Abstract
Background
Urinary Kidney Injury Molecule 1 (KIM-1) is a proximal tubular injury biomarker for early detection of acute kidney injury (AKI), with variable performance characteristics depending on clinical and population settings.
Methods
Meta-analysis was performed to assess the diagnostic value of urinary KIM-1 in AKI. Relevant studies were searched from MEDLINE, EMBASE, Pubmed, Elsevier Science Direct, Scopus, Web of Science, Google Scholar and Cochrane Library. Meta-analysis methods were used to pool sensitivity and specificity and to construct summary receiver operating characteristic (SROC) curves.
Results
A total of 2979 patients from 11 eligible studies were enrolled in the analysis. Five prospective cohorts, two cross-sectional and four case-control studies were identified for meta-analysis. The estimated sensitivity of urinary KIM-1 for the diagnosis of AKI was 74.0% (95% CI, 61.0%–84.0%), and specificity was 86.0% (95% CI, 74.0%–93.0%). The SROC analysis showed an area under the curve of 0.86(0.83–0.89). Subgroup analysis suggested that population settings and detection time were the key factors affecting the efficiency of KIM-1 for AKI diagnosis.
Limitation
Various population settings, different definition of AKI and Serum creatinine level used as the standard might have influence on AKI diagnosis. The relatively small number of studies and heterogeneity between them also affected the evaluation.
Conclusion
Urinary KIM-1 may be a promising biomarker for early detection of AKI with considerable predictive value, especially for cardiac surgery patients, and its potential value needs to be validated in large studies and across a broader scope of clinical settings.
Introduction
Acute kidney injury (AKI) is a common and serious condition recognized in nearly all fields of medical practice. It is characterized as a rapid and intensive decline of renal function associated with series of clinical syndrome which account for high morbidity and mortality [1], [2]. The latest survey reported that almost 2 million people died of AKI every year and the survivors had an enhanced risk of chronic kidney disease [3]. Early diagnosis and intervention of AKI could effectively prevent the occurrence of the outcome. Despite the advanced progress made in etiology and pathology of AKI, the clinical detection and diagnosis was still in controversy. Nowadays, the most widely used and commonly accepted clinical standard for the definition and diagnosis of AKI usually relied on the increase of serum creatinine or decrease of urine output which was proposed by both AKIN (acute kidney injury network) and RIFLE (risk, injury, failure, loss, and ESRD) [4]. Unfortunately, due to the poor sensitivity and specificity and 48 h–72 h time needs, serum creatinine was incapable to comprehensively reflect the time and type of renal injury. Moreover, serum creatinine was also affected by some other factors, such as age, acute and chronic renal failure [5]. These studies suggested that more accurate and efficient measure for AKI diagnosis was urgently required [6]. Lines of evidence showed that urinary NGAL, IL-18, Cys-C, KIM-1 and some other candidate molecules were believed as potential markers to diagnosis of AKI [7], [8]. But until now, none of them are currently established well enough to replace serum creatinine as a marker of renal function. Among various kinds of these markers, growing evidence showed that KIM-1 performed significantly superiority in early detection of AKI than others, especially within 24 hours, well before serum creatinine increase, which made it possible to conduct prevention or treatment strategies at a very early stage of AKI [9], [10].
KIM-1, a type-1 transmembrane protein, was originally found as a putative epithelial cell adhesive molecule containing a novel immunoglobulin domain, which was absent in normal condition but elevated in the proximal tubule apical membrane cells after injury [11], [12], [13]. Previous reports had proved Kim-1 in rat model as an outstanding indicator of kidney injury better than serum creatinine to predict proximal tubule injury [13]. Urinary KIM-1 levels are strongly related to tubular KIM-1 expression in experimental and in human renal disease [12]. Studies in human also indicated that urinary KIM-1 was sensitive and specific marker of injury as well as predictors of outcome [14]. Recently, two systematic reviews had been reported that KIM-1 was an efficient novel urinary biomarker in diagnosis of AKI within 24 hours after kidney injury [15], [16], especially in the diagnosis of ischemic ATN [15]. Although the extensive analyses have been carried out, owing to the limitation of relatively small population settings, heterogeneous patient type, less clinical trial and different detection time, the application of KIM-1 in early diagnosis of AKI still needs to be validated and thoroughly investigated in larger studies. Moreover, since adults are a different population to the children and rare studies were involved in evaluating age effect on urinary KIM-1 level, age might be an important influencing factor which needs to be studied.
To fully understand the diagnostic and predictive performance of urinary KIM-1 of AKI, we conducted a meta-analysis based on 11 original articles, which will be helpful for evaluate their roles on early clinical detection and diagnosis of AKI.
