Abstract
Colorectal cancer is among the leading causes of cancer death in the USA. The polycomb repressive complex 2 (PRC2), including core components SUZ12 and EZH2, represents a key epigenetic regulator of digestive epithelial cell physiology and was previously shown to promote deleterious effects in a number of human cancers, including colon. Using colon cancer stem cells (CCSC) isolated from human primary colorectal tumors, we demonstrate that SUZ12 knockdown and treatment with DZNep, one of the most potent EZH2 inhibitors, increase apoptosis levels, marked by decreased Akt phosphorylation, in CCSCs, while embryonic stem (ES) cell survival is not affected. Moreover, DZNep treatments lead to increased PTEN expression in these highly tumorigenic cells. Taken together, our findings suggest that pharmacological inhibition of PRC2 histone methyltransferase activity may constitute a new, epigenetic therapeutic strategy to target highly tumorigenic and metastatic colon cancer stem cells.
Keywords: Polycomb, Apoptosis, DZNep, PTEN, Stem cells, Colon cancer
Introduction
Colorectal cancer is the second leading cause of cancer death in the USA [1]. Even after chemotherapy, greater than 50% of patients will experience relapse, mostly associated with metastasis [2]. Advanced stage colorectal cancers are particularly resistant to chemotherapy and show a dismal, 10% 5-year survival [1]. Therefore, it is imperative that new therapeutic approaches, particularly for advanced disease, be developed. Recent studies have provided evidence that colorectal carcinomas are hierarchically organized and contain a small subset of self-renewing and highly tumorigenic units termed colon cancer stem or initiating cells (CCSCs/CCICs) [3-6]. These putative CCSCs, which can be identified by marker expression (e.g. CD44, CD133), are thought to be responsible for relapse and subsequent metastasis, in part because of their capacity to survive in non-adherent conditions [3-5, 7].
Polycomb group proteins (PcGs) are epigenetic, transcriptional repressors for differentiation-associated genes and play key roles in embryonic stem (ES) cell self-renewal and pluripotency [8]. Briefly, PcGs form multiprotein complexes that possess histone methyltransferase (HMTase) activity responsible for the trimethylation of lysine 27 of histone H3 (H3K27me3) [8]. PcGs are divided into two main transcriptional repressive complexes, PRC1 and PRC2. EED, EZH1/2, and SUZ12 proteins constitute the core components of the PRC2 complex [8]. Specifically, the PRC2 complex plays an initiating role in PcG-mediated epigenetic repression by generating specific H3K27me3 marks associated with subsequent PRC1 and DNA methyltransferase (DNMT) recruitment [9]. Dysregulated expression of several PcG proteins, including SUZ12 and EZH2, occurs in different types of human tumors, including colon cancer, and is potentially associated with a poor prognosis and the persistence of stem-like cancer cells [9-11]. Here we report that inhibition of the PRC2 HMTase activity, either by depleting SUZ12 or by indirectly inhibiting EZH2 using 3-deazaneplanocin A (DZNep) [12, 13], leads to increased apoptosis in human colorectal cancer cells.
Material and Methods
1-Tissue culture and Reagents
Human colon cancer HT29 cells were authenticated and obtained from ATCC, and were cultured as previously described [5]. Human colon cancer stem cell populations (hCCSC #1 and hCCSC #2) were isolated from stage-2 human sporadic colorectal tumors and have been previously described [5]. Human Intestinal Epithelium Crypt cells were derived from fetal ileum by one of us, and cultured as previously described [14, 15]. Mouse ES cells were established and cultured as described [16]. Suz12 knockdown (shRNA sequence TRCN0000038728) and control shLuciferase (shLuc) human CCSC populations were established as described [10]. Control (shLuc) and Suz12 knockdown (shSUZ12) CCSCs were collected at 48 h post-infection for further analysis. 3-deazaneplanocin A (DZNep) from the National Cancer Institute (Bethesda, MD), was used at 5 μM in water. VO-OHpic trihydrate (V8639, Sigma) was used at 300 nM in DMSO.
2-Tumor growth assays
In vivo xenograft formation assays were performed in 6-week-old NOD/SCID mice (Charles River Laboratories) as previously described [17]. Briefly, 1 × 106 HT29 cells (control and 5 μM DZNep) were injected subcutaneously in the flanks of each mouse. Mice were monitored daily for palpable tumors. Because of rapid growth, tumors were dissected out 3 weeks after injection and were analyzed. The care and use of animals in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medical College.
3-Western Blot analysis
Proteins were extracted in SDS final sample buffer, separated by SDS-PAGE, and transferred onto nitrocellulose membranes as previously described [14]. Membranes were blocked in PBS containing 5% skim milk and 0.1% TWEEN 20 (BioRad). Primary antibodies used are described (Table 1). For blots using antibodies targeting phospho/total Akt or different histone marks, membranes were stripped using a 0.2 M glycine (Sigma), 1% TWEEN 20, 0.1% SDS solution and re-probed using a different primary antibody. For quantitative optical densitometry analysis, Western blot bands were scanned and analyzed using Image J software (National Institutes of Health).
