Abstract
Sixteen years ago, the Nomenclature Committee of the International Union of Pharmacology approved a system for naming human seven-transmembrane (7TM) G protein-coupled chemokine receptors, the large family of leukocyte chemoattractant receptors that regulates immune system development and function, in large part by mediating leukocyte trafficking. This was announced in Pharmacological Reviews in a major overview of the first decade of research in this field [Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, and Power CA (2000) Pharmacol Rev 52:145–176]. Since then, several new receptors have been discovered, and major advances have been made for the others in many areas, including structural biology, signal transduction mechanisms, biology, and pharmacology. New and diverse roles have been identified in infection, immunity, inflammation, development, cancer, and other areas. The first two drugs acting at chemokine receptors have been approved by the U.S. Food and Drug Administration (FDA), maraviroc targeting CCR5 in human immunodeficiency virus (HIV)/AIDS, and plerixafor targeting CXCR4 for stem cell mobilization for transplantation in cancer, and other candidates are now undergoing pivotal clinical trials for diverse disease indications. In addition, a subfamily of atypical chemokine receptors has emerged that may signal through arrestins instead of G proteins to act as chemokine scavengers, and many microbial and invertebrate G protein-coupled chemokine receptors and soluble chemokine-binding proteins have been described. Here, we review this extended family of chemokine receptors and chemokine-binding proteins at the basic, translational, and clinical levels, including an update on drug development. We also introduce a new nomenclature for atypical chemokine receptors with the stem ACKR (atypical chemokine receptor) approved by the Nomenclature Committee of the International Union of Pharmacology and the Human Genome Nomenclature Committee.
I. Introduction
The chemokine signaling system consists of chemokine ligands and 7TM receptors that coordinate leukocyte trafficking in the vertebrate immune system. First appearing in teleost fish, chemokines constitute the largest family of cytokines, and chemokine receptors constitute the largest branch of the γ subfamily of rhodopsin-like 7TM receptors. Chemokine receptors are differentially expressed by all leukocytes and many nonhematopoietic cells, including cancer cells, and can be divided into the following two groups: G protein-coupled chemokine receptors, which signal by activating Gi-type G proteins (see section II), and atypical chemokine receptors, which appear to shape chemokine gradients and dampen inflammation by scavenging chemokines in a G protein-independent, arrestin-dependent manner (see section III). A key structural determinant that distinguishes these two groups is the sequence motif DRYLAIV, located at the end of transmembrane domain 3, which is well conserved in most G protein-coupled chemokine receptors, but is poorly conserved in atypical chemokine receptors. G protein-coupled chemokine receptors have been reported to activate a variety of downstream phospholipid-modifying enzymes, including PI3K, phospholipase Cβ2 and β3, phospholipase A2, and phospholipase D; mitogen-activated protein kinases (MAPK); and tyrosine kinases. Further downstream, low molecular weight G proteins such as Rac, Rho, and cdc42 may be activated, which mediate specific aspects of cell migration, including actin polymerization, adhesion, and membrane protrusion. The relative importance of each of these mediators may vary for each receptor and may be context- and cell type-dependent.
Vertebrate G protein-coupled chemokine receptors represent the largest group of chemokine receptors, which is subdivided into four subgroups, defined by which of four subgroups of chemokines is bound. Chemokine subgroups are structurally defined and named by the number and arrangement of conserved cysteines (Fig. 1). Vertebrate G protein-coupled chemokine receptors can also be classified loosely into three functional groups as follows: homeostatic, inflammatory, and dual inflammatory/homeostatic subtypes, according to whether they are used for immune system development and basal leukocyte trafficking (homeostatic) or emergency trafficking of leukocytes to sites of infection or tissue injury (inflammatory) or both (inflammatory/homeostatic).
Fig. 1.
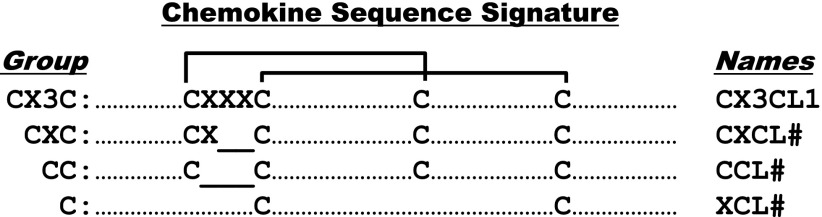
Chemokine primary structure. Chemokines are defined by structure, not function. They are >20% identical for any pairwise protein sequence comparison, and after processing most are 70–80 amino acids long. Four subdivisions are named according to the number and spacing of conserved N-terminal cysteines, as shown; all but three of the human chemokines are in the CXC and CC groups. The cysteines form disulfide bonds as shown by the brackets. Amino acid sequence identity is <30% between members of the four major chemokine groups, but ranges from ∼30 to 99% among members of the same group, indicating separate evolutionary histories. Most chemokine receptors are restricted by group. Most neutrophil-targeted chemokines are in the CXC group, and most monocyte/macrophage-targeted chemokines are in the CC group. Major T and B-cell-targeted chemokines can be found in both groups. The leukocyte target specificity of a chemokine may be narrow or broad and is defined by the expression pattern of its cognate receptor(s).
Functional chemokines and chemokine receptors are also encoded by herpesviruses and poxviruses, which appear to have obtained them by copying genes from their hosts (see section IV.A). Viral chemokine receptors are 7TM proteins that may signal constitutively and in response to binding host chemokines, often by activating a diverse repertoire of G proteins. Soluble non–7TM chemokine-binding proteins, typically with broad specificity for inflammatory chemokines, have also been found in Herpesviruses and Poxviruses, as well as in tick saliva, and may act as anti-inflammatory immune evasion factors (see sections IV.A and V).
Chemokine receptors may function beneficially, for example, in antimicrobial host defense, or harmfully, for example, in the setting of chronic inflammation, autoimmunity, infectious disease, and cancer. Some pathogens, most notably HIV and Plasmodium vivax, exploit host chemokine receptors as key cell entry factors by deploying chemokine mimics. Chemokines may also have nonimmunologic functions, including regulation of organ development.
Because inflammation is important in many diseases and because chemokine receptors are GPCRs that mediate inflammatory responses, identifying diseases in which specific chemokine receptors play an important role in susceptibility and/or outcome for targeting with drugs has been a logical and attractive goal ever since the discovery of the chemokine system. To date, however, despite massive effort, only two drugs, maraviroc and plerixafor, targeting chemokine receptors CCR5 and CXCR4, respectively, have been approved by the United States Food and Drug Administration (FDA), neither of which targets inflammation as an indication. This experience, potential explanations for the failure to develop chemokine receptor-targeted medicines in inflammation to this point, and potential ways forward have been discussed recently in several excellent reviews (Schall and Proudfoot, 2011; Pease and Horuk, 2012). In the present article, we provide an update of the basic, translational, and clinical advances made for each chemokine receptor in the past decade. General principles of chemokine structure and function are summarized in Tables 1 and 2 and Figs. 2–6, and specific details for each receptor are described in the individual sections.
TABLE 1.
Chemokine nomenclature and key immunoregulatory functions
| Standard Name | Common Aliases | Accession Number |
Key Immunoregulatory Functions | |
|---|---|---|---|---|
| Human | Mouse | |||
| CXCL1 | GROα, MGSA Mouse: KC | P09341 | P12850 | Neutrophil trafficking |
| CXCL2 | Groβ; MIP-2α Mouse: MIP-2 | P19875 | P10889 | Neutrophil trafficking |
| CXCL3 | Groγ; MIP-2β, | P19876 | Q6W5C0 | Neutrophil trafficking |
| CXCL4 | Platelet Factor-4 | P02776 | Q9Z126 | Procoagulant |
| CXCL4L1 | PF4V1 | P10720 | Procoagulant | |
| CXCL5 | ENA-78 | P42830 | P50228 | Neutrophil trafficking |
| Mouse: LIX | ||||
| CXCL6 | GCP-2 | P80162 | NA | Neutrophil trafficking |
| CXCL7 | NAP-2 | P02775 | Q9EQI5 | Neutrophil trafficking |
| CXCL8 | IL-8 | P10145 | NA | Neutrophil trafficking |
| CXCL9 | Mig | Q07325 | P18340 | Th1 immune response |
| CXCL10 | γIP-10 | P02778 | P17515 | Th1 immune response |
| CXCL11 | I-TAC | O14625 | Q8R392 | Th1 immune response |
| CXCL12 | SDF-1α a | P48061 | P40224 | Myelopoiesis; B lymphopoiesis; |
| HPC, neutrophil homing to marrow | ||||
| CXCL13 | BLC | O43927 | O55038 | B and T-cell trafficking in lymphoid tissue |
| CXCL14 | BRAK | O95715 | Q6AXC2 | Macrophage migration |
| Cxcl15 | lungkine | NA | Q9WVL7 | Neutrophil trafficking |
| CXCL16 | SR-PSOX | Q9H2A7 | Q8BSU2 | NKT cell trafficking and survival |
| CXCL17 | Q6UXB2 | Q8R3U6 | Mo and DC chemotaxis | |
| CCL1 | I-309 | P22362 | P10146 | Th2 response |
| CCL2 | MCP-1 | P13500 | P10148 | Innate immunity |
| Mouse: JE | Th2 response | |||
| CCL3 | MIP-1α | P10147 | P10855 | T cell and monocyte/macrophage trafficking |
| CCL3L1 | P16619 | P10855 | Innate immunity | |
| CCL3L3 | P16619 | Th1 and Th2 immune responses | ||
| CCL4 | MIP-1β | P13236 | P14097 | T/DC interaction |
| CCL4L1 | Q8NHW4 | NA | HIV suppression | |
| CCL4L2 | Q8NHW4 | NA | ||
| CCL5 | RANTES | P13501 | P30882 | innate and adaptive immunity |
| Ccl6 | C10, MRP-1 | NA | P27784 | ND |
| CCL7 | MCP-3 | P80098 | Q03366 | Th2 immune response |
| CCL8 | MCP-2 | P80075 | Q9Z121 | Th2 immune response |
| Ccl9 | MRP-2, MIP-1γ | NA | P51670 | ND |
| CCL10 (reserved) | NA | NA | NA | |
| CCL11 | Eotaxin | P51671 | P48298 | Th2 immune response |
| Ccl12 | Mcp-5 | NA | Q62401 | Eo, Ba, MC trafficking, and degranulation |
| CCL13 | MCP-4 | Q99616 | NA | ND |
| CCL14 | HCC-1 | Q16627 | NA | ND |
| CCL15 | HCC-2 | Q16663 | NA | ND |
| CCL16 | HCC-4 | O15467 | NA | DC maturation factor |
| CCL17 | TARC | Q92583 | Q9WUZ6 | Th2 immune response |
| CCL18 | PARC | P55774 | NA | DC attraction of T and B cells |
| Hematopoiesis | ||||
| CCL19 | ELC | Q99731 | O70460 | T cell and DC homing to lymph node |
| CCL20 | MIP-3α, LARC | P78556 | O89093 | GALT development |
| B and DC homing to GALT | ||||
| Th17 immune response | ||||
| IgA humoral response in gut | ||||
| CCL21 | SLC | O00585 | P84444 | T cell and DC homing to lymph node |
| CCL22 | MDC | O00626 | O88430 | Th2 immune response |
| CCL23 | MPIF-1 | P55773 | NA | ND |
| CCL24 | Eotaxin-2 | O00175 | Q9JKC0 | Eo migration |
| CCL25 | TECK | O15444 | O35903 | Thymocyte migration |
| Homing of memory T cells to gut | ||||
| CCL26 | Eotaxin-3 | Q9Y258 | Q5C9Q0 | Th2 immune response |
| CCL27 | CTACK | Q9Y4X3 | Q9Z1X0 | Homing of T cells to skin |
| CCL28 | MEC | Q9NRJ3 | Q9JIL2 | Homing of T cells to mucosal surfaces |
| XCL1 | Lymphotactin α | P47992 | P47993 | Ag cross-presentation by CD8+ DCs |
| XCL2 | Lymphotactin β | Q9UBD3 | NA | Ag cross-presentation by CD8+ DCs |
| CX3CL1 | Fractalkine | P78423 | O35188 | NK, Monocyte, MΦ and Th1 cell migration |
Stromal cell-derived factor-1 (SDF-1) α, β, γ, δ, ε and θ are splice variants of the same human gene. IP-10, interferon-induced protein of 10 kDa; I-TAC, interferon-inducible T-cell α-chemoattractant; PF, platelet factor; TECK, thymus expressed chemokine; Ag, antigen; Ba, basophil; Eo, eosinophil; GALT, gut-associated lymphoid tissue; GCP, granulocyte chemotactic protein; HPC, hematopoietic progenitor cell; Mo, monocyte; MΦ, macrophage; MC, mast cell; NA, not applicable; NAP, neutrophil-activating protein; ND, not determined; Th1, type 1 helper T cells.
TABLE 2.
Chemokine receptor nomenclature and key immunoregulatory functions
| Name | CD# | Common Aliases | Accession Number |
Key Immunoregulatory Functions | |
|---|---|---|---|---|---|
| Human | Mouse | ||||
| G Protein-Coupled Chemokine Receptors | |||||
| CXCR1 | CD181 | IL8RA | P25024 | Q810W6 | Neutrophil trafficking |
| CXCR2 | CD182 | IL8RB | P25025 | P35343 | B-cell lymphopoiesis |
| Neutrophil egress from bone marrow | |||||
| Neutrophil trafficking in innate immunity | |||||
| CXCR3 | CD183 | IP10/Mig R | P49682 | O88410 | Type 1 adaptive immunity |
| CXCR4 | CD184 | fusin | P61073 | P70658 | Hematopoiesis |
| Organopoiesis | |||||
| Adaptive Immunity | |||||
| CXCR5 | CD185 | BLR-1 | P32302 | Q04683 | B and T-cell trafficking in lymphoid tissue to B-cell zone/follicles |
| CXCR6 | CD186 | BONZO, STRL33 | O00574 | Q9EQ16 | Innate lymphoid cell function |
| Adaptive immunity | |||||
| CCR1 | CD191 | CC CKR1, MIP-1α/RANTES R | P32246 | P51675 | Innate Immunity |
| Adaptive Immunity | |||||
| CCR2 | CD192 | CC CKR2, MCP-1-R | P41597 | P51683 | Monocyte trafficking |
| Type 1 adaptive immunity | |||||
| CCR3 | CD193 | CC CKR3, Eotaxin receptor | P51677 | P51678 | Type 2 adaptive immunity |
| Eosinophil distribution and trafficking | |||||
| CCR4 | CD194 | CC CKR4 | P51679 | P51680 | Homing of resident memory T cells to skin |
| Thymopoiesis; Th2 immune response | |||||
| CCR5 | CD195 | CC CKR5 | P51681 | P51682 | Type 1 adaptive immunity |
| CCR6 | CD196 | P51684 | O54689 | iDC trafficking; GALT development | |
| Th17 adaptive immune responses | |||||
| CCR7 | CD197 | EBI-1, BLR-2 | P32248 | P47774 | mDC, and B and T-cell trafficking in lymphoid tissue to T-cell zone |
| Egress of T cells from tissue | |||||
| CCR8 | CDw198 | P51685 | P56484 | Thymopoiesis | |
| Immune surveillance in skin | |||||
| Type 2 adaptive immunity | |||||
| CCR9 | CDw199 | P51686 | Q9WUT7 | Thymopoiesis; Homing of T cells to gut. | |
| GALT development and function | |||||
| CCR10 | P46092 | Q9JL21 | Humoral immunity at mucosal sites | ||
| Immune surveillance in skin | |||||
| XCR1 | P46094 | Q9R0M1 | Ag cross-presentation by CD8+ DCs | ||
| CX3CR1 | Fractalkine receptor | P49238 | Q9Z0D9 | Patrolling monocytes in innate immunity | |
| Microglial cell and NK cell migration | |||||
| Type 1 adaptive immunity | |||||
| Atypical Chemokine Receptors (New Nomenclature) | |||||
| ACKR1 | CD234 | DARC; Duffy | Q16570 | Q9QUI6 | Chemokine transcytosis |
| Chemokine scavenging | |||||
| ACKR2 | D6, CCR9 (unofficial), CCR10 (unofficial) | O00590 | Y12879 | Chemokine scavenging | |
| ACKR3 | CXCR7; RDC1 | P25106 | P56485 | Heart valve development | |
| Shaping chemokine gradients for CXCR4 | |||||
| ACKR4 | CCRL1; CCX-CKR, CCBP2, CCR11 | Q9NPB9 | Q924I3 | Chemokine scavenging | |
| CCRL2 (ACKR5) | CKRX, CRAM-A, | O00421 | O35457 | Not defined | |
| L-CCR, CRAM-B, HCR, CCR11 | |||||
| PITPNM3 (ACKR6) | Nir1 | AAI28584.1 | Breast cancer metastasis | ||
iDC, immature dendritic cell; mDC, mature dendritic cell.
Fig. 2.
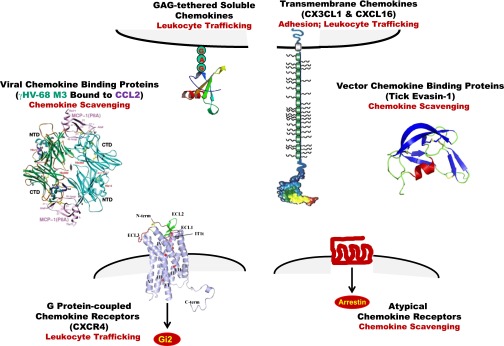
Tertiary structure of chemokines, chemokine receptors, and soluble chemokine-binding proteins. Chemokines have a common fold, and are presented as GAG-tethered molecules on the plasma membrane to leukocytes (upper left [Handel et al., 2005]). The chemokine core, which contains three β sheets arranged in the shape of a Greek key, is overlaid by a C-terminal α-helical domain and is flanked by an N-terminal domain that lacks order. Forced chemokine monomers are active but dimer and tetramer structures may occur, and complex quaternary structures bound to GAGs on the surface of cells may be important for function in vivo. Chemokine heterodimers have been described, both CC/CC and CC/CXC. Although in separate groups as defined by cysteine motifs, CXCL16 and CX3CL1 also form a unique multimodular subgroup (upper right [Imai et al., 1997b]). The model shown for these two chemokines depicts a typical chemokine domain, a mucin-like stalk, a transmembrane domain, and a C-terminal cytoplasmic module. They can exist as membrane-bound or cleaved forms, mediating direct G protein-independent cell-cell adhesion and chemotaxis, respectively. Two G protein-coupled chemokine receptors, CXCR1 and CXCR4, have been structurally defined. CXCR4 (lower left) resolves as a dimer (Wu et al., 2010). Atypical chemokine receptors, which do not appear to signal through G proteins, have not yet been defined structurally but are predicted to be 7TM proteins (lower right). Soluble chemokine-binding proteins are produced by microbes (middle left [Alexander et al., 2002]) and invertebrates (middle right [Dias et al., 2009]).
Fig. 6.
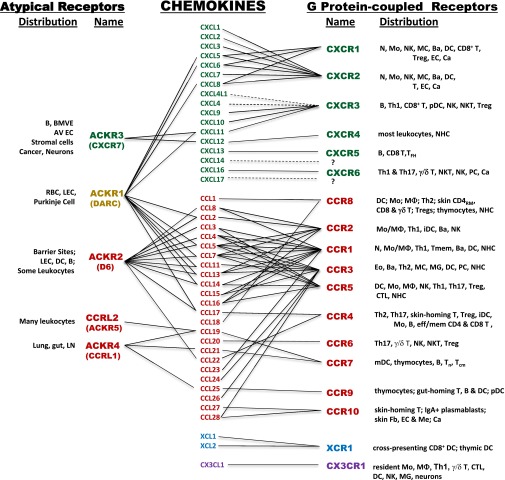
Chemokine receptor specificity for ligands and leukocytes. Abbreviations: Ba, basophil; Ca, cancer; CD4RM, resident memory CD4 T cell; EC, endothelial cell; Eo, eosinophil; Fb, fibroblasts; iDC, immature DC; MC, mast cell; Me, melanocyte; MG, microglial cell; Mo, monocyte; MΦ, macrophage; N, neutrophil; NHC, nonhematopoietic cells; PC, plasma cell; pDC, plasmacytoid DC; Tcm, central memory T cell; Th1, type 1 helper T cell; Tn, naive T cell; eff/mem, effector/memory; thym, thymocytes.
II. Host G Protein-Coupled Chemokine Receptors
A. CXC Chemokine Receptors
1. CXCR1 and CXCR2.
The first chemokine receptors defined at the molecular level were human CXCR1 and CXCR2 (Holmes et al., 1991; Murphy and Tiffany, 1991), the prototypic neutrophil chemotactic receptors for a group of CXC chemokines distinguished by the presence of the amino acid motif ELR in the N-terminal domain. The corresponding genes are clustered on chromosome 2q35, along with a pseudogene for CXCR2 named CXCR2P1 (IL8RBP). The chromosomal location of this cluster and all other human chemokines and chemokine receptors is summarized in Fig. 3. Expression of CXCR1 and CXCR2 is tightly regulated in neutrophils by external signals such as tumor necrosis factor (TNF)-α, lipopolysaccharide (LPS), Toll-like receptor (TLR) agonists, and nitric oxide (Khandaker et al., 1999; Alves-Filho et al., 2009).
Fig. 3.
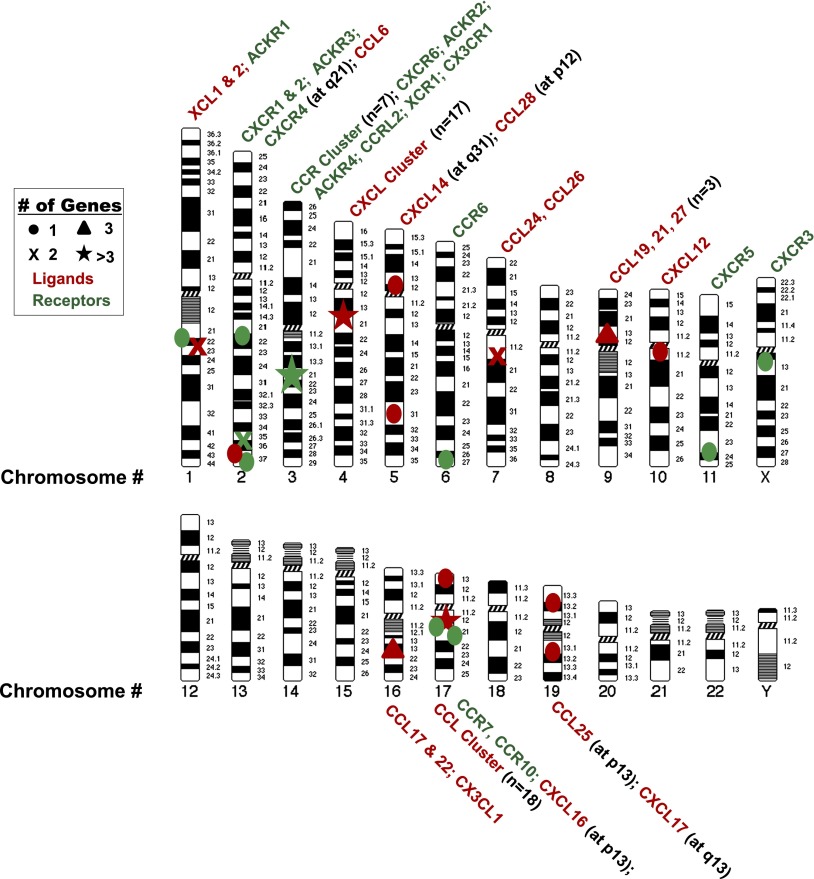
The human chemokinome. Genes for chemokines and chemokine receptors each have common ancestors but are distributed on many chromosomes. The two main chemokine gene clusters on chromosomes 4 and 17 (★) contain most of the chemokines that mediate inflammatory responses. Most inflammatory chemokine receptor genes are on chromosomes 2 and 3. Homeostatic chemokine and chemokine receptor genes are scattered on other chromosomes.
