Abstract
We present a patient with metastatic BRAF-mutated melanoma who achieved long-term stabilization of leptomeningeal disease with sequential whole-brain radiation therapy and vemurafenib. A 53-year-old woman with melanoma that harboured the BRAF V600E mutation and had that metastasized to multiple lymph nodes, both lungs, one breast, and subcutaneous tissue had developed symptomatic leptomeningeal disease 16 months after starting vemurafenib treatment despite achieving a substantial response at the existing metastatic sites. Vemurafenib was discontinued for 7 days, she received whole-brain radiation therapy (30 Gy in 10 fractions), and 7 days after completing the radiation therapy, she resumed vemurafenib therapy. The neurologic symptoms improved significantly, and a cerebrospinal fluid examination revealed disappearance of melanoma cells. She remained alive with radiologically stable leptomeningeal disease for at least 18 months after the whole-brain radiation therapy.
Keywords: metastatic melanoma, leptomeningeal disease, radiation therapy, vemurafenib
Introduction
Leptomeningeal disease (LMD) affects about 5% of cancer patients [1] and is more prevalent among those with certain cancer types, including small-cell lung cancer, breast cancer, and melanoma [2]. The common signs and symptoms of LMD include headache, nausea, vomiting, back or neck pain, and weakness [3]. Magnetic resonance imaging (MRI) of the brain and/or spine is widely used to detect LMD, and cerebrospinal fluid (CSF) analysis confirms the diagnosis. The presence of LMD indicates a very advanced stage of cancer with a grave prognosis; the median survival time of patients with LMD is 4–6 weeks without treatment but may be increased to 4–6 months with treatment in patients with breast cancer [4]. However, the survival of patients with LMD from melanoma is more dismal. In patients with metastatic melanoma, the median survival time is 8–10 weeks [3,5]. No known therapy is effective for LMD in patients with melanoma. In this report, we present the first case, to our knowledge, of long-term stabilization of LMD in a patient with metastatic melanoma treated sequentially with vemurafenib, whole-brain radiation therapy, and vemurafenib again.
Case Presentation
An otherwise healthy 53-year-old woman was diagnosed with a 6-mm-thick, Clark level IV, extensively ulcerated melanoma on the left heel in May 2009. She underwent a wide local excision of the melanoma and intraoperative lymphatic mapping and sentinel node biopsy in the left inguinal region, which revealed one lymph node positive for metastatic melanoma. She subsequently underwent a lymph node dissection of the left groin 2 of 16 lymph nodes removed were positive for metastatic melanoma.
In September 2009, computed tomography of the whole body revealed metastatic lesions in the both lungs, the right breast, the subcutaneous tissue of the back, and the left inguinal and external iliac node basins. A biopsy of an enlarged external iliac node confirmed metastatic melanoma. The patient received two cycles of biochemotherapy consisting of cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon-α before her disease progressed.
A pyrosequencing analysis showed that her melanoma lesion harboured the BRAF V600E mutation. In January 2010, she started treatment with vemurafenib, 960 mg twice per day, as part of a clinical trial. She had a substantial response with a reduction in sizes of the target lesions by 88%. She continued to receive vemurafenib until March 2011, when MRI of the brain showed leptomeningeal enhancement in the right occipital lobe suspicious for LMD.
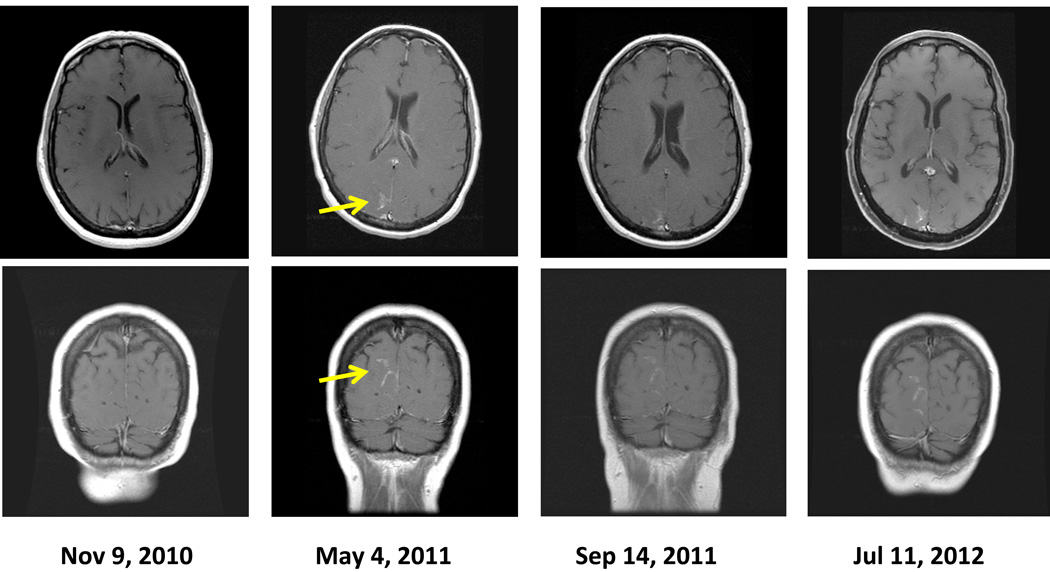
By May 2011, she developed severe dizziness, nausea, and increasing headaches and was hospitalized. MRI of the brain demonstrated progressive enlargement of the area of enhancement, indicating worsening LMD (Fig. 1). A CSF analysis revealed the presence of melanoma cells. Vemurafenib treatment was interrupted, and dexamethasone was initiated, resulting in improvement of her symptoms. In May 2011, she was treated with whole-brain radiation therapy using opposed lateral beams with 6-MV photons; a total dose of 30 Gy was given in 10 fractions. Seven days after whole-brain radiation therapy, she resumed vemurafenib therapy, 960 mg twice per day, while dexamethasone was tapered off.
