Abstract
Background
Helicobacter pylori is an important global pathogen infecting approximately 50% of the world’s population. This study was undertaken in order to estimate the prevalence rate of Helicobacter pylori infections among adults living in Turkey and to investigate the associated risk factors.
Method
This study was a nationally representative cross sectional survey, using weighted multistage stratified cluster sampling. All individuals aged ≥18 years in the selected households were invited to participate in the survey. Ninety two percent (n = 2382) of the households in 55 cities participated; 4622 individuals from these households were tested with the 13C-Urea breath test. Helicobacter pylori prevalence and associated factors were analysed by the t test, chi square and multiple logistic regression with SPSS11.0.
Results
The weighted overall prevalence was 82.5% (95% CI: 81.0-84.2) and was higher in men. It was lowest in the South which has the major fruit growing areas of the country. The factors included in the final model were sex, age, education, marital status, type of insurance (social security), residential region, alcohol use, smoking, drinking water source. While education was the only significant factor for women, residential region, housing tenure, smoking and alcohol use were significant for men in models by sex.
Conclusion
In Turkey, Helicobacter pylori prevalence was found to be very high. Individuals who were women, elderly adults, single, had a high educational level, were living in the fruit growing region, had social security from Emekli Sandigi, were drinking bottled water, non smokers and regular alcohol consumers, were under less risk of Helicobacter pylori infection than others.
Keywords: Helicobacter pylori prevalence, Risk factors of helicobacter pylori infection, Smoking, Alcohol use
Background
Helicobacter pylori was first discovered in 1983, and eleven years later in 1994 the International Agency for Research on Cancers (IARC) classified H.pylori as a definite class 1 carcinogen [1,2]. It is a small, spiral, gram-negative bacillus which inhabits the mucus layer overlying the gastric epithelial cells in humans. It produces a potent urease. The isolation of H.pylori from the human gastric mucosa and the demonstration of its involvement in gastritis, peptic ulcer disease and gastric cancers have radically changed our perception of these diseases. Development of atrophy and metaplasia of the gastric mucosa are strongly associated with H.pylori infection [2-5].
The greatest risk for infection appears to be during childhood and early adult years [6]. Although infected individuals often have histological evidence of gastritis, the vast majority of infections are asymptomatic [2]. Current evidence indicates that disparate disease outcomes are not related solely to the genetic diversity of H.pylori, but also to host factors and environmental agents [7]. Further delineation of the host response to infection, to specific environmental exposures or to bacterial virulence factors is required to identify which patients infected with H.pylori are at greatest risk of developing disease. Identifying and understanding such interactions should promote the development of optimal outcomes.
H.pylori is a public health problem in both developed and developing countries [8]. The IARC has stressed that the need for effective, population based screening programs is essential for tackling cancer [9].
Most previous studies have been carried out in clinical settings on small samples. There is limited evidence concerning the prevalence, determinants and mode of infection in representative population samples. This is the first population based study of a country-wide representative sample with a high response rate using the most sensitive and specific test the 13Carbon Urea Breath Test (13C-UBT) to have been carried out in Turkey. The aim of this survey was to estimate the prevalence rate of H.pylori infection among adults aged ≥18 years and to investigate the factors associated with an H.pylori infection in Turkey.
Methods
Study population
A study of the prevalence and risk factors of H.pylori infection in Turkey (TURHEP) was a nationally representative, population based cross-sectional screening with the 13C-Urea Breath Test. A weighted, multistage, stratified cluster sampling approach was used in the selection of the sample. For this study, 100 different residential areas were selected as clusters for an optimal distribution with a target sample size of 2500 selected households based on the results of the General Population Count of Turkey held in 2000 (Additional file 1: Figure S1). Households which were to be visited in each cluster were selected randomly by the Turkish Statistical Institute.
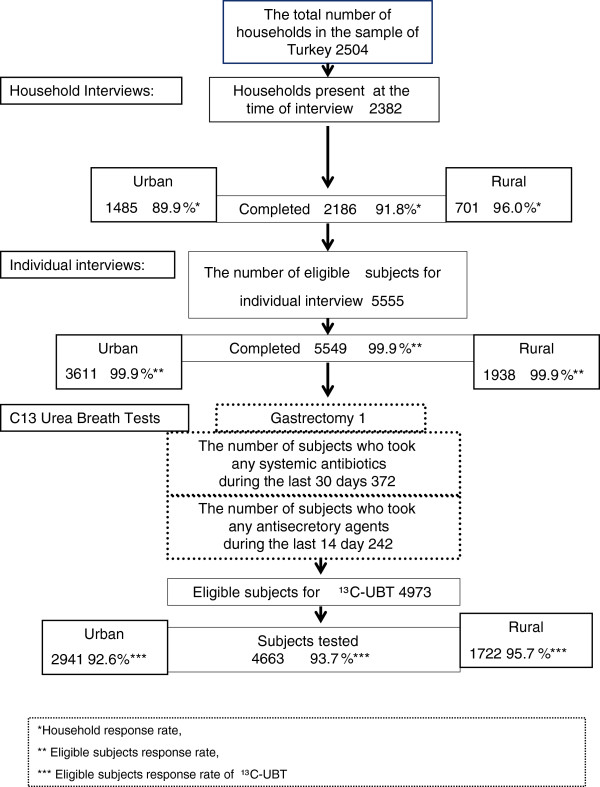
The eligible individuals were all those aged ≥ 18 who had been present in the selected household on the night before the day of the visit. Among the individuals interviewed those who had undergone a gastrectomy, who had used antibiotics during the preceding 30 days or who had used any proton pump inhibitors during the preceding 14 days were excluded from the survey. The next step was the performance of the 13C-UBT on those who accepted this test [10,11] (Figure 1).
Figure 1.
Flowchart of TURHEP study in Turkey.
Breath sample collection
At the first visit, eligible and willing people were informed about a required minimal six-hour period of fasting. At the second visit, after ensuring that they had fasted, two breath samples were collected as first samples. The test solution, 75 mg 13C-urea in 30 ml drinkable water (Helicobacter Test INFAI, Germany), was given after 200 ml of standard orange juice had been drunk. Thirty minutes later two breath samples were taken. Samples were measured by isotope ratio mass spectrometry (IRMS) in Istanbul between August 2003 and February 2004.
The test results were evaluated as H.pylori-negative when the 13C difference between 0th minute sample and 30th minute sample was lower than 4.00 and as H.pylori-positive when it was equal to or higher than 4.00.
Ethical issues
The study protocol was reviewed and approved by the Research Ethics Committee of the School of Medicine of Marmara University. All participants signed a written informed consent.
IRMS measurements were performed in the University and a trained technician employed by Marmara Health Education and Research Foundation measured the samples during the period of the study.
Variable definitions
The primary outcome variable, the results of the H.Pylori UBT were categorized as positive or negative. Demographic variables (age, sex, residential region, geographic region, marital status, education), economic status (occupation, social security status 1a(Emekli Sandigi, SSK, BAG-KUR, Green Card, private insurance, foreign insurance or none), housing tenure, environmental condition (number in household, bedrooms, source of drinking water, type of toilet system, source of heating) in or out of the home as well as cigarette and alcohol consumption were considered in the analysis.
