Abstract
Objective
Because early estrogen deficiency may increase the susceptibility of the optic nerve to glaucoma, we studied the association of early bilateral oophorectomy with glaucoma.
Methods
We studied the risk of glaucoma in the Mayo Clinic Cohort Study of Oophorectomy and Aging, by comparing all women who underwent bilateral oophorectomy from 1950 to 1987 with age-matched referent women who did not undergo unilateral or bilateral oophorectomy. Glaucoma diagnostic codes were searched in the records-linkage system of the Rochester Epidemiology Project. Hazards ratios (HR) were calculated over a median follow-up of 25.5 years. Analyses were stratified by age at the time of bilateral oophorectomy (in tertiles).
Results
Of 1,044 women who underwent bilateral oophorectomy before menopause, 147 developed glaucoma. Of 1,070 referent women, 133 developed glaucoma. Women who underwent bilateral oophorectomy showed no increased risk of glaucoma in the overall group (HR 1.12, 95% CI 0.89–1.42). However, women who underwent oophorectomy before age 43 years (n=344; the first tertile) had a significantly increased risk of glaucoma (HR 1.60, 95% CI 1.15–2.23). The results did not change after adjustment for hypertension, obesity, diabetes, or disorders of lipid metabolism at baseline. Approximately 11% of women with bilateral oophorectomy before age 43 years were treated with estrogen to age 50 years; however, treatment did not reduce the association (HR 1.59, 95% CI 0.81–3.13).
Conclusions
Bilateral oophorectomy before age 43 years may increase the risk of glaucoma, and estrogen treatment does not appear to attenuate the risk.
Keywords: Glaucoma, Aging, Menopause, Oophorectomy, Estrogen, Hormone therapy
Glaucoma is the second leading cause of blindness in the United States and worldwide.1,2 Even in individuals with normal visual acuity, glaucoma-related visual loss can lead to significant functional visual impairment.3, 4 Functional vision loss from glaucoma not only impairs important activities of daily living, such as reading, walking, and driving, but is also associated with higher rates of secondary health conditions including depression, falls, and motor vehicle accidents.3, 4 Approximately 3% of the world population aged 40 years and older has glaucoma. About 74% of these individuals have primary open-angle glaucoma (POAG), a condition expected to increase in incidence by 28% per decade over the next 40 years in the US.5 POAG is an age-dependent, intraocular pressure related type of neurodegeneration, and can be regarded as accelerated aging of the optic nerve.1, 3, 6–9 Worldwide, approximately 59% of patients with glaucoma of all types combined are women. Women have higher rates of visual impairment due to glaucoma than men, but they are 24% less likely to receive medical, laser, and surgical treatment than men.10, 11 Therefore, it is important to understand sex-specific risk factors associated with glaucoma.1, 2
The neuroprotective effects of estrogen on the optic nerve are supported by clinical, epidemiological, and basic science evidence.8, 12–19 Thus, early estrogen deficiency may increase the susceptibility of the optic nerve to glaucoma-related damage. Surrogates for a lifetime decrease in estrogen exposure were measured in several population-based studies, showing that late menarche, early menopause, or shorter length of time from menarche to menopause were associated with the development of glaucoma.12–16
Early estrogen deficiency following oophorectomy has profound effects on women’s health, including increased risks of overall mortality, cardiovascular mortality, cognitive impairment or dementia, parkinsonism, depression, and anxiety.20–24 However, the effects of early oophorectomy on the optic nerve and on the risk of glaucoma have not been investigated. Therefore, we used data from the Mayo Clinic Cohort Study of Oophorectomy and Aging, to determine whether early estrogen loss due to bilateral oophorectomy increased the risk for later development of glaucoma.
METHODS
Bilateral oophorectomy cohort
The Mayo Clinic Cohort Study of Oophorectomy and Aging is a population-based historical cohort study of all women who underwent unilateral or bilateral oophorectomy in Olmsted County, Minnesota, between 1950 and 1987. Because the study was originally designed and funded to study Parkinson’s disease outcomes, women were included only if they were 40 years or older by January 1, 2002. In our analyses, we included only those women who underwent bilateral or second unilateral oophorectomy. In addition, we included only those women who underwent bilateral oophorectomy before menopause; if age at menopause was not known, we included women who underwent bilateral oophorectomy before 56 years of age.22 Thus, most women were presumed to have been premenopausal at the time of surgery. We also excluded women who underwent bilateral oophorectomy for an estrogen-related cancer (usually ovarian or breast); thus, most women were presumed to be free of estrogen-related cancers at the time of inclusion in the study. Finally, we excluded women who received any glaucoma diagnostic code prior to the year of oophorectomy.
