Abstract
In the fields of virology and innate immunity, BST-2/tetherin is well known for its ability to block the egress of enveloped viruses from infected cells. This appears to be accomplished by ‘tethering’ virions to the cell surface, thereby limiting virion release. In the past year, several groups have discovered that BST-2/tetherin can activate NF-κB, a transcriptional activator that leads to the rapid expression of both proinflammatory cytokines and proteins involved in cell survival. While this new BST-2 function has been interpreted as a possible viral-sensing mechanism, there may also be broader implications for HIV gene regulation. This article reviews the evidence for BST-2-dependent NF-κB activation, and explores the significance of these exciting new results.
Keywords: BST-2, CD317, egress, HIV, NF-κB, SIV, tetherin, viral sensor
What was BST-2 before it became a famous viral restriction factor?
BST-2 was first identified in 1994 as a marker of terminally differentiated B cells [1], and was later named for its expression in bone marrow stromal cells [2] (other names include CD317 and HM1.24). Due to its expression on multiple myeloma cells, anti-BST-2 antibodies were briefly considered as agents for cancer immunotherapy [3]. However, BST-2 was later identified in a search for mouse plasmacytoid dendritic cell surface markers, and was also found to have widespread tissue/cell type expression upon both type I and II interferon (IFN) stimulation [4]. BST-2 was first introduced to the field of virology when it was found to be targeted for destruction by the K5 ubiquitin ligase encoded by Kaposi’s sarcoma-associated herpesvirus [5]. Up until this time, BST-2 was merely a biomarker with no known function. Recently, BST-2 has also been implicated in the actin cytoskeleton organization of polarized epithelia [6], as well as overall membrane lipid raft organization [7].
BST-2 is a viral restriction factor
Over the same 10-year period, HIV researchers were hunting for an elusive host factor that blocked viral egress and was overcome by the HIV accessory protein known as Vpu. In 2008, BST-2 was identified as that restriction factor, ending the search and finally revealing an important function for BST-2 (now called tetherin) [8]. In the last 5 years, numerous mechanistic studies have attempted to both understand how BST-2 restricts enveloped virus egress, and to determine how these viruses overcome that restriction. For more details on this topic we refer you to [9–12] and the references within. Briefly, BST-2 is a transmembrane protein that primarily localizes to the cell surface. It is thought that in the absence of an antagonist, BST-2 is incorporated into budding viral particles, and via homo-oligomerization with other BST-2 molecules still residing at the cell surface, those viral particles become ‘tethered’ to the cell [8,13–15]. To counteract this restriction, HIV-1 encodes the accessory protein Vpu, which interacts with BST-2, leading to BST-2 downregulation and degradation (reviewed in [11]). Other lentiviruses, including HIV-2 and the majority of SIV strains, do not encode Vpu. Instead, they have evolved alternative mechanisms to counteract BST-2. For example, HIV-2 Env interacts with and sequesters BST-2 away from the cell surface [16,17]. By contrast, certain strains of SIV utilize their Nef proteins to enhance the AP-2-dependent endocytosis of BST-2 from the cell surface [18–21].
Could BST-2 be more than a viral tether?
While BST-2 restricts viral release into the extracellular milieu, there are conflicting data as to whether BST-2 limits cell-to-cell spread of the virus. Two studies have found that BST-2 restricts cell-to-cell virus spread [22,23], while another suggests that BST-2 enhances cell-to-cell spread [24]. Therefore, it remains unclear whether viral tethering significantly inhibits overall viral dissemination. Unfortunately, testing this in vivo has not been trivial, and so it is not yet clear when and if cell-to-cell viral spread is favored or required during the HIV life cycle, or if the mode of viral transfer is cell type dependent. However, the fact that many enveloped viruses express some form of BST-2 antagonist suggests that BST-2 is indeed detrimental to the virus. This has caused many to hypothesize that BST-2 may serve other antiviral purposes in addition to the physical restriction of viral egress. Such a situation is not without precedent, as other viral restriction factors that both directly inhibit viral replication and activate inflammatory gene expression have been described. For example, TRIM5α is a host E3 ligase that was initially characterized as a restriction factor that binds to viral capsids and promotes their disassembly. More recently, it has been found that in response to capsid binding, Trim5α also synthesizes free, Lys63-linked ubiquitin chains, leading to NF-κB activation (reviewed in [25]). IFN-induced protein with tetratricopeptide repeat (IFIT) proteins are another class of multifunctional innate immune regulators. Human IFIT proteins have been shown to impose blocks at numerous steps in the translation of viral transcripts, in addition to affecting inflammatory gene expression (reviewed in [26]).