The geographic regions defined five major regions of the country (West, South, Central, North and East) (Additional file 1: Figure S1).
Statistical methods
All analyses incorporated sampling weights that were adjusted for the complex study design of TURHEP.
The characteristics of H.pylori-positive and H.pylori-negative participants were compared using the chi-square test for categorical variables and the two sample t-test for continuous variables.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between H.Pylori infection and each potential risk factor were estimated using multivariable logistic regression models. The covariates included in the models were those significantly associated with H.Pylori in the univariate analyses (p < 0.05). The group presenting the lowest infection risk was chosen as the indicator. The final model was developed using a stepwise procedure with backward elimination, with inclusion and exclusion criteria set at the significance level of 0.05 and 0.10 respectively. The multiple logistic regression model fit was determined by the Hosmer-Lemeshow test statistic. A model fits the data if the Hosmer-Lemeshow statistic has a p > 0.05. Significant predictors were identified and ORs calculated with 95% CIs. The following variables were considered in the model: sex, age, region (West, South, Central, North, East), residence (urban, rural), marital status (never married, currently married, widowed/divorced), education level (no education, primary complete, secondary complete, high school +), type of insurance (Emekli Sandigi, private/foreign, BAG KUR, SSK, none, green-card), occupation (employed/unemployed) housing-tenure (owned by a household-member, lodging/no-rent paid), household population per bedroom, source of drinking-water (bottled-water, piped-water, public-fountain, others:‘river/rain-water/etc.’, smoking (never, current-nonsmoker, current-occasional-smoker, regular-smoker) and alcohol consumption (regular drinker, current occasional-drinker, current non-drinker, never).
Results
In TURHEP, 2382 households in 100 clusters from 55 cities (Among 81 cities) were available for interview (Additional file 1: Figure S1) and 91.8% were successfully interviewed. The household response rate for urban areas were 89.9% and for rural areas 96.0% (Table 1, Figure 1). The main reasons that the field teams were unable to interview was that some of the houses were vacant at the time of the interview or household members were away for an extended period.
Tables 1.
Results of the household, individual interviews and breathe samples
| |
Residence |
|
|
|---|---|---|---|
| Results | Urban | Rural | Total |
|
Household Interviews |
|
|
|
| Dwellings sampled |
1753 |
751 |
2504 |
| Households found |
1652 |
730 |
2382 |
| Households interviewed |
1485 |
701 |
2186 |
|
Household Response Rate (%) |
89.9 |
96.0 |
91.8 |
|
Individual Interviews |
|
|
|
| Eligible individual |
3616 |
1939 |
5555 |
| Eligible individual interviewed |
3611 |
1938 |
5549 |
|
Eligible Individual Response Rate (%) |
99.9 |
99.9 |
99.9 |
|
13
C-UBT’s |
|
|
|
| Number of people who had gastrectomy |
0 |
1 |
1 |
| Number of the people who had antibiotic treatment during the last 30 days |
294 |
78 |
372 |
| Number of people who used PPI during the last 14 days |
174 |
68 |
242 |
| Eligible people for test |
3174 |
1799 |
4973 |
| Eligible people tested |
2941 |
1722 |
4663 |
| Eligible people tested rate (%) | 92.6 | 95.7 | 93.7 |
Among 5555 eligible individuals in households, 5549 were successfully interviewed (99.9%). The total number of eligible people for breath test was 4973 and of these 4663 breath samples were collected (93.7%). Three hundred and seventy two individuals who had used antibiotic therapy for any reason during the last 30 days, 242 individuals who had used proton- pump inhibitors during the last 14 days and 1 person who had had a gastrectomy were excluded. The main reason for failure to collect breath samples from the eligible people was that they could not stand the 6-hour fast or were unwilling to undertake the 6-hour fast. Also, a number of eligible individuals were obliged to be outside or working after 6 hrs and a few people did not agree to give breath samples although they gave no reason.
Of the 4663 breath samples, 4622 were measured (99.1%). Forty one breath-samples could not be measured for technical reasons (Table 1).
The basic socio-demographic characteristics and H. pylori infection
The H.pylori infection prevalence was 82.5% in the population aged ≥18. It was more prevalent in men than women after controlling for confounding factors (Tables 2 and 3, Additional file 2: Figure S2). There was an inverse association between age and H.pylori infection (OR:0.98, 95%CI 0.97-0.99) (Tables 2 and 3). Those living in Central or Eastern Turkey were more at risk than those living in Southern Turkey (Tables 2 and 3, Additional file 2: Figure S2).
Table 2.
Socio-demographic factors associated with Helicobacter pylori infection
|
Socio-demographic factors |
Hp positive |
Hp negative |
|
|||
|---|---|---|---|---|---|---|
| n | (%)* | n | (%)* | Total | P** | |
|
Sex |
|
|
|
|
|
|
| Female |
2075 |
(81.4) |
457 |
(18.6) |
2532 |
0.014 |
| Male |
1777 |
(83.9) |
313 |
(16.1) |
2090 |
|
|
Age groups |
|
|
|
|
|
|
| 18–24 |
736 |
(79.6) |
170 |
(20.4) |
906 |
0.000 |
| 25–34 |
957 |
(86.3) |
145 |
(13.7) |
1102 |
|
| 35–44 |
746 |
(84.2) |
123 |
(15.8) |
869 |
|
| 45–54 |
599 |
(83.7) |
108 |
(16.3) |
707 |
|
| 55–64 |
372 |
(78.9) |
99 |
(21.1) |
471 |
|
| 65 + |
442 |
(78.6) |
125 |
(21.4) |
567 |
|
|
Region |
|
|
|
|
|
|
| West |
1027 |
(80.3) |
247 |
(19.7) |
1274 |
0.000 |
| South |
444 |
(78.7) |
118 |
(21.3) |
562 |
|
| Central |
1089 |
(85.0) |
192 |
(15.0) |
1281 |
|
| North |
369 |
(82.3) |
83 |
(17.8) |
452 |
|
| East |
923 |
(88.1) |
130 |
(11.9) |
1053 |
|
|
Residence |
|
|
|
|
|
|
| Urban |
2411 |
(81.7) |
509 |
(18.3) |
2920 |
0.020 |
| Rural |
1441 |
(84.0) |
261 |
(16.0) |
1702 |
|
| Total | 3852 | (82.5) | 770 | (17.5) | 4622 | |
*weighted , **p based on X2 test.
Table 3.
