Abstract
Ageing and cancer is often associated with altered T cell distributions and this phenomenon has been suggested to be the main driver in the development of immunosenescence. Memory phenotype PD-1+ CD4+ T cells accumulate with age and during leukemic development, and they might account for the attenuated T cell response in elderly or diseased individuals. The transcription factor C/EBPα has been suggested to be responsible for the accumulation as well as for the senescent features of these cells including impaired TCR signaling and decreased proliferation. Thus modulating the activity of C/EBPα could potentially target PD-1+ CD4+ T cells and consequently, impede the development of immunosenescence. To exploit this possibility we tested the importance of C/EBPα for the development of age-dependent PD-1+ CD4+ T cells as well as its role in the accumulation of PD-1+ CD4+ T cells during leukemic progression. In contrast to earlier suggestions, we find that loss of C/EBPα expression in the lymphoid compartment led to an increase of PD-1+ CD4+ T cells specifically in old mice, suggesting that C/EBPα repress the accumulation of these cells in elderly by inhibiting their proliferation. Furthermore, C/EBPα-deficiency in the lymphoid compartment had no effect on leukemic development and did not affect the accumulation of PD-1+ CD4+ T cells. Thus, in addition to contradict earlier suggestions of a role for C/EBPα in immunosenescence, these findings efficiently discard the potential of using C/EBPα as a target for the alleviation of ageing/cancer-associated immunosenescence.
Introduction
Immunosenescence is a phenomenon commonly observed in elderly people, cancer patients and individuals with chronic infections such as HIV. This condition is due to gradual deterioration of the immune system and causes attenuated response to infections and vaccinations [1], [2], [3]. One of the main contributors to immunosenescence is the functional changes that occur within the T cell compartment, which results in an inefficient immune response. In the immune system of elderly people there is a shift in the CD4+ T cell populations, which leads to fewer naïve T cells and more memory phenotype (MP) T cells and is suggested to be part of the delayed and diminished immune response often found in elderly people [4], [5], [6].
There is compelling evidence that a potent immune response is crucial in protecting and preventing tumor formation [7]. For example, mice deficient in Perforin or INF-γ are more susceptible to tumor formation upon carcinogen exposure, which suggests that an efficient immune response is critical in order to protect against carcinogenesis [8], [9]. A better understanding of the factors involved in the age-dependent and tumor promoting defects in immune cells are important as this may lead to the development of strategies aimed at improving the immune response in the elderly.
The programmed cell death (PD)-1-expressing MP CD4+ T cells have recently drawn some attention, since this population is increasing both during ageing and disease. Furthermore, these cells respond poorly to stimulation [10], [11], [12], [13] and it has therefore been suggested that the attenuated immune response in elderly is a consequence of the accumulation of MP PD-1+ CD4+ T cells. In accordance, blockade of the PD-1 pathway or CTLA-4, another T cell inhibitory molecule, rejuvenates the immune response and improves the overall survival in certain settings [14], [15], [16], [17]. With this in mind, targeting the PD-1+ CD4+ T cell population potentially holds great promise for restoring the immune system in elderly.
Recently, PD-1+ CD4+ T cells were shown to display high expression of the transcription factor CCAAT/enhancer-binding protein alpha (C/EBPα) [12], [18], which is primarily expressed in common myeloid progenitors (CMPs) and required for their differentiation into granulocyte/monocyte progenitors (GMPs) [19], [20]. C/EBPα drives myeloid differentiation by inducing lineage-affiliated gene expression programs and by promoting cell cycle exit [21], [22], [23], [24], 25,26,27. Through these dual activities C/EBPα have the functional properties to act as a master switch between uncommitted proliferating progenitors and cell cycle arrested differentiated cells [28], [29].
In addition to its expression in PD-1+ MP CD4+ T cells, which is suggestive of a function in age/cancer-induced immunosenescence, C/EBPα is also expressed in double negative (DN) 1–4 T cells [18], [30]. However, the overall importance of C/EBPα in T cell development or function has not been addressed previously.
In the present work, we set out to explore the possibility of rejuvenating the immune system by targeting C/EBPα in the PD-1+ CD4+ T cell compartment. In order to do so, we investigated the importance of C/EBPα in lymphopoiesis and in particular in the development of PD-1+ CD4+ T cells. In addition, as the frequencies of PD-1+ CD4+ T cells have previously been suggested to affect the development of leukemia, we tested if leukemic progression was altered in a C/EBPα-deficient context.
Materials and Methods
Mice
Animals were maintained at the Department of Experimental Medicine at University of Copenhagen and housed according to institutional guidelines. Cebpa fl/fl and CD2iCre mice have been described previously [31], [32]. All experimental animals had been backcrossed for at least 10 generations to the C57BL/6 background.
Ethics Statement
All animal work was done with approval from the Danish Animal Ethical Committee. This study was approved by the review board at the Faculty of Health Sciences, University of Copenhagen (P12-049).
Flow Cytometry and Cell Sorting
Thymi from 7–9 weeks old mice were collected and homogenized in PBS +3% FCS. 10×106 cells were incubated with 2 µL Fc receptor block (anti-CD16/32, BD Biosciences) in 100 µL PBS +3% FCS on ice for 5 min, washed in cold PBS +3% FCS and stained with antibodies for flow cytometry. T cell progenitors were stained with antibodies against lineage (Ter119, Mac1, Gr1, B220, CD19, NK1.1, CD3e, CD4, and CD8; e-Bioscience), CD44 (e-Bioscience), and CD25 (BD Biosciences). Mature T cells were stained with CD4, CD3e, and CD8a (e-Bioscience).
BM cells were collected from femur and tibiae by crushing the bones in PBS +3% FCS. Spleens were homogenized in PBS +3% FCS and red blood cells were lysed in BD PharmLyse (BD Biosciences) according to manufactures instructions. B cell progenitors in the BM were stained with antibodies against lineage (Ter119, Gr1, Mac1, CD3e, CD4, NK1.1 (e-Bioscience)), B220 (e-Bioscience), CD43 (BD Biosciences), CD19 (BD Biosciences), IgM (BD Biosciences), AA4.1 (e-Bioscience) and 7-AAD (1 µg/mL, Invitrogen). To detect mature hematopoietic cells, BM and spleen cells were stained with antibodies against Ter119, NK1.1, Mac1, B220, CD8a, CD4, PD-1, CD44, CD62L (e-Bioscience) and DAPI (0,2 µg/mL, Invitrogen). Spleens from leukemic mice were stained with antibodies against CD4 and PD-1 (e-Bioscience), and DAPI (0,2 µg/mL, Invitrogen) was used to discriminate live from dead cells. Samples were run on a LSRII (BD Biosciences) or sorted on a FACSAria (BD Biosciences). Analyses were performed using the software FlowJo (Tree Star Inc.).
