Abstract
Introduction:
Smoking initiation usually begins in adolescence, but how and for whom nicotine dependence emerges during this period is unclear. The cue-reactivity paradigm is well suited to examine one marker of dependence: craving-related stimulus control, i.e., the ability of environmental cues to elicit craving to smoke. This study examined the effects of both level of smoking involvement (daily vs. occasional smoking) and gender on reactivity to both smoking and alcohol cues.
Methods:
Young (age range 16–20; 42% female) daily (n = 55) and occasional (n = 52) smokers were exposed to each of three counterbalanced cues: (a) in vivo smoking (e.g., sight, smell, lighting of cigarette), (b) alcohol (e.g., opening, pouring, and smell of preferred beverage), and (c) neutral cue.
Results:
Daily smokers exhibited higher levels of tonic (i.e., noncue-elicited) craving than did occasional smokers. Both groups showed significant increases in craving in response to cues (i.e., cue-elicited craving), with little evidence that cue-elicited craving differed between groups. Females were more cue reactive to both the alcohol and smoking cues than males, particularly for the positively reinforced aspects of smoking (i.e., hedonic craving). There were no gender × group interaction effects in response to either the alcohol or the smoking cue.
Conclusions:
Findings show the presence of cue-elicited craving even among occasional smokers and are consistent with literature demonstrating heightened sensitivity to environmental cues among females. Cue-elicited craving may be one mechanism that contributes to the maintenance of smoking behavior and perhaps to the development of nicotine dependence within early stage smokers.
INTRODUCTION
In the United States, it is well accepted that smoking begins in adolescence (Backinger, Fagan, Matthews, & Grana, 2003; Chassin et al., 2008). It is equally understood that smoking initiation follows a pattern of experimentation, occasional smoking, and ultimately daily smoking (Breslau, Fenn, & Peterson, 1993; Lewinsohn, Brown, Seeley, & Ramsey, 2000), although this course is not universal, and the trajectory is variable (Brook et al., 2008; Chassin et al., 2008; Costello, Dierker, Jones, & Rose, 2008). Much less understood, and of modest debate, is the timing and course of onset of nicotine dependence among early stage smokers.
Young occasional smokers typically do not exhibit withdrawal (Panday, Reddy, Ruiter, Bergström, & de Vries, 2007; Rose & Dierker, 2010; Rubinstein, Benowitz, Auerback, & Moscicki, 2009; Wileyto et al., 2009), and thus may not yet show evidence of physiological dependence on nicotine. However, young adult smokers may show other nonphysiologic signs of dependence that may influence smoking. Stimulus control of responses to smoking-related cues, that is, cue-elicited craving (Tiffany, 1990; Tiffany, Warthen, & Goedeker, 2009), may be a marker or precursor of dependence among early stage smokers.
Although there is ample research to support the existence of stimulus control among established, that is, daily young smokers (Rubinstein, Luks, Moscicki, et al., 2011; Thomas, Drobes, & Deas, 2005; Upadhyaya, Drobes, & Thomas, 2004; Upadhyaya, Drobes, & Wang, 2006), few studies have taken the more narrow focus to examine stimulus control among young people who smoke occasionally. Occasional smoking, particularly during adolescence and young adulthood, is often situation specific, and although a number of contexts may be associated with occasional smoking (e.g., social settings, specific mood states, other smoker), one of the strongest cues is alcohol (Brown, Carpenter, & Sutfin, 2011; Lewinsohn et al., 2000; McKee, Hinson, Rounsaville, & Petrelli, 2004; Moran, Wechsler, & Rigotti, 2004; Presson, Chassin, & Sherman, 2002; Rubinstein et al., 2009). For example, in a comparison of adolescent smokers who have versus have not smoked 100 cigarettes in their lifetime (a proxy for a comparison of daily vs. occasional), those without an extensive history of smoking reported a greater likelihood of smoking while drinking (86%) than did those smokers with an extensive history of smoking (63%; McKee et al., 2004). We are aware of only two studies to examine stimulus control among adolescent light smokers. The first study (Rubinstein, Luks, Moscicki et al., 2011) demonstrated that environmental cues produce brain activation within a group of early stage smoker and also suggests that nicotine dependence can begin early. However, without comparison to heavier smokers, it is difficult to interpret these findings. The second study (Curtin, Barnett, Colby, Rohsenow, & Monti, 2005) showed heightened craving to smoke in response to cigarette cues among adolescents who smoke more frequently. There were no differences between frequent and infrequent smokers in craving for alcohol in response to alcohol cues; there was no test of craving to smoke in response to alcohol cue.
If stimulus control is one marker of early dependence, it might be expected to increase as smokers progress from occasional to daily smoking. However, the generalization gradient likely flattens as smoking expands from a few restricted contexts to many, and if so, this would suggest heightened stimulus control among occasional smokers. Two studies support this notion. The first, conducted within our group (Watson, Carpenter, Saladin, Gray, & Upadhyaya, 2010), demonstrated greater increases in craving in response to smoking cues among less versus more dependent smokers. In the second, based on ecological momentary assessment methods (Shiffman & Paty, 2006), smoking patterns of “chippers” (adult light smokers) were associated with consummatory behaviors (e.g., drinking alcohol or coffee, eating). For example, smoking was 4.5 times more likely when chippers engaged in such behaviors but only 1.7 times more likely when daily smokers did so. In contrast, a recent comparison of adult occasional and daily smokers by the same study group did not demonstrate heightened craving response across a broad range of cues (Shiffman et al., 2013). Thus, the small extant literature on stimulus control among and between groups of occasional versus daily smokers is mixed, and in the case of adolescent smokers, almost nonexistent.
