Abstract
Objectives
We previously reported survival trends among patients with inflammatory breast cancer (IBC) over a 30-year-period before 2005. Here we evaluated survival outcomes for women with IBC diagnosed before or after October 2006, in the era of HER2-directed therapy and after opening a dedicated multidisciplinary IBC clinic.
Methods
We retrospectively identified and reviewed 260 patients with newly diagnosed IBC without distant metastasis, 168 treated before October 2006 and 92 treated afterward. Most patients received anthracycline and taxane-based neoadjuvant chemotherapy, mastectomy, and postmastectomy radiation. Survival outcomes were compared between the two groups.
Results
Median follow-up time was 29 months for the entire cohort (39 mo and 24 mo for patients treated before and after October 2006). Patients treated more recently were more likely to have received neoadjuvant HER2-directed therapy for HER2-positive tumors (100% vs. 54%, P<0.001). No differences were found in receipt of hormone therapy. Three-year overall survival (OS) rates were 63% for those treated before and 82% for those treated after October 2006 (log-rank P=0.02). Univariate Cox analysis demonstrated better OS among patients treated after October 2006 than among those treated beforehand (hazard ratio [HR] 0.5, 95% confidence Interval [CI] 0.34-0.94); a trend toward improved survival was noted in the multivariate analysis (HR=0.47, 95% CI 0.19-1.16, P=0.10). Significant factors in the multivariate model included HER2-directed therapy (HR=0.38, 95% CI 0.17-0.84, P=0.02) and estrogen receptor positivity (HR=0.32, 95% CI 0.14-0.74, P=0.01).
Conclusions
Survival improved in the context of the IBC clinic and prompt initiation of neoadjuvant Her-2 directed therapeutics.
Keywords: Inflammatory breast cancer, survival, multidisciplinary clinic, targeted therapy
INTRODUCTION
Inflammatory breast cancer (IBC) is a rare but aggressive form of invasive breast cancer, contributing 1%-5% of all new breast cancer cases in the United States.1 Patients with IBC have lower overall survival (OS) rates than do those with stage III non-IBC,2, 3 and the median survival time is 3.5 years shorter for patients with IBC than for those with locally advanced non-IBC.2 A population-based study of data from the Surveillance, Epidemiology, and End Results program showed that the incidence of IBC is increasing over time, from 2.0 per 100,000 women per year in 1988-1990 to 2.5 per 100,000 in 1997-1999.2
A multidisciplinary approach is crucial for management of IBC. Even so, the survival rates for women with IBC are still poor, ranging from 35%-40%, significantly lower than those for other breast cancers.4 A prospective observational study found that the 15-year OS rate was 20% for patients with IBC versus 50% for patients with stage IIIA non-IBC and 23% for those with stage IIIB non-IBC, even with the use of a multimodality treatment approach.3 Studies published in the early to mid-2000s indicated that combined anthracycline and taxane chemotherapy may improve survival outcomes in IBC.5-7 Another substantive advance in the effectiveness of therapy for breast cancer were the findings from NSABP B-31, NCCTG N9831, and BGIRG 006, which led to adjuvant trastuzumab becoming standard treatment for all patients with operable HER2-positive breast cancer.8 Since that time, the use of neoadjuvant trastuzumab was also shown to improve pathologic complete response (pCR) rates among patients with HER2-positive IBC9 and locally advanced non-IBC as well.10
At The University of Texas MD Anderson Cancer Center, we have used a multidisciplinary approach to manage IBC for decades; we opened the first protocol dedicated exclusively to IBC in 1974.11 Although multidisciplinary care improved outcomes over those of historical series at the time,3, 4 significant advances beyond this were not forthcoming in subsequent decades.12 In a systematic attempt to address this lack of progress, a dedicated multidisciplinary IBC clinic was opened at MD Anderson Cancer Center in October 2006. We report here early findings from a comparison of patients with IBC diagnosed and treated before and after the opening of this clinic to evaluate survival outcomes.
