Abstract
Signal transduction and activator of transcription (STAT) proteins are extracellular ligand-responsive transcription factors that mediate cell proliferation, apoptosis, differentiation, development and the immune response. Aberrant signals of STAT induce uncontrolled cell proliferation and apoptosis resistance and are strongly involved in cancer. STAT has been identified as a promising target for antitumor drugs, but to date most trials have not been successful. Here, we demonstrated that a novel STAT inhibitor, OPB-31121, strongly inhibited STAT3 and STAT5 phosphorylation without upstream kinase inhibition, and induced significant growth inhibition in various hematopoietic malignant cells. Investigation of various cell lines suggested that OPB-31121 is particularly effective against multiple myeloma, Burkitt lymphoma and leukemia harboring BCR–ABL, FLT3/ITD and JAK2 V617F, oncokinases with their oncogenicities dependent on STAT3/5. Using an immunodeficient mouse transplantation system, we showed the significant antitumor effect of OPB-31121 against primary human leukemia cells harboring these aberrant kinases and its safety for normal human cord blood cells. Finally, we demonstrated a model to overcome drug resistance to upstream kinase inhibitors with a STAT inhibitor. These results suggested that OPB-31121 is a promising antitumor drug. Phase I trials have been performed in Korea and Hong Kong, and a phase I/II trial is underway in Japan.
Keywords: novel STAT inhibitor, OPB-31121
Introduction
Signal transduction and activator of transcription (STAT) proteins are extracellular ligand-responsive transcription factors that mediate a wide range of biological processes such as cell proliferation, apoptosis, differentiation, development and the immune response.1, 2 Stimulation with cytokines or growth factors results in the tyrosine phosphorylation of STAT proteins via the activation of upstream tyrosine kinases such as JAK family kinases (JFKs) and Src family kinases (SFKs).3 Activated STAT proteins translocate to the nucleus and regulate gene expression through direct binding to the promoters of responsive genes.4, 5
Out of 7 STAT family members, STAT3 and STAT5 are widely recognized as being master regulators of the cellular functions that lead to the cancer phenotype. Constitutive STAT3 activation is required for oncogenic transformation by oncokinases such as v-Src,6, 7 v-Eyk8 and v-Ros.9 In addition, constitutive STAT3 activation is associated with transformation by tumor viruses, including HTLV-110 and EBV.11 Constitutive activation of STAT5 is essential for oncogenesis by the v-Abl tyrosine kinase,12, 13 BCR–ABL fusion protein,14, 15, 16 FLT3 with internal tandem duplication (FLT3/ITD)17, 18 and JAK2 V617F mutation.19 Moreover, a constitutive activation mutant of STAT3 or STAT5 alone is enough to induce oncogenic transformation.20, 21 These results indicate that STAT3 and STAT5 have intrinsic oncogenic potential and are strongly associated with cancer development.
Considering the strong association of STAT signaling with cancer development and the observed constitutive activation of STAT3/5 in various cancers, STAT3/5 have been identified as promising targets for antitumor drugs; however, to date most trials to block STAT signaling have not been fully successful.22 Many trials aimed to inhibit upstream kinases such as JAK2; however, specific JAK2 inhibition was overcome by alternative activation of other JFKs.23 Several JFK inhibitors are under development, but no significant clinical effect has been achieved. Other approaches that directly inhibit STAT function, such as STAT dimerization inhibitors and STAT phosphorylation inhibitors, are under development, but none has undergone a clinical trial yet.
Here, we demonstrated that a novel STAT3 inhibitor, OPB-31121, strongly inhibited not only STAT3 but also STAT5 phosphorylation. OPB-31121 did not inhibit activities of kinases including JFKs and SFKs and its exact mechanism of action is under investigation; however, it induced significant growth inhibition in a wide range of hematopoietic malignant cells. Investigation among various cell lines indicated that this compound was particularly effective against multiple myeloma and Burkitt lymphoma, and leukemia harboring BCR–ABL, FLT3/ITD and JAK2 V617F, oncogenic kinases with their oncogenicities dependent on STAT3/5. Using an immunodeficient mouse transplantation system, we also showed the significant antitumor effect of this compound against primary human leukemia cells harboring these aberrant kinases and its safety for normal human cord blood cells. Finally, we demonstrated a model to overcome drug resistance to upstream kinase inhibitors with a STAT inhibitor. These results suggested that OPB-31121 is a promising antitumor drug. Phase I trials have been performed in Korea (NCT00955812) and Hong Kong (NCT00511082), and a phase I/II trial is underway in Japan (NCT1406574).
Materials and methods
Cells and reagents
TCC-Y/sr was described previously.24 OCI-Ly1, OCI-Ly3, OCI-Ly7 and OCI-Ly10 were kind gifts from Dr K Takeyama (Dana-Farber Cancer Institute, MA, USA) and were cultured in Iscove's modified Dulbecco's medium supplemented with 20% fetal bovine serum. Other cells were purchased from the American Type of Culture Collection (ATCC, Rockville, MD, USA) and cultured according to the recommendation of ATCC. Sunitinib was purchased from Wako Chemicals (Osaka, Japan). OPB-31121 was described previously25 and provided by Otsuka Pharmaceuticals Co. Ltd. (Tokushima, Japan).
Antibodies
The following antibodies were purchased from Cell Signaling Inc. (Beverly, MA, USA): anti-phospho-STAT5 (Y694) antibody, anti-phospho-JAK2 antibody, anti-phospho-Src antibody, anti-phospho-Akt antibody, anti-phospho-MAPK p44/p42 antibody, anti-phospho-NFκB antibody, anti-STAT3 antibody, anti-STAT5 antibody, anti-JAK2 antibody, anti-Src antibody and anti-IKBα antibody. Anti-phospho-STAT3 (Y705) antibody was obtained from EPIT MICS (Burlingame, CA, USA). Anti-human CD45 antibody and anti-mouse CD45 antibody were from Becton Dickinson (San Jose, CA, USA).
Cell proliferation assay
Cell proliferation was analyzed by the MTT assay using Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan) or TetraColor One (Seikagaku Co., Tokyo, Japan) according to the manufacturer's instructions.
