Abstract
Objective
The potentially detrimental effects of cancer and related treatments on cognitive functioning have emerged as key foci of cancer survivorship research, but little is known about how psychological variables other than depression influence these relationships. To illustrate the potential of social psychological perspectives, we examine how a self-regulatory analysis and specific self-regulatory challenges of contending with cancer-related expectancies and stereotypes provide conceptual frameworks for understanding some of the potential causes and consequences of cancer-related cognitive deficits.
Methods
Literatures on cancer-related cognitive deficits, self-regulatory ego depletion, expectancy-stereotypes and their points of convergence are briefly reviewed.
Results
A review and conceptual integration of relevant literatures suggests that coping with cancer can impair self-regulatory capacity, there is overlap between cognitive deficits associated with self-regulatory challenge and with cancer and its treatment, and restoring self-regulatory resources can attenuate cancer-related cognitive deficits. Examination of specific regulatory challenges of contending with expectancies and stereotypes related to treatment suggest insights that can inform when and among whom cognitive deficits may most likely emerge.
Conclusions
Integrating social psychological ideas with a substantial knowledge base can illustrate novel research trajectories that can deepen our understanding of cancer-related cognitive deficits and their impact on psychosocial well-being.
Keywords: cancer, oncology, cognitive deficit, self-regulation, expectancies
Introduction
Initially inspired by a groundswell of complaints from cancer survivors, the detrimental effects of cancer and related treatments on cognitive functioning has emerged as a key focus of cancer survivorship research [1]. This research has focused primarily on biological factors (e.g., neuroanatomical changes, genetic influences) as explanatory mechanisms [2]. Much less is known about psychological factors affecting the occurrence and maintenance of cognitive symptoms following chemotherapy and other cancer treatments. In fact, studies generally conceptualize psychological factors such as negative affect and fatigue as confounds influencing the relationship between chemotherapy and cognitive functioning [3–5].
Rather than relegating psychological factors to the realm of confounds, we propose their integration may hold considerable generative potential. Studies in other areas increasingly reveal decrements in cognitive functioning resulting from challenges to basic self-regulatory processes [6] that include dealing with expectancies and stereotypes such as those associated with cancer patients [7]. In this paper, we examine the potential for theory and research on specific self-regulation and expectancy processes to increase understanding of potential causes and consequences of cancer-related cognitive deficits. We raise the possibility that for certain patients, self-regulatory and expectancy processes may also play a role – as contributing, additive, or mediational influences – in cognitive functioning. Our goal is to show how integration of psychological theory and research can help uncover novel research trajectories that may inform influences on cancer-related cognitive deficits and their impact on psychosocial well-being.
Cancer-related cognitive deficits: A brief overview
Over the last 10–15 years, evidence reveals acute and long-term cognitive problems for many patients after chemotherapy for non-central nervous system (CNS) malignancies. Cognitive problems may arise – or worsen from pre-chemotherapy problems – during treatment, and may continue long after treatment cessation [8–10]. The observed cognitive deficits are indicative of a frontal-subcortical profile, with core impairments related to learning and memory, executive functions and psychomotor speed [11–14]. Preclinical studies have demonstrated adverse neurological effects of many chemotherapeutic agents, and chemotherapy-induced damage of mature post-mitotic oligodendrocytes and immature progenitor cell populations required for ongoing neurogenesis, gliogenesis, and maintenance of white matter integrity are emerging as important factors in neurotoxicity development. Also, neuroimaging studies reveal structural changes in the brain from pre- to post chemotherapy [15–21], especially in white matter microstructures.
Importantly, however, not all patients seem to be affected; an observation that highlights the need for continued exploration of individual risk factors for, and other contributing influences to, treatment-related cognitive decline [22]. Examinations of these risk factors are particularly critical in light of evidence that cognitive problems can significantly undermine quality of life and daily functioning among cancer survivors [8–10, 23]. This holds for both actual deficits and the perceptions of such deficits [24–25].
We suggest that it is important to consider the phenomenological states of the patient during the cancer treatment trajectory – following diagnosis, during treatment, and post-treatment. Specifically, we argue that factors traditionally considered under the domain of social psychological research may further our understanding of cognitive difficulties observed among some patients with non-CNS cancers.
