Abstract
Introduction:
The Heaviness of Smoking Index (HSI) is validated to measure nicotine dependence in nonpregnant smokers, and in these smokers, mean salivary and serum cotinine levels are related by a ratio of 1.25. However, as nicotine metabolism increases during gestation, these findings may differ in pregnancy. We investigated the validity of HSI in pregnancy by comparing this with 3 biochemical measures; in a search for a less-invasive cotinine measure in pregnancy, we also explored the relationship between mean blood and salivary cotinine levels.
Methods:
Cross-sectional analyses using baseline data from the Smoking, Nicotine, and Pregnancy Trial. Participants were 16–46 years old, 12–24 weeks gestation, smoked more than 5 cigarettes per day, and had exhaled carbon monoxide (CO) readings of at least 8 ppm. Linear regression was used to examine correlations between HSI and blood cotinine and salivary cotinine and exhaled CO. Correlation between blood and salivary cotinine was investigated using linear regression through the origin.
Results:
HSI scores were associated with blood cotinine (R 2 = 0.20, n = 662, p < .001), salivary cotinine (R 2 = 0.11, n = 967, p < .001), and exhaled CO (R 2 = 0.13, n = 1,050, p < .001). Salivary and blood cotinine levels, taken simultaneously, were highly correlated (R 2 = 0.91, n = 628, p < .001) and the saliva:blood level ratio was 1.01 (95% CI 0.99–1.04).
Conclusions:
Correlations between HSI and biochemical measures in pregnancy were comparable with those obtained outside pregnancy, suggesting that HSI has similar validity in pregnant smokers. Salivary and blood cotinine levels are roughly equivalent in pregnant smokers.
INTRODUCTION
Smoking in pregnancy is a modifiable risk factor for much morbidity, which carries a significant risk to both the mother and fetus, including long-term child health (Albrecht et al., 1999; Fang, Dukic, Pickett, Wakschlag, & Espy, 2012; Ward, Vander Weg, Sell, Scarinci, & Read, 2006). A large American retrospective cohort study found infant mortality rates to be 40% higher in pregnant smokers than nonsmokers (Salihu, Aliyu, Pierre-Louis, & Alexander, 2003). Despite this, studies in the United States (Colman & Joyce, 2003; Mathews & Rivera, 2004) and United Kingdom (Health and Social Care Information Centre, 2012) showed that at least one third to one half of female smokers continue to smoke during pregnancy.
Nonpregnant smokers with higher levels of nicotine dependence find it harder to quit smoking (Chaiton, Cohen, McDonald, & Bondy, 2007; Courvoisier & Etter, 2010) and nicotine replacement therapy can assist with this (Tang, Law, & Wald, 1994). Hence, nicotine dependence may be a crucial factor in the maintenance of smoking behavior in pregnant women (Albrecht et al., 1999). One measure of nicotine dependence, the six-item Fagerstrom Test for Cigarette Dependence (FTCD) has been validated and widely used outside of pregnancy (Burling & Burling, 2003; Carpenter, Baker, Gray, & Upadhyaya, 2010; Fagerström, 2012; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Of the six items included in FTCD, “How soon after you wake up do you smoke your first cigarette?” and “How many cigarettes/day do you smoke?” have been shown to account for most of the FTCD predictive value for smoking cessation (Burling & Burling, 2003; Chabrol, Niezborala, Chastan, & de Leon, 2005; Chaiton et al., 2007; Etter, Duc, & Perneger, 1999; Fagerström, Russ, Yu, Yunis, & Foulds, 2012; Haberstick et al., 2007; Heatherton et al., 1991; Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994; Pérez-Ríos et al., 2009) and correlate most strongly with biochemical measures of tobacco smoke exposure (Etter et al., 1999; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989). Hence, these two items have been combined in a shorter alternative measure of nicotine dependence, the Heaviness of Smoking Index (HSI), which has also been used widely outside of pregnancy (Borland, Yong, O’Connor, Hyland, & Thompson, 2010; Chaiton et al., 2007; Etter et al., 1999; John et al., 2004; Kozlowski et al., 1994; Lim et al., 2012). The notion that nicotine dependence is variable and can be measured (Burling & Burling, 2003) is important for characterizing smoking populations; measures of nicotine dependence are frequently used to describe participants in research studies and to guide smoking cessation treatment used by smokers (Tang et al., 1994).
Nicotine metabolism increases during gestation (Rebagliato et al., 1998; Tricker, 2006), and this is likely to affect smoking behaviors that the HSI measures, such as the number of cigarettes smoked. However, the timing and magnitude of gestational changes in nicotine metabolism are not known, and it is also not known if the relationship between smoking behaviors and nicotine metabolism remains constant as the latter changes. Consequently, it is not certain that the HSI, which has been validated to measure nicotine dependence in nonpregnant smokers, remains valid in pregnancy. After a review of the literature, we found no studies investigating the validity of HSI in pregnancy. However, we did find two studies that assessed two other nicotine dependence measures in pregnancy; one studied the FTCD (Panaretto et al., 2009) and the other, the Fagerstrom Tolerance Questionnaire (FTQ), a predecessor of FTCD. The first study (Panaretto et al., 2009) examined the use of FTCD in 152 urban, indigenous pregnant smokers in Australia. This found a statistically significant relationship between increasing FTCD scores and urinary cotinine concentrations. The other study (Albrecht et al., 1999) found a significant relationship between increasing FTQ scores and salivary cotinine levels in 42 pregnant adolescents recruited from three different smoking cessation studies. Although these studies suggest that both questionnaire-based measures may have some validity for measuring nicotine addiction in pregnancy, their sample sizes were small and more evidence is needed, particularly regarding the utility of the HSI in pregnancy.