The indication specified by the surgeon at the time of surgery and categorized as prophylactic or non-prophylactic (to remove a benign ovarian condition), and the use of estrogen after bilateral oophorectomy, were abstracted from the medical records stored within the records-linkage system of the Rochester Epidemiology Project (REP, www.rochesterproject.org). In brief, the REP is a collaboration between health care providers in Olmsted County, Minnesota, including Olmsted Medical Center, Mayo Clinic, Rochester Family Medicine Clinic, and other care providers. By sharing medical information across providers for research purposes, nearly all data on medical diagnoses and procedures are available for the population of Olmsted County. The REP is funded by the NIH and has made possible hundreds of population-based research projects. Additional details related to the cohort design and follow-up have been reported elsewhere.21, 22 Additional details about the population of Olmsted County and about the Rochester Epidemiology Project have also been published.25–28
Referent cohort
The study also included a group of age-matched referent women who had not undergone oophorectomy during the same time period. In brief, for each woman in the bilateral oophorectomy cohort, we defined the index year as the year of surgery. A simple random sampling method was then used to select one woman from the complete Olmsted County population with the same year of birth who had not undergone unilateral or bilateral oophorectomy before the index year. This referent group included women who underwent hysterectomy without oophorectomy; and women were not required to be premenopausal in the index year. Indeed, menopausal status of referent women who did not undergo oophorectomy was not known. However, we excluded women who received any glaucoma diagnostic code prior to the index year.
Our referent cohort was population-based because all women who resided in Olmsted County in the index year and met the inclusion criteria were considered eligible, regardless of menopausal status and of any other conditions or risk factors. The REP provided the list of potential referent women within Olmsted County, and this list was shown to be complete as compared with a random-digit-dialing telephone sample and with the US Decennial Census28.
Follow-up and glaucoma outcomes
Using the complete set of electronic medical diagnoses available as part of the REP infrastructure, we were able to passively follow women in both the bilateral oophorectomy and referent cohorts through onset of glaucoma, death, last medical contact with the REP, or June 30, 2010 (end of study). However, we excluded women who did not receive a medical diagnosis of any kind after the year of oophorectomy (or index year for women in the referent cohort) because they were not informative.
The year of onset of glaucoma was determined by searching for 131 unique glaucoma-related diagnostic codes within the three diagnostic coding systems historically utilized by the REP. The Berkson coding system, a historical classification system developed at the Mayo Clinic, was used to code diagnoses from 1935 to 1975; the hospital adaptation of the International Classification of Diseases - 8th Revision (H-ICDA), was used to code diagnoses from 1975 to 2004; and the International Classification of Diseases – 9th Revision (ICD-9) was used to code diagnoses after 2004.25
The 131 diagnostic codes were grouped by an ophthalmologist (TSV), who was kept unaware of the oophorectomy status, into 5 hierarchical categories based on etiology or type of glaucoma: 1) secondary glaucoma; 2) angle closure/narrow angle glaucoma; 3) open-angle glaucoma (OAG) including POAG, exfoliation, and pigmentary glaucoma; 4) ocular hypertension (OHTN); and 5) unspecified glaucoma. Women having diagnostic codes from more than one of the 5 categories were assigned to a primary group in descending order of priority from category one (highest priority) through category 5 (lowest priority). Our analyses considered the first occurrence of diagnostic codes for any glaucoma and separately for specific types of glaucoma (OAG or OHTN, and OAG alone).
Collection of other clinical information
Diagnostic codes stored electronically in the records-linkage system were used to study the occurrence of hypertension, obesity, diabetes, or disorders of lipid metabolism at baseline (index year). We also searched for codes related to cardiovascular events preceding baseline (myocardial infarction and stroke). The diagnostic codes were grouped using the clinical classification software (CCS) of the Agency for Healthcare Research and Quality - Healthcare Cost and Utilization Project.29, 30 For obesity, we used a subset of codes from the original CCS group (other nutritional, endocrine, and metabolic disorders) to be more specific.
A woman was considered to have one of the metabolic risk factors at baseline if she received at least two codes within the same CCS group before baseline. These same CCS groups were also used to study the de novo appearance of metabolic outcomes between the index year and onset of glaucoma (restricted to women who did not have the condition at baseline). A woman was considered to develop de novo metabolic syndrome if she developed any 3 or more of the 4 components of the syndrome (hypertension, obesity, diabetes, and disorders of lipid metabolism).
Validation of screening
To determine the validity of using diagnostic codes to identify women with glaucoma, we reviewed the complete medical records of 60 randomly chosen women who received a code for glaucoma and 40 who did not receive a code for glaucoma (records reviewed by TSV). We also investigated the agreement separately for women who received a code for glaucoma and had at least one OAG-type code, and those who did not receive any OAG code.
For medical record review, OAG was defined as a combination of any 2 of the following 4 criteria: 1) intraocular pressure > 24 mmHg; 2) optic nerve cup to disc ratio > 0.6; 3) optic nerve cup to disc ratio rim width < 0.1; and 4) optic nerve cup to disc ratio asymmetry > 0.2, or any reliable visual field defects consistent with glaucoma (including nasal steps, arcuate defects, altitudinal defects, advanced tunnel vision, or advanced cases with a remaining temporal island).
Statistical analyses
The pre-specified outcomes were analyzed using time-to-event Cox proportional hazards models. The year of onset of glaucoma was derived from the first occurrence in the REP system of any glaucoma diagnosis code or of a glaucoma code within a specified glaucoma type (OAG or OHTN, and OAG alone). Women with none of the codes for glaucoma were followed passively from the index year through death, last medical contact with the REP, or June 30, 2010. We used age as the time scale for all analyses, and women entered the risk set at their respective index ages.