A psychological perspective on factors contributing to cognitive deficits: The role of self-regulation and expectancies
Basic connections between self-regulation and executive function
Research in social psychology consistently shows that exerting self-regulatory control in one domain can subsequently impair performance on unrelated tasks [26]. For example, persons who initially inhibit desires to eat tempting food in favor of healthier, but less desirable, food later spend less time on problem-solving tasks than those who were not taxed with self-control demands [27]. The theoretical framework that has generated a majority of these findings posits that one’s ability to regulate behavior (i.e., make adjustments to attain desired goals) depends on a generalized psychological resource that can be construed as mental energy, self-regulatory capacity, or ego strength [28–29]. From this view, individuals have a limited stock of mental energy to expend fon the wide range of self-regulatory behaviors (e.g., emotion regulation, attention, decision-making). Overuse of this resource for one self-regulation task reduces what is available for engagement in other tasks. For example, decision-making regarding cancer treatment options may lead to a state of ego depletion that negatively affects one’s ability to engage in other tasks requiring self-regulatory skills, such as planning meals or solving work-related problems. Recently, it has been argued that initial self-control demands may undermine the motivation to exert self-control, or attention to self-control cues, in subsequently encountered tasks [30]. This may manifest when coping with cancer undermines the motivation to expend the cognitive effort required to keep up with other life tasks. Both processes may be relevant to cancer patients: living with cancer may deplete the ability or the motivation to attend to other responsibilities.
Self-control demands do not merely elicit a general state of fatigue. Research directly contrasting regulatory demands (i.e., the suppression of unpleasant emotions) with sleep deprivation does not show parallel effects on behavior [31]. Thus, while self-regulatory demand may share many features with fatigue, it is differently centered on the individuals’ mental energy, attention, and motivation to exert self-control. Although we discuss the applicability of these processes to cancer experiences more fully below, the point here is that while cancer patients can suffer from fatigue, they can also face numerous other regulatory demands (e.g., inhibiting distress associated with physical changes such as hair loss).
Perhaps especially interesting for the present focus are findings linking self-regulatory depletion to impaired executive functioning [32–33]. Across a series of laboratory experiments [34], regulatory demands (e.g., inhibiting the expression of distressing emotions) led to worse performance on tasks requiring the use of logic, reasoning, and cognitive extrapolation; however, there were no adverse effects on rote memory or general knowledge. These findings are consistent with literature indicating that self-regulatory capacity is linked to specific aspects of executive functioning, such as information organization and retrieval from memory [35–38]. That attenuation of self-regulatory control may be linked to executive functioning deficits opens the door to potential insights into cognitive decline in cancer patients and survivors, where problems with executive functioning is one area of documented deficit.
Self-regulation and cancer-related cognitive deficits
Self-regulatory efforts and impaired cognitive capacity may operate in a dialectical series of dynamic, recursive relationships within the context of cancer survivorship. Recent models on the connection between executive function and self-regulation [32,39], as well as attention restoration theory [33], converge to suggest that executive function skills facilitate self-regulation efforts, and effective self-regulation facilitates executive function. Considering this framework in understanding cancer survivors’ experiences, as depicted in Figure 1, survivors may experience demands on their self-regulatory capacities due to efforts to cope with cancer and associated treatments. This may also occur through neurological, immune (e.g., inflammatory responses), emotional (e.g., depression or anxiety), and somatic (e.g., pain, sleep disruption) effects of cancer and its treatment. Additional deficits in self-regulatory resources may stem from attentional demands such as keeping track of medications and dosage regimes. These self-regulatory burdens may contribute to the observed deficits in cognitive function. Simultaneously, these cognitive deficits may then further impair self-regulatory capacities and motivation, thereby exacerbating cognitive decline and possibly a range of other psychosocial consequences. Further, the biology of aging (e.g., increased DNA damage, shortened telomeres, reduced stem cell reserve, etc.) increases risk of cancer and reduced cognitive capacity, and these same pathways are negatively impacted by cancer treatments. Thus, age-associated changes in brain structure and function could further increase vulnerability to cognitive decline and reduced self-regulatory capacity [40]. Below we outline some of the conceptual ways in which this analysis might inform research on cancer-related cognitive deficits.
Figure 1.
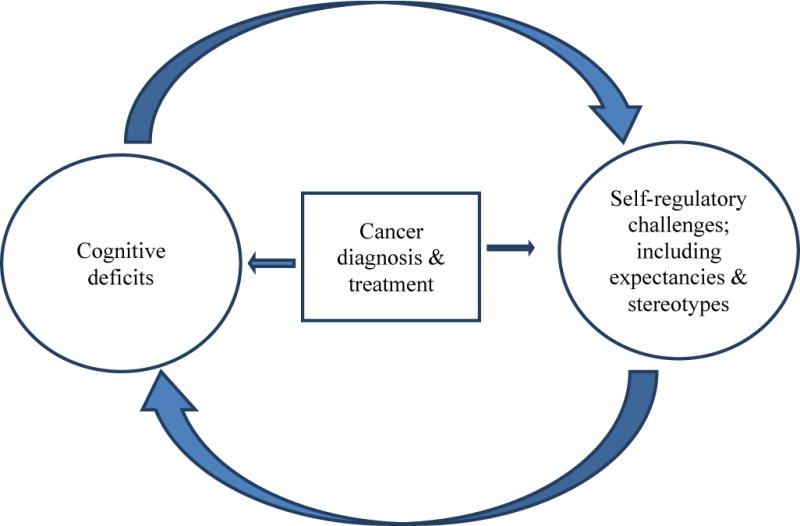
Heuristic model of bi-directional relationships between cognitive deficits and self-regulatory challenge following cancer
Although research has yet to examine effects of cancer on self-regulatory resources, considerable work indicates that coping with illness consumes self-control capacity. Stress has been implicated as a catalyst of self-regulatory depletion [41], and it also predicts cognitive deficits reported by cancer patients [42–43]. In addition, an extensive literature has shown that multi-symptom illnesses (e.g., those involving chronic pain) demand effortful regulation of emotions, thoughts, and behaviors; the tax upon coping processes, and the fatigue and sleep dysfunction that can occur, further lessen self-regulatory resources and motivation [39]. Persons dealing with cancer also have the additional burden of coping with concerns about a shortened life-span: cancer is a disease strongly linked to thoughts about death, both among lay people [44–45] and patients [46–47]. Studies with non-patient samples suggest that people may suppress the death-related cognitions with which cancer is associated [44], and that managing awareness of mortality consumes self-regulatory resources [48].