Methods
Data Sources and Search Strategy
This meta-analysis was performed in accordance with the Quality of Reporting of Meta-analysis (QUOROM) consensus guidelines and according to a protocol that pre-specified outcomes, search strategies, inclusion criteria, and statistical analysis [17]. Studies were identified by a literature search of the PubMed, MEDLINE, ISI Web of Science, EMBASE, Google Scholar, Scopus, Science direct and Cochrane library up to June 2013 with the following key words: “kidney injury molecule 1” or “KIM-1” plus “acute kidney injury” or “acute renal failure”, without language restriction. Besides, we checked the reference lists of retrieved articles to identify additional studies.The searches were performed independently by 2 investigators (Shao X and Tian L).
Study Selection
We chose articles and conference papers that had a prospective design or case-control design or cross-sectional design and explored the performance of urinary KIM-1 for the detection of AKI without language or sample size restrictions. Two reviewers (Shao X and Tian L) used the EndNote bibliography manager to check the titles and abstracts of all citations and then retrieved and rescreened full-text articles. The reference lists of reviewed full-text articles were checked for fear losing additional relevant studies.
Data extraction and quality assessment
Data were extracted by two authors (Shao X and Tian L). From each study, the following information was received: first author, country of origin, year of publication, study design, sample size, population setting (patients after cardiopulmonary bypass surgery, patients after cardiac catheterization, patients admitted to the intensive care unit and patients admitted to emergency department), and patient characteristics (age, sex, and baseline serum creatinine), as well as definition of AKI and number of patients who developed AKI. In addition, data extraction regarding KIM-1 including the laboratory assay used, the reported biomarker value unit (ng per milliliter vs ng per milligram of creatinine), and the timing of the measurement. In relation to the outcomes of interest, the optimal cutoff thresholds, as defined by the authors of the individual studies, the area under the curve (AUC) for the receiver operating characteristic (ROC) and the true-positive, true-negative, false-positive, and false-negative values were recorded.
The methodological quality of studies was individually evaluated by two authors (Shao X and Tian L, two doctors in department of nephrology and were systematically trained for meta-analysis) pivoting on the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) instrument [18], a quality assessment tool specifically developed for systematic reviews of diagnostic accuracy studies to assess bias in the study [19], including 14 questions (each of which is scored as yes, no, or unclear).
Data Synthesis and Analysis
We conducted STATA version 12 and Meta-Disc to analysis the data [20]. Summary sensitivities, specificities, positive and negative likelihood ratio and diagnostic odds ratios (DOR) with their 95% confidence intervals (95% CIs) were obtained using random effect models with DerSimonian Laird methods or fixed effect models depending on the level of heterogeneity of the study group [21]. Forest plots of sensitivities, specificities and DORs were presented. Moreover, AUC-ROC values with 95% CI were combined. An AUC-ROC>0.70 defines a useful risk predictor [22].
Heterogeneity in meta-analysis indicates the degree of variability in results across studies. It was appraised using Q test P value and the I2 index which revealed thresholds for low (25%–49%), moderate (50%–74%), and high (>75%) values [23]. When substantial heterogeneity was found to be present (I2>50%), There were three strategies used to assess possible heterogeneity: Spearman correlation coefficient test which can reveal the presence of threshold effect (differences in sensitivities and specificities occurring because of different cut-offs used in different studies to define a positive test result), subgroup analysis and summary ROC analysis [23].
In addition, we used a funnel plot of effect size against its SE to evaluate the publication bias [24]. The funnel plot should be asymmetric when there is publication bias and symmetric in the case of on publication bias. Since the funnel plot approach is limited by the requirement for a range of studies with varying size, we adopted Egger's linear regression test, which measures the funnel plot asymmetry on the natural logarithm scale of DOR (p<0.05 was believed representative of statistically significant publication bias).
Results
Search Results and Study Characteristics
The primary search revealed 2887 publications from variable databases. Firstly, 339 repeated studies were rejected in our research. Then the majority was sifted out based on titles or abstracts. There were 97 articles evaluated in detail. Finally, 11 studies were accepted [9], [10], [25], [26], [27], [28], [29], [30], [31], [32], [33] (Figure 1). Characteristics of the individual studies are listed in Table 1. There were 5 prospective cohort studies, 4 case-control studies and 2 cross-sectional studies adopted in this meta-analysis. Among these studies, Han [10], Genc [32] and Sarafidis [33] studies enrolled children or infants and others all enrolled adults [10], [32], [33]. All these studies were published from 2008 to 2013, varied in sample size (from 40 to 1635), and involved patients in different clinical settings. Four studies focused on patients underwent cardiopulmonary bypass surgery, and the other included cardiac catheterization, ICU patients, emergency department patients and critically ill patients. All samples are mentioned to store at −80°C and blinding of investigators was documented in seven studies with four articles not known. As listed in Table 1, variable definitions of AKI were adopted in the individual studies. Urinary KIM-1 level was measured in all studies by a commercial enzyme-linked immune sorbent assay (ELISA) or microbead-based ELISA and micro-sphere-based Luminex xMAP technology.