Table 1.
Antibody used for Western blot and ChIP assays
| Target | Application | Company | Clone/Catalog # | Source |
|---|---|---|---|---|
|
| ||||
| PARP | Wb | Cell Signaling | 9542 | Rabbit polyclonal |
| SUZ12 | Wb | Cell Signaling | 3737 | Rabbit monoclonal |
| PTEN | Wb | Millipore | 04-409 | Rabbit monoclonal |
| p-Akt (ser473) | Wb | Cell Signaling | 4051 | Mouse monoclonal |
| Akt | Wb | Cell Signaling | 9272 | Rabbit polyclonal |
| Actin | Wb | Millipore | MAB1501 | Mouse monoclonal |
| H3K27me3 | Wb, ChIP | Millipore | 07-449 | Rabbit polyclonal |
| H3K36me2 | Wb | Millipore | 07-369 | Rabbit polyclonal |
| Rabbit IgG | ChIP | Santa Cruz Biotech | Sc 2027 | --- |
4-Terminal deoxynucleotidyl dUTP Nick End Labeling (TUNEL) assays
Briefly, non-adherent CCSCs were centrifuged and fixed using 4% (w/v) paraformaldehyde (Sigma) and membrane permeabilization was performed with 0.3% (w/v) Triton-X 100 (Sigma). Cells were incubated with In Situ Cell Death Detection Kit Fluorescein® enzymatic reaction mixture (Roche) for 1 hour at 37°C according to the manufacturer’s instructions (www.roche-applied-science.com). TUNEL positive and DAPI stained cells were counted using NIS-Elements Advanced Research software (Nikon).
5-Indirect Immunofluorescence staining
Immunofluorescence staining of cells was performed as described [18]. Briefly, cells were seeded on serum-pretreated glass coverslips (Fisher) 24 hours prior to treatment. Cell monolayers were fixed using 4% (w/v) formaldehyde (Sigma) and membrane permeabilization was performed with 0.3% (w/v) Triton-X 100 (Sigma). 2% BSA (Sigma) was used for blocking for 30 minutes at room temperature prior to incubation with rabbit polyclonal anti-SOX2 (01438, 1:1000, STEMCELL Technologies Inc) primary antibody. Phalloidin-TRITC (Millipore, FAK100, 1:1000) was used to stain actin stress fibers (F-actin). Nuclei were stained using DAPI contained in Vectashield® mounting medium for fluorescence (Vector labs). For SOX2/TUNEL co-labeling, In Situ Cell Death Detection Kit Fluorescein® enzymatic reaction mixture (Roche) was applied on cells for 1 hour at 37°C, prior to primary antibody incubation.
6-Chromatin immunoprecipitation (ChIP) assays
A one-step ChIP protocol involving formaldehyde cross-linking was used for histone chromatin immunoprecipitation assays, as previously described [16, 19]. Briefly, immunoprecipitations (IP) of sonicated chromatin were performed using 2 μg of specific antibodies (Table 1). qPCR amplifications of a PTEN promoter fragment (Forward 5′-AGCAAGCCCCAGGCAGCTACACT-3′ and reverse 5′-GGTAGGAGGGGGCAGAGCGGTA-3′), as well as of a control intergenic region (Forward 5′-GTGAGTGCCCAGT TAGAGCATCTA-3′ and reverse 5′-GGAACCAGTGGGTCTTGAAGTG-3′) [20] were carried out. The size of each PCR product was confirmed by electrophoresis on a 1.5% agarose gel.
7-Statistical analysis
All experiments were performed at least 3 times using independent, biological triplicates. Results are presented as means ± SEM. All statistical tests were performed using GraphPad InStat software version 3.10. For all data, the two-tailed unpaired t-test was applied. P values of ≤ 0.05 were considered significant.
Results and Discussion
Human colorectal tumors are heterogeneous, hierarchically organized, and contain a subpopulation of tumorigenic stem cells, characterized by extensive self-renewal capacity, that gives rise to non-tumorigenic, differentiated cancer progeny [3-5]. PcG proteins are key regulators of apoptosis in cancer cells of different origins [11]. Importantly, the reactivation and/or increased expression of PcGs described in several types of solid tumors is required for the formation and maintenance of malignant stem cells [21]. Thus, we addressed the question of whether pharmacological inhibition of PRC2 affects the survival of CCSCs. Although we and other groups have reported the anti-tumor effects of PcG protein inhibition in diverse cancers [11, 12, 22, 23], little is known about the specific impact of such inhibition on colon cancer stem cells.
