Abstract
Comorbid depression and chronic pain are highly prevalent in individuals suffering from physical illness. Here, we critically examine the possibility that inflammation is the common mediator of this comorbidity, and we explore the implications of this hypothesis. Inflammation signals the brain to induce sickness responses that include increased pain and negative affect. This is a typical and adaptive response to acute inflammation. However, chronic inflammation induces a transition from these typical sickness behaviors into depression and chronic pain. Several mechanisms can account for the high comorbidity of pain and depression that stem from the precipitating inflammation in physically ill patients. These mechanisms include direct effects of cytokines on the neuronal environment or indirect effects via downregulation of G protein–coupled receptor kinase 2, activation of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase that generates neurotropic kynurenine metabolites, increased brain extracellular glutamate, and the switch of GABAergic neurotransmission from inhibition to excitation. Despite the existence of many neuroimmune candidate mechanisms for the co-occurrence of depression and chronic pain, little work has been devoted so far to critically assess their mediating role in these comorbid symptoms. Understanding neuroimmune mechanisms that underlie depression and pain comorbidity may yield effective pharmaceutical targets that can treat both conditions simultaneously beyond traditional antidepressants and analgesics.
I. Introduction: Comorbid Depression and Pain
Physical illness is accompanied by a wide variety of symptoms, including fatigue, anorexia, lack of motivation, reduced sex drive, depressed mood, heightened pain sensitivity, apathy, and low sociability, among others. Many of these can be classified as typical sickness responses or behaviors, which often dissipate along with the disease. However, all too often clusters of these symptoms remain even after recovery from the original disease or illness. In primary care, the most common physical symptom is pain, and the most common psychological symptom is depression (Kroenke et al., 2009). The onset of each of these symptoms has been closely linked with inflammation, which may represent a common mechanism. Depression and chronic pain are two of the most debilitating disorders in the Western world, limiting quality of life and employment opportunities for those who suffer from the disorders as well as those individuals within their support networks. The lifetime prevalence of depression has been reported to affect approximately 17% of the population (Kessler et al., 2005) and costs an estimated $80 to $100 billion annually in the United States (Greenberg et al., 2003). Similarly, a recent report by the Institute of Medicine of the National Academies (2011) announced that at least 116 million U.S. adults currently suffer from chronic pain, with the associated national economic costs ranging from $560 to $635 billion annually. Thus, the consequences of depression and chronic pain have proven to be exorbitantly costly to the individual sufferers, their families, and society as a whole. The clustering of chronic pain with depression also occurs with alarmingly high prevalence, ranging from 30 to 60% (Arnow et al., 2006; Bair et al., 2008), and a number of studies have investigated common treatment strategies that may alleviate both conditions, suggestive of a common mechanism. A prime example of this is the use of antidepressants to treat chronic pain conditions. Many publications report that typical antidepressant medications are successful in treating chronic pain (Finnerup et al., 2005; Goldstein et al., 2005; Krell et al., 2005; Dharmshaktu et al., 2012). Although these studies are compelling, they typically focus on using antidepressants to treat pain in the context of chronic pain and not in the context of inflammation. Many reviews have already dealt with this subject matter, and therefore will not be the focus of this review. Here, we will focus on chronic pain and depression which often arise on the background of physical illness. Thus, we will target the potential underlying common mechanisms and pathways that give rise to the clustering of depression and chronic pain.
Comorbid depression and pain occur within clinical settings with extremely high prevalence, whereby patients present with chronic levels of inflammation, such as with rheumatoid arthritis and cancer (Gureje et al., 1998; Rakoff-Nahoum, 2006; Reyes-Gibby et al., 2006; Isik et al., 2007; Mao et al., 2007). Such high co-occurrence of pain and depression in the context of inflammation is suggestive of a commonality of mechanisms. One possibility is that the mechanisms that link the immune system and the central nervous system, the so-called neuroimmune mechanisms, are involved in the pathogenesis of both pain and depression in these individuals. Recognition of neuroimmune-mediated mechanisms responsible for the comorbidity of pain and depression could push the therapeutic options beyond the traditional antidepressants which have revolved around the targeting of monoamine deficiencies, such as the use of selective serotonin reuptake inhibitors (SSRIs) and the newer selective norepinephrine reuptake inhibitors and dopamine reuptake inhibitors for depression, as well as the traditional analgesics deriving from the opioid family for pain—the efficacy of each yielding success rates lower than 50% (Fava and Davidson, 1996; Kroenke et al., 2009). Furthermore, under conditions of inflammation, the claimed antalgic effects of antidepressants are less convincing in guarding against the affective component of pain (Boyce-Rustay et al., 2010a), which is likely to sit at the intersection of pain and depression. Examination of common neuroimmune-mediated mechanisms could provide novel targets for pharmaceutical interventions that relieve both the depression and pain symptoms experienced during illness as opposed to a range of medications that individually target each symptom. Therefore, it is important to understand depression and pain in the context of inflammation.
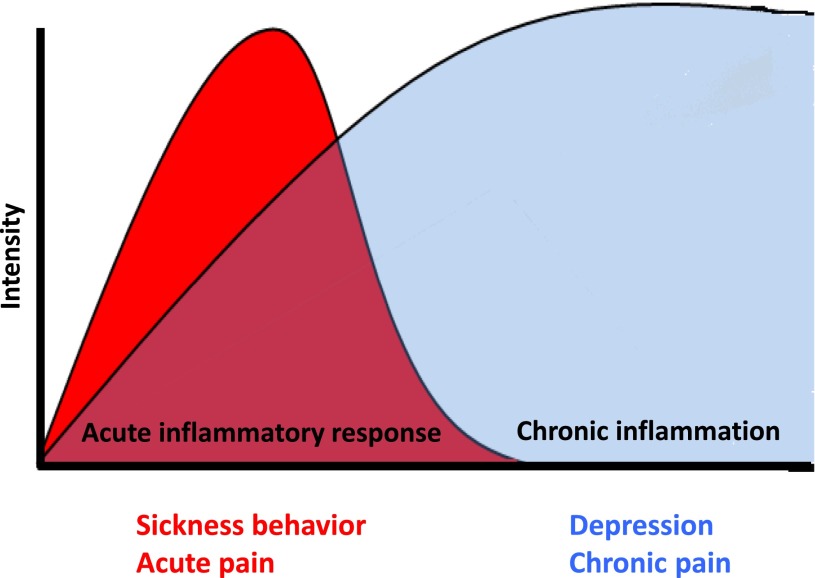
Inflammation gives rise to an intricate network of immune-to-brain signaling, which governs behavioral output during sickness. Cytokines themselves are known to directly interact with the neuronal environment, and thus modulation of the duration of inflammation can work to alleviate the depressive and pain symptoms that result. Inflammatory signals also target downstream pathways which can disturb neural function such as the excitation of the N-methyl-d-aspartate (NMDA) receptor and excessive levels of glutamate or the switch of GABAergic neurotransmission from inhibitory to excitatory. Chronic inflammation can lead to a permanent restructuring of these neurotransmitter pathways and to the transition from sickness to depression and from acute to chronic pain, even after the acute inflammatory response has dissipated (Fig. 1). Therefore, in this review, we outline the typical immune-brain communication pathways, the known mediators responsible for the transition from sickness to depression and from acute to chronic pain, and finally propose common mechanisms that may account for such a high degree of co-occurrence during physical illness.
Fig. 1.
Timing and overlap of sickness and acute pain with depression and chronic pain. Sickness during acute inflammation represents an adaptive response to infection or tissue damage. These acute symptoms include enhanced pain sensitivity, neurovegetative symptoms (e.g., fatigue, reduced appetite, sleep disorders), and malaise, among others. However, intense chronic inflammation can lead to a transition from these adaptive normal responses to inflammation to chronic conditions of depression and chronic pain that can remain even after the inflammation has subsided. During the period of typical sickness behavior, the evidence suggests that the pain and depressive phenomena are reversible. However, if the transition to depression and chronic pain has already taken place, then it may be too late for intervention using drugs that target merely sickness and acute pain.
II. Peripheral Inflammation and Its Propagation to the Brain
A. Initiation of Inflammation: The Role of Damage-Associated Molecular Patterns and Pathogen-Associated Molecular Patterns.
Peripheral inflammation has been well documented to result in both pain and depression (reviewed in Watkins et al., 1995b and McNally et al., 2008). In order for the immune signal initiated in the periphery to regulate nociceptive and depressive output, this information needs to be transmitted to the central nervous system (CNS). This involves an exquisite series of immune-mediated pathways through which the peripheral immune system and CNS communicate.
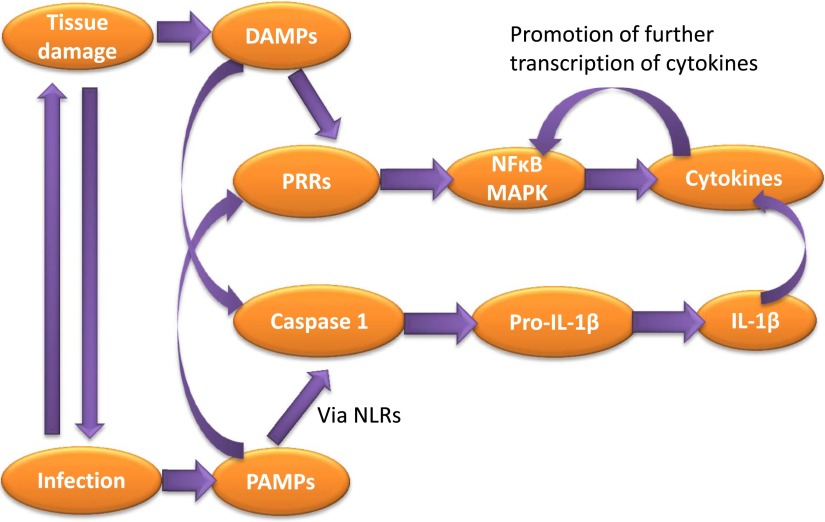
Tissue damage can occur by cells being ripped, squashed, or damaged by heat, chemicals, or oxygen deprivation, as well as by infectious pathogens—each of which triggers inflammation. Therefore, trauma and infection can both trigger similar responses and lead to immune activation, underscoring that the comorbidity of inflammation-induced depression and neuropathic pain is no coincidence. The early response to tissue damage or pathogens is mediated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) (Fig. 2). PAMPs consist of a range of microbial molecules with common recognizable biochemical features that signal pathogen intrusion (Bianchi, 2007; Piccinini and Midwood, 2010). These include, among others, the cytokine inducer lipopolysaccharide (LPS), carrageenan, and complete Freund’s adjuvant (CFA), which are commonly used in many of the models of inflammatory pain and depression to be discussed in this review. DAMPs are endogenous molecules released in response to cellular damage contributing to so-called sterile inflammation. DAMPs include, among others, ATP, interleukin (IL)-1α, uric acid, and high-mobility group protein 1. DAMPs and PAMPs activate the immune system primarily by binding to a vast array of receptors, of which Toll-like receptors (TLRs) have probably been most extensively studied. TLRs recognize bacterial and viral PAMPs in the extracellular environment, leading to activation of nuclear factor κB (NF-κB) and mitogen-activated protein kinases (MAPKs). This results in the activation of the transcription of a wide range of genes, including those for proinflammatory cytokines. PAMP and DAMP activation of the inflammasome (the myeloid cell–expressed oligomer which permits the production of IL-1β and IL-18) occurs via NOD-like receptors, which engage with caspase 1 adaptor protein ASC, resulting in caspase 1 activation (Newton and Dixit, 2012). Caspase 1 cleaves pro–IL-1β for the secretion of its biologically active form. IL-18 is also produced in a similar fashion; however, it is the AIM2 protein that engages with ASC for its secretion. PAMP and DAMP activation of cytokine production and release can, in turn, activate transcription factors for the regulation of further gene transcription, further promoting the inflammatory response (Newton and Dixit, 2012). Thus, the cytokine cascade has been initiated. This begs the question: how does this cascade signal the CNS for the regulation of pain and depression?
Fig. 2.
PAMPs and DAMPs trigger the inflammatory response to infection and tissue damage. Tissue damage can often lead to infection and vice versa, thus the activation of DAMPs and PAMPs usually co-occur. These in turn activate pattern recognition receptors such as TLRs, which induce the transcription of proinflammatory cytokines by factors such as NF-κB and MAPKs, which can then promote the further transcription of cytokines. IL-1β is produced in response to PAMPs via NOD-like receptors (NLRs), which ultimately activate caspase-1, resulting in the cleavage of Pro-IL-1β to mature IL-1β.