Immunohistochemistry, immunoblotting, immunofluorescence and flow cytometry
Subcutaneous xenotransplantation of cell lines into SCID mice
This was performed as described previously28 except that 8-week-old male SCID mice purchased from Clea Japan (Tokyo, Japan) were used. Tumor size was monitored twice a week. Tumor volume was calculated using the following formula: Tumor volume (mm3)=(d2 × D)/2, where D (mm) and d (mm) are the longest and shortest diameters of the tumor, respectively.
Primary leukemia cell xenotransplantation into NOD/SCID/IL2-Rγc−/− (NOG) mice
Primary leukemia cells from patients were collected after obtaining written informed consent, preserved and transplanted into NOG mice as described previously.27 Patients' characteristics are shown in Supplementary Table 2.
Human cord blood cells
Human cord blood cells were obtained from RIKEN BRC (Tsukuba, Japan). The use of human cord blood cells in this study was permitted by the ethics committee of Nagoya University Graduate School of Medicine.
Results
OPB-31121 selectively inhibits STAT phosphorylation without upstream kinase inhibition
OPB-31121 was identified by Otsuka pharmaceuticals Co. Ltd. as a chemical that induced strong growth inhibition of various kinds of tumor cell lines. It was reported that OPB-31121 inhibited growth of gastric cancer cell lines and phosphorylation of STAT1, STAT3 and STAT5 in those cell lines; however, the exact mechanism of action is yet to be clarified.25 In the phase I study performed in Korea, 21 patients with advanced solid tumor were enrolled. The most common toxicities were nausea, vomiting, diarrhea, fatigue and anorexia. Those were predominantly grade 1 or grade 2.29
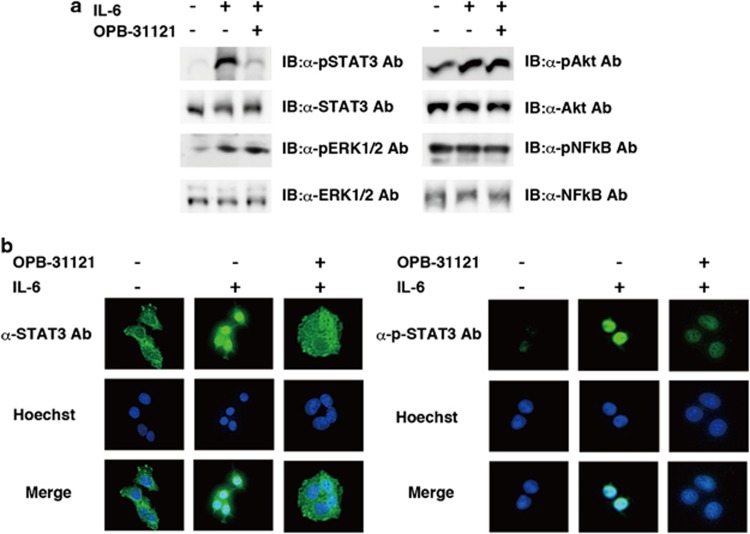
We first analyzed the signal transduction pathway inhibited by OPB-31121. Four major growth signal components, STAT3, ERK1/2, Akt and NFk-B, were analyzed. Among them, tyrosine phosphorylation of STAT3 was selectively inhibited by this compound (Figure 1a). Inhibition of STAT3 nuclear translocation by this compound was investigated by immunofluorescent staining. Inhibition of STAT3 nuclear translocation was observed by immunofluorescent staining with anti-STAT3 antibody (Figure 1b, upper-right corner panel of left panels). On the other hand, residually phosphorylated STAT3 completely translocated to the nucleus under OPB-31121 treatment (Figure 1b, upper-right corner panel of right panels), indicating that this compound did not inhibit nuclear translocation of phosphorylated STAT3. Observed inhibition of nuclear translocation seemed to be the consequence of the inhibition of STAT3 phosphorylation by this compound.
Figure 1.
OPB-31121 selectively inhibited STAT. (a) Selective inhibition of STAT. Hep G2 cells were treated with or without 100 nM OPB-31121 for 4 h and stimulated with or without 100 ng/ml IL-6 for 10 min as indicated. Then, cells were lysed and subjected to immunoblotting (IB) with the indicated antibodies. (b) OPB-31121 did not inhibit nuclear translocation of phosphorylated STAT3. Hep G2 cells were treated with OPB-31121 and IL-6 as in (a). Cells were fixed and subjected to immunofluorescent staining with the indicated antibodies. Nuclear translocation of residually phosphorylated STAT3 was not inhibited on immunofluorescence by anti-phospho-STAT3 antibody (upper-right corner panel).
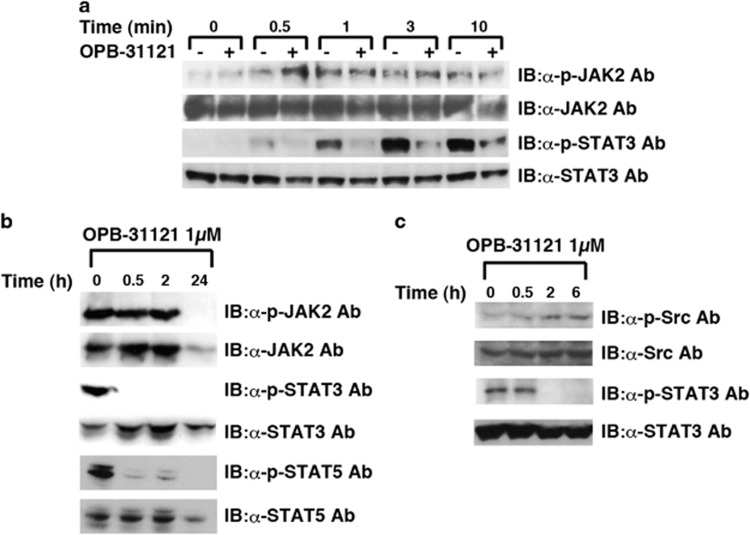
Next, we examined whether OPB-31121 inhibited the upstream kinases of STAT. In Hep G2 cells, JAK2 phosphorylation was induced by IL-6 stimulation and was not inhibited by this compound, whereas STAT3 phosphorylation was strongly inhibited (Figure 2a). In HEL cells with active mutation of JAK2, phosphorylation of STAT3 and STAT5 was inhibited at early time points when JAK2 phosphorylation was not inhibited, although phosphorylated JAK2 was reduced 24 h after OPB-31121 administration, probably due to cell death-related degradation of JAK2 (Figure 2b). In H1650 cells, where mutated epidermal growth factor receptor (EGFR) constitutively activated STAT3 via SFKs, this compound reduced STAT3 phosphorylation without reduction of SFK phosphorylation, indicating that this compound could inhibit STAT3 phosphorylation independently of the type of upstream kinases (Figure 2c). These results strongly suggested that this compound was not an inhibitor of upstream kinases such as JFKs and SFKs. Consistent with this, in vitro screening of kinase inhibitory activity demonstrated that this compound had almost no kinase inhibitory activity against any of the 31 kinases examined (Supplementary Table S1). To further investigate whether STAT was directly inhibited by this compound, we set up in vitro kination assays using STAT3 immunoprecipitated from cells as a substrate and recombinant JAK2 or Lyn as a kinase, and examined whether this compound could inhibit STAT3 phosphorylation in vitro; however, this compound did not inhibit STAT3 phosphorylation in vitro, suggesting that another cellular protein was required for this compound to inhibit STAT phosphorylation (Supplementary Figure S1).