1-Effect of DZNep on a HT29 SOX2 high-expressing cell sub-population
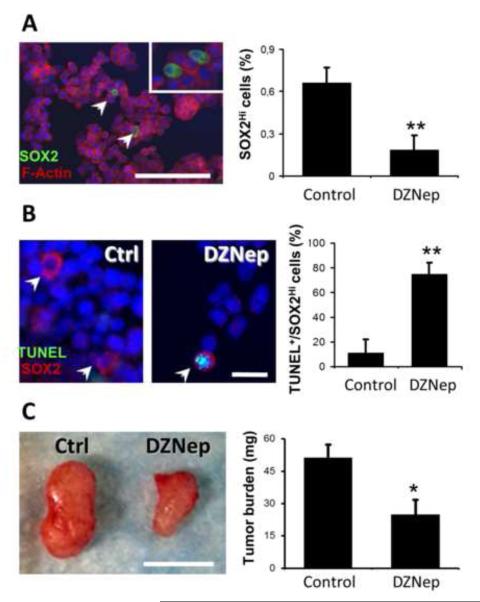
In a previous study, we showed that inhibition of PRC2 histone methyltransferase activity, either by knocking down SUZ12 expression or by using 3-deazaneplanocin A (DZNep) to block EZH2 activity, poises human colon cancer cells for TRAIL-mediated apoptosis [22]. However, PRC2 inhibition alone was not sufficient to induce apoptosis in cultures of HT29 and SW480 human colon cancer cells [22]. Recent studies reported the presence of cancer stem cell sub-populations within human colon cancer cell lines, including HT29 [24-26]. In these cases, cell sorting experiments based on high expression of CCSC markers, including CD133 and CD44, led to an enrichment in drug resistant, highly tumorigenic cells expressing stem cell pluripotency factors, such as SOX2 [25, 26]. We showed by indirect immunofluorescence staining the presence of SOX2 high-expressing (SOX2Hi) cells in HT29 monolayers, at an average abundance of 0.66% (±0.11%) (Figure 1A). DZNep treatment of HT29 cells in monolayer culture caused a decrease in this proportion of SOX2Hi cells to ~0.19% (Figure 1A). We noted no significant impact on cell survival upon DZNep treatment of global HT29 cultures [22], but decided to assess apoptosis incidence in the sub-population of SOX2Hi cells present in HT29 cultures following PRC2 HMTase inhibition. SOX2 immunofluorescence and TUNEL co-labeling experiments were performed on control and DZNep-treated HT29 cultures. For these experiments, cells were exposed to DZNep for a period of 24 hours only to obtain a better representation of apoptotic events before complete resorption of the dying cells. Interestingly, we observed an increased rate of apoptosis in SOX2Hi cells in DZNep treated cultures (74.7% ±9.5%) compared to controls (11.1% ±11.1%) (Figure 1B). By using xenograft tumor formation assays in NOD/SCID mice we observed that HT29 treated with DZNep formed smaller tumors (~50% ±6,8%) than the control group, suggesting a reduced in vivo tumorigenic potential (Figure 1C). This observation further supports the depletion of a tumor-initiating cell population within HT29 cultures following DZNep treatment. Considering that DZNep was identified as a potent EZH2 inhibitor [12, 23, 27], these results suggest a potential role for the polycomb group proteins in the regulation of CCSC survival.
Figure 1. Effect of DZNep on the HT29 SOX2Hi sub-population.
(A) Immunofluorescence staining of SOX2 (green) was performed to highlight a cancer stem cell subpopulation in the human colon cancer cell line HT29 (left panel) (Bar =50 μm). Percentages (%) of SOX2Hi cells present in control (vehicle) and DZNep treated (5 μM, 48 hours) HT29 cultures are represented in the histogram (n=7; **: p=0.008). (B) SOX2 (red) and TUNEL (green) co-labeling was performed on control (vehicle) and DZNep treated (5 μM, 24 hours) HT29 cultures (Bar = 10 μm). The histogram shows the percentages of SOX2Hi cells that are TUNEL-positive in control and DZNep treated HT29 cultures (n=5; **: p=0.0055). (C) Representative picture of 21 days post-injection tumor xenografts from NOD/SCID mice injected with control (vehicle) and DZNep treated (5 μM) HT29 cells (Bar =3 mm). Tumor burden measurements are represented as a histogram (n=6; *: p=0.0444).
2-Effect of DZNep on human CCSC survival
Putative CCSC markers, including CD133, CD44, and EpCAM, allow the efficient isolation of malignant stem cells from primary tumors [3, 5, 6]. To increase the relevance of our investigation for human therapies, we used an innovative in vitro model of CCSCs isolated from human primary colorectal tumors. The tumorigenicity, self-renewal capacity, and specific marker expression of these CCSCs in culture are consistent with an important role for CCSCs in the formation of primary tumors and secondary metastases [6]. As previously described, the CCSCs used in our study exhibit long-term expression of CD133, CD44, and EpCAM, and show high ALDH1 activity [5, 6]. These CCSCs also exhibit self-renewal capacity for more than 1 year [5]. When injected into NOD/SCID mice, these human CCSCs form xenografts that maintain the histopathology of primary human colorectal tumors [5]. The capacity of these cells to grow in non-adherent conditions is typical of highly metastatic colon cancer stem cells [28, 29].