CXCL8 [known previously as interleukin (IL)-8] binds with high affinity to and potently activates both receptors. CXCR1 also binds CXCL6 (granulocyte chemotactic protein-2) and possibly CXCL7 (neutrophil-activating protein-2), whereas CXCR2 binds promiscuously to all seven ELR+ CXC chemokines (Murphy et al., 2000; Stillie et al., 2009). The specificity of these and all other chemokine receptors for ligands and leukocytes is summarized in Fig. 6. The ELR motif partly determines receptor specificity (Clark-Lewis et al., 1993), and other modifications of the N′-terminal region [e.g., CXCL8(3–73)K11R)] may increase receptor affinity (Li and Gordon, 2001). Post-translational citrullination was reported to control the activities of CXCL5 and CXCL8 (Proost et al., 2008). Both monomers and dimers of CXCL8 induce neutrophil migration in vivo, but distinct equilibria exist between them in different tissues possibly as a result of regulation by glycosaminoglycan (GAG) binding (Tanino et al., 2010; Gangavarapu et al., 2012). GAG binding maps in CXCL8 to the C′-terminal helix of the chemokine and to the proximal loop around residues 18–23 (Kuschert et al., 1998).
Several studies have been published identifying nonchemokine ligands for CXCR1 and/or CXCR2, although the actual significance of this is not known. Some of these, including the collagen breakdown product N-acetyl-proline-glycine-proline, macrophage migration inhibitory factor, the N′-terminal domain of human tyrosyl-tRNA synthetase, Brugia malayi asparaginyl-tRNA synthetase, and the HIV matrix protein p17, were suggested to have sequence/charge/structure similarities to ELR+ CXC chemokines, whereas LL-37, an α-helical peptide derived by cleavage of cathelicidin, does not (Bernhagen et al., 2007; Giagulli et al., 2012). The viral chemokine vCXCL1 of human cytomegalovirus (CMV) binds to both CXCR1 and CXCR2 (Luttichau, 2010) but activates neutrophils, mainly through CXCR2 (Penfold et al., 1999). Whether the neutrophil migration-inducing activity of N-acetyl-proline-glycine-proline and its role in chronic lung inflammation are mediated directly by CXCR1 and CXCR2 or indirectly is currently unsettled (Snelgrove, 2011).
ELR+ CXC chemokines are categorized as inflammatory because they recruit neutrophils from blood to sites of infection and inflammation, but they may also have a homeostatic role in regulating neutrophil egress from bone marrow to blood (Kohler et al., 2011) (Fig. 7). ELR+ CXC chemokines also bind to the atypical chemokine receptor ACKR1 (also known as the Duffy antigen receptor for chemokines or DARC) and the virally encoded chemokine receptors Kaposi’s sarcoma-associated herpesvirus-GPCR encoded by ORF 74 of human herpesvirus 8 (HHV8) and ECRF3 of Herpesvirus saimiri (Rosenkilde et al., 1999) (see below). Many members of the CXCR1/CXCR2-ELR+ CXC chemokine axis have been identified in other species (Stillie et al., 2009); however, a murine ortholog of CXCL8 does not exist (Fig. 4) (Zlotnik et al., 2006). The best characterized mouse ELR+ CXC chemokines are KC and macrophage inflammatory protein-2 or MIP-2 (now named Cxcl1 and Cxcl2, respectively), which bind to mouse Cxcr2. Cxcr1 has been reported to respond to mouse Cxcl5 (LIX, a mouse counterpart of human CXCL6) (Fan et al., 2007; Stillie et al., 2009) (Fig. 4); however, native Cxcr1 on mouse leukocytes has not been characterized yet. A Cxcr1 knockout mouse has been generated, but its distinct phenotype is unclear (Clarke et al., 2011; Sakai et al., 2011).
Fig. 7.
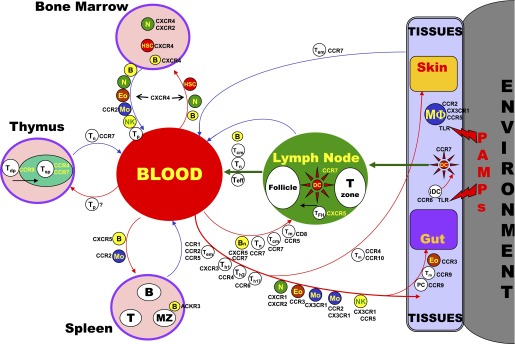
Chemokine receptors important in leukocyte trafficking pathways. Arrows demarcate major leukocyte traffic routes between major tissue and hematopoietic compartments. Cells along the arrows identify some of the cells that follow these routes. The receptors listed for each cell either mark the cell or are used by the cell for trafficking on the route shown. Abbreviations: HSC, hematopoietic stem cell; Tem, effector memory T cell; Teff, effector T cell; Tdp, double positive thymocytes; Tsp, single positive thymocytes; TFH, follicular help T cells.
Fig. 4.
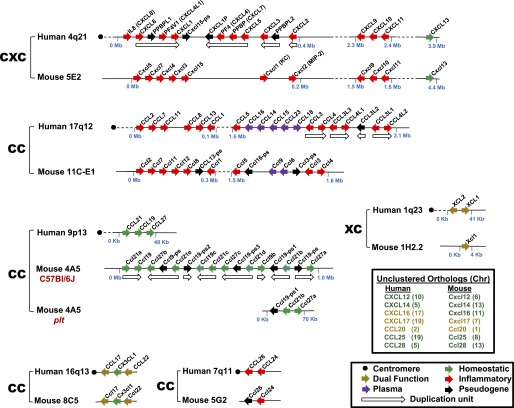
The human and mouse chemokine gene repertoires are distinct. The syntenic positions of chemokine genes located in clusters are shown schematically and aligned for mouse and human. Chromosome assignments of unclustered genes are listed in the upper box inset. See lower box inset for functional codes. Updated and modified from Nomiyama et al. (2010).
A two-step model of ligand binding and receptor activation has been proposed for CXCR4 (see below) that may generally be relevant for other chemokine receptors, including CXCR1 and 2. In particular, the strong interaction of CXCL8 with the N′-terminal domain of CXCR1 may lead to dissociation of this receptor domain from the membrane with which it interacted, followed by transition of the chemokine to a second binding site composed of the extracellular loops and transmembrane residues (Joseph et al., 2010; Park et al., 2011, 2012). This step then induces a conformational change on the receptor, allowing subsequent activation of heterotrimeric G proteins. Homodimerization of CXCR1 and CXCR2 and heterodimerization with other receptors (for CXCR2) have been demonstrated in transfected cells and may regulate the activation properties of the different partners (Martinez Munoz et al., 2009; Stillie et al., 2009). CXCR1 is the first GPCR whose unmodified structure has been solved in the absence of ligand or antibody and the first to be solved by NMR spectroscopy (Park et al., 2012). Several significant differences were observed relative to the crystal structure of CXCR4 (see below), including a monomeric structure, the presence of a C-terminal α-helix, and shifts of the transmembrane domains.
After ligand binding to CXCR1 and CXCR2 in neutrophils, the receptors primarily activate Gi proteins (Damaj et al., 1996). Gαi2 coupling determinants on the receptors include intracellular loop 2 (ICL2) and ICL3; the C′-terminal domain regulates receptor activation and desensitization (Sai et al., 2006). G protein βγ subunits appear to be required for CXCL8-mediated phagocyte migration (Neptune et al., 1999).
Many downstream mediators are induced after CXCR1 and CXCR2 activation [e.g., PLC, phospholipase D (PLD), MAPK and signal transducer and activator of transcription 3 (STAT3)], with key roles identified for PI3Kγ (Waugh and Wilson, 2008; Stillie et al., 2009). However, agonist-specific signaling has been described. For instance, although CXCL1 (GROα), CXCL7, and CXCL8 all induce Ca2+ mobilization in neutrophils, CXCL8 is the most potent chemotactic factor and the only activator of PLD (L'Heureux et al., 1995). Both receptors activate Ca2+ flux and neutrophil exocytosis; however, respiratory burst and PLD activation has been reported to depend exclusively on CXCR1 (Jones et al., 1996). CXCR2 may mediate migration far from the inflammatory focus where CXCL8 concentrations may be at low levels (Chuntharapai and Kim, 1995; McDonald et al., 2010). Murine models have shown that ELR+ CXC chemokines induce selectin-dependent neutrophil rolling on activated endothelium, followed by integrin-mediated firm adhesion and transendothelial migration to inflamed sites (Huber et al., 1991; Zhang et al., 2001a). CXCL8 also triggers firm adhesion of monocytes to vascular endothelium under shear flow (Gerszten et al., 1999).
CXCR1- and CXCR2-dependent migration requires activation of many proteins that may form a “chemosynapse” with the receptors (Raman et al., 2009), including Rho, cdc-42, focal adhesion kinase, paxillin, proline-rich tyrosine kinase 2, PAK1, and Rac2, as well as vasodilator-stimulated phosphoprotein, LASP-1, and IQGAP1. Cytoskeletal elements differentially regulate CXCR1- and CXCR2-induced focal adhesion kinase activation and migration (Cohen-Hillel et al., 2006).
Agonists at high concentrations induce phosphorylation of CXCR1 and CXCR2, leading to homologous desensitization, receptor internalization, and partial degradation. Such processes are regulated by the C′-terminal PDZ ligand binding motif of the receptor, by C′-terminal phosphorylation, and by the LLKIL motif in the C′ terminus (Baugher and Richmond, 2008). Receptor dephosphorylation fosters recycling back to the plasma membrane after ligand removal. Many proteins that mediate these processes have been identified, including arrestins, G protein-coupled receptor kinases, clathrin, dynamin, AP-2, Hip, Rab proteins, and actin filaments. CXCR1 and CXCR2 cross-regulate each other’s downregulation in a process mediated by positive and negative elements in their C′-terminal domains (Attal et al., 2008). CXCR1 and CXCR2 have also been shown to undergo and induce cross or heterologous desensitization, sometimes differentially, with other GPCRs and their ligands, C5a, fMLF, PAF, and other chemokines (e.g., CCR1 and CCR5 agonists) (Nasser et al., 2005); by opiates; and by a 120-kDa fibronectin fragment. CXCR2 may also cross-talk with nucleotide receptors (Werry et al., 2003).
The precise physiologic relevance of desensitization and receptor internalization is unclear. Several studies have suggested that CXCR2 internalization is required for receptor recycling and resensitization. Others have claimed that receptor endocytosis terminates the migration of cells when they reach sites of inflammation. β-Arrestin-2 has been reported to induce and strengthen integrin-mediated leukocyte adhesion during CXCL2-CXCR2-driven extravasation in one study (Molteni et al., 2009) but to be a negative regulator of migration to CXCL1 in another (Su et al., 2005). In other studies, high blood levels of murine Cxcl1 cause Cxcr2 desensitization and arrest of neutrophil migration (Wiekowski et al., 2001). Moreover, cross-desensitization of CXCR2 by formyl peptide receptor signaling has been reported to attenuate neutrophil migration into inflamed airways (Sogawa et al., 2011).
In addition to neutrophils, CXCR1 and CXCR2 are both expressed by CD14+ monocytes, CD56dim CD16+ natural killer (NK) cells, mast cells, basophils, dendritic cells, and freshly isolated T cells (Robertson, 2002; Geissmann et al., 2003). On T cells, CXCR1 is detected mainly on effector CD8+ cells (Takata et al., 2004), and CXCR1 but not CXCR2 is functional on CD4+Foxp3+ regulatory T cells (Tregs). Many types of nonhematopoietic cells also express one or both receptors (endothelial cells, epithelial cells, neurons, mesenchymal stem cells). As a result, the ELR+ CXC chemokine system has been implicated in diverse pathologies, including infectious diseases, cardiovascular disease (Aukrust et al., 2008; Zernecke et al., 2008), cancer (see references below), central nervous system pathologies, and pain regulation (Rittner et al., 2008), as well as morphogenesis (Ueland et al., 2004).
Gene targeting in mice has revealed that Cxcr2 negatively regulates expansion and development of B cells and myeloid progenitors and mediates neutrophil-mediated inflammatory responses to both bacteria and parasites as well as during wound healing (Cacalano et al., 1994; Frendeus et al., 2000). In addition, Cxcr2-mediated neutrophil migration promotes septic injury, autoantibody- and Lyme-mediated arthritis, lung inflammation, and dextran sodium sulfate (DSS)-induced colitis (Buanne et al., 2007). In contrast, Cxcl1−/− mice were more susceptible to DSS colitis (Shea-Donohue et al., 2008).
In mouse, neutralization of Cxcr2 attenuates neutrophil-mediated host defense in a model of ascending urinary tract infection with Escherichia coli (Olszyna et al., 2001). Both CXCR1 and CXCR2 are reduced on neutrophils from patients with hyper-immunoglobulin E syndrome, who are susceptible to bacterial infections. CXCR2 may also mediate protection against pulmonary infection by Nocardia asteroides and Aspergillus. ELR+ CXC chemokines have also been implicated in accumulation of neutrophils, CD4+ T cells, and monocytes at sites of allergic inflammation and pulmonary diseases such as chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis (Mukaida, 2003; Francis et al., 2004; Traves et al., 2004; Bizzarri et al., 2006).
In neuropathology, the CXCR2/CXCL8 axis has been implicated in diverse conditions, including ischemic injury, trauma, and multiple sclerosis (MS) (Semple et al., 2010). CXCR2 has been found on astrocytes, neurons, and oligodendrocyte progenitor cells in the setting of MS and experimental autoimmune encephalomyelitis (EAE) (Semple et al., 2010); however, its exact role in disease is controversial. For example, it has been proposed that the concurrence of CXCR2 on oligodendrocytes and CXCL1 induction in astrocytes is an essential prerequisite for lesion repair (Semple et al., 2010); however, other studies have shown that blocking Cxcr2 has enhanced recovery in chronic models of EAE (Liu et al., 2010).
In tumors, constitutive production of ELR+ CXC chemokines, such as CXCL1 and CXCL8, has been reported for many cancer cell types, and they can be induced by inflammatory cytokines, microbial products, and hypoxia. The chemokines are also expressed by associated stromal cells, endothelial cells, and leukocytes. CXCR1 and CXCR2 may be expressed by cancer cells, leukocytes, and endothelial cells (Waugh and Wilson, 2008; Lazennec and Richmond, 2010; Sharma et al., 2010) and may promote (1) recruitment to tumors of neutrophils, which are protumorigenic in some tumor systems but antitumorigenic in others (Fridlender and Albelda, 2012); (2) tumor cell proliferation, survival, and chemoresistance (Dhawan and Richmond, 2002); (3) osteoclastogenesis (Pathi et al., 2010); and (4) angiogenesis (Addison et al., 2000; Li et al., 2005; Keeley et al., 2010). ELR+ CXC chemokines promote angiogenesis by upregulating proliferation, survival, and migration of endothelial cells and fostering formation of capillary-like structures. Both CXCR1 and CXCR2 may be expressed by endothelial cells and mediate angiogenesis (Li et al., 2005); however, CXCR2 is considered to be more important in vivo (Addison et al., 2000; Li et al., 2005; Keeley et al., 2010). In addition to tumors, ELR+ CXC chemokines may also induce angiogenesis in inflammatory conditions, such as allergen sensitization (Jones et al., 2009), and even in the absence of any evident inflammatory insult. Unlike ELR+ CXC chemokines, most ELR-negative CXC chemokines are not angiogenic and some are angiostatic (see below).
Drug development.
Numerous CXCR1 and CXCR2 blocking agents as well as CXCL8 inhibitors have been evaluated preclinically (Stadtmann and Zarbock, 2012); however, few have reached clinical trials (Tables 3 and 4). ABX-IL-8 (Abgenix, Fremont, CA) is a fully humanized antibody against CXCL8 that reached phase II clinical trials in psoriasis but was stopped because of lack of efficacy. In retrospect, psoriasis may not have been a good target, because neutrophils are not prominent in this disease and the receptor is not prominent on T cells, which drive the disease. Nevertheless, ABCream (Anogen, Missassauga, ON, Canada) is a topical formulation marketed in China of a CXCL8-blocking monoclonal antibody reported to be effective in psoriasis (Bizzarri et al., 2006). Anogens has indicated that ~50% of patients achieved a greater than 60% improvement, and up to 15% of patients achieved a greater than 90% improvement in disease scores after a 6-week treatment cycle.
TABLE 3.
Summary of clinical development of drug candidates targeting chemokine receptors
See Figs. 8–10 for structures of representative clinical candidates.