A separate literature, again based mostly on adults, suggests that cue-elicited craving may be higher among women (Perkins, Donny, & Caggiula, 1999). For example, prior research from within our group (Saladin et al., 2012) suggests that women express heightened craving in response to stress-related cues compared with men. Women within this same study also trended (i.e., nonsignificant difference) toward heightened responses to smoking cues. Similar research from others (Colamussi, Bovbjerg, & Erblich, 2007; Field & Duka, 2004; Tong, Bovbjerg, & Erblich, 2007) has demonstrated heightened craving in response to smoking and/or stress cues among women. However, in one study most similar to ours, adult smokers administered a cue-exposure session were tested for the effects of gender, smoker group (regular smokers vs. chippers), and their interaction. This underpowered study (n = 22) demonstrated heighted craving for regular smokers, but no effects of gender or any interaction.
We are aware of no studies specifically comparing craving stimulus control among occasional versus daily young smokers, with attention to the potentially moderating role of gender. The primary purpose of the current study was to test stimulus control, using laboratory-based cue-reactivity procedures, among and between young occasional and daily smokers. A secondary purpose was to examine possible gender differences in cue-elicited craving among early stage smokers. We specifically hypothesized that (1) daily smokers would show greater tonic craving (i.e., background, noncue-elicited craving) than occasional smokers, (2) both daily and occasional smokers would show significant increases in craving in response to smoking- and alcohol-related cues, (3) the change in craving would be greater for occasional versus daily smokers, but that given the likelihood of diminished withdrawal response within occasional smokers, this would be specific to hedonic craving (i.e., smoking for pleasurable purposes) and not negative reinforcement craving (i.e., smoking to relieve withdrawal), and (4) young female smokers would evince greater increases in cue-elicited craving than males.
METHODS
Participants
Young smokers (there is no widely accepted terminology used to reference either “adolescents” or “young adults,” so we have elected to use the designation “young smokers” throughout) were recruited from the general community via radio, print, and social media advertising. Eligibility criteria included (a) age 16–20 (to control for legal drinking status), (b) current occasional or daily smoker not currently trying to quit, (c) no use of non-cigarette tobacco within past 6 months, (d) ingestion of alcohol at least once in the past 30 days, and (e) does not meet criteria for past or current alcohol abuse/dependence. Participants under age 18 provided parental consent to participate. Occasional smokers were defined as (a) currently smoking (having at least one cigarette in each of the past 8 weeks) and (b) having never smoked daily for a week or more. Thus, we screened out those individuals who were experimenting and had very low levels of smoking, and also those who have previously smoked regularly and who were currently smoking less. Daily smokers were defined as (a) smoking daily ≥5 cigarettes per day, (b) on at least 26/30 days of each month for at least 6 months, and (c) providing an afternoon carbon monoxide upon entry of >8 ppm.
General Procedures
Study eligibility, informed consent, and baseline assessments were administered during the initial study session. Cue-reactivity testing was administered in a second and final study session, 6.1 days on average (SD = 3.2) following the first, >90% of which were scheduled after 1:00 p.m. At the outset of the cue-reactivity procedures, all participants smoked one cigarette 30min prior to testing procedures to equate groups for time since last cigarette and to minimize the confounding effects of withdrawal (i.e., overnight deprivation would have led to differential withdrawal between groups). Prior to formal cue-reactivity testing, participants viewed a 10-min slideshow of nature scenes to acclimate them to the environment and settle any autonomic responding. Cue-reactivity testing consisted of visual and auditory presentation of in vivo cues (see below). Subjective measures of craving are listed below. Participants received payment ($50) upon session completion, and up to $75 overall for completion of both study visits. The Medical University of South Carolina and the College of Charleston granted the appropriate approvals for Protection of Human Subjects.
Cues
Each cue was 120 s in duration and consisted of in vivo handling of objects, accompanied by audio instructions provided via headphones. Order of cue presentation was counterbalanced, and a 10-min washout consisting of nature scenes was administered between each. Cues were initially presented via covered box, and participants were instructed to uncover cues and manipulate them accordingly.
The smoking cue consisted of the participant’s preferred brand pack of cigarettes and lighter, with instructions to open the pack, remove, smell, and light one cigarette. One puff only was allowed (to light the cigarette) to minimize the effect of priming (Dawkins, Powell, West, Powell, & Pickering, 2006).
The alcohol cue was generally derived from prior research (Monti et al., 1987; Saladin, Drobes, Coffey, & Libet, 2002), in which the participant’s preferred alcoholic beverage (wine, beer, or liquor) was presented. Beer was presented in the original packaging (can/bottle); wine and liquor were presented in appropriate glassware. Cue manipulation consisted of the pour (beer only), sight, and repeated smell of the desired beverage. The cue involved no ingestion, verified via video monitoring.