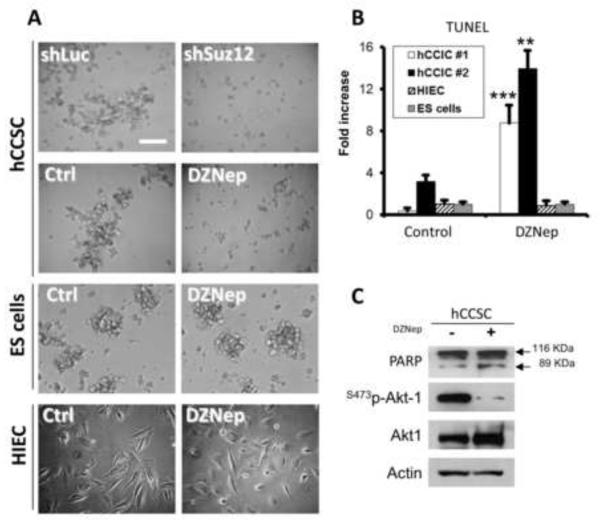
PRC2 HMTase activity was inhibited in non-adherent CCSC cultures using two different approaches. First, a knockdown SUZ12, which represents a core component of the PRC2 complex assembly [9, 10, 30], was achieved using the previously validated shRNA sequence TRCN0000038728 [22, 31]. Second, DZNep, which provides a pharmacological approach to inhibit EZH2 function [12, 22, 23, 27], was used to treat human CCSC cultures. DZNep was shown to reduce H3K27 trimethylation in various cancer cell types and in ES cells [11]. Phase contrast microscopy revealed that both DZNep treatment and SUZ12 depletion decreased the formation of multicellular, sphere-like clusters in non-adherent CCSC cultures (Figure 2A), suggesting either a decrease in cell-cell adhesion and/or a modulation of pathways mediating apoptosis. In contrast, DZNep had no effect on ES cell clustering in non-adherent conditions (Figure 2A). Such a result is in accord with previous observations made by Tan et al., in which DZNep treatment induced apoptosis in human breast cancer cells, but not in normal cells [27].
Figure 2. Effect of DZNep on human CCSC survival.
(A) Human colorectal cancer stem cells (CCSCs) derived from primary tumors (hCCSC #1 line) were infected with shLuc and shSUZ12 (TRCN0000038728) lentiviral particles, or treated with DZNep (5 μM) for 48 hours or vehicle (Ctrl) in low attachment conditions. Phase contrast microscopy pictures show the differences in cell aggregation between shLuc and shSUZ12 CCSC, as well as between DZNep treated (5 μM, 48h) and control (Ctrl) CCSC. Wild type ES cells cultured in non-adherent conditions, as well as Human Intestinal Epithelial Crypt (HIEC) monolayers were used as non-cancer controls for DZNep treatment (Bar = 200 μm). (B) TUNEL analyses were performed on control and DZNep treated (5 μM, 48h) hCCSC #1 and hCCSC #2 lines, on Human normal intestinal epithelial crypt cells (HIEC) and on mouse ES cells (n≥3; **: p=0.0043; ***: p<0.0001). (C) Representative Western blot analysis of PARP (native 116 KDa and 89 KDa cleaved bands), Ser473-phospho-Akt, and total Akt performed on control and DZNep treated hCCSC #1. Actin was used as loading control. The experiments were performed 3 times with very similar results.
To test whether DZNep treatment affects the survival of human CCSCs, we used two independent primary cultures of non-adherent, highly tumorigenic cells (hCCSC #1 and hCCSC #2) isolated from human patients. Human normal intestinal crypt cells (HIEC), as well as wild-type ES cells were used as non-cancer cell controls. HIEC cells were originally isolated from human fetal intestine and exhibit stem/progenitor characteristics, such as the expression of the intestinal stem cell markers BMI1, DCAMKL1, and Musashi1, as well as the potential to differentiate along the enterocytic lineage [14]. In both CCSC cultures tested, we showed by TUNEL assays that DZNep treatment caused a strong (≥9-fold) increase in the number of apoptotic cells compared to vehicle treated CCSC and ES cells (Figure 2B). In contrast, no changes in HIEC and ES cell apoptosis levels occurred in response to DZNep treatment (Figure 2B). However, a marked decrease in Sox2 expression was observed in ES cells following DZNep treatment (not shown), which is in accord with previous studies [32]. Western blot analyses also revealed an increase in the 89 kDa, cleaved form of PARP following DZNep treatment of the CCSC cultures (Figure 2C). In fact, PARP (116 KDa: native form) constitutes a specific substrate of active Caspase-3, and is cleaved into 89 KDa and 27 KDa fragments in apoptotic cells [33]. Taken together, the TUNEL and PARP cleavage measurements support the thesis that apoptosis is induced following PcG/PRC2 inhibition in non-adherent CCSCs.
The PI3K/Akt pathway constitutes a key pathway in the regulation of apoptosis. Specifically, PI3K/Akt signaling was shown to be crucial for survival of undifferentiated colorectal cancer and intestinal epithelial progenitor cells [34, 35]. By western blot analyses performed on DZNep treated CCSC we showed a 96% decrease (±0.2%, p=0.017) in Akt phosphorylation on serine 473 compared to that in untreated controls (Figure 2C). This observation from DZNep treated CCSCs indicates that PcG proteins have a pivotal role in maintaining the sustained, pro-survival signals from the PI3K/Akt pathway previously reported to be responsible for apoptosis resistance in diverse types of cancers, including colon cancer [35-39].