Pre-specified analyses were performed overall and separately within strata defined by age at the time of oophorectomy divided in tertiles, and in strata by indication. Analyses stratified by indication were performed to investigate a possible confounding effect by surgical indication. 31 Additional primary analyses were performed to determine whether estrogen treatment modified the risk of glaucoma. Of note, information about the indications for oophorectomy and estrogen treatment was abstracted from medical records.22
RESULTS
Validity of glaucoma outcome
We randomly selected 60 women who had at least one glaucoma diagnosis, including 30 with an OAG-type code and 30 with another type of glaucoma diagnosis, and 40 women with no glaucoma diagnoses in the REP system. Of these 100 women, 7 (5 with and 2 without glaucoma diagnoses) had withdrawn consent to use their medical records for research at the time of the validation study, thus we were unable to review their full medical histories.
Forty-three of the 55 women with at least one glaucoma diagnosis code had some type of glaucoma documented in their ophthalmic records (positive predictive value (PPV) = 78.2%). Only one of the 38 women without glaucoma diagnosis codes had glaucoma documented in their ophthalmic records (negative predictive value (NPV) = 97.4%). Of the 28 women with one or more diagnosis codes of OAG-type, 27 had some type of glaucoma documented in their ophthalmic records (PPV = 96.4%). However, only 17 of these women met the clinical criteria for OAG (PPV for glaucoma subtype = 60.7%). Among the 27 women with non-OAG glaucoma diagnosis codes, only 16 had some type of glaucoma in their medical records (PPV = 59.3%), and 5 (18.5%) had clinically defined OAG.
Study sample
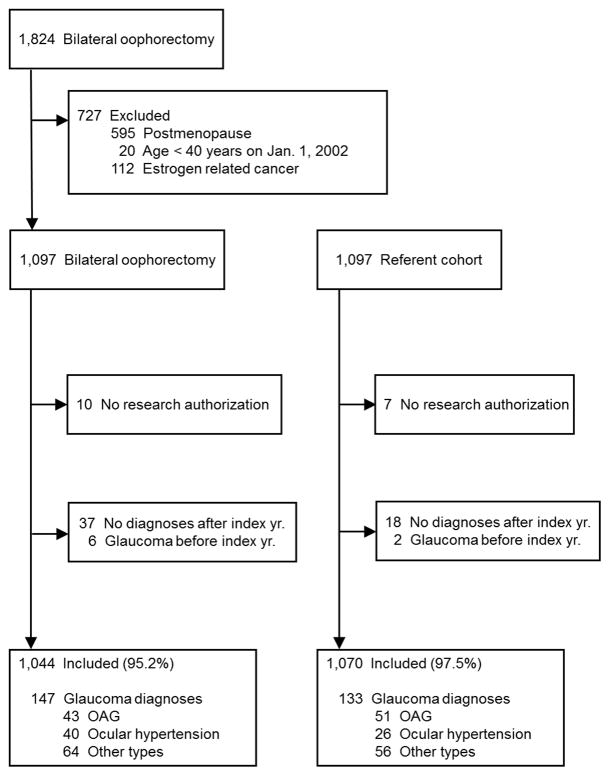
Of the 1,097 women who underwent bilateral oophorectomy, we excluded 10 women who had not given consent to use their medical records for research, 37 with no electronic diagnostic codes after the year of surgery, and 6 with at least one glaucoma diagnosis before oophorectomy (total = 53). Of the 1,097 matched referent women, we excluded 7 women who had not given consent to use their medical records for research, 18 with no electronic diagnostic codes after the index year, and 2 with at least one glaucoma diagnosis before index date (total = 27). Thus, 1,044 women who underwent bilateral oophorectomy and 1,070 referent women were included in the final analyses. Figure 1 shows the flowchart of inclusion in the study, and the numbers of women found to have glaucoma diagnoses during follow-up.
Figure 1.
Flowchart of study cohorts. OAG = primary open-angle glaucoma including exfoliation and pigmentary glaucoma.
The median age (interquartile range) at index year in the bilateral oophorectomy group was 45.9 years (41.0 – 49.7 years). Due to the matched design, the median age at index year was also 45.9 years in the referent group (40.8 – 49.7 years; P = 0.83). The median follow-up durations (interquartile range) were 25.5 years (18.9 – 33.4 years) in the bilateral oophorectomy group and 25.5 years (17.9 – 33.6 years) in the referent women (P = 0.78). Indications for bilateral oophorectomy were a benign ovarian condition in 530 women (50.8%) and prophylaxis in 514 (49.2%).
Risk of glaucoma
A total of 147 women (14.1%) who underwent bilateral oophorectomy and 133 referent women (12.4%) had at least one glaucoma diagnosis code after the index year. Of these 280 women, 94 had OAG including exfoliation and pigmentary glaucoma, 66 had OHTN, 13 had angle closure glaucoma, 6 had secondary glaucoma, and 101 had unspecified glaucoma. Table 1 shows results of cohort analyses for glaucoma of all types, for OAG or OHTN, and for OAG alone. The risk of glaucoma of all types was similar in women who underwent bilateral oophorectomy and in referent women overall (HR 1.12, 95% CI 0.89–1.42). However, the risk of glaucoma was significantly higher in women who underwent bilateral oophorectomy before age 43 years (first tertile of age distribution; HR 1.60, 95% CI 1.15–2.23, Figure 2). The results did not change after adjustment for hypertension, obesity, diabetes, or disorders of lipid metabolism at baseline (HR 1.64; 95 CI 1.17–2.23). None of the women in the bilateral oophorectomy or the referent cohort who were younger than age 43 years at baseline had experienced a myocardial infarction or a stroke.
