Abstract
Objective
To determine how often unexpected FDG PET-CT findings result in change of management of stage IV and clinically evident stage III melanoma patients with resectable disease based on conventional imaging.
Materials and methods
32 oligometastatic patients with stage IV and clinically evident stage III melanoma were identified by surgical oncologists based on the results of conventional imaging, which included contrast-enhanced CT of the chest, abdomen and pelvis and MRI of the brain. The surgical plan included resection of known metastases and/or isolated limb perfusion with chemotherapy. 33 FDG PET-CT scans were performed within 36 days of their contrast-enhanced CT. The impact of PET-CT was defined as the percentage of cases in which a change in surgical plan resulted from the unanticipated PET-CT findings.
Results
PET-CT demonstrated unexpected melanoma metastases in 12 % of scans (4 out of 33). As a result the surgery was cancelled in two patients, and the planned approach was altered in another two patients to address the unexpected sites‥ In 6 % of scans (2 out of 33) the unexpected metastases were detected in the extremities, not included in conventional imaging. Three scans (9%) showed false positive FDG avid findings which proved to be benign by subsequent stability or resolution with no therapy.
Conclusion
In patients with surgically-treatable metastatic melanoma, FDG PET-CT can detect unexpected metastases which are missed or not imaged with conventional imaging, and can be considered as part of preoperative workup.
Keywords: FDG-PET, PET-CT, melanoma, imaging, additive value
Background
Advanced stage malignant melanoma (AJCC stage III and IV) bears a grave prognosis, in part due to a lack of effective systemic therapy. Surgery is one of the most effective tools for local, regional, and distant control of disease, and may prolong survival in selected patients[2]. However, patient selection requires very accurate staging information to confirm the limited nature of metastatic disease which may be most amenable to surgical resection, i.e. confined to the proposed surgical field.
Conventional imaging routinely used for staging of potential surgical candidates with melanoma includes contrast-enhanced CT of the chest, abdomen and pelvis and MRI of the brain. CT of the neck may also be performed for primary melanoma of the scalp, face and neck. In addition, ultrasound of regional nodal basins is often used for diagnostic and screening purposes and as a guide for biopsies.
Positron emission tomography with x-ray computed tomography (PET-CT) performed with 18F-fluorodeoxyglucose (FDG) is a well-established imaging modality used for staging of melanoma patients, and melanoma metastases are typically well-visualized on PET[3]. The value of PET-CT is highest in the setting of macroscopic metastatic disease (stage IIIB and higher). The impact of PET-CT has been shown to be lower in the evaluation of patients with stage I-II melanoma[4], and also in stage IIIA with microscopic metastasis in the sentinel lymph nodes[5]. Similarly contrast-enhanced CT has not proven accuracy in preoperative detection of microscopic metastases in early stage melanoma[6]. PET-CT offers several potential advantages over conventional CT imaging, including imaging broader field, higher sensitivity for intramuscular and bone metastases, and a high specificity for the evaluation of recurrence within postsurgical sites. Despite these advantages, falsely negative PET-CT scans can occur in the setting of small-volume disease or non-FDG avid tumors [7], and falsely positive PET-CT scans can occur due to presence of benign inflammatory conditions or additional primary tumors (such as thyroid nodules). The impact of false positive PET-CT scans is higher in patients with early-stage disease than in those with advanced disease.
Despite the reported accuracy of FDG-PET-CT, this modality has not become a part of conventional imaging for melanoma staging. FDG-PET-CT is not routinely used for the presurgical evaluation of patients with advanced melanoma at our institution. A short survey among the group of our surgical oncologists revealed a low usage of PET-CT in the preoperative setting. In contrast to medical oncologists in melanoma group, the majority of our surgeons did not feel that PET-CT was an important tool for the clinical decision making. Many patients with clinically evident stage III melanoma and some of the oligometastatic patients with stage IV disease referred to surgery based on conventional imaging findings proceed with resection without additional preoperative evaluation by PET-CT. Reluctance to use PET-CT originates mainly from a lack of confidence that PET-CT will add important information not already apparent on high-quality conventional imaging. There is also concern that false positive results could needlessly delay surgery and lead to additional expensive and invasive procedures including biopsies and endoscopies.