Figure 1. Flow diagram for the review process and outcomes of inclusion and exclusion.
Table 1. Characteristics of Studies Included in the Meta-analysis.
| Study | Country | Design | N | Patients with AKI | Population settings | Age (y) | Males | Baseline Scr (mg/dL) | Definition of AKI | Blinding of investigators |
| Genc (2013)[32] | Turkey | Prospective cohort study | 48 | 18 | NICU | 29.9bw | 27 | 1.01a | Scr levels after hour 60 of life >1.3 mg/dL or an increase inScr by either >0.3 mg/dL or an increase of ≥50% from baseline | NR |
| Nickolas (2012)[31] | USA | A Multicenter Prospective Cohort Study | 1635 | 96 | Emergency department | 64.4 | 855 | 0.9 | ≥50% increase in SCr more than 3 days and patients exposed to stimuli | YES |
| Naggar (2012)[27] | Egypt | case-control study | 40 | 21 | Critically ill patients | 51.75a | 16 | 0.9a | RIFLE criteria | NR |
| Sarafidis (2012)[33] | Greece | case-control study | 35 | 8 | NICU | 38.3bw | 21 | 1.13a | sCr ≥1.5 mg/dl for >24 h or rising values >0.3 mg/dl from day of life 1 | YES |
| Endre (2011)[30] | New Zealand Australia USA | Prospective observational study | 528 | 147 | patients in ICU | 60 | 210 | 1.0 | ≥50% or 0.3mg/dL above baseline pCr of the first sample in the ICU | YES |
| Ferguson (2010)[28] | USA | cross-sectional study | 134 | 92 | General hospital ward; critical care setting; Precatheterization | 62.6a | 81 | NR | ≥50% increase in SCr | NR |
| Liang (2010)[29] | China | case-control study | 122 | 30 | CPB surgery | 30a | NR | 1.01a | RIFLE criteria | YES |
| Liangos (2009)[9] | USA | Prospective cohort study | 103 | 13 | CPB surgery | 68 | 74 | 1.1 | ≥50% increase in SCr within 72 h | YES |
| Han (2009)[26] | USA | prospective cohort study | 90 | 36 | CPB surgery | 63.6a | 61 | 1.04a | increase in Scr of ≥0.3 mg/dl or 2- to 3-fold within 72 h | YES |
| Han (2008)[10] | USA | case-control study | 40 | 20 | CPB surgery | 3.2a | 60 | 0.425a | ≥50% increase in SCr | YES |
| Vaidya (2008)[25] | USA | a cross-sectional study | 204 | 102 | General hospital ward; critical care setting; cardiac catheterization | 56.9a | 102 | AKI 1.7–10.0 non-AKI 0.4–1.4 | ≥50% increase in SCr | NR |
Abbreviations: AKI, acute kidney injury; CPB, cardiopulmonary bypass; CS, cardiac surgery; SCr, serum creatinine; ICU, intensive care unit; NR, not reported; NICU, neonatal intensive care unit; KIM-1, kidney injury molecule 1; RIFLE, risk, injury, failure, loss, end-stage renal disease; USA, United States of America.
a Mean baseline SCr level (mg/dL) or age (y,year).
b Gestational age (w,week).
Quality Assessment
Spearman correlation coefficient of these 11 articles was 0.082 (P = 0.811), suggesting there was no significant threshold effect. The methodological quality of the studies according to the QUADAS tool is summarized in Table S1 (provided as supplementary material). Egger test showed p = 0.003, suggesting there was publication bias. Index test results were interpreted with knowledge of the results of the reference standard for the clinical diagnosis of AKI (based on serum creatinine).
