were evaluable for toxicity, and 25 patients were evaluable for response.
Table 1.
Patient Characteristics
| Characteristic | No. of patients by treatment
|
|
|---|---|---|
| 500-mg dose n=14 (%) | 2,000-mg dose n=12 (%) | |
| Sex | ||
| Male | 7 (50) | 8 (67) |
| Female | 7 (50) | 4 (33) |
| Age (years) | ||
| Median | 57 | 61 |
| Range | 38-85 | 35-76 |
| ECOG performance status | ||
| 0 | 11 (79) | 7 (58) |
| 1 | 3 (21) | 4 (33) |
| 2 | 0 (0) | 1 (8) |
| Disease stage | ||
| III | 3 (21) | 4 (33) |
| IV | ||
| M1a | 3 (21) | 1 (8) |
| M1b | 6 (43) | 1 (8) |
| M1c | 2 (14) | 6 (50) |
| Serum lactate dehydrogenase level | ||
| Normal | 11 (79) | 10 (83) |
| Higher than upper limit of normal | 3 (21) | 2 (17) |
| Prior treatment | ||
| None | 5 (36) | 3 (25) |
| Interferon-alfa therapy (adjuvant) | 5 (36) | 3 (25) |
| Isolated limb perfusion | 0 | 1 (8) |
| Biologic | 2 (14) | 2 (17) |
| Chemotherapy | 6 (43) | 6 (50) |
| Melanoma αvβ3 expression | ||
| Negative (0, 1+, 2+) | 9 (64) | 8 (67) |
| Positive (3+, 4+) | 3 (21) | 3 (25) |
| Insufficient cells to analyze | 2 (14) | 1 (8) |
| Site of metastases | ||
| Dermis/subcutaneous tissue | 13 (93) | 11 (92) |
| Lymph nodes | 7 (50) | 5 (42) |
| Lung | 5 (36) | 4 (33) |
| Liver | 1 (7) | 3 (25) |
| Bone | 1 (7) | 1 (8) |
| Brain | 0 (0) | 1 (8) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group;
Melanoma αvβ3 expression: 0, 0%; 1+, 1% to 10% cells staining positive; 2+, 11% to 25% cells staining positive; 3+, 26% to 75% cells staining positive; 4+, 76% to 100% cells staining positive
Treatment
The 26 patients received a total of 56 cycles of cilengitide (median, two cycles per patient; range, < 1-to 6). One patient in the 500-mg dose group was unable to complete the first cycle of therapy because of rapid disease progression. In the 500-mg dose group, one patient received less than one cycle, three received one cycle, eight received two cycles, one received four cycles and one received six cycles. In the 2,000-mg dose group, four patients received one cycle, seven received two cycles and one received six cycles. None of the patients required dose reductions.
Progression-free Survival and Responses
Table 2 summarizes PFS and tumor response. Only three patients remained free of disease progression per RECIST at 8 weeks. The PFS durations of these three patients were 10.6, 5.5 and 3.9 months, respectively. A 71-year-old Caucasian woman with melanoma metastases in the lungs and with numerous cutaneous in-transit melanoma lesions in her left lower extremity had a prolonged partial response after initial enlargement of her cutaneous lesions while receiving 2,000 mg of cilengitide. After one treatment cycle, the sum of the largest diameters of five target lesions on her leg increased by 28%. Although the disease progressed per RECIST, she was allowed to continue treatment under the protocol. After the second cycle, her disease began to regress. She completed six cycles and remained free of disease progression for 5 months afterward until the disease progressed in the lungs. The in-transit lesions in her leg remained stable without progression at the last follow-up evaluation at the 14th month (Figure 1). The best response in the other two patients who were free of disease progression at 8 weeks was stable disease.
Table 2.
PFS and the Best Tumor Response by Dose
| 500-mg dose (n=14) | 2,000-mg dose (n=12) | Overall patients (n=26*) | |
|---|---|---|---|
| Best tumor response | |||
| CR | 0 | 0 | 0 |
| PR | 0 | 1 (8%)† | 1 (4%)† |
| SD | 2(14%) | 0 | 2(8%) |
| Response rate (CR+PR) | 0% | 8% | 4% |
| PFS rate at 8 weeks** (95% CI) | 14% (4-52%) | 8% (1-54%) | 12% (4-33%) |
| Median PFS in weeks** (95% CI) | 8.0 (8.0-NA) | 7.9 (4.7-NA) | 8.0 (7.9-8.1) |
Abbreviation: PFS, progression-free survival; CR, complete response; PR, partial response; SD, stable disease; Cl, confidence intervals; NA, not available
Of 26 treated patients, 25 were evaluable for response because one patient lost to follow up after 1 cycle due to financial difficulty.
