Abstract
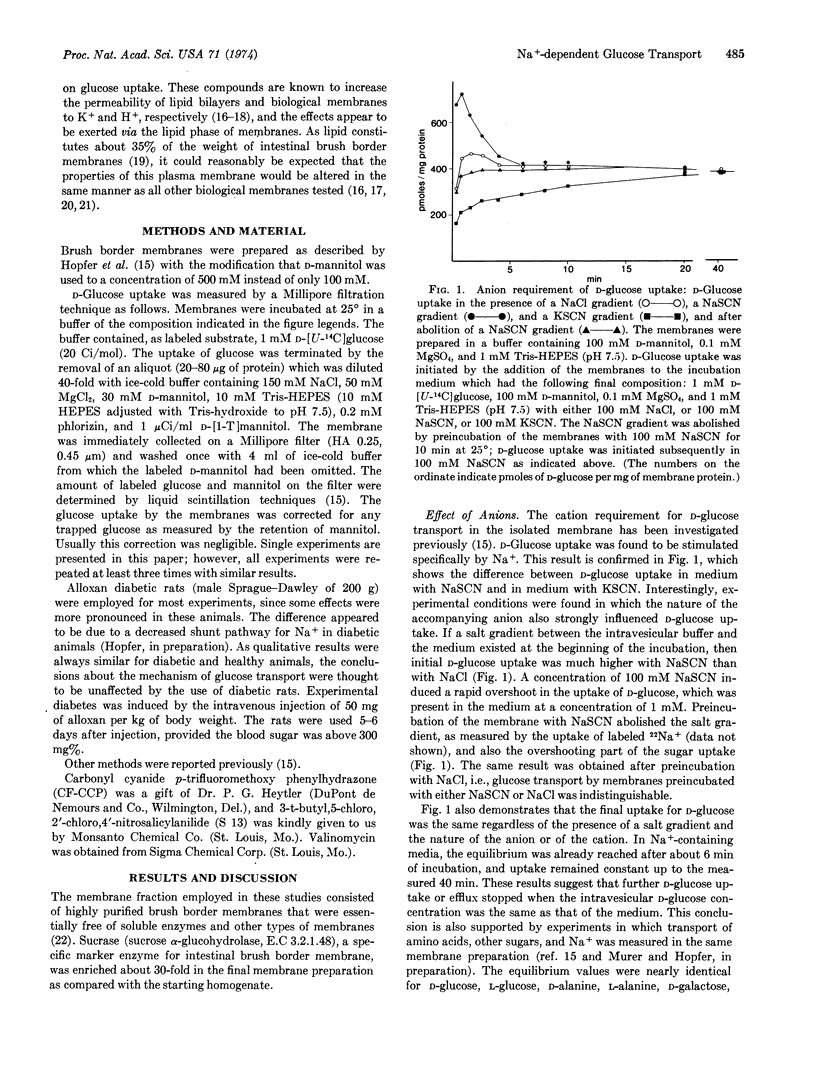
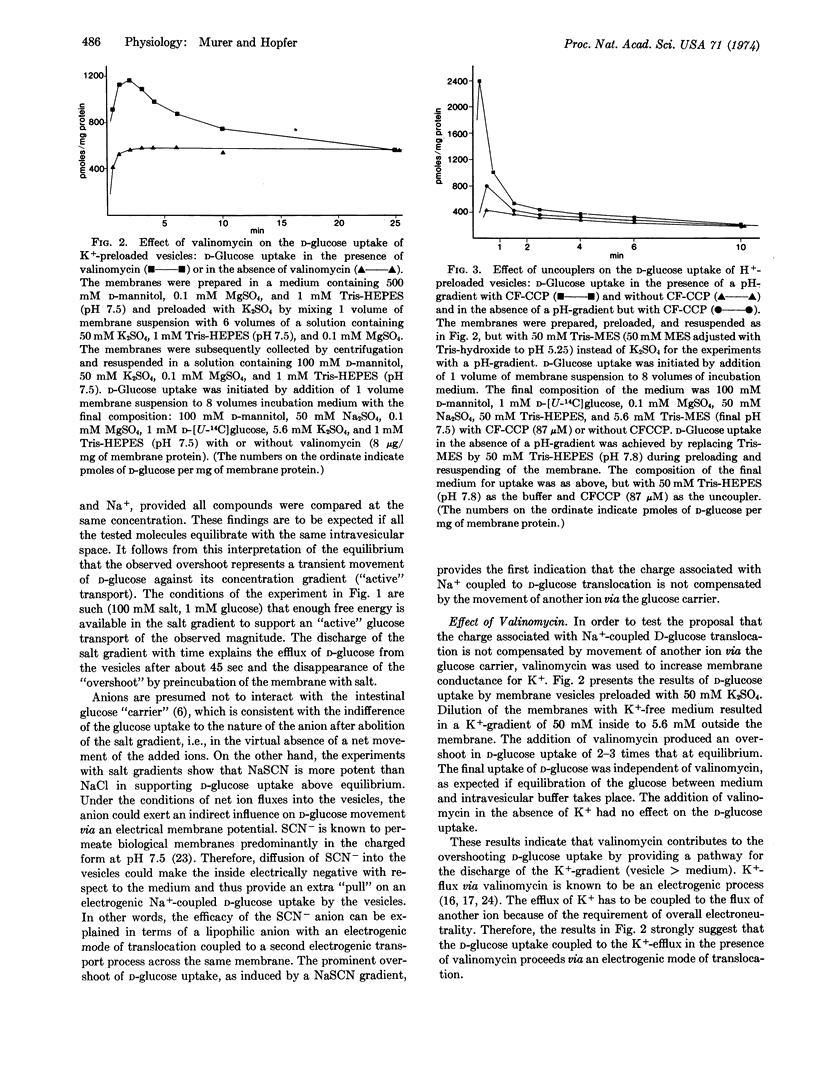
Na+-coupled D-glucose transport was studied in isolated membrane vesicles from intestinal brush borders. Concentration gradients of SCN-, K+, and H+ were established between the intravesicular solution and the incubation medium and their influence on D-glucose uptake from the medium was measured. A gradient (medium > vesicle) of NaSCN, but not of KSCN, produced a transient overshoot of D-glucose uptake above the equilibrium level. Similarly, an increase of the membrane conductance with valinomycin (K+-conductance) or with uncoupling agents of oxidative phosphorylation (H+-conductance) induced an overshooting D-glucose uptake, provided a (vesicle > medium) K+-gradient or a H+-gradient, respectively, was present in each case. The transient overshoot is evidence that D-glucose was taken up against its concentration gradient (up to 10-fold). The gradients of SCN-, K+ (in the presence of valinomycin), and H+ (in the presence of uncouplers) are thought to contribute to the “driving” force for this “active” D-glucose transport by changing the electrical potential across the vesicle membrane and thus making the inside more negative (with respect to the medium). These experiments, therefore, provide evidence that the Na+-coupled D-glucose translocation across the brush border membrane is an electrogenic process, i.e., the positive charge associated with Na+ is not compensated by the co-movement of an anion or the counter-movement of a cation via the glucose “carrier”. The results imply that an electrical potential across the brush border membrane may play an important role in determining the transport of D-glucose by intact cells.
