Abstract
Expansins are cell wall proteins that promote cell wall loosening by inducing pH-dependent cell wall extension and stress relaxation. Expansins are required in a series of physiological developmental processes in higher plants such as seed germination. Here we identified an Arabidopsis expansin gene AtEXPA2 that is exclusively expressed in germinating seeds and the mutant shows delayed germination, suggesting that AtEXP2 is involved in controlling seed germination. Exogenous GA application increased the expression level of AtEXP2 during seed germination, while ABA application had no effect on AtEXP2 expression. Furthermore, the analysis of DELLA mutants show that RGL1, RGL2, RGA, GAI are all involved in repressing AtEXP2 expression, and RGL1 plays the most dominant role in controlling AtEXP2 expression. In stress response, exp2 mutant shows higher sensitivity than wild type in seed germination, while overexpression lines of AtEXP2 are less sensitive to salt stress and osmotic stress, exhibiting enhanced tolerance to stress treatment. Collectively, our results suggest that AtEXP2 is involved in the GA-mediated seed germination and confers salt stress and osmotic stress tolerance in Arabidopsis.
Introduction
The dominant phase of the life cycle of higher plants initiates from seed germination. The embryo of the Arabidopsis seed is surrounded by a single-cell endosperm layer and testa (seed coat) [1]–[2], thus, the emerging radicle needs to overcome the dual constraint of these structures to complete germination, therefore seed germination is defined as a two stage process with testa rupture followed by endosperm rupture [1], [3]. Physiologically the rupture of endosperm and testa is associated with cell division and enlargement, which are accompanied by cell wall expansion and loosening [4]–[5]. The cell wall is an extracellular layer surrounding the cell that plays an important role in maintaining cell shape and provides mechanical strength and rigidity [4]. The cell wall is composed of cellulose microfibrils, hemicellulose, pectin, lignin, and proteins, these cell wall polymers interact together to form a polymeric network then to confer the structural rigidity to cell wall [4]. Therefore, cell wall enlargement needs to destroy this structural rigidity by modifying cell wall extensibility.
Many enzymes are involved in modifying matrix polysaccharides including endo-β-mannanase and other endoglucanases [6]. In addition to those enzymes, expansins are involved in cell wall extensibility modification [7]–[8]. Expansin was firstly identified from young cucumber seedlings for its ability to mediate the acid-induced extension of cucumber hypocotyl walls [7], [9]. Expansins induce cell wall extension by disrupting non-covalent linkages between cellulose microfibrils and the cross-linking matrix glycans in cell wall [8]–[10].
Expansins are encoded by a multigene family and highly conserved in gymnosperms and angiosperms [10]–[12]. Expansins can be divided into four divergent subfamilies based on genomic and phylogenetic analyses, denoted as α-expansin (EXPA), β-expansin (EXPB), EXP-like A (EXLA) and EXP-like B (EXLB) [10], [13], and there are thirty-six expansins encoding by 26 EXPAs, 6 EXPBs, 3 EXLAs and 1 EXLBs in Arabidopsis (http://www.personal.psu.edu/fsl/ExpCentral).
The extensive occurrence of the expansins superfamily in plants suggests that expansins play multiple roles during the plant life cycle [14]–[15], and in the past several decades numerous studies have accumulated evidence about their involvement in diverse developmental processes, including seed germination [16]–[17], seedling morphogenesis [18], root architecture [19]–[26], leaf development [27]–[30], fruit ripening [31]–[32], stoma opening [33]–[34], pollination [35], abscission, stress responses [36]–[43] and others. However, those characterized expansins only represent a small proportion of the expansins superfamily in the plant kingdom, and the individual functions of other expansin coding genes still remains to be elucidated.
In the present study, we investigated the expression pattern of AtEXP2 in Arabidopsis, found that the expression of AtEXP2 is associated with seed germination and subject to GA and stress regulation. Our molecular analyses demonstrate that AtEXP2 plays a key role in controlling seed germination through GA signaling.
Materials and Methods
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Columbia-0 and Landsberg erecta were used as the wild types in this study. The T-DNA insertion mutant exp2 (Salk_117075) was obtained from the Nottingham Arabidopsis Stock Centre (NASC). The ga1-3, rgl1-1, rgl2-1, rga-t2, gai-t6 and della multiple mutants have been described previously [44]–[46]. The plants were grown in a growth room under a 16-h-light (100 µmol·m−2·s−1, 22°C) and 8-h-dark (19°C) photoperiod.
Germination Assay and Stress Treatment
For germination assays, Arabidopsis seeds were surface-sterilized with 5% (v/v) NaClO solution for 10 min and washed five times with sterile water, then sown on 1/2 MS medium containing 0.8% (w/v) agar supplemented with or without additional sucrose (150 or 250 mM), NaCl (100 or 200 mM), mannitol (200 or 400 mM), paclobutrazol (1 or 5 µM), then plates were transferred to a tissue culture room at 22°C under a 16 h-light/8 h-dark photoperiod. Germination rates were scored daily until the 7th day after sown based on radicle tip emergence. Experiments were repeated three times on five plates with about 100 seeds for each genotype.
GA and ABA Treatments
For measuring the effect of GA applications on gene expression, Col-0 wild type seeds were imbibed in 10 µM GA3 (Sigma-Aldrich), then were harvested 4 h, 6 h and 8 h after treatment for RNA extraction respectively. For measuring the effect of ABA applications on gene expression, Col-0 seeds were imbibed in different concentrations of ABA (0.1, 0.3, 1, 3, 10 µM) (Sigma-Aldrich) and harvested 16 h after treatment for RNA extraction.
Construction of Transgenic Lines
All the constructs (35S:AtEXP2, pAtEXP2:GUS) were prepared using the Gateway technology (Invitrogen). For construct 35S: AtEXP2, the EXP2 coding region was amplified from cDNA using primers 5′-CGGTCGACTACTCATCCCCTTTTCCAC-3′ and 5′-AAGCGGCCGCCTAAAATTGTCCGCCTTC-3′. The PCR products were digested with Sal I and Not I and cloned into pENTR-1A vector (Invitrogen), then recombined into destination vector pK2GW7 using the Gateway LR reaction (Invitrogen). To construct pEXP2:GUS, the 1.8 kb genomic fragment upstream of the start codon in EXP2 was amplified with the primers 5′-CGGTCGACAAGAAGTATCTGGGTGGG-3′ and 5′-AAGCGGCCGCATGGGCTAAAGAGGAGGA-3′, the digested PCR product was cloned into pENTR-1A and recombined into pHGWFS7. All binary vector constructs were introduced into Agrobacterium strain GV3101 and transformed into Arabidopsis Columbia-0 using the floral dip method [47]. All the transgenic lines were first selected based on their antibiotics resistance and further confirmed by expression level of the EXP2 or histochemical GUS assays.