Our prospective study was designed to investigate the additive value of PET-CT in the clinical management of patients with clinically evident AJCC stage III and IV metastatic melanoma considered for surgical treatment. Our working hypothesis was that the ability of PET-CT to show management-changing findings in the preoperative setting was relatively low. We felt the need to test our existing imaging ordering practice. In case our hypothesis had proven wrong, this could change the approach of melanoma surgical oncologists to preoperative imaging work up.
Materials and Methods
This prospective study was approved by the Institutional Review Board at MD Anderson Cancer Center. Adult melanoma patients with stage IV or clinically evident stage III with nodal metastases or in transit disease considered candidates for surgery and/or locoregional invasive therapy (isolated limb perfusion) were enrolled into the study. All patients underwent initial conventional imaging which included contrast-enhanced CT of chest, abdomen and pelvis and MRI of the brain. Contrast-enhanced CT of the neck was performed in all patients with a primary melanoma of the head and neck or with clinical evidence of metastases in the neck. CT was performed on 16 slice scanners (General Electric, Milwaukee, WI) with oral and rectal barium based contrast, with 125 ml of intravenous contrast containing 300 mg/ml of iodine administered at the rate of 3 ml/sec. The images were reconstructed at 2.5 mm intervals.
All patients were examined by one of the four surgical oncologists taking part in our study and were determined to have metastatic disease that was amenable for resection based on the combined results of physical examination and the findings on conventional CT imaging. For every patient, the goal of surgical resection was to render the patient free of all detectable metastasis. At this point the patients could proceed with surgical resection, according to the current standard of care accepted within the surgical oncology group.
After surgical candidates were identified, patients were invited to participate in the study exploring potential additive value of preoperative PET-CT. Candidates with microscopic nodal metastases limited to sentinel lymph nodes were excluded from the study. Patients with prior PET-CT performed within 60 days were also excluded, to prevent bias.
Written informed consent was obtained from all eligible patients explaining that FDG-PET-CT will be performed according to standardized protocol, but PET-CT findings will be analyzed and compared with conventional imaging and physical examination findings in the setting of our research. Prior to PET-CT imaging, the responsible surgical oncologist completed a patient management form which detailed the proposed surgical plan.
FDG PET-CT was performed within 36 days of conventional CT imaging. PET-CT scans were performed on a dedicated scanner (General Electric, Milwaukee, WI), of either 8-, 16-, or 64-slice CT. Scans were acquired in 3D mode, from the vertex of the skull to the feet, with the arms along the sides of the body, approximately 1 hour after intravenous administration of 8–12 mCi of 18F-fluorodeoxyglucose. CT scans were acquired without intravenous or gastrointestinal contrast and reconstructed at 3 mm intervals. PET-CT was interpreted by one of three experienced radiologists who were not blinded to the patient’s clinical information, or to the results of conventional imaging. Questionable and indeterminate scans were reviewed by all three radiologists and interpreted by consensus. In addition to the regular clinical report the standardized research form was filled by a radiologist outlining metastatic sites found on PET-CT, and the presence of absence of unexpected FDG-avid findings. The unexpected findings were rated into highly suspicious for metastasis or possibly benign based on visual inspection. Maximum SUV was not utilized as a criterion for lesion characterization.
Suspicious FDG-avid unexpected findings outside of the field of the presumed surgery were either biopsied or followed for 6 months. After 6 months follow-up, all stable non biopsy-proven suspicious sites were considered benign.
After reviewing PET-CT results, the surgical oncologist completed Part II of the patient management form which detailed the final treatment plan based on the combined results of the CT and PET-CT imaging. The number of cases in which the treatment plan was changed based on PET-CT findings was registered.
Statistical Analysis
The number of cases in which PET-CT changed the surgical plan was divided by the number of patients who underwent PET-CT to estimate the proportion of patients from this population whose surgical plan would be changed by PET-CT. Clopper-Pearson exact 95% confidence intervals were calculated based on the observed number of changed cases. Summary statistics were calculated to compare the attributes of those patients whose surgical plans were changed to those whose plans were not changed.
Results
38 patients were initially enrolled in our study from 2009 to 2011. Of these, 6 patients were withdrawn because of incomplete CT imaging of the chest, abdomen and pelvis in 4 patients, or interval between CT and PET-CT exceeded 36 days in 2 patients.