| Receptor | Company | Compound | Affinity | Indication | Clinical Phase | Status |
|---|---|---|---|---|---|---|
| nM | ||||||
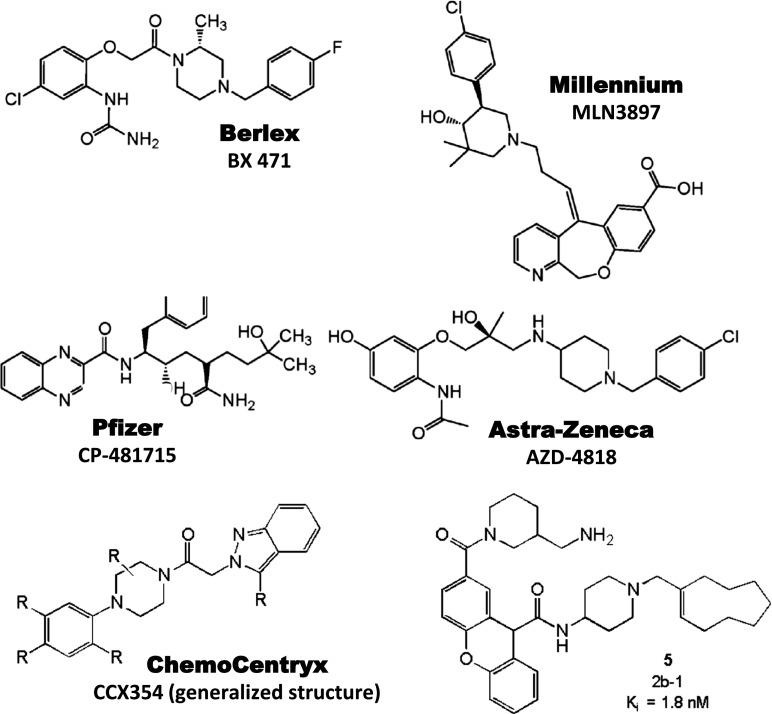
| CCR1 | Schering AG (Berlex) | BX 471 | 1.0 | MS, Psoriasis endometriosis | II | No efficacy |
| CCR1 | Millennium | MLN 3701 | MS, Multiple myeloma | II | No longer reported | |
| CCR1 | Millennium | MLN 3897 | 2.3 | RA, Multiple myeloma | II | No efficacy in RA |
| CCR1 | Pfizer | CP-481,715 | 64 | RA | II | No efficacy |
| CCR1 | AstraZeneca | AZD4818 | 5.0 | COPD | II | No efficacy |
| CCR1 | ChemoCentryx/GSK | CCX354 | 1.5 | RA | II | Ongoing |
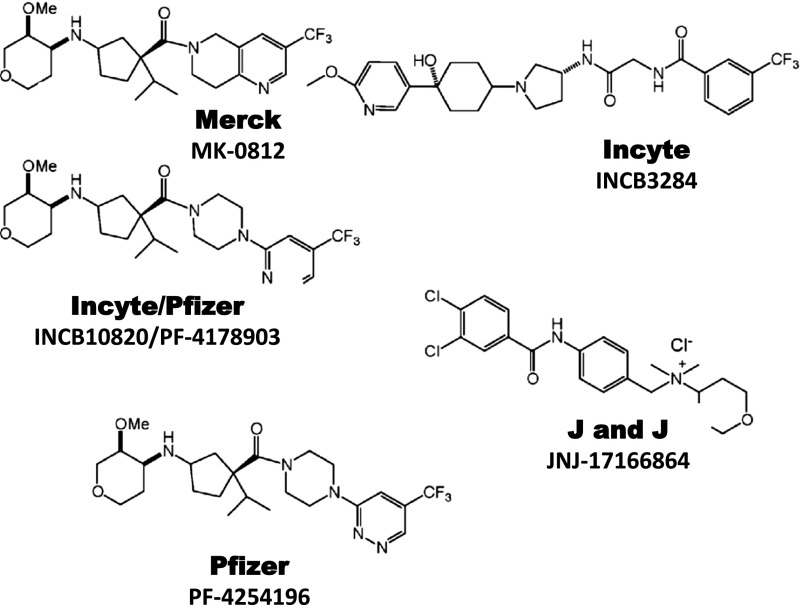
| CCR1 | Merck | C-4462 | RA | II | No efficacy | |
| CCR1 | Merck | C-6448 | MS | II | No efficacy | |
| CCR2 | Millennium | MLN 1202a | RA | II | No efficacy | |
| Atherosclerosis, MS | II | Ongoing | ||||
| CCR2 | Incyte | INCB8696 | MS, Lupus | I | No longer reported | |
| CCR2 | Incyte | INCB3284 | 3.7 | RA, Type II diabetes | II | No longer reported |
| CCR2 | ChemoCentryx | CCX915 | MS | I | Terminated | |
| CCR2 | ChemoCentryx | CCX140 | 2.3 | Diabetic nephropathy | II | Ongoing |
| CCR2 | Merck | MK-0812 | 5.0 | RA, MS | II | No efficacy |
| CCR2 | Pfizer | PF-4136309 | Pain | II | No longer reported | |
| CCR2 | BMS | BMS-741672 | Diabetic neuropathy | II | Ongoing | |
| CCR2 | Johnson & Johnson | JNJ-17166864 | 20.0 | Allergic rhinitis | II | No efficacy |
| CCR3 | Pharmaxis | ASM8b | Asthma | II | Ongoing | |
| CCR3 | GlaxoSmithKline | GSK766994 | 10.0 | Asthma and allergic rhinitis | II | No efficacy |
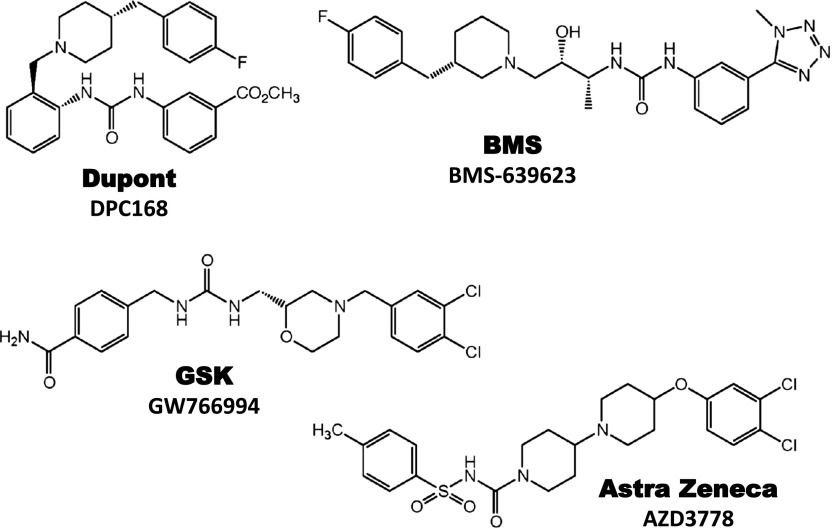
| CCR3 | Dupont | DPC168 | 2.0 | Asthma | I | Development halted |
| CCR3 | BMS | BMS-639623 | 0.3 | Asthma | I | Ongoing |
| CCR3 | Novartis | QAP-642 | Allergic rhinitis | I | Development halted | |
| CCR3 | AstraZeneca | AZD3778 | 8.1 | Allergic rhinitis | II | No longer reported |
| CCR4 | Amgen | KW-0761a | Oncology | II | Ongoing | |
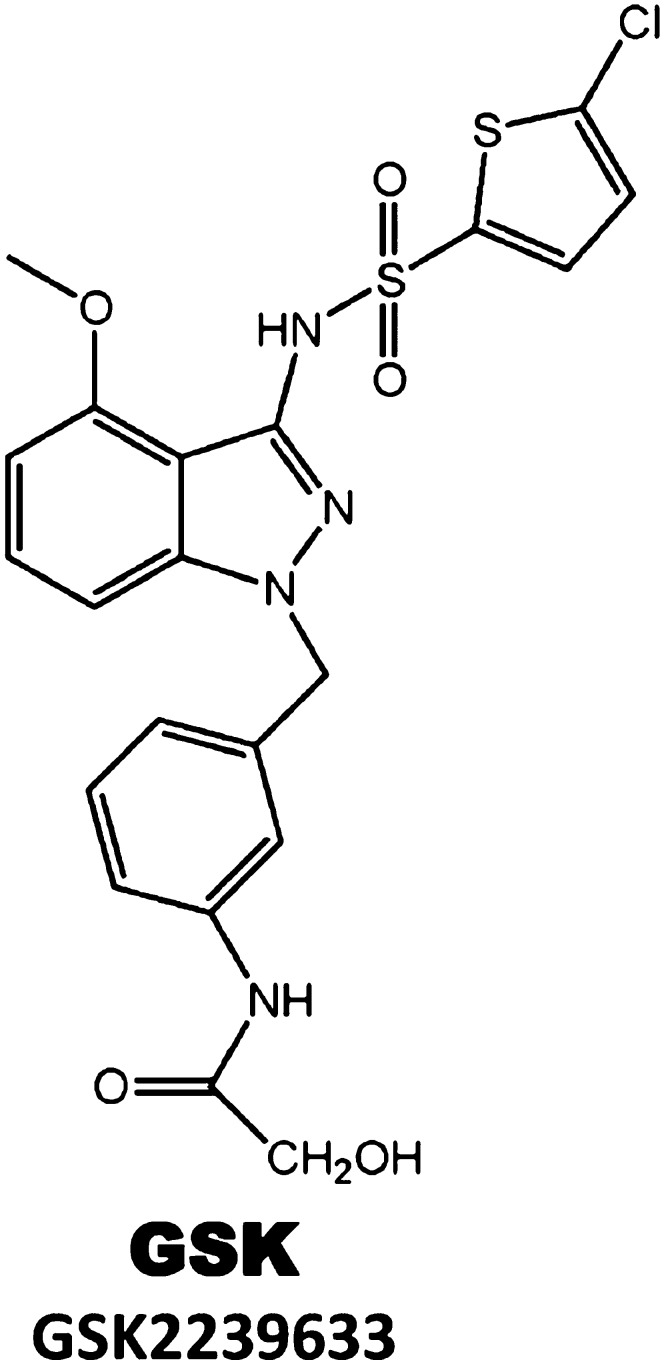
| CCR4 | GSK | GSK2239633 | 10.0 | Asthma | I | Ongoing |
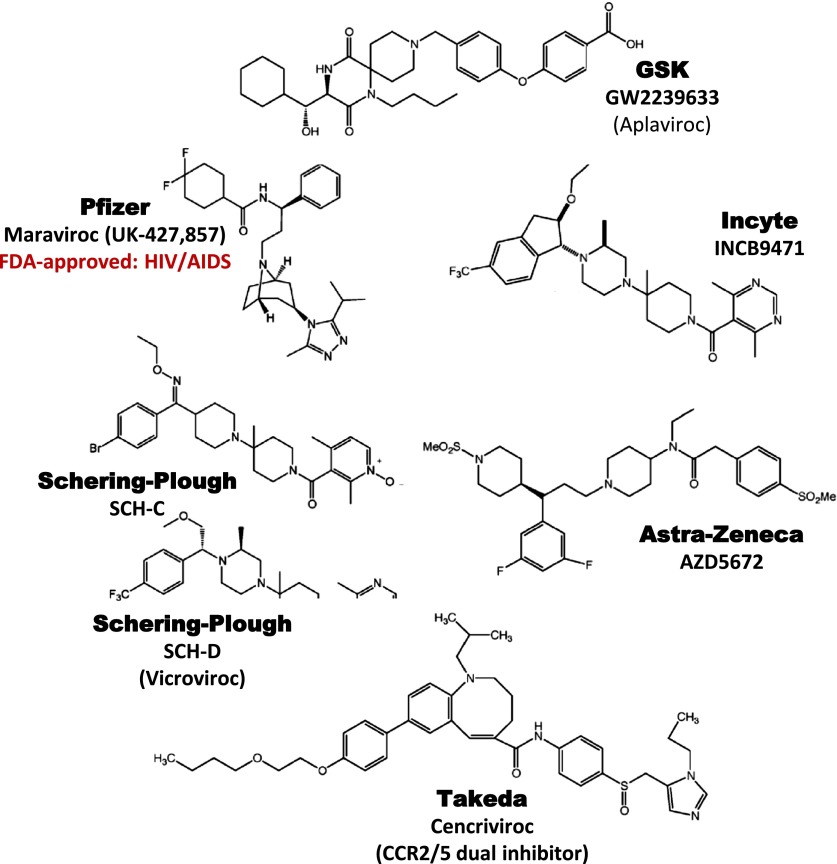
| CCR5 | Pfizer | UK-427,857 (Maraviroc) | 3.0 | RA | II | No efficacy |
| AIDS | Approved | Registered Drug | ||||
| CCR5 | Schering-Plough | SCH-C | 2.0 | RA | II | No efficacy |
| AIDS | I | Development halted | ||||
| CCR5 | Schering-Plough | SCH-D | 0.45 | AIDS | II | Development halted |
| CCR5 | GlaxoSmithKline | GW2239633 | 3.0 | AIDS | III | Development halted |
| CCR5 | Incyte | INCB9471 | 3.1 | AIDS | II | Development halted |
| CCR5 | Progenics | Pro 140a | AIDS | II | Ongoing | |
| CCR5 | Tobira | TAK652 (cenicroviroc) | 3.1e | AIDS | II | Ongoing |
| CCR5 | AstraZeneca | AZD5672 | 0.26 | RA | II | No efficacy |
| CCR5 | Novartis | NIBR-6465 | 0.8 | AIDS | I | Ongoing |
| CCR5 | Sangamo | SB-728c | AIDS | II | Ongoing | |
| CCR5 | HGS | HGS004a | AIDS | I | Ongoing | |
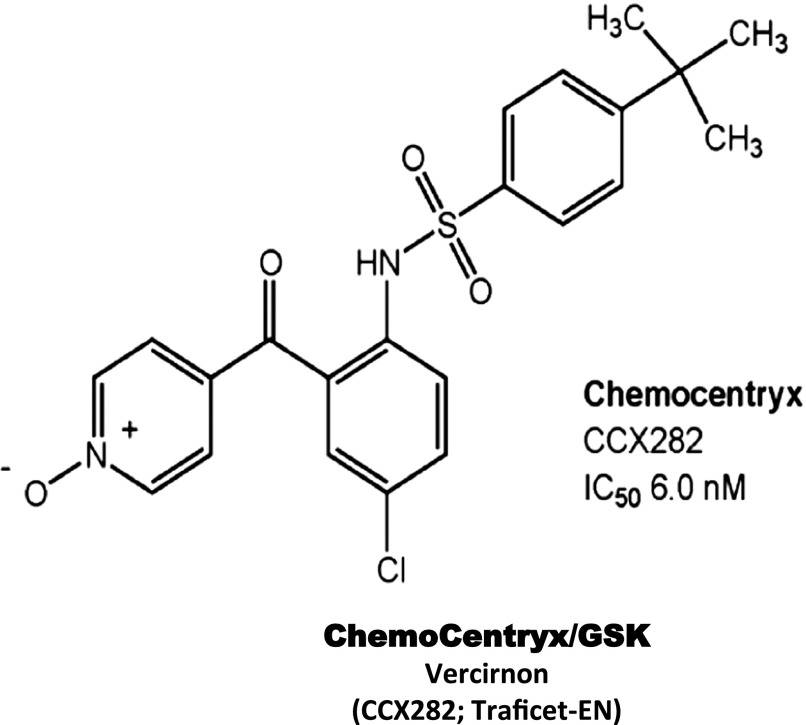
| CCR9 | ChemoCentryx/GSK | CCX282/vercirnon | 6.0 | IBD, Crohn’s | III | Terminated |
| CXCR1/ CXCR2 | Schering-Plough | SCH 527123 | 3.9 | COPD | II | Ongoing |
| 0.049 | ||||||
| CXCR1/ CXCR2 | Dompé | Reparixin | 1.0 (CXCR1) | Pancreatic islet transplantation | III | Ongoing |
| 100 (CXCR2) | ||||||
| CXCR2 | GlaxoSmithKline | SB-656933 | 5.1 | COPD, Cystic fibrosis | I | Ongoing |
| CXCR2 | GlaxoSmithKline | GSK-1325756B | COPD? | I | Ongoing | |
| CXCR2 | AstraZeneca | AZD-5069 | Bronchiectasis | II | Ongoing | |
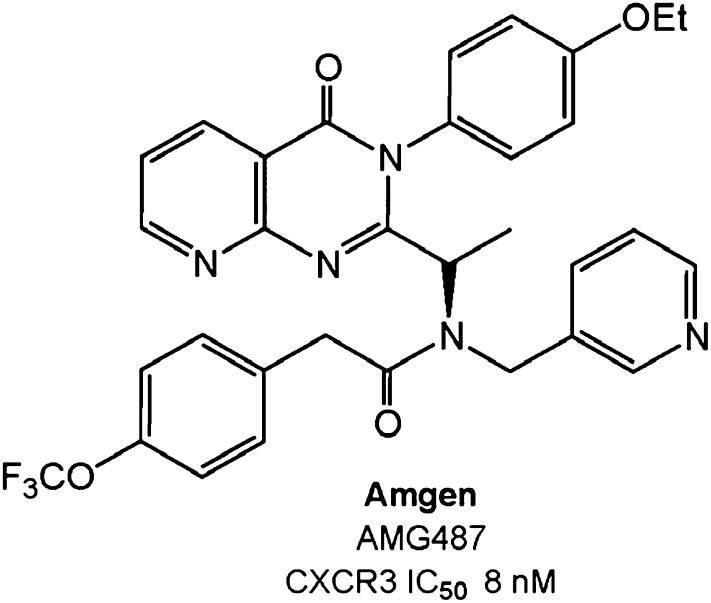
| CXCR3 | Amgen | AMG487 | 8.0 | Psoriasis | II | No efficacy |
| CXCR4 | Genzyme/Sanofi-Aventis | Plerixafor (AMD3100) | 74 | Stem cell mobilization for transplantation in cancer (MM, Non-Hodgkins lymphoma) | Approved | Registered Drug |
| CXCR4 | TaiGen | Burixafor | Stem cell transplantation | II | Ongoing | |
| CXCR4 | Polyphor | POL6326 | Stem cell transplantation | II | Ongoing | |
| CXCR4 | Medarex | MDX-1338a | Multiple myeloma | I | Ongoing | |
| CXCR4 | Biokine | BKT140d | Stem cell transplantation | I | Ongoing |
IBD, inflammatory bowel disease; MM, multiple myeloma; RA, rheumatoid arthritis.
Neutralizing monoclonal antibodies.
Antisense oligonucleotide.
Zinc finger nuclease;
Peptide.
Also has potent antagonist activity at CCR2.
TABLE 4.
Summary of clinical development of drug candidates targeting chemokines
Neutralizing monoclonal antibodies unless otherwise noted.
| Chemokine | Company | Compound | Affinity | Indication | Clinical Phase | Status |
|---|---|---|---|---|---|---|
| pM | ||||||
| CCL2 | Millennium | ABN-912 | NA | RA | II | No efficacy |
| CCL2 | Centocor | CNTO 888 | 22 | Cancer | I | Ongoing |
| CCL2a | Noxxon | NOX-E36a | NA | Diabetic nephropathy | II | Ongoing |
| CXCL8 | Abgenix | ABX-IL8 | NA | Psoriasis | II | No efficacy |
| CXCL8 | Anogen | ABCream | NA | Psoriasis | Marketed in China | |
| CXCL10 | Medarex | MDX-1100 | NA | Ulcerative colitis | II | Ongoing |
| Rheumatoid arthritis | II | Ongoing | ||||
| CXCL12a | Noxxon | NOX-A12 | NA | Multiple myeloma, CLL | II | Ongoing |
CLL, chronic lymphocytic leukemia; NA, not available.
Oligonucleotide.
Several small molecule antagonists of CXCR1 and CXCR2 have also reached the clinic (Bizzarri et al., 2006). The first to be described in the literature was the CXCR2-selective phenol-containing diaryl urea named SB 225002 (GlaxoSmithKline, London, UK) (White et al., 1998) (Fig. 8). However, this compound and some others from this series were not developed further because of undesirable pharmacokinetics (Widdowson et al., 2004). Extensive structure-activity relationship analysis yielded the compound SB 656933 with an IC50 of 22 nM for binding to CXCR2 (Fig. 8). This compound entered clinical trials in patients with cystic fibrosis and chronic obstructive pulmonary disease (Lazaar et al., 2011), where it was found to be safe and well tolerated at all doses (2–100 mg).
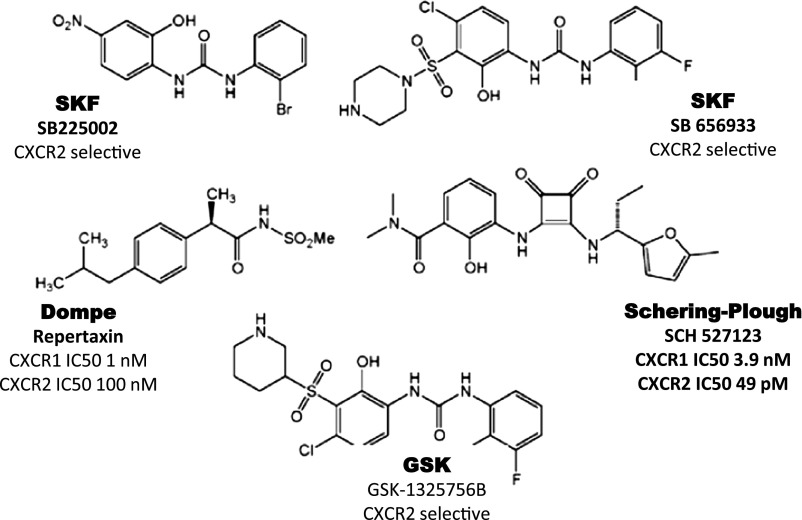
Fig. 8.
Clinical candidates active at CXCR1 and CXCR2.
The observation that 2-arylpropionic acids such as ibuprofen were able to potently inhibit CXCL8-induced chemotaxis in neutrophils prompted scientists at Dompé to screen for novel inhibitors of CXCL8-induced chemotaxis (Allegretti et al., 2005; Zarbock et al., 2008). A class of derivatives of 2-arylphenylpropionic acids was extensively investigated, leading to the selection of an acyl methane sulfonamide derivative named reparixin (Dompe, Milan, Italy) (Fig. 8) as the lead compound (Allegretti et al., 2005). This compound blocks both CXCR1 and CXCR2 but is more potent at CXCR1 and inhibits CXCL8-induced neutrophil chemotaxis with an IC50 of 1 nM. It is noteworthy that it does not inhibit chemokine binding (Allegretti et al., 2005), thus, its mechanism of action may involve allostery. Two distinct allosteric sites have been proposed for CC and CXC chemokine receptors, and several preclinical allosteric antagonists or inverse agonists have been experimentally demonstrated to act through an intracellular allosteric site on CXCR2 close to the G protein-coupling region (Bradley et al., 2009; Salchow et al., 2010). Two phase II clinical trials of reparixin in kidney and lung transplantation were negative. However, after evidence of preclinical activity in islet cell transplantation, a small phase II randomized, open-label pilot study found that reparixin improved outcome with a single infusion of allogeneic islets (Citro et al., 2012); phase 3 trials are under way in the European Union for allogeneic islet transplantation and in the United States for autologous islet transplantation. In addition, a recent report suggests that it may have some utility in certain forms of breast cancer (Ginestier et al., 2010).
Structure-activity studies of a lead cyclobutenedione compound enabled scientists at Schering-Plough (Kenilworth, NJ) to identify SCH-527123 (Fig. 8) as a potent, orally bioavailable dual CXCR1/CXCR2 receptor antagonist (Dwyer et al., 2006). The compound had good pharmacokinetic properties and oral bioavailability in rat and was recently tested in an ozone-induced airway neutrophilia clinical study in healthy subjects (Holz et al., 2010). The drug significantly lowered sputum neutrophil counts compared with prednisolone or placebo. Comparable results were obtained for total cell count, percentage of sputum neutrophils, and for interleukin-8 and myeloperoxidase in sputum supernatant. All treatments were safe and well tolerated. Further evaluation in a large trial of patients with pulmonary disorders is planned (Holz et al., 2010).
GlaxoSmithKline (GSK) has disclosed a CXCR2 antagonist GSK-1325756B (Danarixin; Fig. 8) as a competitive, selective, and potent inhibitor that has just completed phase I studies in healthy volunteers in the United Kingdom. AstraZeneca (London, UK) disclosed an interest in CXCR2 antagonists, and their clinical compound AZD-5069 recently completed a Phase II trial in patients with bronchiectasis in February 2012 in the UK, Poland, and the Czech Republic. Interim results were summarized in Sept. 2011 in the 21st Annual Congress of the European Respiratory Society, Abstract no. P3984. A second compound, AZD-8309, was been tested in healthy volunteers in an LPS airway challenge (Virtala et al., 2012). PA401, an inhibitor of the GAG-mediated step involved in CXCL8-induced CXCR1/CXCR2 activation, is a CXCL8 variant discovered by ProtAffin (Graz, Austria) and is in development for COPD.
2. CXCR3.
CXCR3 is an inflammatory chemotactic receptor specific for CXCL9 (also known as monokine induced by γ-interferon), CXCL10 (interferon-induced protein of 10 kDa), and CXCL11 (I-TAC, interferon-inducible T-cell α-chemoattractant) (Loetscher et al., 1996a, 1998a; Cole et al., 1998; Lu et al., 1999). Although they share one receptor, these three ligands have nonredundant actions in vivo, the result of multiple factors, including differential ligand expression, differential binding to the receptor, and possibly additional nonshared binding sites (Groom and Luster, 2011).
With regard to expression, interferon (IFN)-γ induces production of all three ligands in many cell types (Luster et al., 1985; Farber, 1990; Cole et al., 1998), but they are also differentially regulated by other stimuli, such as the type I interferons (IFNα/β) and nuclear factor κB. CXCL10 is more sensitive to innate stimuli that activate Toll-like receptor-IRF3-dependent induction of type I interferon. It is also preferentially induced by hypoxia-reperfusion injury via nuclear factor κB activation (Medoff et al., 2006) and has been shown to play an early role in the hypoxia-induced inflammation associated with solid organ transplantation, such as heart and lung (Hancock et al., 2001; Medoff et al., 2006). In contrast, CXCL9 is more dependent on and more strongly induced by IFNγ.
With regard to receptor binding, there is a hierarchy of affinity and agonist potency at CXCR3, with CXCL11 > CXCL10 > CXCL9 (Cole et al., 1998; Weng et al., 1998; Cox et al., 2001; Meyer et al., 2001). Moreover, different regions of CXCR3 mediate receptor binding, activation, and internalization for each ligand. CXCR3 is tyrosine-sulfated on its N terminus, and this is required for receptor binding and activation for all three ligands, whereas the proximal 16 amino acid residues of the N terminus are required for CXCL10 and CXCL11 binding and activation, but not for CXCL9 activation (Colvin et al., 2006). Two distinct domains control internalization of CXCR3 (Colvin et al., 2004). The carboxyl-terminal domain and β-arrestin 1 are predominantly required for CXCL9- and CXCL10-directed internalization, whereas ICL3 is required by CXCL11 (Colvin et al., 2004). Structure-activity studies with CXCR3 ligands have identified unique regions in each protein that are important for binding to CXCR3 and to heparin (Campanella et al., 2003; Clark-Lewis et al., 2003; Rosenkilde et al., 2007; Severin et al., 2010). Binding of CXCL9, CXCL10, and CXCL11 to CXCR3 elicits increases in intracellular Ca2+ levels and activates PI3K and MAPK (Smit et al., 2003), and cellular responses include integrin activation, cell adhesion, cytoskeletal changes, and directed cell migration (Piali et al., 1998).