RNA Extraction and Real-time RT-PCR
Total RNAs were isolated from Arabidopsis seeds according to the protocol described previously [48], and total RNAs from other tissues were extracted using TRIZOL reagent (Invitrogen) following the manufacturer’s instructions. cDNA was synthesized using the M-MLV Reverse Transcriptase (Promega) from 2 µg of total RNA in a 25 µl reaction, and diluted 4-fold with water. Quantitative real-time RT-PCR was performed using SYBR-green as described in previous study [49]–[51]. TUB2 was used as an endogenous control gene to normalize expression of the other genes. Primers used in real-time RT-PCR as follows : EXP2-F: 5′-CATAAACTCCGACGACAACG-3′, EXP2-R: 5′-TACCCACAAGCACCACCCAT-3′; TUB2-F: 5′-ATCCGTGAAGAGTACCCAGAT-3′, TUB2-R: 5′-AAGAACCATGCACTCATCAGC-3′ [52]. The PCR program was as follows: 30 s at 95°C, followed by 40 cycles of 5 s at 95°C, 30 s at 60°C.
GUS Activity Analysis
Tissues were prefixed in 90% acetone on ice for 20 min and incubated in GUS staining buffer [50 mM sodium phosphate (pH 7.0), 10 mM EDTA, 1 mg/ml 5-bromo-4-chloro-3-indoyl-β-D-glucuronide, 0.5 mM potassium ferricyanide, and 0.5 mM potassium ferrocyanide, 0.1%(v/v) Triton X-100] at 37°C overnight. Stained samples were then cleared of chlorophyll in an ethanol series and photographed by light microscopy.
Results
AtEXP2 is Mainly Expressed in Germinating Seeds
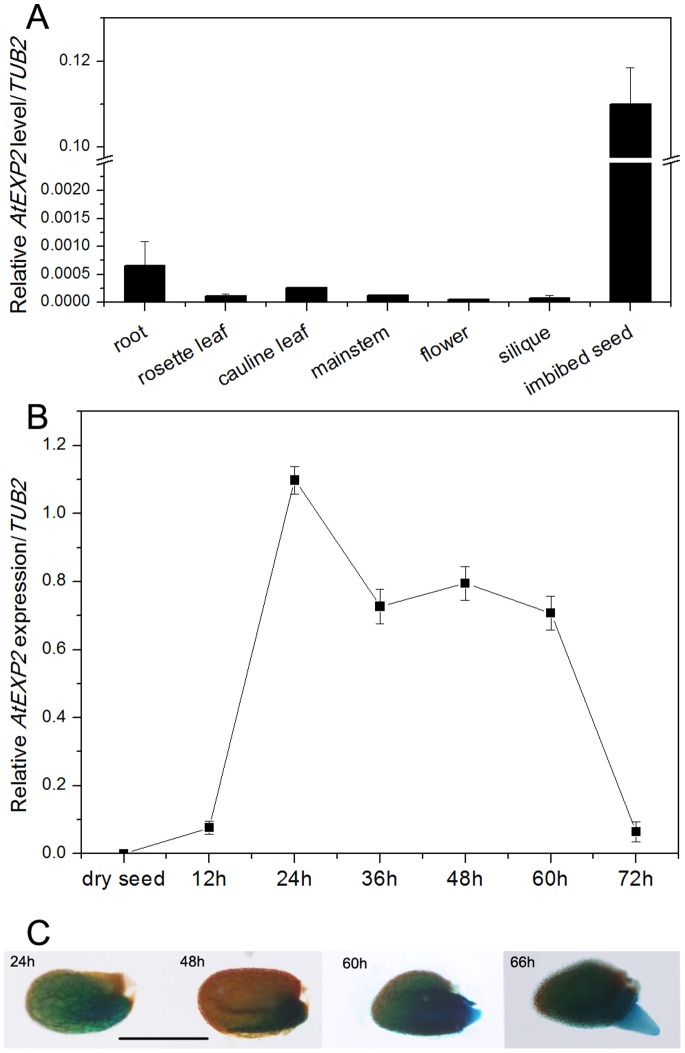
By analyzing the available public Arabidopsis microarray database (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi), we discovered that only AtEXP2 was exclusively expressed in imbibed seeds among thirty-six Arabidopsis expansin coding genes, which implies a probable role of AtEXP2 in seed germination. Therefore we first examined the expression pattern of AtEXP2 in Arabidopsis by real-time RT-PCR, and we found a high expression level in germinating seeds, but very low level in other tissues including roots, rosette leaves, cauline leaves, mainstem, flowers and siliques (Figure 1A). Further analysis showed that the expression of AtEXP2 was not detected in dry seeds, but the transcript abundance in seeds began to accumulate after imbibition in water, and the expression level reached a peak after 24 h imbibition, and then remained at high levels 2–3 days after imbibition (Figure 1B). These observations suggest that AtEXP2 is a seed-specific gene and may be involved in the seed germination process.
Figure 1. Expression pattern of AtEXP2 gene.
(A) Tissue-specific expression of AtEXP2. Various tissues of Arabidopsis wild type plants were harvested for RNA extraction. (B) Time course of AtEXP2 expression. Dry seeds and imbibed seeds of Col-0 were harvested for RNA extraction. Transcript levels of AtEXP2 were measured by real-time RT-PCR, and the values were normalized against the levels of TUB2 as a control. Error bars represent SD. (C) GUS staining in germinating seeds of the pAtEXP2:GUS transgenic line. Seeds from T3 homozygous plants of the pAtEXP2:GUS transgenic line were analyzed. Bar = 1 mm.
Next, we cloned the 1.8 kb genomic sequence upstream of transcription start site of AtEXP2 and generated the pAtEXP2:GUS construct. The β-glucuronidase (GUS) assays in this transgenic line shows AtEXP2 promoter activity continuously detectable during seed imbibition in water (Figure 1C). Later, GUS staining was observed in the radicle. These results are consistent with the RT-PCR assays and further suggests that AtEXP2 plays a role in seed germination and possibly root function.