Figure 2.
OPB-31121 inhibited STAT3 and STAT5 phosphorylation without upstream kinase inhibition. (a) OPB-31121 inhibited IL-6-induced STAT3 phosphorylation without JAK2 inhibition. Hep G2 cells were treated with OPB-31121 and IL-6, as described in Figure 1a, except that the concentration of OPB-31121 was 1 μM. Cells were lysed at the indicated time after IL-6 stimulation and subjected to IB with the indicated antibodies. (b) OPB-31121 inhibited constitutive activation of STAT3 and STAT5 without JAK2 inhibition. HEL cells were lysed at the indicated time after 1 μM OPB-31121 treatment and subjected to IB with the indicated antibodies. (c) OPB-31121 inhibited constitutively activated STAT3 without Src inhibition. H1650 cells were analyzed as in (b).
OPB-31121 had a strong growth inhibitory effect against a wide range of hematopoietic malignant cells
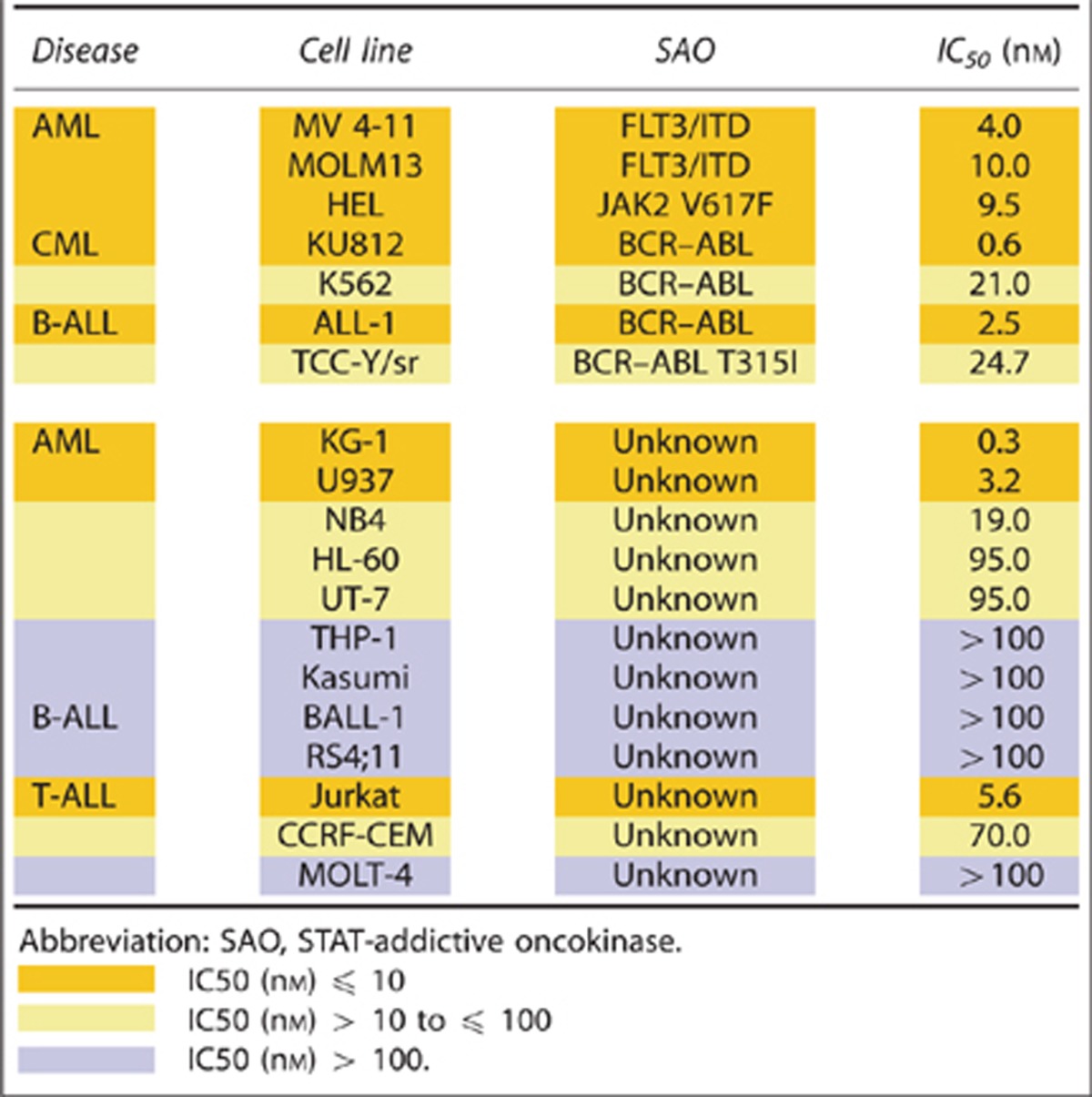
We next investigated the effect of OPB-31121 on the cell growth of various hematopoietic cell lines. Among 35 hematopoietic cell lines, IC50 of OPB-31121 was ⩽10 nM in 20 cell lines (57%), but >100 nM in 8 cell lines (23%, Table 1). Multiple myeloma, Burkitt's lymphoma and chronic myeloid leukemia (CML) seemed to be generally sensitive to this compound. Concerning to other leukemias and lymphomas, all disease types contained both sensitive and insensitive cell lines. Sensitivity to this compound was independent of the strength of STAT3/5 phosphorylation detected by immunoblotting (data not shown); however, looking at gene aberrations, leukemia cells harboring gene aberrations such as BCR–ABL, FLT3/ITD and JAK2 V617F were all sensitive to this compound (Table 2). It has been established that these three mutated kinases are oncokinases and cause constitutive activation of STAT3 and/or STAT5, and that the oncogenicities of these kinases depend on STAT3/5 signal.17, 30, 31 We designated these oncokinases as STAT-addictive oncokinases (SAO).