3-Impact of PRC2 inactivation on epigenetic marks and PTEN expression in human CCSCs
To obtain insight into the effects of PRC2 inhibition on human CCSC epigenetics, we performed western blot analyses assessing the levels of key histone marks in both SUZ12 knockdown and DZNep treated hCCSC #1 compared to controls.
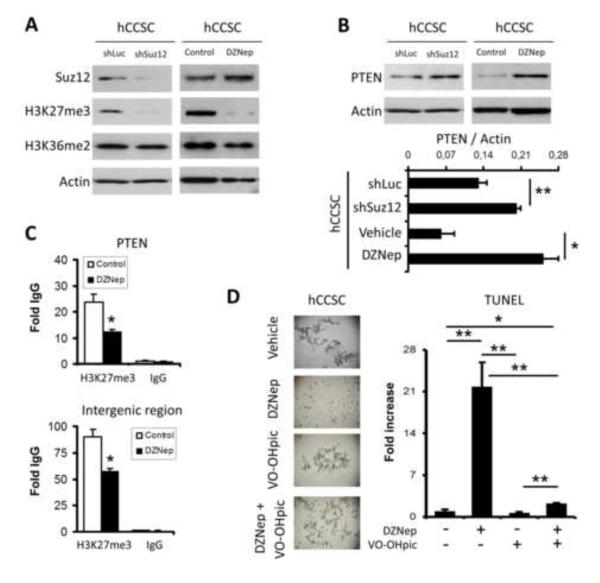
These experiments revealed that both SUZ12 knockdown and DZNep treatment caused a major decrease in H3K27 trimethylation in CCSCs, while H3K36me2, an epigenetic mark associated with transcriptional elongation [40] and DNA repair [41], was unchanged (Figure 3A). This observation differs from data from another study, which reported that DZNep acts as an untargeted, global HMTase inhibitor [42] in cancer cell lines. Our data suggest that DZNep is selective for PcG/PRC2 inhibition in human CCSCs.
Figure 3. Impact of PRC2 inactivation on epigenetic marks and PTEN expression in human CCSCs.
(A) Western blot analyses of H3K27me3, and H3K36me2 histone marks were performed on shLuc, shSUZ12, control/vehicle, and DZNep (5 μM, 48h) treated CCSCs. SUZ12 and PTEN protein levels were also evaluated for each condition. Actin was used as loading control. (B) Densitometry analysis of PTEN protein levels relative to actin levels for each condition tested in (A) is presented as a histogram (n=4; *: p=0.0417, **: p=0.007). (C) Promoter specific chromatin immunoprecipitations (ChIPs) were quantified using qPCR in control and DZNep treated (5 μM, 48h) hCCSC #1. Antibody directed against H3K27me3 or rabbit IgG was used for immunoprecipitation. Fragments of the human PTEN promoter and a random intergenic region were amplified from immunoprecipitated chromatin. Data are presented in histograms as fold over IgG (n=3; *: p<0.03). (D) Representative phase contrast microscopy pictures and TUNEL analyses of control (vehicle), DZNep (5 μM, 48h), VO-OHpic (300 nM, 48h), and DZNep + VO-OHpic treated hCCSC #1 line (n=3; *: p=0.0138, **: p≤0.0089).
PTEN expression has been closely related to apoptosis regulation in a variety of cellular systems [43]. Recently, PcGs were shown to repress PTEN expression in nasopharyngeal cancer cells, leading to the activation of PI3K/Akt [44]. While inherited PTEN mutations were associated with colorectal carcinogenesis in patients with Cowden syndromes, loss-of-function of PTEN caused by mutations was rarely reported [45]. PTEN repression in sporadic colorectal cancer cases was rather associated with epigenetic promoter silencing [45, 46]. By western blot analyses we showed an increase in PTEN protein level in SUZ12 knockdown (~1.7-fold, p=0.077), and in DZNep treated (~4-fold, p=0.0417) hCCSC #1 (Figure 3B). To determine if the PTEN gene constitutes a direct target of PRC2 in CCSC, we performed ChIP assays in DZNep treated and control/vehicle treated human CCSC cultures. We observed a decrease in the PRC2-associated H3K27me3 mark at the PTEN promoter (~48%, p=0.03) in DZNep-treated as compared to untreated CCSC (Figure 3C). DZNep treatment also caused a decrease in the H3K27me3 mark at a random intergenic region (~37%, p=0.013) in human CCSC, suggesting that the effects of DZNep on PRC2 inhibition is epigenome-wide and not exclusively targeted to specific subsets of promoters (Figure 3C). Thus, our data indicate that targeting PRC2 HMTase activity in CCSCs leads to a global decrease in the inhibitory H3K27me3 epigenetic mark affecting the PTEN promoter, which leads to an associated increase in PTEN protein (Figure 3).