AtEXP2 is Required in Seed Germination
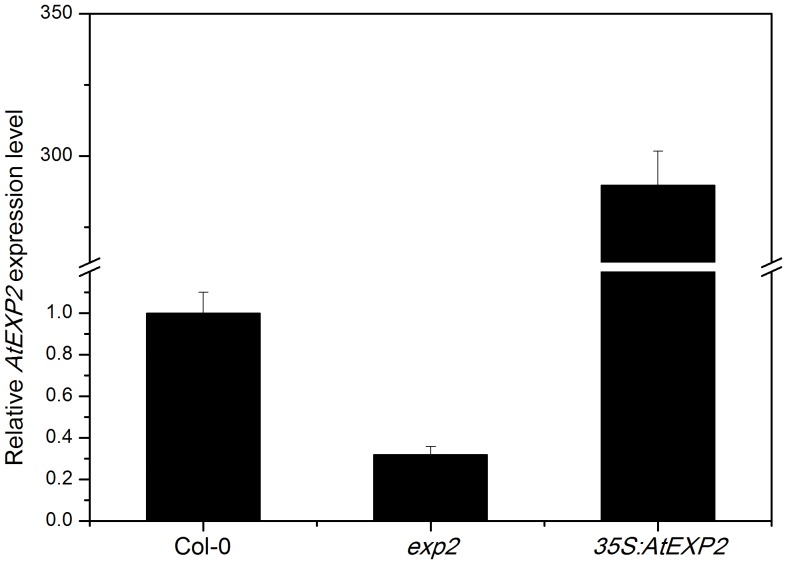
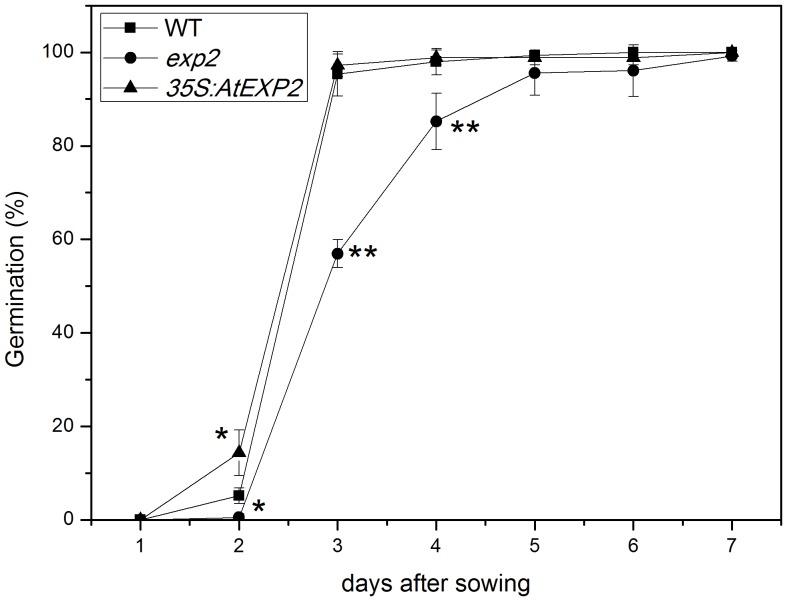
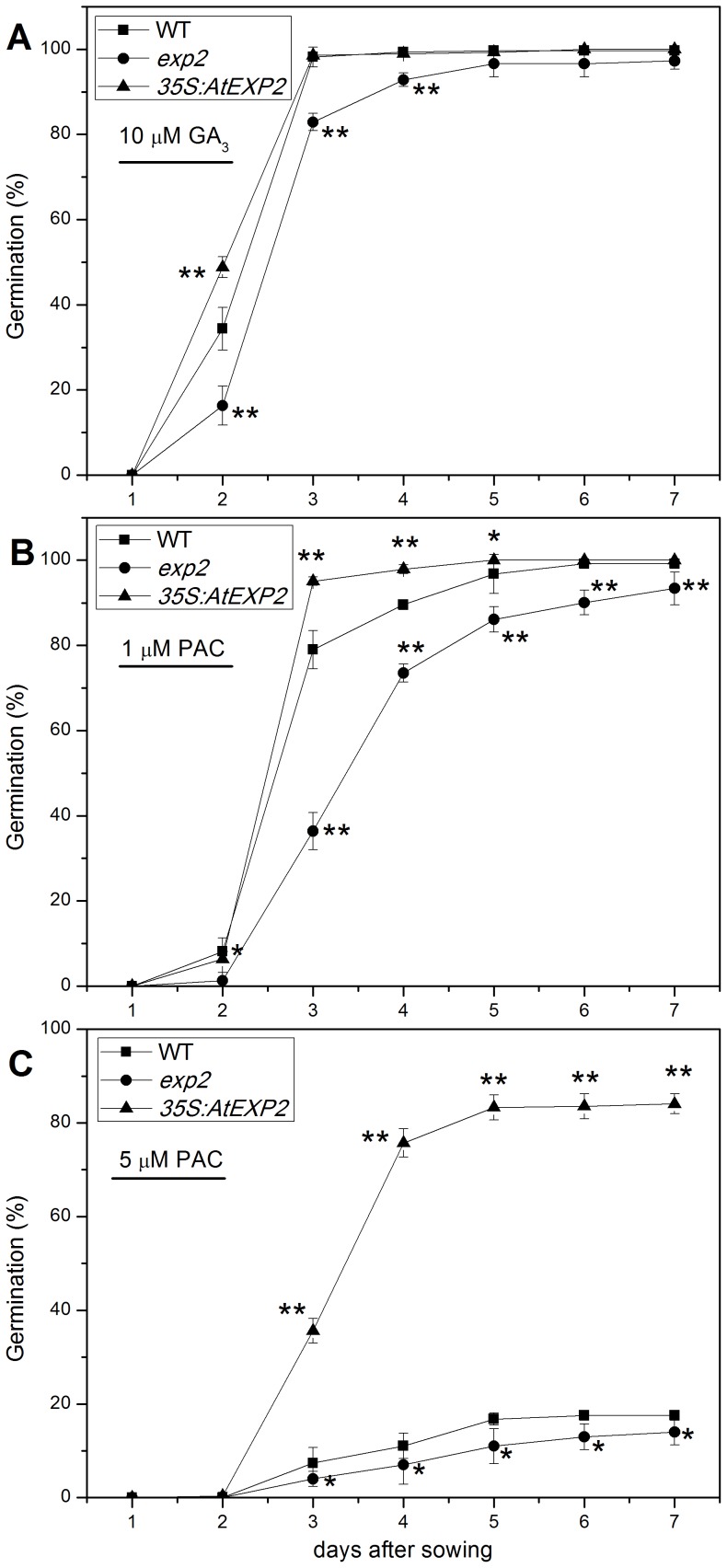
In order to investigate whether AtEXP2 plays a role in seed germination, we isolated an exp2 mutant (Salk_117075) carrying a T-DNA insertion in the AtEXP2 promoter region, which almost completely suppresses AtEXP2 expression (Figure 2). We also created 35S:AtEXP2 overexpression transgenic lines and generated nine independent transgenic lines, from which we selected a representative transgenic line exhibiting significantly higher AtEXP2 expression level compared to wild type (Figure 2). Then we performed the germination assay using seeds of homozygous exp2 mutant and 35S:AtEXP2 line, the results showed that 35S:AtEXP2 line germinated much earlier than wild type on the second day after sown on plates, while the rate of germination in exp2 mutant was significantly delayed compared to wild type (Figure 3), suggesting that AtEXP2 is required for seed germination in Arabidopsis.