What suggested that BST-2 might have NF-κB signaling capability?
Several years prior to its identification as a viral restriction factor, BST-2 was identified in a cDNA library screen for activators of NF-κB [27]. This information went largely unnoticed until recently, when three laboratories published studies confirming that BST-2 can mediate NF-κB activation and may therefore have additional antiviral properties [28–30]. The following discussion will focus mainly on the data presented in two of these papers regarding the potential mechanism of BST-2-dependent NF-κB activation and how BST-2 might therefore act as a viral sensor [28,29].
Discussion
What conditions illustrate that BST-2 mediates NF-κB activation?
All three studies show that when human BST-2 is transiently overexpressed in 293T cells, it can efficiently activate a NF-κB reporter construct in a dose-dependent manner [28–30]. By itself, this observation could be interpreted to mean that BST-2 overexpression leads to an unfolded protein response (UPR), which is known to trigger NF-κB activation. In support of this idea, previous studies have shown that when overexpressed, the majority of BST-2 accumulates within the cell as a 28-kDa ‘high mannose’ form that appears to be trapped within the endoplasmic reticulum (ER) [31]. Indeed, Tokarev et al. show that this BST-2-dependent NF-κB signaling is blocked by calcium sequestration in the ER, a hallmark of UPR-dependent NF-κB activation [29]. However, Galão et al. go on to demonstrate that no significant NF-κB activation is detected in 293 cells that stably express BST-2 from a lentiviral vector. Interestingly, in these cells, NF-κB was activated upon treatment with a BST-2 antibody, or via transfection with Vpu-deleted HIV proviral clones (HIV ΔVpu) [28]. In contrast, a Vpu-expressing HIV proviral clone did not activate NF-κB. This led the authors to suggest that the NF-κB signaling might be initiated through the crosslinking of surface BST-2 molecules resulting from either antibody binding or virion tethering. This phenomenon was not limited to HIV, as virus-like particles derived from both Ebola and murine leukemia virus also triggered NF-κB activation. Thus, while there is general agreement that BST-2 activates NF-κB, there is not a consensus regarding the nature of the signal.
Can this BST-2-mediated NF-κB activation be recapitulated in an actual infection of a relevant cell type?
To determine whether BST-2-dependent NF-κB activation occurs under more physiologically relevant conditions, Galão et al. infected primary human CD4+ T cells with HIV encoding either wild-type (WT) Vpu, ΔVpu or Vpu A14L (a Vpu mutant defective for BST-2 interaction) [28]. NF-κB activity was measured indirectly by analyzing mRNA and secreted protein levels for the downstream targets CXCL10, IL-6 and IFN-β. Although not as clear as their data from 293 cells, in the majority of cases the ΔVpu and A14L viruses induced cytokine production above the levels observed for mock or WT HIV infections. To confirm that this cytokine induction was dependent on BST-2, they used shRNA to knock down BST-2 expression in the T cells prior to HIV infection. This decreased the induction of CXCL10 and IFN-β mRNA to levels comparable to those observed for the ΔVpu and A14L viruses. Interestingly, they were not able to recapitulate the IFN-β activation in their 293-cell-based system. In agreement with this result, Tokarev et al. observed no BST-2-dependent induction of type I IFN or IFN-stimulated genes in their own 293T transient expression system [29]. These results suggest that BST-2 can induce a proinflammatory response that varies depending on both the target cell type and the expression of a functional Vpu.
What regions of BST-2 are necessary for NF-κB activation?