If self-regulatory exertion contributes, in part, to cognitive deficit subsequent to cancer, then there may be interesting parallels between the nature of cognitive deficits observed in laboratory studies of self-regulatory challenge and those documented following cancer treatment. As noted previously, performance of laboratory-based self-control tasks leads to poorer performance on subsequent tasks reliant on executive functioning [34–35]. Providing converging evidence, Schmeichel [6] reported that a variety of self-control experiences (e.g., exaggerating emotional expressions) led to reduced performance on memory updating tests (e.g., a reverse digit span task in which one recalls a string of numbers in reverse order of presentation). These self-control challenges have also been found to disrupt later performance on fluency tests [49]. Although more research is clearly needed, these findings suggest that individuals whose self-regulation is challenged and persons coping with cancer may experience comparable changes in cognitive performance.
If cancer-related cognitive deficits stem in part from self-regulatory fatigue, then efforts to strengthen self-regulatory resources should help to attenuate these deficits. Research from attention restoration theory [33] supports this possibility [50–51]. Attention restoration theory posits that time spent in natural environments that invoke involuntary attention provides directed attention mechanisms the opportunity to recover and replenish. Accordingly, among breast cancer patients with cognitive deficits post-surgery, those in attention restoration conditions (e.g., connecting with nature by walking, gardening multiple times per week) manifested steady improvement over 12 weeks (e.g., on digit span backwards tasks), whereas those in control groups did not. Given the current lack of insight into the exact workings of attention restoration activities, one must be cautious with regard to their widespread potential to attenuate cognitive complaints among cancer patients and survivors; nonetheless, the possibility is encouraging and warrants further investigation.
Implications and future directions for a self-regulatory analysis of cancer-related cognitive deficits
Integrating a self-regulatory analysis into research on cancer-related cognitive change has potential to provide novel insights into the nature and treatment of these problems. First, use of measures such as those commonly used to index depletion (e.g., persistence) or self-report measures of self-control capacity [52] may shed light on whether cognitive deficits can be explained in part by reduced abilities to exert mental control to regulate behavior. A second research agenda might explore the effects of cancer and its treatment on self-regulatory outcomes, by examining potential mediating effects of cognitive deficits on deficits in performance in other self-regulatory domains. Together such research would speak to the possibility that cognitive functioning and regulatory fatigue have reciprocal influences.
In addition, a self-regulatory perspective suggests ways in which cognitive deficits related to cancer might be attenuated. Although attention restoration is one example [50–51], the self-regulatory literature also suggests other avenues. These include the affirmation of important self-values, exercise, and relaxation training [53]. Further, forming implementation intentions (specific plans of how one will respond in a given scenario) can eliminate the impairing effects of self-regulatory demand on cognitive performance [54]. Of course, there are challenges in moving from single shot manipulations to the long term multitasking situations that patients generally confront. Nevertheless, given the relative ease with which these strategies might be integrated into a multi-faceted treatment protocol and their potential for mitigating cognitive deficits, these possibilities should be examined.
Specific self-regulatory challenges: The potential role of expectancies and stereotypes
For at least some cancer patients, self-regulatory challenges can include contending with expectations that the treatment process will impair their cognitive capacities. The broader psychological and medical literature on such phenomena as “nocebo” effects illuminates how expectancies can bring to life the very outcomes, including symptom reports, about which a person is concerned [55–56]. Expectancies can also accentuate the effects of self-regulatory demands among certain individuals. Research suggests that prior self-regulatory demands most strongly affect performance among people who believe that their ability to exert self-control – their willpower – is in fact a limited resource [57]. Yet, encouragingly from a treatment perspective, Job et al. [57] found that they could alter peoples’ implicit beliefs about willpower being limited, and doing so negates the depletion effects. This invites the provocative possibility that self-regulatory fatigue might have more adverse effects on cognitive performance among cancer patients who expect cognitive decline, and that these expectations could be targeted in treatment approaches.