Figure 2. Relative expression levels of AtEXP2 in exp2 and 35S:AtEXP2 line.
Seeds of wild type, exp2 and 35S:AtEXP2 overexpression line were harvested for RNA extraction after 24 h imbibition in water. Transcript abundance was measured by real-time RT-PCR and the values were normalized against the levels of TUB2 as a housekeeping gene. Error bars represent SD.
Figure 3. Germination phenotype of the wild type, exp2 and 35S:AtEXP2 line.
Non-dormant seeds of wild type, exp2 and 35S:AtEXP2 overexpression line were employed in the germination assay. The germination frequencies were scored daily until the 7th day after sown. Error bars represent SD. A Student’s t-test was calculated at the probability of either 5% (*P<0.05) or 1% (**P<0.01).
The Participation of AtEXP2 in Seed Germination is Regulated by GA
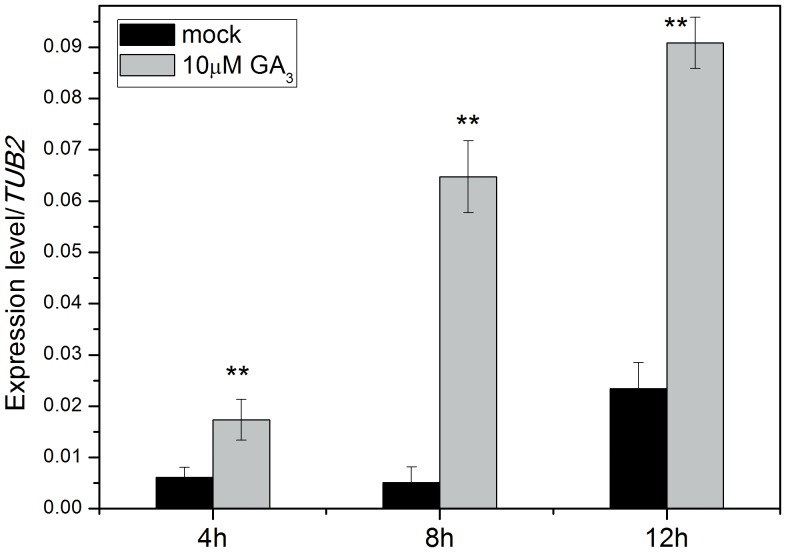
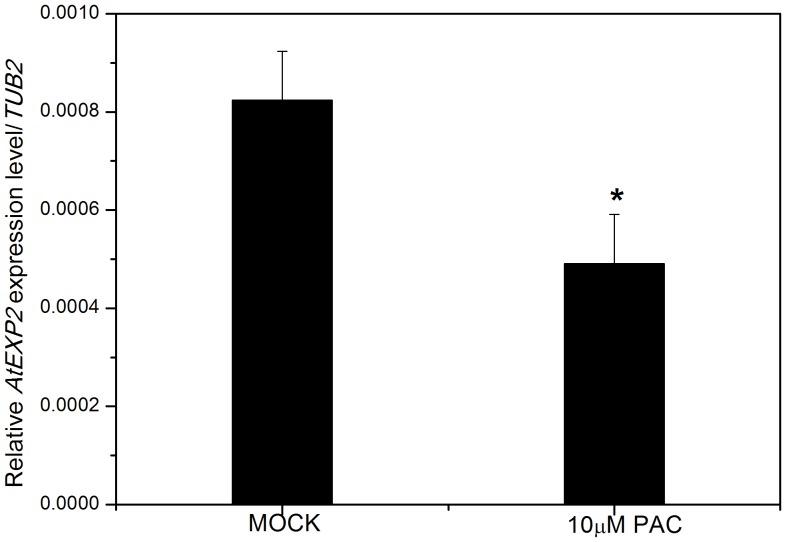
Since seed germination is largely controlled by phytohormones GA and ABA [2], [53], we next determined whether the expression of AtEXP2 was regulated by GA and ABA. As shown in Figure 4, the expression of AtEXP2 was significantly higher in GA3-treated wild type seeds than in mock-treated seeds. After treating seeds with 10 µM paclobutrazol (PAC), a gibberellin biosynthesis inhibitor, the expression of AtEXP2 was significantly decreased compared to the mock treatment (Figure 5), suggesting that the expression of AtEXP2 is induced by GA in germinating seeds. In contrast to GA induction of AtEXP2 expression, the expression of AtEXP2 was not significantly affected when the seeds were treated with different concentration of ABA (data was not shown).
Figure 4. Expression of AtEXP2 in response to exogenous GA application.
Col-0 wild type seeds were harvested 4 h, 6 h and 8 h after imbibition in 10 µM GA3 solution for RNA extraction respectively. Transcript levels were measured by real-time RT-PCR, and the values were normalized against the levels of TUB2 as a control. Error bars represent SD. A Student’s t-test was calculated at the probability of 1% (**P<0.01).
Figure 5. Expression of AtEXP2 in response to paclobutrazol treatment.
AtEXP2 expression was determined by quantitative real-time RT-PCR in 24 h imbibed seeds treated with 10 µM PAC or without (Mock). Error bars represent SD. A Student’s t-test was calculated at the probability of 5% (*P<0.05).
In order to investigate whether AtEXP2 is involved in seed germination in response to GA, we examined the germination phenotype of wild type, exp2 mutant and overexpression line in the present of GA3 and paclobutrazol, as shown in Figure 6A, the germination rate of exp2 seeds was significantly lower than that of wild type seeds on the second day after sown on plates when exogenous GA was applied, while the 35S:AtEXP2 line exhibited significant higher germination rate than wild type, indicating that AtEXP2 likely controls seed germination through GA signaling. However, in the presence of 1 µM and 5 µM paclobutrazol, the exp2 seeds still showed significant lower germination rate than wild type, while the germination of 35S:AtEXP2 line was less inhibited by paclobutrazol compared to wild type (Figure 6B and 6C), implied that factors other than GA would be involved in the AtEXP2-mediated seed germination.
Figure 6. Germination phenotype of the wild type, exp2 and 35S:AtEXP2 line in response to GA and PAC treatment.
Seeds of wild type, exp2 and 35S:AtEXP2 line were treated with 10 µM GA3 (A), 1 µM (B) or 5 µM (C) paclobutrazol (PAC). Error bars represent SD. A Student’s t-test was calculated at the probability of either 5% (*P<0.05) or 1% (**P<0.01).