The final study group included 32 patients (23 men, 9 women) with an average age of 61 years (range: 39–82). Thirty patients had primary cutaneous melanoma; anatomic sites for the primary tumor included extremities (n=17), trunk (n=4), and head/neck region (n=5) and unknown primary (n= 4). Pathology results for the known primary melanomas were available in 22 of 32 patients, summarized in Table 1. Two patients had primary mucosal anal melanoma. Based on physical examination and conventional imaging, 24 patients were diagnosed with stage IIIC melanoma and 8 patients with stage IV melanoma. Metastases determined to be amenable for surgical resection were found in the lymph nodes and soft tissue (n=28) spleen (n=3), and in the adrenal gland (n=2). The maximum size of melanoma metastasis ranged from less than 1 mm to 90 mm, with an average of 24 mm. 5 patients received biochemotherapy within 2 months prior to PET-CT imaging with temozolomide, ciplatin, vinblastin and interleukin 2 followed by pegylated interferon alpha-2b.
TABLE I.
Comparison of Study Subgroups With and Without Unexpected Findings on Preoperative PET/CT
| Characteristic | Total (n = 32) |
Patients With No Unexpected Findings (n=28) |
Patients With Unexpected Findings (n = 4) |
|---|---|---|---|
| Age (y), median (range) | 61 (39–82) | 62 (39–82) | 56.7 (45–70) |
| Sex, no. of patients | |||
| Male | 23 | 19 | 4 |
| Female | 9 | 9 | 0 |
| Stage of melanoma, no. of patients | |||
| IIIC | 24 | 21 | 3 |
| IV | 8 | 7 | 1 |
| Primary melanoma Breslow thickness (mm), median (range)a | 2.7(0.2–19) | 1.7(0.2–5.9) | 8.8(1.8–19) |
| Primary melanoma Clark level, no. of patientsa | |||
| 4 | 8 | 6 | 2 |
| 3 | 11 | 10 | 1 |
| 2 | 2 | 3 | 0 |
| Primary melanoma mitotic figures/mm2, median (range)a | 7(1–24) | 7(1–24) | 5(1–12) |
| Primary melanoma vascular invasion present, no. of patients/totala | 3/20 | 2/17 | 1/3 |
| Size of metastasis amended for resection (mm), median (range) | 24 (0–90) | 23 (0–90) | 34 (13–53) |
| Time between the primary melanoma and enrollment in our study (months), median (range) | 38 (1–176) | 40 (1–176) | 31 (3–68) |
| Biochemotherapy before study enrollment, no. of patients/total | 6/32 | 4/28 | 2/4 |
| Time between PET/CT and CECT (months), median (range) | 12 (1–36) | 12 (1–36) | 15 (2–30) |
Not all characteristics of primary melanoma were available for all 32 study patients because of unknown primary, mucosal melanoma, or incomplete records of cutaneous primary melanoma excisional biopsy. Median value was calculated according to the available incomplete data.
Thirty-three PET-CT scans were performed in 32 study patients at 12 days following CT on average (range: 1 to 36 days). One patient had two PET-CTs on two different occasions prior to two planned surgical resections.
Four PET-CT studies revealed unexpected FDG-avid metastases not identified by clinical examination and conventional imaging (Table 2). In 2 of 4 cases, unexpected metastases were included within the scanning region but not detectable on CT, even upon retrospective review, including stomach (Fig. 1) and bones (Fig. 2). In the other 2 cases, soft tissue metastases were identified in the lower extremities, which were not included in conventional imaging (Fig. 3). Each of these 4 PET-CT scan results led to change in patient management, including the cancellation of surgical resection in 2 patients, and amending the resection plan in 2 patients to include resection of an additional metastasis and adjuvant isolated limb perfusion. The group of 4 patients with a change in management had similar characteristics as the group of 27 patients with no unexpected findings on PET-CT (Table 1), except larger metastases (34 mm versus 23 mm) and higher Breslow thickness of the primary melanomas (8.8 mm versus 1.7 mm).
TABLE 2.