N-terminal processing of CXCR3 ligands by CD26/dipeptidyl peptidase IV results in reduced CXCR3 binding, loss of calcium-signaling capacity through CXCR3, and more than 10-fold reduced chemotactic potency (Proost et al., 2001). Moreover, CXCL10 and CXCL11 cleaved by CD26/dipeptidyl peptidase IV can act as a chemotaxis antagonist of CXCR3. However, the physiologic significance of this is not known, especially because the CXCR3 binding affinity of the truncated forms is ∼10-fold less than the unprocessed forms of the CXCR3 ligands. Nonetheless, the levels of N-terminally processed CXCL10 in the peripheral blood are inversely correlated with the ability of patients to control Hepatitis C virus infection, and it has been suggested that these processed forms of CXCL10 are acting as CXCR3 antagonists and interfering with the host response to Hepatitis C (Casrouge et al., 2011). Several CC-chemokines, particularly CCL11 (eotaxin-1) and CCL13 (MCP-4), also compete with moderate affinity for the binding of CXCL10 to CXCR3 (Weng et al., 1998). CCL26 does not activate CXCR3 but, in CXCR3-transfected cells, can block CXCL10-mediated receptor activation and may therefore be a natural CXCR3 antagonist, although this has not been demonstrated in vivo. Murine CCL21 has also been shown to induce a weak calcium flux in CXCR3 transfected cells, although the physiologic significance of this interaction is not known and human CCL21 does not interact with human CXCR3 (Soto et al., 1998).
CXCR3 is expressed on CD4+ Th1 cells and CD8+ cytotoxic T lymphocytes (CTL) (Loetscher et al., 1996a, 1998a; Yamamoto et al., 2000; Kim et al., 2001b). Early studies showed that T cells from inflamed peripheral tissues in human autoimmune disease are highly enriched in CXCR3 compared with circulating T cells (Loetscher et al., 1998a; Qin et al., 1998; Shields et al., 1999). Moreover, CXCR3 ligands are highly expressed in the same diseased tissues. CXCR3 is not expressed on naive T cells, but is rapidly upregulated after dendritic cell (DC)-induced T-cell activation (Sallusto et al., 1998b; Kim et al., 2001b; Xie et al., 2003). CXCR3+ cells comprise 60–90% of CD8+ memory T cells (Guarda et al., 2007; Hikono et al., 2007) and 40% of CD4+ memory T cells (Kim et al., 2003; Rivino et al., 2004). T-bet, the master transcription factor of Th1 and CTL commitment, directly transactivates CXCR3 (Lord et al., 2005; Beima et al., 2006). Mouse models have verified that CXCR3 and its ligands regulate the migration of Th1 cells into sites of Th1-driven inflammation (Khan et al., 2000; Xie et al., 2003, Campanella et al., 2008a).
CXCR3 is also highly expressed on innate lymphocytes, such as NK cells and NK T cells (NKT), where it may mediate early recruitment to sites of infection and inflammation (Qin et al., 1998; Thomas et al., 2003). It is also expressed on plasmacytoid DCs and subsets of B cells, where it may direct migration to inflamed lymph nodes (Cella et al., 1999; Nanki et al., 2009). Tregs that accumulate at sites of Th1 cell-mediated inflammation have been reported to express the signature Th1 transcription factor T-bet, which is required for CXCR3 expression by these cells and for regulatory function (Koch et al., 2009). This may partially explain modest decreases in T-cell entry in mouse models in which CXCR3 is genetically or pharmacologically inactivated, despite high expression of CXCR3 receptor and ligands in the target tissue.
Cxcr3 is required on CD8+ cells for infiltration into the brain during Plasmodium berghei ANKA infection for the development of cerebral malaria symptoms (Campanella et al., 2008b; Miu et al., 2008). Cxcr3−/− mice are protected from cerebral malaria because of reduced CD8+ CTL sequestration in the brain. The CXCR3 system also participates in the acute response in the brain to Toxoplasma gondii (Khan et al., 2000) as well as in CD8+ T-cell-mediated immunosurveillance of the brain during the chronic phase (Harris et al., 2012). T-cell infiltration of mucosal tissues is also highly dependent on CXCR3. This is true during herpes simplex virus-2 infection of the vaginal mucosa (Thapa et al., 2008; Nakanishi et al., 2009; Thapa and Carr, 2009) and during colitis. In the IL-10 null inflammatory bowel disease model, Cxcl10 and Cxcr3 are highly expressed at sites of colitis because of local production of the ligands, leading to the recruitment of Cxcr3+ T cells. In this model, Cxcl10 neutralization was beneficial (Singh et al., 2008b). In the adoptive transfer model of colitis, CD4+CD25 T cells require expression of Cxcr3 to cause disease in Rag1−/− mice. Interestingly, transfer of Tregs for disease protection in this model does not require Cxcr3, indicating that these different subsets gain access to different locations during disease (Kristensen et al., 2006). Accumulation of effector T cells at sites of autoimmune inflammation is strongly correlated with CXCR3 expression. In addition to autoimmune rheumatoid arthritis (RA) synovium where CXCR3-expressing cells were first characterized in a human disease (Qin et al., 1998) and subsequently shown to regulate T-cell recruitment in murine models (Salomon et al., 2002; Mohan and Issekutz, 2007), deficiency in CXCR3 also reduces autoimmune diabetes and infiltration of T cells into the kidney in systemic lupus erythematosus (Frigerio et al., 2002; Menke et al., 2008; Steinmetz et al., 2009).
More recent data have also indicated an important role for CXCR3 in primary and secondary lymphoid organs. CXCR3 ligands are highly upregulated in the lymph node after infection and immunization, and recent studies have demonstrated a role for CXCR3 in the movement of recently activated CD4+ T cells and central memory CD4+ T cells out of the T-cell zone and into the interfollicular and medullary regions of lymph nodes where they come in contact with antigen-activated innate immune cells (Groom et al., 2012; Sung et al., 2012; Kastenmuller et al., 2013). Similar results have been seen in the spleen where Cxcr3 plays an important role in bringing CD8+ T cells into contact with antigen and inflammatory cytokines after lymphocytic choriomeningitis infection and vaccinia virus infection (Hu et al., 2011; Kurachi et al., 2011). In these models, Cxcr3 deficiency of CD8+ T cells leads to ineffective effector T-cell generation and a resultant expansion of the memory pool.
The CXCR3 ligands are basic proteins that bind avidly to negatively charged glycosaminoglycan (GAG) molecules both on the surface of cells and in the extracellular matrix (Luster et al., 1995; Campanella et al., 2003; Severin et al., 2010). GAG binding is thought to be important for the retention and presentation of chemokines to their chemokine receptors in vivo. Although the in vitro chemotactic activity of CXCL10 and CXCL11 was shown to be GAG binding-independent, the ability of these chemokines to induce CXCR3-dependent T-cell migration in vivo was shown to be dependent on their ability to bind GAGs (Campanella et al., 2006; Severin et al., 2010). The ability of CXCR3 ligands to influence the behavior of certain nonimmune cells, such as endothelial cells and fibroblasts, that do not express CXCR3, has been shown to be a function of the ability of these chemokines to bind to cell surface GAGs (Luster et al., 1998; Proost et al., 2001; Tager et al., 2004; Campanella et al., 2010; Jiang et al., 2010). However, this conclusion is controversial. The identification of an alternative splice variant of CXCR3, termed CXCR3-B, specifically in human endothelial cells, was suggested as a possible explanation for CXCL10’s angiostatic effects (Lasagni et al., 2003). Translation of the putative human CXCR3-B splice variant results in an extracellular N terminus that is 48 amino acids longer than the originally described CXCR3 receptor (referred to as CXCR3-A), with the remaining sequence identical to CXCR3-A. CXCL9, 10, and 11 were shown to bind to CXCR3-B. In addition, CXCL4 (platelet factor 4) was shown to weakly bind CXCR3-B, although subsequent studies using transfected cells found that it binds CXCR3-A with the same low affinity as CXCR3-B and that binding was chiefly mediated by cell surface GAGs (Mueller et al., 2008). Furthermore, although CXCL4 induced intracellular calcium mobilization and Akt and p44/p42 extracellular signal-regulated kinase phosphorylation in activated human T lymphocytes, it failed to elicit migratory responses and did not lead to loss of surface CXCR3 expression, raising doubt about the in vivo functional significance of this interaction (Korniejewska et al., 2011).
CXCR3-B has been described to mediate the angiostatic effect of its ligands, being the preferential CXCR3 receptor reported to be expressed on endothelial cells. Strikingly, overexpression of CXCR3-B in an endothelial cell line resulted in CXCL10 inhibiting proliferation, whereas overexpression of CXCR3-A in the same cell line resulted in CXCL10 augmenting proliferation (Lasagni et al., 2003).
Although the existence of an alternative splice variant of CXCR3 provides a possible explanation for the different functions of CXCL10, it is unclear how a difference in only the N-terminal extracellular domain of CXCR3-A results in intracellular signaling that was purported to oppose CXCR3-A signaling. In addition, although CXCL10’s antiproliferative effects on endothelial cells have been described in mice, the alternative CXCR3-B variant does not exist in mice, as an in-frame stop codon before the conserved sequence would terminate an analogous CXCR3-B splice variant in mice (Campanella et al., 2010). Furthermore, CXCL10 is capable of inhibiting the proliferation of murine endothelial cells that were deficient in CXCR3, and the presence of CXCR3 protein on the surface of human endothelial cells is controversial. Experiments with human endothelial cells also demonstrate that CXCL10 can inhibit endothelial cell proliferation independent of CXCR3 (Campanella et al., 2010). CXCR3-alt is a polymerase chain reaction (PCR)-generated splice variant of CXCR3 encoding a truncated receptor that has not been shown to signal or to be expressed in primary cells (Ehlert et al., 2004). Thus, the existence, relevance, and importance of putative alternate splice forms of CXCR3 remain to be established.
Drug development.
Several synthetic CXCR3-specific small molecule antagonists have been developed that show efficacy in animal models. SCH 546738 from Merck binds to human CXCR3 with high affinity (kD = 0.4 nM) and displaces radiolabeled CXCL10 and CXCL11 from human CXCR3, with an IC50 ranging from 0.8 to 2.2 nM in a noncompetitive manner (Jenh et al., 2012). SCH 546738 potently and specifically inhibits CXCR3-mediated chemotaxis of human activated T cells, with an IC90 of ~10 nM. SCH 546738 attenuated disease development in a mouse collagen-induced arthritis model and reduced disease severity in rat and mouse EAE models. Furthermore, SCH 546738 alone achieved dose-dependent prolongation of rat cardiac allograft survival, and similar to what was seen with the Cxcr3−/− mouse, SCH 546738 in combination with CsA supported permanent engraftment. Amgen Pharmaceuticals has developed small (aza)quinazolinone-based CXCR3 antagonists, the best characterized of which is AMG487, a noncompetitive antagonist (Liu et al., 2009) (Table 3; Fig. 9). It potently inhibits CXCL11-mediated cell migration (IC50 = 15 nM) and calcium mobilization (IC50 = 5 nM) and exhibits >1000-fold selectivity over a panel of other chemokine receptors. In preclinical studies, AMG 487 blocked immune cell migration and demonstrated excellent potency, high selectivity, and good oral bioavailability (Johnson et al., 2007). The drug dose-dependently inhibited cellular infiltration of immune cells into the lungs in a bleomycin-induced model of inflammation in mice. A twice daily dose of 3 mg/kg s.c. was as effective in inhibiting immune cell migration into the lungs as genetic inactivation of Cxcr3. The compound entered phase II clinical trials for the treatment of psoriasis but failed to demonstrate any signs of efficacy, and the trial was terminated (Horuk, 2009).
Fig. 9.
Clinical candidate active at CXCR3.
An analog of AMG487 prolonged cardiac allograft survival in a mouse model and decreased the frequency of interferon-γ-producing cells in the recipient spleen (Rosenblum et al., 2009). CXCR3 blockade for 30 days synergized with short-term, low-dose anti-CD154 monoclonal antibodies to prolong survival past 50 days in 75% of grafts and past 80 days in 25% of the cases.
Medarex has generated a neutralizing monoclonal antibody, MSX-1100, to the CXCR3 ligand CXCL10. The drug had low nanomolar affinity for CXCL10 and was safe in humans. In phase II clinical trials, it demonstrated efficacy in rheumatoid arthritis (Yellin et al., 2012) but not in ulcerative colitis (Bosworth, 2010).
3. CXCR4.
CXCR4 and ACKR3 (CXCR7) are the two most highly conserved chemokine receptors among vertebrates and are essential for life in mice (Fig. 5) (Tachibana et al., 1998; Zou et al., 1998; Sierro et al., 2007). They share the key homeostatic ligand CXCL12, also known as stromal cell-derived factor-1 (SDF-1), and in some settings act cooperatively. CXCR4 is a classic GPCR, whereas ACKR3 (CXCR7) is an atypical receptor signaling in a non–G protein-dependent manner (Fig. 6; see below).
Fig. 5.
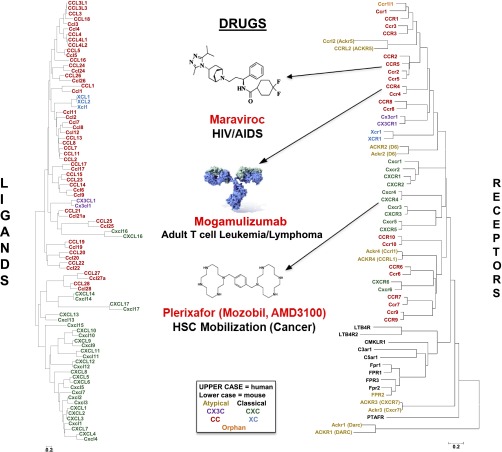
Structural relationship of chemokine ligand and receptor proteins in mouse and human. See color code in box inset at the bottom middle. Structures and disease indications are listed above and below, respectively, the names of marketed drugs acting at the three indicated receptors. Dendrograms prepared by S. Tsang, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
CXCR4 is the only known G protein-coupled chemokine receptor for CXCL12, which is constitutively secreted by bone marrow (BM) stromal cells and many other cell types in many other tissues. CXCL12 binding to CXCR4 can activate all signal transduction pathways typical for chemokine receptors, including adhesion, chemotaxis, survival, and proliferation (Busillo and Benovic, 2007). Six different splice variants of CXCL12 have been reported, which all vary exclusively in the extreme C terminus. The differences in the C termini, not being involved in either binding site one or two of CXCR4, have minor effects on receptor interaction. Most common are CXCL12α and CXCL12β. The extended C terminus of the γ-isoform contains several basic amino acids, has a marked affinity for GAGs, which fosters efficient formation of chemokine gradients (Rueda et al., 2008), and is important for revascularization and infiltration of cells into ischemic tissue (Rueda et al., 2012). The other CXCL12 variants (δ–θ) are poorly characterized.
Several nonchemokine ligands also bind CXCR4. Most important is the envelope protein gp120 of CXCR4 (X4)-tropic HIV. gp120 binds sequentially to CD4 and CXCR4 to allow gp41-guided virus entry. Accordingly, infection with X4-tropic HIV strains is abolished by downregulation of CXCR4 on CD4+ cells (Wilen et al., 2012) and inhibited by CXCL12 (Oberlin et al., 1996). Thus, CXCR4 is referred to as an HIV coreceptor. HIV-1 Env and its subunit gp120 can elicit a complex cellular response that mimics the effects of a chemokine, but whether Env-mediated signaling affects HIV infection and pathogenesis remains unknown (Balabanian et al., 2004; Melar et al., 2007; Wu and Yoder, 2009).
Inflammatory cytokines and danger molecules released from damaged cells or tissues can bind and activate CXCR4, including the pleotropic cytokine macrophage migration inhibitory factor (Bernhagen et al., 2007), extracellular ubiquitin (Saini et al., 2011), and high mobility group protein B1 (HMGB1). HMGB1 is a highly conserved nuclear protein known to act as a damage-associated molecular pattern after release from dead cells. It binds CXCL12 and shifts the efficiency of CXCR4 activation to lower concentrations of CXCL12 (Schiraldi et al., 2012). CXCR4-mediated signal transduction induced by its nonchemokine ligands triggers chemotaxis and conforms to the classic chemokine signal transduction pathway (Thelen, 2001).
CXCR4 is the first chemokine receptor for which highly diffracting crystals have been reported. A heptahelical structure was confirmed from five different crystal structures of the receptor in complex with a small-molecule antagonist (IT1t, an isothiourea derivative) and/or with the cyclic peptide antagonist CVX15 derived from Limulus polyphemus (Wu et al., 2010) (Fig. 2). Overall the conformation of the core of CXCR4 in the crystals bound to IT1t is highly conserved (less than 0.6Å root mean square deviation), but shows slight differences when in complex with the larger molecule CVX15. Compared with other available GPCR structures, CXCR4 displays some unique structural characteristics, which cautions modeling other chemokine receptors on available GPCR structures. Most important is the relative orientation of the helices with their extension into the extracellular and intracellular space. The extracellular end of helix VII reaches two turns longer into the extracellular space than in other GPCRs and ends with a cysteine that forms a second extracellular disulfide bridge with Cys28 located in the N terminus. The distinct extracellular architecture of CXCR4 is consistent with the large size of its ligand CXCL12 compared with ligands of other crystallized GPCRs. In particular, ECL2 makes extensive contact with CVX15 in the crystal structure. The interaction with CVX15 presumably mimics the binding of CXCL12 where the N terminus of the chemokine falls deeply into the binding pocket.
In addition, CXCR4 lacks the short helix VIII located in the C terminus proximal to helix VII of other crystallized GPCRs. The region in question of CXCR4 shows some homology to the canonical sequence, leaving the possibility that the C terminus might fold into a short helix depending on the local environment, as exemplified recently for the three-dimensional structure of CXCR1 in a phospholipid bilayer by NMR spectroscopy (Park et al., 2012). Moreover, CXCR4 lacks a palmitoylation consensus in the C terminus, which in other class A receptors hooks the C terminus to the membrane (Wu et al., 2010).
In most available crystal structures, GPCRs orient in a nonfunctional manner (e.g., antiparallel), precluding any conclusions about possible receptor dimerization. However, in the case of CXCR4, a distinct contact site comprising helix V and VI is found in five different crystal packings. The strongest interaction of two receptor protomers is mediated by hydrophobic side chains of amino acids located in helix V with some participation of Lys267 from helix VI (Wu et al., 2010). The proposed homodimeric structure of CXCR4 is consistent with predictions made from detergent-solubilized receptor (Babcock et al., 2003). Many reports using overexpression systems suggest that CXCR4 arranges in heterodimers with some, but not all, chemokine receptors; however, whether this occurs in primary cells for receptors at natural abundance remains unknown (Thelen et al., 2010). The hydrophobic side chains, which form the dimer interface in CXCR4, are poorly conserved in other chemokine receptors, so it is unlikely that potential heterodimers, including CXCR4 would display a structure similar to the CXCR4 homodimer.
The X-ray data of CXCR4 provide further support for the “two-step” binding model of CXCL12, where the core domain of the chemokine binds to site one in the N terminus of CXCR4 and the N terminus of the chemokine binds site two (Crump et al., 1997). NMR studies of the N terminus of CXCR4 (p38) in complex with CXCL12 are consistent with site one. Of note is the role of the tyrosine residues in the N terminus of CXCR4 (particularly Tyr21 and to a lesser extend Tyr12 and Tyr7), which can undergo post-translational sulfation in the Golgi apparatus. The acidic modification of the tyrosine residues enhances the affinity for the basic chemokine (Seibert et al., 2008). Fully sulfated CXCR4 p38 tends to promote CXCL12 dimerization, but the dimeric chemokine is only a partial agonist at CXCR4, not inducing chemotaxis but maintaining the ability to mobilize calcium (Drury et al., 2011). The crystal structure of CXCR4 can accommodate several receptor-chemokine combinations, including monomeric (CXCL12:CXCR4), dimeric CXCL122:CXCR42), or mixed (CXCL12:CXCR42) conformations (Wu et al., 2010), thus leaving open the exact stoichiometry of a functional receptor ligand complex.
Taken together, the X-ray data unveil multiple potential receptor conformations and ligand interactions, which add to the complexity of context-dependent CXCR4-mediated signal transduction. Some prudence should be exercised in interpretation of the structural data, insofar as all crystal structures of GPCRs, including CXCR4, are obtained with extensively modified receptors, i.e., truncation at the N and C termini as well as insertion of stabilizing amino acids and sequences in the third intracellular loop. This could explain the differences noted in the structure of CXCR1, solved by NMR spectroscopy for an unmodified unliganded receptor in liposomes (Park et al., 2012).
In jawed vertebrates, CXCR4 is expressed throughout development and is the only G protein-coupled chemokine receptor essential for life. The protein is widely expressed on nondifferentiated and differentiated tissues. CXCR4 is found on almost all hematopoietic cells, vascular endothelial cells, in neurons of the central and peripheral nervous system, microglia, and astrocytes (Murphy et al., 2000). It is also functionally expressed by many cancer cells of hematopoietic and nonhematopoietic origin (Balkwill, 2004). Presumably in context with specific adhesion molecules, CXCR4, in addition to promoting survival and growth, may direct metastasis to selected CXCL12-rich organs, e.g., osteosarcoma to the lungs; breast cancer to BM, lung, and liver; and prostate cancer mostly to BM. A role of CXCR4 in lymph node metastasis is not clear despite the pronounced expression of the chemokine. It appears that other chemokine receptors, such as CCR7, may play a more decisive role there (Balkwill, 2004).
The main activities associated with CXCR4 are cell migration (homing) and positioning (homeostasis), neovascularization, survival, and growth. This wide spectrum of activities is unique for chemokine receptors and suggests distinctive signal transduction properties. However, CXCR4 shares the activation of downstream signaling pathways with other typical chemokine receptors. The receptor couples to pertussis toxin sensitive Gi proteins, stimulates phospholipase C leading to calcium mobilization, triggers the MAPK cascade and the protein kinase B/PI3K pathway, and activates arrestin-dependent signaling (Busillo and Benovic, 2007). The unique signaling properties probably do not depend on the activation of these common pathways, but instead on the context of CXCR4, differences in length of stimulation and coupling efficiency, and the interaction with specific proteins (Thelen and Stein, 2008). The proposed dimeric architecture may not be shared by other chemokine receptors and thus may provide a unique docking platform. In addition, CXCR4 signaling is context-dependent, e.g., in B-cell subsets the receptor does not trigger calcium mobilization or chemotaxis, despite surface expression and signaling competence of the cells. The concentration and aggregation state of CXCL12 also contributes to the signaling quality (see above). Context might also be given by neighboring chemokine receptors that might even form functional heterodimers, as recently proposed in support of the observation that small molecule antagonists can inhibit the function of chemokine receptors on which they do not directly bind (Sohy et al., 2009). Molecular characterization of potential receptorsomes from primary cells remains important work for the future.
CXCR4 plays key roles in immune system development during both lymphopoiesis and myelopoiesis (Fig. 7). The receptor retains hematopoietic precursors in the BM, mediates B-cell segregation in lymphoid organs and mediates neutrophil egress from BM and BM homing of senescent neutrophils (Murphy et al., 2000). Cxcr4-deficient mice exhibit defective bone marrow myelopoiesis and B-cell lymphopoiesis, as well as developmental defects in the brain, heart, and stomach vasculature. CXCR4 signaling may also be important in naive and memory B-cell trafficking to germinal centers. Mice harboring a CXCL12-promoted gain of function for Cxcr4 exhibited abnormal compartmentalization of B cells in the periphery, with a reduction of primary follicles in the spleen and their absence in lymph nodes (Balabanian et al., 2012).