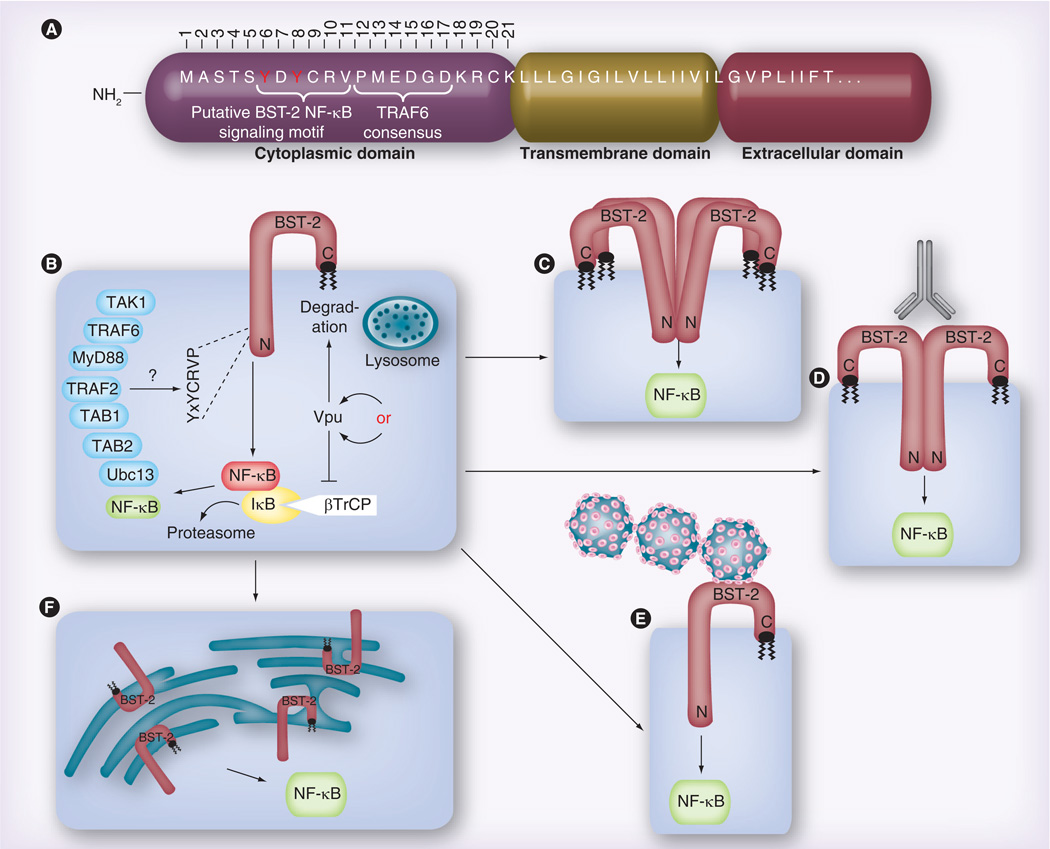
Using reporter assays, all three studies determined that the YxY motif located in the short BST-2 cytoplasmic tail is necessary for NF-κB activation [28–30]. This motif had previously been identified as an endocytosis signal [32,33]. Through more extensive mutagenesis, Galão et al. found that substitutions for the four amino acids immediately downstream of YxY also prevented NF-κB activation, thereby extending the newly identified NF-κB signaling motif to YxYCRVP (Figure 1A) [28]. The NF-κB activation-defective BST-2 mutants each retained their tethering ability, suggesting that tethering does not depend upon NF-κB activation. Through further mutational analysis, Galão et al. found that alanine substitutions for the cytoplasmic residues (DKRCK, amino acids 17–21) immediately proximal to the TM domain also prevented NF-κB activation [28]. However, these particular mutations gave rise to BST-2 proteins that were improperly processed, suggesting that their impact upon NF-κB signaling is merely an indirect consequence of their negative effects on BST-2 secretion. Interestingly, while this report was under review, another study was published that identified a single residue (R19H) within this same region as being critical for BST-2-dependent NF-κB activation [34]. Therefore, it would appear that the identity of all BST-2 cytosolic residues that are critical for NF-κB signaling awaits further experimentation. To further investigate the relationship between BST-2’s viral tethering and NF-κB activation functions, Galão et al. evaluated several known tethering-defective mutants: ΔGPI (a mutation that prevents the addition of the BST-2 GPI anchor), L123P (a mutation in the BST-2 coiled-coil domain) and C3A (alanine substitutions for extracellular cysteines, which eliminates BST-2 dimerization) [28]. Each of these mutants was defective for NF-κB activation in transfected 293 cells. The fact that these authors did not find a tethering mutant that was still able to activate NF-κB strengthened their conclusion that NF-κB signaling is dependent upon restriction of viral egress. In contrast, similar studies by Tokarev et al. showed that tethering and NF-κB signaling are not always linked [29]. For example, a mutation in the BST-2 extracellular domain (L70D), which prevents BST-2 tetramerization but only moderately decreases tethering function, was completely defective for NF-κB activity. This suggested a possible correlation between BST-2 multimerization and NF-κB signaling. Interestingly, this group also assessed a tethering-defective ΔGPI mutant, but in contrast to Galão et al., found it to activate NF-κB just as well as WT BST-2, indicating that NF-κB activation is not dependent upon tethering. This discrepancy may be explained in part by the fact that the two ΔGPI mutants are not the same. The mutant used by Galão et al. has a stop codon that terminates translation just upstream of the GPI anchor, and results in a BST-2 protein that is still localized to the plasma membrane [28]. On the other hand, Tokarev et al. used a mutant with an in-frame deletion of the GPI anchor signal peptide cleavage site, which leads to a BST-2 protein that appears to be retained in the ER [29]. This led Tokarev et al. to question whether the subcellular localization of BST-2 could impact NF-κB signaling. Because their ER-localized ΔGPI mutant retained normal levels of NF-κB activity, Tokarev et al. surmised that ER stress (via calcium release) might be responsible for NF-κB activation [29]. To confirm this hypothesis, they performed experiments demonstrating that the calcium chelator BAPTA-AM could block WT BST-2-induced NF-κB activation. Further analysis will therefore be necessary to confirm that this stress response is not just an artifact of BST-2 overexpression due to transfection conditions. In summary, while it seems clear that the BST-2 YxY motif plays a role in NF-κB activation, the relationship between BST-2-dependent restriction of viral egress and BST-2-dependent NF-κB activation remains unclear.