Table 1. List of IC50 of OPB-31121 and gene aberrations in various cell lines.
| Disease | Cell line | IC50 (nM) | Fusion gene | Gene mutation | Gene deletion |
|---|---|---|---|---|---|
| AML | KG-1 | 0.3 | NRAS, p53 | ||
| U937 | 3.2 | CALM-AF10 | p53 | ||
| MV4-11 | 4.0 | MLL-AF4 | FLT3 | ||
| HEL | 9.5 | JAK2 | CDKN2A , CDKN2B | ||
| MOLM13 | 10.0 | MLL-AF9 | FLT3 | CDKN2A , CDKN2B | |
| NB4 | 19.0 | PML-RARα | CDKN2A , CDKN2B | ||
| HL-60 | 95.0 | p53 | |||
| UT-7 | 95.0 | p53 | CDKN2B | ||
| THP-1 | >100 | MLL-AF9 | NRAS, p53 | CDKN2A , CDKN2B | |
| Kasumi-1 | >100 | AML1-ETO | |||
| CML | KU812 | 0.6 | BCR–ABL | p53 | |
| K562 | 21.0 | BCR–ABL | p53 | CDKN2A , CDKN2B | |
| B-ALL | ALL-1 | 2.5 | BCR–ABL | ||
| TCC-Y/sr | 24.7 | BCR–ABL T315I | |||
| BALL-1 | >100 | IgH-Myc | CDKN2B | ||
| RS4;11 | >100 | MLL-AF4 | CDKN2A , CDKN2B | ||
| B-lymphoblast | CRL8062 | 7.0 | |||
| T-ALL | Jurkat | 5.6 | p53 | CDKN2A , CDKN2B, IFNA1 | |
| CCRF-CEM | 70.0 | SIL-SCL | p53 | CDKN2B, IFNA1, IFNB1 | |
| MOLT-4 | >100 | NRAS | CDKN2B | ||
| DLBCL | OCI-Ly10 | 2.1 | |||
| RL | 2.9 | IgH-BCL2 | p53 | ||
| OCI-Ly3 | 4.0 | ||||
| OCI-Ly7 | 7.8 | ||||
| RC-K8 | 7.9 | ||||
| IM-9 | 10.9 | ||||
| OCI.LY1 | >100 | IgH-BCL1 | |||
| SU-DHL4 | >100 | IgH-BCL2 | p53 | ||
| WILL2 | >100 | Igλ-Myc, IgH-BCL2 | |||
| BL | Ramos | 2.4 | p53 | ||
| Raji | 3.1 | IgH-Myc | p53 | ||
| Daudi | 4.5 | IgH-Myc | p53 | ||
| MM | U266 | 3.9 | p53 | ||
| LICR-LON-Hmy2 | 6.0 | ||||
| WI-L2-729HF2 | 7.0 | p53 |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BL, Burkitt's lymphoma; CML, chronic myeloid leukemia; DLBCL, diffuse large B cell lymphoma; MM, multiple myeloma.
Table 2. Comparison of OPB-31121 effect between STAT-addictive oncokinase-positive and unknown leukemia.
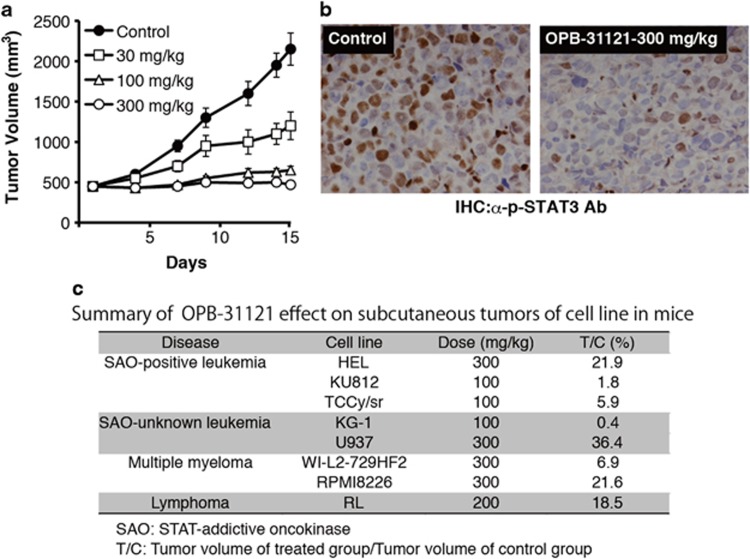
We further investigated the growth inhibitory effect of OPB-31121 against tumors of cell lines inoculated into SCID mice. Orally administered OPB-31121 suppressed the tumor growth of HEL cells significantly in mice, and consistently, STAT3 phosphorylation was strongly inhibited in the tumors (Figures 3a and b). We further examined using OPB-31121-sensitive cell lines containing myeloma and SAO-positive leukemia cell lines. OPB-31121 demonstrated significant tumor growth suppression or even regression in mice inoculated with these cell lines (Figure 3c). These results indicated that this compound was effective against myeloma and SAO-positive leukemia in vivo.
Figure 3.
