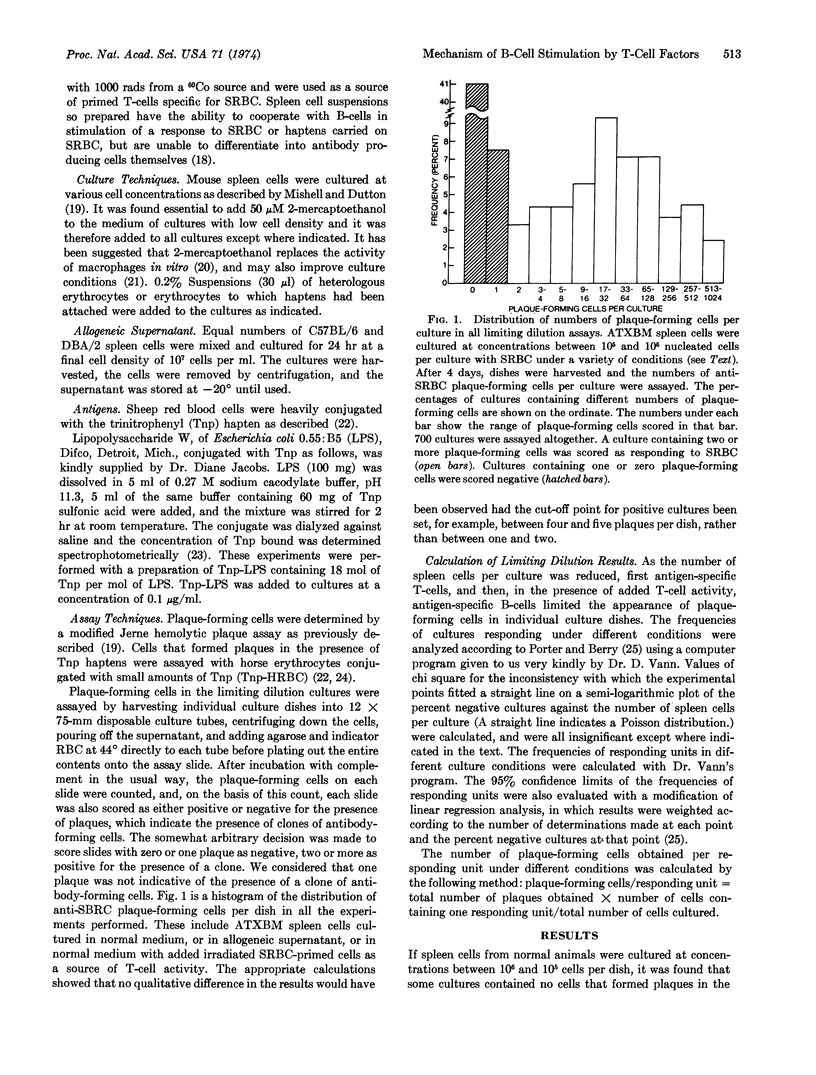
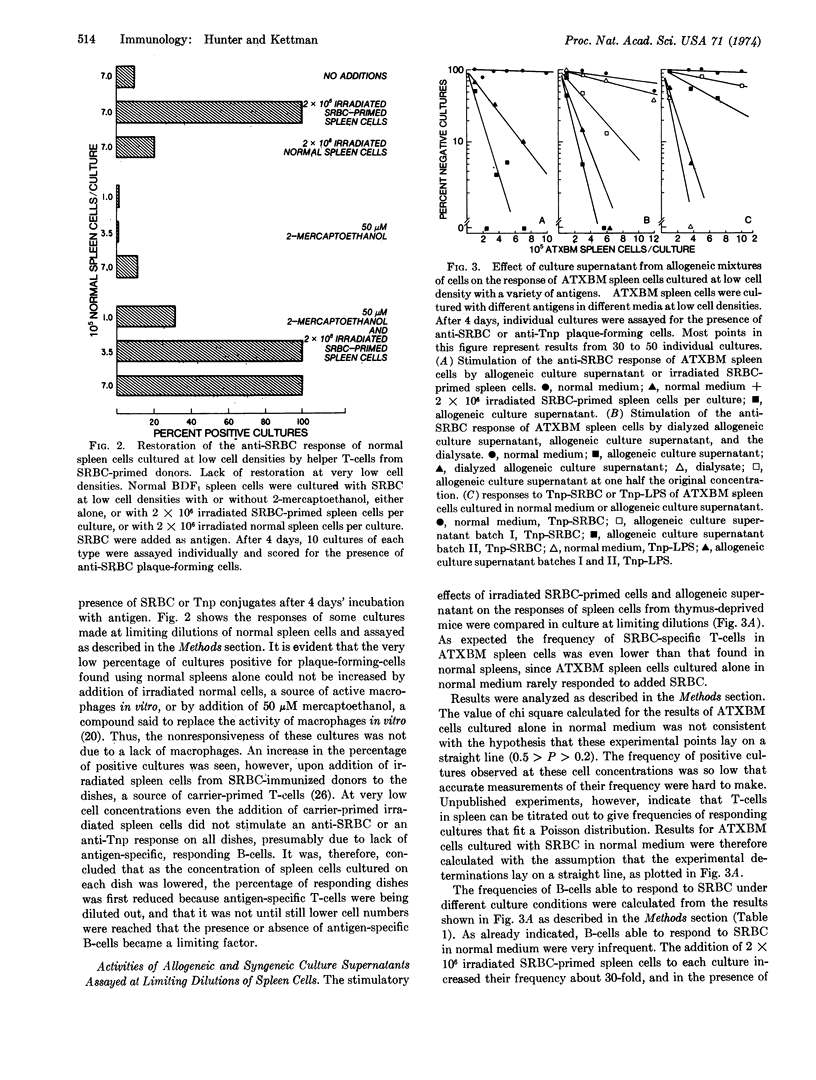
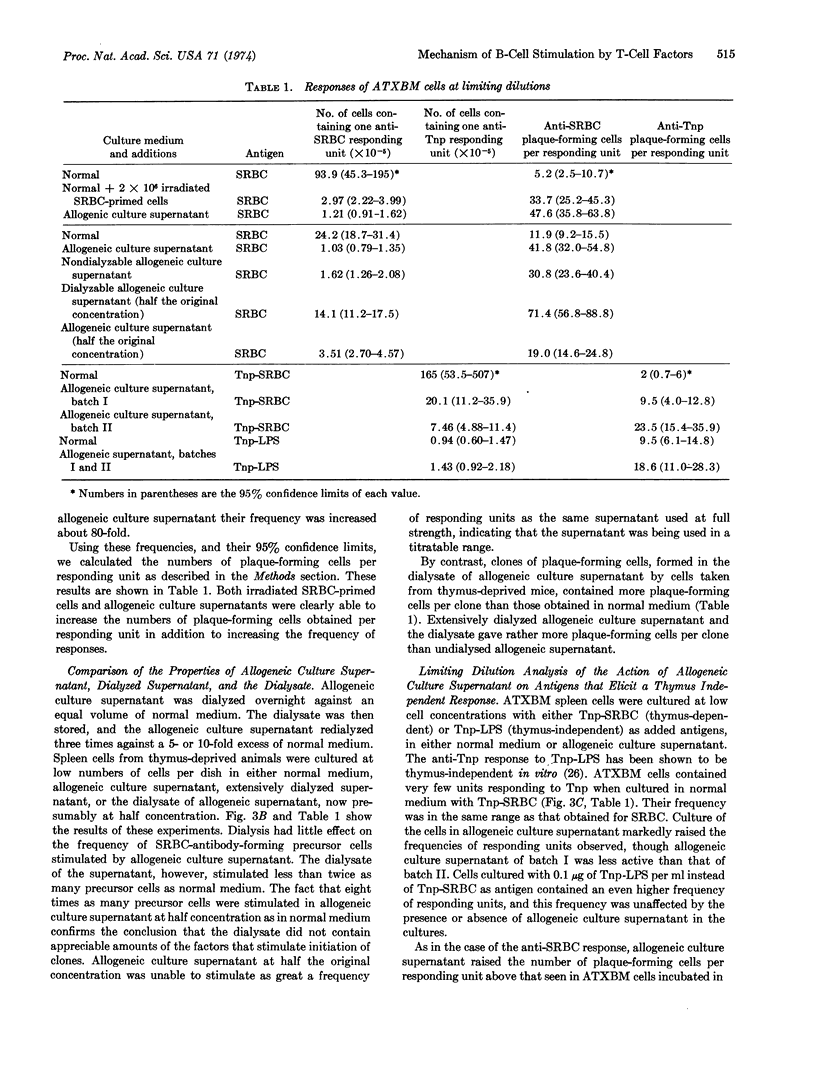
Abstract
The mode of action of “allogeneic supernatant” (the culture supernatant of a 24-hr mixedlymphocyte reaction), has been studied. This factor stimulates the response of spleen cell cultures depleted in thymus-derived lymphoid cells (T-cells) to antigens that elicit a thymus-dependent response. We used a limiting dilution analysis, in which the frequency and size of response of individual bone-marrow-derived lymphoid cells (B-cells) could be measured. In confirmation of other reports, the occurrence of B-cells responding to antigen under different conditions was shown to follow a Poisson distribution in mouse spleen cell suspensions. Allogeneic supernatant increased responses to thymus-dependent antigens, both by increasing the frequency of B-cells whose response is initiated and by increasing the numbers of antibody-forming cells obtained from each responding B-cell. Two fractions were obtained by dialysis of the supernatant. The nondialyzable fraction contained factors able to increase both the frequency of B-cells responding to sheep erythrocytes, and the size of the responding unit. The dialysate contained factors that were only able to increase the numbers of antibody-forming cells obtained per responding B-cell from B-cells whose response had already been initiated by antigen-specific T-cells.