Data synthesis
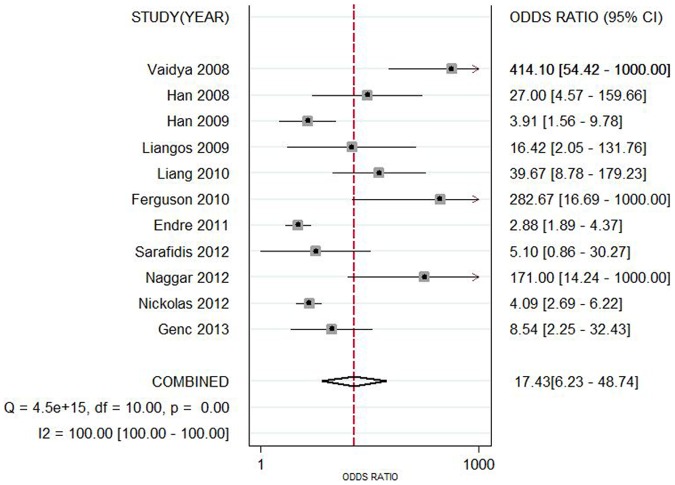
Data in the 11 eligible studies were extracted and showed in Table 2, including true-positive, false-positive, false-negative, and true-negative values; various optimal cutoff values for urinary KIM-1; sensitivities; specificities; AUC-ROC (95% CI); Assess method; time of measurement for the diagnosis of AKI. The estimated sensitivity of urinary KIM-1 for the diagnosis of AKI was 74.0% (95% CI, 61.0%–84.0%), and specificity was 86.0% (95% CI, 74.0%–93.0%), with a DOR of 17.43(95% CI, 6.23–48.74) shown as Figure 2 and Figure 3. There was strong heterogeneity both in sensitivity and specificity between studies as evidenced by an I2 indexes of 88.54% (83.04–94.04%) and 93.62% (91.04–96.20%) respectively. SROC results showed AUC of urinary KIM-1 was 0.86(0.83–0.89), suggesting that efficiency of KIM-1 for AKI diagnosis was considerable (Figure 4). Funnel plots showed there was publication bias with significant difference (Figure 5).
Table 2. Performance of Urinary KIM-1 for AKI diagnosis in Studies Included in the meta-analysis.
| study | NO. of patients | Assay method | Timing of measurement | KIM-1 Cutoff | Sensitivity (95%CI) | Specificity (95%CI) | AUC-ROC (95% CI) | |||
| TP | FP | FN | TN | |||||||
| Genc (2013)[32] | 13 | 7 | 5 | 23 | ELISA | within day of life 2 | ≥0.5 ng/mg increase with 3 days | 0.733 (NR) | 0.769 (NR) | 0.791 (NR) |
| Nickolas (2012)[31] | 50 | 323 | 46 | 1216 | chemiluminescent microparticle immunoassay | within 12 h after patient enrollment | 2.817 ng/ml | 0.52 (NR) | 0.79 (NR) | 0.71 (0.65–0.76) |
| Naggar (2012)[27] | 19 | 1 | 2 | 18 | ELISA | within 1 day after patient enrollment | NR | 0.909 (NR) | 0.9254 (NR) | NR |
| Sarafidis (2012)[33] | 6 | 10 | 2 | 17 | ELISA | within day of life 1 | 0.928 ng/mg | 0.8 (NR) | 0.625 (NR) | 0.608 (NR) |
| Endre (2011)[30] | 59 | 72 | 88 | 309 | microsphere-based Luminex xMAP technology | on entry to ICU | 1.86 ng/mg | 0.40 (0.32–0.48) | 0.81 (0.77–0.85) | 0.66 (0.61–0.72) |
| Ferguson (2010)[28] | 71 | 0 | 21 | 42 | microbead-based sandwich ELISA | NR | 1.7 ng/mg | 0.77 (0.67–0.85) | 1 (0.92–1) | 0.89 (0.82–0.94) |
| Liang (2010)[29] | 28 | 24 | 2 | 68 | ELISA | 6 h post-CPB | 1.5 ng/mg | 0.933 (NR) | 0.739 (NR) | 0.881 (0.810–0.933) |
| Liangos (2009)[9] | 12 | 38 | 1 | 52 | ELISA | 2 h post-CPB | 0.42 ng/mg | 0.92 (NR) | 0.58 (NR) | 0.78 (0.64–0.91) |
| Han (2009)[26] | 19 | 12 | 17 | 42 | ELISA | post-operation immediately | 1.2 ng/mg | 0.5143 (NR) | 0.7778 (NR) | 0.68(0.58–0.78) |
| Han (2008)[10] | 15 | 2 | 5 | 18 | ELISA | 12 h post-CPB | 2.0 ng/mg | 0.74 (NR) | 0.9 (NR) | 0.83 (0.67–0.96) |
| Vaidya (2008)[25] | 82 | 1 | 20 | 101 | microbead-based assay | NR | 1.73 ng/mg | 0.8 (NR) | 0.99 (NR) | 0.93 (NR) |
Abbreviations: AKI, acute kidney injury; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; CPB, cardiopulmonary bypass; FN, false negative; FP, false positive; ICU, intensive care unit; KIM-1, kidney injury molecule 1; NR, not reported; TN, true negative; TP, true positive; ELISA, enzyme-linked immunosorbent assay.