Transplantation Assays
Sublethally irradiated (500 Gy) 12–15 weeks old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice were transplanted intravenously through the tail vein with 10.000 GFP positive MLL-ENL primary leukemia cells. Recipient mice were maintained on antibiotics for 2 weeks after transplantation.
Recombination PCR
To detect the extent of recombination, DNA was purified from relevant cell types and genotyped using the following primers: 5′-CCGCGGCTCCACCTCGTAGAAGTCG-3′, 5′-CCACTCACCGCCTTGGAAAGTCACA-3′ and 5′-GTCCTGCAGCCAGGCAGTGTCC-3′. Band size of 355 bp indicates floxed allele and band size of 560 bp indicates deleted allele.
qRT-PCR
Total RNA was isolated from PD1+ CD4+ and PD1- CD4+ spleen cells using the RNeasy Mini Kit (Qiagen) and cDNA was generated using the Superscript III Kit (Invitrogen). Gene expression was quantified with real-time quantitative PCR (LightCycler 480, Roche) using Sybr Green (Invitrogen). Expression levels of target genes were normalized to β-actin. Primers used: Ccnd1 sense 5′-GAACAAGCTCAAGTGGAACC-3′, Ccnd1 antisense 5′-CTTCAATCTGTTCCTGGCAG-3′, Cebpa sense 5′-TGAGAAAAATGAAGGGTGCAG-3′, Cebpa antisense 5′-CGGG ATCTCAGCTTCCTGT-3′, c-Myc sense 5′-CGAAACTCTGGTGCATAAACT G-3′, c-Myc antisense 5′-GAACCGTTCTCCTTAGCTCTCA-3′, Satb1 sense 5′-ACTGAAACGAG CCGGAATC-3′, Satb1 antisense 5′-CGGAGGATTTCAGAAAGCAA-3′, Sostdc1 sense 5′-AACAGCACCCTGAATCAAGC-3′, Sostdc1 antisense 5′-CAGCCCACTTGAACTCGAC-3′, Spp1 sense 5′-CCCGGTGAAAGTGACTGATT-3′, Spp1 antisense 5′-TTCTTCAGAGGACACAGCATTC-3′, β-actin sense 5′-TCTTCCAGCCTTCCTTCCT-3′ and β-actin antisense 5′-TGCTAGGGCTGTGAT CTCCT-3′, Ifng sense 5′- ATCTGGAGGAACTGGCAAAA –3′, Ifng antisense 5′-TTCAAGACTTCAAAGAGTCTGAGG-3′, Il2 sense 5′- GCTGTTGATGGACCTACAGGA-3′, Il2 antisense 5′-TTCAATTCTGTGGCCTGCTT-3′, Prf1 sense 5′-GCTCCCACTCCAAGGTAGC-3′, Prf1 antisense 5′-TTTGTACCAGGCGAAAACTGT-3′, Gzmb sense 5′- TGCTGCTAAAGCTGAAGAGTAAG-3′, Gzmb antisense 5′-CGTGTTTGAGTATTTGCCCATTG-3′.
T cell Proliferation Assay
Spleen cells were harvested and red blood cells were lysed with PharmLyse (BD Biosciences) according to manufactures protocol. The splenocytes (50*106/mL) were resuspended in RPMI 1640 medium containing 5 µM Carboxy Fluoroscein Sucinimidyl Ester (CFSE; CellTrace, Invitrogen), incubated for 5 min at room temperature, and then washed 3 times with RPMI1640+10% FCS. Next, the splenocytes were washed in PBS, resuspended in 0,5 mM EDTA and incubated for 5 min at room temperature. Cells were then washed in PBS and resuspended in RPMI 1640 medium supplemented with 10% FCS and 2 µg/mL anti-CD28 antibody (Clone 37.51; e-Bioscience). Subsequently, 1–2×105 cells were seeded in round-bottomed 96-well plates, which had been coated with 1 µg/mL anti-CD3e antibody (Clone 145-2C11; e-Bioscience) for 2 hours at 37°C and washed with PBS. Following 72-hours incubation at 37°C, the cells were washed in PBS, incubated in 0,5 mM EDTA for 5 min at room temperature to remove aggregates, and washed again in PBS. The splenocytes were then stained with antibodies against CD4, washed, and resuspended in PBS +3% FCS prior to flow cytometry analysis on a FACSCalibur (BD Biosciences). Analysis was performed using the software FlowJo (Tree Star Inc.).
In vivo BrdU Incorporation Assay
Mice were injected with 2 mg BrdU (BD Biosciences) and three hours later the spleens were harvested. Splenocytes were stained with antibodies against CD4 (BD Biosciences) and BrdU according to manufactures protocol (BD Biosciences), run on a LSRII and analyzed using the FlowJo software (Tree Star Inc.).
Results
Age- and Leukemia-dependent Increase of C/EBPα Expressing PD-1+ CD4+ T cells
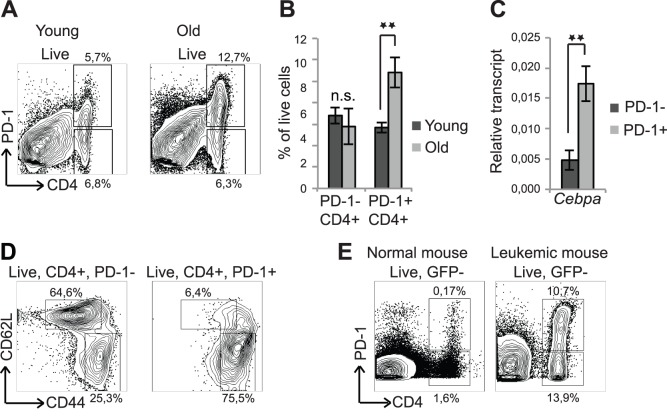
To test the involvement of PD-1+ CD4+ T cells in the depression of the T cell immune response, we first investigated the occurrence of the PD-1+ CD4+ T cells during ageing and leukemia in mice. We therefore harvested spleens from 2- and 14 months old C57BL/6 mice and found the frequencies of PD-1+ CD4 T cells to be increased by 2-fold when comparing old (14 months) with young (2 months) animals, whereas the frequencies of PD-1- CD4+ T cells remained constant (Figure 1A,B). Furthermore, we found the Cepba transcript to be prominently upregulated in PD-1+ CD4 vs. PD-1- CD4+ T cells (Figure 1C). The PD-1+CD4+ T cell population was mainly restricted to the CD4+, CD44high, CD62Llow MP population, whereas the PD-1- CD4+ T cells predominantly were CD44low, CD62Lhigh (Figure 1D).