Adjusted odds ratios for Helicobacter pylori positivity for various risk factors in final model
| Variable and categories | B | P | OR | 95% CI | |
|---|---|---|---|---|---|
|
Sex |
|
|
|
|
|
| Female |
|
|
1.0 |
|
|
| Male |
0.217 |
0.035 |
1.242 |
1.015, |
1.519 |
|
Age |
-0.015 |
0.000 |
0.986 |
0.979, |
0.992 |
|
Education |
|
|
|
|
|
| High school + |
|
|
1.0 |
|
|
| No education |
0.484 |
0.003 |
1.623 |
1.176, |
2.239 |
| Primary complete |
0.511 |
0.000 |
1.666 |
1.333, |
2.083 |
| Secondary complete |
0.405 |
0.013 |
1.499 |
1.091, |
2.059 |
|
Marital status |
|
|
|
|
|
| Never married |
|
|
1.0 |
|
|
| Widowed/Divorced |
0.425 |
0.030 |
1.530 |
1.042, |
2.246 |
| Currently married |
0.554 |
0.000 |
1.739 |
1.378, |
2.197 |
|
Social security |
|
|
|
|
|
| Emekli sandigi |
|
|
1.0 |
|
|
| Private/foreign |
-0.532 |
0.187 |
0.587 |
0.266, |
1.295 |
| BAG-KUR |
0.009 |
0.952 |
1.009 |
0.757, |
1.345 |
| SSK |
0.450 |
0.001 |
1.568 |
1.213, |
2.026 |
| None |
0.439 |
0.002 |
1.550 |
1.174, |
2.048 |
| Green Card |
0.383 |
0.077 |
1.467 |
0.960, |
2.241 |
|
Source of drinking water |
|
|
|
|
|
| Bottled water/demijohn/pet water |
|
|
1.0 |
|
|
| Piped water (in house/garden/outside) |
0.572 |
0.000 |
1.772 |
1.404, |
2.236 |
| Spring/public fountain |
0.517 |
0.002 |
1.677 |
1.218, |
2.308 |
| Other (river. rain water etc.) |
0.495 |
0.008 |
1.640 |
1.135, |
2.371 |
|
Smoking |
|
|
|
|
|
| Never |
|
|
1.0 |
|
|
| Tried at past. currently non-smoker |
0.038 |
0.750 |
1.039 |
0.821, |
1.316 |
| Tried at past. currently occasional smoker |
-0.003 |
0.985 |
0.997 |
0.703, |
1.413 |
| Regular smoker |
0.350 |
0.005 |
1.419 |
1.113, |
1.808 |
|
Alcohol |
|
|
|
|
|
| Regular consumer |
|
|
1.0 |
|
|
| Tried at past. currently drinking occasionally |
0.586 |
0.028 |
1.798 |
1.066, |
3.032 |
| Tried at past. currently non-drinker |
0.687 |
0.012 |
1.988 |
1.161, |
3.403 |
| Never |
0.692 |
0.010 |
1.997 |
1.182, |
3.374 |
|
Region |
|
|
|
|
|
| South |
|
|
1.0 |
|
|
| West |
0.186 |
0.147 |
1.204 |
0.937, |
1.549 |
| North |
0.172 |
0.343 |
1.188 |
0.832, |
1.696 |
| Central |
0.382 |
0.007 |
1.466 |
1.111, |
1.934 |
| East |
0.563 |
0.001 |
1.756 |
1.264, |
2.439 |
| Constant | -0.473 | 0.173 | 0,623 | ||
Variables entered in the model: sex, age, residence, region, marital status, education, social security, occupation, housing tenure, source of drinking water, the number of the household per sleeping room, smoking, and alcohol consumption.
Socio-economic status and H.pylori infection
A current H.pylori infection was associated with education, social-security status and water supply (Tables 4 and 3). Occupation, the number in the household, the source of heating and the total monthly family income were not in the final logistic regression models. Housing tenure was the only significant factor in the men’s model (Table 5). There was an inverse association of educational level and H.pylori infection; individuals with lower educational levels had a higher risk than high school graduates and those with a higher education.
Table 4.
Socio-economic factors associated with Helicobacter pylori infection
|
Socio-economic Factors |
Hp positive |
Hp negative |
|
|
||
|---|---|---|---|---|---|---|
| n | (%)* | n | (%)* | Total | p | |
|
Education (n = 4577) |
|
|
|
|
|
|
| No education |
747 |
(82.6) |
163 |
(17.4) |
910 |
0.000 |
| Primary complete |
1801 |
(86.0) |
276 |
(14.0) |
2077 |
|
| Secondary complete |
355 |
(85.2) |
59 |
(14.8) |
414 |
|
| High school + |
913 |
(75.4) |
263 |
(24.6) |
1176 |
|
|
Social security** (n = 4573) |
|
|
|
|
|
|
| SSK |
1266 |
(83.9) |
228 |
(16.1) |
1494 |
0.000 |
| Emekli sandigi |
431 |
(74.5) |
139 |
(25.5) |
570 |
|
| BAG-KUR |
546 |
(78.5) |
148 |
(21.5) |
694 |
|
| Green Card |
324 |
(87.2) |
42 |
(12.8) |
366 |
|
| Private/foreign |
21 |
(63.9) |
10 |
(36.1) |
31 |
|
| None |
1223 |
(85.4) |
195 |
(14.6) |
1418 |
|
|
Housing tenure (n = 4597) |
|
|
|
|
|
|
| Owned by a household member |
2761 |
(81.6) |
583 |
(18.4) |
3344 |
0.05 |
| Rented |
762 |
(84.5) |
134 |
(15.5) |
896 |
|
| Lodging/no money paid |
306 |
(85.3) |
51 |
(14.7) |
357 |
|
|
Occupation (n = 4503) |
|
|
|
|
|
|
| Agriculture & animal husbandry |
516 |
(86.0) |
83 |
(14.0) |
599 |
0.000 |
| Industry |
228 |
(86.0) |
35 |
(14.0) |
263 |
|
| Construction |
111 |
(92.7) |
9 |
(7.3) |
120 |
|
| Service |
360 |
(78.7) |
87 |
(21.3) |
447 |
|
| Housewife/retired/unemployed |
522 |
(78.7) |
120 |
(21.3) |
642 |
|
| Other |
2021 |
(82.9) |
411 |
(17.1) |
2432 |
|
|
Household population |
|
|
|
|
|
|
| 1–3 person/home |
1000 |
(79.3) |
250 |
(20.7) |
1250 |
0.000 |
| 4–5 person/home |
1592 |
(81.4) |
334 |
(18.6) |
1926 |
|
| 6 + person/home |
1260 |
(87.3) |
186 |
(12.7) |
1446 |
|
|
Rooms for sleeping (n = 4575) |
|
|
|
|
|
|
| 1–2 |
2354 |
(82.2) |
482 |
(17.8) |
2836 |
0.06 |
| 3–4 |
1382 |
(82.5) |
277 |
(17.5) |
1659 |
|
| 5 + |
73 |
(93.0) |
7 |
(7.0) |
80 |
|
|
Source of the drinking water (n = 4594) |
|
|
|
|
|
|
| Piped water (in house/garden/outside) |
2342 |
(83.6) |
445 |
(16.4) |
2787 |
0.00 |
| Spring/public fountain |
726 |
(83.8) |
141 |
(16.2) |
867 |
|
| Bottled water/demijohn/pet water |
327 |
(72.9) |
109 |
(27.1) |
436 |
|
| Other ((river, rain water etc.)) |
430 |
(85.4) |
74 |
(14.6) |
504 |
|
|
Type of toilet system (n = 4598) |
|
|
|
|
|
|
| Connected to drainage system |
2778 |
(82.6) |
551 |
(17.4) |
3329 |
0.76 |
| Closed pit |
1033 |
(81.9) |
215 |
(18.1) |
1248 |
|
| Other (No facility) |
18 |
(86.7) |
3 |
(13.3) |
21 |
|
|
Source of heating (n = 4553) |
|
|
|
|
|
|
| Radiator (Central heating) |
366 |
(75.0) |
102 |
(25.0) |
468 |
0.00 |
| Radiator (Private) |
147 |
(71.0) |
62 |
(29.0) |
209 |
|
| Natural gas stove |
83 |
(84.8) |
14 |
(15.2) |
97 |
|
| Stove (Cool/wood) |
2985 |
(84.4) |
536 |
(15.6) |
3521 |
|
| Animal excrement |
103 |
(86.4) |
17 |
(13.6) |
120 |
|
| Electricity |
85 |
(83.7) |
18 |
(16.3) |
103 |
|
| Gas stove |
28 |
(78.0) |
7 |
(22.0) |
35 |
|
|
Family income (USD/month)*** (n = 4194) |
|
|
|
|
|
|
| 14–179 |
874 |
(84.6) |
147 |
(15.4) |
1021 |
0.00 |
| 183–394 |
1472 |
(83.7) |
272 |
(16.3) |
1744 |
|
| 398–538 |
503 |
(83.8) |
103 |
(16.2) |
606 |
|
| 541 + |
632 |
(75.1) |
191 |
(24.9) |
823 |
|
| Total | 3852 | (82.5) | 770 | (17.5) | 4622 | |
*Weighted, **The group (disabled / orphan hood payment by government, n = 29) is excluded. ***1 USD = 1 395 000 TL.
