Abstract
BACKGROUND
Management of patients with thyroid nodules is based on establishing an accurate diagnosis; however, differentiating benign from malignant lesions preoperatively is not always possible using current cytological techniques. Novel molecular testing on cytological material could lead to clearer treatment algorithms. B-RafV600E mutation is the most common genetic alteration in thyroid cancer, specifically found in papillary thyroid cancer (PTC), and usually reported to be associated with aggressive disease.
DATA SOURCE
A literature search using PubMed identified all the pertinent literature on the identification and utilization of the B-RafV600E mutation in thyroid cancer.
CONCLUSIONS
The utility of using B-Raf mutation testing for nodules with indeterminate cytology is limited since many of those nodules (benign and malignant) do not harbor B-Raf mutations. However, when the pathologist sees cytological features suspicious for PTC, B-RafV600E mutation analysis may enhance the assessment of preoperative risks for PTC, directing a more aggressive initial surgical management when appropriate.
Keywords: B-Raf, Fine-needle aspiration biopsy, Thyroid nodules, Papillary thyroid cancer
Thyroid nodules are common and are being increasingly detected. While most thyroid nodules are benign, surgical treatment remains common. Differentiating benign from malignant lesions preoperatively is not always possible using current imaging and cytological techniques. Novel molecular testing on cytological material could lead to clearer treatment algorithms for those with thyroid nodules and increase the efficacy of novel targeted therapies in those with malignancy. Surgeons, endocrinologists, cytologists, and medical oncologists should be familiar with recent studies on the incidence and role of B-RafV600E mutation in thyroid neoplasms, including implications for mutational analysis in fine-needle aspirations and potential alterations in therapy that may result based on this finding. Information on this mutation and its clinical role is limited, as many studies are small, retrospective, and lack controls.
Fine-needle aspiration biopsy (FNAB) is currently considered the most important and rational component of our diagnostic armamentarium for decision making in any given thyroid nodule. Its main purpose is to provide more information on the risk of malignancy in any given nodule, thus helping guide the clinician to surgical therapy (lobectomy vs total thyroidectomy, ± central compartment lymph node dissection) if necessary. The FNAB diagnostic scheme consists of 4 major categories, including benign, indeterminate, malignant, and unsatisfactory, while indeterminate cytology includes 3 subcategories, including lesion of undetermined significance, follicular neoplasm, and lesion with suspicious cytology.1,2 Although FNAB cytology has high sensitivity and specificity, the rate of specimens that are inadequate for cytological diagnosis (unsatisfactory or nondiagnostic) ranges between 10% and 20% and in another 20% it is not possible to determine with certainty whether the nodule is an adenoma or carcinoma (indeterminate category).3 Indeterminate cytology may either stem from modest cellular or nuclear changes that may be minimal but indistinguishable from findings of papillary thyroid cancer (PTC), or from the inability to determine capsular and vascular invasion on FNAB specimen findings that are required to define follicular thyroid carcinoma (FTC).2 Currently, nondiagnostic biopsies are often repeated. For those with indeterminate cytology, surgery with at least a thyroid lobectomy is often recommended to definitively diagnose the nature of these nodules; however, the vast majority prove to be benign. Traditional thyroid FNAB diagnostic capabilities have been expanded by adding immunohistochemical staining for visualization of cancer-associated antigens, measurement of tissue-specific proteins, and most importantly by analyzing DNA cell content.4 The 2 most promising applications of molecular pathology techniques in thyroid FNAB are cDNA microarray analysis and the determinations of specific gene mutations.
Of all gene alterations identified in thyroid cancer, the B-RafV600E mutation is the most common and is specifically found in PTC. It has a fundamental role in thyroid tumori-genesis through aberrant activation of the mitogen-activated protein kinase (MAPK) pathway, and is generally correlated with tumor aggressiveness. The detection of B-RafV600E mutation in thyroid cells is thought to perhaps increase the diagnostic utility of thyroid FNABs, to help predict thyroid malignancy with better specificity, and to potentially guide the extent of initial surgical treatment. Here we review the significance of screening for B-RafV600E mutation in thyroid FNABs, and discuss possible implications of its identification on the initial surgical treatment of thyroid nodules with PTC cytology.
B-RafV600E Activation of the MAPK Pathway
Since the initial discovery of B-RafV600E mutation in human cancer,5 mutations were identified in approximately 29% to 83% of PTCs, 66% of melanomas, and in a smaller percentage of other tumors, including colonic and ovarian carcinoma and some sarcomas.6 B-Raf is a member of the Raf family of protein kinases. The Raf gene products are RAS effectors, and regulate the MAPK signaling pathway, which connects extracellular signals to transcriptional regulation.6 Among the 3 known Raf kinases (A-Raf, B-Raf, and C-Raf), B-Raf is the most potent activator of the MAPK pathway.7 The gene encodes a cytosolic serine–threonine protein kinase that is also expressed in thyroid follicular cells.8 B-Raf protein is activated at the membrane level through a complex process that involves multiple phosphorylation events and protein/lipid interactions. It subsequently activates MAPKK (MEK), which then phosphorylates MAPK (ERK).9,10 Both B-Raf and MAP kinases are dependent on upstream Ras and tyrosine kinase receptor activation.11-13
B-RafV600E is an oncogenic protein with markedly elevated kinase activity that overactivates the MAPK pathway.5,14,15 There have been more than 40 mutations identified in the B-Raf gene; however, more than 80% of these mutations correspond to a T→A transversion at nucleotide position 1796 that results in the substitution of valine by glutamate at position 600 of the kinase activation segment5,7,16 (Fig. 1).