Figure 1. Data and models proposed in the Galão et al. and Tokarev et al. papers regarding the BST-2-dependent activation of NF-κB.
(A) Overview of the putative domain structure for the BST-2 NH2-terminus, including the approximately 21-residue cytoplasmic domain (numbered), the transmembrane domain and the first few residues of the extracellular domain. (B) Both groups demonstrate that, when overexpressed via transient transfection, BST-2 alone can activate NF-κB, and that Vpu expression reduces this activation. Data shown by Tokarev et al. suggest that Vpu accomplishes this via sequestration of the ubiquitin ligase component βTrCP, which promotes NF-κB activity, while Galão et al. assume that Vpu degrades BST-2, thereby eliminating the source of signaling. Both groups also show a role for the BST-2 YxY motif, which was variously observed to be required for binding to TRAF6 (Galão), or TAB1 and TAK1 (Tokarev). The two groups identified partially overlapping sets of other NF-κB signaling intermediates, which were found to be either directly bound to BST-2, or necessary for BST-2 signaling to NF-κB. (C) Tokarev et al. find that BST-2 must be capable of multimerization in order to activate NF-κB, while (D) Galão et al. show that antibody crosslinking of surface-associated BST-2 can further enhance BST-2 activation of NF-κB. (E) Similarly, Galão et al. also find that surface-associated (‘tethered’) virus enhances BST-2-dependent NF-κB activation. (F) Tokarev et al. provide evidence that BST-2-dependent endoplasmic reticulum stress leads to NF-κB activation.
Is endocytosis of ‘tethered’ virus responsible for NF-κB signaling?
Because residues within the newly established NF-κB signaling YxYCRVP motif had previously been implicated in BST-2 endocytosis, Galão et al. investigated whether virion endocytosis could be the trigger for NF-κB activation [28]. They showed that the BST-2 Y(6,8) A mutant partially blocked Gag:GFP particle uptake, suggesting a positive correlation between endocytosis of tethered virions and NF-κB activation. However, when they completely prevented virion endocytosis by either knocking down the clathrin adapter protein AP2M1 or expressing a dominant-negative Rab-5a endocytic GTPase, BST-2-mediated NF-κB activation increased. They inferred from these seemingly conflicting data that preventing endocytosis results in the accumulation of virions at the cell surface, which in turn induces more BST-2 crosslinking with a concomitant increase in NF-κB activation. Therefore, they concluded that the lack of NF-κB activation by the Y(6,8) A mutant is due to a signaling defect, not a block in virion endocytosis.
What are the components of the BST-2 dependent NF-κB signaling pathway?
In their efforts to define the particular pathway utilized by BST-2 to activate NF-κB, Galão et al. [28] and Tokarev et al. [29] were both able to implicate TGF-β-activated kinase 1 (TAK1), but they did not identify the same intermediate pathway components. Galão et al. found that knockdown of TAK1, TNF receptor-associated factor (TRAF)6, TRAF2 or ubiquitin conjugating enzyme 13 (Ubc13) via siRNA all blocked BST-2-dependent NF-κB activation. In addition, they showed via co-immunoprecipitation that TRAF6 interacts with WT BST-2, but not Y(6,8)A BST-2, suggesting that the YxY signaling motif may be a TRAF6 binding site. However, their 10–12A mutant, which also prevented NF-κB activation, still interacted with TRAF6, leading them to conclude that TRAF6 is necessary but not sufficient for BST-2-dependent NF-κB signaling. Of note, an apparent consensus TRAF6-binding motif (PxExx[Ω/D/E], amino acids 12–17) overlaps the BST-2 YxYCRVP signaling motif. However, mutations in this region had no impact on NF-κB activation (Figure 1A) [28]. By contrast, Tokarev et al. probed BST-2 co-immunoprecipitations for known NF-κB pathway members and observed no interaction with either TRAF3 or TRAF6 [29]. However, they did find that MyD88, TRAF2, TAB1, TAB2 and TAK1 all coimmunoprecipitate with BST-2. In addition, BST-2 Y(6,8)A exhibited reduced binding to both TAK1 and TAB1 (this was not tested by Galão et al.). Given that large complexes of ubiquitinated and phosphorylated proteins are involved in NF-κB signaling, it is unsurprising that independent laboratories would identify different components. A comprehensive, focused analysis of the pathway using tangential approaches will be necessary to resolve these apparent discrepancies.