OPB-31121 inhibited STAT3 phosphorylation and growth of subcutaneous tumors of cell lines in mice. (a) Dose-dependent tumor growth suppression in mice. HEL cells (4 × 107 cells/body) were subcutaneously inoculated into SCID mice. Oral administration of OPB-31121 or 5% gum arabic for control was started 14 days after inoculation when tumors had developed detectably. The average tumor volume of five mice was plotted with standard deviation. (b) STAT3 inhibition by OPB-31121 in mouse tumor. HEL cells were inoculated into SCID mice as in (a). After tumor development, 300 mg/kg OPB-31121 or 5% gum arabic was administered daily for 3 days. On the 4th day, tumors were resected and subjected to immunohistochemistry with anti-phospho-STAT3 antibody. (c) Summary of OPB-31121 effect on cell line tumors in mice.
OPB-31121 caused strong growth inhibition of primary SAO-positive leukemia cells but did not affect the growth of normal cord blood cells in mice
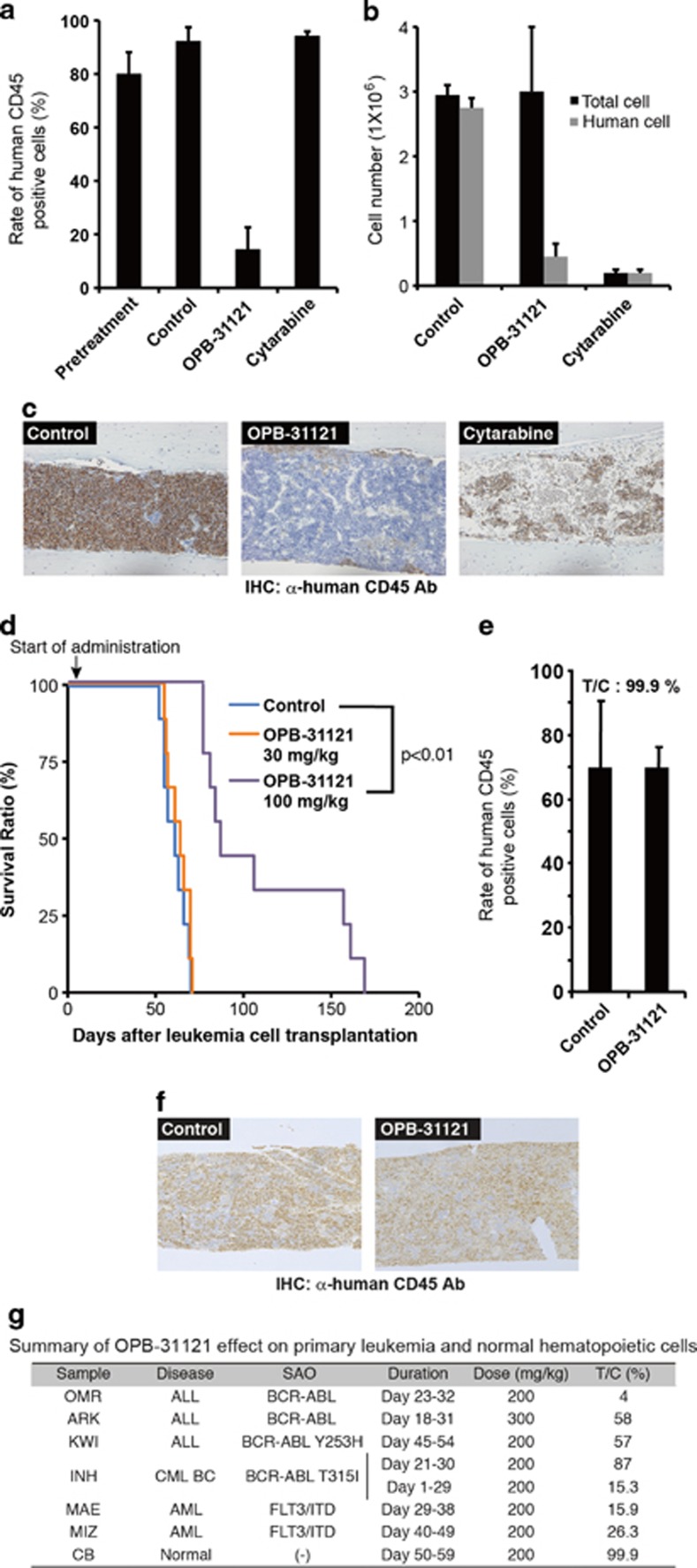
For further analyses, we used a primary leukemia cell xenotransplantation system in which primary human leukemia cells were maintained by serial transplantation into NOG mice. In this system, we could test the drug effect on primary leukemia cells, whose phenotype and heterogeneity mainly maintained their original status.27 Oral administration of OPB-31121 induced significant reduction of the leukemia cell rate in mice transplanted with primary acute myeloid leukemia cells with FLT3/ITD, whereas cytarabine treatment hardly affected it. The treated group/control group (T/C) tumor cell rate were 15.9 and 102.1%, respectively (Figure 4a). Analyzing in detail, cytarabine reduced the number of both human leukemia cells and mouse normal hematopoietic cells, so the rate of leukemia cells did not change by this treatment. On the other hand, OPB-31121 treatment induced selective leukemia cell reduction and the recovery of mouse hematopoietic cells (Figure 4b). These could be clearly observed by immunohistochemistry of bone marrow (Figure 4c). Representative images of flow cytometric analysis of the leukemia cell rate and larger images of the immunohistochemistry of bone marrow are shown in Supplementary Figure S2A and B.
Figure 4.