Table 5.
Adjusted odds ratios for Helicobacter pylori positivity for various risk factors by sex
| |
Sex |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Men |
Women |
||||||||
| Variables | B | P | OR | CI 95% | B | P | OR | CI 95% | ||
|
Age |
-0.013 |
0.009 |
0.987 |
0.977, |
0.997 |
-0.013 |
0.005 |
0.987 |
0.978, |
0.996 |
|
Education |
|
|
|
|
|
|
|
|
|
|
| High school + |
|
|
|
|
|
|
|
1.0 |
|
|
| No education |
|
|
|
|
|
0.813 |
0.000 |
2.254 |
1.517, |
3.350 |
| Primary complete |
|
|
|
|
|
0.762 |
0.000 |
2.142 |
1.593, |
2.880 |
| Secondary complete |
|
|
|
|
|
0.679 |
0.006 |
1.971 |
1.213, |
3.203 |
|
Marital Status |
|
|
|
|
|
|
|
|
|
|
| Never married |
|
|
1.0 |
|
|
|
|
1.0 |
|
|
| Widowed/Divorced |
0.450 |
0.242 |
1.568 |
0.738, |
3.335 |
0.294 |
0.209 |
1.342 |
0.848, |
2.122 |
| Currently married |
0.551 |
0.005 |
1.735 |
1.182, |
2.547 |
0.537 |
0.000 |
1.712 |
1.272, |
2.304 |
|
Social Security |
|
|
|
|
|
|
|
|
|
|
| Emekli sandigi |
|
|
1.0 |
|
|
|
|
1.0 |
|
|
| Private/foreign |
0.541 |
0.428 |
1.718 |
0.451, |
6.546 |
-1.216 |
0.023 |
0.296 |
0.104, |
0.848 |
| BAG-KUR |
0.385 |
0.083 |
1.469 |
0.951, |
2.269 |
-0.186 |
0.339 |
0.831 |
0.568, |
1.215 |
| SSK |
0.768 |
0.000 |
2.155 |
1.478, |
3.140 |
0.276 |
0.110 |
1.317 |
0.940, |
1.847 |
| None |
0.894 |
0.000 |
2.444 |
1.632, |
3.661 |
0.178 |
0.340 |
1.194 |
0.829, |
1.720 |
| Green Card |
0.593 |
0.068 |
1.809 |
0.956, |
3.422 |
0.302 |
0.290 |
1.353 |
0.773, |
2.369 |
|
Housing Tenure |
|
|
|
|
|
|
|
|
|
|
| Owned by household members |
|
|
1.0 |
|
|
|
|
|
|
|
| Rented |
0.276 |
0.109 |
1.317 |
0.940, |
1.845 |
|
|
|
|
|
| Lodging/no money paid |
0.692 |
0.034 |
1.997 |
1.055, |
3.779 |
|
|
|
|
|
|
Source of Drinking Water |
|
|
|
|
|
|
|
|
|
|
| Bottled water/demijohn/pet |
|
|
1.0 |
|
|
|
|
1.0 |
|
|
| Piped water |
0.418 |
0.023 |
1.518 |
1.060, |
2.174 |
0.684 |
0.000 |
1.981 |
1.477, |
2.657 |
| Spring/public fountain |
0.073 |
0.763 |
1.076 |
0.669, |
1.728 |
0.901 |
0.000 |
2.461 |
1.644, |
3.684 |
| Other (river. rain water etc.) |
0.808 |
0.012 |
2.244 |
1.198, |
4.204 |
0.438 |
0.050 |
1.550 |
1.001, |
2.402 |
|
Smoking |
|
|
|
|
|
|
|
|
|
|
| Never |
|
|
1.0 |
|
|
|
|
|
|
|
| Tried at past. currently non-smoker |
0.167 |
0.381 |
1.182 |
0.813, |
1.719 |
|
|
|
|
|
| Tried at past. currently occasional smoker |
-0.073 |
0.792 |
0.929 |
0.539, |
1.602 |
|
|
|
|
|
| Regular smoker |
0.449 |
0.017 |
1.566 |
1.083, |
2.264 |
|
|
|
|
|
|
Alcohol |
|
|
|
|
|
|
|
|
|
|
| Regular consumer |
|
|
1.0 |
|
|
|
|
|
|
|
| Tried at past. currently drinking occasionally |
0.731 |
0.009 |
2.078 |
1.202, |
3.590 |
|
|
|
|
|
| Tried at past. currently non-drinker |
0.764 |
0.009 |
2.148 |
1.214, |
3.799 |
|
|
|
|
|
| Never |
0.779 |
0.007 |
2.180 |
1.243, |
3.822 |
|
|
|
|
|
|
Region |
|
|
|
|
|
|
|
|
|
|
| South |
|
|
1.0 |
|
|
|
|
|
|
|
| West |
0.423 |
0.032 |
1.526 |
1.037, |
2.246 |
|
|
|
|
|
| North |
0.451 |
0.118 |
1.570 |
0.891, |
2.764 |
|
|
|
|
|
| Central |
0.653 |
0.002 |
1.922 |
1.258, |
2.936 |
|
|
|
|
|
| East |
0.816 |
0.002 |
2.262 |
1.353, |
3.782 |
|
|
|
|
|
| Constant | -0.404 | 0.388 | 0.668 | 0.335 | 0.172 | 1.398 | ||||
Variables entered in the model: age, residence, region, marital status, education, social security, occupation, housing tenure, source of drinking water, the number of the household per sleeping room, smoking, alcohol consumption.
Social security status was the only socio-economic status indicator in the final models. Those who had SSK and no social security were at greater risk than those who had insurance of Emekli Sandigi (Table 3).
The source of drinking water was a significant factor in the final models. The people who used piped water, spring/public fountain and other (river, rain water etc.) were at greater risk than those who used bottled water/demijohn/PET bottled water as drinking water (Table 3).