The neutral cue was designed to have many of the stimulus features of the smoking cue and consisted of pencil and eraser, with comparable instructions as above. We did not include a control for the alcohol cue per se (e.g., water) due to concerns of extending session length, which would increase the likelihood of differential increase in withdrawal among daily smokers.
Assessments
Basic demographics and smoking history were assessed at the initial study visit. Dependence was measured via the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) and the Autonomy over Smoking Scale (AUTOS; DiFranza, Wellman, Ursprung, & Sabiston, 2009). Subjective craving was assessed at the outset of the testing session (but after the smoked cigarette) and again pre- and postcue using the Questionnaire of Smoking Urges-Brief (QSU; Cox, Tiffany, & Christen, 2001; Davies, Willner, & Morgan, 2000). This 10-item self-rating questionnaire assesses two aspects of craving that correspond to two factors (1) urge for cigarettes in anticipation of positive outcomes and (2) urge for cigarettes in anticipation of relief from negative affect/withdrawal (Davies et al., 2000; Tiffany & Drobes, 1991). Items are rated on a 1–7 ordinal scale and then averaged for a total and factor-specific score (i.e., possible range for each: 1–7, with higher ratings indicating greater craving).
Data Analysis
We first compared baseline characteristics of occasional and daily smokers, both on demographic and smoking history items, although we expected group differences on the latter. Analysis of between-group differences in tonic (Hypothesis 1) was based on analysis of variance (ANOVA). Tonic craving was assessed as the first assessment of craving (pre first cue). To verify a basic cue-reactivity effect within each smoker group (Hypothesis 2), we separately applied paired t-tests to compare pre- vs. postcue craving for each cue. For Hypothesis 3 (between smoker group comparisons) and Hypothesis 4 (between gender comparisons), we tested pre/post differences in QSU scores for both the alcohol and smoking cue while controlling for both (a) pre/post change in craving in response to neutral cue and (b) precue differences between groups. Hypotheses 3 and 4 were combined into one factorial ANOVA, testing main effects of smoker group and gender, as well as their interaction.
RESULTS
Sample Characteristics
Demographic and smoking history characteristics are shown in Table 1. The study sample (N = 107) was predominantly young adult (mean age 18.9, SD = .9), with over half (58%) male. There were significant smoker group differences on age and race, with more Caucasians and females within the occasional smoker group. As expected, there were significant smoker group differences on all measures of smoking, including number of days smoking in past month (p < .001), mean number of cigarettes smoked per day (p < .001), number of quit attempts (p = .04), and measures of dependence (p < .001 for both FTND and AUTOS, as well self-perception of addiction). Aside from smoking frequency, carbon monoxide, and perceived addiction, there were few differences across gender.
Table 1.
Sample Characteristics
| Smoker group | Gender | |||||
| Occasional (n = 52) | Daily (n = 55) | p value | Male | Female | p value | |
| Female | 55% | 31% | .01 | – | – | |
| Occasional smoker | – | – | 38% | 62% | .01 | |
| Age (SD) | 18.8 (0.8) | 19.1 (1.0) | .08 | 19.1 (0.9) | 18.7 (0.9) | .02 |
| Race | .02 | .2 | ||||
| White | 93% | 72% | 78% | 87% | ||
| Black | 6% | 19% | 22% | 13% | ||
| Addicted to smoking (self report) | 6% | 77% | <.001 | 54% | 28% | .007 |
| Concerned about health effects of smoking | 62% | 61% | .93 | 65% | 57% | .4 |
| Smoke a particular brand | 57% | 93% | <.001 | 80% | 70% | .2 |
| Number of days smoked in past 30 days | 14.1 (6.0) | 28.5 (4.9) | <.001 | 22.3 (9.1) | 19.7 (8.7) | .06 |
| Mean cigarettes/day—weekday | 1.7 (1.3) | 12.4 (6.9) | <.001 | 7.9 (7.1) | 6.3 (7.7) | .3 |
| Mean cigarettes/day—weekend | 3.9 (2.6) | 15.1 (7.6) | <.001 | 10.3 (8.0) | 8.7 (8.0) | .3 |
| Carbon monoxide, ppm | 3.5 (6.2) | 11.7 (4.9) | <.001 | 9.4 (7.5) | 5.4 (5.5) | .004 |
| Age started smoking | 15.9 (1.7) | 15.0 (2.2) | .01 | 15.5 (2.2) | 15.4 (1.6) | .8 |
| Number of serious quit attempts | 0.7 (1.6) | 1.4 (1.9) | .04 | 1.3 (2.1) | 0.8 (1.4) | .2 |
| FTND | .25 (0.9) | 3.2 (1.9) | <.001 | 2.0 (2.0) | 1.6 (2.3) | .3 |
| AUTOS | 9.6 (7.4) | 20.7 (8.1) | <.001 | 15.9 (9.4) | 14.7 (9.7) | .5 |
| Drinking days per month | .005 | .5 | ||||
| <10 | 51% | 77% | 67% | 61% | ||
| ≥10 | 49% | 23% | 33% | 39% | ||
| How often get drunk per week | .07 | .14 | ||||
| None | 26% | 46% | 36% | 37% | ||
| Once per week | 34% | 32% | 27% | 41% | ||
| Twice or more per week | 40% | 23% | 38% | 22% | ||
Note. AUTOS = Autonomy over Smoking Scale; FTND = Fagerström Test for Nicotine Dependence.
