Abstract
Introduction:
Prenatal tobacco exposure, through maternal smoking during pregnancy, has been associated with adverse mental health outcomes in childhood. However, the mechanisms by which prenatal tobacco exposure compromises mental health later in life are unclear. We hypothesized that sensitized reactivity to stressful life events in early childhood mediates the effect of prenatal tobacco exposure on mental health outcomes in middle childhood, after accounting for earlier mental health outcomes.
Methods:
Data were from 12,308 mothers and their children drawn from the Avon Longitudinal Study of Parents and Children, a large prospective population-based study. Mothers’ self-reports of smoking during pregnancy, mothers’ ratings of their child’s reactivity to stressful life events, and teachers’ and mothers’ ratings of the Strengths and Difficulties Questionnaire assessing 5 domains of mental health outcomes were measured.
Results:
A positive association was found between prenatal tobacco exposure and stress reactivity between the ages of 2 and 6. In turn, stress reactivity was positively associated with peer (isolation), hyperactivity, conduct, and emotional problems (but not prosocial behaviors) between the ages of 7 and 11, after accounting for the mental health outcome at age 4 and other confounders.
Conclusions:
Heightened stress reactivity in preschool ages mediated the effect of prenatal tobacco exposure on adverse mental health outcomes between the ages of 7 and 11. Interventions to assist children exposed to tobacco smoke during gestation in coping with stressful life events may help mitigate psychiatric symptoms in this population.
Despite public awareness of its adverse health effects, 11%–12% of pregnant women in the United States report smoking (Martin et al., 2007). This is probably an underestimate because there is thought to be substantial underreporting of smoking during pregnancy and a subset of women who quit smoking early in pregnancy relapse later (Warland & McCutcheon, 2011). Health consequences of prenatal tobacco exposure include reduced birth weight and increased risk of preterm delivery and still births (Salihu & Wilson, 2007). In addition to these physiological effects, children exposed to tobacco smoke in utero also have an increased risk of psychiatric morbidity (Ekblad, Gissler, Lehtonen, & Korkeila, 2010). Prenatal smoke exposure has been associated with diverse externalizing problems including attention deficit hyperactivity disorder, conduct disorder, antisocial behavior, and substance abuse (Brennan, Grekin, Mortensen, & Mednick, 2002; Button, Maughan, & McGuffin, 2007; Ernst, Moolchan, & Robinson, 2001; Linnet et al., 2003; Nomura, Marks, & Halperin, 2010; Rogers, 2009). Although less studied, an association of prenatal tobacco exposure with internalizing problems has been found (Ashford, van Lier, Timmermans, Cuijpers, & Koot, 2008). However, results from recent genetically informed studies using twin (Button, Thapar, & McGuffin, 2005; Knopik et al., 2005; Maughan, Taylor, Caspi, & Moffitt, 2004; Thapar et al., 2003), children-of-twins (D’Onofrio et al., 2003; Knopik et al., 2006), and prenatal cross-fostering (via in vitro fertilization; Rice et al., 2010; Thapar et al., 2009) study designs indicate that once genetic and familial environmental factors were accounted for, the effect of prenatal tobacco exposure became substantially reduced. These findings highlight the importance of rigorous and comprehensive control for confounding factors in the association of prenatal tobacco exposure and subsequent mental health outcomes.
Although the association between prenatal tobacco exposure and adverse mental health outcomes is well documented, the mechanisms by which tobacco exposure in utero compromises mental health later in life are not well characterized. Animal studies have suggested that nicotine exposure during gestation can alter stress reactivity. For example, fetal nicotine exposure had little effect on radial arm maze performance in rats, unless the animals were stressed by exposure to an altered environment (Levin, Wilkerson, Jones, Christopher, & Briggs, 1996). Mice, that were exposed to nicotine either throughout development or just during the postnatal period, also showed increased reactivity to a stressful stimulus as measured by avoidance of a mild footshock (Heath, King, Gotti, Marks, & Picciotto, 2010). Mouse studies (Heath et al., 2010; King et al., 2003) showed that the hyperreactivity to shock occurred only when nicotine exposure or manipulation of the nicotinic system coincided with maturation of corticothalamic circuits and that nicotinic modulation only in cortical layer VI pyramidal neurons is sufficient to alter sensitivity to the mild stressor. The ability of nicotine to bind to, activate, and desensitize nAChRs on neurons (which normally transduce endogenous acetylcholine signals) likely disrupts the normal development of neuronal circuits modulated by release of acetylcholine in response to salient environmental events, thus likely resulting in long-term changes that alter responses to stressful stimuli (Heath & Picciotto, 2009; Navarro et al., 1989; Roy, Seidler, & Slotkin, 2002; Sobrian, Ali, Slikker, & Holson, 1995). However, these animal studies on the role of stress reactivity used nicotine (as opposed to tobacco, which includes over 4000 chemicals besides nicotine), and thus, these results from animal studies likely represent a subset of the effects of prenatal tobacco exposure in humans.
The current study had two aims. First, we aimed to replicate the previous findings showing an association between prenatal tobacco exposure and mental health outcomes in a large prospective population-based sample. We hypothesized that prenatal tobacco exposure would be associated with greater levels of externalizing and internalizing problems in middle childhood. Second, we aimed to characterize a potential mechanism underlying the association between prenatal tobacco exposure and childhood mental health outcomes. We examined whether sensitized reactivity to stressors at ages 2–6 mediates the effect of prenatal tobacco exposure on mental health outcomes at ages 7–11. Specifically, we hypothesized that prenatal tobacco exposure would be associated with sensitized reactivity to stressors. We further hypothesized that the sensitized stress reactivity would, in turn, be associated with externalizing and internalizing problems in middle childhood. Given the previous findings of systematic differences in socioeconomic background and psychiatric histories between mothers who smoke during pregnancy and mothers who do not, we statistically controlled for those potential confounders. Also, due to the concern of potential report biases in children’s mental health outcomes rated by a mother and a teacher as a function of the reporters’ own mental health status (Briggs-Gowan, Carter, & Schwab-Stone, 1996; Youngstrom, Loeber, & Stouthamer-Loeber, 2000; see De Los Reyes & Kazdin, 2005 for a review), we statistically controlled for mothers’ and teachers’ depression and anxiety at the time of their ratings. Finally, to test a unique effect of stress reactivity on the later mental health outcome over and above the effect of the earlier mental health outcome, we statistically controlled for children’s mental health outcome at age 4, as well as children’s gender, intelligence, and stressful life events experienced that have been shown to affect childhood mental health outcomes (Fergusson, Woodward, & Horwood, 1998; Wakschlag & Keenan, 2001).
METHODS
Participants
Data were drawn from the Avon Longitudinal Study of Parents and Children, a large, prospective, population-based study. Information regarding participant selection and assessment procedures for this study is discussed in detail elsewhere (Golding, Pembrey, & Jones, 2001) and is briefly described below. All pregnant women who had an expected delivery date between April 1st, 1991, and December 31st, 1992, in the Avon area of the United Kingdom were invited to participate in the study. Mothers who agreed to participate, their partners, and their children arising from the index pregnancy were followed. Data about the health and development of offspring were collected via postal questionnaires. Ethical approval was obtained from local research ethics committees and from the ALSPAC Ethics & Law committee.