OPB-31121 selectively reduced human primary leukemia cells in mice. MAE cells (1 × 106), primary FLT3/ITD-positive human AML cells, were intravenously transplanted into NOG mice. On day 29, the rate of human leukemia cells in femoral bone marrow of the pretreatment mice was measured by flow cytometry using anti-human CD45 antibody, and daily oral administration of 5% gum arabic or OPB-31121 (200 mg/kg) for 10 days was started. On day 32, daily intraperitoneal injection of cytarabine (400 mg/kg) for 7 days was started. On day 38, bone marrow cells were collected from one femur and the other femur was fixed and subjected to immunohistochemistry with anti-human CD45 antibody. The rate of human leukemia cells in bone marrow was analyzed as above. (a) The average human leukemia cell rate of three mice was plotted on the bar chart with standard deviation. (b) Bone marrow cells collected from a femur were counted. The leukemia cell number was calculated by multiplying the total cell number by the human CD45-positive cell rate. The average value of three mice was plotted on the bar chart with standard deviation. OPB-31121 did not reduce the total cell number. (c) Immunohistochemistry of bone marrow with anti-human CD45 antibody. Cells stained in brown and blue were human CD45-positive cells (leukemia cells) and negative cells (mouse normal hematopoietic cells), respectively. Normal hematopoietic cells recovered by OPB-31121 treatment. (d) Survival benefit of OPB-31121 treatment in mice transplanted with primary CML BC cells harboring BCR–ABL T315I. INH cells (5 × 106), primary BCR–ABL T315I-positive CML BC cells, were intravenously transplanted into NOG mice. Daily oral administration of 5% gum arabic or the indicated dose of OPB-31121 was started on day 2 and continued until mice died. Each group consisted of nine mice. Survival curve were plotted according to the Kaplan–Meier method. Statistical difference of survival was analyzed by the log-rank test. (e) OPB-31121 did not affect the growth of normal hematopoietic cells. CD34-positive human cord blood cells (1 × 105) were transplanted, treated and analyzed as described above except that mice were irradiated (2.5 Gy) the day before transplantation and that OPB-31121 and gum arabic were administered daily from days 50–59. The average human cell rate of two mice was plotted on the bar chart with standard deviation. (f) Immunohistochemistry of bone marrow of mice transplanted with cord blood cells were performed as in (c). (g) Summary of OPB-31121 effect on primary leukemia and normal hematopoietic cells.
We examined the effect of this compound on other primary leukemia cells. This compound induced significant reduction of the leukemia cell rate of another FLT3/ITD-positive AML (T/C: 26.3%), three BCR–ABL -positive ALL (T/C: 4–58%) and one CML blast crisis (BC) with T315I mutation in BCR–ABL (T/C: 87%, Figure 4g). The effect of OPB-31121 on the CML BC sample was relatively weak, when drug administration was started after leukemia engraftment; however, starting administration earlier, this compound showed stronger tumor growth inhibition (T/C: 15.3%, Figure 4g) and a significant survival benefit (Figure 4d). These results further confirmed the effectiveness of this compound on SAO-positive leukemia and suggested that the growth inhibitory effect of this compound was selective to tumor cells.
To further confirm the safety of this compound for normal hematopoiesis, we performed colony formation assay using CD34+ human cord blood cells. OPB-31121 did not significantly affect the number and composition of colonies (Supplementary Figure S3A). Furthermore, we used NOG mice transplanted with healthy human cord blood cells. OPB-31121 administration did not affect the percentage of human CD45+ cells (T/C: 99%, Figures 4e–g). The rate of stem cell fraction (CD34+/CD38−), monocytic progenitor fraction (CD13+/CD14−) and granulocytic progenitor fraction (CD13+/CD14+) in CD45+ cells were not significantly changed by this compound (Supplementary Figure S3B). These results indicated the safety of this compound for normal hematopoiesis. Taken together, OPB-31121 is a promising antitumor drug for Burkitt's lymphoma, multiple myeloma and SAO-positive leukemia.
OPB-31121 overcame autocrine-induced FLT3 inhibitor resistance by STAT signal inhibition
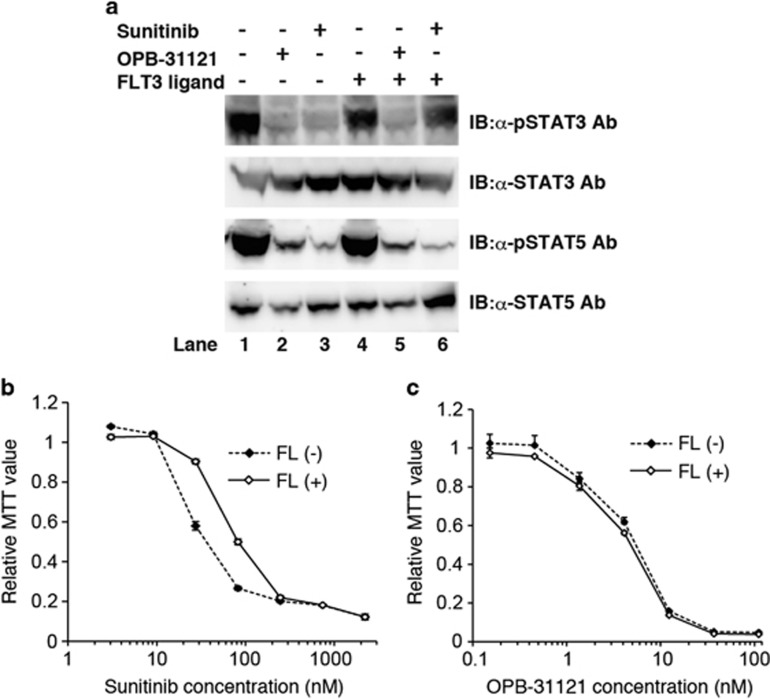
T315I mutation in BCR–ABL causes strong ABL kinase inhibitor resistance in cells. OPB-31121 conquered this mutation-induced kinase inhibitor resistance in TCC-Y/sr cells (Table 2 and Figure 3c) and primary CML cells (Figures 4d and g); therefore, we tried to see whether OPB-31121 overcame another type of kinase inhibitor resistance by inhibition of the downstream signal. According to the previous report, a FLT3 inhibitor-resistant subclone of MV4-11, the FLT3/ITD-positive AML cell line, expressed FLT3 ligand. This autocrine signaling enhanced not only STAT5 phosphorylation but also STAT3 phosphorylation. Additional STAT3 phosphorylation seemed to have an important role in FLT3 inhibitor resistance.32 We therefore examined whether OPB-31121 overcame FLT3 ligand-induced FLT3 inhibitor resistance. Consistent with the previous report, under culture with FLT3 ligand, STAT3 phosphorylation became resistant to sunitinib in MOLM13 cells, another FLT3/ITD-positive AML cell line (top panel of Figure 5a lane 4 and 6 vs lane 1 and 3), although STAT5 phosphorylation was inhibited similarly to the condition without FLT3 ligand (third panel of Figure 5a lane 4 and 6 vs lane 1 and 3). Consistently, the sensitivity of MOLM13 cells to sunitinib fell with FLT3 ligand (Figure 5b). On the other hand, OPB-31121 induced strong inhibition of both STAT3 and STAT5 phosphorylation independently of FLT3 ligand presence (Figure 5a lane 1 and 2 vs lane 4 and 5), and the growth inhibitory effect of this compound on MOLM13 was hardly affected by the addition of FLT3 ligand (Figure 5c). These results indicated that this compound overcame FLT3 ligand-induced FLT3 inhibitor resistance.