Four Patients in Whom PET/CT Showed Unexpected Metastases and Altered Surgical Management
| Patient Enrollment No. |
Known Metastatic Site |
Proposed surgery Before PET/CT |
Maximal Size of Known Metastasis (mm) |
Location of Unexpected Metastasis on PET/CT |
Change in Management After PET/CT |
Figure |
|---|---|---|---|---|---|---|
| 32 | Right adrenal gland | Right adrenalectomy | 22 | Stomach | Addition of neoadjuvant chemotherapy; surgical plan changed to adrenalectomy and partial gastrectomy | Fig. 1 |
| 13 | Axillary lymph nodes | Axillary lymphadenectomy | 53 | Bones | Cancellation of surgery | Fig. 2 |
| 7 | Soft tissue in the arm | Wide local excision | 13 | Soft tissue in the leg | Cancellation of surgery | Fig. 3 |
| 31 | Pelvic and inguinal lymph nodes | Pelvic and inguinal lymph node dissection | 48 | Soft tissue in the leg below the knee | Addition of adjuvant isolated limb perfusion | Not applicable |
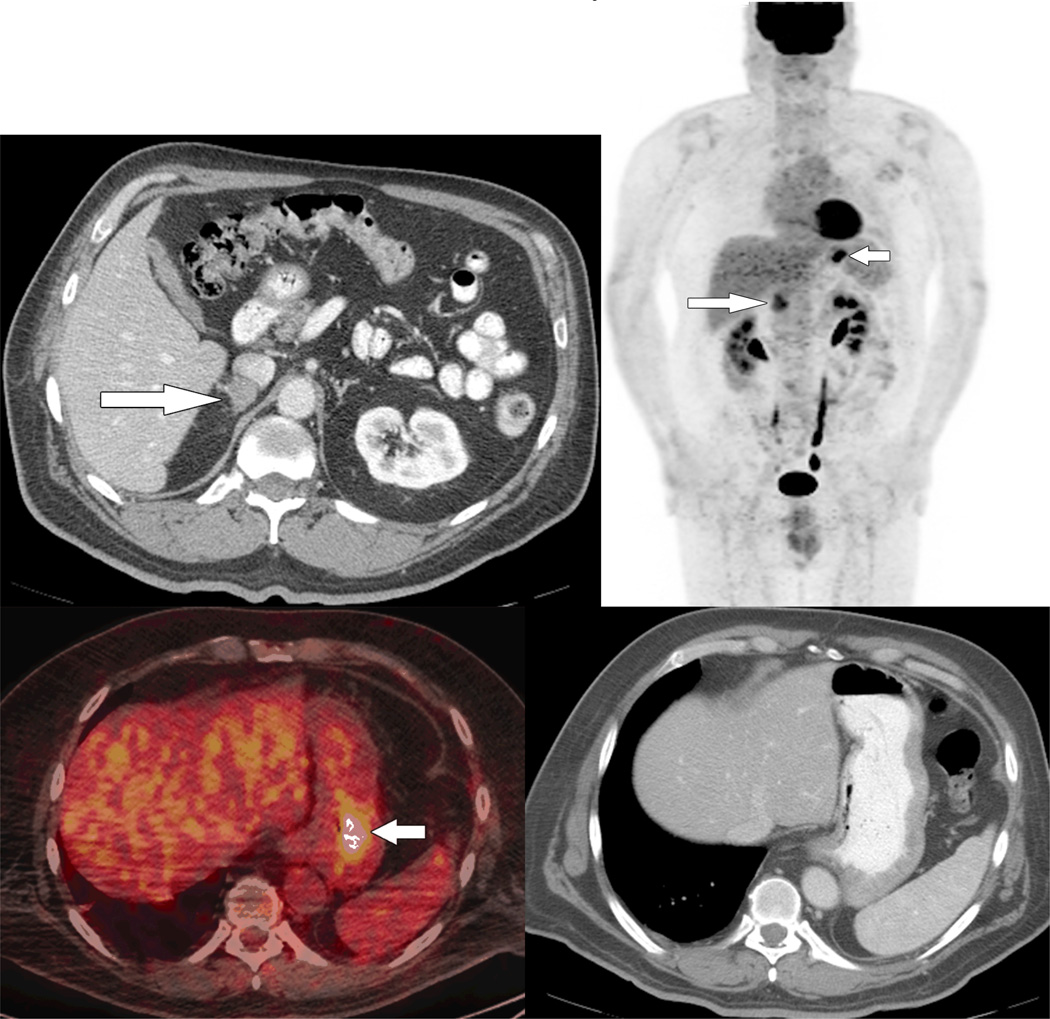
Fig 1.
63 year old male with melanoma metastasis to the right adrenal, candidate for right adrenalectomy.