Cotinine is the primary metabolite of nicotine and is often used as an objective biochemical measure of nicotine exposure to investigate validity of nicotine dependence measures (Benowitz, Hukkanen, & Jacob, 2009; Tricker, 2006). Cotinine levels can be measured in blood or saliva samples; however, the relationship between blood and salivary cotinine in pregnancy remains unclear due to the changes in nicotine metabolism (Dempsey, Jacob, & Benowitz, 2002; Rebagliato et al., 1998) and saliva composition during gestation (Eliasson, Birkhed, Osterberg, & Carlén, 2006; Guidozzi, Maclennan, Graham, & Jooste, 1992; Laine et al., 1988). Epidemiological studies of nonpregnant smokers found that mean salivary cotinine levels are closely related to serum cotinine but are around 25% higher (Jarvis, Primatesta, Erens, Feyerabend, & Bryant, 2003). Quantification of the relationship in pregnancy would enable researchers to compare findings from studies of pregnant smokers, which use either serum or saliva samples and would permit researchers the flexibility to be pragmatic when deciding whether to use saliva or blood samples for cotinine estimation in research studies (Tricker, 2006).
We aimed to investigate the validity of HSI as nicotine dependence measure in pregnancy by comparing this to three biochemical measures of nicotine and smoking exposure: blood cotinine, salivary cotinine, and exhaled carbon monoxide (CO); and we also investigated the relationship between blood and salivary cotinine samples taken simultaneously from pregnant smokers.
METHODS
Data for analyses in this report were collected at baseline in the Smoking, Nicotine and Pregnancy (SNAP) multicenter randomized trial that investigated the efficacy and safety of nicotine replacement patches in pregnancy (Coleman et al., 2012). Full methods and the study protocol were published elsewhere (Coleman et al., 2012, 2007).
Sample Population
From May 2007 to February 2010, 1,050 pregnant women who attended antenatal ultrasonography appointments at seven hospital sites in East Midlands, England, were recruited. Trial participants were pregnant smokers aged 16–46 years and 12–24 weeks of gestation. They smoked 10 or more cigarettes daily before pregnancy and five or more cigarettes daily at trial enrolment, as well as provided an exhaled CO sample of at least 8 ppm.
Measures
During enrolment, trial participants gave demographic information including age, height, weight, ethnicity, age at leaving full-time education, weeks of gestation, and parity. They were also asked the number of cigarettes smoked per day before pregnancy, the time which had elapsed since smoking their last cigarette, their partner’s smoking status, whether they had used nicotine replacement therapy during their current pregnancy, and if so, when they had last used it.
Heaviness of Smoking Index
The two following HSI questions were asked by trained research midwives during trial enrolment: “How soon after you wake up do you smoke your first cigarette?” (TTFC) and “How many cigarettes per day do you currently smoke?” (CPD). The actual value of TTFC in minutes and number of CPD were then categorized to derive HSI scores as suggested by previous study (Heatherton et al., 1989).
Biochemical Measures of Nicotine and Tobacco Exposure
Research midwives obtained biochemical samples from participants; labeled blood and saliva samples were placed in a −20°C freezer on the day of collection. The samples were later taken to the University of Nottingham where they continued to be stored at −20°C before being collated and transferred in three batches (November 2007, December 2008, and July 2010) to the University of Dundee for further storage at −80°C until analysis. Freezers were regularly checked with records kept of minimum and maximum temperatures, and when being transported, samples were placed in insulated containers, using dry ice where necessary, to ensure they remained frozen.
Blood samples used to derive blood cotinine levels were taken in BD Vacutainer Gold top tubes. Due to the samples being hemolyzed, the University of Dundee was unable to analyze these and transferred them to ABS Laboratories, Hertfordshire, in November 2011. They were subsequently analyzed between December 2011 and January 2012 and the stability of cotinine while frozen was assured by the laboratory.
For salivary cotinine analysis, at least 5ml of saliva were collected unstimulated by drooling into a sterile polypropylene container after rinsing mouth with cold water 5min prior to sampling. A strict protocol for saliva collection was adhered to. Samples were not taken if participants admitted to having drunk grapefruit juice within the previous week or if they had eaten, drunk, chewed gum, or brushed their teeth in the hour prior to sampling. The saliva samples were analyzed by the University of Dundee in batches from July 2009 to August 2009 and December 2010 to January 2011.
Both the salivary and blood cotinine levels were measured with a widely used liquid chromatography tandem mass spectrometry assay (Bernert et al., 2009; Doig et al., 2012). The blood cotinine analyses were performed blind to the results of salivary cotinine analyses. Exhaled CO levels were obtained using Micro 4 Smokerlyzer (Bedfont Scientific Limited, Rochester, Kent).
Statistical Analysis
Trial data collected at enrolment prior to randomization were used in all analyses. For blood and salivary cotinine analyses, women who did not provide samples were excluded as were those who reported having used nicotine replacement therapy (NRT) in the previous 7 days. NRT used by this latter group would potentially affect cotinine levels (Benowitz et al., 2009).
Demographics were compared between pregnant women included and excluded from each analysis using Mann–Whitney test for continuous data and Pearson chi-square test for categorical data. Internal reliability of HSI was examined using Cronbach’s alpha.
Linear regression was used to investigate the associations between HSI with each of the biochemical measures: blood cotinine, salivary cotinine, and exhaled CO. Assumptions of linear regression for each of the analyses were found to be adequately met. The relationships modeled appeared linear, and the residuals of the regression were normally distributed, showing no heteroscedasticity. Previous studies have found that age (Benowitz et al., 2009; Boyd, Windsor, Perkins, & Lowe, 1998), ethnicity (Benowitz et al., 2009; Boyd et al., 1998; Tricker, 2006), body mass index (BMI) (Jarvis et al., 2003; Tricker, 2006), and weeks of gestation (Benowitz et al., 2009; Dempsey et al., 2002; Rebagliato et al., 1998) affect nicotine metabolism and hence could be expected to influence salivary and blood cotinine levels. Having a smoking partner exposes the women to passive smoking, which would also affect the cotinine or CO levels (Boyd et al., 1998; Gilligan et al., 2010). As CO has a short half-life of 2–3hr, CO levels are relatively easily affected by the time elapsed since smoking prior to measuring the CO level (Javors, Hatch, & Lamb, 2011; Marrone, Paulpillai, Evans, Singleton, & Heishman, 2010). Hence, adjustment for these a priori factors was performed. The linearity of the association between HSI with each of the biochemical measures was assessed by fitting the effect of HSI in seven quantiles and compared with a trend through these categories using likelihood ratio test. In order to compare the coefficient of determination (R 2) between associations of HSI with different biochemical measures, sensitivity analyses using subgroup of pregnant smokers who provided all three biochemical measures were done.