For occasional smokers, the top three smoking situations were while drinking alcohol (49.1%), when stressed (13.2%), and when at a party (11.3%). For daily smokers, the top three smoking situations were while drinking alcohol (36.4%), when stressed (20.0%), and after a meal (20.0%). Occasional smokers reported more days of drinking alcohol per month and a trend for more drunken episodes per week than did daily smokers; there were no gender differences on drinking frequency or drunk drinking.
Tonic (i.e., Background, Noncue-Elicited) Craving: Comparisons of Smoker Group and Gender
In confirmation of the first hypothesis, daily smokers reported significantly greater tonic craving than occasional smokers, as measured by QSU total (2.1 [SD = 0.9] vs. 1.6 [SD =0.6]; p < .001), QSU Factor 1 (2.5 [SD = 1.0] vs. 1.8 [SD = 0.8]; p < .001) and QSU Factor 2 (1.7 [SD = 0.8] vs. 1.3 [SD = 0.5]; p = .001) scores. There were no differences in tonic craving across gender.
Within-Group Verification of Cue-Elicited Craving
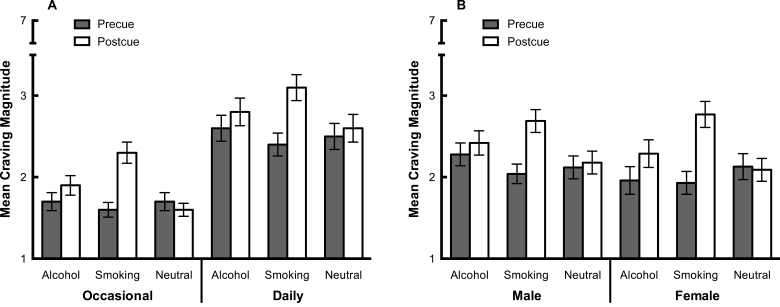
Figure 1 depicts levels of pre- and postcue craving (QSU total) split by both smoker group and gender. For occasional smokers, postcue craving (QSU total) was significantly higher than precue craving, for both the alcohol (p < .001) and smoking (p < .001) cues but not the neutral cue, (p = .2). For daily smokers, postcue craving was significantly higher than that at precue for the alcohol (p < .001), smoking (p < .001), and neutral (p = .02) cues. The increase in craving in response to the neutral cue was unexpected but might be a reflection of the higher tonic craving observed for daily smokers. Both males and females demonstrated significant increases in craving in response to both alcohol (male: p = .005; female: p < .001) and smoking cues (male: p < .001; female: p < .001) but not the neutral cue (male: p = .2; female: p = .4).
Figure 1.
Pre-/post-QSU craving to smoke (possible range 1–7) in response to cues: (A) occasional versus daily smokers; (B) male versus female smokers. Error bars represent SE.
Between-Group Comparisons of Cue-Elicited Craving
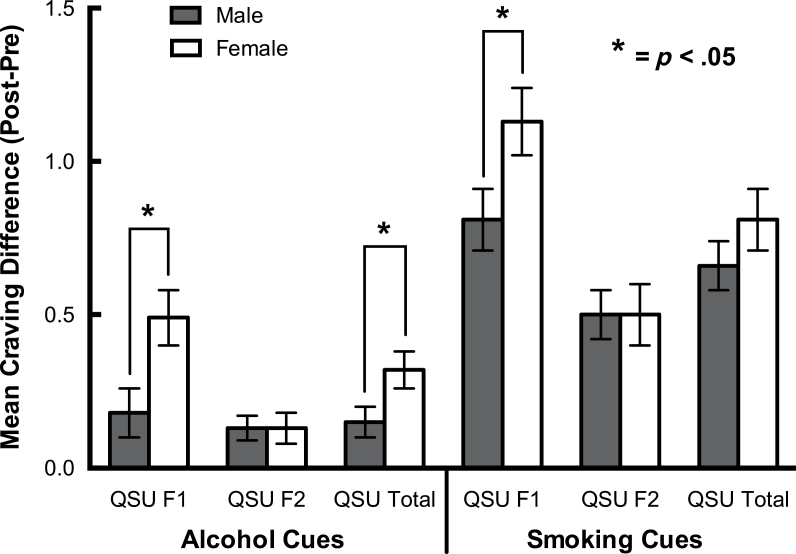
Factorial analysis of variance tested pre/post increases in craving in response to both alcohol and smoking cues, wherein the effects of smoker group and gender were entered as fixed factors, and both precue craving and pre/post changes in response to neutral cues were entered as covariates. Across both cues, and across all measures of craving, there was no effect of smoker group, with the exception of a trend level effect (p = .07) on QSU Factor 2 where the daily smokers reported a larger response to the alcohol cues than the occasional smokers. There were, however, significant effects of gender, in response to both the alcohol cue (QSU total and QSU Factor 1) and smoking cue (QSU Factor 1), with females exhibiting the larger response in each case (Figure 2). There were no significant smoker group × gender interactions.
Figure 2.
Pre-/postcue-elicited increases in craving by gender, controlling for both precue craving for each cue (alcohol and smoking) and pre/post increases in response to neutral cue. Error bars represent SE.