A. Right adrenal metastasis appears as a solitary lesion on contrast-enhanced CT (arrow)
B. Preoperative PET-CT shows right adrenal metastasis (long arrow) and an unexpected gastric metastasis (short arrow).
C. Axial fused PET-CT image shows abnormal focal FDG uptake in the stomach (arrow).
D. No abnormality is evident in the gastric wall on contrast enhanced CT.
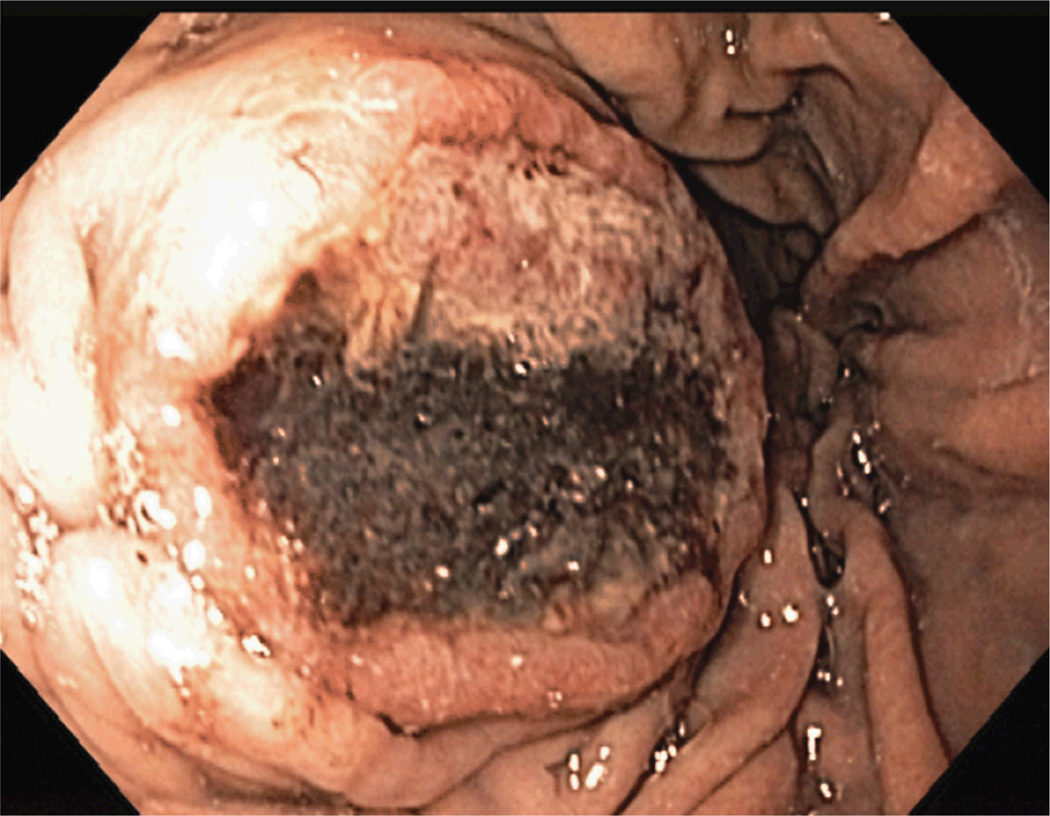
E. Gastric metastasis confirmed on endoscopy. The surgical plan changed to adrenalectomy and partial gastrectomy after neoadjuvant chemotherapy.