Of the data from 15,211 children that were available for analyses, data from 12,308 children (81% of the total sample) were used for the current analyses, after applying two exclusion criteria. First, we excluded 70 children who were both born prematurely (prior to 37th week of pregnancy) and exposed to tobacco after birth from parental smoking. These children were excluded because postnatal tobacco exposure among prematurely born infants could be considered to be analogous to prenatal exposure (in terms of their rate of brain development) but may have different kinetics than in utero exposure. Second, we excluded an additional 2,833 children with missing data for prenatal tobacco exposure. This final sample consisted of 97% Caucasian mothers, 68% in their first marriage, with the mean age at delivery of 28.16 years (SD = 4.91, range = 15–44). This sample also consisted of 13% mothers with a university degree or higher, 23% with an A-level qualification (equivalent to grades 11/12 in the United States), 35% with an O-level qualification (grades 10/11 in the United States), 10% with a vocational qualification (an apprenticeship), and 19% with the Certificate of Secondary Education (General Educational Development in the United States) or no qualification. Children were 52% female and 95% Caucasian.
Measures
Prenatal Tobacco Exposure
At three assessment points during their pregnancy, mothers were asked about their smoking behavior (cigarette, cigar, and chewing tobacco) during the first 3 months, 16–17 weeks, and the last 2 months of pregnancy. A dichotomous variable of prenatal tobacco exposure status (0 = no exposure throughout three trimesters; 1 = exposure during at least one of three trimesters) was computed. Mothers who enrolled in the study after 23 weeks of gestation were given the question about smoking during the third trimester but not during the first and second trimesters; if they reported smoking at the third trimester, they were coded as 1, whereas if they reported nonsmoking at the third trimester, they were coded as missing (because there was no way to determine their smoking at the other trimesters).
Stress Reactivity Measured at Ages 2–6
Stress reactivity was assessed when the child was 2, 3, 4, 5, and 6 years old. Mothers were given a list of 15–16 potentially stressful life events (An event of “the child started school” was added to the assessments at ages 5 and 6). Mothers were asked to report whether their child experienced each event during the past 12 or 15 months, and if so, how upset their child became in response to the events. Mothers’ responded to each event based on a 0 (No, did not happen), 1 (Yes, not upset), 2 (Yes, a bit upset), 3 (Yes, quite upset), and 4 (Yes, very upset) scale. Reactivity scores were computed by summing reactivity scores for 12 stressful life events at ages 2–4 and 13 stressful life events at ages 5 and 6; three items measuring experience of sexual and physical abuse and neglect were dropped from the calculation because these events are considered traumatic (based on criteria for posttraumatic stress disorder the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision; American Psychiatric Association, 2000), and our goal was to study reactivity to ordinary stressful life events. Because the focus of the measure relates to how much the child reacted to stressors (not confounded with the child’s experiences of stressors), when mothers reported that no stressors happened to the child, the score was treated as missing for the given measurement point. Accordingly, a score of reactivity potentially ranged from 1 to 48 for the assessments at ages 2, 3, and 4 and 1–52 for the assessments at ages 5 and 6. This scale has been used successfully to measure life stress in childhood (Araya et al., 2009; Enoch, Steer, Newman, Gibson, & Goldman, 2010).
Mental Health Outcomes Measured at Ages 7–11
Mothers completed the parental version of the Strengths and Difficulties Questionnaire (SDQ; Goodman, 1997, 2001) when their child was 7, 10, and 11 years old. In addition, teachers completed the teacher version of the SDQ when a child was 8 and 11 years old. The SDQ consists of five, 5-item subscales: hyperactivity symptoms (e.g., “restless, overactive, cannot stay still for long,” “easily distracted, concentration wanders”), emotional symptoms (e.g., “has many worries, often seems worried,” “often unhappy, downhearted or tearful”), conduct problems (e.g., “often lies or cheats”), peer problems (e.g., “rather solitary, tends to play alone”), and prosocial behaviors (e.g., “kind to young children”). Each item was measured based on a 0 (not true) to 2 (certainly true) scale, resulting in each subscale score potentially ranging from 0 to 10. For the current analysis, five subscale scores at each of the five assessment points (three from maternal ratings and two from teacher’s ratings) were used. There were small to medium associations between maternal and teacher’s ratings on the same mental health domain (bivariate correlation coefficients [rs] ranging from .18 for emotional problems to .49 for hyperactivity symptoms). This brief behavioral screening questionnaire has been validated against well-established measures including Rutter Children’s Behaviour questionnaires (Goodman, 2001), the Child Behavior Checklist (Goodman & Scott, 1999), and clinicians’ ratings (Mathai, Anderson, & Bourne, 2004).
Potential Confounding Variables
A number of maternal variables were included as covariates (and thus their effects were statistically controlled for), including age at delivery, education, alcohol and illicit drug use during pregnancy, and depression and anxiety status determined using the Edinburgh Postnatal Depression Scale (Cox, Holden, & Sagovsky, 1987), the Anxiety subscale of the Crown-Crisp Experiential Index (CCEI; Crown & Crisp, 1979), and the S-Anxiety subscale of the State-Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) over the three trimesters of pregnancy, 1–5 years after delivery, and 6–11 years after delivery (which corresponded to the time points when mothers rated children’s stress reactivity and mental health outcomes). In addition, mothers’ and their current partners’ self-report histories of alcoholism, drug addiction, and severe depression measured during pregnancy were controlled for. Teachers’ anxiety and depression status was determined using the Depression and Anxiety subscales of the CCEI when children were 8 and 11 years old (which corresponded to the time points when teachers rated children’s mental health outcomes). Child variables also were controlled for, including gender, intelligence quotient measured by an abbreviated form of the Wechsler Intelligence Scale for Children IIIUK (Wechsler, 1992) at age 8, the total number of stressful life events experienced at ages 2, 3, 4, 5, and 6, assessed with the stress reactivity measure described above, and mental health outcomes reported by mothers using the parental version of the SDQ (Goodman, 1997, 2001) when children were 4 years old.
Data Analysis Strategies
Descriptive statistics, including correlation analysis, were analyzed using SPSS, Version 19. A structural equation model was estimated using Mplus, Version 6 (Muthén & Muthén, 1998–2010). Due to the multiple assessment points of stress reactivity and mental health outcomes and to the five different types of mental health outcomes, it was not feasible for us to test mediation using each observed variable, which would yield a total of 125 tests (five measurement points of stress reactivity × five types of mental health outcomes × five measurement points of each mental health outcome). Given the concerns of an inflated Type I error rate, we used structural equation modeling to estimate a latent factor of stress reactivity across ages 2–6 and a latent factor of each mental health outcome across ages 7–11. The use of the structural equation modeling allowed us not only to control for measurement errors inherent in each observed variable (by explicitly estimating latent factors of residual/error terms for observed variables; Tomarken & Waller, 2005) but also to obtain better interpretable results on whether stress reactivity at early childhood mediated the effect of prenatal tobacco exposure on mental health outcomes at middle childhood. Estimating a single latent variable (representing a traitlike factor of a construct across time) using multiple observed variables at different time points (representing time-specific states of a construct) has been used in various forms of state-trait factor models for longitudinal data analysis (Hertzog & Nesselroade, 1987; Kenny & Zautra, 1995; Steyer, Ferring, & Schmitt, 1992).
Because stress reactivity and mental health outcome variables were not normally distributed, maximum likelihood parameter estimates with standard errors and a chi-square test statistic that are robust to nonnormality were used. Maximum likelihood estimation also deals with missing data by determining the parameters that maximize the probability of the sample data on the basis of all the available data (Graham, Cumsille, & Elek-Fisk, 2003). Although analysis with complete data (after removing cases with missing data) had been one of the most frequently used approaches to missing data, list-wise deletion is associated with biased estimates and loss of power (Graham et al., 2003). To evaluate the fit of the model, cutoff values of multiple model fit indices (Hu & Bentler, 1999) were used: 0.95 or above for the Comparative Fit Index, 0.08 or below for the Standardized Root Mean Square Residual, 0.05 or below for point estimates of the Root Mean Square Error of Approximation (RMSEA), and 0.00–0.08 for 90% Confidence Intervals of the RMSEA.