Relating PcGs to malignant stem cell apoptosis regulation is not without precedent. Previous studies on the “death-from-cancer” gene signature in therapy-resistant and metastatic cancer samples from patients revealed essential functions of PcGs in the emergence of the cancer stem cell phenotype and the acquisition of apoptosis [28]. PTEN, an extensively characterized tumor suppressor gene which is also a PcG target, is well known for its inhibitory role in the PI3K/Akt pathway [47]. We showed that PTEN protein levels increase in SUZ12 knockdown and DZNep treated CCSCs, which suggests the participation of PTEN in the decrease in Akt phosphorylation and the increase in subsequent apoptosis. To confirm such a role for PTEN in PRC2-mediated apoptosis we used VO-OHpic, a potent inhibitor of PTEN activity [48], combined with DZNep treatments to rescue CCSCs from apoptosis (Figure 3D). Immunoblot experiments showed a ~46% increase in Akt phosphorylation (serine 473) levels in CCSCs following VO-OHpic treatment (48 h, 300 nM) (not shown). Moreover, combining VO-OHpic treatment with DZNep in CCSCs resulted in a strong reduction (~19.5 fold, p=0.0089) in apoptosis incidence (Figure 3D). This supports the specific involvement of PTEN in PRC2-regulated cell survival. It is also noteworthy that apoptosis can also be initiated by a down-regulation of cell-cell interaction proteins. Although E-cadherin levels were not affected by DZNep treatment of CCSCs (not shown), other junctional complex members, such as EpCAM, could be involved. EpCAM is known to be positively regulated by PcGs in hepatocellular carcinomas, intestinal epithelial progenitors, and ES cells [10, 12].
Conclusions
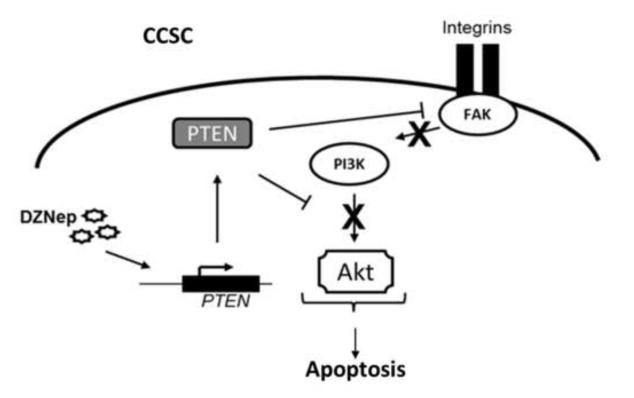
Taken together, our data suggest that DZNep treatment of human CCSCs causes an increase in expression of PTEN, which may in turn repress the pro-survival signaling pathway PI3K/Akt, thereby leading to apoptosis (summarized in Figure 4). Therefore, pharmacological inhibition of PRC2 HMTase activity may constitute a new epigenetic, therapeutic strategy to suppress apoptosis resistance in highly tumorigenic and metastatic human CCSCs. Although other groups have suggested a similar strategy in other types of cancers [12, 23], additional experiments in animal models will be required to determine DZNep metabolism, in vivo effectiveness, and toxicity.
Figure 4. Pharmacological inhibition of PRC2 activity induces apoptosis in human colon cancer stem cells.
Schematic representation of proposed DZNep effects on apoptosis regulation in human CCSCs. PRC2 inhibition increases PTEN expression, which in turn inhibits the PI3K/Akt pro-survival signaling pathway. The final outcome of PRC2 pharmacological inhibition in CCSCs is programmed cell death.
Highlights.
PRC2 inactivation results in apoptosis in human colon cancer stem cells (CCSC).
PRC2 inactivation increases PTEN expression in human CCSCs.
DZNep treatment decreases H3K27me3 levels at the PTEN promoter in human CCSCs.
PTEN increased expression in CCSC is associated with reduced Akt phosphorylation.
Acknowledgments
This work was supported by Weill Cornell funds, the National Institutes of Health [grants number RO1 CA0043796-22 (to LJG), DE010389-17 (to LJG), R21 CA153049 (to SML), R21 CA162483 (to SML), R01 CA098626 (to SML)]; and the Fonds de Recherche en Santé du Québec [PF1-Benoit-25389 (to YDB)]. The authors thank Matthew Bell for a generous donation, and Drs. Victor E. Marquez and Joseph J. Barchi at NIH/NCI for providing 3-deazaneplanocin A. Thanks to Christine Langlois for technical assistance and Tamara Weissman for editing the manuscript.