Figure 1. Increase in PD-1+ CD4+ T cells during ageing and in development of AML.
(A) Spleen cells from 2 months old and 14 months old mice were stained with antibodies against CD4 and PD-1. (B) Quantification of the data in (A) is presented as mean +/− SD, (young: n = 3, old: n = 7). (C) PD-1- CD4+ and PD-1+ CD4+ splenic T cells from 14 months old mice were analyzed for expression of Cebpa normalized to β-actin by qRT-PCR. Data are presented as mean +/− SEM, (n = 7). (D) Spleens from 3 months old mice were stained for CD4, PD-1, CD44 and CD62L. A representative example is shown (n = 5). (E) The spleens from healthy (age-matched, non-transplanted) and leukemic mice were analyzed for PD-1+ CD4+ T cells. **P<0.01; n.s.: not significant.
Mice with BCR/ABL driven chronic myeloid leukemia display an increase in PD-1+ CD4+ T cells [18] and to test whether this observation could be expanded to other myeloid malignancies such as acute myeloid leukemia (AML) we transplanted bone marrow (BM) cells from an MLL-ENL driven AML mouse into sublethally irradiated recipients. Analysis of the T cell compartment of these animals showed that, similar to CML, AML led to an increase of PD-1+ CD4+ T cells in the spleen (Figure 1E).
Collectively, these findings support previous observations [18] by demonstrating that the accumulation of C/EBPα-expressing PD-1+ CD4+ T cells is a general phenomenon in ageing as well as in leukemia, and therefore implicate C/EBPα as a potential driver of this process.
C/EBPα is not Important for Maturation of T cells in Young Mice
Whereas C/EBPα is known to play an essential role in the myeloid compartment, its function in the lymphoid lineage has not been investigated in great detail presumably due to its low expression in these cells (Figure S1A) [33], [34]. We therefore generated Cebpa fl/fl;CD2iCre mice in which C/EBPα is selectively ablated in B- and T cells starting from the common lymphoid progenitor [31], [32] (Figure S1B). The lymphoid compartment of these animals developed normally as assessed by body weight, spleen weight and the cellularities of hematopoietic organs in both young and old mice (Figure S1C,D), suggesting that C/EBPα is dispensable for lymphopoiesis.
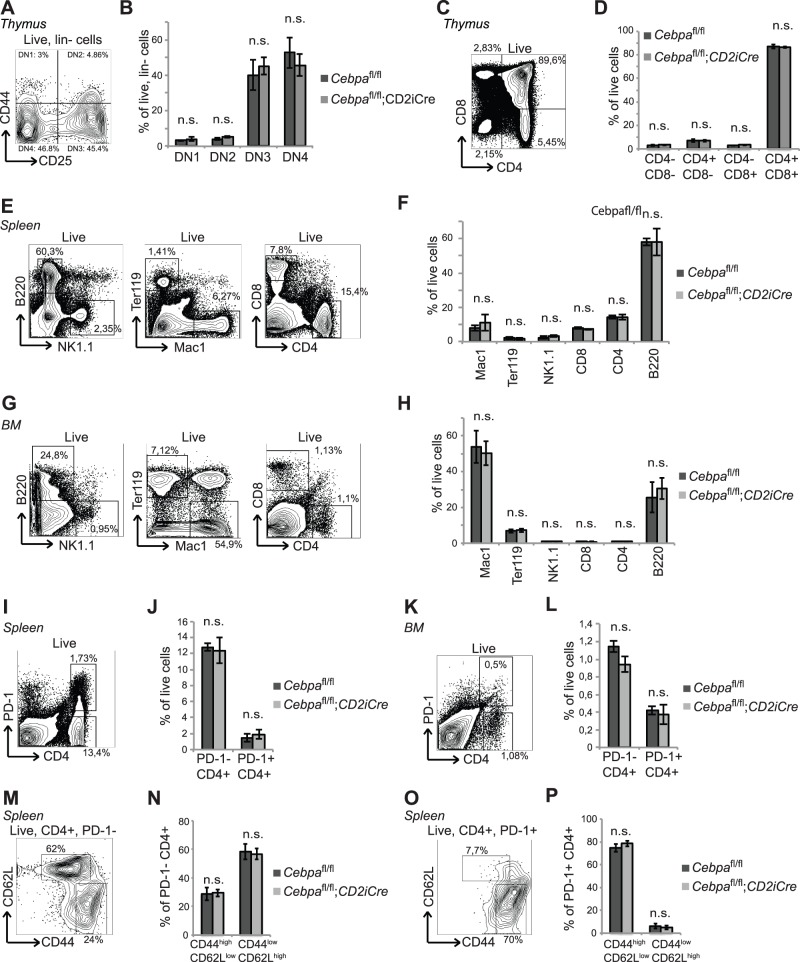
To test if loss of C/EBPα impacted on the early events of T cell development, we isolated thymi from 2 months old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice and analyzed the distribution of thymic T cell subsets. We were unable to detect any alternations in the frequencies of DN1-4 cells, CD3+ as well as single and double positive CD4+/CD8+ T cells (Figure 2A–D and Figure S1E), indicating that C/EBPα is dispensable for early T cell development.
Figure 2. C/EBPα is dispensable for the differentiation of lymphoid cells in young mice.
(A and B) Analysis of DN1-DN4 T cells and (C and D) CD4+ and/or CD8+ T cells in thymi from 2 months old Cebpa fl/fl (n = 4) and Cebpa fl/fl;CD2iCre (n = 5) mice. (E and F) Analysis of mature hematopoietic lineages in spleens from 2 months old Cebpa fl/fl (n = 3) and Cebpa fl/fl;CD2iCre (n = 4) mice. (G and H) Analysis of the mature hematopoietic lineages in BMs from 2 months old Cebpa fl/fl (n = 5) and Cebpa fl/fl;CD2iCre (n = 6) mice. (I and J) Analysis of the PD-1+ CD4+ T cells in spleens from 2 months old Cebpa fl/fl (n = 3) and Cebpa fl/fl;CD2iCre (n = 4) mice. (K and L) Analysis of the PD-1+ CD4+ T cells in BMs from 2 months old Cebpa fl/fl (n = 5) and Cebpa fl/fl;CD2iCre (n = 6) mice. (M–O) Analysis of CD44 and CD62L subsets within PD-1- and PD1+ CD4+ T cells in spleens from 3 months old Cebpa fl/fl (n = 5) and Cebpa fl/fl;CD2iCre (n = 5) mice. The contour plots are examples from Cebpa fl/fl mice. Mean +/− SD; n.s. = not significant.