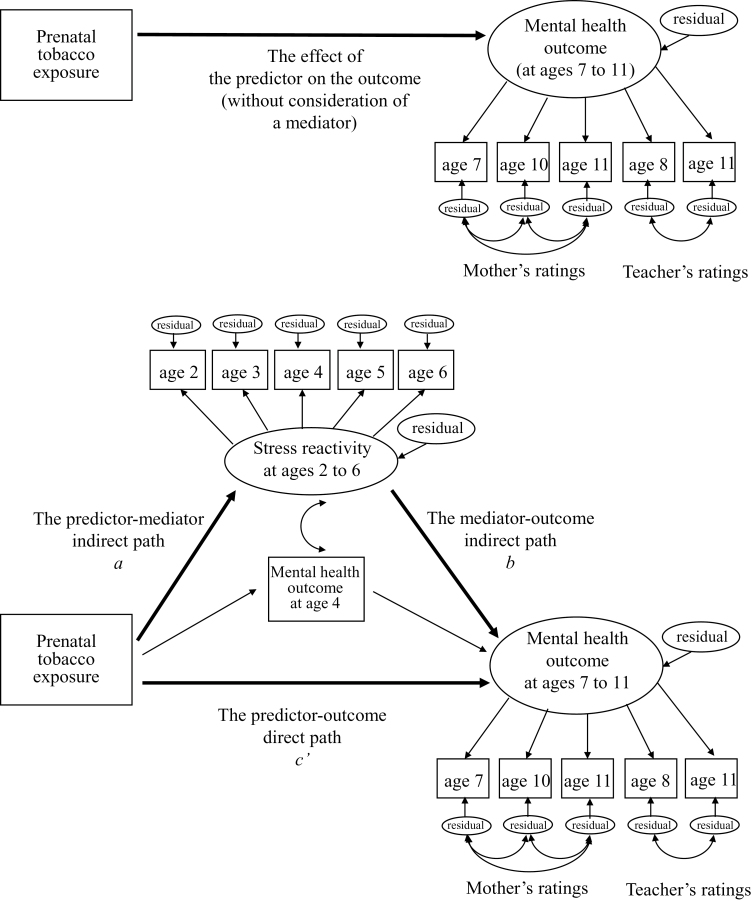
For the first study aim to test the overall/total effect of prenatal tobacco exposure on childhood mental health outcomes without consideration of a mediator, a separate structural equation model for each of the five mental health outcomes was estimated (shown in the panel of Figure 1). In each of these five models, a latent factor of the mental health outcome was estimated with five observed variables—the mental health outcome reported by a mother at ages 7, 10, and 11 and by a teacher at ages 8 and 11. Thus, the latent mental health outcome factor was set to account for covariance among all the five maternal and teachers’ ratings (i.e., the maternal and teachers’ ratings were assumed to correlate with each other because those ratings measured the same mental health outcome of the same child). Factor loadings of those five observed variables were set to be equal so that each variable had an equal weight to the latent mental health outcome factor. In addition, to account for potential residual associations among the observed variables reported by the same reporter (a mother vs. a teacher), error variance of three observed variables based on maternal ratings were allowed to correlate with each other and error variances of two observed variables based on teachers’ ratings also were allowed to correlate with each other. A path from prenatal tobacco exposure to the single latent mental health outcome factor represented the total effect of prenatal tobacco exposure on the mental health outcome (without consideration of a mediator).
Figure 1.
Path diagrams of structural equation models to test the effect of prenatal tobacco exposure on the specific mental health outcome at ages 7–11, shown in the upper panel and to test a mediating role of stress reactivity at ages 2–6 in the effect of prenatal tobacco exposure on the specific mental health outcome at ages 7–11 (that is, a mediating effect), after controlling for the same mental health outcome at age 4, shown in the lower panel. A separate model was estimated for each of the five mental health outcomes. Potential confounding effects of maternal, paternal, teacher, and child variables on the stress reactivity and the mental health outcome were controlled for (paths are not shown for simplicity).
For the second study aim to test a mediating role of stress reactivity in the effect of prenatal tobacco exposure on childhood mental health outcomes, a separate structural equation model for each of the five mental health outcomes was estimated (shown in the lower panel of Figure 1). To each of the above five models for the first study aim, a latent factor of stress reactivity was added; this factor was estimated with five observed variables of stress reactivity measured at ages 2, 3, 4, 5, and 6. Factor loadings of those five observed variables were set to be equal so that each measurement point had an equal weight to the latent stress reactivity factor. Then, we added two directional paths. First, the predictor–mediator indirect path was represented by a path from prenatal tobacco exposure to the stress reactivity factor. Second, the mediator-outcome indirect path was represented by a path from the stress reactivity factor to the mental health outcome factor at ages 7–11. An indirect/mediated effect was calculated by multiplying the unstandardized coefficients of these two indirect paths (i.e., the predictor–mediator indirect path and the mediator-outcome indirect path; MacKinnon, Fairchild, & Fritz, 2007). A path from prenatal tobacco exposure to the mental health outcome factor at ages 7–11 represented a direct effect of prenatal tobacco exposure on the mental health outcome, after accounting for the indirect/mediated effect. To test the unique effect of stress reactivity on the mental health outcome at ages 7–11 over and above the effect of the earlier mental health outcome, an observed variable of the specific mental health outcome at age 4 (that corresponded to the mental health outcome at ages 7–11 under investigation) was added into the model as a covariate. The stress reactivity factor at ages 2–6 and the mental health outcome at age 4 were allowed to correlate with each other to account for the potential association between stress reactivity and mental health outcome in early childhood. Significance tests for mediation were conducted by the Sobel first-order test (Sobel, 1982). Based on the study by Mackinnon, Warsi, and Dwyer (1995), the proportion of the total effect of prenatal tobacco exposure on each of the mental health outcome factors that was mediated by stress reactivity (i.e., proportion mediated; the ratio of the indirect effect to the total effect) was used as an effect size measure of the entire mediating effect. In addition, for the stability and potential true range of the mediating effect, we presented 95% confidence intervals of the mediating effect. In all models, we controlled for the effects of maternal, teacher, and child confounding variables on both stress reactivity and the mental health outcome factors by including them as covariates (for simplicity, these paths are not shown in Figure 1).
RESULTS
Descriptive Analyses
Table 1 shows the percentages and means (and SDs) of all study variables in all participants and as a function of prenatal exposure status. Thirty percent of the participants were classified as prenatally exposed to tobacco.
Table 1.