Additionally, research suggests that shared societal expectancies, such as common stereotypes, may constitute a self-regulatory burden and also contribute to cognitive deficits [58]. This idea is derived from social psychological research on stereotype threat, which refers to an individual’s fear of confirming a stereotype [59–60]. The basic effect, which has been researched extensively in the areas of racial and gender stereotypes, is that activation of a stereotype often triggers behavior in accordance with that stereotype. For instance, priming women with gender-stereotypical information that ‘women are bad at math’ lowers their scores on a math test, compared with women who do not receive this information [61–62]. Stereotype threat effects on cognitive performance have also been observed following information on age-based cognitive decline [63–64], and the relation between ecstasy use and cognitive deficits [65].
Stereotype threat affects performance through numerous mechanisms that may include undermining cognitive resources. Schmader and colleagues [66] propose, for example, that both the distress elicited by stereotype exposure and the constant monitoring process reflecting concern over confirming the stereotype increase mental load and suppression efforts; this, in turn, undermines working memory efficiency. Research increasingly shows that stereotype threat can undermine cognitive and memory resources [58,67], and moreover, that such decrements mediate stereotype threat effects on performance outcomes [68]. These findings invite consideration of how stereotype threat processes may contribute to cancer-related cognitive problems.
Stereotype threat and cancer-related cognitive deficits
Important exceptions to the neglect of stereotype threat research in cancer-relevant clinical settings are studies by Schagen, Das, and colleagues. Their studies followed previous research on stereotype threat in rendering temporarily accessible the relevant stereotype, in this case the connection between chemotherapy and cognitive problems. The study capitalizes on, and then activates, patient concerns which are increasingly publicized in the media that chemotherapy can indeed degrade their cognitive abilities. A first study showed that merely adding one sentence that “some patients treated with chemotherapy experience cognitive problems” to instructional information provided to breast cancer patients increased their reports of cognitive complaints, particularly in patients without firsthand chemotherapy experience [7]. A second study extended stereotype threat effects to cognitive performance on a memory task for individuals with cancer who had received chemotherapy, but not for those who had not received chemotherapy [69].
This line of research has also begun to examine the role of individual differences. Stereotype threat effects appear stronger among people who are especially cognizant of the particular stigma [70], and participants who self-identify more strongly with a stereotyped group show stronger stereotype threat effects on cognitive function [71]. This vulnerability is often measured by degrees of stigma consciousness, the level at which individuals are chronically aware of their stigmatized status [72], for example of being a cancer patient. Recently, Das et al. [73] directly examined the possible role of stigma consciousness in the relationship between stereotype threat and cognition in women with breast cancer. Receipt of stereotypical information about the occurrence of medical problems experienced by cancer patients primed the cognitive accessibility of constructs related to cognitive problems (e.g., brain, dumb) and differentially affected women’s cognitive complaints and test scores depending on their level of consciousness of cancer patient stigma. Specifically, stereotypical information increased cognitive complaints for women high in stigma consciousness, and decreased cognitive complaints for women low in stigma consciousness. While this moderating effect of stigma consciousness amplified priming effects for self-reported cognitive problems, it seemed to decrease priming effects for cognitive task performance, suggesting both stereotype assimilation and contrasts effects depending on the degree of conscious control on the outcome measure.
In sum, emerging evidence suggests that informing women with breast cancer about the relationship between chemotherapy and cognitive problems increases cognitive complaints and decreases cognitive performance. Some patients appear more vulnerable to stereotype priming effects; in particular, patients who are highly aware of the ‘chemobrain’ stereotype – e.g., through firsthand experience or high levels of stigma consciousness – may be motivated to ‘undo’ the stereotype, which may be successful in the short run but may deplete patients’ resources and therefore backfire in the longer run [74].
Implications of a stereotype threat analysis of cancer-related cognitive deficits
Though more research is certainly needed in clinical settings, the work of Schagen, Das and colleagues offers a generative perspective. Like a broader self-regulatory analysis, integrating a stereotype threat analysis into research on cancer-related cognitive change can reveal novel insights into the nature and treatment of these problems. The hundreds of studies that have focused on the mechanisms underlying stereotype threat effects and ways to reduce them may offer a recipe for further understanding the adverse cognitive affects that people report subsequent to chemotherapy.
In terms of mechanisms, future research should assess how exactly stereotype priming affects cancer patients. Empirical findings outside the clinical setting link the negative effects of stereotype priming on test performance to anxiety, ruminative thought [61], attentional distraction [75], and decreased working memory capacity [68]. Other studies found that stereotype priming negatively affects performance due to changes in motivation and effort. For example, the ‘mere effort’ hypothesis suggests that during task performance, an individual under threat tries hard to disprove a negative stereotype, which ironically leads to worse performance [76–77]. Each of these mechanisms may provide insights into psychological processes contributing to cognitive deficits in cancer patients.