TABLE 1.
Risk of glaucoma in women with and without bilateral oophorectomy
| Cohort of stratum | Women at risk | Person-years of follow-up | All glaucoma diagnoses
|
OAG or OHTN
|
OAG alone
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | HR (95% CI) | P | N (%) | HR (95% CI) | P | N (%) | HR (95% CI) | P | |||
| Overall analyses | |||||||||||
| Referent women | 1,070 | 26,615 | 133 (12.4) | 1.00 (reference) | --- | 77 (7.2) | 1.00 (reference) | --- | 51 (4.8) | 1.00 (reference) | --- |
| Bilateral oophorectomy | 1,044 | 26,326 | 147 (14.1) | 1.12 (0.89–1.42) | 0.33 | 83 (8.0) | 1.10 (0.80–1.49) | 0.56 | 43 (4.1) | 0.86 (0.57–1.29) | 0.47 |
| Analyses stratified by indication | |||||||||||
| Benign conditions | 530 | 13,813 | 77 (14.5) | 1.20 (0.91–1.59) | 0.20 | 37 (7.0) | 1.00 (0.67–1.47) | 0.98 | 21 (4.0) | 0.86 (0.52–1.43) | 0.57 |
| Prophylactic | 514 | 12,513 | 70 (13.6) | 1.05 (0.79–1.40) | 0.74 | 46 (9.0) | 1.19 (0.83–1.72) | 0.35 | 22 (4.3) | 0.86 (0.52–1.42) | 0.55 |
| Analyses stratified by age at oophorectomy or index year | |||||||||||
| Before age 43 y | 344 | 9,120 | 50 (14.5) | 1.60 (1.15–2.23) a | 0.01 | 25 (7.3) | 1.40 (0.88–2.23) | 0.15 | 11 (3.2) | 0.96 (0.50–1.86) | 0.90 |
| Benign conditions b | 247 | 6,786 | 33 (13.4) | 1.37 (0.93–2.02) | 0.11 | 13 (5.3) | 0.95 (0.52–1.72) | 0.86 | 7 (2.8) | 0.79 (0.36–1.75) | 0.56 |
| Prophylactic b | 97 | 2,335 | 17 (17.5) | 2.40 (1.44–4.01) | 0.001 | 12 (12.4) | 3.01 (1.61–5.60) | 0.001 | 4 (4.1) | 1.57 (0.56–4.38) | 0.39 |
| No ET to age 50 y c | 307 | 7,850 | 41 (13.4) | 1.60 (1.12–2.30) | 0.01 | 21 (6.8) | 1.44 (0.88–2.37) | 0.15 | 10 (3.3) | 1.08 (0.54–2.15) | 0.83 |
| ET to age 50 y c | 37 | 1,271 | 9 (24.3) | 1.59 (0.81–3.13) | 0.18 | 4 (10.8) | 1.23 (0.45–3.37) | 0.69 | 1 (2.7) | 0.46 (0.06–3.33) | 0.44 |
| Ages 43_48 y | 316 | 8,084 | 47 (14.9) | 1.13 (0.81–1.57) | 0.48 | 26 (8.2) | 1.08 (0.69–1.69) | 0.72 | 16 (5.1) | 1.00 (0.57–1.75) | 0.99 |
| After age 48 y | 384 | 9,122 | 50 (13.0) | 0.83 (0.60–1.15) | 0.26 | 32 (8.3) | 0.92 (0.61–1.39) | 0.68 | 16 (4.2) | 0.68 (0.39–1.20) | 0.18 |
OAG, open-angle glaucoma including primary open-angle glaucoma, exfoliation, and pigmentary glaucoma; OHTN, ocular hypertension; ET, estrogen treatment; HR, hazards ratio; CI, confidence interval
The analyses adjusted by hypertension, obesity, diabetes, or disorders of lipid metabolism yielded similar results (HR 1.64; 95% CI 1.17–2.29; P=0.004).
There was no significant difference between the HR for women who underwent bilateral oophorectomy before age 43 years for benign conditions or for prophylaxis for all glaucoma diagnoses (P for interaction = 0.06) or for OAG alone (P for interaction = 0.27). However, in analyses for OAG and OHTN diagnoses, there was a significant difference in risk between the indication groups (P for interaction = 0.004).
A test for interaction between bilateral oophorectomy and ET was not significant for all glaucoma diagnoses (P for interaction = 0.98), for OAG and OHTN (P for interaction = 0.77), or for OAG alone (P for interaction = 0.42). We stratified the sample in women who received estrogen treatment to age 50 years or older and women who did not receive any estrogen treatment or who interrupted treatment before age 50 years (age 50 years was used as an approximate mean age at the time of natural menopause). The onset of estrogen treatment was invariably proximate to the oophorectomy.