In humans, WHIM syndrome, a rare immunodeficiency disorder, is the only disease shown to be caused by Mendelian inheritance of mutations in a chemokine system element (Table 5). WHIM is an acronym for warts caused by human papillomavirus infection, hypogammaglobulinemia, infections, and myelokathexis (abnormal retention of senescent neutrophils in the BM, which is associated with panleukopenia). Almost all known WHIM mutations result in partial truncation of the C terminus of CXCR4, which leads to agonist-stimulated gain-of-function for the receptor (Hernandez et al., 2003). The mechanism involves enhanced G protein coupling as well as arrestin-dependent signaling (Lagane et al., 2008) and loss of phosphorylation sites on the C terminus important for receptor desensitization and internalization (Busillo and Benovic, 2007), causing retention of mature leukocytes in the BM and possibly in other immune organs (Hernandez et al., 2003; Gulino et al., 2004; Balabanian et al., 2005b; Kawai et al., 2007). However, patients are able to mobilize leukocytes to the blood during infections and therefore develop recurrent bacterial infections that are usually not life threatening, and may survive into adulthood. Thus, the apparent paradox of patients with WHIM syndrome is to exhibit a profoundly altered immune function and yet a limited susceptibility to viral and bacterial pathogens, with the notable exception of human papillomavirus (Beaussant Cohen et al., 2012), the signature pathogen in WHIM syndrome. This may eventually be a consequence of altered immune responses (Tassone et al., 2010) and the hijacking of the CXCL12/CXCR4 signaling axis as a host susceptibility factor for the virus (Chow et al., 2010).
TABLE 5.
Important chemokine system mutations strongly associated with human disease
| Molecule | Mutation | Disease | Phenotype | Mechanism |
|---|---|---|---|---|
| CCR5 | CCR5Δ32 (loss of function) | HIV/AIDS | Resistance to initial infection (−/−) | Loss of HIV coreceptor CCR5 prevents cell entry by R5-tropic HIV-1 |
| Delayed disease progression (+/−) | ||||
| West Nile virus infection | Increased risk of symptomatic infection (−/−) | Reduced leukocyte trafficking to brain | ||
| Rheumatoid arthritis | Reduced risk (+/−) | Reduced inflammation | ||
| Chronic renal allograft rejection | Reduced risk (−/−) | Reduced inflammation | ||
| CXCR4 | C-tail truncations (gain of function) | WHIM Syndrome | Warts | Impaired egress of leukocytes from bone marrow |
| Hypoglobulinemia | ||||
| Infections | ||||
| Myelokathexis (Mendelian AD) | ||||
| CX3CR1 | CX3CR1-M280 | Cardiovascular disease | Reduced risk (+/−) | Reduced foam cell accumulation in vessel wall |
| Age-related macular degeneration | Increased risk (+/−) | Microglial cell accumulation in the subretinal space | ||
| ACKR1 | FYB(ES) (loss of function) | Plasmodium vivax malaria | Resistance to initial infection (−/−) | Promoter mutation abolishes RBC expression of ACKR1, which is required for cell entry by P. vivax |
| CCL26 | CCL26 GG | Eosinophilic esophagitis | Increased risk (GG) | CCR3-dependent eosinophil trafficking |
−/−, Homozygous; +/−, heterozygous; AD, autosomal dominant; GG, genotype GG; RBC, red blood cell.
Drug development.
Given the importance of CXCR4 in HIV infection and its putative involvement in cancer, attempts have been made to identify inhibitors (Table 3). The class of peptide-based CXCR4 inhibitors consists of antibodies, chemokine analogs, derivatives of the horseshoe crab protein polyphemusin, and endogenous defensins. From the latter group of molecules several can block HIV replication, but only human β defensin-3 efficiently competes for CXCL12 binding at CXCR4 (Feng et al., 2006). Human β defensin-3 downregulates CXCR4 without activating canonical Gi-coupled signal transduction, a rare property for a GPCR.
Cyclic polyphemusin peptide inhibitors include T22 ([Tyr5,12, Lys7]-polyphemusin II), T134, T140, and their derivatives (Liang, 2008). From this group, the 14-amino acid polypeptide 4F-benzoyl TN14003 (BKT140) (Beider et al., 2011) is currently in phase I/II clinical trials for multiple myeloma after chemotherapy and BM transplantation.
Inhibitory CXCL12 analogs, designed based on the importance of the N terminus for receptor activation but not for binding (Liang, 2008), include P2G-CXCL12 (where Pro2 is replaced with Gly) (Crump et al., 1997), (1–9)P2G2 (a short dimeric peptide that lacks the chemokine core domain (Loetscher et al., 1998b), and a 10-residue substituted dimer derived from the amino acid 5–14 sequence of CXCL12 (Heveker et al., 2001).
From the panel of available antibodies that efficiently block CXCR4, the best characterized is the mouse mAb 12G5. This antibody binds to a conformational epitope on human CXCR4 comprising ECL2 and ECL3 and inhibits HIV and CXCL12 binding. For clinical applications, the fully human anti-CXCR4 (IgG4) from Bristol-Myers Squibb (BMS; Princeton, NJ) (BMS) and Medarex (Princeton, NJ) (BMS-936564/MDX-1338) is currently being tested for treatment of multiple myeloma (phase 1) after proof-of-principle studies indicating that detachment from bone marrow stromal cells may sensitize cancer cells to chemotherapy (Azab et al., 2009; Kuhne et al., 2013). Recently, a phase I clinical trial in healthy volunteers was completed by Ablynx (Ghent, Belgium) to test the safety of a llama-derived CXCR4-targeted nanobody, ALX-0651, after proof-of-principle studies of this approach in blocking chemotaxis and HIV and mobilizing stem cells (Jahnichen et al., 2010).
Virus-derived chemokine-binding proteins (vCKBP) display diverse mechanisms of interfering with chemokine receptor signaling. The herpes simplex virus 1 and 2 proteins gG1 and gG2 bind CXCL12α and β, thereby enhancing chemokine-induced CXCR4 signaling. Both vCKBPs increase the chemotactic potency and directionality of CXCL12-induced migration (Viejo-Borbolla et al., 2012). By contrast, ectromelia virus-derived E163 does not interfere with binding and triggering of CXCL12 at CXCR4, but instead masks the GAG binding site of the chemokine, preventing adhesion to cell surfaces, which is important for the formation of chemotactic gradients (Ruiz-Arguello et al., 2008). Kaposi's sarcoma-associated herpesvirus (HHV8)-encoded vMIP-II (vCCL2) is a strong antagonist of CXCR4, preventing binding of CXCL12 and activation of the receptor. vMIP-II also inhibits CXCR4-mediated cell entry of HIV (Kledal et al., 1997).
Low molecular weight antagonists of CXCR4 (Liang, 2008) include the bicyclam AMD3100 (plerixafor or Mozobil; Sanofi, Paris, France), which reversibly binds CXCR4 and blocks CXCL12 binding and HIV infection (De Clercq, 2009) (Fig. 5). It also promotes leukocytosis, including release of precursors from bone marrow niches. In this regard, in 2009 the FDA approved plerixafor in combination with granulocyte macrophage colony-stimulating factor (GM-CSF) for mobilization of stem cells for BM transplantation in patients with multiple myeloma and non–Hodgkins’ lymphoma receiving cytoreductive therapy (Pusic and DiPersio, 2010). Currently, another clinical trial is underway testing small doses of plerixafor as mechanism-based treatment to dial down hyperfunctional CXCR4 signaling in WHIM syndrome. Repeated administration of plerixafor is well tolerated and leads to decreased leukopenia (Dale et al., 2011; McDermott et al., 2011). A related substance of lower molecular weight and charge, the monocylam AMD3465, also potently inhibits CXCR4 (Bodart et al., 2009).
The non–cyclam AMD11070 (ritonavir) is the first orally bioavailable small molecule CXCR4 inhibitor introduced to the clinic. Early results indicate that it is well tolerated (Moyle et al., 2009). Another unrelated chemical structure, the chalcones, also inhibit CXCL12 binding and activation of CXCR4 but by neutralizing CXCL12 (Hachet-Haas et al., 2008).
The success of plerixafor has paved the way for a number of companies to target CXCR4. Among these is TaiGen with their CXCR4 antagonist TG-0054 (burixafor). The structure of the drug has not yet been disclosed, but TaiGen has disclosed polyamine and pyrimidines as CXCR4 antagonists in patent applications. Phase I clinical trials revealed that single-dose administration of the antagonist more than increased the number of circulating stem cells to the numbers required for a successful transplant. Currently, the antagonist is undergoing several phase II clinical trials for the treatment of multiple myeloma and non–Hodgkin’s lymphoma.
Isothioureas have better biopharmaceutical properties than plerixafor and are orally bioavailable. A primary compound (IT1a, NIBR1816) and several chemical derivatives, the most potent being IT1t, which were used for CXCR4 crystallization (Thoma et al., 2008), have potent anti-HIV activity, compete with CXCL12 binding in the nanomolar range, and mobilize hematopoietic stem cells (Fiorina et al., 2011).
Other reported CXCR4 inhibitors are the polyarginine ALX40-4C; the peptoids CGP64222, KRH-1636, and KRH-2371 (Murphy et al., 2000; Liang, 2008); POL2438 and POL3026, which are polyphemusin II-derived sequences incorporated into a macrocyclic template-bound β-hairpin mimetic (DeMarco et al., 2006); and SPC3, a branched peptide consisting of eight GPGRAF motifs that are derived from the consensus sequence in the V3 loop of gp120 (Liang, 2008)
The CXCR4 antagonist described by Polyphor, POL6326, is derived from their proprietary technology, known as Protein Epitope Mimetics (DeMarco et al., 2006). According to the company, POL6326 has successfully completed phase I clinical trials in the United Kingdom with 74 healthy volunteers. The drug was safe and well tolerated and is currently being investigated in a phase II clinical trial for safety and efficacy in transplantation of autologous hematopoietic stem cells in patients with multiple myeloma after chemotherapy.
Two other CXCR4-blocking approaches in the clinic are worth mentioning. The first is a peptide, BKT140, that is reported to block CXCR4 and is in phase I clinical trials (Burger et al., 2011). The second is NOX-A12, which is a mirror image RNA of the RNA for the chemokine CXCL12, thus blocking expression of the protein and preventing receptor engagement. Phase I clinical studies were safe, and the company is currently assessing this molecule in phase II clinical trials for the treatment of multiple myeloma and chronic lymphocytic leukemia.
4. CXCR5.
CXCR5 is a homeostatic receptor that binds monogamously with the chemokine CXCL13 and regulates adaptive immunity (Gunn et al., 1998; Legler et al., 1998). It was the first chemokine receptor shown to be involved in lymphocyte homing and development of normal lymphoid tissue (Forster et al., 1996) and the first B-cell-selective chemokine receptor (Forster et al., 1994; Gunn et al., 1998; Legler et al., 1998).
CXCR5 has been detected on all peripheral blood and tonsillar B cells, but not on plasmablast and plasma cells and only on a fraction of cord blood and bone marrow B cells (Wehrli et al., 2001; Shaffer et al., 2002). It is also present on a small subset of peripheral blood memory CD4+ and CD8+ T cells. In contrast, in secondary lymphatic tissue, the majority of CD4+ T cells are positive, and in cord blood T cells are negative (Kaiser et al., 1993; Forster et al., 1994). Similar to other chemokine receptors, CXCR5 is dynamically regulated on T cells. After TCR stimulation, CXCR5 is upregulated on memory/effector T cells, whereas IL-2 causes downregulation (Sallusto et al., 1999).
CXCL13 is produced selectively by high endothelial venule endothelial cells and follicular stromal cells and is constitutively displayed on follicular HEV. The CXCL13/CXCR5 axis supports ∼50% of the signaling required for B-cell entry from blood to Peyer’s patch; CXCR4 may also contribute (Okada et al., 2002, 2005). In Cxcr5−/− mice, B cells do not migrate to lymph node, Peyer’s patches are abnormal, and inguinal lymph nodes are absent. CXCL13 is also required for B1 cell homing, natural antibody production, body cavity immunity, and lymphoneogenesis in the setting of autoimmunity (Luther et al., 2000; Ansel et al., 2002). Cxcr5−/− mice still can produce antibody, perhaps in part because B cells and follicular DCs, by an unknown mechanism, are able to form ectopic germinal centers within T zones of the periarteriolar lymphocyte sheath of spleen.
Drug development.
No CXCR5 antagonists have been developed yet; however, targeting this receptor in autoimmunity is an attractive direction for the future.
5. CXCR6.
Human CXCR6 is located on chromosome 3 (Liao et al., 1997b; Loetscher et al., 1997) at 3p21.31, between CCR9 and XCR1, in the cluster that contains CC, XC, and CX3C (but not CXC) chemokine receptors (Fig. 3). It is notable that in phylogenetic analysis, CXCR6 is most closely related not to other CXC receptors but instead is in a cluster with CCR6, CCR7, and CCR9 and is closest either to CCR6 (Lio and Vannucci, 2003) or CCR9 (Nomiyama et al., 2011), depending on the analysis. CXCR6 has orthologs in multiple species, including reptiles (Nomiyama et al., 2011). Human (and mouse) CXCR6 are unusual in having a DRF sequence in place of the canonical DRY motif at the end of the third transmembrane helix. CXCR6 has a monogamous relationship with CXCL16, which binds with a Kd of 1 nM (Matloubian et al., 2000; Wilbanks et al., 2001). Analogous to its receptor, CXCL16 is an unusual chemokine. CXCL16 is found at 17p13.2, separate from the genes for other chemokines, and phylogenetic analysis shows that CXCL16 is not closely related to other CXC chemokines (Fig. 4) (Huising et al., 2003). CXCL16’s most striking feature is a transmembrane domain (Fig. 2), and CXCL16 can be found in both membrane-bound and soluble forms, with the latter generated from the former by the proteolytic activity of ADAM10 (Abel et al., 2004). Immobilized CXCL16 has been reported to mediate CXCR6-dependent adhesion in the absence of CXCR6 signaling in some systems (Nakayama et al., 2003; Shimaoka et al., 2004), although in other cases adhesion required CXCR6-induced integrin activation (Heydtmann et al., 2005). CXCL16 is additionally notable in that it was discovered independently of its chemokine activity as a scavenger receptor and was originally designated as SR-PSOX (an acronym for “scavenger receptor for phosphatidyl serine and oxidized low-density lipoprotein”) (Shimaoka et al., 2000). CXCR6 is expressed on T cells, including γ/δ T cells (Deng et al., 1997; Liao et al., 1997b; Unutmaz et al., 2000), as well as on NKT cells (Kim et al., 2002), NK cells, and plasma cells (Nakayama et al., 2003). Outside of the hematopoietic compartment, CXCR6 has also been found on epithelial and nonepithelial cancers (Wang et al., 2008; Darash-Yahana et al., 2009; Gao et al., 2012). CXCL16 is expressed on antigen-presenting cells such as dendritic cells, monocyte/macrophages, and possibly B cells (Matloubian et al., 2000; Shimaoka et al., 2000; Wilbanks et al., 2001), as well as on fibroblastic reticular cells, activated T cells (Darash-Yahana et al., 2009), endothelial cells (Abel et al., 2004), and cancer cells (Hojo et al., 2007; Darash-Yahana et al., 2009; Gao et al., 2012).
CXCR6 was initially identified as an entry coreceptor on T cells, along with CD4, for strains of HIV-1 and simian immunodeficiency virus (Alkhatib et al., 1997; Deng et al., 1997; Liao et al., 1997b), and also can be used by primary isolates of HIV-2, particularly those isolated from individuals without plasma viremia (Blaak et al., 2005). Although studies have reported primary isolates of HIV-1 that are able to use CXCR6 in virus entry (Isaacman-Beck et al., 2009; Zhang et al., 2001b) and infection assays (Sharron et al., 2000), there is no established role for CXCR6 in supporting viral infection in vivo. Nevertheless, a polymorphism that results in a nonconservative change in the CXCR6 N terminus was associated with improved survival after infection with Pneumocystis carinii in a group of HIV-infected African-American intravenous drug users (Duggal et al., 2003). In addition, a single nucleotide polymorphism in the 3′-nontranslated region of CXCR6 has been associated with long-term nonprogression to AIDS in several cohorts (Limou et al., 2010). Whether these associations are related to CXCR6 activity as an HIV coreceptor or, perhaps more likely, its activity in the host immune system remains unresolved.
CXCR6 expression on human CD4+ T cells is restricted to the effector/memory population and has been associated with the Th1 and Th17 phenotypes (Kim et al., 2001a; Annunziato et al., 2007; Singh et al., 2008a). CXCR6 is significantly enriched on both CD4+ and CD8+ T cells found in inflamed tissue, such as joints in rheumatoid and psoriatic arthritis (Kim et al., 2001a). CXCR6 is expressed on mouse (Johnston et al., 2003) and human (Kim et al., 2002) NKT cells and is important for survival and activation of liver NKT cells in mice (Geissmann et al., 2005; Germanov et al., 2008). CXCR6 is also important for the maintenance of “memory” NK cells in the liver, which can confer pathogen-specific protection (Paust et al., 2010). Recent work suggests that CXCR6/CXCL16 are important for regulating NKT cell accumulation in colon and lung in mice and that CXCL16 expression at these sites is downregulated by neonatal colonization with bacterial flora (Olszak et al., 2012). Germ-free mice showed increased numbers of NKT cells at these sites that led to exaggerated susceptibilities in models of colitis and asthma (Olszak et al., 2012).
A number of studies have suggested roles for CXCR6 and CXCL16 in cancer, although the data suggest that the roles differ depending on tumor type. Expression of CXCR6 and/or CXCL16 is increased in prostate cancer, with expression correlating with aggressiveness (Lu et al., 2008; Wang et al., 2008; Darash-Yahana et al., 2009; Ha et al., 2011). Similarly, expression of CXCR6 is associated with increased inflammation and poor prognosis in hepatocellular carcinoma (Gao et al., 2012). The cancer-promoting effects of CXCR6/CXCL16 have been postulated to result from direct effects on cancer cells and/or the tumorigenic activities of recruited leukocytes and/or angiogenesis. On the other hand, expression of CXCL16 in tumors has been associated with improved outcomes and increased antitumor immune responses in cancers of the colon, breast, and kidney (Hojo et al., 2007; Matsumura et al., 2008; Gutwein et al., 2009).
Both the proinflammatory activity of CXCR6/CXCL16 and the activity of CXCL16 as a scavenger receptor for oxidized low-density lipoprotein have led to interest in roles for CXCL16/CXCR6 in atherosclerosis. However, data from mouse models have suggested two apparently contradictory effects. In its activity as a scavenger receptor, CXCL16 is atheroprotective (Aslanian and Charo, 2006; Barlic et al., 2009), whereas in its activity in T-cell recruitment, CXCR6 (and presumably CXCL16, possibly in a soluble form) is proatherogenic (Galkina et al., 2007).
Drug development.
Notwithstanding the evidence linking CXCR6 to a number of important diseases, there are no published data on the development of CXCR6 antagonists or neutralizing reagents for clinical use.
6. CXCR7 (now ACKR3, see Section III.A.3)
B. CC Chemokine Receptors
1. CCR1.
CCR1 is broadly expressed on both hematopoietic and nonhematopoietic cells and binds promiscuously to the inflammatory CC chemokines CCL3 (also known as macrophage inflammatory protein-1α or MIP-1α), CCL5 (previously named reduced upon activation, normal T expressed and secreted or RANTES), CCL6, CCL9, CCL15, and CCL23 (see nonstandard names in Table 1). All CCR1 ligands also bind other chemokine receptors; thus, CCR1 lacks a selective chemokine ligand. The full-length forms of CCL6, CCL9, CCL15, and CCL23 bind with low affinity, but proteolytic modification at the N terminus during inflammatory responses in vivo may increase the affinity (Berahovich et al., 2005). Although full-length CCL4 (MIP-1β) is not a CCR1 ligand, activated human peripheral blood lymphocytes secrete an N-terminally truncated form named CCL4 3-69, which is a low-affinity CCR1 ligand, although it functions as an antagonist. This form is truncated by CD26, a membrane-bound ectopeptidase with dipeptidyl peptidase IV activity (Guan et al., 2004).
CCR1 activates the classic chemokine signaling pathway through the Gi/o class of G proteins (Murphy et al., 2000), causing inhibition of adenylyl cyclase and activation of phospholipase C, protein kinase C, calcium flux, and PLA2 (Nardelli et al., 1999). However, not all CCR1 ligands appear to mediate the same functions. For example, although CCL3, CCL5, and CCL16 are all able to transduce signals through CCR1 in a human osteosarcoma line via Gi/Go, phospholipase C, and protein kinase Cδ-mediated pathways, CCL16 can also signal via p38 MAPK, and in contrast to the other two ligands does not induce calcium transients (Kim et al., 2005). The CCR1 ligands CCL3, CCL7, CCL5, and CCL15 can all inhibit adenylyl cyclase activity in cells transiently transfected with CCR1. However, only CCL15 was unable to signal via Gα14- or Gα16-coupled pathways (Tian et al., 2004).
CCR1 is expressed on monocytes, memory T cells, basophils, and dendritic cells (Murphy et al., 2000). It is also constitutively expressed on murine neutrophils, whereas little to no expression is observed on unstimulated human neutrophils. With regard to nonhematopoietic cells, CCR1 is expressed on lung airway smooth muscle cells; normal neurons and dystrophic neurons from patients with Alzheimer's dementia; astrocytes; endothelial cells, which suggest a possible role in angiogenesis; and in multiple myeloma cells and osteoclasts, hinting at a role in bone cancer (Horuk, 2001).
CCR1 is rapidly upregulated and functional in human neutrophils treated with GM-CSF (Horuk, 2001). Conversely, CCR1 (and other CCRs) on dendritic cells and monocytes can be deactivated by LPS and other activating agents in the presence of IL-10 (D'Amico et al., 2000). In this state, the receptor may scavenge inflammatory chemokines and suppress inflammation. CCR1 can also be silenced by drugs, including statins and estrogens. Statins also reduce expression of many chemokines (CCL2, CCL3, and CCL4) and other chemokine receptors (CCR2, CCR4, and CCR5) in endothelial cells and/or macrophages stimulated with TNF-α or IFN-γ, respectively. This may explain in part their anti-inflammatory activity (Veillard et al., 2006). The mechanism may involve inhibition of geranylgeranylation. Estrogens may reduce relapses in patients with multiple sclerosis (Soldan et al., 2003), in part by inhibiting expression of CCR1 and its ligands and that of other chemokines and chemokine receptors (Matejuk et al., 2001).