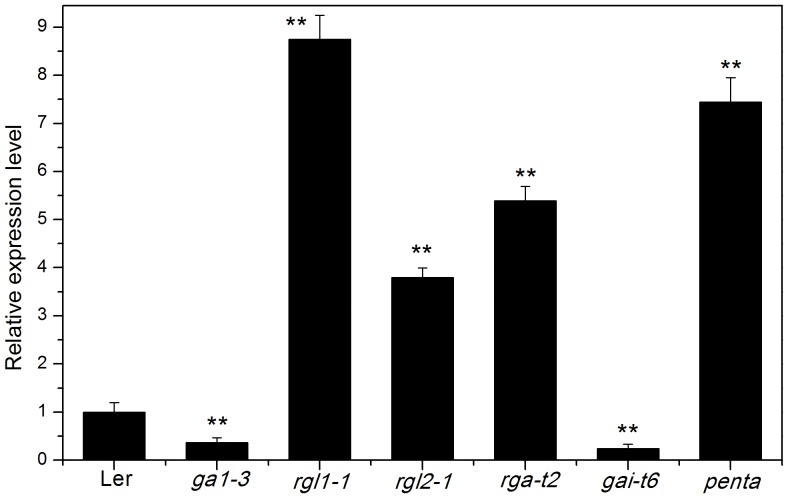
To further elucidate the way AtEXP2 participates in GA signaling during seed germination, we next examined the expression level of AtEXP2 in the GA-deficient mutant ga1-3 and various DELLA mutants. As shown in Figure 7, AtEXP2 expression was significantly reduced in ga1-3, confirming our previous conclusion that the AtEXP2 expression was induced by GA. AtEXP2 expression was also reduced in semi-dominant gain-of-function DELLA mutant gai-t6 seeds compared to wild type, while other three DELLA mutants rgl1-1, rgl2-1 and rga-t2 all exhibited significant higher expression of AtEXP2 than wild type, with rgl1-1 shown the highest expression level. These results suggest that all four DELLA genes tested in the study contribute to the repression of AtEXP2 expression and RGL1 plays the principal role in controlling AtEXP2 expression. However in the penta mutant, which lacks all four DELLA proteins activities in ga1-3 background, the expression of AtEXP2 was lower than that in rgl1-1 (Figure 7), indicating that other regulators besides these four DELLA proteins would be involved in the GA-mediated AtEXP2 expression.
Figure 7. Effects of DELLA on AtEXP2 expression during seed germination.
Expression level of AtEXP2 was measured in 24 h imbibed seeds of wild type, ga1-3, and various DELLA mutants. penta indicates the ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1 mutant. Error bars represent SD. A Student’s t-test was calculated at the probability of 1% (**P<0.01).
AtEXP2 is Involved in Response to Salt Stress and Osmotic Stress in Seed Germination
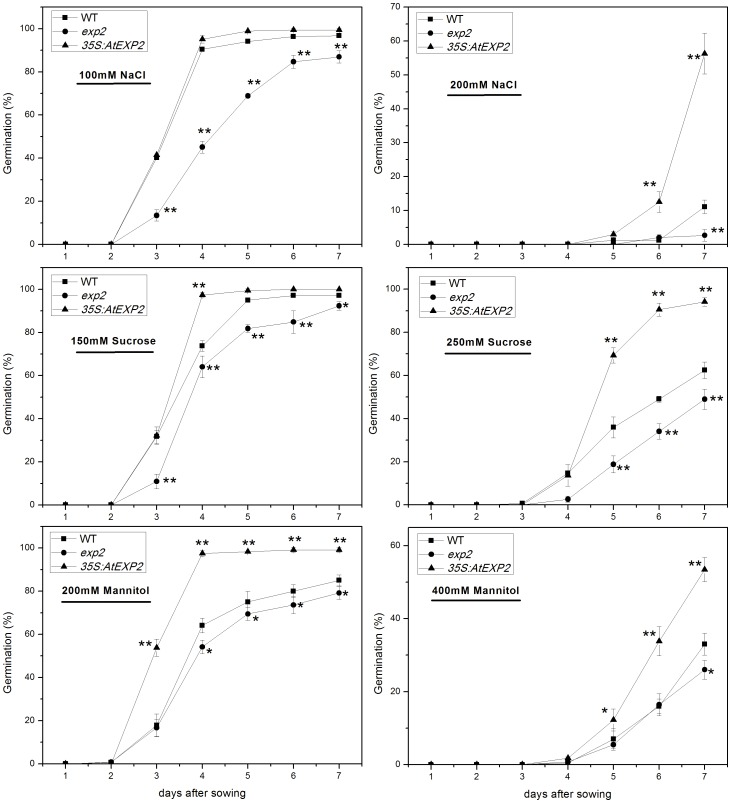
The expression of expansin coding genes are not only regulated by developmental signals, but also affected by environmental cues [17], [54]. In order to investigate whether AtEXP2 is involved in response to abiotic stress, we performed germination tests under salt stress and osmotic stress condition. As shown in Figure 8, when exposed to 100 mM NaCl, the germination frequency of exp2 mutant seeds was much lower than wild type and overexpression line, and ultimately reached a proportion less than 90% at seven days after sown. In the high salt condition (200 mM), germination of all genotypes was severely affected, with less than 15% germination frequency in wild type, and less than 5% germination frequency in exp2. Interestingly, the germination frequency of 35S:AtEXP2 line still reached approximately 60% seven days after sowing, suggesting that AtEXP2 was involved in seed germination in response to salt stress.
Figure 8. Germination phenotype of the wild type, exp2, and 35S:AtEXP2 line in response to abiotic stresses.
Seeds of wild type, exp2 and 35S:AtEXP2 line were treated with different concentrations of NaCl (100 or 200 mM), sucrose (150 or 250 mM) and mannitol (200 or 400 mM). Error bars represent SD. A Student’s t-test was calculated at the probability of either 5% (*P<0.05) or 1% (**P<0.01).
Next, we considered the possible effect of osmotic stress on AtEXP2 expression. Including various concentrations of sucrose and mannitol to the MS plates, we observed significantly lower germination frequency in the exp2 mutant compared to the wild type. The overexpression line was much higher in these conditions, moreover, the differences were more evident when under higher concentrations of sucrose and mannitol (Figure 8). Together, these observations indicate that exp2 mutant is more sensitive to salt stress and osmotic stress than wild type, while the 35S:AtEXP2 overexpression line is less sensitive than wild type, showing elevated tolerance to salt stress and osmotic stress.