Figure 2. Forest plots of the pooled sensitivity (A) and specificity (B) of urine kidney injury molecule 1 level in predicting acute kidney injury across all settings.
The black squares in the gray squares and the horizontal lines represent the point estimate and 95% confidence interval (CI), respectively. The dotted line represents the pooled estimate, and the diamond shape represents the 95% CI of the pooled estimate.
Figure 3. Forest plot of the pooled diagnostic odds ratio of urine kidney injury molecule 1 level in predicting acute kidney injury across all settings.
The black squares in the graysquares and the horizontal lines representthe point estimate and 95% confidence interval(CI), respectively. The dotted line represents the pooled estimate, and the diamond shape represents the 95% CI of the pooled estimate.
Figure 4. Hierarchical summary receiver perating characteristic (SROC) plots of urine kidney injury molecule 1 level to predict acute kidney injury across all settings.
The curve is represented by the straight line; each of the analyzed studies is represented by a circle; the point estimate to which summary sensitivity (SENS) and specificity (SPEC) correspond is represented by the diamond shape, and the respective 95% confidence intervals, by the dashed line, whereas the 95% confidence area in which a new study will be located is represented by the dotted line. Abbreviation: AUC, area under the curve.
Figure 5. Funnel plot for the evaluation of potential publication bias in diagnosis of KIM-1 for AKI.
We also performed subgroup analysis by population settings, study design, age, measurement method, and blinding or not. Data showed that consistency of non-prospective studies had significantly been decreased, which because half of them enrolled established AKI patients (Table 3). Detection by ELISA was much more sensitive than no-ELISA method with significantly decreased consistency coefficient, as shown in Table 3. Moreover, age could also affect the consistency, studies in infants or children showed only 23.8% of I2 index (Table 3). With regard to the patient population settings, patient underwent CPB showed remarkably increased sensitivity compared with ICU and other kinds of patients. Three studies tested KIM-1 between 2 h to 12 h after CPB, except one immediately after CPB. The sensitivity of combined three studies was 87.0% (77.0%–94.0%), and specificity was 68.0% (61.0%–75%), as shown in Table 4. There was moderate heterogeneity between studies as evidenced by an I2 index of 46.2% and Q test P = 0.1559, indicating right detection time was the most important factor affecting diagnosis of AKI (Figure 4).
Table 3. Subgroup analysis based on different standard.
| Studies | Sensitivity(95%CI) | Specificity(95%CI) | +LR(95%CI) | −LR(95%CI) | DOR(95%CI) | AUC | |
| All studies(11) | 0.74(0.61–0.84) | 0.86(0.74–0.93) | 5.29(2.59–10.79) | 0.30(0.19–0.48) | 17.43(6.23–48.74) | 0.86 | |
| I-square(%) | 88.54% | 93.62% | 85.26% | 91.08% | 100% | ||
| Patient population | Cardiac surgery(4) | 0.75(0.65–0.83) | 0.70(0.64–0.76) | 2.88(1.91–4.35) | 0.25(0.08–0.72) | 14.4(3.95–52.54) | 0.8512 |
| I-square(%) | 83.20% | 76.90% | 64.30% | 82.10% | 66.20% | ||
| ICU and others(7) | 0.62(0.57–0.66) | 0.81(0.79–0.82) | 4.31(2.19–8.48) | 0.35(0.21–0.57) | 16.15(5.41–48.20) | 0.7703 | |
| I-square(%) | 91.30% | 90.70% | 88.70% | 92.20% | 88.10% | ||
| Study design | prospective(5) | 0.49(0.44–0.55) | 0.78(0.77–0.80) | 2.33(2.03–2.69) | 0.62(0.49–0.77) | 3.86(2.72–5.47) | 0.7947 |
| I-square(%) | 80.60% | 81.20% | 0.00% | 55.90% | 22.20% | ||
| non-prospective(6) | 0.81(0.76–0.85) | 0.87(0.83–0.91) | 10.41(2.44–45.00) | 0.21(0.17–0.28) | 59.05(15.02–232) | 0.9229 | |
| I-square(%) | 24.90% | 91.00% | 92.10% | 2.70% | 64.70% | ||
| Age | Adults(8) | 0.63(0.58–0.67) | 0.79(0.78–0.81) | 2.96(1.95–4.49) | 0.36(0.23–0.57) | 12.44(4.87–31.55) | 0.8459 |