CCR1 has a number of nonredundant functions in host defense and inflammation (Domachowske et al., 2000; Murphy et al., 2000; Hickey et al., 2007; Liehn et al., 2008). Ccr1−/− mice are more susceptible to chronic demyelination after challenge with mouse hepatitis virus and to Aspergillus fumigatus and T. gondii (Murphy et al., 2000), but are less susceptible to granuloma formation in response to Schistosoma mansoni eggs. The precise cellular mechanisms of action are undefined. Both Ccr1 and Ccl3 appear to also be important for protection against infection with pneumonia virus of mice, a highly virulent mouse paramyxovirus (Domachowske et al., 2000). In mice lacking Ccl3 or Ccr1, virus-induced neutrophil recruitment to the airway is blunted, the eosinophil response is blocked, and mortality is markedly increased. Similar to a number of other immune proteins, CCR1 has been pirated by a virus, human cytomegalovirus, to create the receptor US28 (see below).
Ccr1 also plays an important role beyond host defense and infectious disease. Ccr1−/− mice are protected from pulmonary inflammation secondary to acute pancreatitis (Murphy et al., 2000), associated with decreased TNF-α. Thus, Ccr1 may be an early player in the systemic inflammatory response syndrome. Ccr1 also appears to be involved in remodeling after myocardial ischemia (Liehn et al., 2008). Infarct size was decreased and left ventricular function was preserved in Ccr1−/− animals, associated with an altered postinfarct inflammatory cytokine pattern. A role for Ccr1 in pulmonary injury and fibrosis was recently discovered in a model of thoracic radiation using Ccr1−/− mice and the Ccr1 inhibitor BX471 (Yang et al., 2011). By contrast, mice lacking Ccr5 were not protected. CCR1 is expressed in demyelinating lesions in multiple sclerosis (Trebst et al., 2001). Consistent with a functional role, Ccl3 neutralization is completely protective in a relapsing-remitting EAE model of multiple sclerosis in mice (Karpus et al., 1995) and Ccr1−/− mice have decreased incidence and severity of disease in myelin oligodendrocyte glycoprotein-induced EAE (Rottman et al., 2000). The fact that Ccl3 neutralization is more effective than Ccr1 inactivation suggests that other Ccl3 receptors such as Ccr5 may play a role in pathogenesis and could explain in part the failure of CCR1 antagonists in clinical trials in MS. CCR1 has also been reported to mediate neutrophil recruitment to the inflamed joint in a mouse model of rheumatoid arthritis, acting in a temporal sequence with the leukotriene B4 receptor BLT1 (Chou et al., 2010; Sadik et al., 2012) (Figs. 8 and 9).
Drug development.
To date, eight potent and selective CCR1 antagonists have been tested in human clinical trials, but results have all been disappointing (Table 3; Fig. 10). BX471 (Berlex, Richmond, CA) (Liang et al., 2000) was efficacious in preclinical models of multiple sclerosis, heart transplantation, renal fibrosis, and multiple myeloma (Pease and Horuk, 2009) but was ineffective in several phase II clinical trials, including multiple sclerosis, psoriasis, and endometriosis. MLN3897 (Millennium, Cambridge, MA) (Carson et al., 2011) inhibited CCL3-induced immune cell recruitment in a guinea pig skin sensitization model and impaired osteoclastogenesis by multiple myeloma cells (Vallet et al., 2007), but was discontinued after it failed to reach its clinical endpoint in a phase II trial for rheumatoid arthritis (Vergunst et al., 2009). The drug may have failed because of incomplete receptor coverage (Dairaghi et al., 2011; Lebre et al., 2011). CP-481,715 (Pfizer, New York, NY) (Gladue et al., 2003) was effective in models of inflammation in CCR1-humanized mice (Gladue et al., 2006) and was successful in a small phase Ib clinical trial (Gladue et al., 2006). A phase II trial in RA was stopped after 6 weeks because the compound did not demonstrate efficacy (Brown et al., 2007). Although plasma exposure was published for the phase Ib trial, where it was dosed three times a day, the formulation was changed to allow twice a day dosing in the phase II trial. As discussed in Schall and Proudfoot (2011), receptor coverage may have been insufficient; however, plasma exposure was not revealed. CCX354 (ChemoCentryx, Mountain View, CA) is an azaindazole that blocks chemotaxis of THP-1 cells induced by synovial fluid from patients with RA. CCX354 was active in two animal models (Dairaghi et al., 2011), and phase II clinical trial data showed that it reached its clinical endpoints, including a reduction in both disease score and proinflammatory markers. Merck and its subsidiary Banyu developed two clinical CCR1 antagonists, presumably xanthene carboxamides: C-6448 for multiple sclerosis and C-4462 for rheumatoid arthritis (Naya et al., 2003). No efficacy data are available from phase II clinical trials. AZD4818 (AstraZeneca) is a spirocyclic piperidine derivative (Kerstjens et al., 2010) that was effective preclinically and was entered into clinical trials for the treatment of chronic obstructive pulmonary disease (COPD) by inhalation at a dose of 300 μg twice daily for 4 weeks. Although the drug was well tolerated, it failed to meet its clinical endpoints (Kerstjens et al., 2010).
Fig. 10.
Clinical candidates active at CCR1.
2. CCR2.
CCR2 is a high-affinity receptor for members of the monocyte chemotactic protein (MCP) family, including CCL2, CCL7, and CCL13 in humans and the corresponding murine chemokines Ccl2 (also known as JE/MCP-1) and Ccl7 (Charo et al., 1994; Ben-Baruch et al., 1995; Garcia-Zepeda et al., 1996; Gong et al., 1997; Sarafi et al., 1997). CCL11 and the HIV-1 Tat protein also are CCR2 agonists (Albini et al., 1998).
Human CCR2 gives rise to two alternatively spliced transcripts encoding 360 (CCR2a) and 374 (CCR2b) amino acid polypeptides that diverge only in the C-terminal cytoplasmic region (Charo et al., 1994; Wong et al., 1997). In cell transfectants, the two receptors have similar ligand profiles and functional properties (Sanders et al., 2000); however, they may be regulated differentially in vivo (Cho et al., 2007). The function of CCR2a, which is mostly found in intracellular compartments because of the presence of a retention signal located in its unique C-terminal domain (Wong et al., 1997), is presently unknown. Mouse Ccr2 has 80% amino acid identity to CCR2b and a similar expression profile (Kurihara and Bravo, 1996).
CCR2 is expressed by immature dendritic cells, basophils, NK cells, and T lymphocytes, but not neutrophils or eosinophils. In mouse, Ccr2 is also expressed by the inflammatory Ly6Chigh monocyte subset (Ly6Chigh/CCR2+/CX3CR1low), which is actively recruited to inflamed tissues and gives rise to macrophages and antigen-presenting cells. Conversely, nonclassic Ly6Clow "patrolling" monocytes, which emigrate relatively poorly to inflammatory sites and have been proposed to promote tissue healing and angiogenesis, are negative for Ccr2 and instead express high levels of Cx3cr1 (Geissmann et al., 2010). Low levels of CCR2 agonists secreted under normal conditions maintain homeostatic migration of inflammatory monocytes from bone marrow into the circulation and rapidly mobilize them under inflammatory conditions.
CCR2 is induced by IL-2 in T cells and NK cells (Loetscher et al., 1996b; Polentarutti et al., 1997). On the contrary, during monocyte maturation to macrophages and dendritic cells in response to IFNγ (Penton-Rol et al., 1998) and TLR agonists (Sica et al., 1997; Sallusto et al., 1998a; Arias et al., 2007), CCR2 has been reported to be downregulated, through a redox-sensitive mechanism stimulating rapid mRNA deadenylation and degradation (Xu et al., 1997; Saccani et al., 2000) and direct serine protease-dependent degradation of the receptor (Xu et al., 2000), thus allowing activated cells to leave the tissue and be recruited to draining lymph nodes (Cyster, 1999). Opposite regulation has been reported by glucocorticoids and IL-10 (Sozzani et al., 1998; Penton-Rol et al., 1999).
Depending upon the cell type, CCR2 may signal through different G proteins, although in hematopoietic cells Gαi predominates supporting cell migration (Kehrl, 2006). After CCR2 triggering by CCL2 on monocyte/macrophages, the α and βγ subunits dissociate and activate a signaling cascade involving phosphatidylinositol-3-OH kinase γ, phospholipase C activation, and calcium mobilization. Ultimately, small G proteins are activated, leading to lamellipodium protrusion, cell polarization, and chemotaxis (Thelen and Stein, 2008). Through signaling mechanisms independent of G protein activation, activated CCR2 also couples to scaffold proteins, which are required for its internalization. Among others, CCR2 associates with the clathrin heavy-chain repeat homology protein FROUNT, which forms clusters at the cell front during chemotaxis (Terashima et al., 2005). Different CCR2 agonists can trigger different signaling cascades (biased agonism), suggesting that the receptor can adopt different active conformations depending upon the agonist engaged (Ogilvie et al., 2004). Similar to other GPCRs, CCR2 may also form homodimers and heterodimers with other chemokine receptors, at least when expressed in transfected cells (Sohy et al., 2009). CCR2 homodimerization occurs in the absence of the ligand, but CCL2 dimers have been shown to favor CCR2 homodimerization, which may be required for its chemotactic activity (Rodriguez-Frade et al., 1999). CCR2 heterodimerization has complex functional consequences. CCR2/CCR5 heterocomplexes have been shown to activate calcium responses via Gq/11 and to support cell adhesion rather than chemotaxis (Mellado et al., 2001). CCR2 heterodimerization may be at the basis of the synergistic effect of CCL2 with other chemokines (Gouwy et al., 2012), although in the case of CCR2/CXCR4 dimers, CXCL12 has an allosteric transinhibitory effect on CCL2 binding on CCR2 (Sohy et al., 2007). Finally, the ability of CCR2 to dimerize may significantly affect drug selectivity, because the specific antagonist of one receptor may lead to functional cross-inhibition of the other (Sohy et al., 2009).
Mice lacking Ccr2 develop normally but display strong phenotypes in experimental models of disease. A pathogenetic role for Ccr2 in atherosclerosis is clearly established by evidence of sustained reduction in atherosclerostic lesions both in Ccr2-deficient and Ccl2-deficient animals challenged with a Western diet on an ApoE-deficient genetic background (Boring et al., 1998; Gu et al., 1998). Protection is associated with a reduced number of macrophages infiltrating the arterial wall, consistent with the major role of Ccr2/Ccl2 in recruitment of inflammatory Ly6Chigh/CCR2+ monocytes from the subendothelium, which expand and accumulate in lesions (Swirski et al., 2007). An additive effect with Ccr5 and Cx3cr1 has been demonstrated (Combadiere et al., 2008). Recruitment of Ly6Clow/Ccr2− cells depends only upon Ccr5 (Tacke et al., 2007). The role of Ccr2 in atherogenesis and infectious disease models (see below) may involve not only trafficking of monocytes from blood to tissue but also from bone marrow to blood, because Ccr2-deficient mice are profoundly monocytopenic (Serbina and Pamer, 2006; Jia et al., 2008). Consistent with results in experimental models, in the human setting CCL2 plasma levels after balloon angioplasty predict early restenosis (Cipollone et al., 2001), and allelic variants of CCL2 or its promoter were associated with generalized atherosclerosis and the risk of coronary artery disease (Szalai et al., 2001; McDermott et al., 2005; Brenner et al., 2006). CCR2 is also involved in stroke, as recruitment of inflammatory monocytes by postischemia induced Ccl2 results in increased infarct volume (Chen et al., 2003). As a consequence, Ccl2-deficient animals are resistant to middle brain artery occlusion (Hughes et al., 2002), and Ccr2-deficient animals are protected against ischemia-reperfusion injury (Dimitrijevic et al., 2007). Evidence from neutralizing studies and gene-targeted animals also indicate a nonredundant role of Ccr2 in the recruitment of inflammatory infiltrates into the central nervous system and in the development of EAE (Fife et al., 2000; Izikson et al., 2000). In humans, CCL2 haplotypes show a correlation with predisposition to multiple sclerosis (Ockinger et al., 2010). A nonredundant role for Ccr2 on microglia has been demonstrated in neuropathic pain models (Abbadie et al., 2003; Zhang et al., 2007). The relationship of CCR2 to Alzheimer’s disease is unclear. Analysis of CCL2 or CCR2 haplotypes and disease development risk has led to contrasting results (Conductier et al., 2010).
The relevance of CCR2 in autoimmune diseases is controversial. As synovial macrophages are considered key effector cells in the pathogenesis of rheumatoid arthritis, an involvement of CCR2 was expected, but collagen-induced arthritis was accelerated and exacerbated in Ccr2-deleted animals (Quinones et al., 2005). Moreover, CCR2 targeting was not effective in patients with RA (Vergunst et al., 2008), possibly because of compensation by other chemokine receptors or suboptimal timing (Lebre et al., 2011). Similarly, animal models and genetic analysis of CCL2 and CCR2 haplotypes did not support a nonredundant role for CCR2 in other autoimmune diseases, such as systemic lupus erythematosus.
CCR2 triggering induces mast cell degranulation and anti-Ccl2 can reduce eosinophil recruitment in mouse models (Gonzalo et al., 1999). However, analysis of the Ccl2/Ccr2 axis in type 2 polarized immune responses has given conflicting results. Ccl2 was shown to favor Th2 polarization (Gu et al., 2000), and Ccl2-deficient animals have been reported to have reduced airway hyperreactivity and to be resistant to allergen-induced asthma (Campbell et al., 1999a), whereas preferential development of Th2 immune responses was observed in Ccr2-deficient animals, which also had enhanced allergic responses (Kim et al., 2001c). As the reasons for this disparity are still incompletely understood, it is of relevance to note that a recent report showed that CCR2 blockade was effective in reducing bronchial hyperresponsiveness and leukocyte recruitment in a primate model of asthma (Mellado et al., 2008). CCR2 has also been shown to be required for appropriate type 1 polarized immune responses, as embolization of Ccr2-deficient mice with beads coupled to purified protein derivative resulted in smaller granulomas and reduced production of IFNγ in draining lymph nodes (Boring et al., 1997). Consistent with this, during Mycobacterium tuberculosis infection, Ccr2 is required to recruit macrophages and dendritic cells to the lung and to prime a protective adaptive immune response (Peters et al., 2001, 2004). During Listeria monocytogenes infection, Ccr2 triggering by Ccl2 and Ccl7 produced by bone marrow mesenchymal stem cells in response to TLR stimulation is required to mobilize Ccr2+/Ly6Chigh monocytes from the bone marrow and recruit them to infection sites (Serbina and Pamer, 2006; Jia et al., 2008). Similarly, after challenge with Leishmania major in the absence of Ccr2, skewing from a type 1 protective immune response to a susceptible state characterized by a type 2 polarized immune response has been reported (Sato et al., 2000). CCR2 also may play a role in AIDS pathogenesis, because CCR2 has HIV-1 coreceptor activity (Doranz et al., 1996) and the CCR2-64I allelic variant is consistently associated with delayed progression (Smith et al., 1997). Although the mechanism of action has not been defined, it is interesting to note that this receptor variant, but not the wild-type receptor, may heterodimerize with the major HIV-1 coreceptor CXCR4 (Mellado et al., 1999). CCR2+ monocytes recruited in the brain of HIV-infected patients have also been shown to sustain virus replication and HIV encephalitis (Ragin et al., 2006). As mentioned, Tat is also a direct agonist at CCR2, suggesting a second mechanism for further recruitment of target cells.
A relevant role of CCR2 in tumor biology has been suggested by the direct production of CCL2 by tumor cells (Bottazzi et al., 1983), the negative prognostic value of its expression levels (Ueno et al., 2000), and its significant correlation with the number of tumor-associated macrophages, which have been associated with enhanced malignancy and poor prognosis in different human tumors (Steidl et al., 2010). After active recruitment to tumor, tumor-associated macrophages have been demonstrated to be activated by the tumor microenvironment to an alternatively activated M2 phenotype, which supports tumor growth by different mechanisms, including promotion of angiogenesis and matrix remodeling and direct tumor cell growth (Martinez et al., 2008). Recently, CCR2 has been shown to be crucial for recruitment of inflammatory monocytes at metastatic sites, but not to primary mammary tumors, where they develop as metastasis-associated macrophages and promote extravasation, seeding, and persistent growth of tumor cells in a process requiring monocyte-derived vascular endothelial growth factor. Inhibition of Ccr2 signaling blocks their recruitment, inhibits metastasis in vivo, and prolongs the survival of tumor-bearing mice (Qian et al., 2011). Inflammatory monocytes belong to a heterogeneous Gr1+ population of so-called myeloid-derived suppressor cells, which has been shown to support tumor progression via different mechanisms, including suppression of antitumor adaptive immune responses by regulatory T cells (Gabrilovich and Nagaraj, 2009).
CCR2 may also be involved in chronic low-grade inflammation of adipose tissue, suggesting a role in obesity and related metabolic diseases (Kang et al., 2011). It has also been implicated in age-related macular degeneration in humans and in mouse models (Ambati et al., 2003; Combadiere et al., 2008; Raoul et al., 2010). CCR2-mediated macrophage recruitment is also required for efficient muscle regeneration in a skeletal muscle cardiotoxin-induced injury model (Martinez et al., 2010) and for alkali-induced corneal neovascularization (Lu et al., 2009). CCR2 triggering has been shown to sustain RANK (receptor activator of NF-κB) expression on preosteoclasts, and a decrease in number, size, and function of osteoclasts with consequent higher bone mass is evident in Ccr2-deficient mice, which are resistant to ovariectomy-induced bone loss (Binder et al., 2009). The role of CCR2/CCL2 in bone mass balance has recently been supported in humans by association of CCL2 GG and CCR2-64I allelic variants with osteoporosis risk (Eraltan et al., 2012).
Drug development.
Seven CCR2 inhibitors including small molecule receptor antagonists and neutralizing antibodies to the receptor have been evaluated in clinical trials (Table 3; Fig. 11). Merck has disclosed MK-0812, a potent CCR2 antagonist (IC50 of 5.0 nM), which was their clinical candidate in phase II clinical trials for both rheumatoid arthritis and multiple sclerosis (Horuk, 2009). The CCR2 antagonist failed to show any significant improvement compared with placebo for any of the end points studied (Horuk, 2009). Incyte (Wilmington, DE) has disclosed a number of CCR2 antagonists, including INCB3284, which was one of their clinical compounds in phase II clinical studies for multiple sclerosis and lupus (Xue et al., 2011). No data from the clinical trials were ever reported. Pfizer has had an interest in CCR2 compounds, and one of its lead candidates, PF-4136309 (structure not disclosed), has been reported to be in phase II clinical trials for neuropathic pain. PF-4178903 from the same company is a dual CCR2/CCR5 antagonist with an IC50 for inhibition of CCR2 and CCR5 binding of 3.0 and 5.3 nM, respectively (Zheng et al., 2011). It was initially selected as a clinical candidate based on its potency and favorable pharmacokinetic properties, but it had major cardiovascular liabilities that precluded further clinical development (Hughes et al., 2011). Further optimization of this template resulted in PF-4254196, which had no cardiovascular liabilities and is a potent CCR2 inhibitor (IC50 for inhibition of CCR2 binding of 8.1 nM) (Hughes et al., 2011).
Fig. 11.
Clinical candidates active at CCR2.
ChemoCentryx has reported an interest in CCR2 antagonists in several patent applications (Charvat et al., 2013; Krasinski et al., 2011), and two candidate molecules have progressed to clinical trials. The development of CCX915 (structure not disclosed) was terminated because of poor pharmacokinetic properties in phase I clinical trials. Their current clinical candidate, CCX140 (structure not disclosed), is currently being evaluated in clinical trials for the treatment of type II diabetes, and favorable results from a phase II trial were recently reported (Hanefeld et al., 2011).
Bristol-Myers Squibb has been very active in the CCR2 antagonist field. Two phase II clinical trials have been described for BMS-741672 (structure not disclosed). The first was a double-blind study for the treatment of neuropathic pain associated with type II diabetes, and the clinical end points were a reduction in pain score. The second trial was a 12-week randomized double-blind study for the treatment of type II diabetes, in which patients were treated with placebo or with 50 mg of CCR2 antagonist given once a day. The clinical end point was the reduction of glycated hemoglobins (a marker of diabetes). Both clinical studies ended in 2009; however, no reports have been issued.
Johnson & Johnson is interested in CCR2 antagonists, and a recently described approach led to the identification of JNJ-17166864, which was highly selective for CCR2 and had a binding affinity of 20 nM (Lagu et al., 2007). The compound had a 100-fold reduced affinity for rodent CCR2 (IC50 for mouse of 2 μM) but was still able to demonstrate efficacy in two mouse models of inflammation (Lagu et al., 2007). In line with its structure as a quaternary compound, it demonstrated poor oral bioavailability, but dosing by nasal spray allowed it to enter human clinical trials for allergic rhinitis (Hou et al., 2008). No clinical data from this trial have been revealed.
Millennium developed a CCR2 neutralizing antibody named MLN1202 as a therapeutic to treat autoimmune diseases such as rheumatoid arthritis. This approach has had mixed success with positive results in phase II trials for atherosclerosis (Gilbert et al., 2011) and multiple sclerosis (Sharrack et al., 2007) but negative results in a phase II trial for rheumatoid arthritis (Vergunst et al., 2008). It is noteworthy that two neutralizing monoclonal antibodies to the CCR2 ligand CCL2 have been described (Table 4). The first, ABN-912 from Millennium, failed to demonstrate efficacy in phase II clinical trials for rheumatoid arthritis (Haringman et al., 2006). The second, CNTO 888 is a nanomolar affinity antibody from Janssen Biotech (Philadelphia, PA) that is in phase II clinical trials for the treatment of cancer and idiopathic pulmonary fibrosis (Obmolova et al., 2012). Although CCR2 is the predominant receptor on circulating monocytes and this cell type is known to play a deleterious role in RA, it is probably not the right receptor to target for this indication, as discussed in Schall and Proudfoot (2011), hence the failed clinical trials.
An interesting approach by Noxxon (Berlin, Germany) is NOX-E36, which is a mirror image RNA of the RNA for the chemokine CCL2. This molecule has been shown to block expression of the protein and prevent receptor engagement. Phase I clinical studies were safe, and the company is currently assessing this molecule in phase II clinical trials for the treatment of diabetic neuropathy (http://www.noxxon.com/index.php?option=com_content&view=article&id=60&Itemid=98).
3. CCR3.
CCR3 was originally identified as the eosinophil receptor for eotaxin-1 (CCL11) (Combadiere et al., 1995; Daugherty et al., 1996; Kitaura et al., 1996; Ponath et al., 1996). However, it is now known to be highly promiscuous, binding the inflammatory chemokines CCL3L1 (LD78β), CCL5 (RANTES), CCL7 (MCP-3), CCL8 (MCP-2), CCL11, CCL13 (MCP-4), CCL15 (HCC-2), CCL24 (eotaxin-2), CCL26 (eotaxin-3), and CCL28 (MEC). The “eotaxins” CCL11, CCL24, and CCL26 are the most potent and selective (Fig. 6). Of note, mouse Ccl26 appears to be a pseudogene, because there is no corresponding expressed sequence tag (Pope et al., 2005a), and CCL3L1 and CCL13 exist in humans but not in mice (Nomiyama et al., 2010) (Fig. 4).