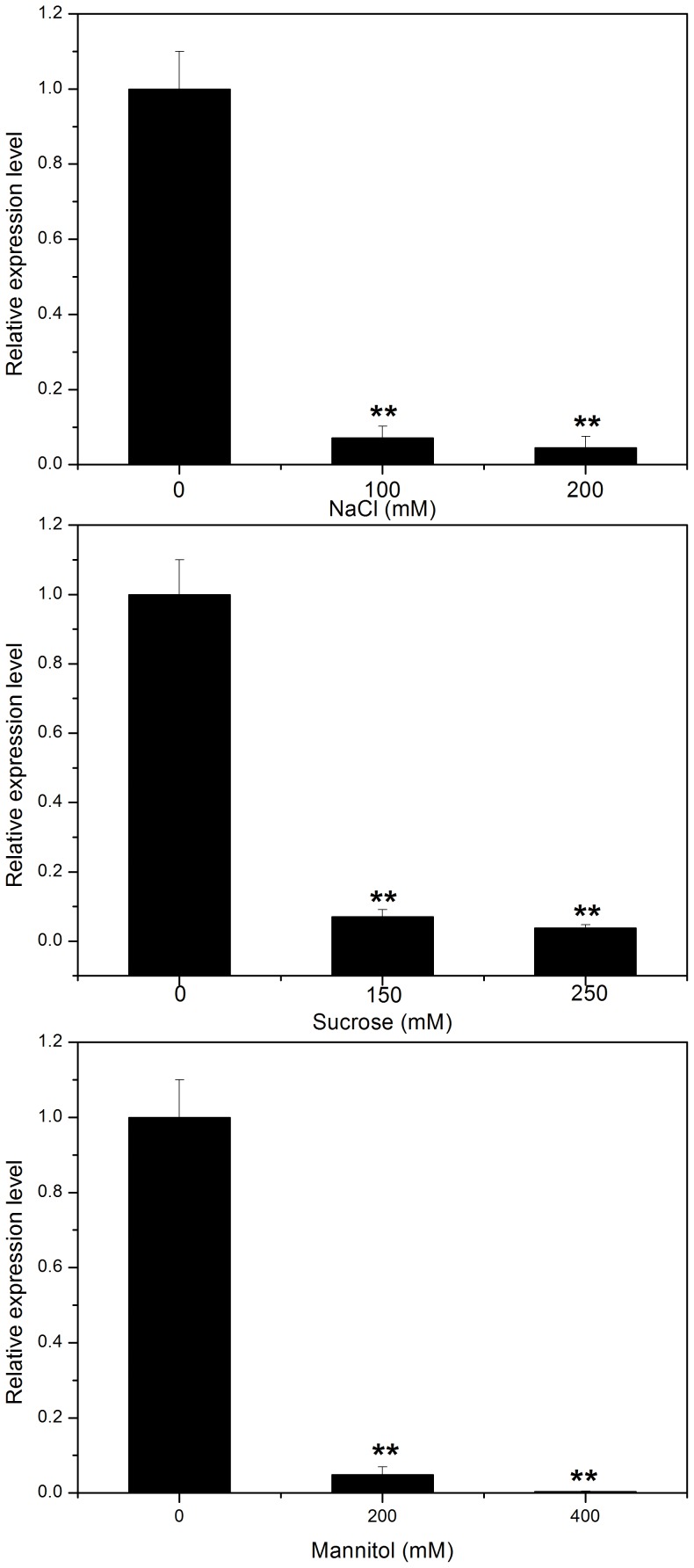
In order to further study the function of AtEXP2 in response to abiotic stress in seed germination, we performed quantitative real-time PCR analysis to evaluate AtEXP2 expression in response to salt stress and osmotic stress in germinating wild type seeds, the results showed that the expression of AtEXP2 were remarkably reduced after NaCl, sucrose and mannitol treatments compared to control treatments, and the reduction of expression levels were more severe when the seeds were treated with increased concentrations of NaCl, sucrose and mannitol (Figure 9), suggested that abiotic stress repressed the AtEXP2 expression in germinating seeds. In summary, AtEXP2 overexpression could confer salt and osmotic stress tolerance in Arabidopsis seed germination, and the salt stress and osmotic stress inhibit seed germination by repressing the expression of AtEXP2.
Figure 9. Expression of AtEXP2 in response to salt and osmotic stresses during seed germination.
Col-0 seeds were collected 24 h after imbibition in different concentrations of NaCl, sucrose and mannitol. Error bars represent SD. A Student’s t-test was calculated at the probability of 1% (**P<0.01).
Discussion
Expansins are encoded by a large gene superfamily and are widely distributed in plant species, consistent with the possibility that expansins perform multiple functions in various aspects of plant life cycle among species. Previous studies have shown that expansins were involved physiologically in almost every developmental process during the plant life cycle, related to cell growth, cell separation and cell wall disassembly [4], [55]–[56], and different expansins would play diverse tissue-specific roles distinct from each other [54]–[55], [57]–[58]. Physiological change during seed germination is associated with cell enlargement and cell wall expansion, and previous studies in tomato have revealed a role in seed germination played by expansins [16]–[17]. Furthermore, LeEXP4 is expressed specifically in the micropylar endosperm cap region and associated with endosperm cap weakening [17]. Another two expansin genes LeEXP8 mRNA is localized to the radicle cortex of the embryo, and LeEXP10 mRNA is expressed throughout the embryo during seed germination, suggesting the specific roles of the two expansin genes during seed germination [16]. In the present study, we first used the public Arabidopsis microarray database to analyze the expression profile of all thirty-six expansin genes in Arabidopsis and found that only AtEXP2 was exclusively expressed in germinating seeds, further real-time RT-PCR analyses confirmed that AtEXP2 was specifically expressed in imbibed seeds and AtEXP2 mRNA amounts peaked after 24 hours in imbibed seeds and maintained a high level during early stage of seed germination, consistent with previous microarray data [59]–[62], Using a β-glucuronidase reporter fusion to the AtEXP2 promoter, we observed signals in the germinating seeds, consistent with the AtEXP2 RNA accumulation pattern.
In order to investigate the role of AtEXP2 during seed germination, we carried out a germination assay using exp2 mutant seeds, which showed that exp2 seeds germinate later than wild type. Further through analyzing promoter sequence of AtEXP2 using PLACE tools (Plant cis-acting regulatory DNA elements) [63] we found there were putative GA-responsive elements (GARE) and ABA-responsive elements (ABRE) in the promoter region of AtEXP2, indicated that AtEXP2 would function downstream of GA and ABA signaling. We then performed GA and ABA treatments and found AtEXP2 expression was GA-inducible, but not affected by ABA treatment, which was consistent with previous studies [1], [17], [60], [64]. GA is well known to be a pivotal phytohormone in breaking seed dormancy and promoting seed germination [1]–[2], [53]. Previous studies showed that active GAs are synthesized mainly in the radicle and micropylar endosperm during germination [60], stimulating growth potential of embryo and inducing hydrolases biosynthesis to weaken endosperm and other structures surrounding the embryo, thus allow the radicle emergence and complete seed germination [65]–[66]. In this context, we suggest that AtEXP2 is involved in GA-mediated promotion of seed germination by weakening cell wall in endosperm and related structures surrounding the embryo.