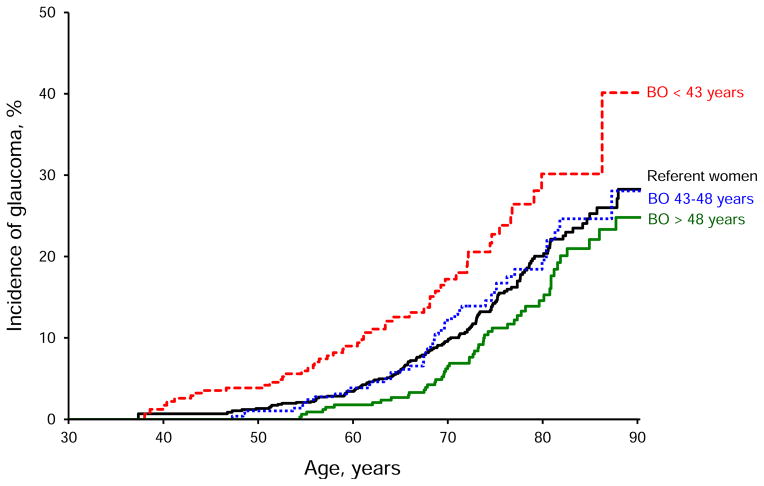
Figure 2.
Incidence of glaucoma in women with bilateral oophorectomy and in women without bilateral oophorectomy (referent women). Separate curves are shown for the three tertiles of age at the time of bilateral oophorectomy (before age 43 years, between age 43 and 48 years, and older than age 48 years). Note the particularly increased risk of glaucoma in women who underwent bilateral oophorectomy before the age of 43 years. BO = bilateral oophorectomy.
In stratified analyses, bilateral oophorectomy before age 43 years was associated with an increased risk of glaucoma diagnosis both in women who had a prophylactic indication (HR 2.40, 95% CI 1.44–4.01) and in women who had a benign ovarian condition (HR 1.37, 95% CI 0.93–2.02; Table 1; P for interaction = 0.06). However, the association was statistically significant only for women who had a prophylactic indication. Of the 344 women who underwent bilateral oophorectomy before age 43 years, only 37 (10.8%) were treated with estrogen to age 50 years or older. The analysis stratified by estrogen treatment showed significant increased risk of glaucoma both in women who did not have estrogen treatment or interrupted treatment before age 50 years (HR 1.60, 95% CI 1.12–2.30) and in women treated with estrogen to age 50 years or older (HR 1.59, 95% CI 0.81–3.13; P for interaction = 0.98).
The risk of OAG or OHTN combined was not higher in women who underwent bilateral oophorectomy than in referent women overall or in strata defined by age (Table 1). In analyses stratified by indication, the risk of OAG or OHTN was higher in women who underwent bilateral oophorectomy before age 43 years for prophylaxis (HR 3.01, 95% CI 1.61–5.60), but not in women who underwent bilateral oophorectomy for a benign ovarian condition (HR =0.95; 95% CI 0.52–1.72; P for interaction = 0.004). In analyses restricted to diagnoses of OAG alone, we found no associations overall or separately within strata defined by age, indication for surgery, or estrogen treatment. However, the number of outcomes within each stratum was small.
DISCUSSION
Major findings
Little is known about the effects of oophorectomy on the risk of glaucoma. The Mayo Clinic Cohort Study of Oophorectomy and Aging provided a unique opportunity to investigate glaucoma in a population-based cohort study with long follow-up. The long follow-up was particularly important because glaucoma is a slow and progressive optic neuropathy and may manifest only many years after the oophorectomy. Our findings suggest that women who undergo bilateral oophorectomy before age 43 years are at 1.6–fold increased risk of developing glaucoma of all types over the next 25 years. The results did not change after adjustment for hypertension, obesity, diabetes, or disorders of lipids metabolism at baseline (index year). Our findings may guide individualized assessment of sex-specific risk factors for glaucoma as well as the risks and benefits of bilateral oophorectomy in younger women.