B. Immune-Brain Interactions.
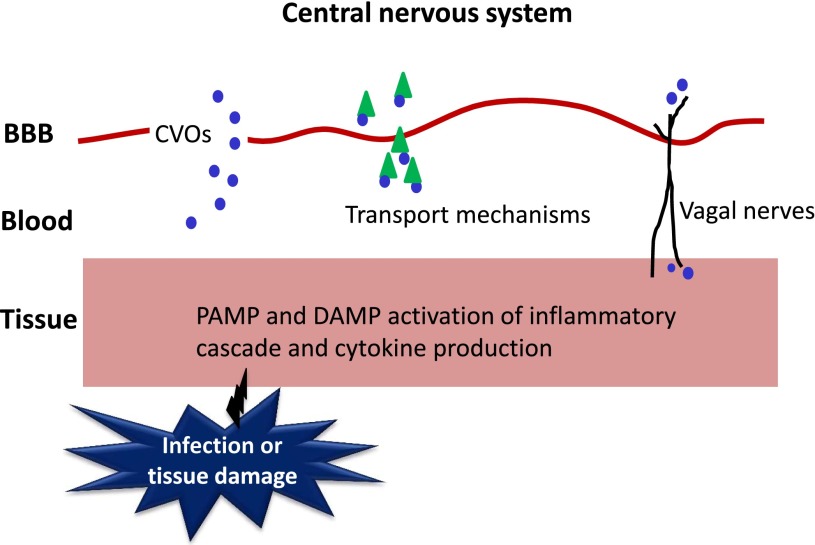
Infection in the CNS is a relatively rare occurrence. Most infections and tissue damage take place in the periphery, and thus inflammation is often initiated here. Therefore, a complex system of immune-brain signaling is required for appropriate centrally mediated sickness and behavioral responses (Fig. 3). Infection and tissue damage stimulate the production of proinflammatory cytokines, including tumor necrosis factor (TNF)-α and IL-1β, by tissue macrophages, liver Kupffer cells, and monocytes. These cytokines can spill over in the circulation. However, as cytokines are large molecules that do not readily cross the blood-brain barrier (BBB), circulating cytokines typically do not reach the brain. Passage into the brain can take place at the level of circumventricular organs, where the BBB is relatively leaky. However, this only occurs when cytokines are produced at very high levels. Thus, there must be mechanisms in place that allow cytokine signaling to the brain over the BBB so that appropriate behavioral responses can occur. Under conditions of systemic inflammation, BBB permeability can be enhanced by specific transport mechanisms (Banks and Kastin, 1991; Gutierrez et al., 1993). Although it was first considered that inflammation led to enhanced leakiness of the BBB, William Banks’ team demonstrated that radio-iodinated IL-1α can cross the rodent BBB 43.9 times more efficiently than can be explained by leakage alone (Banks et al., 1989). Subsequent investigations revealed that murine IL-1α is even more rapidly transported to the brain (Banks et al., 1991), suggestive of the fact that, under conditions of systemic inflammation, active transport of cytokines across the BBB may occur at an even further enhanced rate, ruling out leakage as the predominant transport mechanism. Although the demonstration of transport mechanisms by Banks et al. (1989) represents an extreme case of transportation of the cytokine given that it was injected intravenously, which does not represent normal physiological conditions, it nonetheless demonstrated that the level of cytokine entry to the brain cannot be accounted for merely by nonspecific mechanisms but must also occur via the use of specific transport mechanisms for direct transport across the BBB. Banks’ team later demonstrated that IL-1β also crosses the BBB using either the same or overlapping transporters (Banks et al., 1991). IL-1α and IL-1β appear to cross the BBB via saturable binding of IL-1 to endothelial receptors and internalization by these BBB endothelial cells, indicative of an endocytotic mechanism (Banks et al., 1991, 1993). Active transport mechanisms have since also been identified for TNF-α and IL-6, among others (Watkins et al., 1995a; Osburg et al., 2002). It should be noted that not all cytokines are transported in this way, and transport rates can differ between cytokines (Banks, 2005). Secondary mediators, including nitric oxide and prostaglandin E2s, are also stimulated by peripheral cytokines and readily cross the BBB to induce central cytokine production (Watkins et al., 1995a; Dantzer, 2004).
Fig. 3.
Immune-to-brain signaling pathways. Once PAMPs and DAMPs trigger the production of the inflammatory response, inflammatory signals need to reach the brain to induce sickness responses and behaviors. Cytokines can bypass the BBB via circumventricular organs (CVOs), or are transported across the BBB by specific transport mechanisms. Afferent vagal nerves can send the cytokine signal from peripheral tissue to the base of the brain. Adapted from Quan (2008) with permission from Springer Science and Business Media.
Although immune-brain signaling via the BBB due to active transport mechanisms is possible under systemic inflammation, it does not serve as the primary signaling pathway. This is because, first, circulating cytokine concentrations remain relatively low without infection or supraphysiological inflammation, and even under septic conditions, cytokine concentrations in the blood do not accurately reflect those of the affected tissue. This would make it particularly difficult for BBB-mediated pathways to use circulating cytokines to accurately signal the conditions of inflammation within the tissue to the brain (Quan, 2008). Second, cytokine receptors on brain endothelial cells induce leukocyte infiltration to the CNS following central injection of cytokines (Anthony et al., 1997; Ching et al., 2005). This means that cytokine receptors on endothelial cells play a role in both mediating central inflammation and relaying peripheral to central immune signaling, which can result in misactivation of CNS inflammation under a range of conditions (Quan, 2008). Given that BBB-dependent signaling is not sufficient to adequately transmit subtle changes in inflammatory status to the brain, it would seem that additional pathways of immune-brain communication for modulation of behavior by cytokines under physiological conditions must be in place to supplement the BBB-dependent system. Indeed, cytokines usually act locally, and their action on the CNS is relayed by neural afferents. This notion of a neural pathway emerged following the recognition that heat and pain, which are some of the hallmarks of inflammation, represent sensory components of the peripheral inflammatory response (Kent et al., 1992a). Cytokine signals that are generated in the peritoneal cavity inform the brain via afferent vagal nerves. Activated IL-1β receptors, expressed within the sensory neurons of the hepatic vagus nerve and vagal paraganglia, stimulate vagal sensory nerves which project to the nucleus of the tractus solitarius (NTS) and can lead to activation of catecholaminergic projections rostrally (Goehler et al., 1999).
The first evidence implicating vagal nerves in projecting immune information to the brain came from histological studies demonstrating that peripheral LPS administration in rats results in increased expression of the early activation gene c-fos in the supraoptic nucleus, arcuate nucleus, and ventrolateral region of the brain stem, which is absent when LPS is injected centrally. Vagotomy also prevents such labeling (Wan et al., 1993). Additional approaches have focused on functional consequences of nerve sectioning, whereby subdiaphragmatic vagotomy abrogates peripherally administered LPS-induced hyperalgesia and reductions in social exploration (Bluthé et al., 1994; Watkins et al., 1994). Importantly, elevations in peripheral IL-1β concentrations in plasma and peritoneal macrophages remained unaltered in vagotomized rats (Bluthé et al., 1994).
Once cytokines or their signals reach the brain, they are not restricted to their initial point of contact. Immune-mediated activation of afferent nerves is transmitted to the primary projection area of these nerves, e.g., the NTS for vagal afferents, and propagates from there to secondary projection brain areas such as the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala. In addition, cytokines produced by microglial cells in response to peripheral cytokines diffuse in the brain but via a process known as volume transmission. The earliest studies demonstrating this phenomenon used histological techniques. Brady at al. (1994) examined brain c-fos staining following intraperitoneal administration of IL-1β in rats. Staining patterns differed between 1 and 3 hours post-treatment, with early activation of the area postrema and NTS reflecting entry to the brain, and later activation patterns, including external brain margins, demonstrating diffusion of the signal. Further evidence for volume transmission of IL-1 in the brain came from studies based on central injections of the cytokine. For example, Konsman et al. (2000) injected recombinant rat IL-1β and recombinant human IL-1ra intracerebroventricularly in rats, and used immunocytochemistry to observe transmission of the signal through the extracellular space in the brain. They observed these cytokines to rapidly enter the parenchyma and disperse to other regions, such as the hypothalamus and amygdala.
III. Peripheral Inflammation Is Associated with Pain and Depression
A. Depression.
The immense overlap in the behavioral and affective states of sickness and depression has pushed scientists to consider that depression may stem from immunological roots (Yirmiya, 1996). Certainly this is plausible if we consider depressive behaviors following inflammation to be an extension of certain aspects of the already present sickness behaviors. That is, after sickness has subsided, certain mood, cognitive, and behavioral accompaniments of the sickness response remain and align with the criteria for diagnosable depression. In this way, we can see some patients with physical illness as having transitioned from sickness to depression.
Early studies linking the immune system to depression showed positive correlations between peripheral inflammatory biomarkers and depression indices in the absence of physical illness (Maes et al., 1992). However, major advances in demonstrating a causal relationship between the cytokine cascade which follows inflammation and depression have occurred in patients with a pre-existing medical condition usually undergoing cytokine therapies. Typically, such studies have focused on the effect of systemic interferon-α (IFN-α) therapy on patients with malignant melanoma or hepatitis C viral infection. IFN-α therapy results in increases in depressive symptoms (Capuron et al., 2000; Kraus et al., 2002; Raison et al., 2007) and even clinically diagnosable major depressive disorder (MDD) with rates as high as 45% in several studies (Musselman et al., 2001; Capuron et al., 2002). Two major lines of evidence from these types of studies have been instrumental in validating this hypothesis of sickness to depression transition. The first piece of evidence points to the fact that symptom clusters associated with sickness, such as anorexia, fatigue, and pain, have been reported to commence within a few days of IFN-α therapy, whereas the onset of depressive and cognitive changes appears substantially later (Capuron et al., 2002). This is suggestive of the need for persistent immune activation (common in these patients) in order for the transition from sickness to depression to take place. The second piece of evidence points toward the fact that prophylactic antidepressant treatments have been somewhat efficacious in reducing depressive symptoms in these populations. Musselman et al. (2001) reported that only 11% of patients with malignant melanoma receiving high-dose IFN-α developed MDD under treatment with paroxetine, whereas this number increased to 35% among the placebo controls. Capuron et al. (2002) later reanalyzed these data to show that paroxetine indeed does nothing to the neurovegetative symptoms of depression (akin to sickness behavior) but only alleviates the cognitive and affective symptoms of depression. Kraus et al. (2002) also found reduced depressive symptoms in hepatitis C patients undergoing IFN-α therapy when treated with paroxetine, and as many as 78.6% of these patients were able to complete their cytokine therapy, which is often discontinued due to psychiatric complications. Even in less-convincing studies, antidepressants appear to be, in the very least, beneficial. For instance, although Raison et al. (2007) could not report that paroxetine resulted in significantly reduced rates of MDD in hepatitis C patients undergoing IFN-α therapy, there was a significantly lower percentage of patients meeting the criteria for mild, moderate, or severe depressive symptoms. Notably, antidepressants have also been reported to reduce proinflammatory cytokine profiles and inflammatory markers in patients with depression, in some cases (Basterzi et al., 2005; O’Brien et al., 2006, 2007). In fact, a meta-analytic study looking at antidepressant effects on cytokine concentrations reported consistent reductions in IL-1β following SSRI medication, but failed to observe reductions in TNF-α and IL-6 with any consistency (Hannestad et al., 2011).
Inflammation-induced changes in mood have also been observed in physically healthy individuals given low-dose inflammatory stimuli. These studies often administer infra-septic doses of LPS or typhoid vaccination to healthy volunteers and observe transient changes in mood and cognition. Typhoid vaccination abrogates the normally occurring circadian improvement in mood as the day progresses (Strike et al., 2004), induces negative mood postvaccination (Wright et al., 2005), increases brain activity in depression-related regions such as the subgenual cingulate cortex, and decreases its connectivity to the amygdala, medial prefrontal cortex, and nucleus accumbens (Harrison et al., 2009). Low-dose LPS similarly induces transient increases in anxiety and depressed mood (Reichenberg et al., 2001). Each of these effects has been associated with the cytokine response to these stimuli, with mood and cognitive reductions being correlated with increases in circulating TNF-α, IL-6, IL-1ra, and soluble TNF receptor (Reichenberg et al., 2001; Strike et al., 2004; Wright et al., 2005; Harrison et al., 2009). It is important to note that these changes in mood status occur in the absence of sickness (Reichenberg et al., 2001; Strike et al., 2004).
Finally, the relationship between inflammation and depression has been confirmed in preclinical studies. These studies use as end points behavioral responses that are sensitive to antidepressant actions, such as failure to escape electric shocks after prior exposure to inescapable electric shocks (learned helplessness), increased immobility (the so-called “despair” response) in rodents placed in a container filled with water [the forced swim test (FST)] or suspended by their tails (the tail suspension test), or a decreased preference for a sweet taste solution, a test of “anhedonia.” Systemic injections of LPS and cytokines result in increased depressive-like behaviors in rodents, such as anhedonia, learned helplessness, and reduced social exploration (Kent et al., 1992c; Yirmiya 1996; O’Connor et al., 2009a,b,c). Moreover, these behaviors can be rescued by pretreatment with antidepressant medications (Yirmiya, 1996), reflective of the human studies described earlier.
Such preclinical work has also been instrumental in confirming the notion of symptom transitioning from sickness to depressive-like behavior. This approach stems directly from clinical observations of the early commencement of symptoms, which typify sickness in patients receiving immune therapies and the subsequent emergence of depression symptoms (Capuron et al., 2002). Animal studies often allow for the measurement of depressive-like behavior after sickness behaviors have terminated. For example, peripheral administration of nonseptic doses of LPS induces acute sickness responses and behaviors such as body weight loss and reduced locomotor activity. By 24 hours postinjection, these sickness behaviors have largely dissipated, allowing for a window of opportunity to examine depressive-like behaviors, such as reduced sucrose preference and increased immobility in the forced swim and tail suspension tests (Frenois et al., 2007; Henry et al., 2008; O’Connor et al., 2009a,b,c; Walker et al., 2013). Similar studies using chronic infection models of Bacille Calmette-Guerin have reported findings in kind, whereby sickness behaviors return to normal by a few days after inoculation but depressive-like behaviors persist (O’Connor et al., 2009a,b). These time-course effects have allowed for inflammation-induced depressive-like behavior to be examined in the absence of sickness, which is often difficult to do in the clinic, and have confirmed in controlled settings the notion that depressive-like behavior following inflammation represents a transition from sickness to depression.
B. Pain.
One of the major components of the sickness response is pain. This includes muscle and joint ache and a heightened sensitivity to nociceptive stimuli. Acute pain represents a protective response to infection or tissue damage. In this way, pain can be seen as an adaptive mechanism allowing for the detection of noxious stimuli and, in turn, promoting appropriate behavioral responses to enhance survival. However, when this acute pain response transitions to chronic pain or is no longer related to the tissue damage because healing has occurred, then this response becomes problematic just as depressive symptoms are considered pathological once sickness has abated. What we are specifically interested in here is the immune system’s involvement in the transition from acute to chronic pain and whether this can be linked to the transition from sickness to depression. Examination of the role of the immune system in pain has typically focused on models of inflammatory pain. Injection of carrageenan or CFA into the plantar surface of the hind foot of rats or mice results in inflammation of the hind paw and increased sensitivity to thermal and mechanical stimuli. This represents transient hyperalgesia with increased nociception lasting days to weeks. When injected into the hind paw, formalin causes a very acute biphasic pain response that usually resolves within a day or two but is associated with hyperalgesia at sites remote to the injection site for several weeks (Fu et al., 2000). These inflammatory pain models are described in greater detail in Boyce-Rustay et al. (2010b).