Percentages and Means (and SDs) of Study Variables in All Participants and as a Function of Prenatal Exposure (PAE) Status and Results From Independent-Sample t-Tests for Continuous Study Variables and χ2 Tests for Dichotomous Study Variables Comparing the 2 Groups
| Variable (possible range) | All participants (N = 12,308) | PAE group (n = 3,695) | Non-PAE group (n = 8,613) | Test statistic comparing PAE vs. non-PAE groups |
|---|---|---|---|---|
| PAE status (0 = exposure; 1 = nonexposure) | 30.0% | – | – | – |
| Maternal covariate | ||||
| Age | 28.16 (4.91) | 26.27 (5.12) | 28.97 (4.58) | t(12,289) = −28.88*** |
| Education (0–4) | 1.99 (1.28) | 2.55 (1.22) | 1.78 (1.23) | t(11,547) = 29.78*** |
| Alcohol and illicit drug use during pregnancy | 1.34 (1.17) | 1.37 (1.26) | 1.32 (1.13) | t(12,305) = 2.15* |
| Depression and anxiety during pregnancy (0–6) | 0.70 (1.32) | 1.01 (1.53) | 0.57 (1.20) | t(12,281) = 17.09*** |
| Depression and anxiety 1–5 years after delivery (0–8) | 0.80 (1.57) | 1.02 (1.72) | 0.72 (1.50) | t(11,363) = 9.04*** |
| Depression and anxiety status 6–11 years after delivery (0–6) | 0.64 (1.22) | 0.78 (1.31) | 0.60 (1.18) | t(9,215) = 6.11*** |
| History of drug addiction | 0.5% | 1.3% | 0.2% | χ2(1,11,594) = 61.50*** |
| History of alcoholism | 1.0% | 2.1% | 0.5% | χ2(1,11,594) = 64.27*** |
| History of severe depression | 9.0% | 15.9% | 6.4% | χ2(1,11,594) = 250.95*** |
| Mother’ partner covariate | ||||
| History of drug addiction | 1.2% | 2.6% | 0.8% | χ2(18,035) = 40.04*** |
| History of alcoholism | 2.9% | 6.2% | 1.8% | χ2(17,975) = 98.42*** |
| History of severe depression | 6.1% | 10.1% | 4.9% | χ2(17,974) = 69.83*** |
| Teacher covariate | ||||
| Depression and anxiety at children’s ages 8 and 11 (0–4) | 0.36 (0.70) | 0.38 (0.70) | 0.36 (0.69) | t(8,093) = 1.47 |
| Children covariate | ||||
| IQ | 104.43 (16.44) | 100.69 (16.03) | 105.40 (16.40) | t(6,667) = −9.52*** |
| Female gender (0 = male; 1 = female) | 51.8% | 53.5% | 50.8% | t(12,299) = 2.76** |
| Stressful events experienced at ages 2–6 (0–77) | 5.94 (3.49) | 5.99 (3.82) | 5.92 (3.37) | t(11,144) = 0.85 |
| Mental health outcome | ||||
| Peer (isolation) problem at age 4 (0–10): maternal rating | 1.52 (1.48) | 1.75 (1.53) | 1.45 (1.46) | t(8,849) = 8.26*** |
| Peer (isolation) problem at age 7 (0–10): maternal rating | 1.06 (1.42) | 1.31 (1.57) | 0.99 (1.37) | t(8,099) = 8.48*** |
| Peer (isolation) problem at age 8 (0–10): teacher rating | 1.19 (1.78) | 1.51 (1.95) | 1.07 (1.70) | t(5,557) = 8.43*** |
| Peer (isolation) problem at age 10 (0–10): maternal rating | 1.12 (1.51) | 1.34 (1.61) | 1.06 (1.48) | t(7,379) = 6.54*** |
| Peer (isolation) problem at age 11 (0–10): teacher rating | 1.21 (1.84) | 1.48 (2.01) | 1.10 (1.75) | t(6,410) = 7.61*** |
| Peer (isolation) problem at age 11 (0–10): maternal rating | 1.10 (1.55) | 1.28 (1.63) | 1.06 (1.52) | t(6,742) = 4.78*** |
| Hyperactivity symptoms at age 4 (0–10): maternal rating | 3.96 (2.32) | 4.40 (2.35) | 3.82 (2.30) | t(8,823) = 10.22*** |
| Hyperactivity symptoms at age 7 (0–10): maternal rating | 3.38 (2.37) | 3.84 (2.48) | 3.25 (2.32) | t(8,083) = 9.33*** |
| Hyperactivity symptoms at age 8 (0–10): teacher rating | 2.64 (2.69) | 3.38 (2.90) | 2.35 (2.54) | t(5,557) = 12.96*** |
| Hyperactivity symptoms at age 10 (0–10): maternal rating | 2.94 (2.26) | 3.30 (2.36) | 2.84 (2.22) | t(7,390) = 7.19*** |
| Hyperactivity symptoms at age 11 (0–10): teacher rating | 2.27 (2.65) | 2.96 (2.93) | 1.20 (2.47) | t(6,410) = 13.49*** |
| Hyperactivity symptoms at age 11 (0–10): maternal rating | 2.75 (2.22) | 3.16 (2.33) | 2.64 (2.17) | t(6,722) = 7.77*** |
| Conduct problems at age 4 (0–10): maternal rating | 1.95 (1.41) | 2.31 (1.52) | 1.84 (1.35) | t(8,899) = 13.63*** |
| Conduct problems at age 7 (0–10): maternal rating | 1.61 (1.47) | 1.94 (1.59) | 1.51 (1.41) | t(8,104) = 11.05*** |
| Conduct problems at age 8 (0–10): teacher rating | 0.75 (1.43) | 1.16 (1.79) | 0.59 (1.23) | t(5,555) = 13.65*** |
| Conduct problems at age 10 (0–10): maternal rating | 1.27 (1.42) | 1.57 (1.63) | 1.20 (1.35) | t(7,389) = 9.31*** |
| Conduct problems at age 11 (0–10): teacher rating | 0.83 (1.60) | 1.34 (2.05) | 0.63 (1.32) | t(6,406) = 16.52*** |
| Conduct problems at age 11 (0–10): maternal rating | 1.20 (1.42) | 1.53 (1.60) | 1.11 (1.36) | t(6,738) = 9.77*** |
| Emotional symptoms at age 4 (0–10): maternal rating | 1.45 (1.51) | 1.53 (1.58) | 1.42 (1.48) | t(8,903) = 3.05** |
| Emotional symptoms at age 7 (0–10): maternal rating | 1.51 (1.67) | 1.64 (1.78) | 1.47 (1.64) | t(8,099) = 3.89*** |
| Emotional symptoms at age 8 (0–10): teacher rating | 1.38 (1.94) | 1.63 (2.11) | 1.29 (1.87) | t(5,557) = 5.85*** |
| Emotional symptoms at age 10 (0–10): maternal rating | 1.50 (1.76) | 1.63 (1.88) | 1.47 (1.72) | t(7,375) = 3.25** |
| Emotional symptoms at age 11 (0–10): teacher rating | 1.29 (1.88) | 1.57 (2.08) | 1.17 (1.77) | t(6,409) = 7.70*** |
| Emotional symptoms at age 11 (0–10): maternal rating | 1.46 (1.73) | 1.63 (1.86) | 1.41 (1.69) | t(6,721) = 4.16*** |
| Prosocial behavior at age 4 (0–10): maternal rating | 7.05 (1.98) | 6.97 (1.96) | 7.07 (1.99) | t(8,874) = −2.07* |
| Prosocial behavior at age 7 (0–10): maternal rating | 8.17 (1.76) | 8.11 (1.72) | 8.19 (1.78) | t(8,103) = −1.71 |
| Prosocial behavior at age 8 (0–10): teacher rating | 7.69 (2.44) | 7.21 (2.54) | 7.88 (2.37) | t(5,553) = −9.15*** |
| Prosocial behavior at age 10 (0–10): maternal rating | 8.31 (1.67) | 8.27 (1.64) | 8.32 (1.68) | t(7,396) = −1.02 |
| Prosocial behavior at age 11 (0–10): teacher rating | 7.88 (2.38) | 7.52 (2.52) | 8.03 (2.31) | t(6,410) = −7.88*** |
| Prosocial behavior at age 11 (0–10): maternal rating | 8.33 (1.68) | 8.27 (1.70) | 8.35 (1.68) | t(6,744) = −1.60 |
| Stress reactivity: maternal rating | ||||
| Age 2 (1–48) | 3.19 (2.47) | 3.57 (2.75) | 3.07 (2.36) | t(6,018) = 6.78*** |
| Age 3 (1–48) | 3.87 (2.77) | 4.24 (3.04) | 3.76 (2.66) | t(8,293) = 6.81*** |
| Age 4 (1–48) | 3.04 (2.19) | 3.21 (2.39) | 2.98 (2.12) | t(7,768) = 4.09*** |
| Age 5 (1–52) | 2.98 (2.14) | 3.18 (2.28) | 2.91 (2.08) | t(6,787) = 4.62*** |
| Age 6 (1–52) | 2.59 (1.97) | 2.79 (2.08) | 2.51 (1.92) | t(5,661) = 4.75*** |
Note. *p < .05, **p< .01, ***p < .001.