Figure 5.
OPB-31121 overcame autocrine-induced FLT3 inhibitor resistance. (a) MOLM13 cells were treated with 100 nM sunitinib, 100 nM OPB-31121 and 100 ng/ml FLT3 ligand as indicated for 4 h. Then, cells were lysed and subjected to IB with the indicated antibodies. (b) FLT3 ligand reduced sunitinib sensitivity in MOLM13 cells. MOLM13 cells were cultured with the indicated dose of sunitinib with or without 100 ng/ml FLT3 ligand. Cell proliferation was analyzed by MTT assay after 48 h culture. MTT values were plotted as relative values to the value of the cells without FLT3 ligand and sunitinib. The average value of three experiments was plotted with standard deviation. (c) FLT3 ligand did not affect the sensitivity to OPB-31121 in MOLM13 cells. Sensitivity of OPB-31121 was measured as in (b) using OPB-31121 instead of sunitinib.
Discussion
Constitutive activation of STAT3 and STAT5 has been reported in various cancers and the inhibition of STAT signaling has been thought to be a promising strategy for cancer therapy.33, 34, 35 Many trials using several approaches, such as small molecules including upstream kinase inhibitors, STAT dimerization inhibitors, and STAT phosphorylation inhibitors, neutralizing antibody against upstream receptors and ligands, and decoy oligonucleotides, have been performed; however, very few of them demonstrated an in vivo effect in mouse models and none has undergone clinical trials, except upstream kinase inhibitors such as JAK inhibitors.22 To the best of our knowledge, OPB-31121 is the first STAT inhibitor to undergo phase I trials.
The mechanism of action of OPB-31121 has not been fully revealed. This compound did not have kinase inhibitory activity against any kinase (Supplementary Table S1) and inhibited STAT3 and STAT5 phosphorylation without inhibition of JAK2 and SFKs (Figures 2a–c). This compound did not inhibit nuclear translocation of STAT3 after it was phosphorylated (Figure 1c) and did not disrupt the dimerization of STAT3 (data not shown). In addition, this compound did not induced the protein expression of JAK-STAT pathway negative regulators such as suppressor or cytokine signaling (SOCS)3, protein inhibitor of activated Stats (PIAS)4 and LNK (data not shown). These data indicated that this compound did not inhibit the phosphorylation of upstream kinases but inhibited STAT phosphorylation, most likely by inhibiting the association of STAT with JAK or cytokine receptors; however, this compound could not inhibit STAT3 phosphorylation by JAK2 and Lyn in vitro, suggesting that another cellular protein was required for this compound to inhibit STAT phosphorylation. This compound may indirectly bind to STAT through an unknown protein that interacts with STAT and disrupts the association of STAT with upstream kinases or receptors. We are now searching for the components of STAT complex that interact with this compound. Very recently, another group reported a decrease in the phosphorylation of STAT3 and JAK2 24 or 48 h after treatment with this compound in gastric cancer cell lines, and claimed that this compound was a JAK2 inhibitor;25 however, we clearly demonstrated that the decrease in STAT3 phosphorylation occurred much earlier, within 2 h after treatment with this compound, and before the decrease in JAK2 and c-Src phosphorylation (Figures 2b and c). At 24 h after treatment, we also observed a decrease in JAK2 phosphorylation, probably due to the cell death response (Figure 2b). A similar phenomenon might have been observed in gastric cancer cell lines.
The sensitivity of cell lines to OPB-31121 varied markedly and was independent of the phosphorylation level of STAT (Table 1 and data not shown). This was probably because the survival dependency on STAT signaling varied among cell lines and was independent of the STAT phosphorylation level; therefore, it was difficult to predict OPB-31121-sensitive cells from the phosphorylation level of STAT. On the other hand, it is reasonable to predict a cancer to be addictive to STAT signaling, when the cancer gets addicted to an oncokinase, and the survival supporting activity of the oncokinase depend on STAT signaling. It is also difficult to clarify whether a mutated kinase observed in a cancer is really responsible for cancer cell survival through STAT signaling, but numerous past studies have proved it in some oncokinaes such as SAO (BCR–ABL, FLT3/ITD and JAK2 V617F).17, 30, 31 Therefore, we selected SAO-positive leukemia cells as the target of OPB-31121. Strikingly, SAO-positive leukemia cells, including primary leukemia cells, were generally OPB-31121 sensitive both ex vivo and in vivo. It is intriguing that OPB-31121 did not cause growth inhibition of normal hematopoietic cells (Figures 5b, c, e and f). In fact, the dose-limiting toxicity of this compound did not show hematological toxicity in phase I trial with a maximum dose of 800 mg/kg.29 No hematological toxicity was observed in the toxicity study of this compound in monkeys (1000 mg/kg for 14 days, data not shown). Although the importance of STAT3 and STAT5 in various signals from cytokines such as erythropoietin, thrombopoietin and granulocyte-colony stimulating factor has been established, the dependence on STAT signaling will be lower in normal hematopoietic cells than in malignant cells.
Specific inhibitors of kinases aberrantly activated in tumor cells are powerful tools with high antitumor effects and less toxicity; however, cancer cells sometimes develop resistance in various ways. CML cells are reported to develop the resistance to ABL kinase inhibitor by a drug-resistant mutation in BCR–ABL, overexpression of BCR–ABL,36 overexpression of Lyn as an alternative activator of STAT537 and STAT3 activation through an alternative signal from bone marrow stroma cells.38 In FLT3/ITD-positive AML cells, resistance to FLT3 inhibitor was achieved by a drug-resistant mutation in FLT3, overexpression of FLT3 and autocrine Flt3 ligand stimulation.32, 39 In JAK2 V617F-positive AML or polycythemia vera, specific JAK2 inhibition was overcome by alternative activation of other JFKs.23 The mechanisms of drug resistance are various but many of them finally lead to the maintenance of STAT3/5 activation. This is probably because SAO-positive leukemia cells require the maintenance of STAT3/5 activation for drug-resistant survival; therefore, it is a reasonable strategy to overcome SAO inhibitor resistance by a STAT inhibitor. We showed examples of this strategy in the case of a drug-resistant mutation in BCR–ABL and autocrine-induced FLT3 inhibitor resistance (Figures 4d and 5a–c).