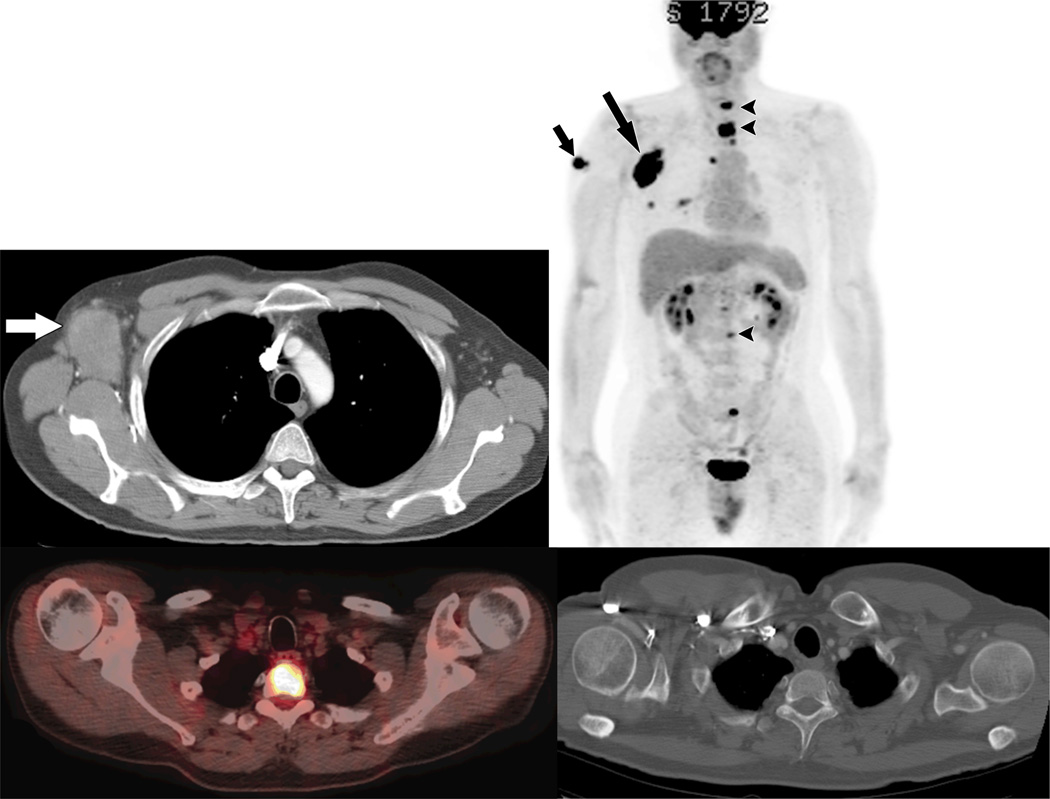
Fig 2.
47 year old male with bulky right axillary metastasis from the right arm primary melanoma (stage IIIC) was a candidate for a wide local excision and right axillary lymph node dissection.
A. Contrast enhanced CT shows large metastatic mass in the right axilla (arrow).
B. Preoperative PET-CT shows known primary melanoma of the right arm (arrow), known right axillary metastasis (long arrow), and multiple unexpected skeletal metastases (arrowheads). The surgery was cancelled.
C. Axial fused PET-CT image shows intense FDG uptake within T2 vertebra (arrow).
D. No obvious skeletal abnormality is seen on contrast enhanced CT.
Fig 3.
71 year old male with in transit metastasis in the right arm from the right arm primary melanoma (A), candidate for wide local excision.
A. Ultrasound of the right arm shows a 1.5cm hypoechoic subcutaneous nodule compatible with melanoma metastasis.
B. Preoperative PET-CT shows 3 FDG-avid intramuscular masses in the left leg (short arrows) in addition to known right arm metastasis (long arrow). The surgery was cancelled.
C. Ultrasound of the left thigh demonstrates 5.9 cm heterogeneous hypoechoic soft tissue mass proven by subsequent biopsy as melanoma metastasis.
Three (9%) PET-CT scans showed increased FDG uptake within benign structures including the lymph nodes (n=2), and the ovary (n=1). These findings which were initially interpreted as suspicious for metastases were proven benign with subsequent resolution on subsequent PET-CT or lack of morphologic progression on follow-up CT exams within 6 months after the enrollment into our study. Neither of these findings required invasive work up or precluded the patient from curative surgery.
In 5 patients, clinically evident and biopsy proven melanoma metastases were not FDG avid on PET-CT, including 3 patients with soft tissue metastasis, and 2 patients with lymph node metastasis. In 4 out of 5 patients, these non-FDG avid metastases were <10 mm in size; in 1 patient a 2 cm metastasis did not show perceptible FDG uptake above background. In one patient with anal melanoma treated by preoperative biochemotherapy, PET-CT did not show abnormal activity in the anal region. Since all of these tumor sites were clinically evident, the lack of FDG uptake on PET-CT did not preclude the patients from metastasectomy. One PET-CT was falsely negative for an 8 mm small bowel metastasis which was incidentally discovered at the time of laparotomy for an adrenal metastasis. Even retrospectively, this small metastasis was not visible on the preoperative PET-CT.
Discussion
According to National Comprehensive Cancer Network guidelines for melanoma imaging work up, cross sectional imaging in form of either CT or PET-CT is encouraged for stage IV and should be considered for stage III patients, with the exception of stage IIIC with clinically evident inguinofemoral lymph nodes, when CT of the pelvis is required[1]. No routine cross sectional imaging is recommended for patients with stages I and II disease.
These guidelines provide oncologists and surgeons with the freedom of choice between contrast-enhanced CT and PET-CT for staging of the advanced disease.