How does Vpu inhibit BST-2-mediated NF-κB signaling?
As discussed above, both groups found that Vpu expression decreases BST-2-dependent NF-κB activation. Galão et al. do not address this question directly [28], presumably taking the stance that this is easily explained by Vpu’s well-characterized ability to downregulate/degrade BST-2. In support of such a mechanism, they found that infection of cells with HIV expressing a WT Vpu prevents NF-κB activation, while HIV expressing the A14L Vpu mutant, which cannot interact with BST-2, does not prevent NF-κB activation. This suggests that, similar to what is observed for Vpu-dependent downregulation of BST-2, Vpu’s ability to interact with BST-2 is necessary for inhibition of BST-2-mediated NF-κB signaling. Surprisingly, Galão et al. did not address a function previously attributed to Vpu, which is the inhibition of NF-κB activation via sequestration of βTrCP [35–37], a component of the Skp/Cullin/F-box E3 ubiquitin ligase complex that is responsible for ubiquitination (and subsequent degradation) of the NF-κB inhibitor IκB [38]. On the other hand, Tokarev et al. compare several Vpu mutants to investigate whether sequestration of βTrCP or degradation of BST-2 is the mechanism whereby Vpu blocks NF-κB activation. Using a co-transfection system to express both Vphu (a highly expressed, codon-optimized Vpu) and BST-2 in 293 cells, they observed that WT Vphu inhibits NF-κB activation, but so does a Vphu ‘3A/F’ transmembrane (TM) mutant that does not bind BST-2 [29]. Therefore, in contrast to Galão et al.’s results, these data indicate that Vpu’s ability to interact with BST-2 is not important for inhibiting NF-κB activation. In addition, Tokarev et al. found that a Vphu 2/6 mutant, which can interact with BST-2, but is unable to interact with βTrCP, did not prevent NF-κB activation [29]. They conclude that high levels of Vphu and Vphu 3A/F titrate βTrCP, thereby stabilizing IκB, which maintains NF-κB in an inactive state. Such a mechanism obviates the need for BST-2 downregulation/degradation.
One possible explanation for this discrepancy in the conclusions reached by the two laboratories is the manner by which Vpu was delivered to the cells. Galão et al. expressed Vpu in the context of a proviral clone [28], while Tokarev et al. expressed exogenous Vphu [29]. The overexpression of Vphu in the latter system could be responsible for the βTrCP sequestration phenotype, which might not occur under more physiological conditions. In support of this, Tokarev et al. repeated the NF-κB reporter assays by transfecting proviral clones into Hela cells that express endogenous BST-2 [29]. In this case, the HIV Vpu 3A/F mutant behaved more similarly to HIV ΔVpu than WT HIV, suggesting that Vpu’s ability to interact with both BST-2 and βTrCP might still be important to prevent NF-κB activation.
Is BST-2-mediated NF-κB signaling conserved throughout evolution?
Because BST-2 proteins from rodents and other nonhuman primates are also known to prevent enveloped virus release, Galão et al. investigated whether these proteins could also activate NF-κB [28]. Surprisingly, none of the BST-2 proteins encoded by other species were able to significantly activate NF-κB. Expression of transfected human BST-2 increased NF-κB activation 30-fold, compared with eightfold for chimpanzee BST-2, and less than fivefold for African green monkey, Rhesus macaque or mouse BST-2. The authors next examined the role of the DDIWK motif that is unique to the cytoplasmic tail of BST-2s encoded by nonhuman primates, and which has been shown to be responsible for BST-2’s sensitivity to SIV-encoded Nef [18,19]. Inserting DDIWK back into human BST-2 completely blocked its ability to activate NF-κB, while removing the motif from chimpanzee BST-2 increased NF-κB activation to levels observed for human BST-2. This prompted the authors to examine whether the DDIWK motif could be inhibiting NF-κB activation by preventing the binding of nonhuman BST-2 proteins to TRAF6. However, the nonhuman BST-2s were subsequently found to efficiently interact with TRAF6, so the reason for the inability of these molecules to activate NF-κB remains unknown. The authors concluded that BST-2-dependent NF-κB activation has been recently acquired, and that this is coincident with the loss of the Nef-specific DDIWK motif.