Housing was a significant factor only in the men’s model. Men lodging/or paying no money for their housing were at more risk than those who lived in a house owned by a household member (Table 5).
Lifestyle factors and prevalence of H.pylori infection
Smoking and alcohol consumption were associated with H.pylori infection. Regular smokers were at higher risk than non smokers. But this association did not hold for females (Tables 6 and 5). In contrast, regular alcohol consumption was a protective factor for H.pylori infection. All of those who never drink alcohol, those who had only tried in the past and the occasional drinkers had a higher risk than regular alcohol consumers (Tables 6 and 5).
Table 6.
Lifestyle factors associated with Helicobacter pylori infection
|
Lifestyle factors |
Hp positive |
Hp negative |
|
|
||
|---|---|---|---|---|---|---|
| n | (%)* | n | (%)* | Total | P | |
|
Smoking cigarettes (n = 4605) |
|
|
|
|
|
|
| Never |
1662 |
(80.8) |
370 |
(19.2) |
2032 |
0.00 |
| Tried at past, currently non-smoker |
745 |
(81.6) |
159 |
(18.4) |
904 |
|
| Tried at past, currently occasional smoker |
245 |
(82.4) |
47 |
(17.6) |
292 |
|
| Regular smoker |
1190 |
(85.9) |
187 |
(14.1) |
1377 |
|
|
Drinking alcohol (n = 4593) |
|
|
|
|
|
|
| Regular consumer |
69 |
(74.7) |
18 |
(25.3) |
87 |
0.03 |
| Others |
3762 |
(82.8) |
744 |
(17.2) |
4506 |
|
| Total | 3852 | (82.5) | 770 | (17.5) | 4622 | |
* Weighted.
Analysis of factors and H.pylori infection by sex
Since sex was a significant factor for H.pylori infection it was necessary to analyze factors separately for each sex. In men, age, marital status, social security status, housing tenure, type of drinking water, smoking, alcohol use and geographic region were factors. However, for women, age, marital status, social security status, type of drinking water and education were factors (Table 5).
Discussion
So far as we know this study is the most representative one that is based on a sample derived from the population of one country, estimating the factors associated with the prevalence of Helicobacter pylori infection and using the 13C-UBT. Furthermore, the response rates were very high. In this study, it was produced highly significant estimates [Design effect (DEFT) = 2.01 and standard error = 0.008].
Awareness of Helicobacter pylori is little more than a decade old. Yet there have been many studies all over the world about its epidemiology. Most prevalence data have used random sampling of blood donors, clinic attendees or industrial employees; none of these groups provides a truly normal population as emphasised by Pounder [12].
Studies that have used labelled breath tests in a normal population to detect Helicobacter pylori infection are very rare. However, they are highly sensitive, specific and are also recommended by the Maastricht 2–2000 Consensus Report and by the Canadian Helicobacter Study Group Consensus conference, 2004 [7,13,14].
When comparing the rates from previous studies directly with our study, it should be kept in mind that other studies also differ from ours in terms of variation by age, type of population, type of diagnostic test and study time at which the study was done.
In TURHEP, the weighted overall prevalence of Helicobacter pylori infection was 82.5% (95% CI 81.0-84.2) with 13C-UBT. Helicobacter pylori prevalence has been reported to reach 70% or more in developing countries and to be less than 40% in developed countries [15-37].
There was an inverse association between age and infection in our study. Earlier studies have shown differing trends regarding age and Helicobacter pylori prevalence. Whereas Helicobacter pylori prevalence increased with age at earlier ages, there was a slight decrease in populations over 60 years of age in France and over 50 years in the other countries (Vietnam, Algeria and Ivory Coast) [15]. Infection increased up to the 40–49 age group, then decreased in analyses for Southern Brazil and Northern India [17,30]. Also, the prevalence peaked at ages 45 to 64 and dropped after the age 65 in Chile and the Czech Republic [31,37]. In Ankara (Turkey), seroprevalence was 58.4% for ages 15–19, 62.6% for ages 20–29, 67.6% for ages 30–39, 81.3% for ages 40–49 and 66.3% for over 50 years [38]. In India also the prevalence was increasing to 100% by 60 then decreasing to 80% by 70 years (n = 238, ages 3–70) and in Athens, whereas the seroprevalence was increasing from 14.2% for ages 15–24 to 67.4% for ages 55–64, it decreased to 57.9% for ages >65 [18,32]. Only in Beninese populations, in 2005 (n = 446, over 2 years old) no association was found between seroprevalence and age [28]. In contrast, some studies claimed that Helicobacter pylori prevalence increased with age [15,16,19,21,24-26,29,33-35,39-43].
We found that men in Turkey were at greater risk than women for Helicobacter pylori infection. Likewise, in Northern California, men had a higher prevalence of antibodies across all strata of race/ethnicity, age, education and income (OR = 2.0, 95% CI 1.2-3.1) [42]. Also, in Northern Ireland, infection was more common in males (60.9%) than females (55.2%, p < 0.01, Or for males versus females was 1.19 (95% CI 1.02-1.40) [35]. In Leeds (UK), Spain and Chile, it was higher in men [31,36,44]. Conversely, in some studies, which mostly had small samples, there was no difference found in Helicobacter pylori prevalence between the sexes [15-18,21,22,24-26,28,29,34,37-39],[43]. To our knowledge, only one study from Israel found that the relative risk of Helicobacter pylori infection was increased in women smokers [19]. We agree with Moayyedi-et-al. that the positive association of Helicobacter pylori with the male sex should probably not be interpreted as a direct causal relationship [36]. The reason for the possible gender difference is unclear but may relate to young boys having poorer hygiene than young girls. Because of social gender roles in Turkey, men seek less healthy facilities for toilet needs than women, and men are outdoors more than women, which brings more risks of infection. Further, men tend to participate in more of the risky behaviours such as smoking, alcohol drinking than women.
The current residential region was found to be a risk factor for H.pylori infection. In Turkey, the western areas are more developed, more crowded, better educated, and have better housing conditions; families are smaller than in the East. The reason why H.pylori infections are lowest in individuals living in the South must be related to this being a major area for growing citrus fruits. These contain high levels of Vitamin C. People in the South can eat oranges, lemons, tangerines or bitter oranges frequently and continuously or drink the juices because citrus fruits are cheap and plentiful all the year round. It is known that Vitamin C is effective in the prevention of most infections. Also H.pylori can be expected not to survive in acidic gastric conditions produced by the acidic citrus fruits. Moreover, for regular smokers the highest H.pylori prevalence may result from an interaction between tobacco and Vitamin C. In contrast, the highest H.pylori infections were found in subjects living in eastern Turkey, which has the least available citrus fruits; they cannot be grown, and snow prevents their transport for several months each year; Besides, this region is the least developed. Although in TURHEP, dietary habits and daily consumptions were not included, supportive studies are available [45-48]. Additionally, garlic is frequently used in southern Turkey. One study presented garlic as a possible protective factor for gastric lesions with H.pylori infection [46].
Some studies with small sample sizes comparing the regions are available from Turkey. H.pylori infection was found to be 73.8% in the West, 48%-81% in the Central, 60%-85.4% in the Eastern parts of the country [38,39,49-51].