Figure 1.
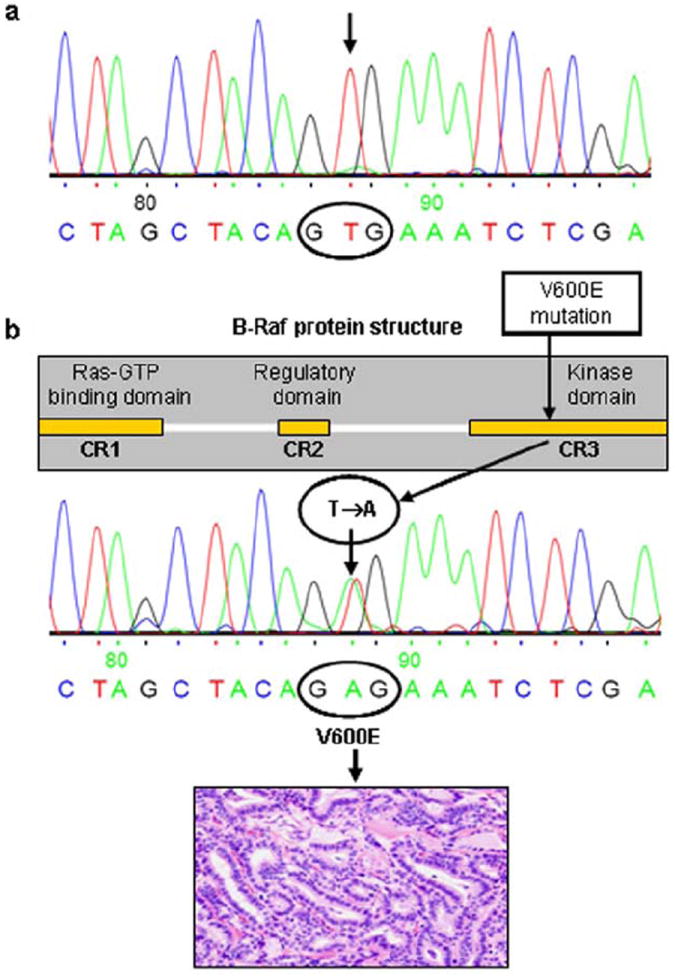
Analysis of B-Raf gene exon-15 by DNA automated sequencing. (A) Electopherogram showing wild-type B-Raf gene (sequence nGTGn at nucleotide 1796) in a human normal thyroid tissue sample. (B) B-Raf gene shows the 3 conserved domains CR1, CR2, and CR3, which are affected by the V600E mutation. The pherogram shows the T1799A (V600E) activating point mutation of the B-Raf gene in a conventional type of human PTC.
Clinicopathological Correlation
B-Raf Mutation in PTC
The B-RafV600E mutation represents the hallmark of PTC, and to date it has not been identified in medullary thyroid carcinomas, FTCs, or benign thyroid neoplasms (adenoma or hyperplasia) (Fig. 2). Of 320 thyroid tumors, Nikiforova et al17 found no mutation in 13 medullary carcinomas, 32 follicular and Hurthle cell carcinomas, 46 follicular and Hurthle adenomas, and 65 hyperplastic nodules. In this study, mutations were found in 38% (45/119) of papillary carcinomas, 13% (2/16) of poorly differentiated carcinomas, and 10% (3/29) of anaplastic thyroid carcinomas. Interestingly, B-Raf mutations also present in some follicular variants of PTC tumors (~12%), but they have not been seen in any of the oncocytic PTCs.18
Figure 2.
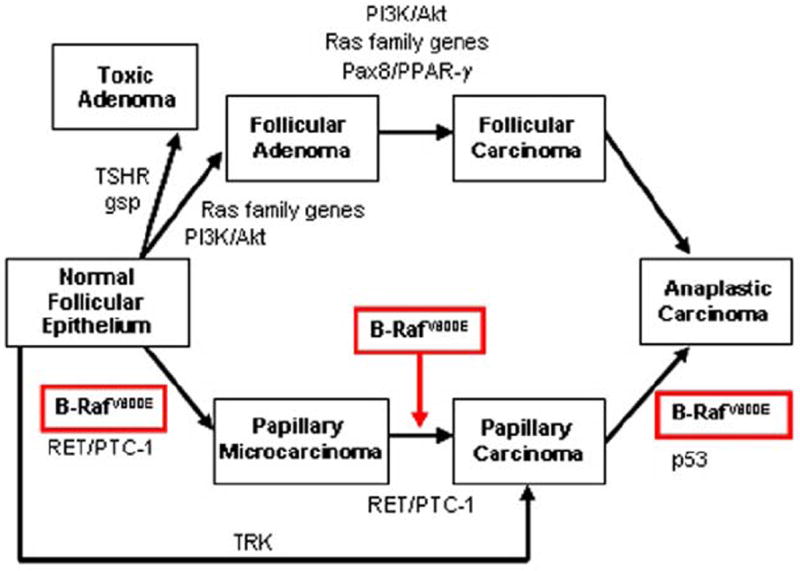
Molecular markers involved in epithelial thyroid tumorigenesis. This model illustrates the 2 major signaling pathways, the PI3K/Akt (includes PIK3CA mutations and amplifications or PTEN mutations) and the B-RafV600E, which, respectively, play a major role in the tumorigenesis of FTC and PTC. B-RafV600E causes transformation of normal thyroid follicular cells, and initiation and progression of papillary thyroid micro- (≤1 cm) and macro-carcinoma (>1 cm) to anaplastic thyroid carcinoma. Other molecular markers of human thyroid tumors include the Ras family genes (H-, K-, and N-Ras) participating in tumorigenesis of follicular thyroid carcinoma, protein-tyrosine kinase oncogenic alterations (RET/PTC-1 or TRK translocations) in papillary carcinoma, and PAX8/PPRA-γ (proliferator-activated receptor gamma) rearrangement in follicular tumors. Activating point mutations of TSH receptor (TSHR) and gsp (subunit of Gs protein) genes can determine the transformation of normal follicular epithelium to toxic adenoma.