PFS rate and median PFS were estimated from the Kaplan-Meier survival curves
This patient initially had disease progression after 4 weeks of treatment, but the tumor lesions regressed to achieve a partial response.
Fig 1.
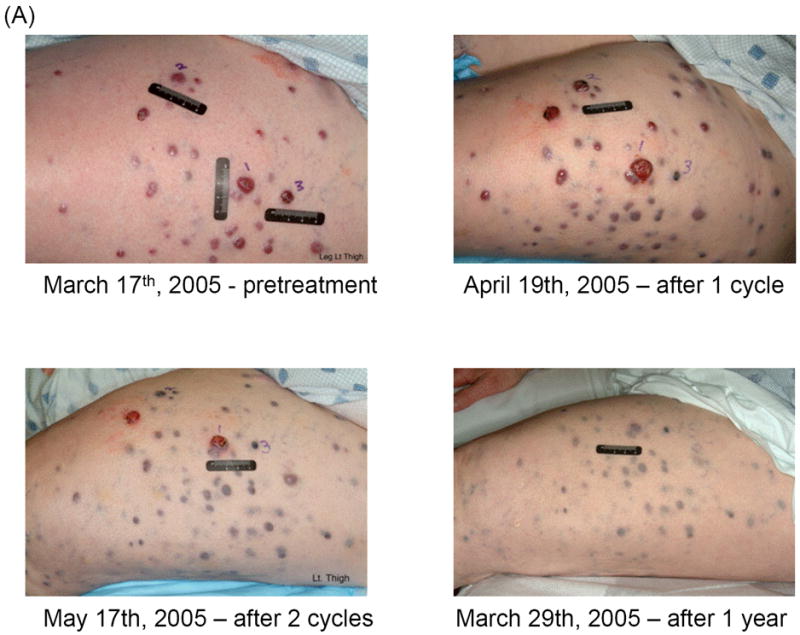
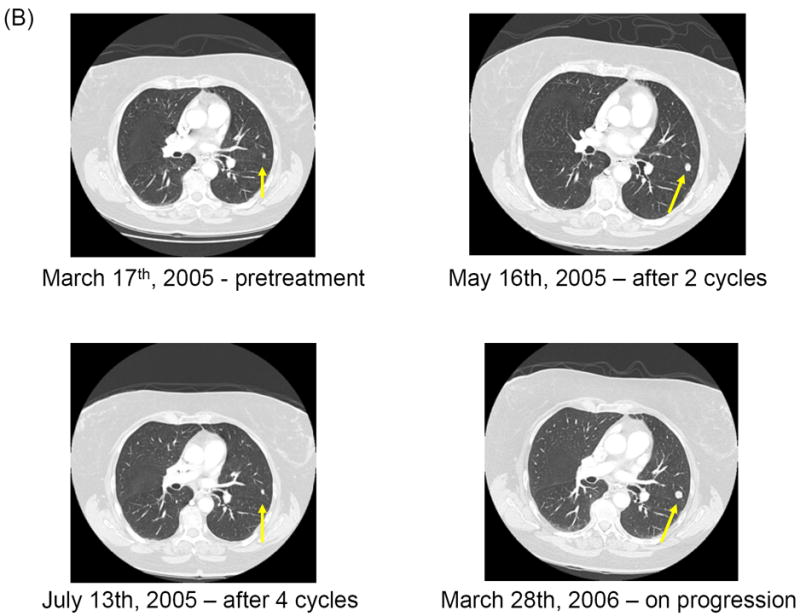
Clinical and radiologic studies of a tumor response to cilengitide. All metastatic lesions decreased in size. It is noteworthy to observe initial disease enlargement before disease regression both in the cutaneous and pulmonary lesions. (A) Regression of in-transit cutaneous metastases on left lower extremity. (B) Computed tomography scan showing response of the lung lesions (arrow).