In most of studies it was found that H.pylori infections were inversely related to level of education [16,22,24,34,37,42,43]. Likewise in TURHEP, the lower the education of the subjects, especially for females, the higher the risk for H.pylori infection. However, two other studies found no association [19,28].
The status of social security was a significant factor in the TURHEP study’s final model and in the models by sex. To our knowledge, this variable has not previously been used as a socio-economic status indicator in any study related with H.pylori infection. Some previous studies have presented an inverse association between H.pylori infection and family income as a socio-economic status indicator [29,38,42,43,52-54]. In TURHEP, the lower the income of the subjects, the higher the infection, but only in a univariate analysis. Some researchers have also studied the association between infection and social class/socio-economical class. In Korea in adults, the rate of infection was high and independent of socio-economic class. However, in children, it was inversely related to the socio-economic class of the child’s family [21]. In Northern Ireland, the adjusted OR of infection in subjects from manual workers relative to those from non-manual occupations was 1.7 (95% CI: 1.47-1.98) [35]. In Northern England, infection was more common in the lower social class groups [36]. In Libya, 91% of a low socio-economic class was H.pylori-positive, while those of middle and high socio-economic classes showed 53% and 57% positivity respectively [24]. In Northern India infection was not associated with socio-economic status [17].
Housing tenure, as another socio-economic indicator was found significant only in a model for males in TURHEP. In contrast, another study, showed no association between prevalence of H.pylori and type of housing (owned/rented)[21].
In TURHEP, a water- H.pylori infection association was found in the final models. This association is a question about H.pylori infection being one of the water-borne contagious diseases. This association was mentioned in many studies from different parts of the world and it has been found that there is mostly a positive significant relation [1,21,25,52]. On the other hand, no association was found in studies from Benin and Turkey [28,39].
Smoking was a significant factor for H.pylori infection in TURHEP except for the female model. Similar results have been presented in some studies [19,35-37]. However smoking was not associated with H.pylori infection in some other studies [15,16,21,23,30,34,43].
In TURHEP, regular alcohol consumption was found to be a protective factor except for the females model. Similar results have also been presented in some earlier studies [53-56]. In a EUROGAST Study, a univariate analysis showed that alcohol consumption was associated with a reduced prevalence of H.pylori, but this effect disappeared completely after adjustment in the multivariate analysis [34]. No association was found between H.pylori and alcohol use in other studies [15,21,30,35,36].
Conclusions
In Turkey, H.pylori prevalence was found to be very high. Individuals, who were women, elderly adults, single, at high educational levels, living in southern Turkey, having social security of Emekli Sandigi, drinking bottled water, non-smokers and regular alcohol consumers, were under less risk of H.pylori infection than others.
In the TURHEP study, whereas prevalence was estimated as 82.5% (95%CI 81.0-84.2) in the adult population, age, sex, education and marital status were suggested as playing critical roles as co-factors for H.pylori infection. Social security, housing tenure and also water have dependant role. Whereas smoking, a common habit especially in men was positively associated, alcohol use, not as common as smoking, was a protective factor for H.pylori. Living in the southern region of Turkey, a citrus fruit growing area, is seen as a protective factor for H.pylori infection and was the most interesting result in TURHEP.
We have presented high quality data from normal, healthy individuals, representative of the whole country, from Turkey. The results of the TURHEP study, offer important public health implications for the prevention of H.pylori. In the future, cohort studies should be implemented to help define more significant risk factors.
Endnotes
aEmekli Sandigi: The pension fund for civil servants, SSK ‘Social Security Institution’ the insurance of employee, BAG-KUR: the insurance of tradesman, artists and other freelance workers, Green-Card: limited insurance of people do not have any other insurance.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NO conceived of the study, developed the questionnaire, monitoring the survey, performed the statistical analysis and finalized the manuscript. ST participated in the study design and helped to draft the manuscript. SC participated in the study design, developed the semi-structural interview guide, and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Distribution of the cities selected for sample for the Helicobacter pylori prevalence study.
Helicobacter pylori prevalence in Turkey by region.
Contributor Information
Nilufer Ozaydin, Email: nozaydin@gmail.com.
Sinan A Turkyilmaz, Email: aturkyil@hacettepe.edu.tr.
Sanda Cali, Email: sandacali@superonline.com.
Acknowledgements and funding
The research leading to these results has received funding from SANDOZ Pharmaceutical Company and Marmara Health Education and Research Foundation.
The authors thank the members of Public Health Department of the School of Medicine of Marmara University, data collectors, all participants, Prof. Dr. Sibel Kalaca for her critical review of the analysis and Prof. Ray W. Guillery for his editing of the manuscript.
References
- Van Duynhoven YT, Jonge R. Transmission of Helicobacter pylori: a role for food? Bull World Health Organ. 2001;13(5):455–460. PMID 11417041. [PMC free article] [PubMed] [Google Scholar]
- Helicobacter pylori: Disease Burden. [Online].[cited2007 Mar 19];[2.screen] Available from: URL: http://www.who.int/vaccine_research/disease/soa_bacterial/en/index1.html.