As the GA signaling pathway is mainly mediated by derepression of DELLA repressors [67]–[68], Stamm et al., (2012) have shown that RGL2 downregulates two expansin encoding genes EXPA3 and EXPA8 in seed germination [69]. In order to study whether AtEXP2 expression is subjected to the regulation of DELLA, we examined the AtEXP2 expression level in germinating seeds of various DELLA mutants and found that all four DELLA genes tested participated in the repression of AtEXP2 expression, including RGL1, RGL2, RGA and GAI. Moreover, RGL1 played the most dominant role in controlling AtEXP2 expression. Taken together, our results suggest that GA promote AtEXP2 expression by removing the repression effect of DELLA on AtEXP2 during seed germination.
In addition to the regulation by phytohormones, expansin genes are also differentially regulated by various environmental cues [54], such as abiotic stresses including salt stress, heat stress, drought stress, and water stress [30], [37], [43], [70]. Here we tested the germination phenotype of exp2 and the overexpression line in response to salt stress and osmotic stress, and we found that exp2 mutant exhibited hypersensitivity to salt stress and osmotic stress compared to wild type, while the overexpression line showed reduced sensitivity to these stress treatments and revealed enhanced tolerance to stress to a certain extent. These results suggest that AtEXP2 may confer salt and osmotic stress tolerance in Arabidopsis seed germination. Real-time RT-PCR analysis showed that salt stress and osmotic stress treatments significantly reduced the expression level of AtEXP2, implying that salt stress and osmotic stress may inhibit seed germination by repressing AtEXP2-mediated cell wall expansion. In contrast to our observation, some other expansin coding genes in other species are subject to upregulation by stress [37], [43], [71]. This variable gene-specific expression pattern of expansin genes under abiotic stress suggest that individual expansin genes are subjected to different regulation by stress conditions and that the mechanisms underlying them are distinct.
Funding Statement
The research was supported by International Scientific and Technological Cooperation Project of Science and Technology Department of Zhejiang Province (grant number 2013C34G2010017), the International Scientific and Technological Cooperation Project of the Ministry of Science and Technology of China (grant number 2010DFA34430), National Natural Science Foundation of China (Grant No. 31370215; 31228002), and Zhejiang Provincial Natural Science Foundation of China (Grant No. Z31100041). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Weitbrecht K, Müller K, Leubner-Metzger G (2011) First off the mark: early seed germination. J Exp Bot 62: 3289–3309. [DOI] [PubMed] [Google Scholar]
- 2. Holdsworth MJ, Bentsink L, Soppe WJJ (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179: 33–54. [DOI] [PubMed] [Google Scholar]
- 3.Bentsink L, Koornneef M (2008) Seed dormancy and germination. The Arabidopsis Book. BioOne. 1–18. [DOI] [PMC free article] [PubMed]
- 4. Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326. [DOI] [PubMed] [Google Scholar]
- 5. Cosgrove DJ (1998) Cell wall loosening by expansins. Plant Physiol 118: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4: 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls (analysis of wall hydrolysis, stress relaxation, and binding). Plant Physiol 107: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Biol 50: 391–417. [DOI] [PubMed] [Google Scholar]
- 10. Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Bio 6: 850–861. [DOI] [PubMed] [Google Scholar]
- 11. Shcherban TY, Shi J, Durachko DM, Guiltinan MJ, McQueen-Mason SJ, et al. (1995) Molecular cloning and sequence analysis of expansins–a highly conserved, multigene family of proteins that mediate cell wall extension in plants. Proc Natl Acad Sci U S A 92: 9245–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutchison KW, Singer PB, McInnis S, Diaz-Sala C, Greenwood MS (1999) Expansins are conserved in conifers and expressed in hypocotyls in response to exogenous auxin. Plant Physiol 120: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kende H, Bradford K, Brummell D, Cho HT, Cosgrove D, et al. (2004) Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol Biol 55: 311–314. [DOI] [PubMed] [Google Scholar]
- 14. Shin JH, Jeong DH, Park MC, An G (2005) Characterization and transcriptional expression of the α-Expansin gene family in rice. Mol Cells 20: 210–218. [PubMed] [Google Scholar]
- 15. Lin Z, Ni Z, Zhang Y, Yao Y, Wu H, et al. (2005) Isolation and characterization of 18 genes encoding α- and β-expansins in wheat (Triticum aestivum L.). Mol Genet Genomics 274: 548–556. [DOI] [PubMed] [Google Scholar]
- 16. Chen F, Dahal P, Bradford KJ (2001) Two tomato expansin genes show divergent expression and localization in embryos during seed development and germination. Plant Physiol 127: 928–936. [PMC free article] [PubMed] [Google Scholar]
- 17. Chen F, Bradford KJ (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao Q, Zhao M, Li F, Guo Q, Xing S, et al. (2008) Expansins and coleoptile elongation in wheat. Protoplasma 233: 73–81. [DOI] [PubMed] [Google Scholar]
- 19. Gal TZ, Aussenberg ER, Burdman S, Kapulnik Y, Koltai H (2006) Expression of a plant expansin is involved in the establishment of root knot nematode parasitism in tomato. Planta 224: 155–162. [DOI] [PubMed] [Google Scholar]
- 20. Wieczorek K, Golecki B, Gerdes L, Heinen P, Szakasits D, et al. (2006) Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana . Plant J 48: 98–112. [DOI] [PubMed] [Google Scholar]
- 21. Kwasniewski M, Szarejko I (2006) Molecular cloning and characterization of β-expansin gene related to root hair formation in barley. Plant Physiol 141: 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Won S-K, Choi S-B, Kumari S, Cho M, Lee S, et al. (2010) Root hair-specific EXPANSIN B genes have been selected for graminaceae root hairs. Mol Cells 30: 369–376. [DOI] [PubMed] [Google Scholar]
- 23. Lin C, Choi HS, Cho HT (2011) Root hair-specific EXPANSIN A7 is required for root hair elongation in Arabidopsis . Mol Cells 31: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ZhiMing Y, Bo K, XiaoWei H, ShaoLei L, YouHuang B, et al. (2011) Root hair-specific expansins modulate root hair elongation in rice. Plant J 66: 725–734. [DOI] [PubMed] [Google Scholar]
- 25. Soltys D, Rudzińska-Langwald A, Gniazdowska A, Wiśniewska A, Bogatek R (2012) Inhibition of tomato (Solanum lycopersicum L.) root growth by cyanamide is due to altered cell division, phytohormone balance and expansin gene expression. Planta 236: 1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma N, Wang Y, Qiu S, Kang Z, Che S, et al. (2013) Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS One 8: e75997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci U S A 98: 11812–11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer TD, Ammerlaan AHM, et al. (2005) Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris . Plant J 43: 597–610. [DOI] [PubMed] [Google Scholar]