Our study provides the first population-based results suggesting that bilateral oophorectomy in women younger than 43 years increases the risk of glaucoma overall, and of OAG or OHTN in particular. These findings are in agreement with previous population-based studies showing that early menopause or late menarche may increase the risks of POAG.12–16 In the Rotterdam Study, the risk of POAG was higher in women who entered menopause before age 45 years (odds ratio [OR] 2.6; 95% CI 1.5–4.8).12 In the Blue Mountains Study, the risk of POAG was significantly higher in women who reported late menarche (after age 13 years, OR 2.0; 95% CI 1.0–3.9), but not in women who underwent early menopause.16 In the Nurses’ Health Study (NHS), the risk of POAG was 50% lower in women who underwent menopause at age ≥ 54 years than at age < 54 years (relative risk [RR] 0.53; 95% CI 0.32–0.89) over more than 20 years of follow-up.15 By contrast, the Aravind Comprehensive Eye Survey did not find significant associations between late menarche, early menopause, or duration of estrogen exposure and the risk of POAG.13 These discrepant findings may be due to the age of the women. The mean age of women in the Aravind Eye Survey was more than 10 years younger than the mean age of the women in other cohorts.15 In addition, although the postmenopausal use of hormones did not decrease the risk of POAG in some studies, the postmenopausal use of combined estrogen and progestin significantly reduced the risk of POAG in women with OHTN in the NHS.15
Consistent with previous epidemiologic evidence, our findings indicate that early loss of female sex hormones caused by bilateral oophorectomy increases the risk for glaucoma development. Estrogen loss may affect the aging of the optic nerve through several alternative mechanisms.8 First, the mechanical theory suggests that mechanical stress from increased intraocular pressure may damage the optic nerve.9 Estrogens may regulate intraocular pressure by influencing the aqueous production and outflow systems.32, 33 The intraocular pressure is higher in postmenopausal than in age-matched premenopausal women, and it decreases 1–4 mmHg in response to postmenopausal hormone therapy in women with and without POAG.32–37 Second, the vascular theory suggests that decreased perfusion or vascular dysregulation of the optic nerve leads to neurodegeneration.9, 38, 39 Estradiol regulates smooth muscle tone and vascular resistance and augments the activity of endothelial-based nitric oxide synthase (eNOS3).40, 41 In addition, animal models and clinical studies have indicated that estradiol increases the perfusion of the optic nerve, retinal ganglion cells, and their supporting structures.17, 32, 42–46 Finally, the neuroprotective effects of estradiol in glaucoma animal models appear to be mediated at least in part through estrogen receptors in the retinal ganglion cells.17–19 Taken together, these findings indicate that estradiol has protective effects on the optic nerve and prevents glaucoma.
The protective effect of estrogen on the optic nerve may be mediated by a reduction in risk of metabolic syndrome or in risk of cardiovascular disease.24, 47, 48 Adjustment by hypertension, obesity, diabetes, or disorders of lipid metabolism at baseline did not modify the results. However, women younger than 43 years at baseline who developed glaucoma after bilateral oophorectomy were more likely to experience a de novo metabolic syndrome between the surgery and the onset of glaucoma compared with referent women of same age at baseline who developed glaucoma (22.0% compared to 4.0% P = 0.04; data not shown). Despite the small numbers, these analyses suggest that there may be a synergistic interaction of bilateral oophorectomy and metabolic syndrome in causing glaucoma.47, 49 A similar interaction was not found for cardiovascular events (myocardial infarction or stroke; data not shown).
Although our analysis failed to demonstrate the benefits of estrogen treatment after oophorectomy, the abrupt loss of estrogens caused by bilateral oophorectomy likely plays a major role. The lack of benefit of estrogen treatment in our analysis may be due to the small number of treated women (11%). On the other hand, early bilateral oophorectomy has been associated with increased risks of a variety of health outcomes including overall mortality, cardiovascular mortality, cognitive impairment or dementia, parkinsonism, depression, anxiety, and glaucoma.20–24 These associations are stronger in women who undergo oophorectomy at younger age. Although estrogen treatment after bilateral oophorectomy has been found to reverse the increased risks of cognitive impairment or dementia and cardiovascular mortality, it did not reduce the risks of parkinsonism, depression, anxiety, or glaucoma. These differences suggest that the causal mechanisms are likely complex and may involve interactions between the effects of reduced concentrations of circulating estrogen, progesterone, or testosterone, or increased release of gonadotropins. In addition, other genetic or non-genetic risk factors may be involved in some women.
Overall, the risk of glaucoma in women who underwent bilateral oophorectomy before age 43 years did not differ significantly by indication. However, the risk of glaucoma was significantly higher in women who underwent bilateral oophorectomy for prophylaxis compared with women with a benign ovarian condition in analyses restricted to OAG or OHTN. These two findings considered together suggest that the benign ovarian conditions that prompted the surgery in a subset of women were not confounding variables in the observed association. Thus, bilateral oophorectomy appears to be a direct causal event leading to glaucoma rather than an event spuriously associated with glaucoma.
Strengths and limitations
This study had several strengths. The year of bilateral oophorectomy, the indication for the oophorectomy, and the use of estrogen after surgery were documented in the medical records, eliminating the need for recall of the surgical event or estrogen use. In addition, we were able to follow women for approximately 25 years to observe incident glaucoma. Finally, age was used as the time scale in the analyses to minimize the confounding effect of age.
However, our study had several limitations. First, glaucoma diagnostic codes were used as glaucoma outcomes, and mixed types of glaucoma were included in our primary analysis. Epidemiological, clinical, and basic science evidence suggests that the effect of early bilateral oophorectomy is restricted to OAG and OHTN. Unfortunately, we were unable to study OAG as our primary outcome because of limited sample size and because of the risk of misclassification. In our validation study, the diagnostic codes for glaucoma of any type had a PPV of 78.2% and a NPV of 97.4%. However, we found misclassifications of OAG vs. other types of glaucoma. Even though the diagnostic codes for OAG had the highest PPV for any type of glaucoma (96.4%), they had a low PPV for OAG in particular (60.7%). This misclassification may be due to changes in the definitions and classifications of glaucoma and OAG over time. In brief, OAG is considered an optic neuropathy and its diagnosis is based on characteristic changes in the optic nerve with or without corresponding visual field defects (perimetric or pre-perimetric glaucoma, respectively). Furthermore, although intraocular pressure remains the major risk factor and the only modifiable factor for OAG, OHTN is considered a separate entity and is no longer one of the criteria for OAG diagnosis.