Since loss of C/EBPα had no effect on the differentiation of early T cell progenitors, we next examined if C/EBPα-deficiency would affect the differentiation or proliferation of mature B- or T cells in spleen or BM. However, no differences in the expansion of CD4+ and CD8+ T cells, B220+ B cells or NK1.1+ natural killer (NK) cells as well as Ter119+ erythroid cells, or Mac1+ granulocytic/monocytic cells were observed in Cebpa fl/fl;CD2iCre mice (Figure 2E–H). In addition, no differences in the distribution of pre-pro B cells, pro B cells, pre B cell and mature B cells were observed when comparing BM from Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice (Figure S2A,B).
Finally, we wanted to investigate whether C/EBPα was responsible for the formation of PD-1+ CD4+ T cells as suggested by Shimatani et al., and therefore analyzed if loss of C/EBPα affected the ontogeny of PD-1+ CD4+ T cells. Surprisingly, we did not detect any changes in the amount of PD-1+ CD4+ T cells (Figure 2I–L), showing that C/EBPα is not required for the formation of PD-1+ CD4+ T cells in neither spleen nor BM of young mice. Furthermore, the frequencies of CD44high/CD62Llow subsets within the PD-1- and PD-1+ CD4+ T cell compartment were unaffected by deletion of Cebpa (Figure 2M–P).
Taken together these results demonstrate that loss of C/EBPα in the lymphoid compartment does not affect the differentiation or distribution of B- and T cells in the thymus, BM, or spleen of young (2 months old) mice.
C/EBPα Inhibits the Accumulation of PD-1+ CD4+ T cells in the Spleen of Old Mice
Given the age-dependent accumulation of PD-1+ CD4+ T cells (Figure 1B), we next tested the possibility of C/EBPα playing a role in the formation or accumulation of PD-1+ CD4+ T cells in old (14 months) mice. Contrary to the observation in young mice, we detected an expansion of PD-1+ CD4+ T cells and a concomitant reduction of the PD-1- CD4+ T cells in the spleen of Cebpa fl/fl;CD2iCre mice (Figure 3A,B), suggesting that C/EBPα constrains the accumulation of PD-1+ CD4+ T cells specifically in aged mice.
Figure 3. C/EBPα restricts the formation of PD-1+ CD4+ T cells in spleens of old mice.
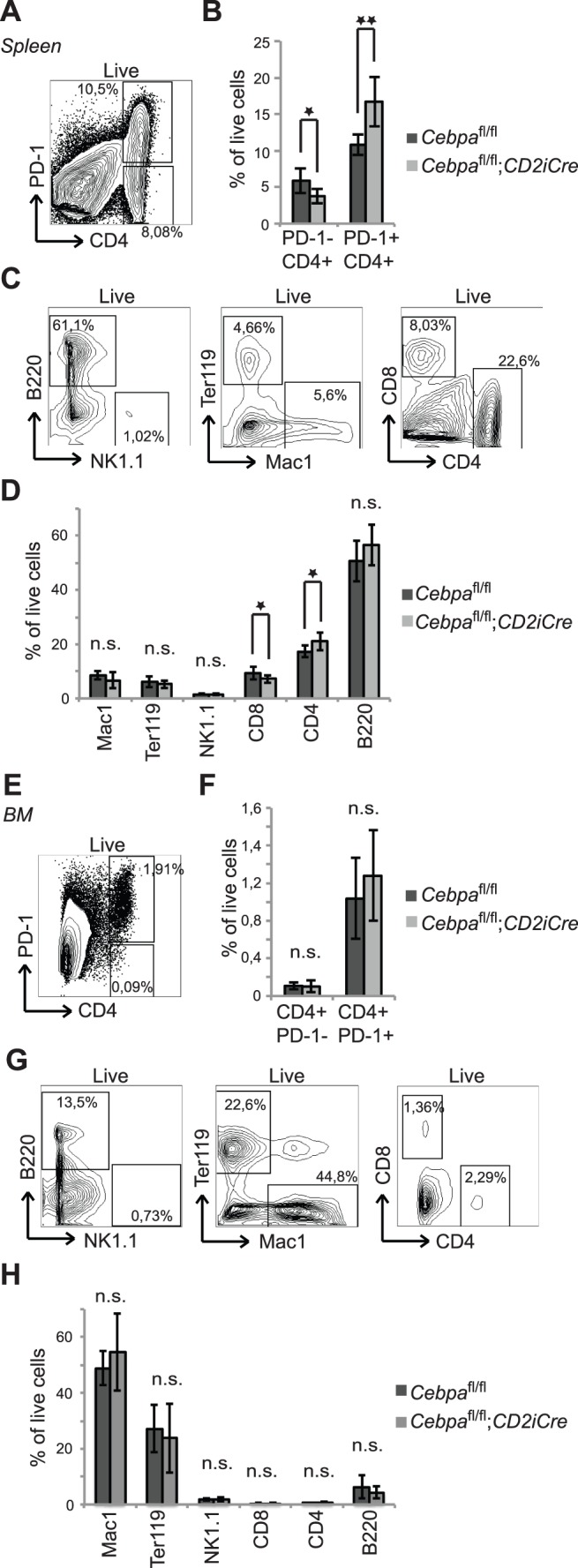
(A and B) Analysis of PD-1+ CD4+ T cells in spleens from 14 months old Cebpa fl/fl (n = 7) and Cebpa fl/fl;CD2iCre (n = 8) mice. (C and D) Analysis of mature hematopoietic lineages in spleens from 14 months old Cebpa fl/fl (n = 7) and Cebpa fl/fl;CD2iCre (n = 8) mice. (E and F) Analysis of the PD-1+ CD4+ T cells as well as the mature hematopoietic lineages (G and H) in BMs from 14 months old Cebpa fl/fl (n = 7) and Cebpa fl/fl;CD2iCre (n = 8) mice. The contour plots are examples from Cebpa fl/fl mice. Mean +/− SD; *P<0.05; **P<0.01; n.s.: not significant.
Since we observed this prominent function of C/EBPα in the PD-1+ CD4+ T cells in aged mice, we next tested if C/EBPα loss may also affect the differentiation of other lymphoid lineages in ageing mice. Interestingly, we find that loss of C/EBPα led to a minor, but significant, increase in CD4+ T cells accompanied by a minor decrease in CD8+ T cells in the spleen of aged mice. In contrast none of the other mature cell populations were affected by loss of C/EBPα (Figure 3C,D). Furthermore, we found the changes in frequencies of PD-1+ CD4+ T cells and the CD4/CD8 ratio to be restricted to the spleen as no differences were observed in BMs of 14 months old Cebpa fl/fl;CD2iCre mice (Figure 3E–H and Figure S2C).