In addition to eosinophils, CCR3 is expressed on basophils, Th2 lymphocytes, and mast cells (Pease, 2011), which all participate in allergic responses (Ying et al., 2006). Interestingly, the CXCR3 agonists CXCL9, CXCL10, and CXCL11, which promote Th1 immunity, are also natural antagonists of CCR3 (Pease, 2006). Thus, CXCR3 agonists may promote Th1 responses not only by recruiting CXCR3-expressing Th1 cells but also by suppressing CCR3-mediated cell recruitment. In vivo, intravenous administration of Cxcl9 before an allergen challenge resulted in strong inhibition of eosinophil recruitment to the mouse lung (Fulkerson et al., 2004). CCL18 (PARC), whose signaling receptors have recently been identified as PITPNM3/Nir1 and CCR8 (see below), is also a natural antagonist of CCR3 (van der Voort et al., 2005).
CCR3-signaling pathways primarily induce cellular chemotaxis and intracellular calcium flux but are also involved in eosinophil and basophil degranulation (Uguccioni et al., 1997), angiogenesis (Salcedo et al., 2001), cell proliferation (Beck et al., 2006), and eosinophil differentiation (Lamkhioued et al., 2003). CCR3 can also act as a coreceptor for HIV infection; however, its role in disease is undefined (Choe et al., 1996; He et al., 1997). CCR3 is most closely related to CCR1, and both genes are located adjacent to each other within the major cluster of chemokine receptors on human chromosome 3 (Nomiyama et al., 2011). CCR3 and CCR1 might have been generated by gene duplication immediately after mammals diverged from other vertebrates.
CCR3 is also highly expressed on microglial cells, dendritic cells, and antibody-secreting plasmablasts and plasma cells (Murphy et al., 2000; Nakayama et al., 2003). Furthermore, constitutive CCR3 expression has been detected on airway structural cells, including epithelial cells (Beck et al., 2006), smooth muscle cells (Joubert et al., 2005), and endothelial cells (Gupta et al., 1998). CCR3 expression on airway epithelial and smooth muscle cells is upregulated by inflammatory signaling molecules such as TNF-α (Joubert et al., 2005). CCR3 expression has been found to be significantly increased in patients with asthma (Koelink et al., 2012) and atopic dermatitis (Homey et al., 2006). A recent study found that CCR3 expression is tightly regulated by the transcription factor GATA-1 (Kim et al., 2010), which has also been implicated in eosinophil development (Rothenberg and Hogan, 2006).
The role of Ccr3 and its ligands in asthma has been difficult to define. In one study, Ccr3 was shown to play an essential role in eosinophil recruitment to the lung in acute models of experimental asthma, and Ccl11 and Ccl24 were implicated as the major ligands involved in this process (Pope et al., 2005a). However, in a second study, mice deficient in Ccl11 had only partially impaired eosinophil recruitment to the lung, probably because of the promiscuity of Ccr3 in ligand binding (Rothenberg et al., 1997; Yang et al., 1998). Differences in the routes of allergen sensitization also produced apparently conflicting data regarding the role of Ccr3 in the development of airway hyperresponsiveness. Thus, Ccr3-deficient mice sensitized epicutaneously with ovalbumin did not develop airway hyper-responsiveness to inhaled allergen, whereas Ccr3-deficient mice sensitized intraperitoneally exhibited increased airway hyperresponsiveness associated with mast cell hyperplasia (Rothenberg and Hogan, 2006). Interestingly, both Ccr3 and Ccl11 but not Ccl24 were involved in basal trafficking of eosinophils to gut mucosa, but not to lung (Rothenberg and Hogan, 2006).
The sources of eotaxins in the asthmatic lung are airway structural cells (epithelial, endothelial, and smooth muscle cells) and inflammatory cells (macrophages, lymphocytes, and eosinophils) (Rothenberg and Hogan, 2006). CCL11 may contribute more to the early phase of allergen-induced recruitment of eosinophils into the airways of patients with allergic asthma, whereas CCL24 and CCL26 mediate subsequent, persistent bronchial eosinophilia (Ying et al., 1999; Berkman et al., 2001; Heiman et al., 2005; Ravensberg et al., 2005). In mice, kinetic induction patterns of Ccl11 and Ccl24 are similar to those observed in asthmatics, and Ccl24 has a central role in the development of airway eosinophilia (Pope et al., 2005b). Alveolar epithelial cells require histamine 2 receptors to produce Ccl24 in mice (Swartzendruber et al., 2012).
CCL26 is the most overexpressed gene in patients with eosinophilic esophagitis (EE), a disease characterized by antigen-driven eosinophil accumulation in the esophagus (Blanchard et al., 2006; Bhattacharya et al., 2007; Bullock et al., 2007; Lucendo et al., 2008), suggesting that CCL26 has a crucial role in eosinophil recruitment and activation in EE. CCL11 and CCL24 were only modestly induced in EE. Consistent with this, Ccr3−/− mice were nearly completely protected from developing EE (Blanchard et al., 2006), whereas Ccl11−/− mice were only modestly protected (Mishra and Rothenberg, 2003).
CCR3 also plays a critical role in age-related macular degeneration (AMD), a leading cause of blindness in the elderly (Takeda et al., 2009). AMD is characterized by choroidal neovascularization (CNV), which is the development of abnormal blood vessels from the choroid. CCR3 is expressed in choroidal neovascular endothelial cells of patients with AMD. The three eotaxins are also expressed in retinal pigmented epithelium located adjacent to choroidal endothelial cells. Notably, however, neither eosinophils nor mast cells are found in human CNV. CNV induced by laser injury was diminished in Ccr3-, Ccl11-, or Ccl24-deficient mice, indicating that the CCR3 axis played an essential role in endothelial cell proliferation (Takeda et al., 2009). In sharp contrast, no CNV inhibition was observed when Ccr3 was blocked in Matrigel CNV models developed in mouse and rat, and Ccr3 expression was not detected in the CNV endothelial cells in the mouse model (Li et al., 2011). The authors argued that this discrepancy was not because of the differences in the CNV models used (Li et al., 2011); thus, this issue is yet to be solved.
Drug development.
To selectively inhibit CCR3 in diseases such as asthma, pharmaceutical companies are conducting research to discover novel small-molecule antagonists (Pease and Horuk, 2009; Willems and Ijzerman, 2010), monoclonal antibodies (Catley et al., 2011), and antisense oligonucleotides. However, none of them has been approved for clinical use (Table 3; Fig. 12). Currently, two small-molecule CCR3 antagonists are being evaluated in clinical trials: GW766944 (GlaxoSmithKline) in phase II trials for asthma (ClinicalTrials.gov identifier NCT01160224) and GW824575 (GlaxoSmithKline) in phase I trials for asthma and AMD (NCT01551771). In a second trial, GW766944 failed to show efficacy in phase III for the treatment of allergic rhinitis.
Fig. 12.
Clinical candidates active at CCR3.
Another drug, TPI-ASM8 (Pharmamix, Sydney, Australia), which is in phase II trials for asthma (NCT00402948), contains two modified phosphorothioate antisense oligonucleotides that target CCR3 and the common β-chain of IL-3, IL-5, and GM-CSF receptors (Gauvreau et al., 2008). Although the trials so far show that the drug is safe and efficacious (Imaoka et al., 2011), the extent to which inhibition of CCR3 by these inhaled drugs contributes to reducing sputum eosinophils is not yet clear. Currently TPI-ASM8 is being evaluated in larger phase II clinical trials for asthma.
Dupont-Merck (Wilmington, DE) disclosed DPC168 as a potent CCR3 inhibitor, but development was discontinued because of potent cytochrome P450 2D6 and hERG (human ether-a-go-go-related gene) activity. BMS has an interest in CCR3 inhibitors and has disclosed a subnanomolar compound named BMS-639623 that is in phase I clinical development for asthma (Santella et al., 2008). AZD3778 is a novel low molecular weight dual CCR3 and histamine H1 receptor antagonist developed by AstraZeneca. The compound has an IC50 of 8.1 nM for the inhibition of eotaxin binding to CCR3 and an IC50 of 40 nM for the inhibition of binding to the H1 histamine receptor (Greiff et al., 2010). A phase II clinical trial in patients with allergic rhinitis revealed that AZD3778 exerted moderately antieosinophilic and symptom-reducing effects thought to be through inhibition of CCR3 rather than through its effects on the histamine receptor (Greiff et al., 2010). Inasmuch as the effects of AZD3778 were only modest, no further development of the compound has been reported.
4. CCR4.
CCR4 was originally cloned from a human basophilic leukemia cell line KU-812. It was shown to be expressed constitutively and IL-5-inducibly by human basophils (Power et al., 1995). However, CCR4 expression by basophils was not reproduced by others (Heinemann et al., 2000). The original report also demonstrated that CCL2, CCL3, and CCL5 were the specific ligands for CCR4 by using assays measuring Ca2+-dependent chloride channel activation in CCR4-transduced frog oocytes (Power et al., 1995). When CCR4-transfected mammalian cells were examined, however, these chemokines did not bind CCR4 or induce chemotaxis or intracellular calcium flux (Imai et al., 1997a). This was also confirmed for mouse Ccr4. Instead, two novel CC chemokines CCL17 (previously called TARC) (Imai et al., 1996; Godiska et al., 1997) and CCL22 (MDC) (Imai et al., 1996; Godiska et al., 1997) were found to be the specific ligands for CCR4 (Imai et al., 1997a, 1998). Although only 37% identical in amino acid sequence, CCL17 and CCL22 are both highly expressed in the thymus (suggesting their roles in T-cell development) and encoded by genes closely mapped on human chromosome 16q13 (suggesting their duplication from a common ancestor) (Nomiyama et al., 1998) (Fig. 4).
In peripheral blood, CCR4 is expressed by platelets and mediates platelet activation in the presence of low levels of primary agonists such as ADP or thrombin (Clemetson et al., 2000; Kowalska et al., 2000). Otherwise, CCR4 expression is highly restricted to T cells (Imai et al., 1997a), and it is the predominant chemokine receptor expressed by Th2 cells (Bonecchi et al., 1998; Sallusto et al., 1998b; Imai et al., 1999; Yamamoto et al., 2000). Although CCR4 expression is transiently induced upon anti-CD3/anti-CD28 stimulation of resting T cells, CCR4 responsiveness is observed later in Th2-polarization (Morimoto et al., 2005). Furthermore, CCR4 expression and responsiveness are strictly dependent on the presence of the IL-4/STAT6 signaling pathways (Morimoto et al., 2005). The local tissue site of helper T-cell differentiation is important for the differential expression of homing molecules. Thus, gut-homing molecules (CCR9, α4β7 integrin) are induced in gut-associated lymph nodes, whereas skin-homing molecules (CCR4, CCR10) are universally induced in all lymph nodes (Alvarez et al., 2007). This may explain the apparent universal expression of CCR4 in Th2 cells.
Consistent with the Th2-dominant expression of CCR4, the importance of the CCR4 axis in allergic diseases such as bronchial asthma and atopic dermatitis has been amply demonstrated using mouse models (Gonzalo et al., 1999; Vestergaard et al., 1999; Lloyd et al., 2000; Kawasaki et al., 2001; Mikhak et al., 2009) and clinical samples from patients with asthma (Panina-Bordignon et al., 2001; Hartl et al., 2005; Ying et al., 2005; Vijayanand et al., 2010) and atopic dermatitis (Nakatani et al., 2001; Wakugawa et al., 2001; Nouri-Aria et al., 2002; Uchida et al., 2002; Zheng et al., 2003). Furthermore, blood levels of its ligands CCL17 and CCL22 correlate well with disease severity for atopic dermatitis and serve as excellent laboratory markers for the disease activity (Kakinuma et al., 2001, 2002; Fujisawa et al., 2002; Horikawa et al., 2002). The results obtained from Ccr4−/− mice were, however, not always consistent with the expected importance of CCR4 in Th2 responses. This may be in part due to less selective expression of CCR4 on Th2 cells in mice (Freeman et al., 2006), differences in strain background, and/or functional complementation by other chemokine receptors.
In addition to Th2 cells, CCR4 expression is also associated with T-cell subsets such as skin-homing T cells (Campbell et al., 1999b; Andrew et al., 2001; Kunkel et al., 2002), T regs (Iellem et al., 2001; Hirahara et al., 2006; Baatar et al., 2007 ), and most recently Th17 cells (together with CCR6) (Acosta-Rodriguez et al., 2007) and Th22 cells (together with CCR6 and CCR10) (Trifari et al., 2009). Accordingly, the crucial role of CCR4 in skin-homing T cells has been amply demonstrated (Campbell et al., 1999b; Andrew et al., 2001; Kunkel et al., 2002; Soler et al., 2003; Baekkevold et al., 2005). Furthermore, CCL17 and CCL22 are not just redundant CCR4 ligands for skin-homing of T cells. It was originally shown that CCL22 was able to fully desensitize CCR4 to CCL17 but not vice versa in intracellular calcium mobilization assays (Imai et al., 1998). Likewise, CCL22 but not CCL17 was shown to efficiently internalize and desensitize CCR4 (Mariani et al., 2004). The dominance of CCL22 over CCL17 in CCR4 desensitization and internalization coupled with their differential expression in inflamed skin (Campbell et al., 1999b; Horikawa et al., 2002; Zheng et al., 2003) may provide coordinated, sequential guidance of CCR4-expressing T cells into inflamed skin; namely, CCL17 acts first at CCR4 at the endothelial surface to promote vascular recognition and emigration by T cells, whereas CCL22 subsequently engages CCR4 within skin tissue to guide T cells to target sites (Mariani et al., 2004).
The importance of CCR4 in Tregs has also been amply demonstrated in humans and mice. For example, tolerance induction in an allogeneic cardiac transplantation model with anti-CD154 plus donor-specific transfusion was shown to be accompanied by dramatic intragraft upregulation of Foxp3 levels together with those of Ccr4 and Ccl22. Furthermore, tolerance induction could not be achieved in Ccr4−/− recipients, suggesting that the recruitment of Foxp3-expressing Treg cells to an allograft tissue was highly dependent on Ccr4 (Lee et al., 2005). Similarly, in a mouse adoptive transfer model of inflammatory bowel disease, Ccr4−/− Treg cells failed to accumulate in the mesenteric lymph nodes and thus were unable to suppress colitis (Yuan et al., 2007). Accumulation of Treg cells in the skin and lung airways was also impaired in Ccr4−/− mice, resulting in severe inflammatory diseases in the skin and lung (Sather et al., 2007).
Drug development.
Obviously, CCR4 is considered to be a highly promising therapeutic target for allergic diseases such as asthma and atopic dermatitis. Thus, a number of small-molecule CCR4 antagonists have been developed and evaluated in animal models of allergic diseases, some giving highly promising results (Pease and Horuk, 2009). Furthermore, because of the dominant expression of CCR4 on Tregs, CCR4 blockade may be useful for enhancing the efficacy of cancer vaccines (Pere et al., 2011). CCR4 is also highly expressed in peripheral T-cell malignancies such as adult T-cell leukemia/lymphoma (ATL) (Yoshie et al., 2002; Ishida et al., 2003) and cutaneous T-cell lymphomas (Ferenczi et al., 2002). CCR4 expression by these peripheral T-cell malignancies may be related to their cellular origin from some CCR4-expressing T-cell subsets. It also explains their frequent involvement in the skin. Furthermore, aberrant expression of the AP-1 family member transcription factor Fra-2 is also in part responsible for the high level expression of CCR4 in ATL and cutaneous T-cell lymphomas (Nakayama et al., 2008). KW-0761, a humanized IgG1 anti-CCR4 with defucosylation to enhance ADCC activity, was developed by Kyowa Hakko Kirin in Japan, and its clinical trials have been conducted against ATL and other peripheral T-cell lymphomas with highly promising results (Antoniu, 2010; Yamamoto et al., 2010; Ishida et al., 2012a). Accordingly, the Japanese Pharmaceutical and Medical Devices Agency has approved KW-0761 (mogamulizumab) in March of 2012 as a therapeutic for ATL, which is notorious for its poor prognosis and strong resistance to conventional chemotherapy. Clinical trials in other peripheral T-cell malignancies are now ongoing. Mogamulizmab also may be applicable to allergic diseases such as asthma and atopic diseases (Antoniu, 2010; Yamamoto et al., 2010; Ishida et al., 2012a) and to cancer adjuvant therapy and is being developed by Amgen (Thousand Oaks, CA).
A number of companies have described CCR4 antagonists, and these were recently reviewed (Pease and Horuk, 2009). All of these molecules are still preclinical, but GSK recently disclosed a series of indazole sulfonamides (Procopiou et al., 2012) and identified a clinical development compound (GSK2239633) that has entered phase I clinical trials, presumably for allergy and asthma (Fig. 13).
Fig. 13.
Clinical candidate active at CCR4.
5. CCR5.
CCR5 is a chemokine receptor ”celebrity,” well known throughout both the scientific community and the general population because of its importance as the major HIV coreceptor controlling susceptibility to HIV infection and disease progression. Once established, the HIV connection immediately catalyzed drug development programs for CCR5, and in 2007, 13 years after the receptor was first identified (Boring et al., 1996; Combadiere et al., 1996; Meyer et al., 1996; Raport et al., 1996; Samson et al., 1996a), resulted in FDA approval of the antagonist maraviroc (Pfizer), the first marketed drug targeting a chemokine receptor. Genetic approaches in mice and humans have also indicated a key role for CCR5 in immunologically mediated disease and have suggested additional disease indications for CCR5 blocking agents.
CCR5 shares 70% amino acid sequence identity with CCR2, its closest GPCR relative; the two genes are adjacent on human chromosome 3p21 (Figs. 3 and 5). Similar to other inflammatory chemokine receptors, CCR5 binds many ligands, including CCL2, 3, 4, 5, 7, 8, 11, 13, 14, and 16 (Fig. 6) (Blanpain et al., 1999; Detheux et al., 2000; Nomiyama et al., 2001). Most of these behave as potent agonists (Gong et al., 1998; Blanpain et al., 1999); however, CCL7 is a natural antagonist (Blanpain et al., 1999). The specificity of ligand-receptor interaction lies within the extracellular loops, particularly ECL2 (Samson et al., 1997). However, additional residues in the amino terminal segment as well as post-translational modifications there, including O-glycosylation (Bannert et al., 2001) and tyrosine sulfation (Farzan et al., 1999; Zhou et al., 2000; Cormier et al., 2001), are important binding determinants.
CCR5 also has several nonchemokine ligands derived from diverse microbes. The first to be characterized was the gp120 envelope glycoprotein of R5 strains of HIV. This followed the seminal discovery that CCL3, 4, and 5 were major HIV-suppressive factors (Cocchi et al., 1995), which, together with the discovery of the T-tropic HIV coreceptor activity of CXCR4, led to the finding that CCR5 is a macrophage-tropic HIV coreceptor (Alkhatib et al., 1996; Trkola et al., 1996) with multiple domains that contact gp120 directly (Rucker et al., 1996), overlapping with chemokine interaction sites (Atchison et al., 1996). Viral macrophage inflammatory protein II (vMIP-II) from human herpesvirus 8 is a potent and efficient CCR5 antagonist (Kledal et al., 1997). More recently, mycobacterial heat shock protein 70 was shown to recapitulate most, if not all, of conventional CCR5 agonist activity (Floto et al., 2006). Other examples of microbial ligands for CCR5 have been reported in poxviruses, T. gondii, and Staphylococcus aureus (Lalani et al., 1999; Aliberti et al., 2003; Alonzo et al., 2013; Golding et al., 2003; Rahbar et al., 2009). The numerous connections of CCR5 with microbial products suggest an important role of CCR5 as a target for exploitation in microbial pathogenesis.
CCR5 is expressed on antigen-presenting cells (both macrophages and DCs) and immune effector cells (T-lymphocytes with memory/effector phenotype and NK cells), as well as on peripheral blood-derived CD34+ hematopoietic progenitor cells (Alkhatib et al., 1996; Dragic et al., 1996; Granelli-Piperno et al., 1996; Bleul et al., 1997; Rubbert et al., 1998; Ruiz et al., 1998). In the CD4+ T-cell lineage, it is preferentially expressed on Th1, Th17, and Treg cells (Bonecchi et al., 1998; Wysocki et al., 2005; Yurchenko et al., 2006; Sato et al., 2007). CCR5 is also expressed on CD8+ T cells (Murai et al., 1999; Nansen et al., 2000) and NK cells (Campbell et al., 2001) and facilitates organ infiltration. Engagement of naive CD4+ T cells with antigen presenting cells (APCs) triggers secretion of CCL3 and CCL4, which favors CCR5-dependent guidance of naive CD8+ T cells toward DC-CD4+ T-cell conjugates and promotes memory CD8+ T-cell survival and function (Castellino et al., 2006). Interestingly, CCR5-dependent recruitment of polyclonal CD8+ T cells to mature DCs engaging antigen-specific CD8+ T cells has been reported to favor naive CD8+ T-cell priming (Hugues et al., 2007), once more highlighting the importance of chemokine-mediated cell-cell interaction in secondary lymphoid organs for generation of immune responses. Interactions between CD8+ tumor-infiltrating lymphocytes and tumor cells trigger CCR5 recruitment at the immunologic synapse, leading to inhibition of T-cell sensitivity to CCL5 chemotactic gradients and T-cell retention (Franciszkiewicz et al., 2009). In addition, APC-derived chemokines can act as costimulatory molecules for engaged T cells through CCR5 relocalized at the immune synapse (Molon et al., 2005). Expression on monocytes and dendritic cell subtypes has been intensely investigated (Combadiere et al., 1996; Rubbert et al., 1998; Sallusto et al., 1998c; Sozzani et al., 1998). Their differentiation and maturation into potent APCs imply downregulation of tissue-specific chemokine receptors such as CCR1, CCR5, and CCR6 and upregulation of CCR7, which guides DCs from sites of Ag exposure to the local lymph nodes via draining afferent lymphatic vessels (Sallusto and Lanzavecchia, 2000).
CCR5 is also expressed on nonhematopoietic cells, including endothelial cells (Edinger et al., 1997), neurons (Galasso et al., 1998), astrocytes (Andjelkovic et al., 1999), epithelial cells (Dwinell et al., 1999), and vascular smooth muscle cells (Schecter et al., 2000). However, functional roles are not well established. Recently, it was shown that CCR5 is a major chemotactic mediator for murine endothelial progenitor cells (EPC), suggesting that it may regulate neovascularization and wound healing (Ishida et al., 2012b).