According to studies of the last two decades, PET-CT surpasses contrast-enhanced CT and MRI in diagnostic accuracy for staging, yet it is unclear if this difference yields any advantage in outcome of melanoma patients[8]. Meta-analyses performed to examine the utility of ultrasonography, computed tomography (CT), and PET-CT for the staging and surveillance of melanoma patients based on 10 528 patients between 1990 and 2009 found PET-CT to be the most accurate modality for detection of the distant metastases, with both a sensitivity and specificity of 95%[3].
A number of studies have reported that PET-CT findings impact surgical decision making in melanoma patients (Table 3). The reported frequency with which PET-CT results in a change in surgical plan has been reported from a low of 10%[9] to a high of 36%[10]. The primary limitations of these studies include the inclusion of heterogeneous patient populations which are not limited to high risk cohorts (stage III and IV)[9–12] and retrospective study designs [12, 13].
TABLE 3.
Effect of PET on Change of Management Choices for Patients With Melanoma Reported in the Literature Since 2000
| Study | Year | Type of study |
No. of PET Scans/No. of Patients |
AJCC Clinical Stage |
Type of FDG-Labeled Imaging |
Overall Change in Management Based on PET/CT(%) |
Change in Surgical Management |
False- Positive Rate (%) |
Method of False-Positive Rate Calculation |
|---|---|---|---|---|---|---|---|---|---|
| Tyleret al.[4] | 2000 | Prospective | 106/95 | IIIC | PET | 15 | Not specifically reported | 56.5 | 39/69 lesions |
| Mijnhout et al.[9]a | 2002 | Prospective | 68/68 | 1–IV | PET | 40 | In 10% of cases, surgery was canceleda | 3 | 2/68 patients |
| Gulec et al.[10] | 2003 | Prospective | 49/49 | II–IV | PET | 49 | In 36% of cases, surgical plan was changed; in 24%, surgery was canceled; in 12%, additional surgery was performed | 8 | 4/49 patients |
| Harris et al. [12] | 2005 | Retrospective | 126/92 | I–IV | PET | 32 | In 15% of cases, surgery was canceled | 1.6 | 2/126 PET scans |
| Brady et al.[11] | 2006 | Prospective | 103/103 | IIC–IV | PET | 33 | In 19% of cases, surgery was canceled (only 5% based on PET alone) | 8 | 5/59 patients |
| Reinhardt et al.[13] | 2006 | Retrospective | 250/64 | I–IV | PET/CT | 48.4b | In 40% of cases, intermo-dality change; in 8.4%, intramodality change | 0.4 | 1/250 patients |
| Pfannenberg et al.[15] | 2007 | Prospective | 64/64 | III–IV | PET/CT | 57.6b | In 19% of cases, surgery was canceled; in 15% of cases, surgical field was amended | 9.4 | 28/297 lesions |
Note—AJCC = American Joint Committee on Cancer.
Imaging workup before PET did not include CT of the chest, abdomen, and pelvis but was variable, ranging from chest x-ray only to CT of two of three body parts.
No baseline conventional imaging was performed before PET/CT. Patients were evaluated only by physical examination and sentinel lymph node biopsy.
In this prospective study, 12% of PET-CT scans (4 out of 33) resulted in changes in surgical management. This finding is in concordance with previously published studies reporting surgical cancellation in 10%[9], 15% [12] and 19%[11] due to unexpected significant findings on PET-CT.
In our study, 6 % of cases (2 out of 32) were found to have unexpected metastases detected by PET-CT located in the lower extremities which are not included in conventional imaging. In one case, these unexpected metastases were clinically occult in transit implants on the same extremity as the location of the primary melanoma. In the other case, large soft tissue masses were incidentally noted in the leg in a patient with a primary melanoma of the contralateral arm. This incidence is higher than the reported 2.7% incidence of unexpected metastases in the extremities reported by Niederkohr in his retrospective review of 296 PET-CT scans in melanoma patients [14]. Since even in these 2.7% of cases there were multiple other metastatic sites, Niederkohr questions the clinical utility of including the extremities in the whole-body PET-CT. However in the subpopulation of melanoma patients who are candidates for surgical resection the inclusion of the legs into the whole-body PET-CT may be of clinical value.