Consider, for example, the integrated process model of stereotype threat [66], which specifies that physiological stress, active monitoring of performance, and suppression of negative thoughts and emotions fuel the experience of being threatened by a stereotype. In the cancer context, a person is likely to experience high levels of stress and anxiety about the disease and its consequences (e.g., “will I be able to continue to work?’). They may also try to remove fatalistic thoughts and emotions from their everyday thinking (e.g., “don’t think this may be my last holiday”), and be vigilant about monitoring symptoms of impairment (e.g., “am I forgetting things more?”). Such concerns may then “spill-over” as stereotype threat is prone to do [78], affecting not just cognitive functioning but also affective and motivational consequences of a cancer diagnosis.
Perhaps the greatest potential of a stereotype threat perspective is its implications for reducing cognitive deficits. Many promising approaches have emerged that generally focus on changing the stereotype, buffering identities from stereotyped expectations, and fostering coping strategies to deal with the threat when it is elicited [79]. Stereotype threat research also points to the pivotal importance of the particular identity to which one subscribes. When, for example, women self-identify more strongly with the stereotyped group, they show stronger stereotype threat effects on cognitive function. When primed with a positive or different identity, however, these women exhibit improvements in working memory capacity [71]. The most recent study by Das et al. [73] showing a moderating effect of women’s self-consciousness of being a cancer patient represents an encouraging step in this direction, at least in terms of initial efforts to disprove the stereotype.
In a related vein, one interesting aspect of cancer patients’ experiences is that they can self-identify in various ways (e.g., as a “survivor” or “warrior”) [80]. An important question, then, is whether certain identities might better insulate patients from vulnerability to stereotypes about cognitive decline. Research might examine whether cognitive performance can be improved if patients receive information that challenges conventional stereotypes about chemotherapy. For example, while simply informing patients that they might experience cognitive deficits can increase self-reported cognitive difficulties [7] and lower performance on cognitive tests [69], arming patients with the knowledge that not all patients experience long-term difficulties and that deficits can be treated effectively [81] may lessen stereotype threat. Additionally, teaching people about stereotype threat processes can help mitigate their manifestation by providing an external attribution that reduces the apprehension and anxiety that can interfere with performance [82]. Thus, cancer patients might be taught about the “chemobrain” stereotype and how it can create expectations that undermine cognitive function. Armed with such information, patients might then better resist succumbing to these effects. Such research should also examine the bi-directional possibility than improved cognitive performance may in turn help to diminish patients’ negative expectancies about cognitive decline.
Conclusion
We have presented two interrelated mechanisms extensively examined in social psychological research that may contribute to the understanding of treatment-related cognitive decline, an important issue in cancer survivorship. We focused on a self-regulatory analysis as well as the specific challenges of expectancies and stereotypes to illustrate the generative potential of a social psychological perspective in cancer research. Rather than suggesting that these processes of self-regulation and stereotype threat are the primary catalysts of cognitive deficits among cancer patients, we propose they may help explain some processes for some patients.
Processes of self-regulation challenge and stereotype threat may affect certain subsets of patients to increase cognitive complaints if not actually degrade cognitive performance, as self-control demands and stereotype threat have been shown to influence cognitive functioning in other, non-patient samples. This raises the possibility that some survivors may experience cognitive changes that are driven primarily by psychological processes, potentially in the absence of biological components. For other survivors, these processes may play more of an additive role, contributing further to the decline precipitated by pharmacological and or biological factors. And for still others, these processes may operate in more of a mediational manner in representing the psychological pathways through which pharmacological and biological causes exert their phenomenological influence. For example, ego depletion might occur more rapidly and be greater for individuals with increased biologically-based vulnerabilities for post-treatment cognitive deficits [83–84]. Moreover, individuals with heightened biologically-based risk might, through their more frequent difficulties with daily cognitive activities, experience greater levels of stereotype threat that in turn exacerbates their cognitive difficulties. These and many other possibilities await further research. Such research may then help to explain some inconsistencies in the cancer and cognition literature and may be critical to more fully understanding this phenomenon, and the treatment approaches that are most appropriate for specific individuals.
To the extent that there are multiple independent and interacting causes of change in cognitive function for cancer survivors [2], the present paper illustrates another set of variables that have only begun to be examined. Different levels of analysis and perspective are vital to a full understanding of most any phenomenon. The present application of self-regulatory and expectancy processes may be one piece of a complex puzzle that positions researchers to better understand, and ultimately enhance, cancer survivorship.
Acknowledgments
This paper was inspired by a National Cancer Institute (NCI) workgroup meeting. We thank the NCI sponsored Cognitive, Affective, and Social Processes in Health Research (CASPHR) workgroup, and Wendy Nelson, for their feedback throughout the preparation of this paper.