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;13(15):1175–1186. doi: 10.1056/NEJMra020542. PMID 12374879. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carsinoma. N Engl J Med. 1991;13(16):1127–1131. doi: 10.1056/NEJM199110173251603. PMID 1891020. [DOI] [PubMed] [Google Scholar]
- Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from prospective investigation. BMJ. 1991;13(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. PMID 2059685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersten O, Wilmoth JR. The cancer transition in Japan since 1951. Demogr Res. 2002;13(5):271–306. Available from URL: http://www.demographic-research.org/volumes/Vol7/5/ [Google Scholar]
- Jones NL, Sherman P, Fallone CA, Flook N, Smaill F. Canadian Helicobacter Study Group Consensus Conference: update on the approach to Helicobacter pylori infection in children and adolescents—an evidence-based evaluation. Can J Gastroenterol. 2005;13(7):399–408. PMID 16010300. [PubMed] [Google Scholar]
- Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;13(2):283–297. doi: 10.1093/oxfordjournals.epirev.a018040. PMID 11218379. [DOI] [PubMed] [Google Scholar]
- IARC. Working Group. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Schistosomes, liver flukes and Helicobacter Pylori, Vol. 61. Lyon, France: IARC; 1994. http://monographs.iarc.fr/ENG/Monographs/vol61/volume61.pdf. [PMC free article] [PubMed] [Google Scholar]
- Leung WK, Hung LC, Kwok CK, Leong RW, Ng DK, Sung JJ. Follow up a serial urea breath test results in patients after consumption of antibiotics for non-gastric infections. World J Gastroenterol. 2002;13:703–706. doi: 10.3748/wjg.v8.i4.703. PMID 12174382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine L, Estrada R, Trujillo M, Knigge K, Fennerty MB. Effect of proton pump inhibitor therapy on diagnostic testing for Helicobacter pylori. Ann Intern Med. 1998;13(7):547–550. doi: 10.7326/0003-4819-129-7-199810010-00007. PMID 9758575. [DOI] [PubMed] [Google Scholar]
- Pounder RE, Nq D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;13(Suppl.2):33–39. PMID 8547526. [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O’Morain C, Hungin APS, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection TheMaastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002;13:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- Bourke B, Ceponis P, Chiba N, Czinn S, Ferraro R, Fishbach L, Gold B, Hyunh H, Jacobson K, Jones NL, Koletzko S, Lebel S, Moavyedi P, Ridell R, Sherman P, van Zanten S, Beck I, Best L, Boland M, Bursey F, Chaun H, Cooper G, Craig B, Creuzenet C, Critch J, Govender K, Hassall E, Kaplan A, Keelan M, Noad G. et al. Canadian Helicobacter Study Group Consensus Conference: update on the approach to Helicobacter pylori infection in children and adolescents – an evidence based evaluation. Can J Gastroenterol. 2005;13(7):399–408. Review. Erratum in:Can J Gastroenterol. 2005 Aug;19(8):478. [PMID 16010300] [PubMed] [Google Scholar]
- Megraund F, Brassens-Rabbe MP, Denis F, Belbouri A, Hoa DQ. Seroepidemiology of campylobacter pylori infection in various populations. J Clin Microbiol. 1989;13(8):1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. PMID 2549098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Moagel MA, Evans DG, Abdulghani ME, Adam E, Evans E, Evans DJ Jr, Malaty HM, Graham DY. Prevalence of Helicobacter (formerly Campylobacter) pylori infection in Saudia Arabia, and comparison of those with and without upper gastrointestinal symptoms. Am J Gastroenterol. 1990;13:944–8. [PubMed] [Google Scholar]
- Singh V, Trikha B, Nain CK, Singh K, Vaiphei K. Epidemiology of Helicobacter pylori and peptic ulcer in India. J Gastroenterol Hepatol. 2002;13(6):659–665. doi: 10.1046/j.1440-1746.2002.02746.x. PMID 12100610. [DOI] [PubMed] [Google Scholar]
- Graham DY, Adam E, Reddy GT, Agarwal JP, Agarwal R, Evans DJ Jr, Malaty HM, Evans DG. Seroepidemiology of H.pylori-infection in India. Dig Dis Sci. 1991;13(8):1084–1088. doi: 10.1007/BF01297451. 1864201. [DOI] [PubMed] [Google Scholar]
- Fich A, Carel RS, Keret D, Goldin E. Sero prevalence of Helicobacter pylori in the Israeli population. Eur J Gastroenterol Hepatol. 1993;13:339–341. doi: 10.1097/00042737-199305000-00007. [DOI] [Google Scholar]
- Zhou D, Yang H. Epidemiology of Helicobacter pylori infection in the People’s Republic of China. Chin Med J. 1995;13(4):304–313. 7789221. [PubMed] [Google Scholar]
- Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol. 1996;13(3):257–262. doi: 10.1093/oxfordjournals.aje.a008736. 8561159. [DOI] [PubMed] [Google Scholar]
- Peach HG, Pearce DC, Farish SJ. Helicobacter pylori infection in an Australian regional city: prevalence and risk factors. Med J Aust. 1997;13(6):310–313. doi: 10.5694/j.1326-5377.1997.tb125076.x. PMID 9322776. [DOI] [PubMed] [Google Scholar]
- Gold BD, Khanna B, Huang LM, Lee CY, Banatvala N. Helicobacter pylori acquisition in infancy after decline of maternal passive immunity. Pediatr Res. 1997;13(5):641–666. doi: 10.1203/00006450-199705000-00007. PMID 9128285. [DOI] [PubMed] [Google Scholar]
- Bakka AS, Salih BA. Prevalence of Helicobacter pylori infection in asymptomatic subjects in Libya. Diagn Microbiol Infect Dis. 2002;13(4):265–268. doi: 10.1016/S0732-8893(02)00411-X. PMID 12151185. [DOI] [PubMed] [Google Scholar]
- Nurgalieva ZZ, Malaty HM, Graham DY, Almuchabetova R, Mahmudova A, Kapsultanova D, Osato MS, Hollinger FB, Zhangabylov A. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. Am J Trop Med Hyg. 2002;13(2):201–206. doi: 10.4269/ajtmh.2002.67.201. PMID 12389948. [DOI] [PubMed] [Google Scholar]
- Sporea I, Popescu A, van Blankenstein M, Sirli R, Focşea M, Dănilă M. The prevalence of Helicobacter pylori infection in western Romania. Rom J Gastroenterol. 2003;13(1):15–18. PMID 12673374. [PubMed] [Google Scholar]
- Malekzadeh R, Merat S, Derakhshan MH, Siavoshi F, Yazdanbod A, Mikaeli J, Sotoudemanesh R, Sotoudeh M, Farashvash MJ, Nasseri-Moghaddam S, Pourshams A, Dolatshahi S, Abedi B, Babaei M, Arshi S, Majidpour A. Low Helicobacter pylori eradication rates with 4 and 7 day regimens in an Iranian population. J of Gastroenterol and Hepatol. 2003;13:13–17. doi: 10.1046/j.1440-1746.2003.02897.x. PMID 12519218. [DOI] [PubMed] [Google Scholar]
- Aguemon BD, Struelens MJ, Massougbodji A, Ouendo EM. Prevalence and risk- factors for Helicobacter pylori infection in urban and rural Beninese populations. Clin Microbiol Infect. 2005;13(8):611–617. doi: 10.1111/j.1469-0691.2005.01189.x. 16008612. [DOI] [PubMed] [Google Scholar]
- Parente JML, Silva BB, Palha-Dias MP, Zaterka S, Nishimura NF, Zeitune JM. Helicobacter pylori infection in children of low and high socioeconomic status in northeastern Brazil. Am J Trop Med Hyg. 2006;13(3):509–512. PMID 16968931. [PubMed] [Google Scholar]