PATIENTS AND METHODS
Patients
This retrospective chart review was approved by the institutional review board of MD Anderson. We reviewed the institutional tumor registry and a multidisciplinary prospective breast cancer database to identify sequential patients presenting with non-stage IV IBC who were treated at MD Anderson between January 2000 and December 2008. A total of 168 women were diagnosed and treated at MD Anderson from January 2000 through September 2006 (before the opening of the dedicated multidisciplinary IBC clinic) and 92 such women were seen from October 2006 through December 2008. Patients who had recurrent disease at first presentation were excluded. For all patients (including those seen before October 2006), the diagnosis and staging of IBC was made jointly by a multidisciplinary team as described elsewhere.12, 13
Diagnosis
Each patient received a thorough physical examination, along with diagnostic mammography, biopsy, sonography, computed tomography (CT), bone scan, positron emission tomography/CT (PET/CT), and brain magnetic resonance imaging (MRI) when appropriate. PET/CT was more routinely recommended as an initial staging tool after the establishment of the IBC clinic. The diagnosis of IBC required pathologic confirmation of the presence of an invasive breast carcinoma by pathologists specializing in breast cancer at MD Anderson. Clinical stage was defined according to the sixth (2002) edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.14 Histologic type was defined according to the World Health Organization’s classification system.15
Pathologic Evaluation
Details of the pathologic evaluation have been described previously.13 Briefly, all cancer diagnoses were confirmed by core biopsy. Expression of the estrogen receptor (ER) and progesterone receptor (PR) by the tumor was tested by immunohistochemical staining of paraffin-embedded tissues with monoclonal antibodies.13 Expression of HER2 was evaluated by immunohistochemical staining, fluorescence in situ hybridization, or both. HER2 positivity was defined as 3+ receptor overexpression (strong membranous staining in ≥10% of cells) or gene amplification (a gene copy ratio of HER2/CEP-17 ≥ 2.0).
Treatment and Follow-Up
Details of the management strategy for non-metastatic IBC at MD Anderson have been described elsewhere.13, 16 The recommended treatment was neoadjuvant chemotherapy, modified radical mastectomy, and postmastectomy radiation to the chest wall and undissected draining lymphatics. The choices of neoadjuvant chemotherapy was at the discretion of the treating medical oncologist and included 5-fluorouracil, doxorubicin, cyclophosphamide, and taxanes. Adjuvant tamoxifen or aromatase inhibitors were used for patients with hormone receptor–positive disease. Neoadjuvant HER2–directed therapy (trastuzumab or lapatinib) for HER2-positive cancer was initiated in 1999 but was used routinely beginning in 2006. Before 2006, many patients received HER2-directed therapy as second-line treatment. Regarding radiation, most patients received 51 Gy in 1.5-Gy fractions delivered twice daily to the chest wall and draining lymphatics, followed by a 15-Gy boost, also in 1.5-Gy fractions delivered twice daily, bringing the total dose to 66 Gy. Some patients received once-daily radiation owing to changes in our treatment guidelines during the study period.17-19 The patients were simulated on a 10-15 degree breast board. The treatment beam arrangement included a medial angled electron beam with a medial border at mid-sternum and a lateral border matched on skin (with a 3 mm overlap) to tangential photon beams covering the lateral chest wall. The internal mammary chain in the first three intercostal spaces were contoured and the electron energies were determined so that the 90% isodose line covered the internal mammary contours. If necessary, the electron beam was split just below the IMC target and a lower energy electron was selected for the inferior field such that the 90% isodose curve covered the anterior aspect of the pectoralis major muscle. The electron/tangent borders were set to exclude the heart from the tangent fields. Since 2007, patients with left-sided disease were simulated with both free-breathing and deep inspiration breath hold technique. Each patient was evaluated individually and treated with breath hold technique when beneficial. Selected patients also received adjuvant chemotherapy. Patients were followed every 6 months for 5 years and then yearly after completion of treatment. At each follow-up visit, a thorough physical evaluation, routine laboratory tests, and imaging studies (mammography, sonography, bone scanning, CT, PET/CT, and brain MRI) were performed.