Together, these results suggest that C/EBPα inhibits the accumulation of PD-1+ CD4+ T cells in an age-dependent manner.
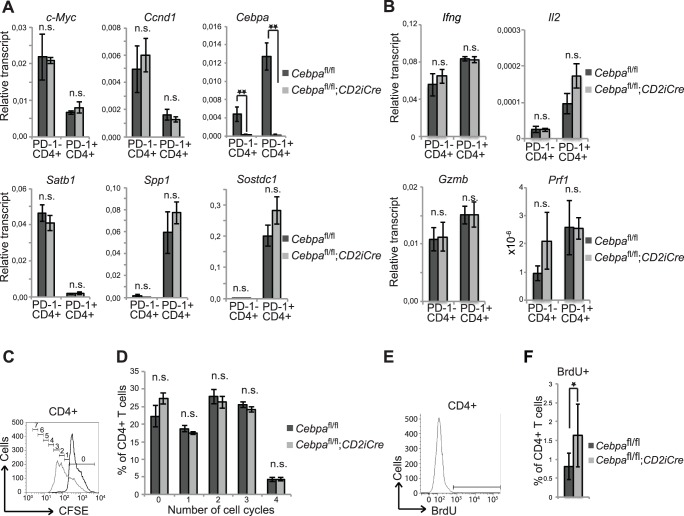
The in vivo Proliferation of Aged CD4+ T cells is Restricted by C/EBPα
The work by Shimatani et al. [18] suggested that the senescent features of PD-1+ CD4+ T cells were driven by a C/EBPα-dependent transcriptional program that included the transcriptional inhibition of the proliferation-promoting factors, c-Myc and Ccnd1, the induction of inflammatory factors such as of Spp1 and Sostdc1 and the inhibition of Satb1, which represses the expression of PD-1. To test this directly we sorted PD-1+ CD4+ T cells and PD-1- CD4+ T cells from 14 months old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice and analyzed the expression of these genes by qRT-PCR. In agreement with Shimatani et al. we observed a reduced expression of Satb1, c-Myc and Ccnd1 as well as an increased expression of Spp1 and Sostdc1 in PD-1+ vs. PD-1- CD4+ T cells, however the expression was not altered when C/EBPα was deleted (Figure 4A). Similarly, the expression of selected cytokines (Interferon-γ, IL-2, Granzyme B and Perforin) in PD-1+ and PD-1- CD4+ T cells were equally unaffected by the presence or absence of C/EBPα (Figure 4B). Hence, these findings clearly demonstrate that C/EBPα is not responsible for the transcriptional changes that distinguish PD-1+ and PD-1- CD4+ T cells.
Figure 4. C/EBPα inhibits proliferation of CD4+ T cells in old mice.
(A and B) Sorted PD-1- CD4+ and PD-1+ CD4+ T cells from 2 months old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice were assessed for transcripts for the indicated genes by qRT-PCR. The relative expression were normalized to β-actin and presented as mean of Cebpa fl/fl n = 7 and Cebpa fl/fl;CD2iCre n = 8+/− SEM. (C) CFSE labeled splenocytes from 2 months old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice were cultured with or without CD3 and CD28 antibodies and after 72 hours after the splenocytes were stained with CD4 antibody and assayed by flow cytometry. Black and grey lines indicate non-stimulated and stimulated cells, respectively. The numbers of cell divisions as given by the Proliferation feature of FlowJo are shown. (D) Quantification of CD4+ T cells in cell cycle 0–4. (Cebpa fl/fl n = 3, Cebpa fl/fl;CD2iCre n = 3). (E and F) Analysis of proliferation of CD4+ T cells in the spleen of 10 to 15 months old Cebpa fl/fl (n = 8) and Cebpa fl/fl;CD2iCre (n = 12) mice. The contour plot and histograms are examples from Cebpa fl/fl mice. Mean +/− SD; *P<0.05; **P<0.01; n.s.: not significant.
Given that C/EBPα can inhibit proliferation through several transcription-independent mechanisms [24], [25], [26], [27] and that ectopic expression of C/EBPα in CD4+ T cells lead to a decreased proliferation [18], we reasoned that C/EBPα might be responsible for the reduced ability of the PD-1+ CD4+ T cells to proliferate upon activation. To test this hypothesis, we harvested spleen cells from Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice, stained them with CFSE and stimulated with anti-CD3 and anti-CD28 antibodies to induce T cell proliferation. After 72-hours, the cells were stained with antibodies against CD4 and analyzed by flow cytometry for proliferating T cells (Figure 4C,D). Surprisingly, the frequencies of proliferating CD4+ T cells were not affected by loss of C/EBPα, showing that the reduced TCR-mediated proliferation of PD-1+ CD4+ T cells compared to PD1- CD4+ T cells reported by Shimatani et al., was not due to the differences in C/EBPα levels.
Finally, we wanted to examine whether C/EBPα had an impact on the proliferation of PD-1+ CD4+ T cells in vivo. We therefore measured the extent of BrdU incorporation in aged Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice and found a slight increase in the frequency of proliferating CD4+ T cells upon Cebpa deletion, which suggests that C/EBPα restricts the proliferation of aged CD4+ T cells (Figure 4E,F).
Taken together, these findings suggest that C/EBPα is responsible for the decreased proliferative capacity of PD-1+ CD4+ T cells, but that it does not affect their expression of signature genes or basal cytokines.
C/EBPα is Dispensable for the Accumulation of Senescent PD-1+ CD4+ T cells during Cancer Progression
One of the hallmarks in the development of leukemia is immunosenescence, which is believed to contribute to the failure of an effective immune response against cancer cells [35], [36], [37]. Since the PD-1+ CD4+ T cell population increases markedly in aged and leukemic animals (Figure 1), it is therefore likely to contribute to the compromised TCR-response in development of cancer and thus to influence the development of leukemia. Because C/EBPα plays a role in the accumulation of PD1+ CD4+ T cells in old mice, we hypothesized that loss of C/EBPα in the lymphoid compartment may affect the leukemia-dependent accumulation of PD-1+ CD4+ T cells and more importantly, the development of leukemia per se.