Footnotes
There are no conflicts of interest with respect to this manuscript.
Contributor Information
Jamie Arndt, University of Missouri.
Enny Das, Radboud University Nijmegen.
Sanne B. Schagen, Netherlands Cancer Institute
Stephanie A. Reid-Arndt, University of Missouri
Linda D. Cameron, University of California-Merced
Tim A. Ahles, Memorial Sloan-Kettering Cancer Institute
References
- 1.Vardy J, Wefle JS, Ahles T, Tannock IF, Schagen SE. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment – Associated cognitive change: an update on the state of the science. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schagen SB, Vardy J. Cognitive dysfunction in people with cancer. Lancet Oncol. 2007;8(10):852–853. doi: 10.1016/S1470-2045(07)70287-5. [DOI] [PubMed] [Google Scholar]
- 4.Shilling V, Jenkins V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Euro J of Oncol Nurs. 2007;11(1):6–15. doi: 10.1016/j.ejon.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 6.Schmeichel BJ. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. J Exp Psych: Gen. 2007;136:241–255. doi: 10.1037/0096-3445.136.2.241. [DOI] [PubMed] [Google Scholar]
- 7.Schagen SB, Das E, van Dam FSAM. The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psycho-Oncol. 2009;18(6):674–678. doi: 10.1002/pon.1454. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich J, Monje M, Wefel J, et al. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90:1691–1696. doi: 10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–75. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 11.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a metaanalysis and review of the literature. J Int Neuropsychol Soc. 2003;9:967–82. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 12.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. 2005;104:2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 13.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips K. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain and Cog. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20:76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 15.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;20:274–81. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 16.de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Map. 2011 doi: 10.1002/hbm.21422. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong X, Schwartz PH, Linskey ME, et al. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76:1126–1134. doi: 10.1212/WNL.0b013e318212a89f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1888-1. Epub Dec 29. [DOI] [PubMed] [Google Scholar]
- 19.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–28. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2011;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neuroscience and Biobehavioral Rev. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Rodin G, Ahles TA. Accumulating evidence for the effect of chemotherapy on cognition. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.43.5776. [DOI] [PubMed] [Google Scholar]
- 23.Reid-Arndt SA. The potential for neuropsychology to inform functional outcomes research with breast cancer survivors. NeuroRehabilitation. 2006;21(1):51–64. [PubMed] [Google Scholar]
- 24.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surv. 2009;3:223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joly F, Rigal O, Noal S, Giffard B. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psycho-oncology. 2011;20(12):1251–1258. doi: 10.1002/pon.1903. [DOI] [PubMed] [Google Scholar]
- 26.Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego-depletion and the strength model of self-control: a meta-analysis. Psychol Bull. 2010;136:496–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- 27.Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? J Pers Soc Psychol. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- 28.Baumeister RF, Heatherton TF. Self-regulation failure: an overview. Psychol Inq. 1996;7:1–15. [Google Scholar]
- 29.Hall PA, Fong GT. Temporal self-regulation theory: a model for individual health behavior. Health Psychol Rev. 2007;1:6–52. [Google Scholar]
- 30.Inzlicht M, Schmeichel BJ. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspec on Psychol Science. 2012;7:450–463. doi: 10.1177/1745691612454134. [DOI] [PubMed] [Google Scholar]
- 31.Vohs KD, Glass BD, Maddox WT, Markman AB. Ego depletion is not just fatigue: Evidence from a total sleep deprivation experiment. Soc Psychol Pers Science. 2011;2:166–173. [Google Scholar]
- 32.Blair C, Ursache A. A bidirectional theory of executive functions and self-regulation. In: Baumeister R, Vohs K, editors. Handbook of Self-Regulation. 2. New York: Guilford; 2011. pp. 300–320. [Google Scholar]
- 33.Kaplan S, Berman MG. Directed attention as a common resource for executive functioning and self-regulation. Perspectives on Psychol Science. 2010;5:43–57. doi: 10.1177/1745691609356784. [DOI] [PubMed] [Google Scholar]