Statistical Analysis
The primary endpoints in this study were locoregional recurrence (LRR), distant recurrence/metastasis (DM), and OS. LRR was defined as any recurrence within the ipsilateral chest wall or regional lymphatics including axillary, supraclavicular, and internal mammary nodes. Recurrences in the contralateral breast were considered distant if contralateral nodes were involved; otherwise locoregional was distinguished from distant recurrence based on the clinical history and distribution of disease according to physical examinations and medical photography. Time to recurrence was computed from the date of diagnosis to the date of first local or distant disease recurrence. Patients without recurrence were censored at the last follow-up date. Patients who died without experiencing disease recurrence were censored at the date of death, except for the overall survival endpoint. Time to recurrence or death was estimated by the Kaplan–Meier method and compared between groups with log-rank tests.20 Univariate and multivariate analyses of time to event were done with a Cox proportional hazards model.21 The variables analyzed were age at diagnosis, race, use of PET/CT for staging, menopausal status (pre- or post-), lymphovascular space invasion (LVSI; present or absent), tumor nuclear grade (grade 3 or grade 1–2), ER expression (positive or negative), HER2 expression (positive or negative), percentage of positive nodes (<20% versus ≥20%), presence of extracapsular extension (ECE; present or absent), margin status (close/positive or negative), pCR (yes or no), neoadjuvant chemotherapy (yes or no), taxane (yes or no), hormone therapy (yes or no), HER2–directed therapy (yes or no), radiation therapy (yes or no), and radiation dose (continuous). An identical method was used for time to LRR, DM, and death. All statistical tests were two-sided, with P values < 0.05 considered significant. All calculations were done with Stata/MP 11.1 statistical software (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
The current study included 168 women who were diagnosed and treated at MD Anderson from January 2000 through September 2006 (before the dedicated multidisciplinary IBC clinic was opened), and 92 women who were treated after October 2006 (after the clinic was opened). The median follow-up time was 29 months for the entire cohort (39 and 24 months for patients treated before and after October 2006, respectively). Table 1 shows the baseline characteristics of the study population. For patients who were alive at the time of analysis, the median follow-up time was 57 months (range 5-127 months) for patients treated before October 2006 and 26 months (range 2-47 months) for those treated after October 2006. The two groups had similar distributions of age at diagnosis, race, hormone receptor status, and HER2 positivity (38% for both groups). The proportions of patients who received neoadjuvant chemotherapy and taxane-based chemotherapy were also comparable between the two groups. Overall, most patients (93%) had surgical margins >2 mm and 18% had pCR after neoadjuvant chemotherapy. All patients with HER2-positive cancer who were diagnosed after October 2006 received neoadjuvant trastuzumab or lapatinib (as part of a protocol), compared with only 54% of patients with HER2-positive cancer who were diagnosed before October 2006 (P=0.001). Fewer patients treated after October 2006 received twice-daily radiation therapy compared with those treated before October 2006 (43% vs. 57%, respectively, P<0.001), which reflected changes in our radiation treatment practice during the study period.17-19 Time from initial diagnosis to treatment was shorter for patients treated after October 2006 than for those treated before October 2005 (94 vs 104 days), although this apparent difference was not statistically significant. Almost all patients with ER-positive tumors received adjuvant hormone therapy (89% overall), and no statistically significant difference was noted between the two groups.
Table 1.