B-Raf mutations and aggressive tumor behavior
B-RafV600E is usually reported to be associated with aggressive disease. The frequency of B-RafV600E has been shown to range from 12% in follicular variants, to 60% in classical PTCs, and to as high as 77% in tall-cell variant, a PTC variant that is associated with more aggressive clinical behavior and loss of responsiveness to radioiodine.19 Further, an association has been reported between B-RafV600E and the presence of both extrathyroidal extension and higher clinical stage.9,20 An association was also demonstrated between B-RafV600E mutation and older age, as well as an association of the mutation with male gender.19 Importantly, patients with PTC positive for the B-RafV600E mutation followed for at least 15 years were shown to have the worst outcome (persistent disease and lower survival rate)21 independent of other negative clinicopathological features. In a recent study by Xing et al22 BRAF mutation examined in FNAB specimens versus the wild-type allele strongly predicted extrathyroidal extension (23% vs 11%, respectively) as well as thyroid capsular invasion (29% vs 16%, respectively). Further, BRAF mutation was strongly associated with PTC persistence or recurrence disease in their study (36% vs 12%, respectively). A significant association was also found between B-RafV600E-positive tumors and distant PTC metastases.23,24 Interestingly, B-RafV600E (and not a RET/PTC translocation) is found in anaplastic thyroid cancers, suggesting that this mutation may be involved in progression of thyroid cancer from PTCs to poorly differentiated and undifferentiated phenotypes.17,25,26 Although found to be less frequent in micro-PTCs compared with PTCs larger than 1 cm (24.3% vs 45.5%, respectively), B-RafV600E was associated with extrathyroidal extension and advanced stage even in micro-PTCs.27,28 These data suggest that B-RafV600E may confer a higher risk of tumor progression and invasiveness in micro-PTCs, potentially serving as a predictive indicator of aggressive behavior in these small tumors. Despite the above data, some controversy remains regarding the association between B-RafV600E mutation and tumor aggressiveness, and a few studies found no correlation between the two.29,30 These conflicting data could stem from several variables, including different patient recruitment strategies, epidemiological factors, small numbers of cases, and the histological tumor classification.
B-Raf mutation and lymph node metastases
Although some studies showed no association between the presence of BRAF mutation and lymph node metastases,29 other studies have shown higher frequency of nodal metastasis in PTC harboring B-RafV600E mutation22,31 and a high prevalence of B-RafV600E mutation in metastasized PTC to lymph nodes.32 Looking at the role of B-RafV600E mutation in the progression of PTC, Kim et al33 assessed B-RafV600E status in 103 papillary tumors and their matched sentinel lymph nodes. They found that in 26 of 34 (76%) patients with B-Raf mutations, concomitant lymph node metastases were detected. This was compared with 12 of 69 (17%) patients with negative B-Raf PTCs that had lymph node metastasis (P <.0001). In a univariate analysis, age, TNM tumor stage, tumor size, and B-Raf mutation were found to be prognostic factors for lymph node metastases; however, on a multivariate analysis, B-Raf gene mutation was the only significant prognostic factor for lymph node metastases, increasing the risk of metastases by nearly 13-fold. Xing et al22 also found BRAF mutation to be significantly associated with lymph node metastasis (38% vs 18% in PTC with wild-type allele). Interestingly, Rodolico et al34 found metastatic PTC lymph nodes bearing the B-RafV600E mutation to be larger in size and exhibit higher prevalence of extracapsular invasion compared with wild-type B-Raf lymph nodes.