DISCUSSION
The overarching purpose of this study was to examine within- and between-group cue reactivity in early stage young smokers. Our primary focus was on smoking intensity (occasional vs. daily smoking), with purposeful recruitment across smoker group; our secondary focus was on gender. Adolescence is a critical developmental period during which initiation of smoking (for those who become eventual smokers) almost universally occurs. It is during this same time period that dependence also unfolds, although the course and trajectory is variable. Several prior studies have examined craving and/or cue reactivity among adolescents or young adults (Bagot, Heishman, & Moolchan, 2007; Curtin et al., 2005; Thomas et al., 2005; Upadhyaya et al., 2006), including two studies comparing adolescent light versus nonsmokers (Rubinstein, Luks, Dryden, Rait, & Simpson, 2011; Rubinstein, Luks, Moscicki et al., 2011). To our knowledge, only one study, offering limited results, has explicitly compared cue-elicited craving to smoke between occasional versus daily smokers during this early developmental period (Curtin et al., 2005), and our results are largely consistent. Controlled examination of early stage processes by which nicotine dependence develops is important to our understanding of the developmental processes in addiction.
Our first aim was to compare tonic craving between daily versus occasional smokers, and between males and females. Affirming our initial hypothesis, daily smokers reported higher levels of all measures of subjective craving than did occasional smokers. This difference was independent of gender, and there were no gender differences in tonic craving. Our second aim was to demonstrate the viability of the cue-reactivity paradigm, as a method to test cue-elicited craving, in both occasional and daily smokers, and again, secondarily, within both males and females. This too was affirmed, as both smoker groups and both gender groups demonstrated a clear response to smoking- and alcohol-related cues. Thus, even early stage smokers demonstrated heightened craving in response to specific environmental stimuli. The fact that these environmental stimuli (other smokers, alcohol) are omnipresent makes this risk all the more meaningful. It is unclear from our data if early stage cue-elicited craving confers risk of further progression to more entrenched patterns of smoking.
The more nuanced focus of our study was based on our third aim (Hypotheses 3 and 4), in which we directly compared daily versus occasional smokers and males versus females. Comparisons of smoker group exist among adults (Shiffman et al., 2013; Shiffman & Paty, 2006) but not among younger smokers early in their smoking trajectory when dependence develops. Given that occasional smoking is often more situation bound than is daily smoking, particularly to alcohol, we initially hypothesized that occasional smokers would demonstrate greater cue-elicited craving than daily smokers and that this response would be specific to hedonic craving (QSU Factor 1). This was not supported, nor we did not demonstrate any smoker group differences for any measure of craving, in response to any cue. Thus, occasional and daily smokers show comparable response to cues. This has important implications, suggesting that cue-elicited craving is already evident in early stage, that is, occasional smoking. Thus, our findings are largely consistent with a growing literature that shows the potential for early onset of nicotine dependence after initiation of smoking (Doubeni, Reed, & DiFranza, 2010; Kandel, Hu, Griesler, & Schaffran, 2007; Ursprung, Morello, Gershenson, & DiFranza, 2011).
The present findings, very much consistent with the adult literature (Field & Duka, 2004; Saladin et al., 2012; Tong et al., 2007), show that adolescent females show stronger increases in craving in response to cues than do males. Although both males and females showed increases in cue-elicited craving, the increase was higher among females. This was true for both alcohol and smoking cues. Although gender has been shown to play a role in nicotine dependence once it has been established (Perkins et al., 1999), our data support the interpretation that gender also may play a role in the early development of this dependence. Heightened craving in response to environmental cues could undermine quitting among women and also suggests that management of smoking-related triggers, such as alcohol and other smokers, may be more particularly beneficial for adolescent females. The influence of gender on cessation outcome is unclear. Although a number of treatment outcome studies show decreased abstinence rates among women (Bjornson et al., 1995; Scharf & Shiffman, 2004; Wetter et al., 1999), recent population-based research among adults (Jarvis, Cohen, Delnevo, & Giovino, 2013) and adolescents (Branstetter, Blosnich, Dino, Nolan, & Horn, 2012) suggest no relationship between gender and cessation.
Several methodological issues from the cue-reactivity literature are relevant to the present discussion (Conklin, Perkins, Robin, McClernon, & Salkeld, 2010; Conklin, Robin, Perkins, Salkeld, & McClernon, 2008; Sayette, Griffin, & Sayers, 2010; Sayette et al., 2000). First, all participants smoked one cigarette prior to testing to equate for time since last cigarette. Overnight abstinence would likely have increased our sensitivity to test cue-elicited craving, but doing so would have started the smoker groups off on different levels of withdrawal, which we wanted to avoid. We also recognize the possible effect of priming (Chiamulera, 2005), in which a presession cigarette may have had a differential effect on smoker group, but in the end, there seems to be no single best approach to manage this. Additionally, our focus was on craving and not on actual smoking behavior although our prior work (Carpenter et al., 2009) and that of others (Van Zundert, Boogerd, Vermulst, & Engels, 2009) suggest a link between the two. Finally, we did not assess craving to drink alcohol as a function of cue presentation. Cross-cue craving was not our focus and has been reported elsewhere (Drobes, 2002).
