Abstract
We report a case in which 18F-FDG PET was able to discriminate pseudoprogression from progression observed on contrast-enhanced (CE) MRI (CE-MRI). A 56-year-old male patient with anaplastic oligodendroglioma demonstrated markedly increased tumor enhancement on CE-MRI 1 month after completing radiation therapy (RT), suggesting radiological progression. However, the patient was clinically improved and therefore received an early-therapy response assessment PET to assess for pseudoprogression. PET showed low tumor uptake indicating stable disease. Follow-up CE-MRI at 3 and 4 months post-RT confirmed stable disease. This case emphasizes the value of 18F-FDG PET when pseudoprogression is clinically suspected.
Keywords: pseudoprogression, anaplastic oligodendroglioma, 18F-FDG, PET, therapy response assessment
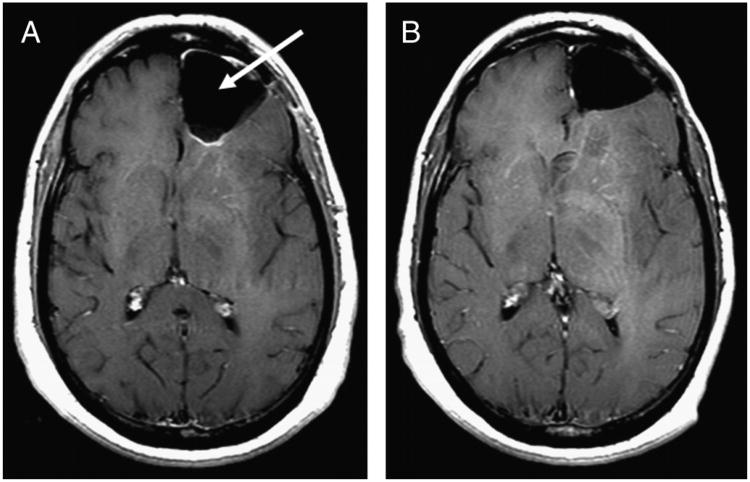
Figure 1.
A 56-year-old male patient with left-frontal anaplastic oligodendroglioma underwent tumor resection (arrow), followed by a gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) CE-MRI scan (A). After completing 9 cycles of temozolomide chemotherapy (75 mg/m2), the patient was clinically in remission and a follow-up CE-MRI showed stable disease (B).
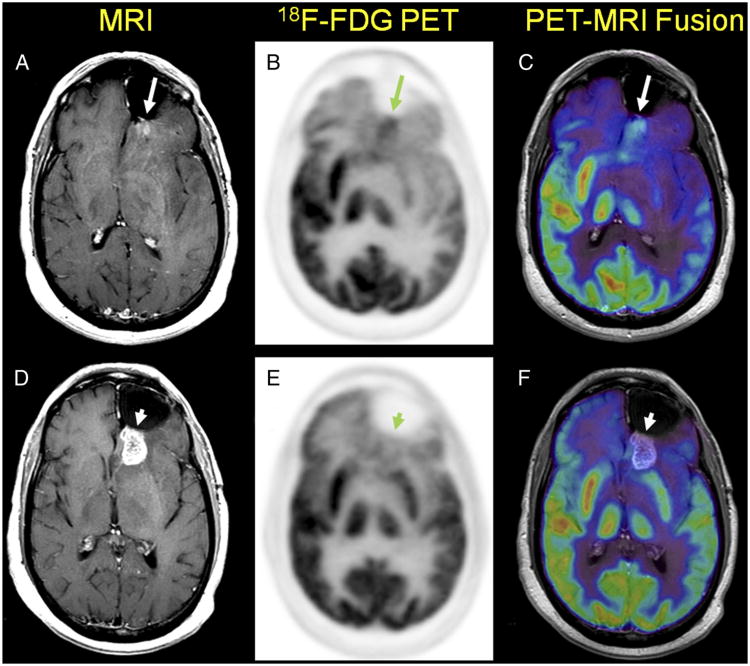
Figure 2.
After completing 3 additional cycles of temozolomide chemotherapy (12 cycles total), the patient presented with a new peripheral region of CE (arrow) on CE-MRI (A) posterior to the resection site suggesting radiologic progression of disease.1-3 18F-FDG PET showed a region of increased tracer uptake (B) anatomically correlating with the observed CE region on MRI (C). Therefore, the patient underwent RT to a total dose of 60 Gy in 2-Gy fractions. One month after completing RT, CE-MRI (D) showed increased enhancement posterior to the prior resection cavity in the left frontal lobe (arrowhead). However, the patient was clinically improved, and therefore an early-therapy response assessment 18F-FDG PET scan was obtained with the intent of differentiating the clinical possibilities of true progression and pseudoprogression4-6 On PET (E), no abnormal areas of increased 18F-FDG uptake in the region of MRI contrast enhancement were identified (F), thus additional therapy was deemed not indicated; the patient was monitored on follow-up CE-MRI scans.
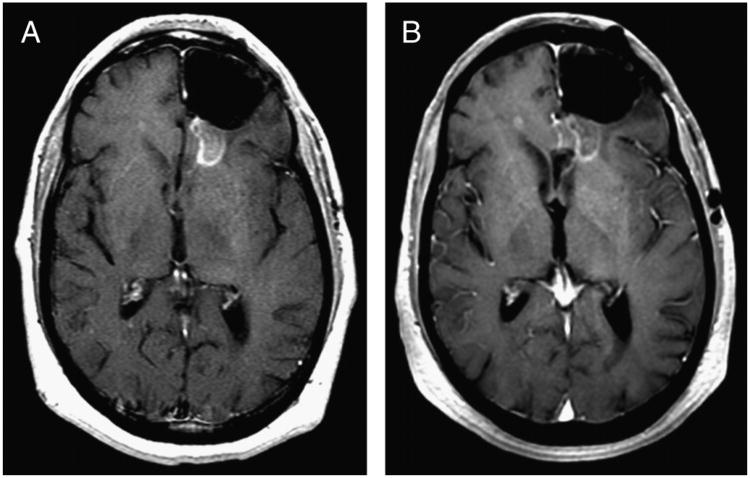
Figure 3.
Follow-up CE-MRI scans obtained at 3 months (A) and 4 months (B) after completion of therapy and the negative 18F-FDG scan. Compared with the CE-MRI obtained at 1 month after RT, the intensity and size of the CE region is observed to decrease and stabilize, and the patient remained in remission. The potential for pseudoprogression limits the use of CE-MRI for early-therapy response assessment in high-grade brain tumors.7,8 Current recommendations aimed at reducing the risk of misdiagnosis due to pseudoprogression include delaying diagnosis of progression for 12 weeks after completion of RT in regions showing CE on MRI,2,9 a significant shortcoming in the management of high-grade gliomas.10,11 Currently, 18F-FDG PET is not a standard-of-care method for evaluating therapeutic response in high-grade gliomas. However, as emphasized by this case, 18F-FDG PET may allow for timely discrimination of pseudoprogression from true progression.
Acknowledgments
Conflicts of interest and sources of funding: This work was supported by the US National Institutes of Health research grant U01 CA140230 and UPCI shared resources award P30CA047904.
Footnotes
Institution where work was performed: Department of Radiology, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 5.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 6.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 7.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32:1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol. 2009;27:2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easaw JC, Mason WP, Perry J, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18:e126–e136. doi: 10.3747/co.v18i3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhard HH, Stelea A, Mundt A. Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis. Surg Neurol. 2003;60:443–456. doi: 10.1016/s0090-3019(03)00167-8. [DOI] [PubMed] [Google Scholar]
- 11.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]