B-Raf mutations and loss of iodine avidity
Iodine 131 continues to be one of the mainstays of therapy for differentiated thyroid cancer of follicular cell origin. Retrospective studies show a significant reduction in the rates of disease recurrence and cause-specific mortality with postoperative radioiodine remnant ablation in high-risk patients.35 In addition, 131I is the main treatment modality in patients with radioiodine uptake (iodine-avid) metastatic disease.36 The metabolism of radioiodine in papillary and follicular carcinoma is profoundly altered mainly due to decreased iodine uptake via the sodium-iodide symporter (NIS), reduced iodine organification, and shorter effective half-life of iodine in tumor tissue.37 An interesting association was found between B-RafV600E mutation in the primary PTC and loss of radioiodine avidity in the recurrent tumor, where recurrent disease in the B-RafV600E-positive patients required more aggressive treatments (surgery and external-beam radiation therapy) than the recurrent disease in the B-RafV600E-negative patients, most of whom were cured by repeating the 131I treatment alone.38 The role of B-RafV600E in thyroid tumor dedifferentiation was further studied through the expression of the Na+/I− symporter (NIS) mediating active I− transport into the thyroid follicular cells. After demonstrating a significant association between B-RafV600E and cancer recurrence, Riesco-Eizaguirre et al39 found that most of the recurrences associated with B-RafV600E were lacking radioiodine (131I) avidity. They further studied the NIS immunoreactivity in 60 tumor samples and found that PTCs harboring the B-RafV600E expressed significantly less immunoreactivity then those without the mutation. Durante et al40 examined the expression of key markers in thyrocyte differentiation in 56 PTCs with B-RafV600E and 37 with wild-type B-Raf. While the mRNA levels for all thyroid-specific genes were reduced in all PTCs versus normal thyroid tissue, the expression of NIS, the apical iodide transporter (AIT-B), Tg (thyroglobulin), as well as TPO (thyroperoxidase) were significantly lower in the B-RafV600E-positive tumors versus the wild-type group. Specifically, the transcript levels of NIS and AIT in B-RafV600E tumors were reduced by 82% and 86%, respectively. Further, the limited amounts of NIS protein that were expressed in these tumors were confined almost exclusively to the cytoplasm rather than targeted to the plasma membrane. Finally, Romei et al41 showed that both the expression of NIS mRNA and the percentage of NIS-positive cells were significantly lower in B-RafV600E-positive PTCs compared with nonmutated samples. These data further suggest that PTCs harboring the B-RafV600E may lose their ability to trap 131I as part of a dedifferentiation process that occurs in B-RafV600E-positive tumors. The precise impact of a B-RafV600E mutation on the diagnostic or therapeutic use of radioiodine has not yet been established.
In summary, B-RafV600E mutations are common and appear to play an important role in the biological behavior of PTCs. It may therefore serve as a good target in thyroid FNABs, with potentially significant implications for clinical management.
Detection of B-RafV600E in Thyroid FNAB
Testing for B-RafV600E mutation in thyroid tumors is simplified by the fact that virtually all mutations are restricted to nucleotide position 1796. Polymerase chain reaction (PCR) amplification followed by direct sequencing is probably the most common method used for B-RafV600E mutation detection to date42,43 (Fig. 1). However, many other methods have been successfully used, including LightCycler PCR (LC PCR) analysis,17 the Colorimetric Mutector assay (Mutector, TrimGen, Sparks, MD) employing a primer extension method,26,44,45 single-strand conformational polymorphism (SSCP) analysis,46 and restriction fragment length polymorphism (PCR-RFLP).47 More recently, Sapio et al48 developed an assay based on mutant allele-specific PCR amplification (MASA) to detect the B-RafV600E mutation, and found it to be a more sensitive method compared with SSCP and direct DNA sequencing. Chung et al49 compared direct DNA sequencing and PCR-RFLP for the detection of B-RafV600E mutation in ex vivo FNABs; with a B-RafV600E mutation rate of 83%, direct DNA sequencing had a sensitivity and specificity of 83% and 96%, while PCR-RFLP had sensitivity and specificity of only 79% and 80%, respectively. Finally, Jin et al42 examined the diagnostic accuracy of B-RafV600E mutation detection in 71 FNA samples (58 PTCs, 13 non-PTC lesions, and 5 indeterminate/suspicious lesions). They used 4 different methods, including direct sequencing, colorimetric Mutector Assay, real-time LC PCR with fluorescence resonance energy transfer probes, and an allele-specific LC PCR with CYBR green 1. The B-RafV600E mutation was detected in 31 of 58 (53%) PTC FNAs but in none of the 13 non-PTCs lesions. They found similar sensitivity (54%) and specificity (100%) for the 4 detection methods, with the CYBR green 1 method being the most rapid, easiest to perform, and least expensive.
The detection of B-RafV600E mutation was also shown to be easily performed in FNAB specimens from thyroid nodules.43,45,46 Xing et al43 used direct DNA sequencing and the colorimetric Mutector technique to evaluate B-RafV600E mutation status in preoperative cytological specimens of patients with thyroid nodules for whom thyroid surgery was planned. Three to 4 passes with a 27- to 22-gauge needle were made to harvest cytological material for analysis, and DNA was isolated. Cytological specimens from all the patients with benign lesions (21 patients), those with FTCs (5 patients), and the 1 patient with Hurthle cell carcinoma were all negative for B-RafV600E mutation, whereas there was a 50% prevalence of B-RafV600E mutation in patients with a histopathological diagnosis of PTC.
B-RafV600E Screening in Preoperative FNAB
Given that FNAB is currently the best diagnostic tool in the clinician’s armamentarium when working up a thyroid nodule, it would be useful if mutational analysis on the FNAB specimen increased the diagnostic yield and accuracy for malignancy in the index nodule. Traditionally, about 10% to 20% of thyroid nodule FNABs prove to be inadequate for cytological diagnosis, with the majority possessing insufficient levels of tumor DNA to undergo nucleic acid preparation. In another 20%, it is not possible to determine with certainty whether the nodule is an adenoma or carcinoma. Ten percent of nodules associated with nondiagnostic FNABs, and 20% of FNABs with indeterminate findings, are ultimately diagnosed as malignant on histological analysis.3 Surgery is generally required in patients with nondiagnostic (after repeated attempts) or indeterminate cytology, even though at most only about 10% to 20% of them eventually prove to harbor malignancy.