In contrast to inflammatory pain, neuropathic pain originates from damage to nerves and is therefore modeled by inducing injury to peripheral nerves, although the location and form of injury may vary (Wang and Wang, 2003). These models include nerve ligation, diabetic neuropathy, and chemotherapy-induced neuropathic pain. A commonly used model of peripheral nerve injury is the chronic constriction injury (CCI) model introduced by Bennett and Xie (1988). The sciatic nerve is loosely tied, which causes local nerve inflammation and ischemic damage to the distal processes. This induces pronounced mechanical allodynia lasting up to 2 months. Thermal hyperalgesia has also been reported but with far less intensity than mechanical allodynia or than is seen under inflammatory model conditions. Similar models of ligation of peripheral nerves work in much the same manner but differ depending on the location of the ligation. The common site is the L5 and L6 spinal nerves, which causes the same effects at the sciatic nerve site and induces chronic mechanical hyperalgesia and allodynia. This model has been developed and characterized by Kim and Chung (1992). In addition to ligation, other models use injury—one of the most commonly used in recent years being the spared nerve injury model developed by Decosterd and Woolf (2000). Two of the three branches of the sciatic nerve (usually the peroneal and tibial) are either crushed or cut, producing mechanical and thermal hyperalgesia lasting around 2 months.
The efficacy of these models in enhancing nociceptive responses to stimuli can be measured using various approaches, the most common of which are the Von Frey filament and Hargreaves test. The Von Frey filament test measures mechanical allodynia. A small filament or hair fiber is presented to the plantar surface of the paw in consecutive forces, and paw withdrawal thresholds are calculated. Thus, the reduction in threshold for evoking a response is measured to quantify the response to nerve damage or inflammation. The Hargreaves assay measures the sensitivity to noxious heat. Paw withdrawal latency is assessed by the time taken to remove the paw from thermal stimulation produced by a light beam shone onto the paw. This test is similar to the hot plate test in which an animal is placed onto a thermal plate of predetermined temperature, and the time taken to withdraw the hind paw or lick the paw is measured.
A great deal of research has identified several spinal inflammatory mechanisms implicated in the sensitization of nociceptors, of which proinflammatory and some anti-inflammatory cytokines appear to play a vital role. Cytokine sensitization of nociceptors following peripheral injury can occur via several pathways in the spinal cord. DAMP activation of TLRs which bind to pathogens or molecules released from damaged cells leads to the activation of NF-κB, in turn, initiating the release of cytokines, chemokines, and other molecules crucial for tissue homeostasis (Guo and Schluesener, 2007). TLR4 knockout mice exhibit reduced mechanical and thermal hypersensitivity, lower expression of microglial activation markers, and reduced proinflammatory cytokines following L5 spinal nerve injury compared with wild-type controls (Tanga et al., 2005). This suggests that TLRs are key regulators of nociception. Similarly, immune cells, such as mast cells residing near nociceptors, degranulate to secrete histamine and bradykinin, causing vasodilation and recruitment of other immune cells such as macrophages to the site of injury and the further release of proinflammatory cytokines such as IL-1β and IL-6. Additionally, bradykinin directly stimulates nociceptors, leading to increased excitability and/or decreased nociceptive thresholds. Cytokines also work to recruit further macrophages to the nociceptor environment, leading to a positive cycle of inflammation, which sensitizes nociceptors. For instance, rats exhibit significant increases in macrophages, NK cells, T lymphocytes, IL-6, and TNF-α in the sciatic nerve following nerve injury, which corresponds to the onset of mechanical allodynia (Cui et al., 2000). A few studies have more directly tested the impact of cytokines on nociception. Reeve and colleagues (2000) reported intrathecal IL-1β, but not TNF-α, to result in mechanical allodynia. However, in other studies, intraneural TNF-α was found to induce thermal hyperalgesia and mechanical allodynia in rats (Zelenka et al., 2005). Indeed, numerous studies have indicated that a range of cytokines can directly stimulate nociceptors (Opree and Kress, 2000; Obreja et al., 2002; Parada et al., 2003).
The cell bodies of nociceptors in the dorsal root ganglion (DRG) meet with further inflammatory mediators, which also sensitize neuronal signaling and elevate nociceptive responses. Satellite glial cells surrounding the neuron reduce K+ buffering following injury, which increases excitation (Ren and Dubner, 2010). In turn, this activation also results in neuronal release of calcitonin gene–related peptide (CGRP), which can elevate IL-1β concentrations, leading to increased prostaglandin E2 activity and further CGRP expression, creating yet another positive feedback loop of cytokine recruitment. Furthermore, these factors alone have direct sensitizing effects on neurons. Injection of CGRP in rat models of temporomandibular joint disorder results in increased neuronal and glial markers of the p38 MAPK and extracellular signal–regulated kinases, which have been highly implicated in the development of neuropathic pain (Cady et al., 2011). The inflammatory signal is sent to the central nervous system via neurotransmission and afferent cytokine signaling of the vagus nerves. Several growth and differentiation factors also sensitize nociceptors directly, as well as indirectly by allowing neurons to signal microglia and regulate cytokine release and chemotaxis of surrounding cells. These include neuregulin-1, nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 (Polazzi and Contestabile, 2002; Calvo et al., 2010, 2011). In response to nerve damage, the brain also stimulates the central cytokine cascade, activating further spinal microglia in pain-related areas and causing a positive cycle of cytokine release. Milligan et al. (2003) reported that sciatic inflammatory neuropathy induced by unilateral localized inflammation to one of the branches of the sciatic nerve in rats, measured using the Von Frey test, was reversed by intrathecal administration of fluorocitrate (a glial and astrocyte metabolic inhibitor), as well as by intrathecal IL-1β, TNF-α, and IL-6 antagonists.
In addition to the proinflammatory cytokine-mediated effects described earlier, anti-inflammatory cytokines have also been implicated in the pathogenesis of pain, with IL-10 having been most extensively studied. Typically, low levels of IL-10 have been reported in patients suffering from chronic pain disorders (Shoskes et al., 2002; Uceyler et al., 2006). Acute intrathecal injection of rat IL-10 protein or an adenoviral vector encoding human IL-10 to rats has been demonstrated to transiently reverse CCI-induced mechanical and thermal allodynia (Milligan et al., 2005), and intrathecal administration of plasmid DNA encoding IL-10 reverses CCI-induced mechanical allodynia acutely with increasing duration following each successive injection (Milligan et al., 2006). Chronic reversal of mechanical allodynia was achievable depending on the treatment regimen. Despite these promising findings implicating low levels of spinal IL-10 with increased pain, clinical findings are not always so clear. One study reported a positive correlation between seminal plasma IL-10 and both pain severity and life interference in patients with chronic prostatitis–chronic pelvic pain syndrome (Miller et al., 2002). However, increased concentrations of proinflammatory cytokines IL-2 and IFNγ were similarly correlated, suggesting that the IL-10 relationship with pain severity may be spurious and represent only an attempt to alleviate the increase in the proinflammatory cytokines in these patients.
Microglia support not only pain-enhancing but also pain-decreasing mechanisms. A key molecule is G protein–coupled receptor kinase 2 (GRK2). GRK2 is ubiquitously expressed in all cell types and regulates desensitization of numerous G protein–coupled receptors (GPCRs). It normally protects cells from overstimulation by uncoupling GPCR from its G protein, and by promoting internalization of agonist-occupied receptors (Aragay et al., 1998). Importantly, GRK2 is involved in regulating the duration of microglial activation in the spinal cord in this manner, which in turn limits microglial cytokine release, and thus the extent of nociceptive sensitization. However, GRK2 can also regulate the duration of microglial activation independently of its role in regulating GPCR signaling by inhibiting the p38 MAPK (Eijkelkamp et al., 2010a). The GRK2 regulatory mechanism will be discussed in greater detail later in this review, and we will posit the notion that a breakdown in the optimal function of GRK2 is responsible for the transmission from acute to chronic inflammatory pain.
The previous evidence represents the majority of research thus far undertaken to understand the inflammatory mechanisms involved in pain sensitization. This evidence is focused around spinal inflammatory mechanisms and enhanced reflexive pain. The problem we face here, however, is that we are interested in potential common mechanisms of inflammation that may be responsible for the clustering of both chronic pain and depression. Hence, for a common immune-mediated mechanism to exist between them, it must reside not in the spinal cord but in the brain. Supraspinal inflammation and pain sensitization have been far less thoroughly investigated. Later, we discuss the available evidence that supraspinal inflammation is responsible for the transition from sickness to depression and acute to chronic pain.
IV. Brain Inflammation Is Associated with Pain and Depression
A. Depression.
So far we have primarily discussed studies that have demonstrated the relationship between peripheral inflammation and the transition from sickness to depression. However, in order for these behavioral, cognitive, and mood changes to take place, immune signaling in the brain is required. Although it is now apparent that inflammatory mediators are critical in signaling the brain to produce the behavioral sequelae typical of sickness in response to inflammation such as fatigue, motivational loss, negative affect, reduced reward and anhedonia, lack of hunger, isolation, pain sensitivity, and so on, this was not always the case. Prior to the cloning of recombinant cytokines in the early 1980s, the brain was considered immune privileged, and thus, inflammatory mediators were considered unable to act in the brain to regulate mood and behavior. This view was held despite the already well known effects of endogenous pyrogens on brain thermoregulation areas (Blatteis et al., 2000). However, in 1989, Dantzer and Kelley (1989) proposed that the development of sickness behaviors during infection is a direct result of cytokines acting in the brain to regulate behavioral responses to infection. It was later confirmed that the administration of cytokines directly to the brain alone can induce sickness behaviors in rodents, including reduced locomotor activity, increased sleep, decreased social exploration, and reduced food and water consumption, with particular involvement of IL-1β and TNF-α (Kent et al., 1992a,b,c; Bluthé et al., 2000; Palin et al., 2009). Similarly, intracerebroventricular injections of LPS or proinflammatory cytokines such as TNF-α and IL-1β are sufficient to produce depressive-like behaviors (Connor et al., 1998; Palin et al., 2008; O’Connor et al., 2009a,b,c; Fu et al., 2010), and intracerebroventricular administration of cytokine antagonists (Kent et al., 1992a,b,c; Bluthé et al., 2000) is able to abrogate sickness and depressive-like behaviors that typically follow systemic inflammation.
B. Pain.
There is also evidence that inflammation in the brain may contribute to pain sensitization and chronification. In regards to neuropathic pain, human studies investigating cytokine profiles in the cerebrospinal fluid (CSF) have indicated that it may be the balance between proinflammatory and anti-inflammatory cytokine profiles here that is important. Backonja et al. (2008) found that CSF soluble tumor necrosis factor receptor and IL-1β were positively correlated with pain intensity in patients with distal painful nondiabetic polyneuropathy or post-traumatic neuralgia, whereas IL-10 was inversely correlated with pain severity. Other studies have reported the severity of neuropathic pain in patients with noninflammatory polyneuropathy and complex regional pain syndrome to be positively correlated with serum and CSF concentrations of proinflammatory cytokines (Alexander et al., 2005; Ludwig et al., 2008).
These findings are in line with animal studies that have also yielded data demonstrating activation of brain cytokine signaling pathways and enhanced pain sensitivity in models of neuropathic pain. For instance, elevated IL-1β and activation of caspase 1, caspase 2, and caspase 8 in the brainstem, thalamus/striatum, and orbitofrontal and prefrontal cortex following spinal nerve injury (SNI) have been reported (Apkarian et al., 2006; Fuccio et al., 2009; Norman et al., 2010a). Furthermore, supraspinal IL-10 has likewise been implicated in preclinical models of neuropathic pain. Spinal nerve injury to rats increases mechanical allodynia and thermal hyperalgesia with concomitant increases in brain NF-κB, IL-1β, and TNF-α—all of which were attenuated in the presence of the anti-inflammatory glucocorticoid betamethasone (Xie et al., 2006). Interestingly, betamethasone not only reduced these proinflammatory cytokines but also induced the expression of brain IL-10.
Numerous studies have also connected brain cytokine concentrations with models of inflammatory pain. As intrathecal injections can spill over into the DRG, these in vivo approaches rely on the injection of cytokines or other inflammatory agents directly into the brain. The results of these studies unequivocally show a role of brain IL-1β and TNF-α in nociception. For instance, intracerebroventricular injection of recombinant human IL-1β and recombinant bovine TNF-α in rats mimicked the elevated and delayed abdominal response to rectal distension seen in response to systemic LPS (Coelho et al., 2000). Moreover, intracerebroventricular injection of IL-1β and TNF-α antagonists attenuated this systemic LPS-induced inflammatory response, suggesting that it is the central action of these proinflammatory cytokines that is critical for inflammatory pain processing. An elegant study recently demonstrated that stereotaxically injected TNF-α nanoplasmidexes restricted to the CA1 region of the hippocampus was sufficient to increase thermal hypersensitivity in rats for up to 3 weeks, and increase mechanical allodynia between day 12 and day 21 postinjection (Martuscello et al., 2012). Other studies have similarly implicated central TNF-α to be instrumental in arthritic pain sensation (Hess et al., 2011).
These studies are certainly convincing, but they still use outcome measures of reflexive pain, much of which is governed in the spinal cord. This issue complicates our current agenda, which is to examine the intersection between pain and depression. One way to avoid this issue is to focus on affective pain rather than reflexive pain. So far, we have focused on the sensory component of pain. However, pain also comprises an aversive, motivational, or emotional component that relies on attention, expectation, and appraisal of the sensory element within a given context. It is the affective component of pain that is likely to be monitored in the clinic, where patients are asked to rate the intensity of their pain, which is discrepant to preclinical models of reflexive pain that focus on targeting changes in response thresholds. Affective pain must be governed by supraspinal mechanisms given that it involves an emotional response and cognitive actions of attention, expectation, and appraisal. It is here that the behavioral, mood, and cognitive symptoms of depression and pain begin to merge, and we can begin to unmask the potential commonalities in the pathogenesis of these conditions. A handful of studies have investigated the activation of inflammatory signaling pathways in brain regions responsible for the affective component of pain. In regards to neuropathic pain, Knerlich-Lukoschus and colleagues (2011) observed increased cannabinoid receptor type-1 coexpression with chemokines CCL2, CCL3, and CCR2 in the hippocampus; coexpression of cannabinoid receptor type-1 with CCR1 and CCR2 in the thalamus; and elevated CCL3 in the periaqueductal gray—areas associated with affective pain—in response to spinal cord injury in rats. Notably, chemokine upregulation was observed only in the late-phase response and not the acute-phase response, suggestive of the involvement of chemokines in pain chronification.