Patient, tumor, and treatment characteristics
| All Patients n=260 (%) |
Patients Seen Before Oct 2006 n=168 (%) |
Patients Seen After Oct 2006 n=92 (%) |
P Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age, median (range) | 50 (24-83) | 50 (24-78) | 52 (26-83) | 0.32 | |||
|
| |||||||
| Race | |||||||
| Caucasian | 198 | (76%) | 128 | (76%) | 70 | (76%) | 0.51 |
| Hispanic | 32 | (12%) | 23 | (14%) | 9 | (10%) | |
| African-American | 24 | (9%) | 12 | (7%) | 12 | (13%) | |
| Other | 6 | (2%) | 5 | (3%) | 1 | (1%) | |
|
| |||||||
| Premenopausal | 112 | (43%) | 73 | (43%) | 39 | (42%) | 0.98 |
|
| |||||||
| Tumor Characteristics | |||||||
| LVSI | 102 | (39%) | 64 | (38%) | 38 | (41%) | 0.344 |
| Grade 3 | 193 | (74%) | 128 | (76%) | 65 | (71%) | 0.37 |
| Nodal Category | |||||||
| N0 | 21 | (8%) | 14 | (8%) | 7 | (8%) | 0.084 |
| N1 | 110 | (42%) | 67 | (40%) | 43 | (47%) | |
| N2 | 24 | (9%) | 21 | (13%) | 3 | (3%) | |
| N3 | 102 | (39%) | 63 | (38%) | 39 | (42%) | |
| NX | 3 | (1%) | 3 | (2%) | 9 | (10%) | |
| ER+ | 99 | (38%) | 57 | (34%) | 42 | (46%) | 0.063 |
| HER2+ | 98 | (38%) | 63 | (38%) | 35 | (38%) | 0.395 |
|
| |||||||
| Treatment and Diagnostics | |||||||
| pCR | 48 | (18%) | 31 | (18%) | 17 | (18%) | 0.90 |
| Surgical margin < 2 mm | 17 | (7%) | 9 | (5%) | 8 | (9%) | 0.29 |
| Extracapsular extension | 62 | (24%) | 40 | (24%) | 22 | (24%) | 0.96 |
| >20% nodes positive | 110 | (42%) | 74 | (44%) | 36 | (39%) | 0.44 |
| >10% nodes removed | 194 | (75%) | 123 | (73%) | 71 | (77%) | 0.30 |
| Radiation received | 226 | (87%) | 146 | (87%) | 80 | (87%) | 0.607 |
| Radiation BID | 135 | (52%) | 95 | (57%) | 40 | (43%) | <0.001 |
| PET/CT in initial disease staging | 72 | (28%) | 21 | (13%) | 51 | (55%) | <0.001 |
|
| |||||||
| Chemotherapy | |||||||
| Neoadjuvant chemotherapy | 257 | (99%) | 165 | (98%) | 92 | (100%) | 0.20 |
| Taxane received | 255 | (98%) | 165 | (98%) | 90 | (98%) | 0.94 |
| n=99 | n=57 | n=42 | |||||
| Hormone therapy in ER+ | 88 | (89%) | 50 | (88%) | 38 | (91%) | 0.66 |
| n=99 | n=63 | n=36 | |||||
| Neoadjuvant trastuzumab in HER2+ | 70 | (71%) | 34 | (54%) | 36 | (100.0%) | 0.001 |
Abbreviations: chemo, chemotherapy; LVSI, lymphovascular space invasion; cN+, ER+, pCR, pathologic complete response; BID, twice a day; PET/CT, positron emission tomography - computed tomography
Factors Associated with Patient Survival
Figure 1 shows OS rates by year of diagnosis and treatment. The 2-year OS rates were 77% for the entire study population, 74% for those treated before October 2006 and 85% for those treated later. The 3-year OS rate was 68% for the entire cohort, 63% for those treated before October 2006 and 82% for those treated later (overall log-rank P=0.02). No significant differences were noted between groups in LRR, DM-free survival, and disease-free survival. The 2- and 3-year rates of LRR-free survival were 83% and 81% for those treated before October 2006 and 89% and 85% for those treated afterwards (overall log-rank P=0.13). The 2- and 3-year rates of DM-free survival were 61% and 58% for those treated before October 2006 and 68% and 60% for those treated afterwards (overall log-rank P=0.29).
FIGURE 1.
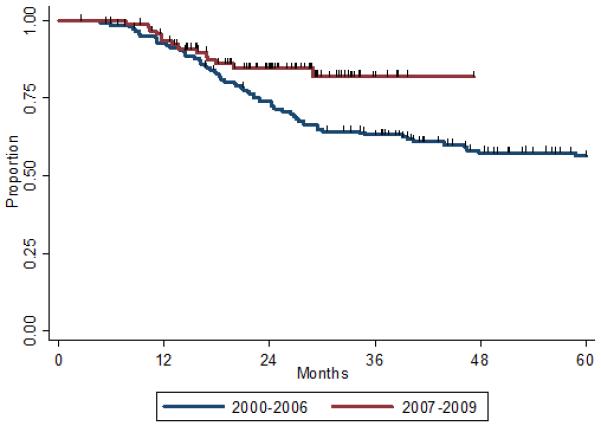
Overall survival according to time of diagnosis and treatment of inflammatory breast cancer (IBC). Blue line indicates patients diagnosed and treated before October 2006 (i.e., before the opening of the multidisciplinary IBC clinic); red line, patients diagnosed and treated after October 2006 at the IBC clinic.