Nondiagnostic FNAB results
Nondiagnostic FNAB usually results from limited cellularity, absence of follicular cells, or poor fixation and preservation.1 Similar to conventional cytological testing, mutation testing may fail if the tumor cell content in the aspirate is inadequate, although amplification techniques may be able to overcome this limitation. In a study by Sapio et al, of 46 inadequate FNAB samples, none were found to harbor B-RafV600E mutations, 26 of whom were operated on and found to be benign.50 Salvatore et al46 found the molecular analysis of B-Raf to be useful in 1 of 4 insufficient FNAB samples that were classic PTC tumors, one of which showed the presence of the B-Raf mutation.
Indeterminate FNAB results
Indeterminate cytological category is composed of follicular lesions of undetermined significance (atypical cells of undetermined significance) and follicular neoplasms (microfollicular pattern), as well as lesions with features of malignancy but not quite meeting the histological criteria of malignancy (suspicious cytology).1,2
The prevalence of B-RafV600E mutation in indeterminate thyroid nodules is not well defined, with published rates of 8% to 27% in a few small studies43,45,46,51 (Table 1). Tumors associated with microfollicular pattern are usually benign, and when malignant (5%–30%) most commonly represent follicular variants of PTC and rarely FTC or other malignancies.52,53 These tumors are therefore only rarely or not at all associated with B-RafV600E mutations, respectively. Suspicious lesions by contrast often turn out to be malignant (50%–75%) with the majority being PTCs. Cohen et al45 analyzed 91 archival preoperative FNA thyroid smears that included 55 nodules with indeterminate cytology. B-Raf mutation was detected in 5 of 32 (16%) carcinomas within the indeterminate group—samples that could not be conclusively diagnosed by FNAB alone. Importantly, a carcinoma was more likely to harbor a B-Raf mutation if the preoperative FNAB sample was interpreted as suspicious for PTC (2 of 6, 33%) than if it was a follicular neoplasm or a follicular neoplasm with cytological atypia suspicious for follicular variant of PTC (3 of 49, 6%). Salvatore et al46 looked at 96 cases of PTC for both the B-RafV600E and RET/PTC mutation in archival FNA thyroid smears. B-RafV600E was detected in 38% of PTC FNAB samples, and the identification of B-RafV600E refined the diagnosis of PTC in 3 of 11 indeterminate cytological samples. Sapio et al50 found that of 21 indeterminate FNABs, none had B-RafV600E mutations; 20 of those had surgery and none were found to have PTC; however, 2 were found to have other cancers—1 follicular carcinoma and 1 medullary thyroid carcinoma. Of 16 specimens that were suspicious for PTC, B-Raf mutation was detected in 4 of 6 (67%) carcinomas.
Table 1.
Frequency of BRAF mutation in indeterminate thyroid nodules
| Authors | Year | Frequency (mutation/total, %)
|
|||||
|---|---|---|---|---|---|---|---|
| PTC | FVPTC | FTC | HCC | Benign neoplasm | Total | ||
| Cohen et al | 2004 | 3/8 (38) | 2/21 (10) | 0/2 (0) | 0/1 (0) | 0/23 (0) | 5/55 (9) |
| Salvatore et al* | 2004 | 2/5 (40) | 1/6 (17) | — | — | — | 3/11 (27) |
| Xing et al | 2004 | 2/7 (29) | 0/5 (0) | 0/1 (0) | 0/12 (0) | 2/25 (8) | |
| Rowe et al* | 2005 | 3/14 (21) | 0/5 (0) | — | — | — | 3/19 (16) |
| Sapio et al | 2007 | — | — | 0/1 (0) | — | 0/18 (0) | 0/20† |
PTC = papillary thyroid cancer; FVPTC = follicular variant of papillary thyroid cancer; FTC = follicular thyroid cancer; HCC = Hurthle cell carcinoma.
Included only indeterminate samples that revealed to be PTC on final histopathology.
One indeterminate nodule turned out to be medullary thyroid cancer.
In summary, with statistical analysis, the small prevalence of B-RafV600E mutation in follicular lesions and follicular neoplasms translates into a low sensitivity of B-RafV600E mutation analysis for diagnosing malignancy. Further, it is important to note that since the prevalence of B-RafV600E mutation in FNAB proven PTCs varies from 29% to 83%, negative FNAB findings do not exclude the presence of a PTC. In a nodule with suspicious FNAB cytology, with traditional cytological testing the risk for cancer is higher (50%–75%)1 and surgery is indicated. Frozen section may sometimes accurately confirm PTC during surgery, and knowledge of the B-Raf mutation status may then serve as a guide for the extent of surgery as summarized below.
Thyroid nodules with PTC cytological diagnosis
Another potential benefit for the detection of B-RafV600E mutation in preoperative FNABs could be selection of a group of patients with potentially increased risk of recurrence. Most patients with differentiated thyroid cancer have an excellent prognosis with an overall 10-year survival of 85% to 90%.54-56 However, 10% to 15% of patients do recur, usually within the neck and the regional lymph nodes.56 Ten-year survival rates after local recurrence range from 49% to 68%, dropping to 25% to 42% for those that recur with distant metastases.56 Neck recurrences alone are responsible for a third of thyroid cancer–related deaths. Therefore, accurate staging is important in determining the prognosis and tailoring the appropriate treatment for the individual patient.