Linear regression through the origin was used to investigate the estimate of ratio between blood and salivary cotinine. Age and BMI had been found to affect the regression coefficient in identical analysis performed in nonpregnant smokers (Benowitz et al., 2009; Jarvis et al., 2003; Tricker, 2006), whereas gestational age affects nicotine metabolism (Benowitz et al., 2009; Dempsey et al., 2002; Rebagliato et al., 1998). Hence, the effects of these a priori factors on the regression coefficient were investigated by interaction testing. All statistical analyses were performed using STATA, version 11.2. All CIs were at 95% and level of significance was at 5%.
RESULTS
Demographics
As all trial participants provided CO readings, all 1,050 pregnant women were included in the analyses that used exhaled CO readings. However, only 970 women provided saliva samples, and three of these had used NRT in the past 7 days, giving a sample size of 967 for the salivary cotinine analyses. Similarly, the sample size for the analyses using blood cotinine samples was 662, where 664 women provided blood samples, but two had used NRT in the previous 7 days. Six hundred twenty-eight women provided both blood and saliva samples and hence were included in the investigation of the relationship between blood and salivary cotinine. Demographics of the participants included in the analyses and the comparison with participants excluded are shown in Table 1.
Table 1.
Baseline Characteristics of Pregnant Women Included and Excluded From the Blood Cotinine (n = 662), Salivary Cotinine (n = 967), and Exhaled Carbon Monoxide (CO) (n = 1050) Analyses
| Characteristics | CO analysis (n = 1050) | Blood cotinine analysis | Salivary cotinine analysis | ||
|---|---|---|---|---|---|
| Included (n = 662) | Excluded (n = 388) | Included (n = 967) | Excluded (n = 83) | ||
| Age in years (M ± SD) | 26.3±6.2 | 26.8±6.2* | 25.6±6.0* | 26.3±6.2 | 26.2±6.0 |
| Gestational age in weeks (M ± SD) | 16.2±3.5 | 16.3±3.5 | 16.1±3.5 | 16.2±3.5 | 16.3±3.3 |
| Body mass index in kg/m2 (M ± SD)a | 27.0±6.2 | 26.8±5.7 | 27.2±7.0 | 27.0±6.2 | 26.0±6.3 |
| Ethnicity, n (%)b | |||||
| White British | 1018 (97.0%) | 643 (97.1%) | 375 (96.6%) | 940 (97.2%) | 78 (94.0%) |
| Others | 32 (3.0%) | 19 (2.9%) | 13 (3.4%) | 27 (2.8%) | 5 (6.0%) |
| Number of cigarettes smoked per day before pregnancy, (n) | |||||
| Median | 20 | 20 | 20 | 20 | 20 |
| Interquartile range | 15–20 | 15–20 | 15–20 | 15–20 | 15–20 |
| Number of cigarettes smoked per day at enrolment (n) | |||||
| Median | 13.5 | 13 | 15 | 13 | 15 |
| Interquartile range | 10–20 | 10–20 | 10–20 | 10–20 | 10–18 |
| Time from awakening to lighting first cigarette (min) | |||||
| Median | 10.0 | 10.0* | 7.0* | 10.0* | 7.0* |
| Interquartile range | 3.0–21.0 | 3.0–31.0 | 3.0–21.0 | 3.0–28.0 | 3.0–21.0 |
| Heaviness of Smoking Index, n (%) | |||||
| 0 | 65 (6.2%) | 45 (6.8%) | 20 (5.2%) | 61 (6.3%) | 4 (4.8%) |
| 1 | 134 (12.8%) | 91 (13.7%) | 43 (11.1%) | 128 (13.2%) | 6 (7.2%) |
| 2 | 259 (24.7%) | 161 (24.3%) | 98 (25.3%) | 238 (24.6%) | 21 (25.3%) |
| 3 | 321 (30.6%) | 202 (30.5%) | 119 (30.7%) | 295 (30.5%) | 26 (31.3%) |
| 4 | 209 (19.9%) | 132 (19.9%) | 77 (19.8%) | 186 (19.2%) | 23 (27.7%) |
| 5 | 52 (5.0%) | 29 (4.4%) | 23 (5.9%) | 50 (5.2%) | 2 (2.4%) |
| 6 | 10 (1%) | 2 (0.3%) | 8 (2.1%) | 9 (0.9%) | 1 (1.2%) |
| Blood cotinine (ng/ml)c | |||||
| Median | 127.1 | 127.1 | 142.3 | ||
| Interquartile range | 88.0–174.1 | 88.8–174.1 | 92.6–176.9 | ||
| Salivary cotinine (ng/ml)d | |||||
| Median | 121.5 | 120.5 | 124.0 | ||
| Interquartile range | 78.1–177.2 | 74.9–180.1 | 81.2–167.7 | ||
| Exhaled carbon monoxide (ppm) | |||||
| Median | 14 | 15 | 14 | 14 | 15 |
| Interquartile range | 11–20 | 11–21 | 11–19.8 | 11–20 | 11–21 |
| Women with partner who smokes, n (%)e | 716 (74.4%) | 439 (71.4%)* | 277 (79.6%)* | 662 (74.3%) | 54 (75.0%) |
| Previous use of nicotine replacement therapy during current pregnancy, n (%) | 47 (4.5%) | 27 (4.1%) | 20 (5.2%) | 41 (4.2%) | 6 (7.2%) |
Note. aBody mass index was not recorded for 22 women in the included group and 34 women in the excluded group (blood cotinine analysis); 47 women in the included group and 9 women in the excluded group (salivary cotinine analysis); 56 women in the overall group (exhaled CO analysis).
bSelf-reported ethnicity was categorized according to standard U.K. census category. “Other” included other White categories (e.g., White Irish or White other).
cThree hundred and thirty-nine women in the included group and 47 women in the excluded group (salivary cotinine analysis); 386 women in the overall group (exhaled CO analysis) did not have blood cotinine collected at the time of enrolment.
dThirty-four women in the included group and 46 women in the excluded group (blood cotinine analysis); 80 women in the overall group (exhaled CO analysis) did not have salivary cotinine collected at the time of enrolment.
eForty-seven women in the included group and 40 women in the excluded group (blood cotinine analysis); 76 women in the included group and 11 women in the excluded group (salivary cotinine analysis); 87 women in the overall group (exhaled CO analysis) did not have a partner at the time of enrolment.