An emerging methodology to measure affective pain in rodents is to use their motivational drive to escape pain. To do this, researchers have used operant conditioning such as conditioned place avoidance, where a rodent will avoid a chamber or environment that it has previously paired with the experience of pain, or conditioned place preference, where a rodent will seek out a chamber or environment that it has paired with the relief of pain (reviewed in Navratilova et al., 2013). Although using this paradigm to study affective pain is, for the most part, relatively new, some studies have investigated inflammatory pain and found an enhanced conditioned place preference for morphine and low-dose NMDA receptor antagonist MK-801 in rats injected with CFA in their hind paws (Sufka, 1994). A similar study, however, observed that morphine-, cocaine-, and methamphetamine-induced place preference place preference was attenuated in the presence of inflammatory nociception by administration of formalin and carrageenan to the hind paw (Suzuki et al., 1996). The place preference and drug administration timelines differed slightly between these experiments, highlighting the importance of the distance, timing, and saliency of the conditioning stimuli. A more recent study has directly measured supraspinal proinflammatory cytokine expression in response to pain-related conditioned place avoidance. Lu et al. (2011) found elevated IL-1β and TNF-α mRNA and protein in the anterior cingulate cortex, an area considered necessary for the affective component of pain (Johansen et al., 2001), in mice exhibiting formalin-induced conditioned place avoidance compared with controls.
V. Possible Molecular Mechanisms
We have explained that chronic pain and depression co-occur with high prevalence in the clinic, and we have presented evidence showing peripheral and central inflammation to be associated with both conditions. In this final section, we will discuss a number of putative candidate mechanisms that may serve as common pathways to both depression and chronic pain. We will discuss only those pertaining to the context of inflammation in an attempt to propose a model(s) to account for the clustering of depression and chronic pain in somatic illness. The majority of the mechanisms described here, with exception to GRK2, which plays a role in regulating the intensity and duration of the inflammatory response, occur downstream of inflammation. Thus, inflammation can be seen as the overarching mechanism that triggers the molecular pathways that follow and have been linked to pain and depression. Table 1 summarizes the known implications of each of these mechanisms for pain and depression.
TABLE 1.
The neuroimmune mechanisms of depression and pain
The potential mediators discussed in this review for the transition from sickness to depression and acute to chronic pain moving from upstream to downstream of the inflammatory cascade. A summary of the evidence associated with these mediators in relation to depression and pain is provided along with key references for each section.
| Depression | Pain | Key References | |
|---|---|---|---|
| GRK2 | Modulates intensity and duration of inflammation. | Modulates intensity and duration of inflammation and thus the sensitization of nociceptors by downstream mediators. | Eijkelkamp et al., 2010a,b; |
| Garcia-Sevilla et al., 2010; | |||
| Kleibeuker et al., 2008; | |||
| The majority of the few clinical studies on MDD have associated low GRK2 with treatment-resistant depression. | Chronic inflammation in patients and mice associated with reduced GRK2 in PBMCs. | Matuzany-Ruban et al., 2010; | |
| Vroon et al., 2005; | |||
| Role of GRK2 in inflammation-induced depression is yet to be confirmed. | Low GRK2 has been associated with the chronification of nociception. | Wang et al., 2011; | |
| Willemen at al., 2010 | |||
| Proinflammatory cytokines | Increased levels of IL-1β, TNF-α, IL-6, and IFN-α associated with depression. | Increased proinflammatory cytokines directly and indirectly promote sensitization of nociceptors and elevate pain sensitivity. | Cui et al., 2000; |
| Harrison et al., 2009.; | |||
| Kleibeuker et al., 2008; | |||
| Activates downstream pathways known to induce inflammation-induced depression such as IDO. | Most evidence for IL-1β and TNF-α. | Milligan et al., 2003; | |
| May reduce KCC2 cotransporter activity. | IL-1β necessary for downstream production of GRK2. | Opree and Kress, 2000; | |
| Reichenberg et al., 2001; | |||
| Strike et al., 2004 | |||
| Anti-inflammatory cytokines | May lead to reduced depressive symptoms likely due to the reduction in inflammation and thus reduced downstream activation of depression-inducing pathways such as IDO. | Reduce inflammation and thus the sensitization of nociceptors. | Backonja et al., 2008; |
| Miller et al., 2002; | |||
| Increasing IL-10 shown to have beneficial effects for nociceptive pain. | Milligan et al., 2005, 2006; | ||
| Shoskes et al., 2002; | |||
| Uceyler et al., 2006 | |||
| IDO | Favors NMDA receptor-mediated glutamatergic neurotransmission via quinolinic acid production in microglia and macrophages under conditions of inflammation. | Role of IDO in increasing nociception during inflammation has been supported in one paper but needs confirmation. | Capuron et al., 2002, 2003; |
| Kim et al., 2012; | |||
| Maes et al., 1991a,b, 1993; | |||
| Associated with inflammation-induced depression. | Could lead to increased NMDA receptor neurotransmission during inflammation, which has been associated with nociception. | O’Connor et al., 2009a,b,c; | |
| Raison et al., 2010; | |||
| Walker et al., 2013 | |||
| Glutamate | Excessive levels associated with increased depression. | Excessive levels associated with pain. | Autry et al., 2011; |
| AMPA receptor-mediated transmission shown to have antidepressant effects. | NMDA, AMPA, and kainite-mediated transmission shown to be associated with pain. | Garcia et al., 2008, 2009; | |
| Mitani et al., 2006; | |||
| Nie and Weng 2009; | |||
| NMDA-mediated transmission shown to have depression-inducing effects. | Upregulation of glutamate transporter shown to be associated with chronification of pain. | Niesters et al., 2013; | |
| NMDA antagonism reduces depression. | Walker et al., 2013; | ||
| Zarate et al., 2006 | |||
| GABA | Associated with anxiety and depression. | Spinal GABAergic disinhibition associated with increased pain. | Huang et al., 2012; |
| Enhancement of GABAergic neurotransmissions by benzodiazepines reduces anxiety and depression. | Intrathecal administration of benzodiazepines can reverse inflammation-induced and neuropathic nociception in rodent models. | Kahle et al., 2008; | |
| Role of GABA in inflammation-induced depression still to be elucidated. | Knabl et al., 2008; | ||
| Li et al., 2010; | |||
| Lin et al., 1994; | |||
| Cation chloride transporters NKCC1 and KCC2 can switch to immature state and excitation of GABA neurons during pathology. | Chloride cation cotransport of GABA neurons by NKCC1 and KCC2 associated with inflammatory and neuropathic pain. | Malan et al., 2002; | |
| Matrisciano et al., 2010; | |||
| Oraifo and Omogbai, 2012; | |||
| Polgár et al., 2003; | |||
| The role of NKCC1 and KCC2 in inflammation-induced depression requires confirmation. | Evidence to indicate a reduction of KCC2 expression and increase in NKCC1 expression. | Rees et al., 1995; | |
| Sluka and Westlund, 1993; | |||
| Sluka et al., 1993 |
A. Modulation of Cytokine Signaling Pathways by G Protein–Coupled Receptor Kinase 2
GRKs represent an important homeostatic regulator of GPCRs, bringing about appropriate desensitization to GPCR agonists, and thus acting as a defense mechanism against acute and chronic hyperstimulation (Vroon et al., 2006). In addition to their direct effects on GPCR signaling, GRKs can also control signal transduction by regulating the activity of signaling proteins such as extracellular signal-regulated kinase 1/2 and p38 MAPK. Of interest in our discussion of the mechanisms underlying depression and chronic pain symptom clustering is the ubiquitously expressed GRK2. GRKs regulate GPCR activity via phosphorylation, and in this manner, GRK2 plays a particularly influential role in the response to inflammatory stimuli.
Research investigating the role of GRK2 in depression is scanty. The majority of studies have focused on the change in GRK2 levels following SSRIs and selective norepinephrine reuptake inhibitors in patient populations of major depressive disorder. Unfortunately, the data remain inconclusive. An early study reported GRK2 protein in the prefrontal cortex of suicidal and nonsuicidal patients with depression to be significantly higher in the absence of antidepressant treatment (Grange-Midroit et al., 2003). The same study showed that those patients who were medicated at the time of death exhibited normalized GRK2 protein levels, suggesting that high GRK2 levels may contribute to the pathogenesis of depression. Conversely, other studies have suggested that low GRK2 may be responsible for the onset and maintenance of depression. For instance, protein and mRNA levels of GRK2 in the mononuclear leukocytes of untreated MDD patients were significantly lower than those of healthy controls. Antidepressant treatment reversed this effect and increased GRK2 levels (Matuzany-Ruban et al., 2010). This effect was similarly reported for platelet GRK2 protein levels (Garcia-Sevilla et al., 2010). Notably, this study also found that nonresponders to antidepressant therapies had significantly lower GRK2 levels compared with responders. Such contradictory findings provide more questions than answers. First, we do not know the exact nature in which GRK2 may contribute to depression, meaning more research is required. Second, peripheral GRK2 may not be reflective of central GRK2 function and cannot be used as a litmus marker for GRK2 in the brain or depression susceptibility. Finally, to our knowledge, no studies have examined GRK2 expression in the context of inflammation-induced depression, which may work independently, to our understanding of the system, in patients with MDD alone. It is likely that GRK2 plays a role in inflammation-induced depression given that GRK2 has been shown to be a potent regulator of the intensity and duration of the inflammatory response, especially in regards to chemokine activity (Vroon et al., 2006). Thus, one would expect that GRK2 activity that regulates inflammation should impact both the depressive and pain-related symptoms of inflammation.
The case is quite different when it comes to pain, however. In recent years, GRK2 has yielded much interest in regards to the pathogenesis of chronic pain. This began with observations that spinal nerve transection decreases spinal GRK2 expression and chronic carageenan-induced hyperalgesia reduces GRK2 levels in the DRG (Kleibeuker et al., 2008; Eijkelkamp et al., 2010b). A number of preclinical studies have demonstrated that low levels of GRK2 prolong mechanical and thermal hyperalgesia in response to a range of stimuli, including epinephrine, and a number of inflammatory mediators, including carageenan, IL-1β, and prostaglandin E2 (Eijkelkamp et al., 2010a,b; Willemen at al., 2010; Wang et al., 2011). Whereas the duration of pain sensitivity is increased under conditions of low GRK2, the acute peak of pain sensitivity does not differ between mice with low or normal levels of GRK2, indicating that GRK2 plays a unique role precisely in the transition from acute to chronic pain. In fact, a 50% knockdown of GRK2 is sufficient (and the effect is equal to that of full deletion) to prolong hyperalgesia up to 21 days in models of inflammatory pain in which wild-type mice appear to recover within 3–4 days (Willemen et al., 2010; Wang et al., 2011).
It also appears that a deletion of GRK2 in specific cells is responsible for prolonged hyperalgesia. For instance, GRK2 depletion in astrocytes does not yield any changes in the severity or duration of the hyperalgesic response to inflammatory or other mediators measured by thermal or mechanical stimulation (Willemen et al., 2010; Wang et al., 2011). GRK2 reduction in nociceptors appears to be able to prolong or increase the hyperalgesic response, depending on the stimulant (Eijkelkamp et al., 2010a,b; Wang et al., 2011). However, it seems that low GRK2 in lysM-positive myeloid cells is necessary to be able to produce the maximal extension of pain duration (Wang et al., 2011). This has been demonstrated in studies in which knockdown of GRK2 in activated microglia/macrophages extends thermal and mechanical hyperalgesia in response to epinephrine and carrageenan (Eijkelkamp et al., 2010a; Wang et al., 2011). Furthermore, this effect is rescued in the presence of the microglial/macrophage inhibitor minocycline (Eijkelkamp et al., 2010a). Finally, this effect cannot be accounted for simply via genotype-specific differences in the peripheral inflammatory response, as GRK2 knockdown mice exhibited carageenan-induced elevations in paw thickness, paw IL-1β, and CCL3 in equal measure to wild-type mice.
Of particular relevance to the clinic is the evidence showing that chronic inflammation in patients coincides with reduced GRK2 levels in peripheral blood mononuclear cells (PBMCs) and increased pain. Lombardi et al. (1999) found reduced GRK activity and reduced GRK2 protein in the leukocytes of rheumatoid arthritis patients compared with healthy controls. Similarly, Vroon and colleagues (2005) investigated patients with relapsing-remitting multiple sclerosis suffering from acute exacerbation of the disease. These patients exhibited approximately 40% lower GRK2 in PBMCs compared with healthy age-matched controls. Furthermore, in a murine model of multiple sclerosis (experimental autoimmune encephalomyelitis), these authors observed advanced onset of the disease and greater relapse rates in GRK2 knockdown mice compared with wild-type controls (Vroon et al., 2005). These data indicate that chronic inflammation may result in reduced PBMC GRK2 levels. In turn, this reduction in GRK2 can impair the regulation of inflammation by GRK2 and potentially lead to the transition from acute to chronic pain in patients.