Univariate Cox regression analysis suggested better survival among patients treated after October 2006 than among patients treated before October 2006 (hazard ratio [HR] 0.5, 95% confidence Interval [CI] 0.34-0.94, P=0.03) (Table 2). Other factors associated with better survival included having non-basal disease, receipt of radiation treatment, <20% positive lymph nodes, no ECE, higher radiation dose, hormone receptor positivity, the absence of LVSI, lower tumor grade, and the presence of pCR (Table 2).
Table 2.
Univariate Cox regression analysis of variables influencing overall survival in patients with inflammatory breast cancer
| Adjusted Hazard Ratio |
95% Confidence Interval |
P value | |
|---|---|---|---|
|
| |||
| Year group | |||
| Before 10/2006 | 1.00 (Reference) | ||
| After 10/2006 | 0.50 | (0.34-0.94) | 0.03 |
|
| |||
| Molecular receptor expression type | |||
| Basal | 1.00 | ||
| Luminal A | 0.23 | (0.12-0.41) | <0.001 |
| Luminal B | 0.37 | (0.17-0.79) | 0.01 |
| HER2 | 0.49 | (0.29-0.83) | 0.008 |
|
| |||
| % nodes positive | |||
| ≥ 20% | 1.0 | ||
| < 20% | 0.42 | (0.25-0.70) | 0.001 |
|
| |||
| Radiation dose (continuous) | 0.94 | (0.90-0.98) | 0.003 |
|
| |||
| Radiation treatment | |||
| Yes | 1.00 | ||
| No | 5.01 | (2.69-9.35) | <0.001 |
|
| |||
| Estrogen receptor+ | |||
| Yes | 1.00 | ||
| No | 2.74 | (1.64-4.57) | <0.001 |
|
| |||
| Hormone therapy for estrogen receptor+ disease |
1.00 | ||
| Yes | 4.61 | (2.54-8.37) | <0.001 |
| No | |||
|
| |||
| Lymphovascular invasion | |||
| Yes | 1.00 | ||
| No | 0.56 | (0.34-0.92) | 0.02 |
|
| |||
| Grade | |||
| Grade 3 | 1.00 | ||
| Grade 1 or 2 | 0.29 | (0.14-0.61) | 0.001 |
|
| |||
| Pathologic complete response | |||
| Yes | 1.00 | ||
| No | 3.55 | (1.55-8.15) | 0.003 |
|
| |||
| Extracapsular extension | |||
| Yes | 1.00 | ||
| No | 0.57 | (0.34-0.94) | 0.03 |
|
| |||
| PET/CT used in initial staging | |||
| Yes | 1.00 | ||
| No | 0.69 | (0.39-1.21) | 0.20 |
|
| |||
| Race | |||
| African-American | 1.00 | ||
| Other | 1.18 | (0.72-1.94) | 0.51 |
|
| |||
| Taxane received | |||
| Yes | 1.00 | ||
| No | 3.47 | (0.85-14.24) | 0.08 |
|
| |||
| HER2 | |||
| Yes | 1.00 | ||
| No | 1.12 | (0.072-1.75) | 0.61 |
|
| |||
| Neoadjuvant trastuzumab in HER2 + | |||
| Yes | 1.00 | ||
| No | 2.16 | (0.66-7.06) | 0.20 |
Abbreviations: PET/CT, positron emission tomography - computed tomography Other factors that were not significant in the univariate analysis were race, age, menopause status, HER2 status, fraction schedule, and surgical margin
On multivariate analysis, diagnosis and treatment after 2006 seemed to be associated with improved survival, but this apparent association was not statistically significant (adjusted HR 0.47, 95% CI 0.19-1.16, P=0.10) (Table 3). Factors that remained significantly associated with OS included receipt of neoadjuvant HER2-directed therapy (HR 0.38, 95% CI 0.17-0.84, P=0.02) and positive ER status (HR 0.32, 95% CI 0.14-0.74, P=0.01). To see if survival after relapse differed between the two groups, we analyzed OS rates among patients after LRR or DM in both groups. In patients with LRR after initial treatment, the 1-year OS rate after recurrence was 38% if they had been treated before October 2006 and 64% if they had been treated afterwards (P=0.79). In patients with DM, the 1-year OS rate was 22% for patients treated before October 2006 and 49% for those treated afterwards (P=0.63). When we combined patients with local and distant failure, the 1-year OS rate after relapse was 25% for patients treated before October 2006 and 52% for those treated afterwards (P=0.72).