*Difference in baseline characteristics between pregnant women included and excluded in the analyses was statistically significant (p < .05).
The 662 women who were included in the blood cotinine analyses were primarily White British with a mean (SD) age of 26.8 (6.2), mean (SD) gestation of 16.3 (3.5) weeks, and mean BMI (SD) of 26.8 (5.7) kg/m2. They smoked a median (interquartile range) of 20 (15–20) and 13 (10–20) cigarettes before pregnancy and at the time of enrolment, respectively. Pregnant women included in the blood cotinine analyses were found to be older (26.8 years in the included group vs. 25.6 years in the excluded group, p = .002) and less likely to have a partner who smokes than women excluded from the analyses (71.4% vs. 79.6%, p = .005). They also took longer to light their first cigarette after waking (10min vs. 7min, p = .02). There were no statistically significant differences between remaining baseline characteristics, including HSI scores (mean HSI of 2.57 and 2.75 in the included and excluded group, respectively, p = .058).
The 967 pregnant women used for salivary cotinine analyses generally had similar characteristics to all women enrolled in the SNAP trial except that pregnant women in this group also took longer to light their first cigarette of the day (10min vs. 7min, p = .04).
Internal Reliability
The Cronbach’s alpha coefficient calculated for HSI in pregnant smokers was .49.
Relationship Between HSI With Biochemical Measures of Nicotine and Tobacco Exposure
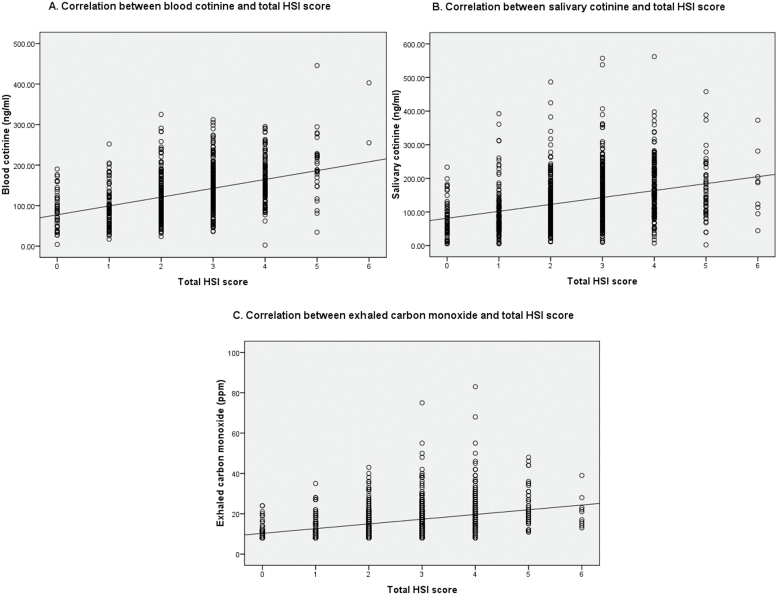
Scatter plots showed a fairly linear relationship between HSI and biochemical measures (Figure 1). Linear regression revealed that HSI accounted for 20%, 11%, and 13% of the variations in blood cotinine, salivary cotinine, and exhaled CO, respectively. After controlling for a priori factors, HSI was still significantly associated with blood cotinine, salivary cotinine, and exhaled CO (all p values <.001). For every 1 unit rise of the HSI score, blood and salivary cotinine increased by 20.4ng/ml and 18.9ng/ml, respectively, whereas exhaled CO increased by 2.16 ppm. Sensitivity analyses using linear regression of only pregnant women who provided all three biochemical measures (n = 628) revealed similar R 2 and regression coefficient for HSI scores for each of the three biochemical measures: blood cotinine (R 2 = 0.21, p < .001), salivary cotinine (R 2 = 0.13, p < .001), and exhaled CO (R 2 = 0.14, p < .001).
Figure 1.
Scatter plots showing correlations between Heaviness of Smoking Index (HSI) score and (A) blood cotinine, (B) salivary cotinine, and (C) exhaled carbon monoxide in 662, 967, and 1,050 pregnant women, respectively, with their relevant linear regression lines.
Likelihood ratio testing revealed no significant improvement in the fit of model using HSI as seven quantiles rather than as a trend across the categories for the salivary cotinine (p = .35) and exhaled CO analyses (p = .28). These indicated that HSI had linear relationships with salivary cotinine and exhaled CO. However, for the analysis using blood cotinine, the model using HSI as seven quantiles was found to fit significantly better than that of a trend across the categories (p = .03). However, there was no obvious nonlinear relationship between HSI and blood cotinine on inspection of the scatter plot (Figure 1).
Relationship Between Blood and Salivary Cotinine
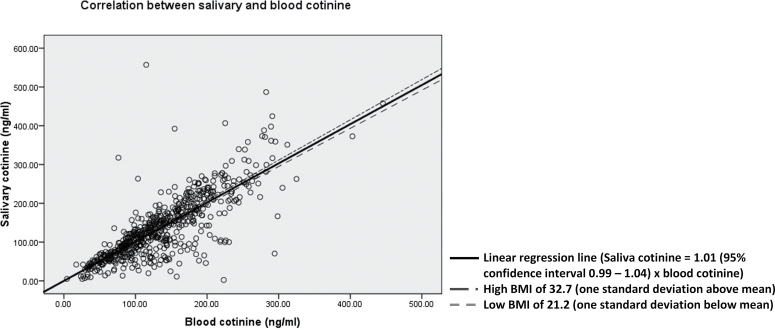
Figure 2 showed a good correlation between blood and salivary cotinine levels (R 2 = 0.91, n = 628, p < .001). Univariate linear regression through the origin provided an estimate of ratio of 1.01 (95% CI 0.99–1.04) for the relationship between mean salivary and blood cotinine. Even after controlling for a priori factors, the regression coefficient for the relationship remained very similar at 1.03 (95% CI 0.97–1.09).