- 34.Schmeichel BJ, Vohs KD, Baumeister RF. Intellectual performance and ego depletion: role of the self in logical reasoning and other information processing. J Pers and Soc Psychol. 2003;85:33–46. doi: 10.1037/0022-3514.85.1.33. [DOI] [PubMed] [Google Scholar]
- 35.Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neuroscience. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair C, Razza RP. Relating effortful control, executive function, and false-belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 37.Gyurak A, Goodkind MS, Madan A, Kramer JH, Miller BL, Levenson RW. Do tests of executive functioning predict ability to down regulate emotions spontaneously and when instructed to suppress? Cog Affect & Beh Neuroscience. 2009;9:144–152. doi: 10.3758/CABN.9.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 39.Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Beh Med. 2009;37:173–83. doi: 10.1007/s12160-009-9096-5. [DOI] [PubMed] [Google Scholar]
- 40.Ahles TA, Root JC, Ryan EL. Cancer – and cancer treatment – associated cognitive change: an update on the state of the science. J of Clin Oncol. Oct;20(30):3675–86. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muraven MR, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol Bull. 2001;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- 42.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22:185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Reid-Arndt SA, Cox CR. Does rurality affect quality of life following treatment for breast cancer? J Rural Health. 2010;26:402–405. doi: 10.1111/j.1748-0361.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arndt J, Cook A, Goldenberg JL, Cox CR. Cancer and the threat of death: the cognitive dynamics of death thought suppression and its impact on behavioral health intentions. J Pers Soc Psychol. 2007;92:12–29. doi: 10.1037/0022-3514.92.1.12. [DOI] [PubMed] [Google Scholar]
- 45.Moser RP, Arndt J, Han P, Waters E, Amsellem M, Hesse B. Perceptions of cancer as a death sentence: prevalence and consequences. J Health Psychol. doi: 10.1177/1359105313494924. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox CR, Reid-Arndt SA, Arndt J. Considering the unspoken: the role of death cognition in quality of life among women with and without breast cancer. J Psychosocial Oncol. 2012;30:128–139. doi: 10.1080/07347332.2011.633980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Little M, Sayers EJ. The skull beneath the skin: cancer survival and awareness of death. Psycho-oncology. 2004;13:190–198. doi: 10.1002/pon.720. [DOI] [PubMed] [Google Scholar]
- 48.Gailliot MT, Schmeichel BJ, Baumeister RF. Self-regulatory processes defend against the threat of death: effects of self-control depletion and trait self-control on thoughts and fears of dying. J Pers Soc Psychol. 2006;91:49–62. doi: 10.1037/0022-3514.91.1.49. [DOI] [PubMed] [Google Scholar]
- 49.Schmeichel BJ, Demaree HA, Robinson JL, Pu J. Ego depletion by response exaggeration. J Exper Soc Psychol. 2006;42:95–102. [Google Scholar]
- 50.Cimprich B. Development of an intervention to restore attention in persons with cancer. Cancer Nurs. 1993;16:83–92. [PubMed] [Google Scholar]
- 51.Cimprich B, Ronis D. An environmental intervention to restore attention in women newly diagnosed with breast cancer. Cancer Nurs. 2003;26:284–292. doi: 10.1097/00002820-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Berman MG, Jonides J, Kaplan S. The cognitive benefits of interacting with nature. Psychol Sci. 2008;19:1207–1212. doi: 10.1111/j.1467-9280.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- 53.Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72:271–322. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 54.Bauer IM, Baumeister RF. Self-regulatory strength. In: Vohs K, Baumeister R, editors. Handbook of Self-Regulation: Research, Theory, and Applications. 2. New York: Guilford; 2011. pp. 64–82. [Google Scholar]
- 55.Webb TL, Sheeran P. Can implementation intentions help to overcome ego-depletion? J Exper Soc Psychol. 2003;39:279–286. [Google Scholar]
- 56.Rief W, Hoffmann G, Nestoriuc Y. The power of expectation: understanding the placebo and nocebo phenomenon. Soc Pers Psychol Compass. 2008;2:1345–1361. [Google Scholar]
- 57.Hahn RA. Expectancies of sickness: concept and evidence of the nocebo phenomenon. In: Kirsch I, editor. How Expectancies Shape Experience. Washington, DC; American Psychological Association: 1999. pp. 333–356. [Google Scholar]
- 58.Job V, Dweck CS, Walton GM. Ego depletion-Is it all in your head? Implicit theories about willpower affect self-regulation. Psychol Sci. 2010;21:1686–1693. doi: 10.1177/0956797610384745. [DOI] [PubMed] [Google Scholar]
- 59.Inzlicht M, McKay L, Aronson J. Stigma as ego depletion: how being the target of prejudice affects self-control. Psychol Sci. 2006;17:262–269. doi: 10.1111/j.1467-9280.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 60.Steele CM. A threat in the air: how stereotypes shape intellectual identity and performance. Am Psychol. 1997;52:613–629. doi: 10.1037//0003-066x.52.6.613. [DOI] [PubMed] [Google Scholar]