Table 1 also shows the results from independent-sample t-tests for continuous study variables and χ2 difference tests for categorical study variables comparing the two groups as a function of prenatal exposure status. There were significant differences between mothers who smoked during pregnancy and mothers who did not in terms of their age, education, alcohol and illicit drug use during pregnancy, depression and anxiety status, and their own and their current partners’ psychiatric histories. Also, there were significant differences between children prenatally exposed to tobacco and children who were not exposed in terms of their IQ, stress reactivity, and adverse mental health outcomes, but not in the number of stressful events experienced, their teachers’ depression and anxiety status, and their prosocial behaviors at ages 7, 10, and 11.
Although a bivariate correlation coefficient as negligible as r = .03 was statistically significant at p < .05 due to our big sample size, negligible to small-sized associations of prenatal tobacco exposure were found with stress reactivity (rs = 0.05–0.09) and with mental health outcomes (rs = −0.01 to 0.13) across measurement points. Also, negligible to small associations of stress reactivity with mental health outcomes at age 4 (based on mothers’ rating) were found: peer problems (rs = 0.04–0.08), hyperactivity symptoms (rs = 0.05–0.07), emotion problems (rs = 0.08–0.17), conduct problems (rs = 0.07–0.11), and prosocial behaviors (rs = 0.001–0.02). Similarly, negligible to small association of stress reactivity at ages 2–6 with mental health outcomes at ages 7–11 (based on mothers’ and teacher’s ratings) were found: peer problems (rs = 0.02–0.12), hyperactivity symptoms (rs = 0.05–0.10), emotion problems (rs = 0.03–0.17), conduct problems (rs = 0.03–0.10), and prosocial behaviors (rs = –0.06 to 0.01).
Structural Equation Models
Effects of Prenatal Tobacco Exposure on Childhood Mental Health Outcomes Without Consideration of a Mediator
Table 2 shows results from five structural equation models estimated to test the relationship between prenatal tobacco exposure and each of the five mental health outcomes at ages 7–11, after controlling for maternal, teacher, and child covariates. As shown in the last column of Table 2, all models showed an excellent fit to the data. As shown in the first column of data in Table 2, prenatal tobacco exposure was significantly and positively associated with peer isolation problems, hyperactivity symptoms, conduct problems, and emotional problems, but it was significantly and negatively associated with prosocial behaviors, ps < .01.
Table 2.
Effects of Prenatal Tobacco Exposure on Mental Health Outcome at Ages 7–11 (Without Consideration of a Mediator)
| b (β) | Model fit indices | |
|---|---|---|
| Peer (isolation) problems | 0.20 (0.09)*** | χ2(88) = 111***; CFI = 1.00; SRMR = 0.02; RMSEA = 0.01 (90% CI = 0.00, 0.01) |
| Hyperactivity symptoms | 0.41 (0.10)*** | χ2(88) = 162***; CFI = 1.00; SRMR = 0.02; RMSEA = 0.01 (90% CI = 0.01, 0.01) |
| Conduct problems | 0.32 (0.18)*** | χ2(88) = 147***; CFI = 0.99; SRMR = 0.02; RMSEA = 0.01 (90% CI = 0.01, 0.01) |
| Emotional symptoms | 0.09 (0.05)** | χ2(88) = 127***; CFI = 0.99; SRMR = 0.02; RMSEA = 0.01 (90% CI = 0.00, 0.01) |
| Prosocial behaviors | −0.29 (−0.10)*** | χ2(88) = 134***; CFI = 0.99; SRMR = 0.03; RMSEA = 0.01 (90% CI = 0.00, 0.01) |
Note. N = 12,308; b = unstandardized coefficients; β = standardized coefficients. Potential confounding effects of maternal, paternal, teacher, and child variables were controlled for in all models. CFI = Comparative Fit Index; SRMR = Standardized Root Mean Square Residual; RMSEA = Root Mean Square Error of Approximation.
**p < .01, ***p < .001.
Mediating Role of Stress Reactivity in the Effect of Prenatal Tobacco Exposure on Childhood Mental Health Outcomes
Table 3 shows results from five structural equation models estimated to test a mediating role of stress reactivity in the effect of prenatal tobacco exposure on each of the five mental health outcomes at ages 7–11, after controlling for the mental health outcome at age 4 as well as other maternal, teacher, and child covariates. As shown in the last column of Table 3, all models showed a good fit to the data. The first through sixth columns of the data in Table 3 show the unstandardized coefficients of the direct path (corresponding to the c′ path in Figure 1), the two indirect paths (corresponding to the a and b paths in Figure 1), and the indirect/mediated effect (calculated as a product of the two indirect paths’ coefficients), the Sobel test statistic for the indirect/mediated effect, and the proportion mediated (calculated as an indirect effect divided by a sum of absolute values of an indirect effect and a direct effect), in order.
Table 3.
Summary of Results for Mediation of Stress Reactivity in the Effects of Prenatal Tobacco Exposure on the Mental Health Outcome at Ages 6–11, After Controlling for the Mental Health Outcome at Age 4
| The direct effect from PTE to an outcome (c′) | The indirect path from PTE to SR (a) | The indirect path from SR to an outcome (b) | The indirect effect (a product of the two indirect paths’ coefficients = a × b) | Sobel test for the indirect effect | Proportion mediated (a × b/|c′+ a × b|) | Model fit indices | |
|---|---|---|---|---|---|---|---|
| Peer (isolation) problems | 0.08* | 0.11** | 0.48** | 0.05* (CI = 0.01, 0.10) | z = 2.37* | 25% | χ2(210) = 897***; CFI = 0.95; SRMR = 0.03; RMSEA = 0.02 (90% CI = 0.02, 0.02) |
| Hyperactivity symptoms | 0.19*** | 0.11** | 0.54** | 0.06* (CI = 0.01, 0.12) | z = 2.15* | 12% | χ2(210) = 1,493***; CFI = 0.94; SRMR = 0.04; RMSEA = 0.02 (90% CI = 0.02, 0.02) |
| Conduct problems | 0.15*** | 0.12** | 0.26* | 0.03* (CI = 0.001, 0.04) | z = 2.03* | 9% | χ2(210) = 1,119***; CFI = 0.94; SRMR = 0.04; RMSEA = 0.02 (90% CI = 0.02, 0.02) |
| Emotional symptoms | −0.07 | 0.13*** | 1.28*** | 0.17** (CI = 0.05, 0.28) | z = 2.79** | 67% | χ2(210) = 1,053***; CFI = 0.94; SRMR = 0.04; RMSEA = 0.02 (90% CI = 0.02, 0.02) |
| Prosocial behaviors | −0.14*** | 0.11** | 0.02 | 0.002 (CI = −0.02, 0.03) | z = 0.16 | 1% | χ2(210) = 1,051***; CFI = 0.95; SRMR = 0.03; RMSEA = 0.02 (90% CI = 0.02, 0.02) |
Note. N = 12,308; unstandardized coefficients are shown. PTE = prenatal tobacco exposure; SR = stress reactivity. CI = 95% confidence interval. Potential confounding effects of maternal, paternal, teacher, and child variables were controlled for in all models. CFI = Comparative Fit Index; SRMR = Standardized Root Mean Square Residual; RMSEA = Root Mean Square Error of Approximation.