Figure 2.
Scatter plot showing correlation between blood and salivary cotinine in 628 pregnant smokers with linear regression line without taking into account of a priori factor, as well as regression lines at high and low body mass index (BMI).
Interaction Testing
Age (p = .74) and gestation (p = .39) were not significant moderators to the relationship between blood and salivary cotinine. A statistically significant interaction effect was found between linear continuous variable of BMI and blood cotinine levels in predicting salivary cotinine levels (p = .02), suggesting that the slope of the regression line between blood and salivary cotinine varies with BMI. However, the estimate of this slope did not alter markedly at the two extremes of BMI of 1 SD below and above the mean, respectively (0.98 [95% CI 0.94–1.02] in low BMI vs. 1.04 [95% CI 1.00–1.08] in high BMI; Figure 2).
DISCUSSION
This study found that in pregnant smokers, higher HSI scores were associated with increasing tobacco exposure as indicated by biochemical measures. The relationships observed were of a similar magnitude to those obtained in studies done in nonpregnant smokers, suggesting that HSI is similarly valid in pregnancy. Our study also found a high correlation between salivary and blood cotinine levels in pregnancy. Unlike the previously observed ratio of 1.25 in nonpregnant smokers (Jarvis et al., 2003), the ratio of mean salivary to blood cotinine levels in pregnancy was 1, even after adjustment for a priori factors.
Study Limitations and Strengths
Smokers included in our study mainly described their ethnic group as “White British” and were all enrolled in a clinical trial, which specifically sought participants who were in their second trimester of pregnancy, smoked at least five cigarettes daily and motivated to quit smoking. Hence, findings in the study may not be generalizable to pregnant smokers of other racial background and who smoke fewer cigarettes or who have not expressed a desire to quit. However, our pregnant women smoked a mean of 14 cigarettes daily with mean HSI of 2.64, which was similar to previous studies in pregnant (England et al., 2001; Pollak et al., 2007; Office for National Statistics, 2013) and nonpregnant smokers (Courvoisier & Etter, 2010; Health and Social Care Information Centre, 2012). It is unclear if similar findings could be extrapolated to the first and third gestation of pregnancy. However, this may not affect the clinical importance of the findings as the first clinical encounter of the majority pregnant women would be during their booking visit when they are in the end of their first or beginning of their second trimester of pregnancy.
Although our study found similar magnitude of the associations between HSI and biochemical markers with other studies of nonpregnant smokers, this was not tested formally using a control nonpregnant group due to study design.
Participants in the study were aware that biological specimens would be used to monitor their smoking behavior. We found some evidence that participants who refused to provide biological specimens may have been more nicotine dependent than others; these women were found to light their first cigarette after waking earlier than those who provided samples. It is not clear how the likelihood that participants who provided samples differed from others might have affected study findings. However, as a sensitivity analysis conducted in the much smaller group of women who provided all three biochemical measures revealed similar findings to those in the larger, less self-selected groups, we believe that any biases in women’s propensity to provide samples are unlikely to have had a major effect on the relationships between HSI and biochemical measures observed in our study.
One of the strengths of our study was the large sample sizes available for analyses and the ability to adjust for key potential confounders of cotinine and CO levels obtained after smoking exposure. As such, the analyses should have had sufficient power and confounder adjustments to permit relationships between HSI and biochemical measures to be accurately quantified. An additional strength was the use of trained research midwives who followed a well-defined protocol to collect all questionnaire data and biological samples. Finally, to our knowledge, this was the first study to investigate the validity of HSI as a self-reported nicotine dependence measure in pregnancy, so findings are likely to be of widespread interest.
Relationship Between HSI With Biochemical Measures of Nicotine and Tobacco Exposure
Cotinine is the primary metabolite of nicotine found in cigarettes. Although there may be a lack of agreement about the gold standard measure for nicotine dependence in pregnancy, blood cotinine is a sensitive and stable biochemical nicotine exposure measure from cigarette smoking (Dempsey et al., 2002; Kvalvik et al., 2012; Tricker, 2006) and has previously been used to validate nicotine dependence measures (Carpenter et al., 2010; SRNT Subcommittee on Biochemical Verification, 2002). In our study, after controlling for factors considered, a priori, to possibly affect cotinine or CO levels, a significant positive correlation was found between HSI scores and the three biochemical measures. Of the three biochemical measures, blood cotinine, which has previously been used as a “gold standard” in similar studies (Carpenter et al., 2010; SRNT Subcommittee on Biochemical Verification, 2002), was found to correlate best with HSI. Although the relationships observed were not strong, associations between salivary cotinine levels and HSI scores were comparable with those obtained in the very few studies conducted within nonpregnant smokers of similar sample size (Table 2). However, we could not find any previous studies comparing HSI with blood cotinine levels for comparison. This similarity in findings was observed despite women in our sample smoking fewer cigarettes per day (mean number smoked daily, 14 compared with 17–27 in Table 2 studies); but lighting their first cigarette of the day was substantially earlier than in other studies (mean time to first cigarette, 21min compared with 43–47min in Table 2 studies). The stability of the relationship between HSI and cotinine measures in these varied samples of smokers suggests that HSI’s validity for measuring nicotine dependence may be generic across smokers with very different characteristics.
Table 2.