| I-square(%) | 91.50% | 91.80% | 86.00% | 91.40% | 86.40% | ||
| Infants or chidren(3) | 0.8(0.67–0.89) | 0.86(0.75–0.93) | 5.86(1.97–417.41) | 0.26(0.14–0.48) | 25.99(5.37–125.8) | 0.8465 | |
| I-square(%) | 23.80% | 45.50% | 56.00% | 30.10% | 56.10% | ||
| Measurement method of KIM-1 | ELISA(8) | 0.77(0.71–0.82) | 0.75(0.70–0.79) | 3.53(2.05–6.08) | 0.27(0.16–0.45) | 18.79(6.74–52.39) | 0.8807 |
| I-square(%) | 66.60% | 85.20% | 81.50% | 71.20% | 66.50% | ||
| non-ELISA(3) | 0.55(0.50–0.61) | 0.80(0.79–0.82) | 3.65(1.59–8.39) | 0.46(0.25–0.83) | 9.30(2.50–34.58) | 0.1599 | |
| I-square(%) | 95.30% | 94.70% | 92.40% | 95.20% | 92.70% | ||
| Blinding or not | Blinding(7) | 0.54(0.49–0.59) | 0.78(0.76–0.80) | 2.48(2.09–2.96) | 0.5(0.35–0.69) | 6.13(3.34–11.28) | 0.8102 |
| I-square(%) | 87.80% | 78.10% | 33.40% | 75.10% | 66.80% | ||
| non-Blinding(4) | 0.79(0.74–0.84) | 0.95(0.91–0.98) | 20.99(1.51–292.37) | 0.22(0.17–0.29) | 100.02(10.12–988.14) | 0.8813 | |
| I-square(%) | 0.00% | 85.80% | 89.90% | 12.30% | 79.30% | ||
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; ICU, intensive care unit; KIM-1, kidney injury molecule 1; ELISA, enzyme-linked immunosorbent assay; +LR, positive likelihood ratio; -LR, negative likelihood ratio; DOR, diagnositic odds ratio.
Table 4. Subgroup analysis based on patient type and detection time.
| Studies | Sensitivity(95%CI) | Specificity (95%CI) | LR+(95%CI) | LR-(95%CI) | DOR(95%CI) | AUC | |
| All studies(11) | 0.74(0.61–0.84) | 0.86(0.74–0.93) | 5.29(2.59–10.79) | 0.30(0.19–0.48) | 17.43(6.23–48.7) | 0.86 | |
| I-square(%) | 88.54% | 93.62% | 85.26% | 91.08% | 100% | ||
| Patient population | Cardiac surgery(4) | 0.75(0.65–0.83) | 0.70(0.64–0.76) | 2.88(1.91–4.35) | 0.25(0.08–0.72) | 14.4(3.95–52.54) | 0.8512 |
| I-square(%) | 83.20% | 76.90% | 64.30% | 82.10% | 66.20% | ||
| 2h after surgery(3) | 0.87(0.77–0.94) | 0.68(0.61–0.75) | 3.21(1.75–5.90) | 0.18(0.07–0.42) | 28.53(10.43–78.07) | 0.9109 | |
| I-square(%) | 46.20% | 82.00% | 79.80% | 32.50% | 0.00% | ||
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; +LR, positive likelihood ratio; -LR, negative likelihood ratio; DOR, diagnositic odds ratio.
Discussion
Recently, the serum creatinine, common standard to test AKI, displayed numerous limitations which affect the early diagnosis and prognosis of AKI [6]. In order to enhance the ability to predict the occurrence of AKI and facilitate timely introduction of AKI-specific therapies, more and more effort were made to discover novel urinary biomarkers prior to serum creatinine [6].
In the present meta-analysis, we generalized all published studies which have examined the performance characteristics of one of the urinary biomarker, KIM-1, to fully evaluate the diagnostic value. Of all the identified cohort studies of patients at risk for AKI, 11 could be meta-analyzed for the diagnosis of AKI, whereby urinary KIM-1 showed good performance characteristics with high sensitivity and specificity, especially in patients undergoing cardiac surgery.
To our knowledge, it might be a novel meta-analysis which assessed the diagnostic value of KIM-1 for AKI. Interestingly, pooled analysis of the studies exhibited relatively much more sensitive role in predicting AKI, with combined 74.0% sensitivity and 86.0% specificity. However, the inconsistency factor was very huge. Accordingly, the source of heterogeneity present in this analysis on AKI early detection was analyzed using subgroup analysis by study design, population settings, time point of measurements of patients and other factors. The results showed that age, population settings and time of measurement of KIM-1 were the main source of heterogeneity. Furthermore, subgroup analysis by other factors did not expressively alter the diagnostic significance of KIM-1.