Table 3.
Multivariate Cox regression analysis of variables influencing overall survival in patients with inflammatory breast cancer
| Adjusted Hazard Ratio |
95% Confidence Interval |
P value | |
|---|---|---|---|
| Year group (<Oct 2006 vs. >Oct 2006) | 0.47 | (0.19, 1.16) | 0.10 |
| % nodes positive (<20% vs. ≥ 20%) | 1.20 | (0.53, 2.71) | 0.66 |
| Radiation therapy (yes vs. no) | 1.95 | (0.56, 6.73) | 0.29 |
| Estrogen receptor status (yes vs. no) | 0.32 | (0.14, 0.74) | 0.01 |
| Neoadjuvant Trastuzumab in HER2 + (yes vs. no) | 0.38 | (0.17, 0.84) | 0.02 |
| Tumor grade (grade 1,2 vs. grade 3) | 0.99 | (0.39, 2.52) | 0.99 |
| Pathologic complete response (no. vs. yes) | 0.56 | (0.19, 1.68) | 0.30 |
DISCUSSION
The current study demonstrated that patients with IBC that had been diagnosed and treated after October 2006 had higher OS rates than did those diagnosed and treated before that time. This improvement undoubtedly reflected several factors, among them the early initiation of aggressive treatments such as neoadjuvant HER2-directed therapy for patients with HER2-positive tumors and other changes in treatment strategies over time. Findings from the current study are different from those of a prior study from our institution that evaluated survival outcomes of 398 patients with IBC by decades of diagnosis/treatment between 1974 and 2005, before the opening of the IBC clinic.12 In that study, which had a 5.8-year median follow-up time, 238 patients developed recurrence and 236 died, for a median OS time of 4.2 years.12 Multivariate analysis did not show any improvement in survival or recurrence according to time of diagnosis and treatment during that 30-year period before 2005, and the authors concluded that recent advancements in systemic and locoregional treatments for non-IBC had not affected the prognosis of IBC since the introduction of multidisciplinary management. In a similar study, Panades et al22 compared 10-year breast cancer–specific survival rates among IBC patients treated in 1980–1990 versus 1991–2000 and found no differences between the two groups (27.4% vs. 28.6%). Although those authors found mastectomy to be associated with improved local control and use of more intensive chemotherapy to be associated with breast cancer–specific survival, those observation did not translate into improved survival over time. Dawood et al.23 also found that even in the modern era of multidisciplinary therapy, patients with IBC continue to have worse survival than those with non-IBC. Factors associated with better survival among patients with IBC were having low-grade tumors, being of white/other race, undergoing surgery, receiving radiation therapy, and having hormone receptor-positive disease.24
The lack of significant improvement in survival of IBC patients over time underscores the need for a dedicated multidisciplinary treatment approach, new therapeutics that specifically target IBC, early initiation of aggressive therapy, and an understanding of the distinctive biology of IBC.4, 25 Our findings, although preliminary and limited by the duration of follow-up, among other things, suggest that being treated in a dedicated IBC clinic, with the use of extensive initial staging evaluation and early initiation of systemic therapy, particularly neoadjuvant HER2-directed therapy, may be beneficial for patients with this relatively rare presentation. Our observations are consistent with an earlier report of increased rates of pathologic response and progression-free survival among patients who received neoadjuvant trastuzumab versus those who did not.9 As was true in the Dawood et al. study,23 hormone receptor positivity was also a significant prognostic factor for survival among our patients with IBC. Results from our study also echo the recently published findings from the NOAH trial, in which event-free survival was significantly improved for patients with HER2-positive IBC treated with both neoadjuvant and adjuvant trastuzumab versus those not given trastuzumab.10