The extent of surgery for PTC remains controversial. Consensus guidelines for the treatment of PTC suggest that patients with tumors displaying high-risk features should undergo a total or near-total thyroidectomy, and patients with single, small (<1 cm), low-risk, intrathyroidal node-negative PTC may be candidates for less extensive surgery such as lobectomy. Routine central compartment neck dissection should be considered for patients with PTC, alternatively, near-total or total thyroidectomy followed by radioactive iodine therapy without central neck dissection may be appropriate.54 Bilimoria et al57 recently reviewed 52,173 patients from the National Cancer DataBase (1985–1998) who underwent surgery for PTC and found that for PTC <1 cm the extent of surgery did not impact recurrence or survival, whereas for tumors ≥1 cm, lobectomy was associated with a 15% higher risk of recurrence and 31% higher risk of death. Since the evidence suggests that B-RafV600E may also confer a higher risk of tumor progression and invasiveness in micro-PTCs, B-RafV600E positive microcarcinomas may warrant a different, more aggressive, initial surgical strategy.
The rate of regional lymph node metastases in patients with PTC varies from between 20% and 90%. Nodal metastasis predominate in the cervico-central and ipsilateral cervico-lateral compartments.58 The lack of reliable criteria for the diagnosis of nodal metastases other than histopathology has greatly contributed to the ongoing controversy about the extent of lymph node dissection. Fewer than 50% of PTC metastases are correctly identified intraoperatively by experienced surgeons.58 When performed, lymph node dissection in thyroid cancer has both a curative intent as well as a role in palliation, helping to prevent the invasion of adjacent structures by disease.59 There are no prospective, randomized trials that evaluate the impact of central lymph node dissection on recurrence or disease specific mortality in PTC.60 Existing data suggest that central lymph node dissection may decrease recurrence of PTC and improve disease-specific survival. There are also limited data to suggest a survival benefit with prophylactic central neck dissection. However, central neck dissection may be associated with higher rates of permanent hypoparathyroidism and permanent nerve injury, especially when performed as a reoperation for recurrence. Given the data shown above indicating higher frequency of nodal metastasis in PTC harboring B-RafV600E mutation and higher rates of recurrence with B-RafV600E-positive tumors, a more aggressive initial operation including prophylactic central neck dissection may be warranted in these cases. Further, in view of the association between B-RafV600E mutation and loss of radioiodine avidity, every attempt should be made to eliminate all macro- and microscopic disease in the initial surgery.
Other molecular biomarkers
The application of other thyroid cancer biomarkers in thyroid tissue are beyond the scope of this review and are well summarized in the review of Shibru et al.61 Apart from B-Raf, the common genetic alterations in thyroid cancer include RET/PTC, NTRK, RAS, and PAX8-PPARγ. These are usually mutually exclusive and will be present in approximately 90% of all thyroid cancer of follicular origin (Fig. 2). Combined testing for these mutations will probably result in higher rates of improving the accuracy of FNAB. In fact, in a recent prospective study of Nikiforov et al, testing for a panel of mutations in preoperative FNAB, including B-Raf, RAS, RET/PTC, and PAX8/PPARγ mutations resulted in a 100% positive predictive value for malignancy when a mutation was detected in a nodule with indeterminate cytology.62
Multiple expression profiling studies have been performed in an attempt to identify differentially expressed genes with an important role in cancer development or progression. Technologies such as cDNA microarrays, oligonucleotide arrays, and serial analysis of gene expression (SAGE) have been utilized. A meta-analysis of thyroid cancer biomarkers using a gene ranking system identified consistent gene expression markers that included MET, TFF3, SERPINA1, TIMP1, FN1, and TPO.63
Recently, studies have demonstrated the potential usefulness of microRNA (miRNA) profiling as a diagnostic aid in the preoperative evaluation of thyroid nodules. A strong correlation was found between the miRNA profile and the mutational status (BRAF, RET/PTC, or RAS), and further, certain miRNAs were found to be upregulated in FNAB samples obtained from nodules found to be malignant after surgery.64
Finally, advances in proteomics have also improved our ability to diagnose malignant conditions. In a study of Linkov et al, a set of 4 serum markers, including interleukin-8, hepatocyte growth, monocyte-induced interferon gamma, and interleukin-12 p40 achieved discrimination between benign and malignant groups in a multivariate analysis, suggesting that multiplex panels of serum biomarkers may improve our ability to diagnose cancer.65
Conclusions
Appropriate management of patients with thyroid nodules is based on an accurate diagnosis. Several diagnostic challenges are often encountered in the clinical management of thyroid nodules. Currently, the risk assessment when dealing with an indeterminate nodule is based on other nodule characteristics, contralateral nodules, patient characteristics and surgeon and patient preference. New advances in gene expression technology have opened a new venue for identifying markers for diagnosing cancerous nodules. The detection of B-RafV600E in routine FNAB may not be cost-effective in all patients undergoing FNA biopsy of a thyroid nodule, and therefore its potential to improve the selection of nodules for surgical removal may currently be limited. However, when the pathologist sees cytological evidence that is positive or suspicious for PTC, B-RafV600E assessment may enhance the preoperative risk and prognostic evaluation. B-RafV600E has several advantages in serving as a diagnostic and prognostic marker for PTC: (1) it represents the most commonly targeted gene in the development of PTC; (2) it is a stable molecular marker with 100% specificity and relatively high sensitivity; and (3) most data show that B-RafV600E lends more aggressive properties to PTC. Given the increasing prevalence of thyroid nodules, thyroid cancer and the widespread popularity of FNA, a significant number of patients with ultimately proven PTCs may benefit from B-Raf mutational analysis in their original FNA material. Management of PTC based on accurate risk stratification and prognostic evaluation is important for reducing recurrences and avoiding the need for reoperations, which carry an increased morbidity. Larger prospective studies are still necessary to calculate the utility of B-RafV600E mutation analysis diagnostically as well as its role in altering treatment regimens. Furthermore, when considering major clinical practice changes, utilization of a wider mutational/genomics or proteomics platform will likely be important in lieu of a single mutation analysis.