The PFS rate at 8 weeks was 12% (95% CI, 4% to 33%) among all 26 patients and 14% (95% CI, 4% to 52%) and 8% (95% CI, 1% to 54%) among the 14 patients at the 500-mg dose and the 12 patients at the 2,000-mg dose, respectively. Therefore, the study (both dose groups) was terminated early. Median OS was 9.6 months (95% CI, 5.2-42.5 months), with six patients still alive after more than 4 years. These 6 patients received various chemotherapy regimens with or without immunotherapy and/or surgical resection. None of the 6 patients received either vemurafenib or ipiliumumab after their disease progressed on cilengitide.
Toxicity
Table 3 lists adverse events. The only adverse event greater than grade 2 was one case of grade 3 lymphopenia in a patient in the 2,000-mg dose group. None of the patients required treatment discontinuation or dose reduction for toxicity. Although both doses of cilengitide were well tolerated, the 2,000-mg dose was associated with higher incidences of grade 2 fatigue, arthralgia, lymphopenia, peripheral neuropathy and gastrointestinal disorders.
Table 3.
Adverse Events Observed with Cilengitide Treatment (Number of patients with adverse events; percentage in parentheses)
| Adverse event | 500-mg Dose (n=14) | 2,000-mg Dose (n=12) | ||
|---|---|---|---|---|
|
| ||||
| Gr 1 n (%) | Gr 2 n (%) | Gr 1 n (%) | Gr 2 n (%) | |
| Nonhematologic | ||||
| Constipation | 8 (57) | 2 (14) | 11 (92) | 6 (50) |
| Fatigue | 10 (71) | 0 | 10 (83) | 5 (42) |
| Anorexia | 5 (36) | 0 | 6 (50) | 4 (33) |
| Nausea | 6 (43) | 0 | 5 (42) | 4 (33) |
| Vomiting | 1 (7) | 1 (7) | 5 (42) | 3 (25) |
| Diarrhea | 3 (21) | 0 | 5 (42) | 2 (17) |
| Arthralgia | 7 (50) | 1 (7) | 3 (25) | 5 (42) |
| Peripheral neuropathy | 7 (50) | 1 (7) | 3 (25) | 5 (42) |
| Cough | 4 (29) | 0 | 6 (50) | 0 |
| Edema | 4 (29) | 0 | 5 (42) | 0 |
| Dyspnea | 0 | 0 | 4 (33) | 0 |
| Skin erythema/pruritis | 6 (43) | 0 | 3 (25) | 0 |
| Fever | 0 | 0 | 3 (25) | 0 |
| Ocular effects | 3 (21) | 0 | 0 | 0 |
| Creatinine elevation | 1 (7) | 0 | 0 | 0 |
| Hematologic | ||||
| Anemia | 10 (71) | 0 | 9 (75) | 0 |
| Lymphopenia | 0 | 1 (7) | 1 (8) | 4 (33) |
| Thrombocytopenia | 1 (7) | 0 | 1 (8) | 1 (8) |
| Neutropenia | 1 (7) | 1 (7) | 1 (8) | 0 |
Abbreviation: Gr, grade
1 patient had Grade 3 lymphopenia in the 2,000 mg arm; No other patient had Grade 3 or 4 adverse events.
Pharmacokinetic Analysis
Plasma samples for the pharmacokinetic analysis were available from eight patients in the 500-mg dose group and from 10 patients in the 2,000-mg dose group. Table 4 shows the mean plasma cilengitide concentration by dose group and treatment cycle. No accumulation was observed in either group after repeated biweekly doses. As anticipated, patients treated at the 2,000-mg dose level had higher cilengitide exposures than patients treated with 500 mg. Increases in plasma concentrations at the given time points were dose proportional, and the data were consistent with findings reported in preclinical studies.
Table 4.