Studies Investigating the Relationship of Heaviness of Smoking Index (HSI) With Biochemical Measures of Nicotine and Tobacco Exposure
| Study | Sample size | Population characteristics | HSI score (M ± SD) | TTFC in minutes (M ± SD) | CPD (M ± SD) | Questionnaire | Biochemical measure | R 2 |
|---|---|---|---|---|---|---|---|---|
| Heatherton et al. (1989): Sample 1 | 168 | Visitors to science centre; 52% male and 48% female; mean age was 30.7±9.6 years | N/A | 43.3±56.9 | 22.1±9.3 | HSI | Salivary cotinine | .30 |
| Exhaled CO | .30 | |||||||
| Heatherton et al. (1989): Sample 2 | 736 | Population recruited by visiting randomly selected addresses; 51% male and 49% female; mean age was 39.6±13.7 years | N/A | 46.4±90.5 | 21.5±11.3 | HSI | Salivary cotinine | .10 |
| Exhaled CO | .07 | |||||||
| Heatherton et al. (1991) | 254 | Visitors to science center; 44% male and 56% female; mean age was 33.5±12.7 years. | N/A | 47.2±86.8 | 20.7±10.5 | HSI | Salivary cotinine | .31 |
| Exhaled CO | .26 | |||||||
| Kozlowski et al. (1994) | 932 | Smoking cessation program participants; 35% male and 65% female. mean age was 40±11.3 years | 3.9±0.05 | N/A | 27.0±12.2 | HSI | Exhaled CO | .06 |
| Burling (2003) | 191 | All male drug/alcohol dependent cigarette smokers in residential rehabilitation; mean age was 40.0±6.7 years | 3.0±1.2 | N/A | 17.2±7.3 | HSI | Urine cotinine | .10 |
| Exhaled CO | .17 |
Note. TTFC = time taken from awakening to first cigarette smoked; CPD = number of cigarettes smoked per day; R 2 = coefficient of determination of linear regression; N/A = data not reported in the study; CO = carbon monoxide.
Our study found a generally linear relationship between HSI scores and biochemical measures of exposure, which was consistent with studies of nonpregnant smokers (Table 2). The nonlinearity of the relationship between HSI scores and blood cotinine as suggested by the likelihood ratio testing must be interpreted with caution. Pregnant smokers with HSI scores of 6 had blood cotinine levels that were substantially higher than those predicted by linear regression (Figure 1), but as there are very few participants who had such high HSI scores, this finding could reflect the very small numbers of participants contributing to this analysis. Internal reliability of HSI in our sample of pregnant women was comparable with studies in nonpregnant smokers, which obtained Cronbach’s α of .49 to .56 (Burling & Burling, 2003; de Meneses-Gaya et al., 2009).
Relationship Between Blood and Salivary Cotinine
Similar to analyses conducted in nonpregnant smokers, blood and salivary cotinine in pregnant smokers were closely related (Jarvis et al., 2003; Tricker, 2006). Although BMI was found to influence the relationship between blood and salivary cotinine in pregnant smokers, it did not alter the regression coefficient substantially. The effect of age on the relationship found in studies of nonpregnant smoker (Jarvis et al., 2003) was not noted in our study; however, as all participants in our study were pregnant, the range of their ages was restricted, which could explain this difference.
Interestingly, the ratio of salivary to blood cotinine levels in our sample of pregnant smokers was around unity. This contrasts to findings of a recent review (Tricker, 2006), which reported the range of salivary:plasma cotinine ratio in nonpregnant smokers as 1.1–1.4, with most empirical studies being small or having wide CIs (Bernert, McGuffey, Morrison, & Pirkle, 2000; Machacek & Jiang, 1986; van Vunakis et al., 1989). The largest study included in the review (Jarvis et al., 2003) included 270 female nonpregnant smokers and found a ratio of salivary to plasma cotinine of 1.23 (95% CI 1.21–1.25). Although this study used plasma cotinine analyzed by gas chromatography rather than blood cotinine measured by liquid chromatography, blood samples from this study and from ours were processed by the same laboratory, and no difference in cotinine levels due to variation in sample analysis method was expected (Bernert et al., 2009). Hence, it seems appropriate that in samples of pregnant women, mean salivary and blood cotinine values can be treated virtually interchangeable.
The closer relationship between salivary and blood cotinine levels observed in our pregnant sample may be due to the effects of hormonal changes in pregnancy on saliva characteristics and composition. The rise in estrogen in pregnancy may lower the pH and buffer capacity of saliva in pregnant women, affecting absorption of cotinine (Laine et al., 1988; Lukacs & Largaespada, 2006; Rebagliato et al., 1998). An alternative explanation may be that the increased nicotine metabolism in pregnancy (Rebagliato et al., 1998; Tricker, 2006) may affect salivary cotinine more than blood cotinine leading to the lower ratio, and further research is required.
SUMMARY
Our study had shown that HSI is a valid, brief measure of nicotine dependence in pregnancy, producing comparable results and psychometric properties to those observed in nonpregnant smokers. Future studies involving pregnant smokers can routinely incorporate HSI as a measure of nicotine dependence and, if preferred, cotinine assays can be performed using salivary samples rather than blood samples because these produce roughly equivalent cotinine values and obtaining saliva samples is less invasive. In pregnancy, when calculating mean salivary cotinine values from mean blood cotinine values, a ratio of 1 should be used instead of 1.25, which has been suggested for nonpregnant smokers.
FUNDING
The original Smoking, Nicotine and Pregnancy (SNAP) randomized control trial was supported by a grant from the National Institute for Health Research (NIHR) Health Technology Assessment Programme (grant number 06/07/01).
DECLARATION OF INTERESTS
The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. T.C. and S.L. are members of the UK Centre for Alcohol and Tobacco Studies; T.C. and S.C. are members of the NIHR National School for Primary Care Research, and J.T. is funded via an NIHR fellowship.
ACKNOWLEDGMENTS
We would like to thank Mira V. Doig and ABS Laboratories for their contribution in blood cotinine analyses and interpretation of the blood cotinine values. We would also like to thank Dr Sheila Sharp and the University of Dundee for their contribution in analyzing the salivary cotinine samples.