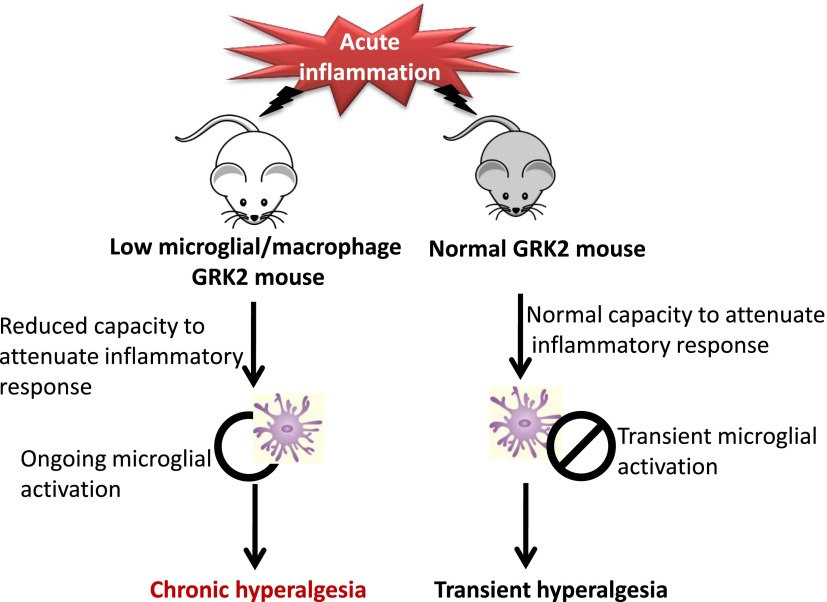
The precise mechanistic pathway through which inflammation gives rise to low levels of GRK2 and thus the inability to efficiently switch off the pain response is still being elucidated. However, it is understood that IL-1β is necessary for the downstream reduction of GRK2 and development of mechanical allodynia in a model of L5 spinal nerve transection given that genetic deletion of the biologically active IL-1 receptor abrogates the development of mechanical allodynia and does not alter GRK2 expression (Kleibeuker et al., 2008). These findings indicate early cytokine signaling to be a requirement for the cascade of effects that lead to low GRK2 and prolonged pain in response to nerve injury. It is also known that mitogen-activated kinase kinase contributes to the prolonged response given that inhibition of mitogen-activated kinase kinase abrogates the prolonged hyperalgesic effects observed in GRK2 knockdown mice, and protein kinase Cε (activated by Epac) inhibition attenuates the duration of hyperalgesia in these mice as well (Eijkelkamp et al., 2010b). Thus, GRK2 appears to be a potent responder to as well as a regulator of inflammation, representing a probable mediator in the transition from acute to chronic pain and a possible contributor to inflammation-induced depression. Figure 4 shows the proposed transition from acute to chronic pain observed in GRK2-deficient mice in models of inflammatory pain.
Fig. 4.
The transition from acute to chronic pain in GRK2-deficient mice. Acute inflammation in mice with normal GRK2 levels results in the typical response and duration of microglial activation, with the ability to readily and quickly attenuate the inflammatory response. Thus, these mice experience hyperalgesia transiently. In mice with low microglial/macrophage GRK2 levels, the response and duration of microglial activation is extended, decreasing the expediency of attenuating the inflammatory response. Thus, these mice experience longer durations of hyperalgesia. Note that low GRK2 mice have not yet been tested in models of neuropathic pain. However, it is known that GRK2 is reduced in microglia in models of neuropathic pain.
B. Cytokines and Cytokine Signaling Pathways
In this section, it is important to focus on some of the fundamental issues involved in the inflammation and cytokine hypothesis. We have already demonstrated in detail the evidence linking proinflammatory and even anti-inflammatory cytokines to depression and to pain in our discussion on peripheral and central inflammation for both conditions. Here, it may be more prudent to evaluate the assumptions we are making to be able to propose a common mechanism stemming from elevated cytokine release. Namely, we must consider that, if inflammation is the source of both depression and chronic pain, the location of cytokine action that leads to these conditions must be the same. That is, even if the source of inflammation is peripheral, propagation of the cytokine signal to the brain is required for a common mechanism to be responsible for the transition from sickness to depression and to chronic pain. This is in line with the argument that the affective component of pain is most troublesome for patients. However, an alternative explanation could be that spinal inflammation is responsible for pain, whereas supraspinal inflammation is responsible for depression. The assumption we are making in this review is that the action of the cytokines that leads to, or in the very least contributes to, both depression and pain occurs supraspinally. Few studies have directly attempted to link cytokine profiles to both pain and depression together. One such example is a study by Ballok and Sakic (2008) who investigated anxiety-like and depressive-like behaviors, which are governed by the brain, in lupus-prone mice. These mice exhibited increased neophobia in the novel object test and anhedonia in the sucrose preference test, which were abrogated in the presence of the P2 receptor antagonist suramin. P2X receptors are involved in nociceptor activation, providing the link between depression and pain. Serum concentrations of IL-1β and TNF-α did not differ among groups, but the authors failed to measure central cytokine levels, nor did they investigate any supraspinal effects of suramin despite observed changes in depressive-like behavior. An interesting study by Norman et al. (2010b) looked at induction of a depressive-like state in a mouse model of neuropathic pain (SNI-induced mechanical allodynia). Social isolation both significantly increased immobility time in the FST and reduced the reflexive threshold in the Von Frey test in mice exposed to SNI. These mice also had significantly greater IL-1β mRNA expression in the prefrontal cortex. These depressive-like effects of SNI were reversed by a daily regimen of oxytocin only in mice that underwent both SNI and social isolation. This combined effect of social isolation and SNI suggests a common cytokine-driven pathway for depression and pain in which one condition feeds the other.
C. Indoleamine 2,3-Dioxygenase, Glutamate, and GABA
1. Indoleamine 2,3-Dioxygenase.
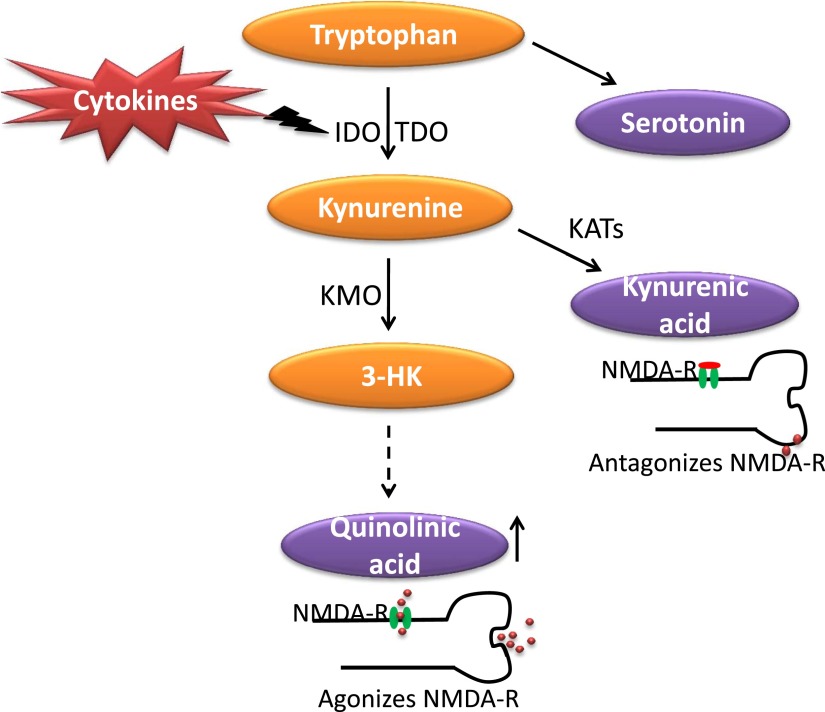
Tryptophan, the biochemical precursor and rate-limiting substrate for the synthesis of serotonin, is used for general protein synthesis, serotonin synthesis, or catabolized via the kynurenine pathway to produce biologically active metabolites by the liver enzyme tryptophan 2,3,-dioxygenase (TDO). However, tryptophan and other indoleamines are also catabolized by an immune-activated enzyme that is ubiquitously distributed in the body, including the brain. This enzyme is known as indoleamine 2,3-dioxygenase (IDO). Its main inducers are IFNγ and TNF-α. Reductions in circulating tryptophan in depressed patients have been published for a long time (Cowen et al., 1989; Maes et al., 1991a,b, 1993; Quintana, 1992), with the hypothesis that they are associated with concomitant decreases in serotonin. Thus, many have argued that reduced tryptophan availability simply results in reduced synthesis of serotonin and, in turn, depression (Moore et al., 2000; Van der Does, 2001). However, evidence indicates that, during inflammation, it is the metabolism of tryptophan down other metabolic pathways of the kynurenine system that results in the generation of neurotoxic metabolites that trigger the onset of depressive symptoms. Tryptophan metabolism involves several enzymes, including 1) tyrosine hydroxylase, which drives the synthesis of serotonin; 2) IDO; and 3) TDO, which result in the synthesis of kynurenine. It is the latter two enzymes that are believed to play an important cell-specific role in the pathogenesis of inflammation-induced depression. IDO and TDO metabolize tryptophan into kynurenine, which can be further metabolized into biologically active metabolites. Kynurenine aminotransferases can metabolize kynurenine into the neuroprotective NMDA antagonist kyunurenic acid, which occurs primarily in astrocytes, brain endothelial cells, and neurons (Guillemin et al., 2005; Kwidzinski and Bechmann, 2007). In microglia and macrophages, however, there is a bias for metabolized kynurenine to be shunted down the pathways, which results in the formation of the neurotoxic NMDA receptor agonist quinolinic acid (Heyes et al., 1996; Guillemin et al., 2005). A diagrammatic representation of the kynurenine pathway in response to inflammation is provided in Fig. 5.
Fig. 5.
Kynurenine pathway of tryptophan metabolism in response to inflammation. Tryptophan is metabolized intracellularly by IDO and TDO to produce kynurenine. In response to inflammation, there is an increase in the metabolism of kynurenine down the kynurenine monooxygenase (KMO) pathways, which results in increased production of quinolinic acid, which agonizes NMDA receptors (NMDA-R). 3-HK, 3-hydroxy kynurenine; KATs, kynurenine aminotransferases.
Clinical studies support the association between depressive symptoms in patients with somatic illness and IDO activation. In many of these studies, the trigger of IDO activation is represented by administration of IFN-α to cancer and hepatitis C patients. IDO activity is measured indirectly by the ratio of kynurenine over tryptophan. Consistently, plasma kynurenine-to-tryptophan ratios are increased in IFN-α–treated patients, which correlate with inflammatory markers and are predictive of depression severity (Capuron et al., 2002, 2003; Wichers et al., 2005; Raison et al., 2010). Although IDO activation is usually measured at the periphery, it can be observed when tryptophan, kynurenine, and kynurenine metabolites are measured in the CSF (Raison et al., 2010). These findings indicate some degree of overlap between IDO activity in the brain and IDO activity in the periphery, although their relationship has not been studied specifically.
This hypothesis that activation of IDO increases the risk of transition from sickness to depression has been confirmed with preclinical models. Acute immune stimulation by LPS or chronic immune stimulation by inoculation of an attenuated form of Mycobacterium bovis, Bacillus Calmette-Guerin, increases peripheral and brain IDO activity, reduces tryptophan concentrations, and increases the formation of kynurenine and kynurenine metabolites, which results in depressive-like behavior (O'Connor et al., 2009b). Moreover pharmacological or genetic deletion of IDO prevents the development of depressive-like behaviors without altering sickness (O’Connor et al., 2009b). The importance of the cytokine cascade in activating microglial IDO has been underscored by the fact that genetic deletion of IFNγR or pharmaceutical blockade of TNF-α attenuates the depressive-like behavioral response in these models (O’Connor et al., 2009a). Finally, it is unlikely that these effects are a result of reduced tryptophan and thus lower serotonin turnover, as these mice display increased central kynurenine levels without central loss of tryptophan, and serotonin turnover actually increases (O’Connor et al., 2009c; Walker et al., 2013).
It is likely that TDO also plays a similar role; however, few studies have examined its involvement in depression. One study has indicated that TDO activity may actually produce behavioral outcomes more akin to anxiety than depression (Funakoshi et al., 2011). Regardless, the data are quite clear that, within the context of inflammation, tryptophan metabolism appears to be shunted down the kynurenine pathway, resulting in the formation of neurotoxic kynurenine metabolites that induce depressive behaviors by agonizing NMDA receptors.
Although there is converging evidence for a role of IDO activation in the development of inflammation-induced depression, the involvement of this mechanism in the transition from acute to chronic pain has received much less attention. Only one published study to our knowledge has attempted a thorough investigation into the kynurenine pathway in relation to pain. Importantly, this study also reported comorbidity of pain with depression. Kim et al. (2012) injected CFA into the tibiotarsal joint cavity of rodents to induce inflammatory arthritis, which was associated with mechanical and thermal allodynia as well as depressive-like behaviors in the FST and open field test. These behavioral outcomes were associated with elevated bilateral hippocampal IDO mRNA and protein, as well as elevated kynurenine/tryptophan ratios and IL-6 concentrations. These findings were supported by clinical studies in which individuals with comorbid depression and pain also demonstrated increased plasma kynurenine/tryptophan ratios. Pharmacological blockade and genetic deletion of IDO were claimed to significantly attenuate both nociceptive and depressive-like behaviors. Although these findings are promising in regards to implicating IDO in both depression and pain, great caution must be taken in the interpretation of these findings, which are therefore in dire need of confirmation. The clinical data, for instance, only compare healthy controls with patients suffering from pain and depression clustering, whereas there are no controls for each disorder individually, making any conclusion about the relative role of kynurenine in depression or pain or both virtually impossible. There are also a number of peculiar findings and methodologies, such as the support of enzymatic activity with protein changes and colocalization of IDO in hippocampal astrocytes, microglia, and neurons, which are not fully supported by the published data set. Hence, although it is promising for a common pathway for pain and depression to arise via IDO metabolism of tryptophan, more research is required to confirm this.