A second limitation of our study was the relatively small number of women who underwent oophorectomy before age 43 years and were treated with estrogen. Therefore, we were unable to adequately explore the effects of estrogen therapy after early bilateral oophorectomy. Third, our outcomes were restricted to glaucoma diagnoses given to women at one of the participating REP providers. Unfortunately, privately practicing optometrists do not currently share data with the REP, and we may have undercounted the diagnoses of glaucoma. However, the potential undercounting of glaucoma outcomes should be equal in the oophorectomy and referent cohorts (non-differential misclassification).50
Fifth, because we did not exclude or stratify referent women based on their menopausal status at the index year, we compared women who underwent oophorectomy to the entire remaining population. Our study was not designed to compare women who underwent premature or early menopause due to a bilateral oophorectomy versus those who experienced premature or early menopause naturally. For the stratum of women younger than 43 years at the time of oophorectomy, very few referent women were expected to be naturally menopausal. Premature natural menopause occurs in approximately 1% of women younger than 40 years and early natural menopause occurs in approximately 5% of women between ages 40–45 years.51
CONCLUSIONS
Early bilateral oophorectomy is associated with an increased risk of a variety of health outcomes, including several conditions related to brain aging. Our study is the first to show that women who underwent bilateral oophorectomy before age 43 years are at increased risk of developing glaucoma. These findings are in agreement with previous epidemiologic, clinical, and animal studies suggesting that estrogen has a neuroprotective effect on the optic nerve.8, 12–19 Our findings provide new evidence to guide the individualized assessment of sex-specific risk factors for glaucoma, and may assist women and their physicians in evaluating the risks and benefits of bilateral oophorectomy.52
Acknowledgments
Funding: This work was supported by the National Institute of Neurological Disorders and Stroke (R01 NS033978), the National Institute on Aging (R01 AG034676), and the National Institute of Child Health and Human Development and the Office of Research on Women’s Health (K12HD055892; support for TSV).
Footnotes
Conflict of interest/financial disclosure: None of the authors have any financial disclosures.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010;21:91–99. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515–522. doi: 10.1016/j.ajo.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20:92–98. doi: 10.1097/ICU.0b013e32832401a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vajaranant TS, Wu S, Torres M, Varma R. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthalmol. 2012;154:303–314. e303. doi: 10.1016/j.ajo.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guedes G, Tsai JC, Loewen NA. Glaucoma and aging. Curr Aging Sci. 2011;4:110–117. doi: 10.2174/1874609811104020110. [DOI] [PubMed] [Google Scholar]
- 7.Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007;48:1201–1211. doi: 10.1167/iovs.06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vajaranant TS, Pasquale LR. Estrogen deficiency accelerates aging of the optic nerve. Menopause. 2012;19:942–947. doi: 10.1097/gme.0b013e3182443137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 10.Ryskulova A, Turczyn K, Makuc DM, Cotch MF, Klein RJ, Janiszewski R. Self-reported age-related eye diseases and visual impairment in the United States: results of the 2002 national health interview survey. Am J Public Health. 2008;98:454–461. doi: 10.2105/AJPH.2006.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DS, Nordstrom B, Mozaffari E, Quigley HA. Variations in treatment among adult-onset open-angle glaucoma patients. Ophthalmology. 2005;112:1494–1499. doi: 10.1016/j.ophtha.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Hulsman CA, Westendorp IC, Ramrattan RS, et al. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am J Epidemiol. 2001;154:138–144. doi: 10.1093/aje/154.2.138. [DOI] [PubMed] [Google Scholar]
- 13.Nirmalan PK, Katz J, Robin AL, et al. Female reproductive factors and eye disease in a rural South Indian population: the Aravind Comprehensive Eye Survey. Invest Ophthalmol Vis Sci. 2004;45:4273–4276. doi: 10.1167/iovs.04-0285. [DOI] [PubMed] [Google Scholar]
- 14.Pasquale LR, Kang JH. Female reproductive factors and primary open-angle glaucoma in the Nurses’ Health Study. Eye. 2011;25:633–641. doi: 10.1038/eye.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquale LR, Rosner BA, Hankinson SE, Kang JH. Attributes of female reproductive aging and their relation to primary open-angle glaucoma: a prospective study. J Glaucoma. 2007;16:598–605. doi: 10.1097/IJG.0b013e318064c82d. [DOI] [PubMed] [Google Scholar]
- 16.Lee AJ, Mitchell P, Rochtchina E, Healey PR. Female reproductive factors and open angle glaucoma: the Blue Mountains Eye Study. Br J Ophthalmol. 2003;87:1324–1328. doi: 10.1136/bjo.87.11.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deschenes MC, Descovich D, Moreau M, et al. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2010;51:2587–2600. doi: 10.1167/iovs.09-3710. [DOI] [PubMed] [Google Scholar]
- 18.Russo R, Cavaliere F, Watanabe C, et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008;173:583–590. doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Li F, Ge J, et al. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol. 2007;67:603–616. doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- 20.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 21.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 22.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 23.Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–1059. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- 24.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Yawn BP, et al. Data Resource Profile: The Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of Epidemiologic Findings and Public Health Decisions: An Illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey HC-120. [Accessed May 20, 2013];Appendix 3: Clinical Classification Software (CCS) to ICD-9-CM Code Crosswalk. Available at: http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h120/h120_icd9codes.shtml.