REFERENCES
- Albrecht S. A., Cornelius M. D., Braxter B., Reynolds M. D., Stone C., Cassidy B. (1999). An assessment of nicotine dependence among pregnant adolescents. Journal of Substance Abuse Treatment, 16, 337–344.10.1016/s0740-5472(98)00074-9 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Hukkanen J., Jacob P., III (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of Experimental Pharmacology, 192, 29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert J. T., Jacob P., III, Holiday D. B., Benowitz N. L., Sosnoff C. S., Doig M. V, … Langman L. J. (2009). Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: A round-robin study. Nicotine & Tobacco Research, 11, 1458–1466.10.1093/ntr/ntp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert J. T., Jr, McGuffey J. E., Morrison M. A., Pirkle J. L. (2000). Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. Journal of Analytical Toxicology, 24, 333–339 [DOI] [PubMed] [Google Scholar]
- Borland R., Yong H. H., O’Connor R. J., Hyland A., Thompson M. E. (2010). The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four Country study. Nicotine & Tobacco Research, 12, S45–S50.10.1093/ntr/ntq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. R., Windsor R. A., Perkins L. L., Lowe J. B. (1998). Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Maternal and child health journal, 2, 77–83.10.1023/a:1022936705438 [DOI] [PubMed] [Google Scholar]
- Burling A. S., Burling T. A. (2003). A comparison of self-report measures of nicotine dependence among male drug/alcohol-dependent cigarette smokers. Nicotine & Tobacco Research, 5, 625–633.10.1080/1462220031000158708 [DOI] [PubMed] [Google Scholar]
- Carpenter M. J., Baker N. L., Gray K. M., Upadhyaya H. P. (2010). Assessment of nicotine dependence among adolescent and young adult smokers: A comparison of measures. Addictive Behaviors, 35, 977–982.10.1016/j.addbeh. 2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol H., Niezborala M., Chastan E., de Leon J. (2005). Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addictive Behaviors, 30, 1474–1477.10.1016/j.addbeh.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Chaiton M. O., Cohen J. E., McDonald P. W., Bondy S. J. (2007). The Heaviness of Smoking Index as a predictor of smoking cessation in Canada. Addictive Behaviors, 32, 1031–1042.10.1016/j.addbeh.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Coleman T., Cooper S., Thornton J. G., Grainge M. J., Watts K., Britton J., Lewis S; Smoking, Nicotine, and Pregnancy (SNAP) Trial Team (2012). A randomized trial of nicotine-replacement therapy patches in pregnancy. The New England Journal of Medicine, 366, 808–818 [DOI] [PubMed] [Google Scholar]
- Coleman T., Thornton J., Britton J., Lewis S., Watts K., Coughtrie M. W. H, … Godfrey C. (2007). Protocol for the Smoking, Nicotine and Pregnancy (SNAP) trial: Double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy. BMC Health Services Research, 7, 2.10.1186/1472-6963-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G. J., Joyce T. (2003). Trends in smoking before, during, and after pregnancy in ten states. American Journal of Preventive Medicine, 24, 29–35.10.1016/s0749-3797(02)00574-3 [DOI] [PubMed] [Google Scholar]
- Courvoisier D. S., Etter J. F. (2010). Comparing the predictive validity of five cigarette dependence questionnaires. Drug and Alcohol Dependence, 107, 128–133.10.1016/j.drugalcdep.2009.09.011 [DOI] [PubMed] [Google Scholar]
- de Meneses-Gaya C., Zuardi A. W., de Azevedo Marques J. M., Souza R. M., Loureiro S. R., Crippa J. A. (2009). Psychometric qualities of the Brazilian versions of the Fagerström Test for Nicotine Dependence and the Heaviness of Smoking Index. Nicotine & Tobacco Research, 11, 1160–1165 [DOI] [PubMed] [Google Scholar]
- Dempsey D., Jacob P., III, Benowitz N. L. (2002). Accelerated metabolism of nicotine and cotinine in pregnant smokers. The Journal of Pharmacology and Experimental Therapeutics, 301, 594–598.10.1124/jpet.301.2.594 [DOI] [PubMed] [Google Scholar]
- Doig M. V., Feyerabend C., Coleman T., Cooper S. (2012). See how sophisticated LC-MS/MS assays are helping smoking research and cessation programme. Poster session presented at 14th Annual Conference of the Society for Research on Nicotine and Tobacco-EuropeChapter; 2012 Aug 30–Sep 2; Helsinki, Finland [Google Scholar]
- Eliasson L., Birkhed D., Osterberg T., Carlén A. (2006). Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. European Journal of Oral Sciences, 114, 494–499.10.1111/j.1600-0722.2006.00413.x [DOI] [PubMed] [Google Scholar]
- England L. J., Kendrick J. S., Wilson H. G., Merritt R. K., Gargiullo P. M., Zahniser S. C. (2001). Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology, 154, 694–701.10.1093/aje/154.8.694 [DOI] [PubMed] [Google Scholar]
- Etter J. F., Duc T. V., Perneger T. V. (1999). Validity of the Fagerström test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction, 94, 269–281.10.1046/j.1360-0443.1999.94226910.x [DOI] [PubMed] [Google Scholar]
- Fagerström K. (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research, 14, 75–78.10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Fagerström K., Russ C., Yu C. R., Yunis C., Foulds J. (2012). The Fagerström Test for Nicotine Dependence as a predictor of smoking abstinence: A pooled analysis of varenicline clinical trial data. Nicotine & Tobacco Research, 14, 1467–1473.10.1093/ntr/nts018 [DOI] [PubMed] [Google Scholar]
- Fang H., Dukic V., Pickett K. E., Wakschlag L., Espy K. A. (2012). Detecting graded exposure effects: A report on an East Boston pregnancy cohort. Nicotine & Tobacco Research, 14, 1115–1120.10.1093/ntr/ntr272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan C., Sanson-Fisher R., Eades S., Wenitong M., Panaretto K., D’Este C. (2010). Assessing the accuracy of self-reported smoking status and impact of passive smoke exposure among pregnant Aboriginal and Torres Strait Islander women using cotinine biochemical validation. Drug and Alcohol Review, 29, 35–40.10.1111/j.1465-3362.2009.00078.x [DOI] [PubMed] [Google Scholar]