Plasma Cilengitide Concentration
| Dose (mg) | Cycle | Time (h) | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 24 | |||
| 500 | 1 | No. of pts | 8 | 8 | 8 | 8 |
| Mean cilengitide concentration, μg/ml | 0 | 18.2 | 8.25 | 0.04 | ||
| SD | 0 | 4.58 | 2.21 | 0.12 | ||
| % CV | -- | 25.1 | 26.8 | 282.8 | ||
| 2 | No. of pts | 4 | 4 | 4 | 4 | |
| Mean cilengitide concentration, μg/ml | 0 | 17.6 | 8.75 | -- | ||
| SD | 0 | 4.18 | 2.88 | -- | ||
| % CV | -- | 23.8 | 32.9 | -- | ||
| 2,000 | 1 | No. of pts | 10 | 10 | 10 | 10 |
| Mean cilengitide concentration, μg/ml | 0 | 73.2 | 35.9 | 0.71 | ||
| SD | 0 | 11,7 | 8.97 | 0.54 | ||
| % CV | -- | 16.0 | 25.0 | 76.7 | ||
| 2 | No. of pts | 9 | 9 | 9 | 9 | |
| Mean cilengitide concentration, μg/ml | 0 | 65.8 | 30.5 | 0.58 | ||
| SD | 0 | 21.1 | 12.9 | 0.52 | ||
| % CV | -- | 32.0 | 42.3 | 90.1 | ||
Abbreviation: pts, patients; SD, standard deviation; CV, coefficient of variation
Correlative Studies
All 26 patients treated in this study underwent tumor biopsy at baseline;18 underwent a repeat tumor biopsy on day 8. At baseline, six patients (23%) had more than 10% of tumor cells stained positive for αvβ3, and of these six patients, five patients (19%) had more than 25% of their tumor cells stained positive for αvβ3 (Table 5). One of the biopsies on day 8 did not yield an adequate amount of tumor tissue to analyze for αvβ3 expression; therefore, 17 matching samples were available for αvβ3 expression analyses. Overall, αvβ3 expression decreased with cilengitide treatment (P=0.05); among eight patients with matching tumor samples with any detectable level of αvβ3 expression at baseline, αvβ3 expression decreased in five and increased in none. The sole responder and one of the two patients with stable disease had no tumoral αvβ3 expression at baseline.
Table 5.
αvβ3 Expression and Clinical Response
| Patient | Baseline αvβ3 expression | Day 8 αvβ3 expression | Change in αvβ3 expression | Best response |
|---|---|---|---|---|
| 1 | 3/2 | 3/2 | ↔ | PD |
| 2 | 0/0 | -- | PD | |
| 3 | 3/2 | 0/0 | ↓ | SD |
| 4 | 1/0 | -- | PD | |
| 5 | 1/1 | 0/0 | ↓ | PD |
| 6 | 1/1 | NA | PD | |
| 7 | 0/0 | 0/0 | PD | |
| 8 | 0/0 | -- | ↔ | PD |
| 9 | 0/0 | 0/0 | ↔ | PD |
| 10 | 1/1 | 0/0 | ↓ | PD |
| 11 | 0/0 | -- | PD | |
| 12 | 0/0 | 0/0 | ↔ | PD |
| 13 | 0/0 | 0/0 | ↔ | PD |
| 14 | 0/0 | 0/0 | ↔ | PR |
| 15 | 1/1 | 0/0 | ↓ | PD |
| 16 | 0/0 | 0/0 | ↔ | PD |
| 17 | 0/0 | 0/0 | ↔ | NE |
| 18 | 1/1 | 1/1 | ↔ | PD |
| 19 | 0/0 | 0/0 | ↔ | PD |
| 20 | 4/2 | 4/2 | ↔ | PD |
| 21 | 4/1 | -- | PD | |
| 22 | 3/2 | 0/0 | ↓ | PD |
| 23 | 0/0 | -- | PD | |
| 24 | 0/0 | 0/0 | ↔ | PD |
| 25 | 0/0 | -- | SD | |
| 26 | 2/2 | -- | PD |
Abbreviations: PD, progressive disease; SD, stable disease; PR, partial response; NA, not adequate (amount of tumor for analysis); NE, not evaluable. The first number is an indicator of the percentage of cells staining positively for αvβ3 expression (0, 0%; 1+, 1% to 10%; 2+, 11% to 25%; 3+, 26% to 75%; 4+, 76% to 100%); the second number denotes the staining intensity (1, weak; 2, moderate; 3, strong).
-- indicates no tissue available for analysis
Only 9, 12 and 9 matching samples were adequate for pERK1/2, pAKT and pFAK expression analyses, respectively (supplemental table). None of them had changes in the levels of phosphorylated proteins significantly correlated with changes in αvβ3 expression (results not shown).