Abbreviations
- CCSC
Colon cancer stem cell
- DZNep
3-deazaneplanocin A
- H3K27me3
Trimethylation of Histone 3 lysine 27
- HIEC
Human Intestinal Epithelial Crypt cells
- HMTase
Histone methyltransferase
- PcG
Polycomb group proteins
- PRC1
Polycomb repressive complex 1
- PRC2
Polycomb repressive complex 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [2].Rodriguez-Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, Batiste-Alentorn E, Lacy AM, Delgado S, Maurel J, Pique JM, Castells A. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–393. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- [3].O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- [4].Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, Maria R. De. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- [5].Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, Lipkin SM. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cammareri P, Lombardo Y, Francipane MG, Bonventre S, Todaro M, Stassi G. Isolation and culture of colon cancer stem cells. Methods Cell Biol. 2008;86:311–324. doi: 10.1016/S0091-679X(08)00014-9. [DOI] [PubMed] [Google Scholar]
- [7].Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Benoit YD, Lepage MB, Khalfaoui T, Tremblay E, Basora N, Carrier JC, Gudas LJ, Beaulieu JF. Polycomb repressive complex 2 impedes intestinal cell terminal differentiation. J Cell Sci. 2012;125:3454–3463. doi: 10.1242/jcs.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crea F, Paolicchi E, Marquez VE, Danesi R. Polycomb genes and cancer: time for clinical application? Crit Rev Oncol Hematol. 2012;83:184–193. doi: 10.1016/j.critrevonc.2011.10.007. [DOI] [PubMed] [Google Scholar]
- [12].Chiba T, Suzuki E, Negishi M, Saraya A, Miyagi S, Konuma T, Tanaka S, Tada M, Kanai F, Imazeki F, Iwama A, Yokosuka O. 3-Deazaneplanocin A is a promising therapeutic agent for the eradication of tumor-initiating hepatocellular carcinoma cells. Int J Cancer. 2012;130:2557–2567. doi: 10.1002/ijc.26264. [DOI] [PubMed] [Google Scholar]
- [13].Glazer RI, Knode MC, Tseng CK, Haines DR, Marquez VE. 3-Deazaneplanocin A: a new inhibitor of S-adenosylhomocysteine synthesis and its effects in human colon carcinoma cells. Biochem Pharmacol. 1986;35:4523–4527. doi: 10.1016/0006-2952(86)90774-4. [DOI] [PubMed] [Google Scholar]
- [14].Benoit YD, Pare F, Francoeur C, Jean D, Tremblay E, Boudreau F, Escaffit F, Beaulieu JF. Cooperation between HNF-1alpha, Cdx2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line. Am J Physiol Gastrointest Liver Physiol. 2010;298:G504–517. doi: 10.1152/ajpgi.00265.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Perreault N, Beaulieu JF. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp Cell Res. 1996;224:354–364. doi: 10.1006/excr.1996.0145. [DOI] [PubMed] [Google Scholar]
- [16].Kashyap V, Gudas LJ, Brenet F, Funk P, Viale A, Scandura JM. Epigenomic reorganization of the clustered Hox genes in embryonic stem cells induced by retinoic acid. J Biol Chem. 2011;286:3250–3260. doi: 10.1074/jbc.M110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen PC, Kuraguchi M, Velasquez J, Wang Y, Yang K, Edwards R, Gillen D, Edelmann W, Kucherlapati R, Lipkin SM. Novel roles for MLH3 deficiency and TLE6-like amplification in DNA mismatch repair-deficient gastrointestinal tumorigenesis and progression. PLoS Genet. 2008;4:e1000092. doi: 10.1371/journal.pgen.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laursen KB, Wong PM, Gudas LJ. Epigenetic regulation by RARalpha maintains ligand-independent transcriptional activity. Nucleic Acids Res. 2012;40:102–115. doi: 10.1093/nar/gkr637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jia L, Berman BP, Jariwala U, Yan X, Cogan JP, Walters A, Chen T, Buchanan G, Frenkel B, Coetzee GA. Genomic androgen receptor-occupied regions with different functions, defined by histone acetylation, coregulators and transcriptional capacity. PLoS One. 2008;3:e3645. doi: 10.1371/journal.pone.0003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benoit YD, Laursen KB, Witherspoon MS, Lipkin SM, Gudas LJ. Inhibition of PRC2 histone methyltransferase activity increases TRAIL-mediated apoptosis sensitivity in human colon cancer cells. J Cell Physiol. 2013;228:764–772. doi: 10.1002/jcp.24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Bitoux M.A. Le, Marino D, Cironi L, Marquez VE, Clement V, Stamenkovic I. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- [24].Fan X, Ouyang N, Teng H, Yao H. Isolation and characterization of spheroid cells from the HT29 colon cancer cell line. Int J Colorectal Dis. 2011;26:1279–1285. doi: 10.1007/s00384-011-1248-y. [DOI] [PubMed] [Google Scholar]