- Guidozzi F., Maclennan M., Graham K. M., Jooste C. P. (1992). Salivary calcium, magnesium, phosphate, chloride, sodium and potassium in pregnancy and labour. South African Medical Journal, 81, 152–154 [PubMed] [Google Scholar]
- Haberstick B. C., Timberlake D., Ehringer M. A., Lessem J. M., Hopfer C. J., Smolen A., Hewitt J. K. (2007). Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction, 102, 655–665 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Rickert W., Robinson J. (1989). Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction, 84, 791–799 [DOI] [PubMed] [Google Scholar]
- Health and Social Care Information Centre (2012). Statistics on Smoking—England, 2012 Retrieved from http://www.hscic.gov.uk/catalogue/PUB07019
- Jarvis M. J., Primatesta P., Erens B., Feyerabend C., Bryant A. (2003). Measuring nicotine intake in population surveys: Comparability of saliva cotinine and plasma cotinine estimates. Nicotine & Tobacco Research, 5, 349–355.10.1080/1462220031000094213 [DOI] [PubMed] [Google Scholar]
- Javors M. A., Hatch J. P., Lamb R. J. (2011). Sequential combination of self-report, breath carbon monoxide, and saliva cotinine to assess smoking status. Drug and Alcohol Dependence, 113, 242–244.10.1016/j.drugalcdep.2010. 07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U., Meyer C., Schumann A., Hapke U., Rumpf H. J., Adam C, … Ludemann J. (2004). A short form of the Fagerström Test for Nicotine Dependence and the Heaviness of Smoking Index in two adult population samples. Addictive Behaviors, 29, 1207–1212.10.1016/j.addbeh. 2004.03.019 [DOI] [PubMed] [Google Scholar]
- Kozlowski L. T., Porter C. Q., Orleans C. T., Pope M. A., Heatherton T. (1994). Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence, 34, 211–216.10.1016/0376-8716(94)90158–9 [DOI] [PubMed] [Google Scholar]
- Kvalvik L. G., Nilsen R. M., Skjærven R., Vollset S. E., Midttun O., Ueland P. M., Haug K. (2012). Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatric Research, 72, 101–107.10.1038/pr.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine M., Tenovuo J., Lehtonen O. P., Ojanotko-Harri A., Vilja P., Tuohimaa P. (1988). Pregnancy-related changes in human whole saliva. Archives of Oral Biology, 33, 913–917.10.1016/0003-9969(88)90022-2 [DOI] [PubMed] [Google Scholar]
- Lim K. H., Idzwan M. F., Sumarni M. G., Kee C. C., Amal N. M., Lim K. K., Gurpreet K. (2012). Heaviness of smoking index, number of cigarettes smoked and the Fagerstrom test for nicotine dependence among adult male Malaysians. Asian Pacific Journal of Cancer Prevention, 13, 343–346.10.7314/apjcp.2012.13.1.343 [DOI] [PubMed] [Google Scholar]
- Lukacs J. R., Largaespada L. L. (2006). Explaining sex differences in dental caries prevalence: Saliva, hormones, and “life-history” etiologies. American Journal of Human Biology, 18, 540–555.10.1002/ajhb.20530 [DOI] [PubMed] [Google Scholar]
- Machacek D. A., Jiang N. S. (1986). Quantification of cotinine in plasma and saliva by liquid chromatography. Clinical Chemistry, 32, 979–982 [PubMed] [Google Scholar]
- Marrone G. F., Paulpillai M., Evans R. J., Singleton E. G., Heishman S. J. (2010). Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Human Psychopharmacology, 25, 80–83.10.1002/hup.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews T. J., Rivera C. C. (2004). Smoking during pregnancy—United States, 1990–2002. Morbidity and Mortality Weekly Report, 53, 911–915 [PubMed] [Google Scholar]
- Office for National Statistics (2013). Chapter 1—Smoking (general lifestyle survey overview—a report on the 2011 General Lifestyle Survey) Retrieved from http://www.ons.gov.uk/ons/dcp171776_302558.pdf
- Panaretto K. S., Mitchell M. R., Anderson L., Gilligan C., Buettner P., Larkins S. L., Eades S. (2009). Tobacco use and measuring nicotine dependence among urban Indigenous pregnant women. The Medical journal of Australia, 191, 554–557 [DOI] [PubMed] [Google Scholar]
- Pérez-Ríos M., Santiago-Pérez M. I., Alonso B., Malvar A., Hervada X., de Leon J. (2009). Fagerstrom test for nicotine dependence vs heavy smoking index in a general population survey. BMC Public Health, 9, 493.10.1186/1471-2458-9-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak K. I., Oncken C. A., Lipkus I. M., Lyna P., Swamy G. K., Pletsch P. K, … Myers E. R. (2007). Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. American Journal of Preventive Medicine, 33, 297–305.10.1016/j.amepre.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliato M., Bolúmar F., Florey Cdu. V., Jarvis M. J., Pérez-Hoyos S., Hernández-Aguado I., Avino M. J. (1998). Variations in cotinine levels in smokers during and after pregnancy. American Journal of Obstetrics and Gynecology, 178, 568–571.10.1016/s0002-9378(98)70440–5 [DOI] [PubMed] [Google Scholar]
- Salihu H. M., Aliyu M. H., Pierre-Louis B. J., Alexander G. R. (2003). Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Maternal and Child Health Journal, 7, 219–227.10.1023/a:1027319517405 [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159.10.1080/ 14622200210123581 [DOI] [PubMed] [Google Scholar]
- Tang J. L., Law M., Wald N. (1994). How effective is nicotine replacement thearpy in helping people to stop smoking. British Medical Journal, 308, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricker A. R. (2006). Biomarkers derived from nicotine and its metabolites: A review. Beitraege zur Tabakforschung International, 22, 147–175 [Google Scholar]
- van Vunakis H., Tashkin D. P., Rigas B., Simmons M., Gjika H. B., Clark V. A. (1989). Relative sensitivity and specificity of salivary and serum cotinine in identifying tobacco-smoking status of self-reported nonsmokers and smokers of tobacco and/or marijuana. Archives of Environmental Health, 44, 53–58 [DOI] [PubMed] [Google Scholar]
- Ward K. D., Vander Weg M. W., Sell M. A., Scarinci I. C., Read M. C. (2006). Characteristics and correlates of quitting among black and white low-income pregnant smokers. American Journal of Health Behavior, 30, 651–662 [DOI] [PubMed] [Google Scholar]