Conclusion
In the model proposed by Galão et al., budding HIV ΔVpu virions are PAMPs that are tethered to BST-2, which, to extend the analogy, acts as a pattern recognition receptor [28]. This results in the BST-2-dependent activation of the NF-κB signaling cascade, which does not occur in the presence of Vpu, presumably because BST-2 is removed from the cell surface and degraded. Mechanistically, they find that a complex of signaling mediators including TRAF6 interacts with BST-2 via a newly identified YxYCRVP motif, thereby triggering the Ubc13-dependent stimulation of TAK1, which ultimately leads to NF-κB activation. They also find that in rodents or other nonhuman primates, BST-2 does not induce NF-κB signaling, and so perhaps this BST-2 function may have arisen later in evolution.
The data presented by Tokarev et al. supports two potential models for BST-2-mediated NF-κB activation [29]. In one model, BST-2 multimerization triggers intrinsic NF-κB signaling, possibly through an ER stress-induced mechanism involving a signaling complex containing TAK1, TAB1, TAB2, MyD88 and possibly TRAF2. In their second model, BST-2 acts as a viral sensor that triggers NF-κB activation dependent upon an unknown step in the viral life cycle such as virus assembly or viral gene expression. While Tokarev et al. were unable to correlate BST-2 tethering function with NF-κB signaling, they did observe an increase in NF-κB activation in the presence of HIV ΔVpu, leading them to conclude that BST-2 might be acting as a viral sensor. In both of the models proposed by Tokarev et al., Vpu disrupts NF-κB signaling by sequestering βTrCP, the E3 ubiquitin ligase that regulates IκB inhibitor of NF-κB.
Despite the differences in mechanistic details between the two studies, the main conclusion that BST-2 can elicit NF-κB activation through a TAK1-dependent pathway that is stimulated further by Vpu-deleted HIV is consistent and compelling. In this way, BST-2 would join TRIM5α and IFIT as host proteins that directly interfere with viral replication, and serve as innate immune signaling molecules. The prospect that BST-2 might act as a viral sensor to stimulate a proinflammatory response that can be counteracted by Vpu is an exciting development that warrants further investigation.
Future perspective
The major issues arising from these studies that need to be resolved are to determine whether viral tethering, BST-2 multimerization and/or ER stress are the actual triggers for NF-κB activation, and exactly which players in the TAK1-dependent pathway are important. Additional questions to be addressed in the future are:
-
▪
Has HIV evolved an antagonist for BST-2 to prevent NF-κB activation, viral tethering or both? That is, is it more advantageous for the virus to prevent a proinflammatory response or to enhance viral release?
-
▪
What is the significance of the observations that nonhuman primate BST-2 does not activate NF-κB, but SIV encodes a BST-2 antagonist? Does this suggest that viral tethering is a more important outcome than NF-κB activation, or that the NF-κB function evolved in humans to counteract a change in the evolution from SIV to HIV?
-
▪
How does the presence of the DDIKW and other cytoplasmic sequences in nonhuman primate BST-2 prevent NF-κB signaling?
-
▪
Does the IFN induction of BST-2 play any role in the NF-κB signaling?
-
▪
Could there be an as yet unidentified BST-2 ligand that triggers the NF-κB signaling?
-
▪
Are tethering and viral sensing really one and the same thing, such that the true function of BST-2 is not to prevent viral egress, but instead is to capture surface-associated viral particles and reintroduce them to the cytosol for the activation of innate immune signaling pathway(s)?
-
▪
Is the BST-2-dependent activation of NF-κB functional in all cell types targeted by HIV, such as macrophages and dendritic cells?
These studies also raise interesting questions about HIV’s overall ability to control NF-κB. For example, a recent study by Postler and Desrosiers shows that HIV Env can induce TAK1-dependent NF-κB activation, which in turn appears to be necessary for viral replication in unstimulated human peripheral blood mononuclear cells [39]. Therefore, an important goal will be to understand how the virus can balance its need for NF-κB activation (to maintain efficient viral replication) on the one hand with its need to prevent an excessive host proinflammatory response on the other. We anticipate a great deal more investigation, including studies of individual proteins and pathways as well as more global/systems approaches, into how the various immune activation and evasion strategies proposed for HIV integrate with one another. A continued effort to understand the intricacies of HIV’s interaction with the host innate immune system will be critical for future vaccine and drug development strategies.
Executive summary.
What conditions illustrate that BST-2 mediates NF-κB activation?