- Santos IS, Boccio J, Santos AS, Valle NCJ, Halal CS, Bachilli MC, Lopes RD. Prevalence of Helicobacter pylori infection and associated factors among adults in Southern Brazil: a population-based cross-sectional study. BMC Public Health. 2005;13:118. doi: 10.1186/1471-2458-5-118. PMID 16283940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Rollan A, Harris PR, Serrano C, Gederlini A, Margozzini P, Gonzalez C, Aquilera X, Venegas A, Jara A. Gastric cancer is related to early Helicobacter pylori infection in a high-prevalence country. Cancer Epidemiol Biomarkers Prev. 2007;13(4):662–667. doi: 10.1158/1055-9965.EPI-06-0514. PMID 17416755. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos P, Vafiadis-Zouboulis I, Tzivras M, Kourtessas D, Katsilambros N, Archimandritis A. Helicobacter pylori (Hpylori) infection in Greece: the changing prevalence during a ten-year period and its antigenic profile. BMC Gastroenterol. 2002;13:11. doi: 10.1186/1471-230X-2-11. PMID 12014991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitas F, Forman D, Yarnell WG, Burr ML, Elwood PC, Pedley S, Marks KJ. Helicobacter pylori infection rates in relation to age and social class in a population of Welsh men. Gut. 1991;13(1):25–28. doi: 10.1136/gut.32.1.25. PMID 1991634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;13(12):1672–1676. doi: 10.1136/gut.34.12.1672. PMID 8282253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LJ, McCrum EE, Evans AE, Bamford KB. Epidemiology of Helicobacter pylori infection among 4742 randomly selected subjects from Northern Ireland. Int J Epidemiol. 1997;13(4):880–887. doi: 10.1093/ije/26.4.880. PMID 9279623. [DOI] [PubMed] [Google Scholar]
- Moayyedi P, Axon AT, Feltbower R, Duffett S, Crocombe W, Braunholtz D, Richards ID, Dowel AC, Forman D. Leeds HELP Study Group. Relation of adult lifestyle and socioeconomic factors to the prevalence of Helicobacter pylori infection. Int J Epidemiol. 2002;13(3):624–631. doi: 10.1093/ije/31.3.624. PMID 12055165. [DOI] [PubMed] [Google Scholar]
- Bures J, Kopacova M, Koupil I, Vorisek V, Rejchrt S, Beranek M, Seifert B, Pozler O, Zivny P, Douda T, Kolesarova M, Pinter M, Palicka V, Holcik J. European Society for Primary Care Gastroenterology. Epidemiology of Helicobacter pylori infection in the Czech Republic. Helicobacter. 2006;13(1):56–65. doi: 10.1111/j.0083-8703.2006.00369.x. PMID 16423091. [DOI] [PubMed] [Google Scholar]
- Us D, Hasçelik G. Seroprevalence of Helicobacter pylori infection in an Asymptomatic Turkish population. J of Infect. 1998;13(2):148–150. doi: 10.1016/S0163-4453(98)80169-2. PMID 9821089. [DOI] [PubMed] [Google Scholar]
- Yilmaz E, Doğan Y, Gürgöze MK, Unal S. Seroprevalence of Helicobacte pylori infection among children and their parents in eastern Turkey. J Paediatr Child Health. 2002;13(2):183–186. 12031003. [PubMed] [Google Scholar]
- Kosunen TU, Höök J, Rautelin HI, Myllyla G. Age dependant increase of Camplylobacter pylori antibodies in blood donors. Scand J Gastroenterol. 1989;13(1):110–114. doi: 10.3109/00365528909092247. PMID 2648556. [DOI] [PubMed] [Google Scholar]
- Perez-Perez GI, Taylor DN, Bodhidatta L, Wongsrichanalai J, Baze WB, Dunn BE, Echeverria PD, Blaser MJ. Seroprevalence of Helicobacter pylori infections in Thailand. J of Infect Dis. 1990;13:1237–1241. doi: 10.1093/infdis/161.6.1237. PMID 2345304. [DOI] [PubMed] [Google Scholar]
- Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;13(8):856–863. doi: 10.1093/oxfordjournals.aje.a117725. PMID 7572962. [DOI] [PubMed] [Google Scholar]
- Graham DY, Malaty HM, Evans DG, Evans DJ Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States: Effect of age, race and socipeconomic status. Gastroenterology. 1991;13(6):1495–1501. doi: 10.1016/0016-5085(91)90644-z. PMID 2019355. [DOI] [PubMed] [Google Scholar]
- Leandro Liberato SV, Hernández Galindo M, Torroba Alvarez L, Sánchez Miramón F, Leandro Ciriza SE, Gómez Abadía A, Chueca Rodriquez P. Helicobacter pylori infection in the child population in Spain: prevalence, related factors and influence on growth. An Pediatr (Barc) 2005;13(6):489––494. doi: 10.1016/s1695-4033(05)70247-2. PMID 16324613. [DOI] [PubMed] [Google Scholar]
- Rokkas T, Liatsos C, Petridou E, Papatheodorou G, Karameris A, Ladas SD, Raptis SA. Relationship of Helicobacter pylori CagA(+) status to gastric juice vitamin C levels. Eur J of Clin İnvest. 1999;13(1):56–62. doi: 10.1046/j.1365-2362.1999.00432.x. PMID 10092990. [DOI] [PubMed] [Google Scholar]
- You WC, Li JY, Zhang L, Jin ML, Chang YS, Ma JL, Pan KF. Etiology and prevention of gastric cancer: a population study in a high risk area of China. Chin J Dig Dis. 2005;13(4):149–154. doi: 10.1111/j.1443-9573.2005.00222.x. PMID 16246221. [DOI] [PubMed] [Google Scholar]
- Kato I, Vivas J, Plummer M, Lopez G, Peraza S, Castro D, Sanchez V, Cano E, Andrade O, Garcia R, Franceschi S, Oliver W, Munoz N. Environmental factors in Helicobacter pylori related gastric precancerous lesions in Venezuela. Cancer Epidemiol Biomarkers Prev. 2004;13(3):468–76. [PubMed] [Google Scholar]
- Simon JA, Hudes ES, Perez-Perez GI. Relation of serum ascorbic acid to Helicobacter pylori serology in US adults: the Third National Health and Nutrition Examination Survey. J Am Coll Nutr. 2003;13(4):283–289. doi: 10.1080/07315724.2003.10719305. PMID 12897042. [DOI] [PubMed] [Google Scholar]
- Akarca US, Aydın A, Ozutemiz O, Musoglu A, Uslu Z. Ege yoresinde Helikobakter pylori infeksiyonunun seroprevalensi. Ege Tıp Derg. 1993;13(1–2):31–35. [In Turkish] [Google Scholar]
- Ozden A, Dumlu S, Soylu K, Samur M, Uzunalimoglu O. The prevalence of Helicobacter pylori infection in a defined healthy population in Turkey [Abstract] Hellenic J Gastroenterol. 1992;13(Suppl):267. [Google Scholar]
- Turkdogan MK, Kaba I, Dulger C, Mete R, Akman N. Epidemiological aspects of Helicobacter pylori infection in Van region of Eastern Turkey. 2004. pp. 29–34. (abstract book of the forth East and Souteast Anatolia Hepato-Gastroenterology symposium).
- Klein PD, Graham DY, Gaillour A, Opekun AR, Smith EO. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet. 1991;13(8756):1503–1506. doi: 10.1016/0140-6736(91)93196-G. PMID 1675369. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Lane AJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Inverse relationship between alcohol consumption and active Helicobacter pylori infection: the Bristol Helicobacter project. Am J Gastroenterol. 2002;13(11):2750–2755. doi: 10.1111/j.1572-0241.2002.07064.x. PMID 12425543. [DOI] [PubMed] [Google Scholar]
- Brenner H, Rothenbacher D, Bode G, Adler G. Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ. 1997;13(7121):1489–1492. doi: 10.1136/bmj.315.7121.1489. PMID 9420488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper-Nybelen J, Rothenbacher D, Brenner H. Relationship between lifetime alcohol consumption and Helicobacter pylori infection. Ann Epidemiol. 2005;13(8):607–613. doi: 10.1016/j.annepidem.2004.11.001. PMID 16118005. [DOI] [PubMed] [Google Scholar]
- Kuepper-Nybelen J, Thefeld W, Rothenbacher D, Brenner H. Patterns of alcohol consumption and Helicobacter pylori infection: results of a population-based study from Germany among 6545 adults. Aliment Pharmacol Ther. 2005;13(1):57–64. doi: 10.1111/j.1365-2036.2004.02276.x. PMID 15644046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of the cities selected for sample for the Helicobacter pylori prevalence study.
Helicobacter pylori prevalence in Turkey by region.