- 30.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Clinical Classification Software (CCS) for ICD-9-CM. [Accessed May 20, 2013];HCUP Website. Available at: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Updated August 30m 2012.
- 31.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altintas O, Caglar Y, Yuksel N, Demirci A, Karabas L. The effects of menopause and hormone replacement therapy on quality and quantity of tear, intraocular pressure and ocular blood flow. Ophthalmologica Journal international d’ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 2004;218:120–129. doi: 10.1159/000076148. [DOI] [PubMed] [Google Scholar]
- 33.Ogueta SB, Schwartz SD, Yamashita CK, Farber DB. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Ophthalmol Vis Sci. 1999;40:1906–1911. [PubMed] [Google Scholar]
- 34.Tint NL, Alexander P, Tint KM, Vasileiadis GT, Yeung AM, Azuara-Blanco A. Hormone therapy and intraocular pressure in nonglaucomatous eyes. Menopause. 2010;17:157–160. doi: 10.1097/gme.0b013e3181b82fb4. [DOI] [PubMed] [Google Scholar]
- 35.Sator MO, Akramian J, Joura EA, et al. Reduction of intraocular pressure in a glaucoma patient undergoing hormone replacement therapy. Maturitas. 1998;29:93–95. doi: 10.1016/s0378-5122(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 36.Sator MO, Joura EA, Frigo P, et al. Hormone replacement therapy and intraocular pressure. Maturitas. 1997;28:55–58. doi: 10.1016/s0378-5122(97)00060-1. [DOI] [PubMed] [Google Scholar]
- 37.Toker E, Yenice O, Temel A. Influence of serum levels of sex hormones on intraocular pressure in menopausal women. J Glaucoma. 2003;12:436–440. doi: 10.1097/00061198-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Harris A, Rechtman E, Siesky B, Jonescu-Cuypers C, McCranor L, Garzozi HJ. The role of optic nerve blood flow in the pathogenesis of glaucoma. Ophthalmol Clin North Am. 2005;18:345–353. doi: 10.1016/j.ohc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Feke GT, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 2008;115:246–252. doi: 10.1016/j.ophtha.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 40.Kang JH, Wiggs JL, Rosner BA, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–979. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohmichi M, Kanda Y, Hisamoto K, et al. Rapid changes of flow-mediated dilatation after surgical menopause. Maturitas. 2003;44:125–131. doi: 10.1016/s0378-5122(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 42.Harris-Yitzhak M, Harris A, Ben-Refael Z, Zarfati D, Garzozi HJ, Martin BJ. Estrogen-replacement therapy: effects on retrobulbar hemodynamics. Am J Ophthalmol. 2000;129:623–628. doi: 10.1016/s0002-9394(99)00468-7. [DOI] [PubMed] [Google Scholar]
- 43.Harris A, Harris M, Biller J, et al. Aging affects the retrobulbar circulation differently in women and men. Arch Ophthalmol. 2000;118:1076–1080. doi: 10.1001/archopht.118.8.1076. [DOI] [PubMed] [Google Scholar]
- 44.Atalay E, Karaali K, Akar M, et al. Early impact of hormone replacement therapy on vascular hemodynamics detected via ocular colour Doppler analysis. Maturitas. 2005;50:282–288. doi: 10.1016/j.maturitas.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Atilla H, Arslanpence A, Batioglu F, et al. Effect of hormone replacement therapy on ocular hemodynamics in postmenopausal women. Eur J Ophthalmol. 2001;11:277–280. doi: 10.1177/112067210101100311. [DOI] [PubMed] [Google Scholar]
- 46.Battaglia C, Mancini F, Regnani G, Persico N, Volpe A, De Aloysio D. Hormone therapy and ophthalmic artery blood flow changes in women with primary open-angle glaucoma. Menopause. 2004;11:69–77. doi: 10.1097/01.GME.0000079741.18541.92. [DOI] [PubMed] [Google Scholar]
- 47.Park BJ, Park JO, Kang HT, Lee YJ. Elevated intraocular pressure is associated with metabolic syndrome in postmenopausal women: the Korean National Health and Nutrition Examination Survey. Menopause. 2013 doi: 10.1097/GME.0b013e31827ce3c6. (In Press) [DOI] [PubMed] [Google Scholar]
- 48.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 49.Pasquale LR. There is more to the relation between intraocular pressure and metabolic syndrome than meets the eye : a connection to estrogen deficiency. Menopause. 2013 doi: 10.1097/GME.0b013e318294096b. (In Press) [DOI] [PubMed] [Google Scholar]
- 50.Porta MS International Epidemiological Association. A Dictionary of Epidemiology. 5. Oxford; New York: Oxford University Press; 2008. [Google Scholar]
- 51.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rocca WA, Ulrich LG. Oophorectomy for whom and at what age? Primum non nocere (Editorial) Maturitas. 2012;71:1–2. doi: 10.1016/j.maturitas.2011.10.006. [DOI] [PubMed] [Google Scholar]