To test this hypothesis, we first generated primary AML by retrovirus mediated expression of the potent fusion oncogene MLL-ENL, and next transplanted the resulting GFP-positive leukemic cells into sub-lethally irradiated 12–15 weeks old Cebpa fl/fl and Cebpa fl/fl;CD2iCre secondary recipients (Figure 5A). Following leukemic development, spleens were harvested and the accumulation of PD-1- CD4+ T cells and PD-1+ CD4+ T cells in the recipients GFP negative immune system was analyzed. We detected no differences in the frequencies of PD-1+ CD4+ T cells between the two genotypes, suggesting that loss of C/EBPα has no impact on the accumulation of PD-1+ CD4+ T cells during leukemic development (Figure 5B,C). Moreover, loss of C/EBPα in the lymphoid compartment did not affect disease latency (Figure 5D). Collectively, these findings suggest that C/EBPα is dispensable for the accumulation of PD-1+ CD4+ T cells during disease development and that its loss have no impact on disease progression.
Figure 5. C/EBPα expression in PD-1+ CD4+ T cells does not affect the development of leukemia.
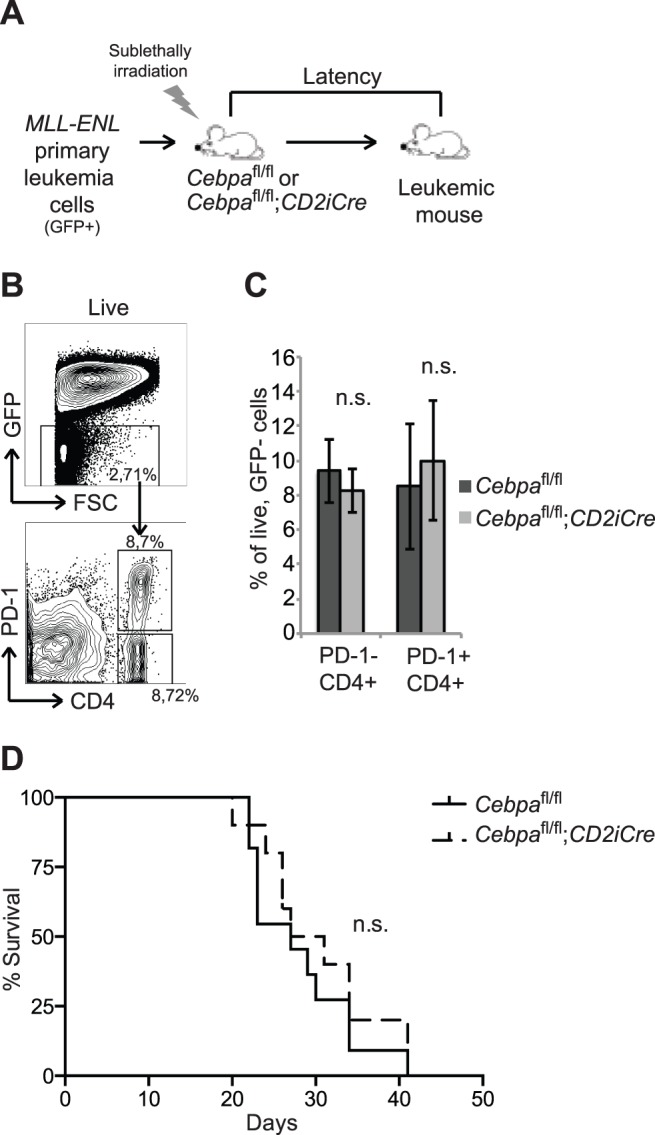
(A) Experimental setup. Sublethally irradiated Cebpa fl/fl and Cebpa fl/fl;CD2iCre recipient mice were transplanted with 10.000 MLL-ENL primary leukemia cells that express GFP. (B) Flow cytometry analysis of GFP- (non-leukemic cells) for the accumulation of PD-1- CD4+ or PD-1+ CD4+ T cells in the spleen as a consequence of leukemic development. (C) Quantification of the data in (B) (Cebpa fl/fl n = 6, Cebpa fl/fl;CD2iCre n = 9). (D) Survival of the leukemia-transplanted Cebpa fl/fl (n = 11) and Cebpa fl/fl;CD2iCre (n = 10) mice. The contour plots are examples from Cebpa fl/fl mice. Mean +/− SD. n.s. = not significant.
Discussion
C/EBPα is generally perceived as a myeloid-specific transcription factor involved in the regulation of myeloid vs. lymphoid lineage choices. Indeed, overexpression of C/EBPα in DN1-4 T cells or pre-B cells leads to their trans-differentiation into macrophages and C/EBPα-deficient hematopoietic stem cells upregulate lymphoid gene expression programs [30], [38], [39], [40]. Moreover, a subclass of leukemia patients with silenced C/EBPα expression develops AML with distinct T cell characteristics [41]. Although these findings are consistent with a requirement for the downregulation of C/EBPα during lymphoid development, C/EBPα has also been reported to be expressed in DN1-4 T cells as well as in PD-1+ CD4+ T cells suggesting that C/EBPα could have a previously unrecognized role in lymphopoiesis [18], [30].
In this study we therefore analyzed the potential function of C/EBPα in lymphopoiesis with particular emphasis on a role of C/EBPα in PD-1+ CD4+ T cells and in age/cancer-dependent immunosenescence. Whereas we were unable to detect any changes in the differentiation of B- and T cells in young C/EBPα-deficient mice, aged animals accumulated splenic CD4+ T cells accompanied by a corresponding reduction in CD8+ T cells upon deletion of Cebpa. Within the CD4+ T cell compartment, we detected a 50% increase of PD-1+ CD4+ MP T cells in aged C/EBPα-deficient mice, which suggests that C/EBPα potentially restricts the accumulation of these cells in elderly. This is most likely not due to its transcriptional activity since the PD-1+ CD4+ T cells in Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice have a similar unique transcriptional profile, but rather that C/EBPα appears to inhibit the in vivo proliferation of splenic CD4+ T cells.
There is substantial evidence that the occurrence of cancer increases with age. This could be attributed to many processes and pathways including a deregulation of the immune system with age. In particular, the T cell compartment is altered during ageing and is associated with the accumulation of PD-1+ CD4+ MP T cells, a cell population with several senescent features including low proliferation and reduced production of T cell lymphokines following TCR stimulation [10], [11], [12], [13]. Given the accumulation of PD-1+ CD4+ T cells in leukemic mice, as well as the finding that the T cell response can be restored using PD-1 blocking antibodies [15], PD-1+ CD4+ T cells have been suggested to be responsible for the increased susceptibility to disease in elderly [10], [17], [18].