Taken together, we conclude that OPB-31121 holds promise as a non-myelosuppressive therapeutic agent against a wide range of hematopoietic malignancies, especially SAO-positive leukemia. As OPB-31121 showed strong antitumor effects also in a wide range of solid tumors (data not shown), two phase I studies have been performed on advanced solid tumors in Korea (NCT00955812) and for non-Hodgkin's lymphoma and multiple myeloma in Hong Kong (NCT00511082), and the results are awaited. A phase I/II study of hepatocellular carcinoma in Japan (NCT1406574) is ongoing.
Acknowledgments
We thank Dr Takeyama for generously providing the cell lines. We are very grateful to Yoko Matsuyama, Asako Watanabe and Chika Wakamatsu for their technical assistance. This work was supported by MHLW KAKENHI Grant Number H22-3jigan-Ippan-010 and JSPS KAKENHI Grant Numbers 23591381, 25293218, and 23659487.
Author Contributions
FH designed the research, performed experiments and wrote the paper. KS designed the research and performed experiments. YH, NH, NO and SK performed experiments. TN designed the research.
TN received research funding from Otsuka Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd., Wyeth and Chugai Pharmaceutical Co., Ltd. KS, YH, NH and NO are employees of Otsuka Pharmaceutical Co., Ltd., whose product was studied in this work. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Hayakawa F, Naoe T. SFK-STAT pathway: an alternative and important way to malignancies. Ann NY Acad Sci. 2006;1086:213–222. doi: 10.1196/annals.1377.002. [DOI] [PubMed] [Google Scholar]
- Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell JE, Kuriyan J., Jr Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser D, Bromberg JF, Darnell JE, Jr, Hanafusa H. A single amino acid substitution in the v-Eyk intracellular domain results in activation of Stat3 and enhances cellular transformation. Mol Cell Biol. 1999;19:1401–1409. doi: 10.1128/mcb.19.2.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong CS, Zeng L, Jiang Y, Sadowski HB, Wang LH. Stat3 plays an important role in oncogenic Ros- and insulin-like growth factor I receptor-induced anchorage-independent growth. J Biol Chem. 1998;273:28065–28072. doi: 10.1074/jbc.273.43.28065. [DOI] [PubMed] [Google Scholar]
- Migone TS, Lin JX, Cereseto A, Mulloy JC, O'Shea JJ, Franchini G, et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Weber-Nordt RM, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart V, Mertelsmann R, et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- Danial NN, Pernis A, Rothman PB. Jak-STAT signaling induced by the v-abl oncogene. Science. 1995;269:1875–1877. doi: 10.1126/science.7569929. [DOI] [PubMed] [Google Scholar]
- Danial NN, Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000;19:2523–2531. doi: 10.1038/sj.onc.1203484. [DOI] [PubMed] [Google Scholar]
- de Groot RP, Raaijmakers JA, Lammers JW, Jove R, Koenderman L. STAT5 activation by BCR-Abl contributes to transformation of K562 leukemia cells. Blood. 1999;94:1108–1112. [PubMed] [Google Scholar]
- Nieborowska-Skorska M, Wasik MA, Slupianek A, Salomoni P, Kitamura T, Calabretta B, et al. Signal transducer and activator of transcription (STAT)5 activation by BCR/ABL is dependent on intact Src homology (SH)3 and SH2 domains of BCR/ABL and is required for leukemogenesis. J Exp Med. 1999;189:1229–1242. doi: 10.1084/jem.189.8.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber C, Gesbert F, Frank DA, Sattler M, Griffin JD. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood. 2000;95:2118–2125. [PubMed] [Google Scholar]
- Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci USA. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Onishi M, Nosaka T, Misawa K, Mui AL, Gorman D, McMahon M, et al. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we. Exp Opin Invest Drug. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotsu Y, Kiyoi H, Ishikawa Y, Tanizaki R, Shimizu M, Umehara H, et al. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim TY, et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013;335:145–152. doi: 10.1016/j.canlet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Kurahashi S, Hayakawa F, Miyata Y, Yasuda T, Minami Y, Tsuzuki S, et al. PAX5-PML acts as a dual dominant-negative form of both PAX5 and PML. Oncogene. 2011;30:1822–1830. doi: 10.1038/onc.2010.554. [DOI] [PubMed] [Google Scholar]
- Tanizaki R, Nomura Y, Miyata Y, Minami Y, Abe A, Hanamura A, et al. Irrespective of CD34 expression, lineage-committed cell fraction reconstitutes and re-establishes transformed Philadelphia chromosome-positive leukemia in NOD/SCID/IL-2Rgammac−/− mice. Cancer Sci. 2010;101:631–638. doi: 10.1111/j.1349-7006.2009.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Hayakawa F, Miyata Y, Watamoto K, Emi N, Abe A, et al. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia. 2007;21:403–410. doi: 10.1038/sj.leu.2404547. [DOI] [PubMed] [Google Scholar]
- Oh D, Han S, Kim TM, Lee S, Kim T, Heo DS, et al. A phase I, open-label, nonrandomized trial of OPB-31121, a STAT3 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2010;28:e13056. [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Carlesso N, Frank DA, Griffin JD. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med. 1996;183:811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG, et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009;113:4052–4062. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]
- Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review. Exp Opin Ther Patent. 2011;21:65–83. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
- Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Update Rev Comment Antimicrob Anticancer Chemother. 2010;13:67–78. doi: 10.1016/j.drup.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Scuto A, Kujawski M, Kowolik C, Krymskaya L, Wang L, Weiss LM, et al. STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res. 2011;71:3182–3188. doi: 10.1158/0008-5472.CAN-10-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- Bewry NN, Nair RR, Emmons MF, Boulware D, Pinilla-Ibarz J, Hazlehurst LA. Stat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistance. Mol Cancer Ther. 2008;7:3169–3175. doi: 10.1158/1535-7163.MCT-08-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.