2. Glutamate.
Inflammation-induced increases in metabolism of tryptophan by IDO ultimately result in the NMDA receptor agonist quinolinic acid. Glutamate and its receptor subtypes, NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), have been clearly implicated in the pathogenesis of both depression and chronic pain. In regards to depression, a great deal of work in recent years has demonstrated that excessive glutamatergic activity can lead to increased depressive symptoms. Mitani and colleagues (2006) observed a positive correlation of plasma glutamate, glutamine, glycine, and taurine with Hamilton Depressive Rating Scale (HDRS) scores in depressed patients, as have others (Kim et al., 1982). Preclinical transgenic mouse models have supported these findings by showing that deletion of the AMPA receptor subunit (GluA1) resulted in increased learned helplessness in a shuttle box paradigm and associated decreases in serotonin. These changes were associated with altered glutamatergic function and increased NMDA receptor expression (Chourbaji et al., 2008). There is even evidence to suggest that traditional antidepressant drugs can also lead to reduced glutamatergic outflow and, via this mechanism, decrease depressive symptomatology (Michael-Titus et al., 2000). This work has led to recent investigations into the efficacy of drugs that more directly target glutamatergic neurotransmission as a potential treatment of depression. Clinical trials with ketamine, an NMDA receptor antagonist used commonly as a dissociative anesthesia-inducing agent, have yielded promising results. Subanesthetic doses of ketamine produce rapid, prolonged antidepressant responses in patients. An early study by Berman and colleagues (2000) used a placebo-controlled, double-blind design looking at the effects of a single intravenous infusion of 0.5 mg/kg ketamine hydrochloride in patients presenting with clinical depression. Scores on the 21-item HDRS indicated alleviation of depressive symptoms within 72 hours of ketamine administration, but not for placebo control patients. A follow-up study conducted by Zarate et al. (2006) examined the effects of the same dose of ketamine administered on two days, a week apart, on treatment-resistant patients with diagnosed major depression. Acute acting and prolonged antidepressant effects were observed for ketamine-treated patients on the HDRS, Beck Depression Inventory, and on visual analog scale depression scores.
Animal models have also demonstrated antidepressant-like effects following ketamine administration. Administration of subanesthetic doses of ketamine to naive rats and mice induces antidepressant-like effects such as reduced immobility in the FST without any apparent changes in locomotor activity (LMA), as examined in novel cages and open field apparatuses (Garcia et al., 2008; Autry et al., 2011; Reus et al., 2011). Reduction of depressive-like behaviors following ketamine administration has also been reported in animal models of stress-induced depressive-like behavior. Autry et al. (2011) demonstrated that C57BL/6 mice treated with ketamine exhibit reduced immobility in the FST, increased sucrose preference, and reduced latency to feed compared with controls following 28 days of chronic mild stress. Similarly, Wistar rats exposed to 40 days of chronic mild stress do not exhibit the expected reduction in sweet food consumption when treated with ketamine (Garcia et al., 2009). Pertinent to this discussion, we have recently published data demonstrating that ketamine treatment can abrogate the onset of LPS-induced depressive-like behaviors in the FST and sucrose preference tests. This effect was specific to ketamine’s NMDA antagonist properties, as it did not alter cytokine and sickness responses and could be reversed by treatment with the AMPA receptor antagonist 3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione (NBQX) (Walker et al., 2013). Thus, the role of NMDA agonism and glutamatergic activity in depression is becoming increasingly apparent.
Glutamate is also a key player in pain sensation. It is the major neurotransmitter of primary nociceptive afferents activating AMPA and NMDA receptors on secondary pain transmission neurons in the spinal cord response to noxious stimuli. Multiple pain models have demonstrated that glutamate is a necessary contributor to pain hypersensitivity and chronic pain. NMDA and AMPA receptor activation in the peripheral nervous system has been strongly implicated in nociception. For example, activation of NMDA, AMPA, and kainate receptors in the hindpaw of rats has been shown to produce mechanical allodynia and hyperalgesia, which were attenuated following administration of their appropriate antagonists (Zhou et al., 1996). Numerous studies have reported similar effects, such as intraplantar injection of glutamate resulting in decreased mechanical paw withdrawal thresholds in rats via activation of peripheral NMDA, kainate, and AMPA receptors (Jackson et al., 1995). Notably, temporomandibular joint injury models in rats have demonstrated that local administration of glutamate, NMDA, and AMPA activates jaw muscles evidenced by increased electromyographic activity (Cairns et al., 1998). Furthermore, these effects were attenuated under NMDA antagonism. Appropriate to the topic of inflammation, this group also demonstrated almost identical findings with the administration of the inflammatory irritant mustard oil to the temporomandibular joint, which was attenuated in the presence of the NMDA receptor antagonist MK-801.
Brain glutamate is also an important contributor to pain sensitivity. NMDA receptor activation in the dorsal horn is connected to most types of pathological inflammation-induced pain (Ren and Dubner, 2007). Pharmacological blockade of glutamate transporters in the dorsal horn increases extracellular glutamate in the spine and results in mechanical and thermal hyperalgesia (Liaw et al., 2005). Similarly, glutamate transporter blockade increases the number and duration of NMDA receptors activated by stimuli to the primary nociceptive afferents (Nie and Weng, 2009), and ligation of spinal nerves L5–L6 in rats results in 70–90% reductions in glutamate uptake in the ipsilateral deep dorsal and ventral horns (Binns et al., 2005). Glutamatergic activity in the CNS as opposed to only in the peripheral nervous system is required to study pain and depression comorbidity, as the brain is the primary regulator of depressive behavior, and thus common mechanisms to account for pain and depression should be localized here.
Finally, it appears that several components of the glutamatergic system are important for pain sensitivity and chronification. Sung and colleagues (2003) found that CCI in rats results in an initial upregulation of the glutamate transporter following surgery, followed by a prolonged downregulation several days thereafter, which may indicate a role for glutamate in producing a transition from acute to chronic pain. Moreover, if the initial upregulation of glutamate transporters was pharmacologically blocked, then the rats displayed exacerbated thermal and mechanical allodynia. This could again be blocked by MK-801 after recovery from the treatment used to inhibit glutamate transporter upregulation.
That glutamate or NMDA receptor activation plays a role in depression and pain perception is clear. One particular study has suggested that it alters both disease states within the one model, whereby ketamine has been shown to reduce pain in a model of neuropathic pain. Blockade of the NMDA receptor by ketamine in a model of spared nerve injury in rats abrogates depressive-like behaviors associated with neuropathic pain, as measured in the FST and sucrose preference test (Wang et al., 2011). Indeed, multiple studies have implicated NMDA receptor blockade by ketamine in attenuating pain. A meta-analysis investigating the analgesic effects of ketamine in studies that tested outcome measures at 1 and 4 weeks post-treatment indicated mean effect sizes for improvement by ketamine of 1.22 and 0.39, respectively, although retreatment with ketamine was indicated to be required from 4 weeks onward (Sigtermans et al., 2009). Ketamine has also been shown to potentiate the efficacy of morphine (Bell et al., 2012). Other studies have yielded less convincing data, however. For example, Niesters et al. (2013) recently reported that low-dose ketamine was able to produce conditioned pain modulation responses in neuropathic pain subjects, but the magnitude of the response did not differ from placebo controls.
3. Loss of GABAergic Inhibition.
We have discussed the role of glutamatergic signaling following inflammation and how this can arise via IDO metabolism of tryptophan and generation of potentially neurotoxic kynurenine metabolites, thus increasing the propensity for depression and pain development. However, another mechanism through which glutamatergic activity can be promoted is in the breakdown of efficient inhibition of the actions of glutamate by GABA. GABAergic transmission is initially excitatory early in fetal development before switching to inhibition later in gestation. This is due primarily to the Na-K-Cl transporters NKCC1 and KCC2. In an immature state, the cotransporter NKCC1 is predominant and increases the internal chloride concentration. This in turn promotes a net efflux of chloride ions from the cell, resulting in depolarization. However, during development, KCC2 becomes the more predominant cotransporter, which removes chloride ions from the cell and promotes the driving force of chloride ions into the cell, resulting in hyperpolarization and inhibiting neuronal activity. Under conditions of pathology, NKCC1 and KCC2 ratios can revert back toward their immature state, thus increasing excitation of GABAergic transmission and reducing its inhibitory influence. This has been implicated in a range of neurological disorders, particularly seizures (Kahle et al., 2008; Huang et al., 2012), schizophrenia (Kalkman, 2011), and anxiety (Krystal et al., 2012). Notably, inflammation has been suggested to also enhance the reversion of these cotransporters to an immature state (Li et al., 2010).
In regards to inflammation-induced depression, there has been no research to our knowledge that has specifically tackled this area of interest. There is evidence that the loop diuretics furosemide and bumetanide, which antagonize the cation chloride cotransporter in GABA neurons, significantly reduce anxiety-related freezing of Long-Evans rats in contextual fear-conditioning paradigms and fear-potentiated startle (Krystal et al., 2012). However, these drugs had no impact on unconditioned anxiety-related behaviors on the elevated plus maze and open field. In regards to depression, furosemide and bumetanide drastically and significantly enhance the antidepressant effects of sertraline in rodents both acutely and chronically, as indicated by delayed onset and reduced duration of immobility in the FST (Oraifo and Omogbai, 2012). Finally, Matrisciano and colleagues (2010) investigated ataxic responses to diazepam in Flinders Sensitive Line rats considered to be a genetic model of unipolar depression. Their findings revealed these rats to be far more sensitive to the ataxic effects of low-dose diazepam compared with controls. Moreover, Flinders Sensitive Line rats exhibited elevated KCC2 expression in the brain, highlighting the relationship of these cotransporters with GABAergic function and behavioral output. These findings clearly implicate Na-K-Cl transport of GABA neurons in the pathogenesis of depression. It is likely that they also play an important role in the pathogenesis of inflammation-induced depression given the literature indicating that inflammation can disrupt the normal NKCC1-to-KCC2 ratio. This literature has primarily come from the area of inflammatory pain.
GABA has been suggested to play a major role in pain and its chronification. Much of this work has indicated that enhanced pain sensitivity may result from a loss of spinal GABAergic tone, especially in the dorsal horn. Intrathecal injection of GABAA and GABAB antagonists in rats results in mechanical allodynia and thermal hyperalgesia, whereas GABAA and GABAB agonists reverse spinal nerve ligation–induced mechanical allodynia and thermal hyperalgesia (Malan et al., 2002). Similarly, Knabl et al. (2008) investigated GABAA point-mutated knockin mice, in which GABAA receptor subtypes could be selectively desensitized to benzodiazepine-site ligands. It was found that, in both a model of inflammatory pain by subcutaneous injection of zymosan A into the hind paw and in a model of neuropathic pain using CCI, significant analgesia and reduced nociceptive signaling to the brain could be achieved by activation of GABAA receptors containing α2 and/or α3 subunits. Despite research demonstrating that abrogation of GABAergic inhibition may be sufficient to enhance pain sensitivity, there are studies that indicate that it may not be necessary. One such study was conducted by Polgár and colleagues (2003). They were unable to observe any differences in GABA or glycine immunoreactivity of laminae I–III between the ipsilateral and contralateral sides of the dorsal horn in rats that had undergone CCI.
GABAergic transmission plays a particularly important role in regulating peripheral inflammation-induced pain via dorsal root reflexes. Experimentally induced arthritis by the injection of carrageenan to the knee joint of rats results in a measurable increase in dorsal root reflexes in the medial articular nerve, which can be inhibited by administration of GABAA and non–NMDA receptor antagonists directly to the superficial dorsal horn. However, GABAB and NMDA receptor antagonists have no effect (Rees et al., 1995). Furthermore, blockade of non-NMDA excitatory amino acid and GABAA receptors also reduces peripheral inflammation (Sluka and Westlund, 1993; Sluka et al., 1993). It has been proposed that the increased antidromic excitation seen in this model of carrageenan-induced arthritis requires activation of dorsal horn GABA interneurons, which occurs via the action of excitatory amino acids on non-NMDA receptors (Rees et al., 1995; Willis, 1999). Curiously, GABAergic transmission in the dorsal horn is also important for periaqueductal gray descending inhibition of neuronal activity in the dorsal horn, as this too can be blocked by antagonism of GABAA receptors and glycine but is likely to produce this effect through interaction with serotoninergic and noradrenergic systems (Lin et al., 1994; Peng et al., 1996).
A great deal of work has also implicated chloride cation cotransport of GABA neurons in the pathogenesis of neuropathic and inflammatory pain. For instance, LPS administration to the tooth pulp of C57BL/6 mice results in more unilateral tongue protrusions toward the treated side compared with the control side of the mouth (Li et al., 2010). This indication of elevated sensitivity coincides with significantly reduced KCC2 mRNA and significantly increased NKCC1 mRNA in the ipsilateral side of the trigeminal subnucleus caudalis compared with the contralateral side. Similarly, formalin-induced orofacial pain in C57BL/6 mice has been linked to reductions in KCC2 mRNA in the ipsilateral, but not contralateral, side of the medullary dorsal horn. NKCC1 levels remain stable, however (Wu et al., 2009). Despite the lack of changes in NKCC1, it should be noted that the relative ratio of NKCC1 to KCC2 will have increased compared with the control tissue, thus affecting appropriate GABAergic inhibition. The downregulation of KCC2 takes place rapidly and is likely to be due to calpain-mediated cleavage of the protein (Puskarjov et al., 2012). Electrophysiological recordings demonstrate almost a complete loss of KCC2-mediated Cl- efflux, and suggest changes at the level of transcription found by others (Rivera et al., 2002) are responsible for long-lasting changes in KCC2 function. Thus, it is conceivable that acutely observed responses to inflammatory pain are driven translationally, whereas transcriptional influences drive the transition from acute to chronic pain. In addition to these proposed mechanisms of change in these cotransporters producing alterations in GABAergic inhibition of glutamate, NKCC1 may upregulate TRPV1, which plays a vital role in heat sensitivity.
Antagonism of NKCC1 or KCC2 has yielded findings supportive of their role in pain development. Injection of NKCC1 inhibitors such as bumetanide and furosemide reduces rapid eye movement sleep deprivation–induced mechanical sensitivity (Wei et al., 2010), decreases formalin-induced paw flinching in Wistar rats in both phases of the response (Granados-Soto et al., 2005), and increases withdrawal latency on the tail-flick and hot-plate tests in C57BL/6 mice under otherwise baseline conditions (Austin and Delpire, 2011). Importantly, intrathecal administration of a recently developed KCC2 antagonist, D4, decreases withdrawal latency of C57BL/6 mice on tail-flick and hot-plate tests. These pharmacological studies have implicated the relative activity of these cotransporters in the development of pain. However, an interesting anomaly comes from a recent transgenic model. Tornberg and colleagues (2005) behaviorally phenotyped KCC2 knockdown mice. Their analysis on the pain responsiveness of these mice revealed them to actually require greater force in the Von Frey test, which is contrary to expectations based on the pharmacological studies. Clearly the role of GABA and its cation cotransporters is in its infancy for both pain and depression. However, they represent an intriguing mechanism, which fits nicely with the already known glutamatergic mechanisms involved in both disease states.