Whereas it is well-established that increased expression of inhibitory molecules, such as PD-1 and CTLA-4 are involved in T cell senescence, the underlying transcriptional mechanisms have not been thoroughly investigated. Recent work has demonstrated a role for the transcriptional repressors BLIMP-1 and FOXP3 in the induction of inhibitory molecules during chronic infections [42], [43], [44], [45]. Thus, deletion of either Blimp-1 or Foxp3 alleviates senescent features of T cells [44], [46] demonstrating that these transcription factors are key regulators of immunosenescence. Apart from these findings, we lack knowledge regarding the transcriptional control of immunosenescence, which is needed if we are to develop new strategies for the restoration of T cell response in elderly. In this context it was recently suggested that immunosenescence, and in particular the senescent features of PD-1+ CD4+ T cells, could be attributed to the expression of C/EBPα in T cell subsets. In line with this it was hypothesized that C/EBPα was responsible for the formation of immunosenescent T cells [18], which in turn advocated for C/EBPα as a potential target for the reversal of immunosenescence.
Here we tested these hypotheses in a proper in vivo setting using genetically modified mice deficient in C/EBPα in the lymphoid compartment. Our data shows that rather than promoting the accumulation of PD-1+ CD4+ T cells in elderly, C/EBPα specifically limits the accumulation of PD-1+ CD4+ T cells by inhibiting their proliferation. Moreover, the status of C/EBPα does not affect the accumulation of PD1+ CD4+ T cells during leukemic progression and it does not affect the susceptibility to cancer. Interestingly, this may suggest that the accumulation of PD-1+CD4+ T cells in ageing and disease occurs through two independent mechanisms.
In addition to the age-related alterations within the CD4 compartment, it has recently been described that regulatory T cells accumulate in aged mice and cancer patients [47], [48], [49]. Regulatory T cells are important for limiting autoimmune responses, but increasing evidence also suggests a role in dampening the immune response against infections and tumour cells. Importantly, this can be overcomed by depleting regulatory T cells [5], [50], [51]. Whether C/EBPα plays a role in inhibiting the proliferation and accumulation of regulatory T cell subsets remains to be established.
In conclusion, we have analyzed the potential role of C/EBPα during lymphoid development and in immunosenescence. Whereas loss of Cebpa only had minor phenotypic impact on general lymphoid development, we find that C/EBPα specifically restricts the accumulation of PD-1+CD4+ T cells during ageing by inhibiting their proliferation. These findings contradict earlier suggestions that C/EBPα promotes immunosenescence and efficiently discard the potential of using C/EBPα as a target for the alleviation of ageing/cancer associated immunosenescence.
Supporting Information
(A) Gene expression of Cebpa in the different hematopoietic lineages. Data were obtained from the HemaExplorer (http://servers.binf.ku.dk/hemaexplorer/) [33], [34]. (B) PCR of recombination in the thymus, spleen and sorted cells from Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice. fl designates floxed allele, Δ designates deleted allele and * designates an unspecific band. (C) Spleen weight and cellularity of BM, thymus and spleen of 2 months old Cebpa fl/fl (n = 4–12) and Cebpa fl/fl;CD2iCre (n = 4–12) mice (D) Body weight, spleen weight and cellularity of BM and spleen of 14 months old Cebpa fl/fl (n = 7) and Cebpa fl/fl;CD2iCre (n = 8) mice. (E) Flow cytometry analysis of immature CD3+ T cells in the thymus from 2 months old Cebpa fl/fl (n = 4) and Cebpa fl/fl;CD2iCre (n = 5) mice. The contour plot is an example from a Cebpa fl/fl mouse. Mean +/− SD. n.s. = not significant.
(EPS)
Loss of C/EBPα does not affect early B cell differentiation. (A) Flow cytometry analysis of immature B cells in BMs of 2 months old or 14 months old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice. (B) Quantification of the data in (A) of 2 months old mice (Cebpa fl/fl n = 5, Cebpa fl/fl;CD2iCre n = 6). (C) Quantification of the data in (A) of 14 months old mice. (Cebpa fl/fl n = 5, Cebpa fl/fl;CD2iCre n = 6). The contour plots are examples from a Cebpa fl/fl mouse. Mean +/− SD. n.s. = not significant.
(EPS)
Acknowledgments
We thank Inge Damgaard for technical assistance and the Porse Lab for fruitful discussions.
Funding Statement
This study was supported through a center grant from the NovoNordisk Foundation - The Novo Nordisk Foundation Section for Stem Cell Biology in Human Disease. Foundation website: http://www.novonordiskfonden.dk; no grant number is available. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Linton PJ, Dorshkind K (2004) Age-related changes in lymphocyte development and function. Nature immunology 5: 133–139. [DOI] [PubMed] [Google Scholar]
- 2. Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, et al. (2009) Vaccination in the elderly: an immunological perspective. Trends in immunology 30: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virgin HW, Wherry EJ, Ahmed R (2009) Redefining chronic viral infection. Cell 138: 30–50. [DOI] [PubMed] [Google Scholar]
- 4. Kovaiou RD, Weiskirchner I, Keller M, Pfister G, Cioca DP, et al. (2005) Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. International immunology 17: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 5. Sharma S, Dominguez AL, Lustgarten J (2006) High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. Journal of immunology 177: 8348–8355. [DOI] [PubMed] [Google Scholar]
- 6. Chiu BC, Stolberg VR, Zhang H, Chensue SW (2007) Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mechanisms of Ageing and Development 128: 618–627. [DOI] [PubMed] [Google Scholar]
- 7. Lustgarten J, Dominguez AL, Thoman M (2004) Aged Mice Develop Protective Antitumor Immune Responses with Appropriate Costimulation. The Journal of Immunology 173: 4510–4515. [DOI] [PubMed] [Google Scholar]
- 8. Street SE, Cretney E, Smyth MJ (2001) Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 97: 192–197. [DOI] [PubMed] [Google Scholar]
- 9. van den Broek ME, Kägi D, Ossendorp F, Toes R, Vamvakas S, et al. (1996) Decreased tumor surveillance in perforin-deficient mice. The Journal of Experimental Medicine 184: 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M (2009) Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Experimental Gerontology 44: 517–522. [DOI] [PubMed] [Google Scholar]
- 11. Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C (2010) Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell 9: 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, et al. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354. [DOI] [PubMed] [Google Scholar]
- 14. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, et al. (2010) Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439: 682–687. [DOI] [PubMed] [Google Scholar]