Keywords: membrane vesicles, active transport, electrogenic
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Tieffenberg M., Tosteson D. C. The effect of valinomycin on the ionic permeability of thin lipid membranes. J Gen Physiol. 1967 Dec;50(11):2527–2545. doi: 10.1085/jgp.50.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Eggenton J. Membrane potentials of epithelial cells in rat small intestine. J Physiol. 1972 Dec;227(1):201–216. doi: 10.1113/jphysiol.1972.sp010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Epstein W., Silver S. Valinomycin-induced uptake of potassium in membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1488–1492. doi: 10.1073/pnas.68.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihler I., Cybulsky R. Sugar transport at the basal and lateral aspect of the small intestinal cell. Biochim Biophys Acta. 1973 Mar 16;298(2):429–436. doi: 10.1016/0005-2736(73)90370-2. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z., THALE M. Effect of ionic environment on intestinal sugar transport. J Physiol. 1960 Apr;151:59–65. [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Curran P. F., Hajjar J. J., Glynn I. M. The sodium-alanine interaction in rabbit ileum. Effect of alanine on sodium fluxes. J Gen Physiol. 1970 Mar;55(3):297–308. doi: 10.1085/jgp.55.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Tanaka K., Isselbacher K. J. Lipid composition of the isolated rat intestinal microvillus membrane. Biochem J. 1968 Aug;109(1):51–59. doi: 10.1042/bj1090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Fujita M., Ota H., Kawai K., Matsui H., Nakao M. Differential isolation of microvillous and basolateral plasma membranes from intestinal mucosa: mutually exclusive distribution of digestive enzymes and ouabain-sensitive ATPase. Biochim Biophys Acta. 1972 Aug 9;274(2):336–347. doi: 10.1016/0005-2736(72)90181-2. [DOI] [PubMed] [Google Scholar]
- Förster H., Hoos I. The excretion of sodium during the active absorption of glucose from the perfused small intestine of rats. Hoppe Seylers Z Physiol Chem. 1972 Jan;353(1):88–94. doi: 10.1515/bchm2.1972.353.1.88. [DOI] [PubMed] [Google Scholar]
- Förster H., Menzel B. Untersuchung der intestinalen Glucoseresorption bei künstlich erhöhter Blutglucosekonzentration. Z Ernahrungswiss. 1972 Mar;11(1):10–23. doi: 10.1007/BF02019580. [DOI] [PubMed] [Google Scholar]
- Förster H., Menzel B. Zur Bestimmung der Michaelis-Konstanten für die intestinale Glucoseresorption bei Untersuchungen in vivo. Z Ernahrungswiss. 1972 Mar;11(1):24–39. doi: 10.1007/BF02019581. [DOI] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Gibb L. E., Eddy A. A. An electrogenic sodium pump as a possible factor leading to the concentration of amino acids by mouse ascites-tumour cells with reversed sodium ion concentration gradients. Biochem J. 1972 Oct;129(4):979–981. doi: 10.1042/bj1290979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kimmich G. A. Active sugar accumulation by isolated intestinal epithelial cells. A new model for sodium-dependent metabolite transport. Biochemistry. 1970 Sep 15;9(19):3669–3677. doi: 10.1021/bi00821a004. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A. Coupling between Na+ and sugar transport in small intestine. Biochim Biophys Acta. 1973 Apr 3;300(1):31–78. doi: 10.1016/0304-4157(73)90011-7. [DOI] [PubMed] [Google Scholar]
- Lyon I., Sheerin H. E. Studies on transmural potentials in vitro in relation to intestinal absorption. VI. The effect of sugars on electrical potential profiles in jejunum and ileum. Biochim Biophys Acta. 1971 Oct 12;249(1):1–14. doi: 10.1016/0005-2736(71)90078-2. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- RIKLIS E., QUASTEL J. H. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958 Mar;36(3):347–362. [PubMed] [Google Scholar]
- Rose R. C., Schultz S. G. Studies on the electrical potential profile across rabbit ileum. Effects of sugars and amino acids on transmural and transmucosal electrical potential differences. J Gen Physiol. 1971 Jun;57(6):639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman D. A., Rector F. C., Jr, Fordtran J. S. The role of intraluminal sodium in glucose absorption in vivo. J Clin Invest. 1972 Apr;51(4):876–885. doi: 10.1172/JCI106882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Frizzell R. A. An overview of intestinal absorptive and secretory processes. Gastroenterology. 1972 Jul;63(1):161–170. [PubMed] [Google Scholar]
- Schultz S. G., Fuisz R. E., Curran P. F. Amino acid and sugar transport in rabbit ileum. J Gen Physiol. 1966 May;49(5):849–866. doi: 10.1085/jgp.49.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. F., Armstrong W. M. Effect of transported solutes on membrane potentials in bullfrog small intestine. Am J Physiol. 1971 Jul;221(1):194–201. doi: 10.1152/ajplegacy.1971.221.1.194. [DOI] [PubMed] [Google Scholar]
- Wright E. M. The origin of the glucose dependent increase in the potential difference across the tortoise small intestine. J Physiol. 1966 Jul;185(2):486–500. doi: 10.1113/jphysiol.1966.sp007998. [DOI] [PMC free article] [PubMed] [Google Scholar]