DISCUSSION
We showed in this trial that at a dose up to 2,000 mg, cilengitide administered intravenously twice weekly is well tolerated, but it does not offer patients with metastatic melanoma significant clinical benefits, despite its ability to decrease tumoral αvβ3 expression. It is interesting that only 42% of the patients had αvβ3 expression in their tumors in our study whereas available literatures report nearly 80-90% of melanomas express αvβ3. It is not clear if different thresholds used in other studies for αvβ3 expression to be labeled positive could explain the discrepancy. Because the tumor of the only responder in our study did not express αvβ3, we speculate that cilengitide exerts its clinical effect mostly through an angiogenic mechanism rather than by directly targeting melanoma cells although it is possible that αvβ5 integrin might have been the main target of the anticancer activity in the sole responder. There are several potential explanations for the lack of clinical activity.
The half-life of cilengitide is less than 5 hours [13], and in our study, the pharmacokinetic analysis demonstrated that the plasma concentration of cilengitide was below the biologically active level of 11-13 μg/mL predicted from preclinical studies after 24 hours of the drug administration, and even after 4 hours in the 500 mg dose; therefore, the dosing schedule used in our study might have been suboptimal. The biweekly schedule of cilengitide administration was selected on the basis of limited preclinical studies and also mainly due to the practicality. However, this dosing schedule is likely to be suboptimal, and a more frequent or continuous infusion schedule may be more appropriate for cilengitide treatment. Cilengitide has been studied in other cancer types with variable results. In particular, it appears to have a meaningful clinical benefit in patients with recurrent glioblastoma multiforme (GBM). In a randomized phase II study of cilengitide using the same dosing schedules as in our study, the 6-month PFS rate was 15%, and the median OS duration was 9.9 months, with a trend for higher antitumor activity in the 2,000 mg dose cohort among 81 patients with GBM [19]. On the basis of the results of that trial, it is likely that a higher dose or more frequent doses of cilengitide would lead to superior clinical benefit in patients with advanced melanoma, which appears to be less sensitive to cilengitide treatment.
Another possible explanation for the poor clinical efficacy is that integrins other than αvβ3 confer a resistance to cilengitide treatment. Although αvβ3 appears to be important for the proliferation, survival and invasion of endothelial cells and the metastasis of melanoma cells, it may not be sufficient to support tumor progression in patients. This conjecture is also suggested by the results of a clinical study of etaracizumab, a specific humanized antibody of integrin αvβ3, in patients with metastatic melanoma [20]. Among 112 patients who were treated with etaracizumab with or without dacarbazine in a randomized phase II study, none of 57 patients who received etaracizumab alone had a clinical response, and only 12.7% of the patients in the combination arm had a clinical response.
It has been demonstrated that other integrins, such as α5β1, are also involved in angiogenesis [21-23]. It is also conceivable that subsets of tumor-associated endothelial cells and melanoma cells may be less dependent on the integrin binding to the extracellular components but rather more dependent on other proangiogenic growth factors, such as interleukin-8, VEGF or bFGF, produced by tumor cells. Early evidence has shown that drugs that target VEGF receptors and/or bFGF receptors can be effective in a subset of patients with metastatic melanoma. In a phase II study of axitinib, a potent inhibitor of VEGF receptor-1, -2 and -3, five (16%) of 32 treated patients with advanced melanoma had an objective clinical response [24]. In a phase I study of E7080, a tyrosine kinase inhibitor of VEGF receptor-1 and -2, bFGF receptor and platelet-derived growth factor receptor-β, in patients with advanced solid tumors, at least two patients with melanoma had a confirmed partial response [25]. Successful antivascular therapy might require a multi-target drug or a combination of multiple drugs that inhibit various angiogenic factors together.
Likewise, the clinical efficacy of cilengitide in patients with advanced melanoma may be enhanced by combining with cytotoxic chemotherapeutic drugs. Tentori and colleagues demonstrated the synergistic antiproliferative effects of the combination of cilengitide and temozolomide against melanoma and endothelial cells in vitro, and the more effective tumor reduction compared with temozolomide alone in vivo [26]. In a phase I/II trial of cilengitide in combination with temozolomide and radiation therapy in patients with glioblastoma, the 6- and 12-month PFS rates were 69% an 33%, respectively, and the median PFS was 8.0 months, suggesting a promising efficacy of this combined modality [27]. These preclinical and clinical findings suggest that the combination of cilengitide with temozolomide may provide superior clinical benefit than either drug alone.