-
▪
When overexpressed in 293T cells, BST-2/tetherin can induce NF-κB activation in reporter assays.
-
▪
Compared with cells infected with HIV encoding WT Vpu, BST-2/tetherin-mediated NF-κB activation is increased in cells infected with Vpu-deleted HIV, suggesting that BST-2/tetherin might act as a viral sensor.
Can this BST-2-mediated NF-κB activation be recapitulated in an actual infection of a relevant cell type?
-
▪
HIV ΔVpu induces proinflammatory cytokines in primary peripheral blood mononuclear cells in a BST-2/tetherin-dependent manner.
What regions of BST-2 are necessary for NF-κB activation?
-
▪
The YxYCRVP sequence in the cytoplasmic domain of BST-2 has been identified as the NF-κB signaling motif.
-
▪
Experimental evidence supports several possible mechanisms for BST-2-induced NF-κB activation, including BST-2 multimerization, antibody-mediated BST-2 crosslinking, virion tethering and endoplasmic reticulum stress.
What are the components of the BST-2-dependent NF-κB signaling pathway?
-
▪
While the current studies agree that BST-2/tetherin accomplishes NF-κB signaling via a TAK1-dependent pathway, the identity of other signaling intermediaries remains unclear.
How does Vpu inhibit BST-2-mediated NF-κB signaling?
-
▪
Vpu may inhibit BST-2-mediated NF-κB activation by either inducing the degradation of BST-2 or sequestering βTrCP, the E3 ubiquitin ligase responsible for degrading IκB.
Is BST-2-mediated NF-κB signaling conserved throughout evolution?
-
▪
BST-2/tetherin from rodents and nonhuman primates are not able to activate NF-κB.
Acknowledgments
JK Gustin and JL Douglas are both supported by the NIH.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Goto T, Kennel SJ, Abe M, et al. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood. 1994;84(6):1922–1930. [PubMed] [Google Scholar]
- 2.Ishikawa J, Kaisho T, Tomizawa H, et al. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26(3):527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki S, Kosaka M, Wakatsuki S, Abe M, Koishihara Y, Matsumoto T. Immunotherapy of multiple myeloma with a monoclonal antibody directed against a plasma cell-specific antigen, HM1.24. Blood. 1997;90(8):3179–3186. [PubMed] [Google Scholar]
- 4.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177(5):3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 5.Bartee E, McCormack A, Früh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2(10):e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rollason R, Korolchuk V, Hamilton C, Jepson M, Banting G. A CD317/tetherin–RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Biol. 2009;184(5):721–736. doi: 10.1083/jcb.200804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billcliff PG, Rollason R, Prior I, Owen DM, Gaus K, Banting G. CD317/tetherin is an organiser of membrane microdomains. J. Cell Sci. 2013;126(Pt 7):1553–1564. doi: 10.1242/jcs.112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425. doi: 10.1038/nature06553. ▪ Primary paper that identifies BST-2 as a tethering molecule that restricts viral egress, but that can be overcome by HIV-1 Vpu.
- 9.Andrew A, Strebel K. The interferon-inducible host factor bone marrow stromal antigen 2/tetherin restricts virion release, but is it actually a viral restriction factor? J. Interferon Cytokine Res. 2011;31(1):137–144. doi: 10.1089/jir.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Tortorec A, Willey S, Neil SJD. Antiviral inhibition of enveloped virus release by tetherin/BST-2: action and counteraction. Viruses. 2011;3(5):520–540. doi: 10.3390/v3050520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas JL, Gustin JK, Viswanathan K, Mansouri M, Moses AV, Früh K. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010;6(5):e1000913. doi: 10.1371/journal.ppat.1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokarev A, Skasko M, Fitzpatrick K, Guatelli J. Antiviral activity of the interferon-induced cellular protein BST-2/tetherin. AIDS Res. Hum. Retroviruses. 2009;25(12):1197–1210. doi: 10.1089/aid.2009.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Damme N, Goff D, Katsura C, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Caballero D, Zang T, Ebrahimi A, et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139(3):499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammonds J, Wang J-J, Yi H, Spearman P. Immunoelectron microscopic evidence for tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 2010;6(2):e1000749. doi: 10.1371/journal.ppat.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Tortorec A, Neil SJD. Antagonism and intracellular sequestration of human tetherin by the HIV-2 envelope glycoprotein. J. Virol. 2009:1–49. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser H, Lopez LA, Yang SJ, et al. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology. 2010;7(1):51. doi: 10.1186/1742-4690-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Wilson SJ, Landford WC, et al. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6(1):54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia B, Serra-Moreno R, Neidermyer W, et al. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F, Landford WN, Ng M, Mcnatt MW, Bieniasz PD, Hatziioannou T. SIV Nef proteins recruit the AP-2 Complex to antagonize tetherin and facilitate virion release. PLoS Pathog. 2011;7(5):e1002039. doi: 10.1371/journal.ppat.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra-Moreno R, Zimmermann K, Stern LJ, Evans DT. Tetherin/BST-2 antagonism by Nef depends on a direct physical interaction between Nef and tetherin, and on clathrin-mediated endocytosis. PLoS Pathog. 2013;9(7):e1003487. doi: 10.1371/journal.ppat.1003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casartelli N, Sourisseau M, Feldmann J, et al. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6(6):e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhl BD, Sloan RD, Donahue DA, Bar-Magen T, Liang C, Wainberg MA. Tetherin restricts direct cell-to-cell infection of HIV-1. Retrovirology. 2010;7:115. doi: 10.1186/1742-4690-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly C, Booth NJ, Neil SJD. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J. Virol. 2010;84(23):12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol. Med. 2011;3(9):513–527. doi: 10.1002/emmm.201100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013;13(1):46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuda A, Suzuki Y, Honda G, et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22(21):3307–3318. doi: 10.1038/sj.onc.1206406. ▪ Describes an expression-based screen that identifies BST-2 as an NF-κB activator.