Our IBC clinic, which we believe to be the first of its kind, is staffed by a multidisciplinary team of physicians with expertise in the diagnosis and management of IBC. We had begun to routinely administer neoadjuvant HER-directed therapy to patients with HER2-positive disease at the IBC clinic before the data from the NOAH trial became available,10 and this approach contributed to the observed improvement in survival. We also routinely used ultrasonography, MRI, and more recently, PET/CT for diagnosis. We recently demonstrated the benefit of PET/CT as a staging tool in IBC for discerning locoregional, mediastinal, and contralateral lymph node involvement, and we found it to be useful for designing radiation treatment fields as well.26 Operable patients could be treated with contralteral axillary lymph node dissection followed by local extension into anterior mediastinal lymph nodes in the comprehensive post-mastectomy radiation therapy. PET/CT is also useful for detecting unsuspected sites of DM; Niikura et al.27, 28 showed that the addition of PET/CT to conventional imaging modalities for IBC could detect metastases that had not been detected by conventional imaging. Furthermore, there has been increased availability and accrual to trials specifically for IBC patients. Currently, there are six trials that examine the role of targeted therapies available to IBC patients. The IBC clinic also has increased, organized patient advocacy support and educational resources, which can potentially increase timely adherence with therapy. It is also worthwhile to mention that while the practicality of such a dedicated multidisciplinary IBC clinic may be less feasible at other institutions due to the rarity of IBC, it might be still beneficial and reasonable to include other high-risk subtypes in a designated multidisciplinary clinic for most large breast cancer programs.
Our study did have some limitations. As tempting as it might be to attribute improvements in OS to the opening of our IBC clinic, many other factors may have been involved as well, including increased awareness of the disease, better patient selection, improvements in the accuracy of diagnosis and exclusion of metastatic disease (stage migration due to use of PET/CT), access to new treatment protocols, and the timeliness of referrals. It is also possible that patients treated at the IBC clinic lived longer after relapse because of the timely detection of recurrence through close surveillance and aggressive salvage therapy. Although the 1-year survival estimates after relapse were no different between the two groups, a two-fold increase in survival was noted among patients with recurrent IBC who had been treated at the IBC clinic compared with their earlier counterparts. With longer follow-up time, this apparent difference may become more prominent.
Other limitations include the retrospective nature of this analysis of disease trends over time, with the attendant variations in patient demographics, disease characteristics, treatment standards, diagnostic approach, and other unmeasured confounders, any of which could bias the results. The implementation of neoadjuvant HER2-directed therapy certainly contributed to the observed results. We had no comparison group that had been treated at another institution with no IBC clinic, which would have strengthened our hypothesis that the IBC clinic, in addition to the timely use of neoadjuvant HER2 therapy, had a major role in improving survival in addition to the timely use of neoadjuvant HER2 therapy. The difference in follow-up time between the two groups is also problematic; however, because the follow-up was shorter for patients treated at the IBC clinic, one could argue that more events would occur with longer follow-up for those patients, which would affect our conclusion.
In summary, the results from this preliminary analysis support the benefit of early initiation of aggressive treatment and neoadjuvant targeted therapy for IBC. Timely diagnosis and treatment, increased awareness of the disease, close follow-up, and aggressive salvage therapy, all of which are more easily achieved in a specialized multidisciplinary IBC clinic, could also contribute to the observed improvement in survival outcome.
ACKNOWLEDGEMENT
The authors thank Christine F Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology, for editorial contributions.
Supported in part by the State of Texas Rare and Aggressive Breast Cancer Research Program Grant and by Cancer Center Support (Core) Grant CA016672 to from the National Cancer Institute to The University of Texas MD Anderson Cancer Center.