Since the nondialyzable factors were active in the absence of detectable functional T-cells, it was concluded that these factors, produced by T-cells, might represent one mechanism whereby T-cells cooperate with B-cells in the initiation or development of a humoral immune response.
Keywords: Poisson distribution, responding B-cell frequency, clone size, dialysis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton S. When allogeneic mouse spleen cells are mixed in vitro the T-cells secrete a product which guides the maturation of B-cells. Scand J Immunol. 1972;1(1):89–98. doi: 10.1111/j.1365-3083.1972.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Enhancement of antibody synthesis in vitro by mercaptoethanol. Cell Immunol. 1972 Jan;3(1):156–160. doi: 10.1016/0008-8749(72)90237-7. [DOI] [PubMed] [Google Scholar]
- Falkoff R., Kettman J. Differential stimulation of precursor cells and carrier-specific thymus-derived cell activity in the in vivo reponse to heterologous erythrocytes in mice. J Immunol. 1972 Jan;108(1):54–58. [PubMed] [Google Scholar]
- Feldman M. Induction fo B cell tolerance by antigen specific T cell factor. Nat New Biol. 1973 Mar 21;242(116):82–84. [PubMed] [Google Scholar]
- Feldmann M., Basten A. Cell interactions in the immune response in vitro. IV. Comparison of the effects of antigen-specific and allogeneic thymus-derived cell factors. J Exp Med. 1972 Oct 1;136(4):722–736. doi: 10.1084/jem.136.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Basten A. Specific collaboration between T and B lymphocytes across a cell impermeable membrane in vitro. Nat New Biol. 1972 May 3;237(70):13–15. doi: 10.1038/newbio237013a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. V. Specific collaboration via complexes of antigen and thymus-derived cell immunoglobulin. J Exp Med. 1972 Oct 1;136(4):737–760. doi: 10.1084/jem.136.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Miller R. G., Phillips R. A. Initiation of antibody production to sheep erythrocytes in vitro: replacement of the requirement for T-cells with a cell-free factor isolated from cultures of lymphoid cells. J Immunol. 1972 Feb;108(2):547–551. [PubMed] [Google Scholar]
- Hartmann K. U. Induction of a hemolysin response in vitro. Interaction of cells of bone marrow origin and thymic origin. J Exp Med. 1970 Dec 1;132(6):1267–1278. doi: 10.1084/jem.132.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J. A., Dutton R. W. Cell components in the immune response. 3. Neonatal thymectomy: restoration in culture. Cell Immunol. 1970 Jul;1(2):190–195. doi: 10.1016/0008-8749(70)90006-7. [DOI] [PubMed] [Google Scholar]
- Hunter P., Munro A., McConnell I. Properties of educated T cells for rosette formation and cooperation with B cells. Nat New Biol. 1972 Mar 15;236(63):52–53. doi: 10.1038/newbio236052a0. [DOI] [PubMed] [Google Scholar]
- Jonard J., Panijel J. In vitro primary response in microcultures containing less than fifty thousand lymphoid cells. Eur J Immunol. 1973 Apr;3(4):245–249. doi: 10.1002/eji.1830030414. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten antibody responses. 3. Stimulation of antibody synthesis and facilitation of hapten-specific secondary antibody responses by graft-versus-host reactions. J Exp Med. 1971 Feb 1;133(2):169–186. doi: 10.1084/jem.133.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. An in vitro primary immune response to 2,4,6-trinitrophenyl substituted erythrocytes: response against carrier and hapten. J Immunol. 1970 Jun;104(6):1558–1561. [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. Radioresistance of the enhancing effect of cells from carrier-immunized mice in an in vitro primary immune response. Proc Natl Acad Sci U S A. 1971 Apr;68(4):699–703. doi: 10.1073/pnas.68.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. F. Studies on mouse leukaemia. The role of the thymus in leukaemogenesis by cell-free leukaemic filtrates. Br J Cancer. 1960 Mar;14:93–98. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER E. H., BERRY R. J. THE EFFICIENT DESIGN OF TRANSPLANTABLE TUMOUR ASSAYS. Br J Cancer. 1963 Dec;17:583–595. doi: 10.1038/bjc.1963.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Amkraut A. A. Immunogenicity of trinitrophenyl-hemocyanin: production of primary and secondary anti-hapten precipitins. J Immunol. 1966 Sep;97(3):421–430. [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G., Connell M. S., Priore R. L. Cellular differentiation of the immune system of mice. I. Separate splenic antigen-sensitive units for different types of anti-sheep antibody-forming cells. J Exp Med. 1968 Sep 1;128(3):437–457. doi: 10.1084/jem.128.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Reconstitution of the antibody response in vitro of T cell-deprived spleen cells by supernatants from spleen cell cultures. J Immunol. 1972 Dec;109(6):1379–1385. [PubMed] [Google Scholar]
- Vann D. C., Kettman J. R. In vitro cooperation of cells of bone marrow and thymus origins in the generation of antibody-forming cells. J Immunol. 1972 Jan;108(1):73–80. [PubMed] [Google Scholar]