- 29. Goh HH, Sloan J, Dorca-Fornell C, Fleming A (2012) Inducible repression of multiple expansin genes leads to growth suppression during leaf development. Plant Physiol 159: 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lü P, Kang M, Jiang X, Dai F, Gao J, et al. (2013) RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta: 1–13. [DOI] [PubMed]
- 31. Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, et al. (1999) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11: 2203–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Budzinski I, Santos T, Sera T, Pot D, Vieira L, et al. (2011) Expression patterns of three α-expansin isoforms in Coffea arabica during fruit development. Plant Biology 13: 462–471. [DOI] [PubMed] [Google Scholar]
- 33. Wei PC, Zhang XQ, Zhao P, Wang XC (2011) Regulation of stomatal opening by the guard cell expansin AtEXPA1 . Plant Signal Behav 6: 740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang XQ, Wei PC, Xiong YM, Yang Y, Chen J, et al. (2011) Overexpression of the Arabidopsis α-expansin gene AtEXPA1 accelerates stomatal opening by decreasing the volumetric elastic modulus. Plant Cell Rep 30: 27–36. [DOI] [PubMed] [Google Scholar]
- 35. Tabuchi A, Li LC, Cosgrove DJ (2011) Matrix solubilization and cell wall weakening by β-expansin (group-1 allergen) from maize pollen. Plant J 68: 546–559. [DOI] [PubMed] [Google Scholar]
- 36. Sabirzhanova IB, Sabirzhanov BE, Chemeris AV, Veselov DS, Kudoyarova GR (2005) Fast changes in expression of expansin gene and leaf extensibility in osmotically stressed maize plants. Plant Physiol Biochem 43: 419–422. [DOI] [PubMed] [Google Scholar]
- 37. Xu J, Tian J, Belanger FC, Huang B (2007) Identification and characterization of an expansin gene AsEXP1 associated with heat tolerance in C3 Agrostis grass species. J Exp Bot 58: 3789–3796. [DOI] [PubMed] [Google Scholar]
- 38. Kwon YR, Lee HJ, Kim KH, Hong SW, Lee SJ, et al. (2008) Ectopic expression of Expansin3 or Expansinβ1 causes enhanced hormone and salt stress sensitivity in Arabidopsis . Biotechnol Lett 30: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 39. Guo W, Zhao J, Li X, Qin L, Yan X, et al. (2011) A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J 66: 541–552. [DOI] [PubMed] [Google Scholar]
- 40. Li F, Xing S, Guo Q, Zhao M, Zhang J, et al. (2011) Drought tolerance through over-expression of the expansin gene TaEXPB23 in transgenic tobacco. J Plant Physiol 168: 960–966. [DOI] [PubMed] [Google Scholar]
- 41. Sasidharan R, Voesenek LA, Pierik R (2011) Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit Rev Plant Sci 30: 548–562. [Google Scholar]
- 42. Dai F, Zhang C, Jiang X, Kang M, Yin X, et al. (2012) RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol 160: 2064–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao MR, Han YY, Feng YN, Li F, Wang W (2012) Expansins are involved in cell growth mediated by abscisic acid and indole-3-acetic acid under drought stress in wheat. Plant Cell Rep 31: 671–685. [DOI] [PubMed] [Google Scholar]
- 44. Josse EM, Gan Y, Bou-Torrent J, Stewart KL, Gilday AD, et al. (2011) A DELLA in disguise: SPATULA restrains the growth of the developing Arabidopsis seedling. Plant Cell 23: 1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cao D, Hussain A, Cheng H, Peng J (2005) Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis . Planta 223: 105–113. [DOI] [PubMed] [Google Scholar]
- 46. Sun T, Goodman HM, Ausubel FM (1992) Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell 4: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 48. Suzuki Y, Kawazu T, Koyama H (2004) RNA isolation from siliques, dry seeds, and other tissues of Arabidopsis thaliana . Biotechniques 37: 542–544. [DOI] [PubMed] [Google Scholar]
- 49. An L, Zhou Z, Sun L, Yan A, Xi W, et al. (2012) A zinc finger protein gene ZFP5 integrates phytohormone signaling to control root hair development in Arabidopsis . Plant J 72: 474–490. [DOI] [PubMed] [Google Scholar]
- 50. Zhou Z, An L, Sun L, Zhu S, Xi W, et al. (2011) Zinc finger protein5 is required for the control of trichome initiation by acting upstream of ZFP8 in Arabidopsis . Plant Physiol 157: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gan Y, Zhou Z, An L, Bao S, Forde BG (2011) A comparison between northern blotting and quantitative real-time PCR as a means of detecting the nutritional regulation of genes expressed in roots of Arabidopsis thaliana . Agric Sci China 10: 335–342. [Google Scholar]
- 52. Xi W, Liu C, Hou X, Yu H (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis . Plant Cell 22: 1733–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bewley JD, Black M (1994) Seeds: physiology of development and germination. New York: Plenum Press.
- 54. Lee Y, Choi D, Kende H (2001) Expansins: ever-expanding numbers and functions. Curr Opin Plant Biol 4: 527–532. [DOI] [PubMed] [Google Scholar]
- 55. Cosgrove DJ (1997) Creeping walls, softening fruit, and penetrating pollen tubes: the growing roles of expansins. Proc Natl Acad Sci U S A 94: 5504–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cho HT, Cosgrove DJ (2000) Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana . Proc Natl Acad Sci U S A 97: 9783–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cosgrove DJ (2000) New genes and new biological roles for expansins. Curr Opin Plant Biol 3: 73–78. [DOI] [PubMed] [Google Scholar]
- 58. Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, et al. (2002) Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol 128: 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Penfield S, Li Y, Gilday AD, Graham S, Graham IA (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, et al. (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Linkies A, Müller K, Morris K, Turečková V, Wenk M, et al. (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: A comparative approach using Lepidium sativum and Arabidopsis thaliana . Plant Cell 21: 3803–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, et al. (2008) Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis . Plant J 53: 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, et al. (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16: 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hooley R (1994) Gibberellins: perception, transduction and responses. Plant Mol Biol 26: 1529–1555. [DOI] [PubMed] [Google Scholar]
- 66. Yamaguchi S, Kamiya Y (2001) Gibberellins and light-stimulated seed germination. J Plant Growth Regul 20: 369–376. [DOI] [PubMed] [Google Scholar]
- 67. Peng J, Harberd NP (2002) The role of GA-mediated signalling in the control of seed germination. Curr Opin Plant Biol 5: 376–381. [DOI] [PubMed] [Google Scholar]
- 68. Schwechheimer C, Willige BC (2009) Shedding light on gibberellic acid signalling. Curr Opin Plant Biol 12: 57–62. [DOI] [PubMed] [Google Scholar]
- 69. Stamm P, Ravindran P, Mohanty B, Tan E, Yu H, et al. (2012) Insights into the molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis thaliana . BMC Plant Biology 12: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu Y, Cosgrove DJ (2000) Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot 51: 1543–1553. [DOI] [PubMed] [Google Scholar]
- 71. Harb A, Krishnan A, Ambavaram MMR, Pereira A (2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 154: 1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]