Footnotes
Conflicts of Interest and Source of Funding: The authors declare no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer (IBC) Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97(13):966–75. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low JA, Berman AW, Steinberg SM, Danforth DN, Lippman ME, Swain SM. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22(20):4067–74. doi: 10.1200/JCO.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 4.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60(6):351–75. doi: 10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 5.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686–96. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 8.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 9.Dawood S, Gong Y, Broglio K, et al. Trastuzumab in Primary Inflammatory Breast Cancer (IBC): High Pathological Response Rates and Improved Outcome. Breast J. 2010 Jul 7; doi: 10.1111/j.1524-4741.2010.00953.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 11.Krutchik AN, Buzdar AU, Blumenschein GR, et al. Combined chemoimmunotherapy and radiation therapy of inflammatory breast carcinoma. J Surg Oncol. 1979;11(4):325–32. doi: 10.1002/jso.2930110407. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Angulo AM, Hennessy BT, Broglio K, et al. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12(8):904–12. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Gonzalez-Angulo AM, Allen PK, et al. Triple-negative subtype predicts poor overall survival and high locoregional relapse in inflammatory breast cancer. Oncologist. 2011;16(12):1675–83. doi: 10.1634/theoncologist.2011-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th Edition Springer; 2002. [Google Scholar]
- 15.The world Health Organization Histological Typing of Breast Tumors--Second Edition. The World Organization. Am J Clin Pathol. 1982;78(6):806–16. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 16.Bristol IJ, Buchholz TA. Inflammatory breast cancer: current concepts in local management. Breast Dis. 2005;22:75–83. doi: 10.3233/bd-2006-22109. [DOI] [PubMed] [Google Scholar]
- 17.Liao Z, Strom EA, Buzdar AU, et al. Locoregional irradiation for inflammatory breast cancer: effectiveness of dose escalation in decreasing recurrence. Int J Radiat Oncol Biol Phys. 2000;47(5):1191–200. doi: 10.1016/s0360-3016(00)00561-7. [DOI] [PubMed] [Google Scholar]
- 18.Ballo MT, Strom EA, Prost H, et al. Local-regional control of recurrent breast carcinoma after mastectomy: does hyperfractionated accelerated radiotherapy improve local control? Int J Radiat Oncol Biol Phys. 1999;44(1):105–12. doi: 10.1016/s0360-3016(98)00545-8. [DOI] [PubMed] [Google Scholar]
- 19.Woodward WA, Debeb BG, Xu W, Buchholz TA. Overcoming radiation resistance in inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2840–5. doi: 10.1002/cncr.25173. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:547–81. [Google Scholar]
- 21.Cox D, Oakes N. Analysis of Survival Data. Chapman and Hall; New York: 1984. [Google Scholar]
- 22.Panades M, Olivotto IA, Speers CH, et al. Evolving treatment strategies for inflammatory breast cancer: a population-based survival analysis. J Clin Oncol. 2005;23(9):1941–50. doi: 10.1200/JCO.2005.06.233. [DOI] [PubMed] [Google Scholar]
- 23.Dawood S, Ueno NT, Valero V, et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer. 2011;117(9):1819–26. doi: 10.1002/cncr.25682. [DOI] [PubMed] [Google Scholar]
- 24.Dawood S, Ueno NT, Valero V, et al. Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdr319. [DOI] [PubMed] [Google Scholar]
- 25.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22(3):515–23. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker GV, Niikura N, Yang W, et al. Pretreatment Staging Positron Emission Tomography/Computed Tomography in Patients With Inflammatory Breast Cancer Influences Radiation Treatment Field Designs. Int J Radiat Oncol Biol Phys 2012. 2012 Aug 1;83(5):1381–6. doi: 10.1016/j.ijrobp.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Niikura N, Liu J, Costelloe CM, et al. Initial staging impact of fluorodeoxyglucose positron emission tomography/computed tomography in locally advanced breast cancer. Oncologist. 2011;16(6):772–82. doi: 10.1634/theoncologist.2010-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niikura N, Costelloe CM, Madewell JE, et al. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. Oncologist. 2011;16(8):1111–9. doi: 10.1634/theoncologist.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]