Acknowledgments
Dr Mekel is a recipient of fellowship grant from the American Physicians Fellowship for Medicine in Israel. Dr Mekel is also supported in part by Rambam-Health Care Campus, Israel, the American Friends of Rambam, Israel Medical Association and the Israel Cancer Association.
References
- 1.Baloch ZW, Livolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–37. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 2.Alexander EK. Approach to the patient with a cytologically indeterminate thyroid nodule. J Clin Endocrinol Metab. 2008;93:4175–82. doi: 10.1210/jc.2008-1328. [DOI] [PubMed] [Google Scholar]
- 3.Puxeddu E, Durante C, Avenia N, et al. Clinical implications of BRAF mutation in thyroid carcinoma. Trends Endocrinol Metab. 2008;19:138–45. doi: 10.1016/j.tem.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Russo D, Arturi F, Pontecorvi A, et al. Genetic analysis in fine-needle aspiration of the thyroid: a new tool for the clinic. Trends Endocrinol Metab. 1999;10:280–5. doi: 10.1016/s1043-2760(99)00169-1. [DOI] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Michaloglou C, Vredeveld LC, Mooi WJ, et al. BRAF (E600) in benign and malignant human tumours. Oncogene. 2008;27:877–95. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 7.Emuss V, Garnett M, Mason C, et al. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 2005;65:9719–26. doi: 10.1158/0008-5472.CAN-05-1683. [DOI] [PubMed] [Google Scholar]
- 8.Kondo T, Nakazawa T, Murata S, et al. Enhanced B-Raf protein expression is independent of V600E mutant status in thyroid carcinomas. Hum Pathol. 2007;38:1810–8. doi: 10.1016/j.humpath.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Brummer T, Martin P, Herzog S, et al. Functional analysis of the regulatory requirements of B-Raf and the B-Raf(V600E) oncoprotein. Oncogene. 2006;25:6262–76. doi: 10.1038/sj.onc.1209640. [DOI] [PubMed] [Google Scholar]
- 10.Leicht DT, Balan V, Kaplun A, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–21. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 12.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 13.Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–53. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 14.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 15.Nucera C, Goldfarb M, Hodin R, et al. Role of B-Raf(V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-raf (V600E) Biochim Biophys Acta. 2009;1795:152–61. doi: 10.1016/j.bbcan.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuveson DA, Weber BL, Herlyn M. BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell. 2003;4:95–8. doi: 10.1016/s1535-6108(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 18.Musholt PB, Musholt TJ, Morgenstern SC, et al. Follicular histotypes of oncocytic thyroid carcinomas do not carry mutations of the BRAF hot-spot. World J Surg. 2008;32:722–8. doi: 10.1007/s00268-007-9431-6. [DOI] [PubMed] [Google Scholar]
- 19.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38–46. doi: 10.1002/cncr.22754. [DOI] [PubMed] [Google Scholar]
- 21.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–9. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 22.Xing M, Clark D, Guan H, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–82. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–7. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 24.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–70. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro M, Carlomagno F, Hay ID, et al. Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest. 1992;89:1517–22. doi: 10.1172/JCI115743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begum S, Rosenbaum E, Henrique R, et al. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–63. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 27.Frasca F, Nucera C, Pellegriti G, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 28.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:4085–90. doi: 10.1210/jc.2007-1179. [DOI] [PubMed] [Google Scholar]
- 29.Puxeddu E, Moretti S, Elisei R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–20. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- 30.Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–95. doi: 10.1007/s00428-005-1236-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim KH, Kang DW, Kim SH, et al. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004;45:818–21. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 32.Vasko V, Hu S, Wu G, et al. High prevalence and possible de novo formation of BRAF mutation in metastasized papillary thyroid cancer in lymph nodes. J Clin Endocrinol Metab. 2005;90:5265–9. doi: 10.1210/jc.2004-2353. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Giuliano AE, Turner RR, et al. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Ann Surg. 2006;244:799–804. doi: 10.1097/01.sla.0000224751.80858.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodolico V, Cabibi D, Pizzolanti G, et al. BRAF V600E mutation and p27 kip1 expression in papillary carcinomas of the thyroid < or = 1 cm and their paired lymph node metastases. Cancer. 2007;110:1218–26. doi: 10.1002/cncr.22912. [DOI] [PubMed] [Google Scholar]