*p < .05, **p < .01, ***p < .001.
There was a significant and positive effect of prenatal tobacco exposure on stress reactivity in all of the five models, bs = 0.11–0.13, ps < .01 (shown in the second column of the data in Table 3). (Note that this coefficient was slightly different in each model because the coefficient was obtained after controlling for the different mental health outcome at age 4.) In turn, stress reactivity was significantly and positively associated with peer isolation, hyperactivity, conduct, and emotional problems, bs = 0.26–1.28, ps < .05; however, stress reactivity was not significantly associated with prosocial behaviors, b = 0.02, p > .05 (shown in the third column of the data). Sobel tests indicated a significant indirect/mediating effect of stress reactivity in the association of prenatal tobacco exposure with peer isolation, hyperactivity, and emotional problems, zs = 2.03–2.79, ps < .05, but not with prosocial behaviors, z = 0.16, p > .05 (shown in the fifth column of the data). Proportions of the total effects of prenatal tobacco exposure on peer isolation, hyperactivity, conduct, and emotional problems that were mediated by stress reactivity ranged from 9% for conduct problems to 67% for emotional problems (shown in the sixth column of the data).
Even after accounting for the significant indirect/mediating effect of stress reactivity, there remained a significant direct effect from prenatal tobacco exposure on peer isolation, hyperactivity, and conduct problems, bs = 0.08–0.19, ps < .05, but not on emotional problems, b = −0.07, p > .05 (shown in the first column of the data in Table 3). These results suggested that the effect of prenatal tobacco exposure on peer isolation, hyperactivity, and conduct problems was partially mediated by stress reactivity (with a significant direct effect remaining even after accounting for the mediating effect), whereas the effect of prenatal tobacco exposure on emotional problems was fully mediated by stress reactivity (with no significant direct effect remaining after accounting for the mediating effect). For prosocial behaviors, a significant and negative direct effect was found, b = −0.14, p < .001, suggesting that the effect of prenatal tobacco exposure was directly and negatively associated with prosocial behaviors, without being mediated by stress reactivity.
As ancillary analyses to test potential reporter biases in the mental health outcomes rated by a teacher versus a mother, each of the above five mediation models was estimated with a latent mental health outcome factor using the two observed variables of teacher’s ratings at ages 8 and 11, after dropping the three observed variables of maternal ratings at ages 7, 10, and 11. Results from the models based only on teachers ratings were remarkably similar to the results from the models including both mothers’ and teachers’ ratings reported above, in terms of significant mediating effects of stress reactivity in hyperactivity symptoms (b = 0.10, z = 2.24, p < .05, proportion mediated = 18%), conduct problems (b = 0.05, z = 2.01, p < .05, proportion mediated = 12%), and emotional problems (b = 0.18, z = 2.44, p < .05, proportion mediated = 83%), but not in prosocial behaviors (b = −0.05, z = −1.65, p > .05, proportion mediated = 13%). The only exception was that the previously significant indirect/mediating effect of stress reactivity in peer (isolation) problems became marginally significant (b = 0.05, z = 1.64, p = .10, proportion mediated = 14%).
DISCUSSION
Our findings replicate the positive relationship between prenatal tobacco exposure and diverse negative mental health outcomes in childhood (Ashford et al., 2008; Brennan et al., 2002; Button et al., 2007; Cornelius & Day, 2009; Ekblad et al., 2010; Ernst et al., 2001; Linnet et al., 2003; Nomura et al., 2010; Rogers, 2009), including externalizing problems of attention deficit hyperactivity and conduct problems and internalizing problems of emotional and peer (isolation) problems; in contrast, prenatal tobacco exposure was inversely associated with a positive mental health outcome of prosocial behaviors. More importantly, we demonstrated that heightened stress reactivity mediated the prospective influence of prenatal tobacco exposure on both externalizing and internalizing problems, even after controlling for an array of potential confounders. Specifically, a small association between prenatal tobacco exposure and heightened stress reactivity at ages 2–6 was found. This heightened stress reactivity, in turn, was associated with greater levels of externalizing and internalizing problems at ages 7–11. Our finding of a mediating role of stress reactivity is significant particularly because of several strengths in the method used in this study: the use of latent factors (to control for measurement errors inherent in assessment measures), statistical control for a wide range of potential confounders (to help rule out alternative explanations), consistent findings obtained from models using mothers’ and teachers’ ratings on mental health outcomes, and the use of a large population-based sample assessed prospectively from gestation period to age 11 (to increase the generalizability of findings).
Our findings demonstrate that one of the potential mechanisms through which prenatal tobacco exposure increases adverse mental health outcomes between the ages of 7 and 11 is heightened stress reactivity. Interestingly, we found that the number of stressful life events experienced at ages 2–6 did not differ, whereas stress reactivity differed as a function of prenatal tobacco exposure status. Of particular note, the mediating role of stress reactivity at ages 2–6 was found after controlling for the level of the mental health outcome of interest at age 4. Together, these results suggest that early stress reactivity may not be a proxy of early mental health outcomes and that early stress reactivity contributes to mental health outcomes in middle childhood over and above earlier mental health outcomes. This finding is consistent with human studies showing that tobacco exposure in utero compromises neurological development underlying stress reactivity, including epinephrine and norepinephrine levels (Oncken et al., 2003) and serotonin and other monoamine systems (Xu, Seidler, Ali, Slikker, & Slotkin, 2001).
It is also noteworthy that prenatal tobacco exposure was associated with childhood emotional problems (reflective of sadness and anxiety) only through stress reactivity; that is, without considering stress reactivity, no association between prenatal tobacco exposure and emotional problems was found. This finding may partially explain the mixed literature on the link between prenatal tobacco exposure and internalizing problems (Xu et al., 2001); prenatal tobacco exposure may increase emotional problems in childhood only in the context of experiences of stressful events.
Results of the current analyses are largely consistent with the animal literature. This study and the preclinical literature demonstrate that prenatal tobacco exposure increases stress reactivity (Heath et al., 2010; King et al., 2003). The current findings support the idea that the nicotine and other tobacco constituents affect multiple neuronal subtypes and brain functions and that multiple neurobiological processes occurring throughout development likely contribute to the etiology of mental health outcomes in humans (Heath & Picciotto, 2009).