- 61.Steele C, Aronson J. Stereotype threat and the intellectual test performance of African Americans. J Pers Soc Psychol. 1995;69(5):797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- 61.Cadinu M, Maass A, Rosabianca A, Kiesner J. Why do women underperform under stereotype threat? Evidence for the role of negative thinking. Psychol Sci. 2005;16(7):572–578. doi: 10.1111/j.0956-7976.2005.01577.x. [DOI] [PubMed] [Google Scholar]
- 62.Good C, Aronson J, Harder JA. Problems in the pipeline: Stereotype threat and women’s achievement in high-level math courses. J Appl Dev Psychol. 2008;29(1):17–28. [Google Scholar]
- 63.Hess T, Auman C, Colcombe S, Rahhal T. The impact of stereotype threat on age differences in memory performance. J Gerontol Psychol Sci. 2003;58(1):3–11. doi: 10.1093/geronb/58.1.p3. [DOI] [PubMed] [Google Scholar]
- 64.Mazerolle M, Regner I, Morisset P, Rigalleau F, Huguet P. Stereotype threat strengthens automatic recall and undermines controlled processes in older adults. Psychol Sci. 2012;23:723–727. doi: 10.1177/0956797612437607. [DOI] [PubMed] [Google Scholar]
- 65.Cole JC, Michailidou K, Jerome L, Sumnall HR. The effects of stereotype threat on cognitive function among ecstasy users. J Psychopharmacol. 2006;20:518–525. doi: 10.1177/0269881105058572. [DOI] [PubMed] [Google Scholar]
- 66.Schmader T, Johns M, Forbes C. An integrated process model of stereotype threat effects on performance. Psychological Review. 2008;115:336–356. doi: 10.1037/0033-295X.115.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kane MJ, Engle RW. Working memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol: Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- 68.Schmader T, Johns M. Converging evidence that stereotype threat reduces working memory capacity. J Pers Soc Psychol. 2003;85(3):440–452. doi: 10.1037/0022-3514.85.3.440. [DOI] [PubMed] [Google Scholar]
- 69.Schagen SB, Das E, Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psycho-Oncol. 2012;21(10):1132–1135. doi: 10.1002/pon.2011. [DOI] [PubMed] [Google Scholar]
- 70.Pinel EC. Stigma consciousness: the psychological legacy of social stereotypes. J Pers Soc Psychol. 1999;76:114–128. doi: 10.1037//0022-3514.76.1.114. [DOI] [PubMed] [Google Scholar]
- 71.Rydell BJ, McConnel AR, Beilock SL. Multiple social identities and stereotype threat: imbalance, accessibility, and working memory. J Pers Soc Psychol. 2009;96:949–966. doi: 10.1037/a0014846. [DOI] [PubMed] [Google Scholar]
- 72.Brown RP, Pinel EC. Stigma on my mind: Individual differences in the experience of stereotype threat. J Exp Soc Psychol. 2003;39:626–633. [Google Scholar]
- 73.Das E, Jacobs W, Monster S, Schagen SB. Priming cognitive problems following chemotherapy: the role of stigma consciousness; ICCTF Cognition Cancer Conference; March 15–17; Paris, France. 2012. [Google Scholar]
- 74.Ståhl T, Van Laar C, Ellemers N. The role of prevention focus under stereotype threat: initial cognitive mobilization is followed by depletion. J Pers Soc Psychol. 2012;102:1239–1251. doi: 10.1037/a0027678. [DOI] [PubMed] [Google Scholar]
- 75.Mrazek MD, Chin JM, Schmader T, Hartson KA, Smallwood J, Schooler J. Mind wandering as a consequence of stereotype threat. J Exp Soc Psychol. 2011 [Google Scholar]
- 76.Jamieson JP, Harkins SG. Mere effort and stereotype threat performance effects. J Pers Soc Psychol. 2007;93(4):544–564. doi: 10.1037/0022-3514.93.4.544. [DOI] [PubMed] [Google Scholar]
- 77.Smith JL. Understanding the process of stereotype threat: A review of mediational variables and new performance goal directions. Educ Psychol Rev. 2004;16(3):177–206. [Google Scholar]
- 78.Inzlicht M, Kang SK. Stereotype threat spillover: how coping with threats to social identity affects aggression, eating, decision making, and attention. J of Per Soc Psychol. 2010;99(3):467–481. doi: 10.1037/a0018951. [DOI] [PubMed] [Google Scholar]
- 79.Schmader T, Hall W, Croft A. Stereotype threat in intergroup relations. In: Simpson J, Dovidio J, editors. APA Handbook of Personality and Social Psychology. Washington, D.C.; APA: in press. [Google Scholar]
- 80.Park C, Zlateva I, Blank TO. Self-Identity after cancer: “Survivor”, “Victim”, “Patient”, and “Person with cancer”. J Gen Internal Med. 2009;24(Supplement 2):S430–S435. doi: 10.1007/s11606-009-0993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psycho-oncology. 2012;21(2):176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johns M, Schmader S, Martens M. Knowing is half the battle: teaching stereotype threat as a means of improving women’s math performance. Psychol Sci. 2005;16(3):175–179. doi: 10.1111/j.0956-7976.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 83.Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 84.Small BJ, Rawson KS, Walsh E, Jim HSL, Hughes TF, Iser L, Andrykowski MA, Jacobsen PB. Cathechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]