VI. Conclusion
Depression is the most common psychological symptom and pain is the most common physical symptom reported in clinical settings. Often these symptoms co-occur and thus represent a common example of symptom clustering. Here we have focused on the notion that such a high degree of clustering between depression and chronic pain in individuals suffering from physical illness is no coincidence. We propose that there are common underlying mechanisms that may be responsible for such clustering. Inflammation is clearly the common denominator to both pain and depression, initiating the activation of several pathways that can trigger the transition from sickness to depression and from acute to chronic pain. Thus, the majority of potential mediators must occur downstream of inflammation. Although there are numerous known pathways through which inflammation can drive the onset of depression and pain, here we have chosen to propose a handful of relatively novel mechanisms we believe may contribute. The evidence thus far would suggest that inflammation-induced depression is primarily dependent on IDO activation, which is biased to metabolize tryptophan down the kynurenine pathway, resulting in excessive production of the NMDA receptor agonist quinolinic acid. This enhances glutamatergic neurotransmission through NMDA receptors. Currently, there are not enough data on the potential involvement of IDO in chronic pain to definitively conclude this as a mechanism for comorbid pain and depression. In the case of GRK2, the situation is just the opposite. There is extensive evidence to indicate that GRK2, which regulates the intensity and duration of inflammation, is a primary regulator of the transition to chronic pain. Given that GRK2 modulates inflammation, one would expect that the downstream consequences for the transition of inflammation-induced sickness to depression and acute to chronic pain should occur equally. However, once again the evidence currently stands for pain but requires greater investigation for depression. An additional mechanism proposed to contribute to the clustering of depression and chronic pain in clinical settings is decreased GABAergic inhibitory tone due to a return of NKCC1 and KCC2 cotransporter ratios to their immature state. Several papers have demonstrated this mechanism to contribute to chronic pain; however, only preliminary evidence and conjecture points toward this mechanism being promising for depression at this stage. We also mentioned the role of anti-inflammatory cytokines downstream of inflammation. There is clear evidence that IL-10 downregulates nociception and sickness, with some data also implicating its involvement in inflammation-induced depression. Clearly some of the proposed mechanisms outlined in this review are worth investigating further. It is for this reason that we believe this review should serve as both a summary of research in this particular field as well as a primer for the instigation of new research avenues—of which there is little research specifically looking at the potential common mechanisms regarding the comorbid relationship between inflammation-induced depression and chronic pain.
As an important final note, we have focused primarily on the convergence of inflammation-induced depression and pain in this review, as the vast majority of research into the comorbidity model of pain and depression has done an impressive job at showing the overlap between the conditions. However, if we are to fully elucidate the nature of the overlap of depression and pain and provide new and effective pharmaceutical targets to treat them, we also need to test the limits of the comorbidity model. That is, where exactly do inflammation-induced pain and depression diverge in their molecular and neuroanatomical etiologies? So far, this has been inadequately tested. As we have mentioned, many of the potential comorbid mechanisms described here show stronger evidence in support of their role in either depression or pain, but not both at this stage. This is exactly why we have focused on these mechanisms. These mechanisms represent likely, but not definite, examples of convergence between depression and pain. By understanding them more fully, and by testing their limits, we can more accurately understand their role in both the convergence of pain and depression as well as their divergence. This will ultimately reveal the specific intersection between pain and depression and inform appropriate development of new pharmaceutical tools.
Acknowledgments
The authors thank Jennifer Hsieh and Aiat Radwan for editorial assistance on this manuscript.
Abbreviations
- ASC stands for ; ASC
apoptosis-associated speck-like protein containing a CARD
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BBB
blood-brain barrier
- CCI
chronic constriction injury
- CFA
complete Freund’s adjuvant
- CGRP
calcitonin gene–related peptide
- CNS
central nervous system
- CSF
cerebrospinal fluid
- D4
Delpire 4
- DAMP
damage-associated molecular pattern
- DRG
dorsal root ganglion
- FST
forced swim test
- GPCR
G protein–coupled receptor
- GRK2
G protein–coupled receptor kinase 2
- HDRS
Hamilton Depressive Rating Scale
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MAPKs
mitogen-activated protein kinases
- MDD
major depressive disorder
- NBQX
3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione
- NF-κB
nuclear factor κB
- NMDA
N-methyl-d-aspartate
- NTS
nucleus of the tractus solitarius
- PAMP
pathogen-associated molecular pattern
- PBMCs
peripheral blood mononuclear cells
- SNI
spinal nerve injury
- SSRI
selective serotonin reuptake inhibitor
- TDO
tryptophan 2,3-dioxygenase
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRPV1
transient receptor potential cation chloride channel subfamily V member 1
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Walker, Kavelaars, Heijnen, Dantzer.
Footnotes
This work was supported by the University of Texas MD Anderson Cancer Center and National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS073939 and R01-NS074999]. The work of A.K. is also supported by a STARS [“Science and Technology Acquisition and Retention”] award of the University of Texas System.
R.D. is a consultant with Ironwood Pharma (Cambridge, MA).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. (2005) Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 116:213–219 [DOI] [PubMed] [Google Scholar]
- Anthony DC, Bolton SJ, Fearn S, Perry VH. (1997) Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain 120:435–444 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Lavarello S, Randolf A, Berra HH, Chialvo DR, Besedovsky HO, del Rey A. (2006) Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett 407:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragay AM, Ruiz-Gómez A, Penela P, Sarnago S, Elorza A, Jiménez-Sainz MC, Mayor F., Jr (1998) G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions. FEBS Lett 430:37–40 [DOI] [PubMed] [Google Scholar]
- Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C. (2006) Comorbid depression, chronic pain, and disability in primary care. Psychosom Med 68:262–268 [DOI] [PubMed] [Google Scholar]
- Austin TM, Delpire E. (2011) Inhibition of KCC2 in mouse spinal cord neurons leads to hypersensitivity to thermal stimulation. Anesth Analg 113:1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja MM, Coe CL, Muller DA, Schell K. (2008) Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol 195:157–163 [DOI] [PubMed] [Google Scholar]
- Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. (2008) Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med 70:890–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballok DA, Sakic B. (2008) Purine receptor antagonist modulates serology and affective behaviors in lupus-prone mice: evidence of autoimmune-induced pain? Brain Behav Immun 22:1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. (2005) Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 11:973–984 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. (1991) Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci 48:PL117–PL121 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Durham DA. (1989) Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull 23:433–437 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. (1993) Interleukin-1 alpha in blood has direct access to cortical brain cells. Neurosci Lett 163:41–44 [DOI] [PubMed] [Google Scholar]
- Banks WA, Ortiz L, Plotkin SA, Kastin AJ. (1991) Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther 259:988–996 [PubMed] [Google Scholar]
- Basterzi AD, Aydemir C, Kisa C, Aksaray C, Tuzer V, Yazici K, Goka E. (2005) IL-6 levels decrease with SSRI treatment in patients with major depression. Human Psychopharmacol 20:473–476 [DOI] [PubMed] [Google Scholar]
- Bell RF, Eccleston C, Kalso EA. (2012) Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev 11:CD003351. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107 [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354 [DOI] [PubMed] [Google Scholar]
- Bianchi ME. (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5 [DOI] [PubMed] [Google Scholar]
- Binns BC, Huang Y, Goettl VM, Hackshaw KV, Stephens RL., Jr (2005) Glutamate uptake is attenuated in spinal deep dorsal and ventral horn in the rat spinal nerve ligation model. Brain Res 1041:38–47 [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Sehic E, Li S. (2000) Pyrogen sensing and signaling: old views and new concepts. Clin Infect Dis 31 (Suppl 5):S168–S177 [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Michaud B, Poli V, Dantzer R. (2000) Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol Behav 70:367–373 [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Walter V, Parnet P, Laye´ S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, and Dantzer (1994) Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III 317:499–503. [PubMed]
- Boyce-Rustay JM, Honore P, Jarvis MF. (2010b) Animal models of acute and chronic inflammatory and nociceptive pain. Methods Mol Biol 617:41–55 [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, Decker MW, Honore P. (2010a) Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacology 58:537–543 [DOI] [PubMed] [Google Scholar]
- Brady LS, Lynn AB, Herkenham M, Gottesfeld Z. (1994) Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci 14:4951–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RJ, Glenn JR, Smith KM, Durham PL. (2011) Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. (1998) Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci 18:8056–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DLH. (2011) Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia 59:554–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DL. (2010) Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci 30:5437–5450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. (2003) Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry 54:906–914 [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. (2000) Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol 18:2143–2151 [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. (2002) Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry 7:468–473 [DOI] [PubMed] [Google Scholar]
- Coelho AM, Fioramonti J, Bueno L. (2000) Brain interleukin-1β and tumor necrosis factor-α are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res Bull 52:223–228 [DOI] [PubMed] [Google Scholar]
- Ching S, He L, Lai W, Quan N. (2005) IL-1 type I receptor plays a key role in mediating the recruitment of leukocytes into the central nervous system. Brain Behav Immun 19:127–137 [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hörtnagl H, Riva MA, Sprengel R, Gass P. (2008) AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J 22:3129–3134 [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. (1998) An assessment of the effects of central interleukin-1β, -2, -6, and tumor necrosis factor-α administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neurosci 84:923–933 [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Parry-Billings M, Newsholme EA. (1989) Decreased plasma tryptophan levels in major depression. J Affect Disord 16:27–31 [DOI] [PubMed] [Google Scholar]
- Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. (2000) Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 88:239–248 [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2004) Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500:399–411 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. (1989) Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci 44:1995–2008 [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158 [DOI] [PubMed] [Google Scholar]
- Dharmshaktu P, Tayal V, Kalra BS. (2012) Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol 52:6–17 [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Heijnen CJ, Willemen HL, Deumens R, Joosten EA, Kleibeuker W, den Hartog IJ, van Velthoven CT, Nijboer C, Nassar MA, et al. (2010a) GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci 30:2138–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. (2010b) Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci 30:12806–12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Davidson KG. (1996) Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 19:179–200 [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. (2005) Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 118:289–305 [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. (2007) Lipopolysaccharide induces delayed Fos B/Delta Fos B immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinol 32:516–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. (2000) Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience 101:1127–1135 [DOI] [PubMed] [Google Scholar]
- Fu X, Zunich SM, O’Connor JC, Kavelaars A, Dantzer R, Kelley KW. (2010) Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3-dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation 7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccio C, Luongo C, Capodanno P, Giordano C, Scafuro MA, Siniscalco D, Lettieri B, Rossi F, Maione S, Berrino L. (2009) A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur J Pharmacol 603:42–49 [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Kanai M, Nakamura T. (2011) Modulation of Tryptophan Metabolism, Promotion of Neurogenesis and Alteration of Anxiety-Related Behavior in Tryptophan 2,3-Dioxygenase-Deficient Mice. Int J Tryptophan Res 4:7–18 [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, et al. (2008) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32:140–144 [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. (2009) Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 33:450–455 [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Alvaro-Bartolomé M, Díez-Alarcia R, Ramos-Miguel A, Puigdemont D, Pérez V, Alvarez E, Meana JJ. (2010) Reduced platelet G protein-coupled receptor kinase 2 in major depressive disorder: antidepressant treatment-induced upregulation of GRK2 protein discriminates between responder and non-responder patients. Eur Neuropsychopharmacol 20:721–730 [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. (1999) Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci 19:2799–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. (2005) Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 116:109–118 [DOI] [PubMed] [Google Scholar]
- Granados-Soto V, Arguelles CF, Alvarez-Leefmans FJ. (2005) Peripheral and central antinociceptive action of Na+-K+-2Cl- cotransporter blockers on formalin-induced nociception in rats. Pain 114:231–238 [DOI] [PubMed] [Google Scholar]
- Grange-Midroit M, García-Sevilla JA, Ferrer-Alcón M, La Harpe R, Huguelet P, Guimón J. (2003) Regulation of GRK 2 and 6, beta-arrestin-2 and associated proteins in the prefrontal cortex of drug-free and antidepressant drug-treated subjects with major depression. Brain Res Mol Brain Res 111:31–41 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. (2003) The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry 64:1465–1475 [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. (2005) Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49:15–23 [DOI] [PubMed] [Google Scholar]
- Guo LH, Schluesener HJ. (2007) The innate immunity of the central nervous system in chronic pain: the role of Toll-like receptors. Cell Mol Life Sci 64:1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Von Korff M, Simon GE, Gater R. (1998) Persistent pain and well-being: A World Health Organization study in primary care. JAMA 280:147–151 [DOI] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. (1993) Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 47:169–176 [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. (2011) The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36:2452–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. (2009) Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 66:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. (2008) Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, Sergeeva M, Saake M, Garcia M, Kollias G, et al. (2011) Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci USA 108:3731–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. (1996) Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J 320:595–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, McMahon J, Yang J, Shin D, Huang Y. (2012) Rapamycin down-regulates KCC2 expression and increases seizure susceptibility to convulsants in immature rats. Neuroscience 219:33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies (2011) Relieving pain in America: A blueprint for transforming prevention, care, education, and research, The National Academies Press, Washington, DC. [PubMed]
- Isik A, Koca SS, Ozturk A, Mermi O. (2007) Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol 26:872–878 [DOI] [PubMed] [Google Scholar]
- Jackson DL, Graff CB, Richardson JD, Hargreaves KM. (1995) Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur J Pharmacol 284:321–325 [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. (2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA 98:8077–8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. (2008) Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol 4:490–503 [DOI] [PubMed] [Google Scholar]