Our findings should be interpreted in the context of several limitations. Maternal self-report was used to assess prenatal smoking exposure. Mothers may have underreported their smoking behavior (Warland & McCutcheon, 2011), which could have reduced the effect sizes of prenatal smoking exposure on stress reactivity and mental health outcomes reported here. Similarly, this study is limited in that children’s stress reactivity was assessed by maternal ratings; future studies need to use objective measures of stress reactivity such as salivary cortisol reactivity. Finally, given the literature indicating potential confounding effects of genetics in the association between prenatal tobacco exposure and behavioral outcomes (see Knopik, 2009, for a review), the current study is limited in that genetic effects were not controlled for. Replications of our findings using a genetically informed study deign and objective measures of mothers’ smoking during pregnancy and children’s stress reactivity are needed.
Because this is the first report of a mediating role of stress reactivity in the behavioral consequences of prenatal tobacco exposure, replication in other prospective population-based samples is crucial. Given the potential interaction between genetics and prenatal and postnatal environment in mental health outcomes, studies using racially and culturally diverse samples would help clarify the boundaries of generalizability of our findings. The current findings highlight the public health benefits of smoking cessation in women of childbearing potential. In addition, stress management programs (Kraag, Zeegers, Kok, Hosman, & Abu-Saad, 2006) designed to help children prenatally exposed to tobacco may be valuable in reducing the development of mental health problems in response to adverse life events.
FUNDING
This work was supported by the National Institutes of Health (DA020515, DA10455, DA00436, AA15632, AA014715, and AA017823) and the State of Connecticut Department of Mental Health and Addiction Services and Transdisciplinary Tobacco Use Research Center.
DECLARATION OF INTERESTS
SSO: member, American College of Neuropsychopharmacogy workgroup, the Alcohol Clinical Trial Initiative, sponsored by Alkermes, Abbott Laboratories, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck, and Schering Plough; partner, Applied Behavioral Research; consultant, Pfizer; Medication supplies, Pfizer; Scientific Panel of Advisors, Hazelden Foundation. MRP: member, Society for Neuroscience Council; Senior Editor, the Journal of Neuroscience; received varenicline from Pfizer for studies of alcohol dependence; patent (Yale University and University of Bonn): “Cytisine analogs and methods of treating mood disorders.” AP and SLK report no financial relationships with commercial interests.
ACKNOWLEDGMENTS
The authors thank all the families who took part in ALSPAC, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The U.K. Medical Research Council, the Wellcome Trust, and the University of Bristol provide core support for ALSPAC. The authors would like to thank Marcus Munafò at the University of Bristol for helpful advice about the ALSPAC database and Laura Miller and Jon Heron for accessing the ALSPAC data set.
REFERENCES
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed, text rev.) Washington, DC: Author [Google Scholar]
- Araya R., Hu X., Heron J., Enoch M. A., Evans J., Lewis G, … Goldman D. (2009). Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 150B, 670–682.10.1002/ajmg.b.30888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford J., van Lier P. A., Timmermans M., Cuijpers P., Koot H. M. (2008). Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 779–787.10.1097/CHI.0b013e318172eefb [DOI] [PubMed] [Google Scholar]
- Brennan P. A., Grekin E. R., Mortensen E. L., Mednick S. A. (2002). Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. The American Journal of Psychiatry, 159, 48–54.10.1176/appi.ajp.159.1.48 [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan M. J., Carter A. S., Schwab-Stone M. (1996). Discrepancies among mother, child, and teacher reports: Examining the contributions of maternal depression and anxiety. Journal of Abnormal Child Psychology, 24, 749–765.10.1007/BF01664738 [DOI] [PubMed] [Google Scholar]
- Button T. M., Maughan B., McGuffin P. (2007). The relationship of maternal smoking to psychological problems in the offspring. Early Human Development, 83, 727–732.10.1016/j.earlhumdev.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button T. M., Thapar A., McGuffin P. (2005). Relationship between antisocial behaviour, attention-deficit hyperactivity disorder and maternal prenatal smoking. The British Journal of Psychiatry, 187, 155–160.10.1192/bjp.187.2.155 [DOI] [PubMed] [Google Scholar]
- Cornelius M. D., Day N. L. (2009). Developmental consequences of prenatal tobacco exposure. Current Opinion in Neurology, 22, 121–125.10.1097/WCO.0b013e328 326f6dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. L., Holden J. M., Sagovsky R. (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150, 782–786.10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Crown S., Crisp A. H. (1979). Manual of the crown-crisp experiential index. London: Hodder & Stroughton [Google Scholar]
- D’Onofrio B. M., Turkheimer E. N., Eaves L. J., Corey L. A., Berg K., Solaas M. H., Emery R. E. (2003). The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. The Journal of Child Psychology and Psychiatry, 44, 1130–1144.10.1111/1469–7610.00196 [DOI] [PubMed] [Google Scholar]
- De Los Reyes A., Kazdin A. E. (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131, 483–509.10.1037/0033-2909.131.4.483 [DOI] [PubMed] [Google Scholar]
- Ekblad M., Gissler M., Lehtonen L., Korkeila J. (2010). Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Archives of General Psychiatry, 67, 841–849.10.1001/archgenpsychiatry.2010.92 [DOI] [PubMed] [Google Scholar]
- Enoch M. A., Steer C. D., Newman T. K., Gibson N., Goldman D. (2010). Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes, Brain, and Behavior, 9, 65–74.10.1111/j.1601-183X.2009.00535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Moolchan E. T., Robinson M. L. (2001). Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child and Adolescent Psychiatry, 40, 630–641.10.1097/00004583-200106000-00007 [DOI] [PubMed] [Google Scholar]
- Fergusson D. M., Woodward L. J., Horwood L. J. (1998). Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry, 55, 721–727.10.1001/archpsyc.55.8.721 [DOI] [PubMed] [Google Scholar]
- Golding J., Pembrey M., Jones R. (2001). ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric and Perinatal Epidemiology, 15, 74–87.10.1046/j.1365-3016.2001.00325.x [DOI] [PubMed] [Google Scholar]
- Goodman R. (1997). The Strengths and Difficulties Questionnaire: A research note. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 38, 581–586.10.1111/j.1469–7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- Goodman R. (2001). Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 40, 1337–1345.10.1097/00004583-200111000-00015 [DOI] [PubMed] [Google Scholar]
- Goodman R., Scott S. (1999). Comparing the Strengths and Difficulties Questionnaire and the Child Behavior Checklist: Is small beautiful? Journal of Abnormal Child Psychology, 27, 17–24.10.1023/A:1022658222914 [DOI] [PubMed] [Google Scholar]
- Graham J. W., Cumsille P. E., Elek-Fisk E. (2003). Methods for handling missing data. In Schinka J. A., Weiner I. B., Velicer W. F. (Eds.), Handbook of Psychology. Research methods in psychology, New York, New York: John Wiley & Sons; 2, 87–114 [Google Scholar]
- Heath C. J., King S. L., Gotti C., Marks M. J., Picciotto M. R. (2010). Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through α4/β2/α5 nicotinic acetylcholine receptors. Neuropsychopharmacology, 35, 2324–2338.10.1038/npp.2010.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath C. J., Picciotto M. R. (2009). Nicotine-induced plasticity during development: Modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology, 56,(Suppl. 1)254–262.10.1016/j.neuropharm.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C., Nesselroade J. R. (1987). Beyond autoregressive models: Some implications of the trait-state distinction for the structural modeling of developmental change. Child Development, 58, 93–109.10.2307/1130294 [PubMed] [Google Scholar]