Urinary KIM-1 was reported to be affected by detection time [34]. As shown in this study, only 50% sensitivity was shown immediately after CPB [26]. Two studies showed more than 90% sensitivity when KIM-1 was tested 2 h and 6 h after CPB [9], [29]. However, the sensitivity was decreased to 74% 12 h after CPB [10]. These results suggested that right time adoption could remarkably increase the success of AKI diagnosis. More studies with a larger sample size are thus needed to further elucidate the diagnostic value of KIM-1 for AKI.
Urinary KIM-1 level also has been shown to correlate with fibrotic changes in experimental models of chronic kidney disease [35]. Seven studies included in this paper were involved in ICU patients, Emergency Department patients and general hospital ward, which might combined some chronic disease or other unknown diseases. Owing to the possibly fundamental expression of KIM-1, the elevation of KIM-1 in AKI detection might be affected to some extent. However, we cannot completely rule out other possibility that some other covariates might potentially account for part of the heterogeneity. Furthermore, patients with AKI in ICU, Emergency Department or general hospital ward had distinct causes including ischemia, sepsis, contrast media and renal toxin, which can affect the expression of KIM-1.Yun Huang et al had revealed that urinary KIM-1 level was the highest in ischemic acute tubule necrosis patients [15]. However, due to scant data we cannot assess the performance of KIM-1 in different causes of AKI.
On the basis of age subgroup analysis, we detected that urinary KIM-1 had better diagnostic accuracy in infants or children than in adults. We deduced the significant comorbid conditions, such as hypertension, diabetes mellitus and atherosclerotic, are more prevailing in adults and may influence urinary KIM-1 concentrations. Therefore, the performance of urinary KIM-1 for AKI in infants/children may be more reliable than in adults. In addition, the measurement of KIM-1 needs standardization as a series of independently assays have been used. The chief methodology of KIM-1 measurement is based on ELISA, however, varible antibodies, reagents and reaction designs lead to a difference of KIM-1 test performance which leads to difficulties in data comparison.
Although we examined the diagnostic value of urinary KIM-1 level as a predictor of early AKI, we were unable to assess whether this marker adds value to other clinical factors or to a panel of urinary markers of kidney injury, which might be a limitation.
Furthermore, the limitations of this meta-analysis cannot be ignored. Limitations of the analysis include the small number of studies, heterogeneity in study populations with a broad range of clinical settings, variable definitions of AKI (reference standard test), variable biomarker cutoff values (index test), variable KIM-1 assays and variable duration of follow-up. One additional important limitation is the current AKI vriteria using serum creatinine as a diagnositc index, which cannot reveal subclinical kidney injury and might underscore the specificity of KIM-1. Moreover, a certain degree of publication bias was found in the analysis concerning KIM-1 for AKI diagnosis according to Egger test and funnel plot. The DOR estimate in our meta-analysis might be overestimated because of publication and reporting bias.
Recently, more and more experts suggested that combination of multiple biomarkers to form a biomarkers panel was an optimal way to detect AKI more efficiently and accurately [36], [37]. One study enrolled in this meta-analysis also reported on the combination application of the urinary biomarkers KIM-1 and interleukin-18 for early detection of AKI [29]. For early predicting progressive AKI, the estimated sensitivity was 81.8% and specificity was 83.8%.
In conclusion, the present meta-analysis demonstrates that measurement of urinary KIM-1, a proximal tubular injury marker, appears to be a relatively good discrimination for the diagnosis of AKI in hospital-based cohorts of patients at risk of AKI, especially in patients underwent cardiac surgery after CPB 2 h to 12 h, suggesting KIM-1 might be a specific predictor for early AKI. The potential diagnostic and prognostic value of this biomarker needs to be validated further in large cohort studies and clinical settings.
Supporting Information
Methodological quality of the 11 studies included in the meta-analysis.
(DOC)
PRISMA statement of the meta-analysis.
(DOC)
Four phase Flow diagram of the meta-analysis.