- 35.Mazzaferri EL, Jhiang SM. Differentiated thyroid cancer long-term impact of initial therapy. Trans Am Clin Climatol Assoc. 1995;106:151–68. [PMC free article] [PubMed] [Google Scholar]
- 36.Maxon HR, 3rd, Smith HS. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am. 1990;19:685–718. [PubMed] [Google Scholar]
- 37.Robbins RJ, Schlumberger MJ. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46(Suppl 1):28S–37S. [PubMed] [Google Scholar]
- 38.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 39.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I-targeting to the membrane. Endocr Relat Cancer. 2006;13:257–69. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 40.Durante C, Puxeddu E, Ferretti E, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–3. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- 41.Romei C, Ciampi R, Faviana P, et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr Relat Cancer. 2008;15:511–20. doi: 10.1677/ERC-07-0130. [DOI] [PubMed] [Google Scholar]
- 42.Jin L, Sebo TJ, Nakamura N, et al. BRAF mutation analysis in fine needle aspiration (FNA) cytology of the thyroid. Diagn Mol Pathol. 2006;15:136–43. doi: 10.1097/01.pdm.0000213461.53021.84. [DOI] [PubMed] [Google Scholar]
- 43.Xing M, Tufano RP, Tufaro AP, et al. Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab. 2004;89:2867–72. doi: 10.1210/jc.2003-032050. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum E, Hosler G, Zahurak M, et al. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod Pathol. 2005;18:898–902. doi: 10.1038/modpathol.3800252. [DOI] [PubMed] [Google Scholar]
- 45.Cohen Y, Rosenbaum E, Clark DP, et al. Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res. 2004;10:2761–5. doi: 10.1158/1078-0432.ccr-03-0273. [DOI] [PubMed] [Google Scholar]
- 46.Salvatore G, Giannini R, Faviana P, et al. Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2004;89:5175–80. doi: 10.1210/jc.2003-032221. [DOI] [PubMed] [Google Scholar]
- 47.Hayashida N, Namba H, Kumagai A, et al. A rapid and simple detection method for the BRAF(T1796A) mutation in fine-needle aspirated thyroid carcinoma cells. Thyroidology. 2004;14:910–5. doi: 10.1089/thy.2004.14.910. [DOI] [PubMed] [Google Scholar]
- 48.Sapio MR, Posca D, Troncone G, et al. Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA) Eur J Endocrinol. 2006;154:341–8. doi: 10.1530/eje.1.02072. [DOI] [PubMed] [Google Scholar]
- 49.Chung KW, Yang SK, Lee GK, et al. Detection of BRAFV600E mutation on fine needle aspiration specimens of thyroid nodule refines cyto-pathology diagnosis, especially in BRAF600E mutation-prevalent area. Clin Endocrinol (Oxf) 2006;65:660–6. doi: 10.1111/j.1365-2265.2006.02646.x. [DOI] [PubMed] [Google Scholar]
- 50.Sapio MR, Posca D, Raggioli A, et al. Detection of RET/PTC, TRK and BRAF mutations in preoperative diagnosis of thyroid nodules with indeterminate cytological findings. Clin Endocrinol (Oxf) 2007;66:678–83. doi: 10.1111/j.1365-2265.2007.02800.x. [DOI] [PubMed] [Google Scholar]
- 51.Rowe LR, Bentz BG, Bentz JS. Utility of BRAF V600E mutation detection in cytologically indeterminate thyroid nodules. Cytojournal. 2006;3:10. doi: 10.1186/1742-6413-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–15. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein RE, Netterville JL, Burkey B, et al. Implications of follicular neoplasms, atypia, and lesions suspicious for malignancy diagnosed by fine-needle aspiration of thyroid nodules. Ann Surg. 2002;235:656–62. doi: 10.1097/00000658-200205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroidology. 2006;16:109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 55.Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 56.Baudin E, Schlumberger M. New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148–56. doi: 10.1016/S1470-2045(07)70034-7. [DOI] [PubMed] [Google Scholar]
- 57.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–81. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machens A, Hinze R, Thomusch O, et al. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002;26:22–8. doi: 10.1007/s00268-001-0176-3. [DOI] [PubMed] [Google Scholar]
- 59.Dralle H, Machens A. Surgical approaches in thyroid cancer and lymph-node metastases. Best Pract Res Clin Endocrinol Metab. 2008;22 doi: 10.1016/j.beem.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 60.White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg. 2007;31:895–904. doi: 10.1007/s00268-006-0907-6. [DOI] [PubMed] [Google Scholar]
- 61.Shibru D, Chung KW, Kebebew E. Recent developments in the clinical application of thyroid cancer biomarkers. Curr Opin Oncol. 2008;20:13–8. doi: 10.1097/CCO.0b013e3282f27e49. [DOI] [PubMed] [Google Scholar]
- 62.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–8. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 63.Griffith OL, Melck A, Jones SJ, et al. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24:5043–51. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- 64.Nikiforova MN, Chiosea SI, Nikiforov YE. MicroRNA expression profiles in thyroid tumors. Endocr Pathol. 2009;20:85–91. doi: 10.1007/s12022-009-9069-z. [DOI] [PubMed] [Google Scholar]
- 65.Linkov F, Ferris RL, Yurkovetsky Z, et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics Clin Appl. 2008;2:1575–85. doi: 10.1002/prca.200780095. [DOI] [PMC free article] [PubMed] [Google Scholar]