- [25].Botchkina GI, Zuniga ES, Das M, Wang Y, Wang H, Zhu S, Savitt AG, Rowehl RA, Leyfman Y, Ju J, Shroyer K, Ojima I. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheroids induced by purified colon tumor-initiating cells. Mol Cancer. 2010;9:192. doi: 10.1186/1476-4598-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, Asanuma H, Nakatsugawa M, Nishizawa S, Tamura Y, Tsuruma T, Terui T, Kondo T, Ishitani K, Hasegawa T, Hirata K, Sato N. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol. 2011;178:1805–1813. doi: 10.1016/j.ajpath.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Glinsky GV. Genomic models of metastatic cancer: functional analysis of death-from-cancer signature genes reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype with altered cell cycle control and activated Polycomb Group (PcG) protein chromatin silencing pathway. Cell Cycle. 2006;5:1208–1216. doi: 10.4161/cc.5.11.2796. [DOI] [PubMed] [Google Scholar]
- [29].Bellizzi A, Sebastian S, Ceglia P, Centonze M, Divella R, Manzillo EF, Azzariti A, Silvestris N, Montemurro S, Caliandro C, Luca R. De, Cicero G, Rizzo S, Russo A, Quaranta M, Simone G, Paradiso A. Co-expression of CD133(+)/CD44(+) in human colon cancer and liver metastasis. J Cell Physiol. 2013;228:408–415. doi: 10.1002/jcp.24145. [DOI] [PubMed] [Google Scholar]
- [30].Kirmizis A, Bartley SM, Kuzmichev A, Margueron R, Reinberg D, Green R, Farnham PJ. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–1605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Benoit YD, Lepage MB, Khalfaoui T, Tremblay E, Basora N, Carrier JC, Gudas LJ, Beaulieu JF. Polycomb repressive complex 2 impedes intestinal cell terminal differentiation. J Cell Sci. 2012 doi: 10.1242/jcs.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Perez S.V. Diaz, Kim R, Li Z, Marquez VE, Patel S, Plath K, Clark AT. Derivation of new human embryonic stem cell lines reveals rapid epigenetic progression in vitro that can be prevented by chemical modification of chromatin. Hum Mol Genet. 2012;21:751–764. doi: 10.1093/hmg/ddr506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Germain M, Affar EB, D’Amours D, Dixit VM, Salvesen GS, Poirier GG. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7. J Biol Chem. 1999;274:28379–28384. doi: 10.1074/jbc.274.40.28379. [DOI] [PubMed] [Google Scholar]
- [34].Gauthier R, Harnois C, Drolet JF, Reed JC, Vézina A, Vachon PH. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell Physiol. 2001;280:C1540–1554. doi: 10.1152/ajpcell.2001.280.6.C1540. [DOI] [PubMed] [Google Scholar]
- [35].Demers MJ, Thibodeau S, Noël D, Fujita N, Tsuruo T, Gauthier R, Arguin M, Vachon PH. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J Cell Biochem. 2009;107:639–654. doi: 10.1002/jcb.22131. [DOI] [PubMed] [Google Scholar]
- [36].Westhoff MA, Fulda S. Adhesion-mediated apoptosis resistance in cancer. Drug Resist Updat. 2009;12:127–136. doi: 10.1016/j.drup.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [37].Benoit YD, Larrivee JF, Groulx JF, Stankova J, Vachon PH, Beaulieu JF. Integrin alpha8beta1 confers anoikis susceptibility to human intestinal epithelial crypt cells. Biochem Biophys Res Commun. 2010;399:434–439. doi: 10.1016/j.bbrc.2010.07.107. [DOI] [PubMed] [Google Scholar]
- [38].Benoit YD, Groulx JF, Gagné D, Beaulieu JF. RGD-Dependent Epithelial Cell-Matrix Interactions in the Human Intestinal Crypt. J Signal Transduct. 2012;2012:248759. doi: 10.1155/2012/248759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- [40].Bell O, Wirbelauer C, Hild M, Scharf AN, Schwaiger M, MacAlpine DM, Zilbermann F, Leeuwen F. van, Bell SP, Imhof A, Garza D, Peters AH, Schübeler D. Localized H3K36 methylation states define histone H4K16 acetylation during transcriptional elongation in Drosophila. EMBO J. 2007;26:4974–4984. doi: 10.1038/sj.emboj.7601926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fnu S, Williamson EA, Haro L.P. De, Brenneman M, Wray J, Shaheen M, Radhakrishnan K, Lee SH, Nickoloff JA, Hromas R. Methylation of histone H3 lysine 36 enhances DNA repair by nonhomologous end-joining. Proc Natl Acad Sci U S A. 2011;108:540–545. doi: 10.1073/pnas.1013571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, Kelly TK, Marquez VE, Jones PA. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther. 2009;8:1579–1588. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- [44].Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, Kong QL, Xu LH, Zhang X, Liu WL, Li MZ, Zhang L, Kang TB, Fu LW, Huang WL, Xia YF, Tsao SW, Li M, Band V, Band H, Shi QH, Zeng YX, Zeng MS. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119:3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang ZJ, Taylor F, Churchman M, Norbury G, Tomlinson I. Genetic pathways of colorectal carcinogenesis rarely involve the PTEN and LKB1 genes outside the inherited hamartoma syndromes. Am J Pathol. 1998;153:363–366. doi: 10.1016/S0002-9440(10)65579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chang JG, Chen YJ, Perng LI, Wang NM, Kao MC, Yang TY, Chang CP, Tsai CH. Mutation analysis of the PTEN/MMAC1 gene in cancers of the digestive tract. Eur J Cancer. 1999;35:647–651. doi: 10.1016/s0959-8049(98)00411-0. [DOI] [PubMed] [Google Scholar]
- [47].Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–2996. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- [48].Mak LH, Vilar R, Woscholski R. Characterisation of the PTEN inhibitor VO-OHpic. J Chem Biol. 2010;3:157–163. doi: 10.1007/s12154-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]