(DOC)
Funding Statement
This study was supported in part by the National Basic Research Program of China 973 Program No. 2012CB517600 (No. 2012CB517602). The study was also sponsored by the National Natural Science Foundation of China (81102700 and 81373865), National “Twelfth Five-Year” Plan for Science & Technology Support No. 2011BAI10B00 and by grants 10JC1410100, 12401906400 and 13401906100 from the Science and Technology Commission of Shanghai Municipality, China. Program ZXSNXD-CC-ZDYJ002 from the Shanghai Health Bureau and Funding scheme for training young teachers in Colleges and Universities in Shanghai were also included. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ronco C, Levin A, Warnock DG, Mehta R, Kellum JA, et al. (2007) Improving outcomes from acute kidney injury (AKI): Report on an initiative. Int J Artif Organs 30: 373–376. [PubMed] [Google Scholar]
- 2. Webb S, Dobb G (2007) ARF, ATN or AKI? It’s now acute kidney injury. Anaesth Intensive Care 35: 843–844. [DOI] [PubMed] [Google Scholar]
- 3. Li PK, Burdmann EA, Mehta RL (2013) Acute kidney injury: Acute kidney injury—global health alert. Nat Rev Nephrol 9: 133–135. [DOI] [PubMed] [Google Scholar]
- 4. Lattanzio MR, Kopyt NP (2009) Acute kidney injury: new concepts in definition, diagnosis, pathophysiology, and treatment. J Am Osteopath Assoc 109: 13–19. [PubMed] [Google Scholar]
- 5. Perrone RD, Madias NE, Levey AS (1992) Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38: 1933–1953. [PubMed] [Google Scholar]
- 6. Slocum JL, Heung M, Pennathur S (2012) Marking renal injury: can we move beyond serum creatinine? Transl Res 159: 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adiyanti SS, Loho T (2012) Acute Kidney Injury (AKI) biomarker. Acta Med Indones 44: 246–255. [PubMed] [Google Scholar]
- 8. Edelstein CL (2008) Biomarkers of acute kidney injury. Adv Chronic Kidney Dis 15: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, et al. (2009) Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 14: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, et al. (2008) Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV (2002) Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244. [DOI] [PubMed] [Google Scholar]
- 12. Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H (2010) Kidney injury molecule-1 in renal disease. J Pathol 220: 7–16. [DOI] [PubMed] [Google Scholar]
- 13. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, et al. (1998) Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142. [DOI] [PubMed] [Google Scholar]
- 14.Bonventre JV (2008) Kidney Injury Molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl 241: 78–83. [DOI] [PubMed]
- 15. Huang Y, Don-Wauchope AC (2011) The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: a systematic review. Inflamm Allergy Drug Targets 10: 260–271. [DOI] [PubMed] [Google Scholar]
- 16. Coca SG, Yalavarthy R, Concato J, Parikh CR (2008) Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, et al. (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 354: 1896–1900. [DOI] [PubMed] [Google Scholar]
- 18. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whiting P, Rutjes AW, Dinnes J, Reitsma J, Bossuyt PM, et al. (2004) Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 8: 1–234. [DOI] [PubMed] [Google Scholar]
- 20. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A (2006) Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, et al. (2002) Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, et al. (2008) Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 1: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han WK, Wagener G, Zhu Y, Wang S, Lee HT (2009) Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naggar GFE, Srogy HAEL, Fathy SM (2012) Kidney Injury Molecule - 1 (KIM- 1): an early novel biomarker for Acute Kidney Injury (AKI) in critically – ill patients. Life Sci J 9: 3937–3943. [Google Scholar]
- 28. Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, et al. (2010) Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int 77: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang XL, Liu SX, Chen YH, Yan LJ, Li H, et al. (2010) Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case-control study. Biomarkers 15: 332–339. [DOI] [PubMed] [Google Scholar]
- 30. Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, et al. (2011) Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 79: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, et al. (2012) Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 59: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Genc G, Ozkaya O, Avci B, Aygun C, Kucukoduk S (2013) Kidney injury molecule-1 as a promising biomarker for acute kidney injury in premature babies. Am J Perinatol 30: 245–252. [DOI] [PubMed] [Google Scholar]
- 33. Sarafidis K, Tsepkentzi E, Agakidou E, Diamanti E, Taparkou A, et al. (2012) Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol 27: 1575–1582. [DOI] [PubMed] [Google Scholar]
- 34. Barrera-Chimal J, Perez-Villalva R, Rodriguez-Romo R, Reyna J, Uribe N, et al. (2013) Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int 83: 93–103. [DOI] [PubMed] [Google Scholar]
- 35. Bhavsar NA, Kottgen A, Coresh J, Astor BC (2012) Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sprenkle P, Russo P (2013) Molecular markers for ischemia, do we have something better then creatinine and glomerular filtration rate? Arch Esp Urol 66: 99–114. [PubMed] [Google Scholar]
- 37. Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, et al. (2013) Performance of Kidney Injury Molecule-1 and Liver Fatty Acid-Binding Protein and Combined Biomarkers of AKI after Cardiac Surgery. Clin J Am Soc Nephrol 8: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methodological quality of the 11 studies included in the meta-analysis.
(DOC)
PRISMA statement of the meta-analysis.
(DOC)
Four phase Flow diagram of the meta-analysis.
(DOC)