Fig. 1.
Magnetic resonance images of the patient’s brain. The yellow arrows indicate the enhancement of the leptomeninges on May 2011 before radiation therapy began. The images at the latest follow-up in July 2012 showed stabilization of the leptomeningeal disease
In September 2011, an MRI scan of the brain showed stabilization of the leptomeningeal enhancement (Fig. 1), and the patient’s symptoms further improved, allowing her to return to her work as a schoolteacher. In April 2012, a CSF analysis finally revealed a complete absence of melanoma cells; her latest CSF examination in July 2012 was also negative. As of the most recent follow-up evaluation in November 2012 (18 months after beginning whole-brain radiation therapy), her neurologic symptoms have remained minimal in severity, and her radiologic LMD findings and extracranial metastatic disease have not progressed while she has continued to take vemurafenib.
Discussion
The incidence of LMD in patients with melanoma is likely to increase as patients survive longer because of more durable control of their extracranial metastasis with newer drugs such as vemurafenib and ipilimumab. Unfortunately, an effective treatment for LMD is practically nonexistent, and accordingly no standard treatment for LMD from melanoma exists. The main goal of the LMD treatment is palliative and includes mitigating symptoms and slowing the rate of tumor progression. Recent literature indicates that the 1-year survival rate of patients with LMD from melanoma is only about 7% [3] and that the median survival time is only 8–10 weeks after diagnosis of LMD in these patients [3,5]. Considering the grave prognosis of melanoma patients with LMD, we find our case very unusual and interesting because of the unexpectedly long duration of LMD control and improvement of symptoms as a result of sequential therapy with vemurafenib, whole-brain radiation therapy, and vemurafenib.
Cases in which patients with LMD from melanoma had prolonged survival have been rarely reported [3,6]. Harstad and colleagues reported a patient with LMD, who was treated with intrathecal interleukin-2 and survived more than 11 years [3]. However, the details regarding the method of diagnosis of the LMD, the symptoms present at the time of the diagnosis and the patient’s cancer type were not reported. Despite the rarity of prolonged control of LMD and the limitations inherent to case reports, it is important to study these cases carefully because they may lead to the generation of hypotheses and the investigation of new therapeutic regimens for these patients.
In our patient, it is possible that sequential treatments with vemurafenib, radiation therapy and then vemurafenib again had additive or synergistic effects in the control of LMD. The fact that melanoma cells were present in the CSF during the lumbar puncture after 16 months of initial vemurafenib treatment suggests that these cells were resistant to vemurafenib. While the patient continued vemurafenib between March 2011, when LMD was initially suspected radiographically, and May 2011, when LMD was confirmed, she developed neurological symptoms suggesting the progression of LMD and the increasing resistance of the melanoma cells in the CSF to vemurafenib. Accordingly, radiation therapy may have sensitized the tumor cells to vemurafenib; however, it is also possible that vemurafenib sensitized the otherwise radioresistant cells to radiation. Sambade and colleagues demonstrated in an in vitro experiment that the inhibition of mutated BRAF kinase rendered melanoma cells more sensitive to the antiproliferative effect of radiation [7] suggesting that a combination approach can improve response in patients receiving a BRAF inhibitor.
Another possible explanation for the prolonged control of LMD in our patient is that the radiation therapy could have disrupted the blood-brain barrier, enabling a higher level of vemurafenib in the CSF. It has previously been shown through in vivo models that whole-brain radiation therapy induces permeability changes in the blood-brain barrier, allowing certain chemicals or drugs to penetrate into the CSF system [8,9]. For example, d’Avella and colleagues demonstrated a significant increase in the transport of 14C-α-aminoisobutyric acid across the blood-brain barrier after rats were exposed to radiation [8].