Our study has several limitations, including a small sample size which limits the ability to perform statistical analyses. With 33 PET-CT scans, the maximum likelihood estimate that PET-CT performed before surgery changes surgical plan has a 95% confidence interval extending from 0.04 to 0.31. Unfortunately, our study encountered difficulty in accrual due to the wide availability of PET-CT in the community which is often performed prior to referral. However, given the highly selective nature of our patient population, these data can be of value to surgical oncologists in their clinical practice. While, the small study group limits our ability to define factors associated with unexpected PET-CT findings. Patients with large metastases and thick primary melanomas may be at particularly high risk for identification of additional lesions by PET-CT, however, larger patient populations are required to confirm this. The increasing number of patients presenting to our Melanoma Center for initial evaluation of metastatic melanoma with pre referral PET-CT imaging demonstrates the popularity that F18-FDG-PET-CT has gained among oncologists. Our study demonstrates that PET-CT can show unexpected metastases missed or not included in the contrast-enhanced CT of the body and the neck in a small fraction of surgical candidates with metastatic melanoma. Similarly small is the fraction of false positive results produced by PET-CT interpretation.
In conclusion, PET-CT can be considered as part of the pre operative imaging evaluation of patients with advanced melanoma when surgical resection with a curative intent is planned.
References
- 1.Coit DG, Andtbacka R, Anker CJ, et al. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network; 2011. Melanoma. [Google Scholar]
- 2.Choi EA, Gershenwald JE. Imaging studies in patients with melanoma. Surg Oncol Clin N Am. 2007;16:403–430. doi: 10.1016/j.soc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Xing Y, Bronstein Y, Ross MI, et al. Contemporary Diagnostic Imaging Modalities for the Staging and Surveillance of Melanoma Patients: a Meta-analysis. J Natl Cancer Inst. 2011;103:129–142. doi: 10.1093/jnci/djq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyler DS, Onaitis M, Kherani A, et al. Positron emission tomography scanning in malignant melanoma. Cancer. 2000;89:1019–1025. [PubMed] [Google Scholar]
- 5.Miranda EP, Gertner M, Wall J, et al. Routine imaging of asymptomatic melanoma patients with metastasis to sentinel lymph nodes rarely identifies systemic disease. Arch Surg. 2004;139:831–836. doi: 10.1001/archsurg.139.8.831. discussion 836–837. [DOI] [PubMed] [Google Scholar]
- 6.Buzaid AC, Sandler AB, Mani S, et al. Role of computed tomography in the staging of primary melanoma. J Clin Oncol. 1993;11:638–643. doi: 10.1200/JCO.1993.11.4.638. [DOI] [PubMed] [Google Scholar]
- 7.Strobel K, Dummer R, Husarik DB, et al. High-risk melanoma: accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. Radiology. 2007;244:566–574. doi: 10.1148/radiol.2442061099. [DOI] [PubMed] [Google Scholar]
- 8.Mohr P, Eggermont AM, Hauschild A, et al. Staging of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi14–vi21. doi: 10.1093/annonc/mdp256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mijnhout GS, Comans EF, Raijmakers P, et al. Reproducibility and clinical value of 18F-fluorodeoxyglucose positron emission tomography in recurrent melanoma. Nucl Med Commun. 2002;23:475–481. doi: 10.1097/00006231-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Gulec SA, Faries MB, Lee CC, et al. The role of fluorine-18 deoxyglucose positron emission tomography in the management of patients with metastatic melanoma: impact on surgical decision making. Clin Nucl Med. 2003;28:961–965. doi: 10.1097/01.rlu.0000099805.36471.aa. [DOI] [PubMed] [Google Scholar]
- 11.Brady MS, Akhurst T, Spanknebel K, et al. Utility of preoperative [(18)]f fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. Ann Surg Oncol. 2006;13:525–532. doi: 10.1245/ASO.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Harris MT, Berlangieri SU, Cebon JS, et al. Impact of 2-deoxy-2[F-18]fluoro-D-glucose Positron Emission Tomography on the management of patients with advanced melanoma. Mol Imaging Biol. 2005;7:304–308. doi: 10.1007/s11307-005-0002-7. [DOI] [PubMed] [Google Scholar]
- 13.Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 14.Niederkohr RD, Rosenberg J, Shabo G, et al. Clinical value of including the head and lower extremities in 18F-FDG PET/CT imaging for patients with malignant melanoma. Nucl Med Commun. 2007;28:688–695. doi: 10.1097/MNM.0b013e32827420cc. [DOI] [PubMed] [Google Scholar]