It is interesting to note that the patient who achieved a partial response in our study had an initial tumor enlargement after 4 weeks of treatment. This phenomenon of late tumor regression has been noted in animal models after treatment with antivascular drugs, such as angiostatin. This result suggests that clinical studies of antivascular drugs should not mandate that treatment be discontinued in patients who have an initial disease enlargement per RECIST. Accordingly, the conventionally accepted methods, such as RECIST or World Health Organization criteria, in cancer therapy trials may not provide the most accurate assessment of the drug effect, especially antiangiogenic drugs. Perhaps, other imaging modalities measuring the metabolic or tumor vascular flow activity of tumor lesions, such as 18 FDG-PET or dynamic contrast MRI scans, may be more appropriate for the evaluation of antiangiogenic agents. The investigation of these imaging modalities in current clinical trials of various antiangiogenic drugs may answer these questions.
In conclusion, single-agent cilengitide administered at a dose up to 2,000 mg twice weekly has minimal clinical efficacy in patients with metastatic melanoma. A prolonged, continuous infusion schedule and the combination of cilengitide with other targeted drugs should be considered to further develop cilengitide as a treatment for advanced melanoma.
Supplementary Material
Acknowledgments
This work was supported by National Cancer Institute grants N01 CM-17003 and N02 CO-12400 and Cancer Center Support Grant CA16672.
Footnotes
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL and at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29 – June 2, 2009, Orlando, FL
References
- 1.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 2.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8(5):724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 3.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182(3):1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 4.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85(5):683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 5.Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996;98(2):426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83(5):715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 7.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol. 1996;135(2):533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103(9):1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Belle PA, Elenitsas R, Satyamoorthy K, Wolfe JT, Guerry Dt, Schuchter L, et al. Progression-related expression of beta3 integrin in melanomas and nevi. Hum Pathol. 1999;30(5):562–567. doi: 10.1016/s0046-8177(99)90202-2. [DOI] [PubMed] [Google Scholar]
- 10.Kageshita T, Hamby CV, Hirai S, Kimura T, Ono T, Ferrone S. Differential clinical significance of alpha(v)Beta(3) expression in primary lesions of acral lentiginous melanoma and of other melanoma histotypes. Int J Cancer. 2000;89(2):153–159. doi: 10.1002/(sici)1097-0215(20000320)89:2<153::aid-ijc9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, et al. Clinical significance of alpha(v)beta3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res. 1997;57(8):1554–1560. [PubMed] [Google Scholar]
- 12.Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, et al. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem. 1999;42(16):3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 13.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39(7):917–926. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 14.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25(13):1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 20.Hersey P, Sosman J, O’Day S, Richards J, Bedikian A, Gonzalez R, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), +/- dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116(6):1526–1534. doi: 10.1002/cncr.24821. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156(4):1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beauvais A, Erickson CA, Goins T, Craig SE, Humphries MJ, Thiery JP, et al. Changes in the fibronectin-specific integrin expression pattern modify the migratory behavior of sarcoma S180 cells in vitro and in the embryonic environment. J Cell Biol. 1995;128(4):699–713. doi: 10.1083/jcb.128.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian F, Zhang ZC, Wu XF, Li YP, Xu Q. Interaction between integrin alpha(5) and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem Biophys Res Commun. 2005;333(4):1269–1275. doi: 10.1016/j.bbrc.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Fruehauf JP, Lutzky J, McDermott DF, Brown CK, Pithavala YK, Bycott PW, et al. Axitinib (AG-013736) in patients with metastatic melanoma: A phase II study. J Clin Oncol. 2008;26(May 20 suppl) Abstr# 9006. [Google Scholar]
- 25.Glen H, Boss DR, Morrison R, Roelvink M, Wanders J, Mazur A, et al. A phase I study of E7080 in patients (pts) with advanced malignancies. J Clin Oncol. 2008;26(May 20 suppl) Abstr # 3526. [Google Scholar]
- 26.Tentori L, Dorio AS, Muzi A, Lacal PM, Ruffini F, Navarra P, et al. The integrin antagonist cilengitide increases the antitumor activity of temozolomide against malignant melanoma. Oncol Rep. 2008;19(4):1039–1043. [PubMed] [Google Scholar]
- 27.Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 28(16):2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