- 16. Velu V, Titanji K, Zhu B, Husain S, Pladevega A, et al. (2009) Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH (2006) Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. The Journal of Experimental Medicine 203: 2223–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N (2009) PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proceedings of the National Academy of Sciences of the United States of America 106: 15807–15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, et al. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci U S A 94: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, et al. (1998) CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Molecular and cellular biology 18: 4301–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Porse BT, Bryder D, Theilgaard-Monch K, Hasemann MS, Anderson K, et al. (2005) Loss of C/EBP alpha cell cycle control increases myeloid progenitor proliferation and transforms the neutrophil granulocyte lineage. The Journal of experimental medicine 202: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porse BT, Pedersen TA, Hasemann MS, Schuster MB, Kirstetter P, et al. (2006) The proline-histidine-rich CDK2/CDK4 interaction region of C/EBPalpha is dispensable for C/EBPalpha-mediated growth regulation in vivo. Mol Cell Biol 26: 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porse BT, Pedersen TA, Xu X, Lindberg B, Wewer UM, et al. (2001) E2F repression by C/EBPalpha is required for adipogenesis and granulopoiesis in vivo. Cell 107: 247–258. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Goode T, Iakova P, Albrecht JH, Timchenko NA (2002) C/EBPalpha triggers proteasome-dependent degradation of cdk4 during growth arrest. The EMBO journal 21: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Iakova P, Wilde M, Welm A, Goode T, et al. (2001) C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Molecular cell 8: 817–828. [DOI] [PubMed] [Google Scholar]
- 26. Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, et al. (1997) CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Molecular and cellular biology 17: 7353–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ (1996) CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes & development 10: 804–815. [DOI] [PubMed] [Google Scholar]
- 28. Johnson PF (2005) Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118: 2545–2555. [DOI] [PubMed] [Google Scholar]
- 29. Schuster MB, Porse BT (2006) C/EBPalpha: a tumour suppressor in multiple tissues? Biochim Biophys Acta 1766: 88–103. [DOI] [PubMed] [Google Scholar]
- 30. Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T (2006) Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity 25: 731–744. [DOI] [PubMed] [Google Scholar]
- 31. de Boer J, Williams A, Skavdis G, Harker N, Coles M, et al. (2003) Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology 33: 314–325. [DOI] [PubMed] [Google Scholar]
- 32. Lee YH, Sauer B, Johnson PF, Gonzalez FJ (1997) Disruption of the c/ebp alpha gene in adult mouse liver. Molecular and cellular biology 17: 6014–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bagger FO, Rapin N, Theilgaard-Monch K, Kaczkowski B, Jendholm J, et al. (2012) HemaExplorer: a Web server for easy and fast visualization of gene expression in normal and malignant hematopoiesis. Blood 119: 6394–6395. [DOI] [PubMed] [Google Scholar]
- 34.Bagger FO, Rapin N, Theilgaard-Monch K, Kaczkowski B, Thoren LA, et al.. (2012) HemaExplorer: a database of mRNA expression profiles in normal and malignant haematopoiesis. Nucleic acids research. [DOI] [PMC free article] [PubMed]
- 35. Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG (2005) Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. The Journal of Clinical Investigation 115: 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Myers CE, Mirza NN, Lustgarten J (2011) Immunity, cancer and aging: lessons from mouse models. Aging and disease 2: 512–523. [PMC free article] [PubMed] [Google Scholar]
- 37. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 38. Di Tullio A, Vu Manh TP, Schubert A, Castellano G, Mansson R, et al. (2011) CCAAT/enhancer binding protein alpha (C/EBP(alpha))-induced transdifferentiation of pre-B cells into macrophages involves no overt retrodifferentiation. Proceedings of the National Academy of Sciences of the United States of America 108: 17016–17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie H, Ye M, Feng R, Graf T (2004) Stepwise reprogramming of B cells into macrophages. Cell 117: 663–676. [DOI] [PubMed] [Google Scholar]
- 40.Hasemann MS, Lauridsen FKB, Waage J, Jakobsen JS, Frank A-K, et al.. (2013) C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors. PLoS genetics In Press. [DOI] [PMC free article] [PubMed]
- 41. Figueroa ME, Wouters BJ, Skrabanek L, Glass J, Li Y, et al. (2009) Genome-wide epigenetic analysis delineates a biologically distinct immature acute leukemia with myeloid/T-lymphoid features. Blood 113: 2795–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Che KF, Shankar EM, Muthu S, Zandi S, Sigvardsson M, et al. (2012) p38 Mitogen-activated protein kinase/signal transducer and activator of transcription-3 pathway signaling regulates expression of inhibitory molecules in T cells activated by HIV-1-exposed dendritic cells. Molecular medicine 18: 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M (2011) Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Molecular medicine 17: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, et al. (2009) A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity 31: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, et al. (2006) FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126: 375–387. [DOI] [PubMed] [Google Scholar]
- 46. Williams LM, Rudensky AY (2007) Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology 8: 277–284. [DOI] [PubMed] [Google Scholar]
- 47. Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S (2006) CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. Journal of immunology 176: 6586–6593. [DOI] [PubMed] [Google Scholar]
- 48. Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, et al. (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. Journal of immunology 169: 2756–2761. [DOI] [PubMed] [Google Scholar]
- 49. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, et al. (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 9: 606–612. [PubMed] [Google Scholar]
- 50. Shimizu J, Yamazaki S, Sakaguchi S (1999) Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. Journal of immunology 163: 5211–5218. [PubMed] [Google Scholar]
- 51. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, et al. (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Research 59: 3128–3133. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Gene expression of Cebpa in the different hematopoietic lineages. Data were obtained from the HemaExplorer (http://servers.binf.ku.dk/hemaexplorer/) [33], [34]. (B) PCR of recombination in the thymus, spleen and sorted cells from Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice. fl designates floxed allele, Δ designates deleted allele and * designates an unspecific band. (C) Spleen weight and cellularity of BM, thymus and spleen of 2 months old Cebpa fl/fl (n = 4–12) and Cebpa fl/fl;CD2iCre (n = 4–12) mice (D) Body weight, spleen weight and cellularity of BM and spleen of 14 months old Cebpa fl/fl (n = 7) and Cebpa fl/fl;CD2iCre (n = 8) mice. (E) Flow cytometry analysis of immature CD3+ T cells in the thymus from 2 months old Cebpa fl/fl (n = 4) and Cebpa fl/fl;CD2iCre (n = 5) mice. The contour plot is an example from a Cebpa fl/fl mouse. Mean +/− SD. n.s. = not significant.
(EPS)
Loss of C/EBPα does not affect early B cell differentiation. (A) Flow cytometry analysis of immature B cells in BMs of 2 months old or 14 months old Cebpa fl/fl and Cebpa fl/fl;CD2iCre mice. (B) Quantification of the data in (A) of 2 months old mice (Cebpa fl/fl n = 5, Cebpa fl/fl;CD2iCre n = 6). (C) Quantification of the data in (A) of 14 months old mice. (Cebpa fl/fl n = 5, Cebpa fl/fl;CD2iCre n = 6). The contour plots are examples from a Cebpa fl/fl mouse. Mean +/− SD. n.s. = not significant.
(EPS)