Among our study limitations, we tested only a limited range of cues. Other cues that may influence craving and thus smoking patterns at these early stages include other smokers, social settings (parties), mood states, stress, or even times of day/week. For example, craving could increase, perhaps more so among occasional smokers, as a function of a social setting on a weekend evening. Second, our study would have been strengthened by inclusion of individuals with limited history of experimental smoking (whom we would expect to be minimally cue reactive, given limited pairing history of smoking/cues) and nonsmokers. Inclusion of these earlier stage groups would allow us to delineate where and when cue reactivity actually starts. Third, although smoker groups were generally equivalent in age, they were not equivalent in terms of number of years smoking. By definition, the groups had nonequivalent smoking history, per amount of consumption, and thus, these differences may be an artifact of our recruitment procedures. Fourth, a small number of cue-reactivity sessions occurred in the morning, for scheduling purposes only, which could reduce response to cues, particularly alcohol cues. Fifth, as with other studies, failure to find between-group differences may be a function of limited statistical power. Finally, the best test of stimulus control and its association with the developmental trajectory of smoking would involve a longitudinal design. In the absence of such studies, the cross-sectional, between-group comparison herein is more feasible.
Given that smoking begins in young people, it is important to understand the processes that underlie transitions in patterns of smoking behavior. Both occasional and daily smokers demonstrated cue-elicited craving, with no apparent between-group differences. Consistent with most of the adult literature, females were more reactive to cues than were males. Stimulus control, as assessed via the cue-reactivity paradigm and other methods, may be one marker of what may become dependence among young people. If and how stimulus control processes influence, or are influenced by, the developmental trajectory of smoking and how gender might moderate this relationship are worthy of further study.
FUNDING
This work was supported by Career Development Awards from NIDA (K23 DA020482 to M.J.C.; K12 DA000357 to K.M.G).
DECLARATION OF INTERESTS
MJC, MES, SDL, EAM, and SS report no conflicts of interest. KMG has received research funding from Merck Inc. and Supernus Pharmaceuticals. HPU, formerly of the Medical University of South Carolina, is an employee and stockholder of Eli Lilly and Company.
ACKNOWLEDGMENTS
All authors contributed to the design, conduct, and interpretation of this study. All authors have approved the final manuscript. The authors thank Nicola Thornley, Amy Boatright, and Elizabeth Byrd, and Ashley McCullough for their contributions in data collection and study management. The authors also thank Drs. Michael Sayette and David Drobes for their advice during study.
REFERENCES
- Backinger C. L., Fagan P., Matthews E., Grana R. (2003). Adolescent and young adult tobac prevention and cessation: Current status and future directions. Tobacco Control, 12(Suppl. 4), 46–53.10.1136/tc.12.suppl_4.iv46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot K. S., Heishman S. J., Moolchan E. T. (2007). Tobacco craving predicts lapse to smoking among adolescent smokers in cessation treatment. Nicotine & Tobacco Research, 9, 647–652.10.1080/14622200701365178 [DOI] [PubMed] [Google Scholar]
- Bjornson W., Rand C., Connett J. E., Lindgren P., Nides M., Pope F. … O’Hara P. (1995). Gender differences in smoking cessation after 3 years in the Lung Health Study. American Journal of Public Health, 85, 223–230.10.2105/AJPH.85.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branstetter S. A., Blosnich J., Dino G., Nolan J., Horn K. (2012). Gender differences in cigarette smoking, social correlates and cessation among adolescents. Addictive Behaviors, 37, 739–742.10.1016/j.addbeh.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Fenn N., Peterson E. L. (1993). Early smoking initiation and nicotine dependence in a cohort of young adults. Drug and Alcohol Dependence, 33, 129–137.10.1016/0376-8716(93)90054-T [DOI] [PubMed] [Google Scholar]
- Brook D. W., Brook J. S., Zhang C., Whiteman M., Cohen P., Finch S. J. (2008). Developmental trajectories of cigarette smoking from adolescence to the early thirties: Personality and behavioral risk factors. Nicotine & Tobacco Research, 10, 1283–1291.10.1080/14622200802238993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Carpenter M. J., Sutfin E. L. (2011). Occasional smoking in college: Who, what, when and why? Addictive Behaviors, 36, 1199–1204.10.1016/j.addbeh.2011.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. J., Saladin M. E., DeSantis S., Gray K. M., LaRowe S. D., Upadhyaya H. P. (2009). Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addictive Behaviors, 34, 536–541.10.1016/j.addbeh.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L., Presson C., Seo D. C., Sherman S. J., Macy J., Wirth R. J., Curran P. (2008). Multiple trajectories of cigarette smoking and the intergenerational transmission of smoking: A multigenerational, longitudinal study of a Midwestern community sample. Health Psychology, 27, 819–828.10.1037/0278-6133.27.6.819 [DOI] [PubMed] [Google Scholar]