- Kalkman HO. (2011) Alterations in the expression of neuronal chloride transporters may contribute to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 35:410–414 [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. (1992b) Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci USA 89:9117–9120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW., Dantzer R. (1992a) Sickness behavior as a new target for drug development. Trends Pharmacol Sci 13:24–28 [DOI] [PubMed] [Google Scholar]
- Kent S, Kelley KW, Dantzer R. (1992c) Effects of lipopolysaccharide on food-motivated behavior in the rat are not blocked by an interleukin-1 receptor antagonist. Neurosci Lett 145:83–86 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602 [DOI] [PubMed] [Google Scholar]
- Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, Rusanescu G, Yang L, Tian Y, Mao J. (2012) Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest 122:2940–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363 [DOI] [PubMed] [Google Scholar]
- Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. (1982) Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr 232:299–304 [DOI] [PubMed] [Google Scholar]
- Kleibeuker W, Gabay E, Kavelaars A, Zijlstra J, Wolf G, Ziv N, Yirmiya R, Shavit Y, Tal M, Heijnen CJ. (2008) IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun 22:200–208 [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, et al. (2008) Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451:330–334 [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, Noack M, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. (2011) Spinal cord injuries induce changes in CB1 cannabinoid receptor and C-C chemokine expression in brain areas underlying circuitry of chronic pain conditions. J Neurotrauma 28:619–634 [DOI] [PubMed] [Google Scholar]
- Konsman JP, Tridon V, Dantzer R. (2000) Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience 101:957–967 [DOI] [PubMed] [Google Scholar]
- Kraus J, Engelhardt B, Chatzimanolis N, Bauer R, Tofighi J, Kuehne BS, Laske C, Stolz E, Frielinghaus P, Schaefer C, et al. (2002) Cell surface bound and soluble adhesion molecules in CSF and blood in multiple sclerosis: correlation with MRI-measures of subclinical disease severity and activity. J Neuroimmunol 122:175–185 [DOI] [PubMed] [Google Scholar]
- Krell HV, Leuchter AF, Cook IA, Abrams M. (2005) Evaluation of reboxetine, a noradrenergic antidepressant, for the treatment of fibromyalgia and chronic low back pain. Psychosomatics 46:379–384 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, Tu W. (2009) Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA 301:2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Sutherland J, Hochman DW. (2012) Loop diuretics have anxiolytic effects in rat models of conditioned anxiety. PLoS ONE 7:e35417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwidzinski E, Bechmann I. (2007) IDO expression in the brain: a double-edged sword. J Mol Med (Berl) 85:1351–1359 [DOI] [PubMed] [Google Scholar]
- Li YQ, Li H, Wei J, Qu L, Wu LA. (2010) Expression changes of K+-Cl- co-transporter 2 and Na+-K+-Cl- co-transporter1 in mouse trigeminal subnucleus caudalis following pulpal inflammation. Brain Res Bull 81:561–564 [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. (2005) Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain 115:60–70 [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng Y, Willis WD. (1994) Glycine and GABAA antagonists reduce the inhibition of primate spinothalamic tract neurons produced by stimulation in periaqueductal gray. Brain Res 654:286–302 [DOI] [PubMed] [Google Scholar]
- Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JWJ, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. (1999) Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J 13:715–725 [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhu L, Gao YJ. (2011) Pain-related aversion induces astrocytic reaction and proinflammatory cytokine expression in the anterior cingulate cortex in rats. Brain Res Bull 84:178–182 [DOI] [PubMed] [Google Scholar]
- Ludwig J, Binder A, Steinmann J, Wasner G, Baron R. (2008) Cytokine expression in serum and cerebrospinal fluid in non-inflammatory polyneuropathies. J Neurol Neurosurg Psychiatry 79:1268–1273 [DOI] [PubMed] [Google Scholar]
- Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, de Jonckheere C, Minner B, Raus J. (1992) Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med 22:45–53 [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Scharpé S, Bosmans E, Suy E, De Meester I, Calabrese J, Cosyns P. (1993) Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res 49:151–165 [DOI] [PubMed] [Google Scholar]
- Maes M, Minner B, Suy E. (1991a) The relationships between the availability of l-tryptophan to the brain, the spontaneous HPA-axis activity, and the HPA-axis responses to dexamethasone in depressed patients. J Amino Acids 1:57–65 [DOI] [PubMed] [Google Scholar]
- Maes M, Schotte C, D’Hondt P, Claes M, Vandewoude M, Scharpe S, Cosyns P. (1991b) Biological heterogeneity of melancholia: results of pattern recognition methods. J Psychiatr Res 25:95–108 [DOI] [PubMed] [Google Scholar]
- Malan T, Mata H, Porreca F. (2002) Spinal GABAA and GABAB receptor pharmacology in a rat model of neuropathic pain. Anesthiol 96:1161–1167 [DOI] [PubMed] [Google Scholar]
- Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. (2007) Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med 20:434–443 [DOI] [PubMed] [Google Scholar]
- Martuscello RT, Spengler RN, Bonoiu AC, Davidson BA, Helinski J, Ding H, Mahajan S, Kumar R, Bergey EJ, Knight PR, et al. (2012) Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms. Pain 153:1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Nasca C, Molinaro G, Riozzi B, Scaccianoce S, Raggi MA, Mercolini L, Biagioni F, Mathè AA, Sanna E, et al. (2010) Enhanced expression of the neuronal K+/Cl- cotransporter, KCC2, in spontaneously depressed Flinders Sensitive Line rats. Brain Res 1325:112–120 [DOI] [PubMed] [Google Scholar]
- Matuzany-Ruban A, Golan M, Miroshnik N, Schreiber G, Avissar S. (2010) Normalization of GRK2 protein and mRNA measures in patients with depression predict response to antidepressants. Int J Neuropsychopharmacol 13:83–91 [DOI] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. (2008) Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr 13:501–510 [DOI] [PubMed] [Google Scholar]
- Michael-Titus A, Bains S, Jeetle J, Whelpton R. (2000) Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex -a possible mechanism of neuroprotection in major depression. Neurosci 100:681–684 [DOI] [PubMed] [Google Scholar]
- Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, Kreutzer DL. (2002) Interleukin-10 levels in seminal plasma: implications for chronic prostatitis-chronic pelvic pain syndrome. J Urol 167:753–756 [DOI] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, et al. (2005) Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21:2136–2148 [DOI] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, et al. (2006) Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 126:294–308 [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. (2003) Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci 23:1026–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. (2006) Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 30:1155–1158 [DOI] [PubMed] [Google Scholar]
- Moore P, Landolt HP, Seifritz E, Clark C, Bhatti T, Kelsoe J, Rapaport M, Gillin JC. (2000) Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology 23:601–622 [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. (2001) Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 344:961–966 [DOI] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, King T, Porreca F. (2013) Evaluation of reward from pain relief. Ann N Y Acad Sci 1282:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dixit VM. (2012) Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. (2009) Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. J Neurophysiol 101:2041–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Martini C, Dahan A. (2013) Ketamine for Chronic Pain: Risks and Benefits. Br J Clin Pharmacol DOI: 10.1111/bcp.12094 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, Courtney DeVries A. (2010b) Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosom Med 72:519–526 [DOI] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, DeVries AC. (2010a) Stress and IL-1β contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry 15:404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obreja O, Rathee PK, Lips KS, Distler C, Kress M. (2002) IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1R1, tyrosine kinase, and protein kinase C. FASEB J 16:1497–1503 [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scott LV, Dinan TG. (2006) Antidepressant therapy and C-reactive protein levels. Br J Psychiatry 188:449–452 [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. (2007) Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res 41:326–331 [DOI] [PubMed] [Google Scholar]
- O’Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. (2009a) Interferon-γ and tumor necrosis factor-α mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 29:4200–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. (2009b) Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J Immunol 182:3202–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. (2009c) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14:511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprée A, Kress M. (2000) Involvement of the proinflammatory cytokines tumor necrosis factor-α, IL-1 β, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci 20:6289–6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oraifo S, Omogbai E. (2012) Effect of sertraline on the antidepressant-like actions of furosemide and bumetanide in the FST and TST in mice. Int Res J Pharm Pharmacol 2:71–75 [Google Scholar]
- Osburg B, Peiser C, Dömling D, Schomburg L, Ko YT, Voigt K, Bickel U. (2002) Effect of endotoxin on expression of TNF receptors and transport of TNF-α at the blood-brain barrier of the rat. Am J Physiol Endocrinol Metab 283:E899–E908 [DOI] [PubMed] [Google Scholar]
- Palin K, Bluthe RM, McCusker RH, Levade T, Moos F, Dantzer R, Kelley KW. (2009) The type 1 TNF receptor and its associated adapter protein, FAN, are required for TNF alpha-induced sickness behavior. Psychopharmacol 201:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K, McCusker RH, Strle K, Moos F, Dantzer R, Kelley KW. (2008) Tumor necrosis factor-α-induced sickness behavior is impaired by central administration of an inhibitor of c-jun N-terminal kinase. Psychopharmacology (Berl) 197:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. (2003) Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci 17:1847–1852 [DOI] [PubMed] [Google Scholar]
- Peng YB, Lin Q, Willis WD. (1996) Effects of GABA and glycine receptor antagonists on the activity and PAG-induced inhibition of rat dorsal horn neurons. Brain Res 736:189–201 [DOI] [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS. (2010) DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polazzi E, Contestabile A. (2002) Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev Neurosci 13:221–242 [DOI] [PubMed] [Google Scholar]
- Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskár Z, Todd AJ. (2003) Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain 104:229–239 [DOI] [PubMed] [Google Scholar]
- Puskarjov M, Ahmad F, Kaila K, Blaesse P. (2012) Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J Neurosci 32:11356–11364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N. (2008) Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Mol Neurobiol 37:142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J. (1992) Platelet serotonin and plasma tryptophan decreases in endogenous depression. Clinical, therapeutic, and biological correlations. J Affect Disord 24:55–62 [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Mol Psychiatry 15:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, Zajecka JM, Bruno CJ, Henderson MA, Reinus JF, et al. (2007) Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther 25:1163–1174 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S. (2006) Why cancer and inflammation? Yale J Biol Med 79:123–130 [PMC free article] [PubMed] [Google Scholar]
- Rees H, Sluka KA, Westlund KN, Willis WD. (1995) The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol 484:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. (2000) Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 4:247–257 [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. (2001) Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58:445–452 [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. (2007) Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: role of BDNF-TrkB signaling and NMDA receptors. Mol Neurobiol 35:224–235 [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. (2010) Interactions between the immune and nervous systems in pain. Nat Med 16:1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, Souza CT, Quevedo J. (2011) Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res 221:166–171 [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Aday LA, Anderson KO, Mendoza TR, Cleeland CS. (2006) Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. J Pain Symptom Manage 32:118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, et al. (2002) BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol 159:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoskes DA, Albakri Q, Thomas K, Cook D. (2002) Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J Urol 168:331–335 [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MCR, Arbous MS, Marinus J, Sarton EY, Dahan A. (2009) Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain 145:304–311 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. (1993) Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain 55:217–225 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD, Westlund KN. (1993) Joint inflammation and hyperalgesia are reduced by spinal bicuculline. Neuroreport 5:109–112 [DOI] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. (2004) Mild acute inflammatory stimulation induces transient negative mood. J Psychosom Res 57:189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufka KJ. (1994) Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain 58:355–366 [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. (2003) Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci 23:2899–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Misawa M. (1996) Formalin- and carrageenan-induced inflammation attenuates place preferences produced by morphine, methamphetamine and cocaine. Life Sci 59:1667–1674 [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. (2005) The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA 102:5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornberg J, Voikar V, Savilahti H, Rauvala H, Airaksinen MS. (2005) Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur J Neurosci 21:1327–1337 [DOI] [PubMed] [Google Scholar]
- Uçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. (2006) Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum 54:2656–2664 [DOI] [PubMed] [Google Scholar]
- Van der Does AJ. (2001) The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord 64:107–119 [DOI] [PubMed] [Google Scholar]
- Vroon A, Heijnen CJ, Kavelaars A. (2006) GRKs and arrestins: regulators of migration and inflammation. J Leukoc Biol 80:1214–1221 [DOI] [PubMed] [Google Scholar]
- Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, Caron MG, Schedlowski M, Heijnen CJ. (2005) G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol 174:4400–4406 [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. (2013) NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Janz L, Vriend CY, Sorensen CM, Greenberg AH, Nance DM. (1993) Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res Bull 32:581–587 [DOI] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, Eijkelkamp N, Garza Carbajal A, Schedlowski M, Kelley KW, Dantzer R, Kavelaars A. (2011) GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain 152:1649–1658 [DOI] [PubMed] [Google Scholar]
- Wang LX, Wang ZJ. (2003) Animal and cellular models of chronic pain. Adv Drug Deliv Rev 55:949–965 [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. (1995a) Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci 57:1011–1026 [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. (1995b) Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 63:289–302 [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. (1994) Characterization of cytokine-induced hyperalgesia. Brain Res 654:15–26 [DOI] [PubMed] [Google Scholar]
- Wei H, Hao B, Huang JL, Ma AN, Li XY, Wang YX, Pertovaara A. (2010) Intrathecal administration of a gap junction decoupler, an inhibitor of Na(+)-K(+)-2Cl(−) cotransporter 1, or a GABA(A) receptor agonist attenuates mechanical pain hypersensitivity induced by REM sleep deprivation in the rat. Pharmacol Biochem Behav 97:377–383 [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpé S, Maes M. (2005) IDO and interferon-α-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 10:538–544 [DOI] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, 2nd, Kelley KW, Heijnen CJ, Kavelaars A. (2010) Microglial/macrophage GRK2 determines duration of peripheral IL-1β-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 150:550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD., Jr (1999) Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124:395–421 [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. (2005) Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun 19:345–350 [DOI] [PubMed] [Google Scholar]
- Wu LA, Huang J, Wang W, Wang W, Wang XJ, Wu SX. (2009) Down-regulation of K+ -Cl- co-transporter 2 in mouse medullary dorsal horn contributes to the formalin-induced inflammatory orofacial pain. Neurosci Lett 457:36–40 [DOI] [PubMed] [Google Scholar]
- Xie W, Luo S, Xuan H, Chou C, Song G, Lv R, Jin Y, Li W, Xu J. (2006) Betamethasone affects cerebral expressions of NF-kappaB and cytokines that correlate with pain behavior in a rat model of neuropathy. Ann Clin Lab Sci 36:39–46 [PubMed] [Google Scholar]
- Yirmiya R. (1996) Endotoxin produces a depressive-like episode in rats. Brain Res 711:163–174 [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864 [DOI] [PubMed] [Google Scholar]
- Zelenka M, Schäfers M, Sommer C. (2005) Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 116:257–263 [DOI] [PubMed] [Google Scholar]
- Zhou S, Bonasera L, Carlton SM. (1996) Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport 7:895–900 [DOI] [PubMed] [Google Scholar]