- 28. Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJD. Innate sensing of HIV-1 assembly by tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe. 2012;12(5):633–644. doi: 10.1016/j.chom.2012.10.007. ▪▪ Provides the first demonstration that human BST-2/tetherin activates NF-κB in response to viral tethering, and maps this function to a novel NF-κB signaling motif, YxYCRVP.
- 29. Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-κB activity by the HIV restriction factor BST2. J. Virol. 2013;87(4):2046–2057. doi: 10.1128/JVI.02272-12. ▪▪ Confirms that BST-2/tetherin activates NF-κB, and that this depends upon the BST-2 YxY motif. Also provides evidence that viral tethering and NF-κB are genetically separable.
- 30. Cocka LJ, Bates P. Identification of alternatively translated tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8(9):e1002931. doi: 10.1371/journal.ppat.1002931. ▪▪ Confirms that BST-2/tetherin activates NF-κB and identifies the BST-2 YxY motif as a potential signaling motif.
- 31.Andrew AJ, Miyagi E, Kao S, Strebel K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology. 2009;6(1):80. doi: 10.1186/1742-4690-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 2007;120(Pt 21):3850–3858. doi: 10.1242/jcs.003343. [DOI] [PubMed] [Google Scholar]
- 33.Masuyama N, Kuronita T, Tanaka R, et al. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J. Biol. Chem. 2009;284(23):15927–15941. doi: 10.1074/jbc.M109.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauter D, Hotter D, Engelhart S, et al. A rare missense variant abrogates the signaling activity of tetherin/BST-2 without affecting its effect on virus release. Retrovirology. 2013;10(1):85. doi: 10.1186/1742-4690-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bour S, Perrin C, Akari H, Strebel K. The human immunodeficiency virus type 1 Vpu protein inhibits NF-kappa B activation by interfering with beta TrCP-mediated degradation of Ikappa B. J. Biol. Chem. 2001;276(19):15920–15928. doi: 10.1074/jbc.M010533200. [DOI] [PubMed] [Google Scholar]
- 36.Akari H, Bour S, Kao S, Adachi A, Strebel K. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappa B-dependent expression of antiapoptotic factors. J. Exp. Med. 2001;194(9):1299–1311. doi: 10.1084/jem.194.9.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besnard-Guerin C, Belaïdouni N, Lassot I, et al. HIV-1 Vpu sequesters beta-transducin repeat-containing protein (betaTrCP) in the cytoplasm and provokes the accumulation of beta-catenin and other SCFbetaTrCP substrates. J. Biol. Chem. 2004;279(1):788–795. doi: 10.1074/jbc.M308068200. [DOI] [PubMed] [Google Scholar]
- 38.Kroll M, Margottin F, Kohl A, et al. Inducible degradation of IkappaBalpha by the proteasome requires interaction with the F-box protein h-betaTrCP. J. Biol. Chem. 1999;274(12):7941–7945. doi: 10.1074/jbc.274.12.7941. [DOI] [PubMed] [Google Scholar]
- 39.Postler TS, Desrosiers RC. The cytoplasmic domain of the HIV-1 glycoprotein gp41 induces NF-βB activation through TGF-β-activated kinase 1. Cell Host Microbe. 2012;11(2):181–193. doi: 10.1016/j.chom.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]