- Chiamulera C. (2005). Cue reactivity in nicotine and tobacco dependence: A “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Research Reviews, 48, 74–97.10.1016/j.brainresrev.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Colamussi L., Bovbjerg D. H., Erblich J. (2007). Stress- and cue-induced cigarette craving: Effects of a family history of smoking. Drug and Alcohol Dependence, 88, 251–258.10.1016/j.drugalcdep.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin C. A., Perkins K. A., Robin N., McClernon F. J., Salkeld R. P. (2010). Bringing the real world into the laboratory: Personal smoking and nonsmoking environments. Drug and Alcohol Dependence, 111, 58–63.10.1016/j.drugalcdep.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin C. A., Robin N., Perkins K. A., Salkeld R. P., McClernon F. J. (2008). Proximal versus distal cues to smoke: The effects of environments on smokers’ cue-reactivity. Experimental and Clinical Psychopharmacology, 16, 207–214.10.1037/1064-1297.16.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello D. M., Dierker L. C., Jones B. L., Rose J. S. (2008). Trajectories of smoking from adolescence to early adulthood and their psychosocial risk factors. Health Psychology, 27, 811–818.10.1037/0278-6133.27.6.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. S., Tiffany S. T., Christen A. G. (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3, 7–16.10.1080/14622200124218 [DOI] [PubMed] [Google Scholar]
- Curtin J. J., Barnett N. P., Colby S. M., Rohsenow D. J., Monti P. M. (2005). Cue reactivity in adolescents: Measurement of separate approach and avoidance reactions. Journal of Studies on Alcohol, 66, 332–343 [DOI] [PubMed] [Google Scholar]
- Davies G. M., Willner P., Morgan M. J. (2000). Smoking related cues elicit craving in tobacco “chippers”: A replication and validation of the two-factor structure of the Questionnaire of Smoking Urges. Psychopharmacology, 152, 334–342.10.1007/s002130000526 [DOI] [PubMed] [Google Scholar]
- Dawkins L., Powell J. H., West R., Powell J., Pickering A. (2006). A double-blind placebo controlled experimental study of nicotine: I–effects on incentive motivation. Psychopharmacology, 189, 355–367.10.1007/s00213-006-0588-8 [DOI] [PubMed] [Google Scholar]
- DiFranza J. R., Wellman R. J., Ursprung W. W., Sabiston C. (2009). The autonomy over smoking scale. Psychology of Addictive Behaviors, 23, 656–665.10.1037/a0017439 [DOI] [PubMed] [Google Scholar]
- Doubeni C. A., Reed G., DiFranza J. R. (2010). Early course of nicotine dependence in adolescent smokers. Pediatrics, 125, 1127–1133.10.1542/peds.2009-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes D. J. (2002). Cue reactivity in alcohol and tobacco dependence. Alcoholism, Clinical and Experimental Research, 26, 1928–1929.10.1111/j.1530-0277.2002.tb02506.x [DOI] [PubMed] [Google Scholar]
- Field M., Duka T. (2004). Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacology, Biochemistry, and Behavior, 78, 647–652.10.1016/j.pbb.2004.03.026 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127.10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Jarvis M. J., Cohen J. E., Delnevo C. D., Giovino G. A. (2013). Dispelling myths about gender differences in smoking cessation: Population data from the USA, Canada and Britain. Tobacco Control, 22, 356–360.10.1136/tobaccocontrol-2011–050279 [DOI] [PubMed] [Google Scholar]
- Kandel D. B., Hu M. C., Griesler P. C., Schaffran C. (2007). On the development of nicotine dependence in adolescence. Drug and Alcohol Dependence, 91, 26–39.10.1016/j.drugalcdep.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. M., Brown R. A., Seeley J. R., Ramsey S. E. (2000). Psychosocial correlates of cigarette smoking abstinence, experimentation, persistence and frequency during adolescence. Nicotine & Tobacco Research, 2, 121–131.10.1080/713688129 [DOI] [PubMed] [Google Scholar]
- McKee S. A., Hinson R., Rounsaville D., Petrelli P. (2004). Survey of subjective effects of smoking while drinking among college students. Nicotine & Tobacco Research, 6, 111–117.10.1080/14622200310001656939 [DOI] [PubMed] [Google Scholar]
- Monti P. M., Binkoff J. A., Abrams D. B., Zwick W. R., Nirenberg T. D., Liepman M. R. (1987). Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology, 96, 122–126.10.1037/0021-843X.96.2.122 [DOI] [PubMed] [Google Scholar]
- Moran S., Wechsler H., Rigotti N. A. (2004). Social smoking among US college students. Pediatrics, 114, 1028–1034.10.1542/peds.2003-0558-L [DOI] [PubMed] [Google Scholar]
- Panday S., Reddy S. P., Ruiter R. A., Bergström E., de Vries H. (2007). Nicotine dependence and withdrawal symptoms among occasional smokers. Journal of Adolescent Health, 40, 144–150.10.1016/j.jadohealth.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Perkins K. A., Donny E., Caggiula A. R. (1999). Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine & Tobacco Research, 1, 301–315.10.1080/14622299050011431 [DOI] [PubMed] [Google Scholar]
- Presson C. C., Chassin L., Sherman S. J. (2002). Psychosocial antecedents of tobacco chipping. Health Psychology, 21, 384–392.10.1037/0278-6133.21.4.384 [DOI] [PubMed] [Google Scholar]