In addition to mechanically disrupting the blood-brain barrier, radiation may hinder the efflux function of multidrug resistance proteins. Mittapalli and colleagues demonstrated that the low concentration of vemurafenib in the central nervous system in those taking the drug is at least partially due to the active efflux by P-glycoprotein and breast cancer resistance protein [10]. It is possible that radiation therapy interferes with the functions of P-glycoprotein and breast cancer resistance protein and thus permits the accumulation of vemurafenib in the central nervous system and the CSF. Of course, we cannot exclude the possibility that the clinical improvement was due to whole-brain radiation therapy alone; the melanoma cells in the CSF might have been particularly sensitive to radiation therapy in our patient. However, this is unlikely because melanoma cells are generally regarded as resistant to radiation therapy unless it is a hypofractionated dosing schedule or stereotactic radiosurgery [11]. In addition, a whole brain radiation therapy has been shown to be ineffective in the treatment of LMD [12]. It is possible that BRAF-mutant melanoma cells are more sensitive to radiation therapy than wild-type melanoma cells, but this is not yet known.
It is important to note that in our patient’s case, vemurafenib therapy was ceased for 7 days before the initiation of whole-brain radiation therapy and resumed 7 days after the completion of the radiation therapy. This was done because concurrent radiation and vemurafenib may exacerbate toxic effects on the scalp and normal brain parenchyma. One of the most common adverse events of vemurafenib is photosensitivity [13], which can be quite severe and disabling; the addition of radiation may increase this effect. It is not known whether vemurafenib’s radiosensitizing effect increases damage to normal brain tissues, but this was a particular concern given that the blood-brain barrier may be disrupted by radiation. The concurrent use of radiation therapy and vemurafenib should be considered only if a carefully designed phase I study of this combination demonstrates its safety.
This particular case suggests that sequential treatment with vemurafenib, whole-brain radiation therapy and vemurafenib again may decrease symptoms and extend survival times in patients with BRAF-mutated melanoma that has metastasized the leptomeninges. This particular therapeutic regime using whole-brain radiation therapy and vemurafenib should be investigated further.
Acknowledgements
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through cancer center support grant CA16672.
References
- 1.Siomin VE, Vogelbaum MA, Kanner AA, Lee SY, Suh JH, Barnett GH. Posterior fossa metastases: risk of leptomeningeal disease when treated with stereotactic radiosurgery compared to surgery. Journal of Neuro-oncology. 2004;67:115–121. doi: 10.1023/b:neon.0000021785.00660.2b. [DOI] [PubMed] [Google Scholar]
- 2.Orphanos G, Ardavanis A. Leptomeningeal metastases from prostate cancer: an emerging clinical conundrum. Clin Exp Metastasis. 2010;27:19–23. doi: 10.1007/s10585-009-9298-z. [DOI] [PubMed] [Google Scholar]
- 3.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro-Oncology. 2008;10:1010–1018. doi: 10.1215/15228517-2008-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruna J, Simó M, Velasco R. Leptomeningal metastases. Curr Treat Options Neurol. 2012;14:402–415. doi: 10.1007/s11940-012-0182-9. [DOI] [PubMed] [Google Scholar]
- 5.Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117:1687–1696. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 6.Walker JG. Diagnosis and management of leptomeningeal disease. Clinical Journal of Oncology Nursing. 2009;13:384–387. doi: 10.1188/09.CJON.384-387. [DOI] [PubMed] [Google Scholar]
- 7.Sambade MJ, Peters EC, Thomas NE, Kaufmann WK, Kimple RJ, Shields JM. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX 4032. Radiother Oncol. 2011;98:394–399. doi: 10.1016/j.radonc.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d'Avella D, Cicciarello R, Albiero F, Mesiti M, Gagliardi ME, Russi E, et al. Quantitative study of blood-brain barrier permeability changes after experimental whole brain radiation. Neurosurgery. 1992;30:30–34. doi: 10.1227/00006123-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mehta M, Bradely K. Radiation therapy for leptomeningeal cancer. Cancer Treat Res. 2005;125:147–158. doi: 10.1007/0-387-24199-x_9. [DOI] [PubMed] [Google Scholar]
- 10.Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J Pharmacol Exp Ther. 2012;342:33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan N, Khan MK, Almasan A, Singh AD, Macklis R. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys. 2011;80:645–654. doi: 10.1016/j.ijrobp.2010.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro Oncol. 2008;10:1010–1018. doi: 10.1215/15228517-2008-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]