- Hu L.-T., Bentler P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55.10.1080/10705519909540118 [Google Scholar]
- Kenny D. A., Zautra A. (1995). The trait-state-error model for multiwave data. Journal of Consulting and Clinical Psychology, 63, 52–59.10.1037/0022-006X.63.1.52 [DOI] [PubMed] [Google Scholar]
- King S. L., Marks M. J., Grady S. R., Caldarone B. J., Koren A. O., Mukhin A. G, … Picciotto M. R. (2003). Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. The Journal of Neuroscience, 23, 3837–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik V. S. (2009). Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology, 34, 1–36.10.1080/87565640802564366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik V. S., Heath A. C., Jacob T., Slutske W. S., Bucholz K. K., Madden P. A, … Martin N. G. (2006). Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine, 36, 1461–1471.10.1017/s0033291706007884 [DOI] [PubMed] [Google Scholar]
- Knopik V. S., Sparrow E. P., Madden P. A., Bucholz K. K., Hudziak J. J., Reich W, … Heath A. C. (2005). Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: A female twin study. Psychological Medicine, 35, 625–635.10.1017/S0033291704004155 [DOI] [PubMed] [Google Scholar]
- Kraag G., Zeegers M. P., Kok G., Hosman C., Abu-Saad H. H. (2006). School programs targeting stress management in children and adolescents: A meta-analysis. Journal of School Psychology, 44, 449–472.10.1016/j.jsp.2006.07.001 [Google Scholar]
- Levin E. D., Wilkerson A., Jones J. P., Christopher N. C., Briggs S. J. (1996). Prenatal nicotine effects on memory in rats: Pharmacological and behavioral challenges. Developmental Brain Research, 97, 207–215.10.1016/S0165-3806(96)00144-7 [DOI] [PubMed] [Google Scholar]
- Linnet K. M., Dalsgaard S., Obel C., Wisborg K., Henriksen T. B., Rodriguez A, … Jarvelin M. R. (2003). Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: Review of the current evidence. The American Journal of Psychiatry, 160, 1028–1040.10.1176/appi.ajp.160.6.1028 [DOI] [PubMed] [Google Scholar]
- MacKinnon D. P., Fairchild A. J., Fritz M. S. (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614.10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon D. P., Warsi G., Dwyer J. H. (1995). A simulation study of mediated effect measures. Multivariate Behavioral Research, 30, 41.10.1207/s15327906mbr3001_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. A., Hamilton B. E., Sutton P. D., Ventura S. J., Menacker F., Kirmeyer S., Munson M. L. (2007). Births: Final data for 2005. National Vital Statistics Reports, 56, 1–103 [PubMed] [Google Scholar]
- Mathai J., Anderson P., Bourne A. (2004). Comparing psychiatric diagnoses generated by the Strengths and Difficulties Questionnaire with diagnoses made by clinicians. The Australian and New Zealand Journal of Psychiatry, 38, 639–643.10.1111/j.1440-1614.2004.01428.x [DOI] [PubMed] [Google Scholar]
- Maughan B., Taylor A., Caspi A., Moffitt T. E. (2004). Prenatal smoking and early childhood conduct problems: Testing genetic and environmental explanations of the association. Archives of General Psychiatry, 61, 836–843.10.1001/archpsyc.61.8.836 [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (1998–2010). Mplus User’s Guide (6th ed). Los Angeles, CA: Muthén & Muthén [Google Scholar]
- Navarro H. A., Seidler F. J., Eylers J. P., Baker F. E., Dobbins S. S., Lappi S. E., Slotkin T. A. (1989). Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. The Journal of Pharmacology and Experimental Therapeutics, 251, 894–900 [PubMed] [Google Scholar]
- Nomura Y., Marks D. J., Halperin J. M. (2010). Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. The Journal of Nervous and Mental Disease, 198, 672–678.10.1097/NMD.0b013e3181ef3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C. A., Henry K. M., Campbell W. A., Kuhn C. M., Slotkin T. A., Kranzler H. R. (2003). Effect of maternal smoking on fetal catecholamine concentrations at birth. Pediatric Research, 53, 119–124.10.1203/00006450- 200301000-00020 [DOI] [PubMed] [Google Scholar]
- Rice F., Harold G. T., Boivin J., van den Bree M., Hay D. F., Thapar A. (2010). The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychological Medicine, 40, 335–345.10.1017/s0033291709005911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. M. (2009). Tobacco and pregnancy. Reproductive Toxicology (Elmsford, N.Y.), 28, 152–160.10.1016/j.reprotox.2009.03.012 [DOI] [PubMed] [Google Scholar]
- Roy T. S., Seidler F. J., Slotkin T. A. (2002). Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. The Journal of Pharmacology and Experimental Therapeutics, 300, 124–133.10.1124/jpet.300.1.124 [DOI] [PubMed] [Google Scholar]
- Salihu H. M., Wilson R. E. (2007). Epidemiology of prenatal smoking and perinatal outcomes. Early Human Development, 83, 713–720.10.1016/j.earlhumdev.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Sobel M. E. (1982). Asymptotic confidence intervals for indirect effects in structural equation models. Sociological Methodology, 13, 290–312. 10.2307/270723 [Google Scholar]
- Sobrian S. K., Ali S. F., Slikker W., Jr., Holson R. R. (1995). Interactive effects of prenatal cocaine and nicotine exposure on maternal toxicity, postnatal development and behavior in the rat. Molecular Neurobiology, 11, 121–143.10.1007/BF02740690 [DOI] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L., Lushene R. E., Vagg P. R., Jacobs G. A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press [Google Scholar]
- Steyer R., Ferring D., Schmitt M. J. (1992). States and traits in psychological assessment. European Journal of Psychological Assessment, 8, 79–98 [Google Scholar]
- Thapar A., Fowler T., Rice F., Scourfield J., van den Bree M., Thomas H, … Hay D. (2003). Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. The American Journal of Psychiatry, 160, 1985–1989.10.1176/appi.ajp.160.11.1985 [DOI] [PubMed] [Google Scholar]
- Thapar A., Rice F., Hay D., Boivin J., Langley K., van den Bree M, … Harold G. (2009). Prenatal smoking might not cause attention-deficit/hyperactivity disorder: Evidence from a novel design. Biological Psychiatry, 66, 722–727.10.1016/j.biopsych.2009.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken A. J., Waller N. G. (2005). Structural equation modeling: Strengths, limitations, and misconceptions. Annual Review of Clinical Psychology, 1, 31–65.10.1146/annurev.clinpsy.1.102803.144239 [DOI] [PubMed] [Google Scholar]
- Wakschlag L. S., Keenan K. (2001). Clinical significance and correlates of disruptive behavior in environmentally at-risk preschoolers. Journal of Clinical Child Psychology, 30, 262–275.10.1207/s15374424jccp3002_13 [DOI] [PubMed] [Google Scholar]
- Warland J., McCutcheon H. (2011). The ‘quit’ smoker and stillbirth risk: A review of contemporary literature in the light of findings from a case-control study. Midwifery, 27, 607–611.10.1016/j.midw.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1992). Manual for the Wechsler Intelligence Scale for Children-Third Edition UK. London: Psychological Corporation [Google Scholar]
- Xu Z., Seidler F. J., Ali S. F., Slikker W., Jr., Slotkin T. A. (2001). Fetal and adolescent nicotine administration: Effects on CNS serotonergic systems. Brain Research, 914, 166–178.10.1016/S0006-8993(01)02797-4 [DOI] [PubMed] [Google Scholar]
- Youngstrom E., Loeber R., Stouthamer-Loeber M. (2000). Patterns and correlates of agreement between parent, teacher, and male adolescent ratings of externalizing and internalizing problems. Journal of Consulting and Clinical Psychology, 68, 1038–1050.10.1037/0022-006X.68.6.1038 [DOI] [PubMed] [Google Scholar]