- Rose J. S., Dierker L. C. (2010). An item response theory analysis of nicotine dependence symptoms in recent onset adolescent smokers. Drug and Alcohol Dependence, 110, 70–79.10.1016/j.drugalcdep.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M. L., Benowitz N. L., Auerback G. M., Moscicki A. B. (2009). Withdrawal in adolescent light smokers following 24-hour abstinence. Nicotine & Tobacco Research, 11, 185–189.10.1093/ntr/ntn028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M. L., Luks T. L., Dryden W. Y., Rait M. A., Simpson G. V. (2011). Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine & Tobacco Research, 13, 751–755.10.1093/ntr/ntr046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M. L., Luks T. L., Moscicki A. B., Dryden W., Rait M. A., Simpson G. V. (2011). Smoking-related cue-induced brain activation in adolescent light smokers. Journal of Adolescent Health, 48, 7–12.10.1016/j.jadohealth.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin M. E., Drobes D. J., Coffey S. F., Libet J. M. (2002). The human startle reflex and alcohol cue reactivity: Effects of early versus late abstinence. Psychology of Addictive Behaviors, 16, 98–105.10.1037/0893-164X.16.2.98 [DOI] [PubMed] [Google Scholar]
- Saladin M. E., Gray K. M., Carpenter M. J., LaRowe S. D., DeSantis S. M., Upadhyaya H. P. (2012). Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. American Journal on Addictions, 21, 210–220.10.1111/j.1521-0391.2012.00232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette M. A., Griffin K. M., Sayers W. M. (2010). Counterbalancing in smoking cue research: A critical analysis. Nicotine & Tobacco Research, 12, 1068–1079.10.1093/ntr/ntq159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette M. A., Shiffman S., Tiffany S. T., Niaura R. S., Martin C. S., Shadel W. G. (2000). The measurement of drug craving. Addiction (Abingdon, England), 95(Suppl. 2), S189–S210.10.1046/j.1360-0443.95.8s2.8.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf D., Shiffman S. (2004). Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction, 99, 1462–1469.10.1111/j.1360-0443.2004.00845.x [DOI] [PubMed] [Google Scholar]
- Shiffman S., Dunbar M. S., Kirchner T. R., Li X., Tindle H. A., Anderson S. J. … Ferguson S. G. (2013). Cue reactivity in non-daily smokers: Effects on craving and on smoking behavior. Psychopharmacology, 226, 321–333.10.1007/s00213-012-2909-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Paty J. (2006). Smoking patterns and dependence: Contrasting chippers and heavy smokers. Journal of Abnormal Psychology, 115, 509–523.10.1037/0021-843X.115.3.509 [DOI] [PubMed] [Google Scholar]
- Thomas S. E., Drobes D. J., Deas D. (2005). Alcohol cue reactivity in alcohol-dependent adolescents. Journal of Studies on Alcohol, 66, 354–360 [DOI] [PubMed] [Google Scholar]
- Tiffany S. T. (1990). A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review, 97, 147–168.10.1037/0033-295X.97.2.147 [DOI] [PubMed] [Google Scholar]
- Tiffany S. T., Drobes D. J. (1991). The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction, 86, 1467–1476.10.1111/j.1360-0443.1991.tb01732.x [DOI] [PubMed] [Google Scholar]
- Tiffany S. T., Warthen M. W., Goedeker K. C. (2009). The functional significance of craving in nicotine dependence. In: Bevins R. A., Caggiula A. R. (Eds), The motivational impact of nicotine and its role in tobacco use (Vol. 55). New York: Springer; [DOI] [PubMed] [Google Scholar]
- Tong C., Bovbjerg D. H., Erblich J. (2007). Smoking-related videos for use in cue-induced craving paradigms. Addictive Behaviors, 32, 3034–3044.0.1016/j.addbeh.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya H. P., Drobes D. J., Thomas S. E. (2004). Reactivity to smoking cues in adolescent cigarette smokers. Addictive Behaviors, 29, 849–856.10.1016/j.addbeh.2004.02.040 [DOI] [PubMed] [Google Scholar]
- Upadhyaya H. P., Drobes D. J., Wang W. (2006). Reactivity to in vivo smoking cues in older adolescent cigarette smokers. Nicotine & Tobacco Research, 8, 135–140.10.1080/14622200500432112 [DOI] [PubMed] [Google Scholar]
- Ursprung W. W., Morello P., Gershenson B., DiFranza J. R. (2011). Development of a measure of the latency to needing a cigarette. Journal of Adolescent Health, 48, 338–343.10.1016/j.jadohealth.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Van Zundert R. M., Boogerd E. A., Vermulst A. A., Engels R. C. (2009). Nicotine withdrawal symptoms following a quit attempt: An ecological momentary assessment study among adolescents. Nicotine & Tobacco Research, 11, 722–729.10.1093/ntr/ntp055 [DOI] [PubMed] [Google Scholar]
- Watson N. L., Carpenter M. J., Saladin M. E., Gray K. M., Upadhyaya H. P. (2010). Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addictive Behaviors, 35, 673–677.10.1016/j.addbeh.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter D. W., Kenford S. L., Smith S. S., Fiore M. C., Jorenby D. E., Baker T. B. (1999). Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology, 67, 555–562.10.1037//0022-006X.67.4.555 [DOI] [PubMed] [Google Scholar]
- Wileyto P., O’Loughlin J., Lagerlund M., Meshefedjian G., Dugas E., Gervais A. (2009). Distinguishing risk factors for the onset of cravings, withdrawal symptoms and tolerance in novice adolescent smokers. Tobacco Control, 18, 387–392.10.1136/tc.2009.030189 [DOI] [PubMed] [Google Scholar]