Genetic approaches studying the null CCR5Δ32 allele were instrumental in determining the pathophysiological relevance of this receptor in human biology (Table 5). CCR5Δ32, the most frequent human CCR5 coding sequence mutation, is a deletion of 32 base pairs between nucleotides 554 to 585 that causes a frame shift after amino acid 184. The truncated protein has only four transmembrane domains and is not expressed on the cell surface. CCR5Δ32 allelic frequencies vary substantially by geographic origin and range from 1% to more than 15% among whites. Approximately 1% of North American Caucasians is homozygous for this null allele yet appears healthy, suggesting compensatory mechanisms to alleviate CCR5 deficiency. Homozygotes are almost totally resistant to HIV-1 infection (Dean et al., 1996; Huang et al., 1996; Liu et al., 1996; Samson et al., 1996b; Zimmerman et al., 1997). Its key role in HIV infection and the relative innocuousness of its deficiency define CCR5 as an ideal target for developing HIV entry blockers. This has been underscored by the “Berlin patient,” a case of acute myeloid leukemia that occurred in the context of pre-existing HIV-1 infection. After chemotherapy in 2008, the patient was transplanted with hematopoietic stem cells from an HLA-matched donor homozygous for CCR5Δ32 and has had no PCR-detectable virus in blood or tissue since then off antiretroviral therapy, suggesting that viral reservoirs may have been eliminated (Hutter et al., 2009). CCR5 blocking agents may also be used to delay HIV disease progression. Since the allele appeared in Europe long before the HIV epidemic, an ancient selective factor, possibly bubonic plague or smallpox, has been suspected (Lucotte and Mercier, 1998; Galvani and Slatkin, 2003). Additional single nucleotide polymorphisms (snp) have been identified in the CCR5 promoter region that form at least 10 haplotypes (Martin et al., 1998) and illustrate cumulative and interactive influences in AIDS progression.
CCR5Δ32 has also been associated with a protective role in HCV infection with mild fibrosis and reduced portal inflammation (Hellier et al., 2003; Goulding et al., 2005). In contrast, homozygous CCR5Δ32 was shown to be a strong risk factor for symptomatic West Nile virus infection (Glass et al., 2006; Lim et al., 2008). In view of the importance of targeting CCR5 in anti-HIV therapies, these findings raise questions about the safe use of CCR5-blocking agents in the context of HIV/West Nile virus coinfection.
Studies using CCR5-deficient mice are complex and reveal that functional roles depend on the nature of the insult (microbial, metabolic, tumor) and the site of the lesion. Ccr5−/− mice appear healthy and develop normally in a pathogen-free environment (Zhou et al., 1998) but have abnormal immune responses in several pathologic conditions. Ccr5−/− mice showed altered T-cell activity (Kohlmeier et al., 2008), impaired macrophage function, and fewer infiltrating leukocytes at infected sites than control mice (Glass et al., 2001, 2005). At the microbiological level, they showed reduced efficiency in clearance of L. monocytogenes infection (Zhou et al., 1998), encephalomyocarditis virus (Christmann et al., 2011), and genital herpes simplex virus (Thapa et al., 2007) and increased mortality rates associated with fatal pneumonitis after influenza infection (Dawson et al., 2000a; Tyner et al., 2005) and West Nile virus infection (Glass et al., 2005). In addition to triggering leukocyte recruitment, the CCR5-CCL5 axis provides antiapoptotic signals for macrophage survival during infection (Tyner et al., 2005). On the other hand, Ccr5−/− mice are able to control M. tuberculosis infection and show an efficient Th1 response (Algood and Flynn, 2004). They are protected from mucosal ulcerations in a model of intestinal inflammation (Andres et al., 2000) and from chronic fungal asthma (Schuh et al., 2002) and behave similarly to control mice in EAE (Tran et al., 2000).
CCR5 deficiency limits atherosclerotic plaque formation in atherosclerosis-prone mice by reducing the systemic immuno-inflammatory response that leads to profound changes in lesion composition during the initial stages of plaque development (Potteaux et al., 2006; Braunersreuther et al., 2007). Furthermore, Ccr5−/− mice were protected from insulin resistance, glucose intolerance, and hepatic steatosis induced by high-fat feeding (Kitade et al., 2012). This protection was associated with both reduction of the macrophages infiltrating the adipose tissue and their polarization into M2 macrophages. In contrast, Ccr5−/− mice showed signs of adverse remodeling and early progression to heart failure in a model of ischemia-reperfusion injury. Impaired myocardial wound healing was concomitant with reduced macrophage activation (Zamilpa et al., 2011).
Drug development.
Many strategies have been developed to block CCR5 therapeutically, including intrakines, which sequester the receptor in the endoplasmic reticulum and prevent surface expression (Yang et al., 1997); chemokine analogs with antagonist activity, such as methionyl-RANTES (Proudfoot et al., 1996); and aminooxypentane-linked-RANTES (Simmons et al., 1997), chemokine analogs with defective glycosaminoglycan binding activity (Johnson et al., 2004); and RNA interference (Martinez et al., 2002). To date, only one CCR5 blocking agent, the small molecule maraviroc (Pfizer), originally known as UK-427,857 (Figs. 5 and 14), has succeeded as an approved drug (Wood and Armour, 2005). Maraviroc is potent against all known CCR5-tropic HIV-1 strains, with a mean IC90 value of 2 nM in antiviral assays (Dorr et al., 2005), and acts by an allosteric mechanism, suggesting that the transmembrane cavity remains accessible for maraviroc in CCL3-bound and gp120-bound CCR5 (Garcia-Perez et al., 2011). Maraviroc was FDA approved in HIV/AIDS for treatment-experienced individuals with HIV-1 strains resistant to multiple antiretroviral drugs. More recently, it was approved for individuals with drug-sensitive HIV-1 strains as a first-line drug in combination with other antiretroviral drugs. The structure of the CCR5-maraviroc complex has recently been solved (Tan et al., 2013).
Fig. 14.
Clinical candidates active at CCR5.
Schering-Plough has disclosed its clinical CCR5 inhibitor SCH 351125, also known as SCH-C. This compound was a potent CCR5 inhibitor, but phase I studies were suspended due to QTc prolongation in patients, increasing their risk of developing ventricular arrhythmias and potentially resulting in sudden death (Este, 2002). Further development of this compound led to SCH-D (vicriviroc), a methoxymethyl analog, which had improved receptor selectivity and no cardiovascular liabilities (Tagat et al., 2004). Unfortunately the development of the drug experienced multiple problems in clinical trials for HIV/AIDS, and the program was terminated.
GSK also had an interest in CCR5 antagonists. Their most advanced compound, GW873410 (aplaviroc), is a spiroketopiperazine (Maeda et al., 2004) and a potent CCR5 inhibitor (Kd of 3.0 nM) (Maeda et al., 2004). Aplaviroc potently blocked the binding of a wide spectrum of laboratory and primary R5 HIV isolates; however, phase II trials were halted in 2005 because of serious liver toxicity (Nichols et al., 2008), and GSK abandoned the program (Moyle, 2006).
Takeda has disclosed a number of CCR5 antagonists, including their clinical candidate TAK-652 (cencriviroc), which is a potent (IC50 of 3.0 nM for CCL3), metabolically stable, and orally bioavailable CCR5 antagonist (Baba et al., 2005). The compound was active against HIV-1 clinical isolates with an EC50 of 61 pM (Baba et al., 2005). Phase I clinical studies in healthy volunteers have shown the drug to be safe, with a mean half-life of 35 to 40 hours, supporting once a day dosing (Baba et al., 2005). Currently TAK-652 is in phase II clinical trials to treat AIDS (Lalezari et al., 2011).
Incyte has disclosed CCR5 antagonists for the treatment of AIDS including INCB9471 (Shin et al., 2011), which is highly selective and potent (IC50 of 16 nM in calcium flux assays). It also has highly potent anti-HIV-1 activity with an IC90 of 9.0 nM for all CCR5-tropic viruses, whereas it is inactive against X4-tropic HIV-1 strains. Phase I studies showed the drug was safe and well tolerated, and phase II studies in patients with HIV who were infected with R5-tropic virus were positive (Cohen et al., 2007). Despite these promising data, Incyte has halted all further internal development of this program.
AstraZeneca has disclosed a number of CCR5 antagonists, some of which had cardiovascular liabilities. Further optimization resulted in their clinical compound AZD567, which had excellent CCR5 potency (binding Ki = 0.17 nM) and no cardiovascular problems. Although the compound had excellent, once daily oral pharmacokinetic properties and exhibited high levels of receptor occupancy and maximal inhibition of CCR5, for unclear reasons it had no efficacy in a phase IIb study in patients with rheumatoid arthritis (Gerlag et al., 2010). Novartis is in phase I clinical trials with a CCR2/CCR5 dual antagonist (Miltz et al., 2008), but the structure has not been disclosed.
Progenics (Tarrytown, NY) is developing a humanized monoclonal antibody to CCR5 (PRO 140) as a potent inhibitor of viral entry for the treatment of R5 HIV-1-infected individuals. A small phase IIa clinical trial showed the drug was safe (Jacobson et al., 2009), and it has been given “fast track” designation by the FDA. Human Genome Sciences has described a fully human IgG4 monoclonal antibody against CCR5, HGS004, that has potent HIV coreceptor blocking activity (Lalezari et al., 2008). Phase 1 clinical studies demonstrated that HGS004 was safe and well tolerated by patients (Lalezari et al., 2008). However, the finding that some patients treated with high doses of HGS004 showed a switch from CCR5- to CXCR4-tropism was of concern.
The natural HIV-1 resistance conferred by CCR5Δ32, both in preventing initial infection and in treating established infection, as in the case of the Berlin patient, has spurred interest in specific zinc finger nuclease targeting of CCR5 in HIV/AIDS (Urnov et al., 2010). Sangamo Biosciences (Richmond, CA) is in phase II clinical trials to treat AIDS using this approach with an agent named SB-728.
6. CCR6.
Human CCR6 is found at 6q27 (Liao et al., 1997c), outside the clusters of chemokine receptor genes, and CCR6 orthologs have been identified in multiple species, including zebrafish. Phylogenetic analysis of human chemokine receptors puts CCR6 in a cluster with CXCR6, CCR7, and CCR9 (Lio and Vannucci, 2003). CCR6 is the only receptor identified thus far for the chemokine CCL20 (previously called MIP-3α) (Baba et al., 1997; Greaves et al., 1997; Liao et al., 1997a; Power et al., 1997), which binds to CCR6 with a Kd of 0.9 nM (Baba et al., 1997). CCR6 has also been reported to be a receptor for human and mouse β-defensins, antibacterial peptides produced at mucosal surfaces (Yang et al., 1999), although there are contradictory data (Soruri et al., 2007). CCL20 is expressed by leukocytes, epithelial cells and endothelial cells, and expression can be induced by cell activation and/or inflammatory cytokines (Schutyser et al., 2003). CCR6 is expressed by subsets of dendritic cells, particularly in their immature state (Dieu et al., 1998), on blood B cells and subsets of effector/memory CD4+ and CD8+ T cells (Liao et al., 1999), including regulatory T cells (Kleinewietfeld et al., 2005), on blood γ/δ T cells (Glatzel et al., 2002), on subsets of NKT cells (Thomas et al., 2003) and on NK cells and other innate lymphoid cells (Cella et al., 2009).
The biology of CCR6 has been best studied for B cells, dendritic cells, and T cells. CCR6 is expressed on virtually all mature human B cells, but is absent from germinal center B cells (Liao et al., 2002). In mice, CCR6 on B cells has been found to be important for gut-associated lymphoid tissue—for formation of isolated lymphoid follicles (McDonald et al., 2007) and for M cells over Peyer’s patches (Ebisawa et al., 2011). Ccr6-deficient mice have also been shown to have defects in numbers of antibody-producing cells in the gut mucosa after oral immunization (Cook et al., 2000). Recent work in sheep has suggested that CCR6 may also be important for trafficking of B cells to the skin (Geherin et al., 2012).
In cultured cells from humans, CCR6 has been found on dendritic cells derived from CD34+ progenitors (Greaves et al., 1997; Power et al., 1997) as well as from monocytes, and has been suggested to be important in the trafficking of Langerhans cell precursors to epidermis (Charbonnier et al., 1999). In mice, Ccr6 is expressed on myeloid, but not CD8a+ dendritic cells, and at low levels on Langerhans cells (Kucharzik et al., 2002). Although Ccr6−/− mice have normal numbers of Langerhans cells (Cook et al., 2000; Varona et al., 2001; Le Borgne et al., 2006), data suggest a role for Ccr6 in recruiting Langerhans cell precursors under inflammatory conditions. Ccr6 was found to be essential (1) for recruiting dendritic cells to the dome of the Peyer’s patch and the subsequent activation of CD4+ T cells after infection with an enteric pathogen (Salazar-Gonzalez et al., 2006), (2) for recruiting monocyte-derived dendritic cells into epithelial sites under inflammatory conditions where they cross-prime CD8+ T cells (Le Borgne et al., 2006), and (3) for priming CD4+ T cells by blood phagocytes after bacterial infection of the skin (Ravindran et al., 2007). Together, the data suggest an important role for CCR6 in the recruitment of dendritic cells to inflammatory tissue sites.
CCR6+ human effector/memory T cells were found to express markers for trafficking to both skin and gut (Liao et al., 1999), and Ccr6 was also found on subsets of T cells in the mouse (Kucharzik et al., 2002). A significant recent discovery is that CCR6 is expressed on all T cells that can express the cytokine IL-17 and is therefore part of the phenotype of Th17 cells (Acosta-Rodriguez et al., 2007; Singh et al., 2008a). Beyond Th17 cells, the relationship between CCR6 expression and IL-17 production extends to CD8+ T cells (Singh et al., 2008a) as well as γ/δ T cells (Gray et al., 2011). The relationship between CCR6 and IL-17 secretion is analogous to that between CXCR3 and IFNγ secretion and between CCR4 and IL-4 secretion: the cytokine-producing cells are subsets of the chemokine receptor expressing cells, although most receptor+ cells are still not able to produce the relevant cytokine.
Genome-wide association studies have revealed possible roles for CCR6 in inflammatory/autoimmune diseases in which Th17 cell-related responses are of potential importance, including Crohn’s disease, rheumatoid arthritis, generalized vitiligo, and Graves’ disease (Wang et al., 2009; Kochi et al., 2010; Quan et al., 2010; Chu et al., 2011 ). Ccr6 has been shown to contribute to the pathogenesis of a number of mouse models of autoimmune disease, including arthritis (Hirota et al., 2007), experimental allergic encephalomyelitis (EAE) (Reboldi et al., 2009), acute graft-versus host disease (Varona et al., 2005), and psoriasis (Hedrick et al., 2009). On the contrary, other studies have shown that Ccr6 is protective in some autoimmune models, including in EAE (Villares et al., 2009) and inflammatory bowel disease (Kitamura et al., 2010), through its role on regulatory T cells. Ccr6 has also been reported to contribute to allergic inflammation through its expression on T cells and/or dendritic cells (Lukacs et al., 2001; Lundy et al., 2005).
Drug development.
Despite the data to date linking CCR6 to a number of important diseases, no publications have described CCR6 antagonists or neutralizing reagents for clinical use.
7. CCR7.
CCR7 regulates trafficking of DCs and B and T lymphocytes and is a major homeostatic chemokine receptor critical for lymph node development and efficient adaptive immune responses. Its only two ligands, CCL19 (previously called ELC) and CCL21 (SLC), are produced constitutively in overlapping microenvironments of lymphoid tissue and are not agonists for other G protein-coupled chemokine receptors (Campbell et al., 1998; Yoshida et al., 1997, 1998a). CCL21 is produced by endothelial cells of afferent lymphatics, endothelial cells in high endothelial venules in lymph node, and fibroblastic reticular cells in T-cell zones of lymph node. CCL19 is produced by afferent lymphatics and stromal cells in lymph node. Neither CCR7 ligand is expressed in B-cell areas or sinuses of lymph nodes or by blood-derived leukocytes (Willimann et al., 1998). CCL21 is immobilized on the reticular network by GAG interaction with its extended C-terminal domain. The chemokine domain appears to be cleaved from the GAG binding domain by an undefined protease. CCL19 lacks a C-terminal domain extension and may preferentially function chemotactically as a soluble ligand, not haptotactically bound to surfaces, as shown for CCL21 (Weber et al., 2013). Ex vivo experiments with lymph node sections suggest that DCs migrate to T-cell zones by using immobilized CCL21 for adhesive random migration and soluble CCL21 for chemotactic steering, both acting through CCR7 activation on DCs (Schumann et al., 2010; Wendland et al., 2011).
CCR7 expression on DCs is dynamically regulated. Immature DCs in peripheral tissues express inflammatory chemokine receptors, but not CCR7. Upon antigen exposure, the cells mature and undergo a receptor switch downregulating inflammatory chemokine receptors and upregulating CCR7, which mediates migration of mature DCs, as well as T cells, via afferent lymphatics to draining lymph nodes in response to CCL21 expressed on lymphatic endothelial cells (Gunn et al., 1998). CCR7 also mediates recirculation of naive and central memory T cells from blood into lymph node T-cell zones across high endothelial venules expressing CCL19 and CCL21 (Gunn et al., 1998; Sallusto et al., 1998b; Yanagihara et al., 1998; Cyster, 1999; Kellermann et al., 1999; Saeki et al., 1999; Sallusto et al., 1999; Sozzani et al., 1999). Both chemokines are displayed on the luminal and abluminal sides of the high endothelial venules and are appropriately positioned to mediate transendothelial migration from the blood of CCR7+ cells (Okada et al., 2002, 2005). In Peyer’s patches, B-cell entry is also dependent on CCR7.
CCR7 is also dynamically regulated during thymocyte maturation and may regulate thymocyte trafficking (Suzuki et al., 1999). Evidence for chemoattraction of activated T lymphocytes by maturing dendritic cells via upregulation of CCR7 ligands has also been reported (Tang and Cyster, 1999). Studies with knockout mice have suggested that CCR7-dependent cortex to medulla migration of positively selected thymocytes may be essential for establishing central tolerance but not for maturation or export of thymocytes in vivo (Kurobe et al., 2006).
Ccr7−/− mice and the plt/plt (paucity of lymph node T cells) mouse, which is naturally deficient in Ccl19 and the Ccl21 isoforms expressed in secondary lymphoid organs (Nakano and Gunn, 2001) (Fig. 4; Table 1), have similar phenotypes: atrophic T-cell zones populated by few naive T cells. This plus the failure of mature DCs to migrate to lymph node from the skin of Ccr7−/−and plt/plt mice, explains why contact sensitivity, delayed type hypersensitivity, and antibody production follow a delayed course and why these mice show increased sensitivity to various pathogens. However, CCR7 expression is not absolutely required for the induction of adaptive immune responses. Moreover, because of the defect in negative selection of thymocytes and in lymph node homing and function of regulatory T cells, immune responses may even take an exaggerated course in Ccr7−/− mice, leading to development of spontaneous autoimmunity (Kurobe et al., 2006; Schneider et al., 2007; Forster et al., 2008).
CCR7 also appears to be important for lymphocyte movement within the lymphoid microenvironment. Follicular help T cells lack CCR7 but express CXCR5, which appears to facilitate migration from the T zone after activation to the follicles in response to CXCL13 where they provide help for B-cell maturation and antibody production. Reciprocally, B cells activated by antigen in the follicles upregulate CCR7 and move toward the T zone in response to CCL21 (Reif et al., 2002). Thus, B-T interaction may be facilitated by reciprocal movement of these cells, which may be determined in part by the balance of chemokines made in adjacent lymphoid zones. Other regulators of B-T contact include oxysterols and their GPCR, EBI2 (Pereira et al., 2009; Gatto et al., 2011; Hannedouche et al., 2011; Liu et al., 2011; Yi et al., 2012).
There is also evidence that T-cell zone chemokines such as CCL21 are bound to the surface of lymph node DCs in vivo and function to capture and prime naive T cells for activation by peptide-MHC. Thus, T cells are costimulated “in trans” and sequentially after initial engagement with their chemokine-rich environment (Friedman et al., 2006).
Upon activation, the classic polarized effector subsets Th1 and Th2 downregulate CXCR5 and CCR7 and upregulate inflammatory chemokine receptors (Sallusto et al., 1998b). This switch facilitates exit from lymph node via efferent lymphatics and homing to inflamed sites. As B cells differentiate into plasma cells they also downregulate CXCR5 and CCR7 and exit lymph node. CCR7+ effector memory cells have been detected in inflamed tissues and CCR7 facilitates their exit into lymphatics (Bromley et al., 2005; Debes et al., 2005; Schneider et al., 2007; Forster et al., 2008).
Drug development.
Antagonists and specific disease indications have not been identified yet for CCR7.
8. CCR8.
Human and mouse CCR8 are 355 and 353 amino acid polypeptides, respectively, that share 71% amino acid identity (Goya et al., 1998). Both genes are encoded on a single exon. Human CCR8 is located on chromosome 3, whereas mouse Ccr8 is on chromosome 9. Human CCR8 was originally molecularly identified as an orphan receptor (Napolitano et al., 1996; Samson et al., 1996c; Zaballos et al., 1996) and subsequently functionally characterized as the sole receptor for CCL1 (previously called I-309) (Roos et al., 1997; Tiffany et al., 1997; Goya et al., 1998). Later, the HHV8-derived viral chemokine vMIP-1 was identified as an agonist (Dairaghi et al., 1999; Endres et al., 1999).
CCL1 is produced by activated monocytes and lymphocytes (Miller et al., 1989, 1990) and is constitutively expressed in dermal microvessels and epidermal antigen-presenting cells (Schaerli et al., 2004). Mouse CCL8 (MCP-2) was recently identified as a second mammalian chemokine ligand for both mouse and human CCR8 (Islam et al., 2011). Although it is a member of the MCP subfamily, mouse Ccl8 does not signal through Ccr2. Human CCL8 in contrast is not a CCR8 agonist (Islam et al., 2011). Moreover, syntenic analysis reveals that mouse Ccl8 and human CCL8 are not orthologs and that mouse Ccl8 lacks a human counterpart (Fig. 4) (Zlotnik and Yoshie, 2012). Thus the nomenclature for these chemokines is misleading. Other anomalies of the CCR8 receptor system include the finding that, although human and mouse CCL1 are both high-affinity ligands and potent agonists for mouse Ccr8, mouse Ccl1 does not signal through human CCR8 and only binds human CCR8 with low affinity (Goya et al., 1998; Dairaghi et al., 1999). Interestingly, human CCL7 (MCP-3), an MCP family chemokine, but not mouse Ccl7, was noted to bind human CCR8 with intermediate affinity and with higher affinity than mouse Ccl1. Furthermore, human CCL7 is an antagonist for vMIP-1 but not for human CCL1 activity on human CCR8 (Dairaghi et al., 1999; Fox et al., 2006). Several other ligands originally assigned to CCR8 have not been confirmed (Bernardini et al., 1998; Howard et al., 2000; Fox et al., 2006). Recently, CCL18 was also found to act at CCR8 (Islam et al., 2013).
In human and mouse tissues, CCR8 is highly expressed constitutively in thymus and at lower levels in spleen (Napolitano et al., 1996; Zaballos et al., 1996; Tiffany et al., 1997). Leukocytes that express CCR8 in the steady state include human and mouse monocytes and macrophages (Zaballos et al., 1996; Tiffany et al., 1997; Hoshino et al., 2007), thymocytes (Annunziato et al., 2002), and regulatory T cells (Iellem et al., 2001; Kremer et al., 2001; Soler et al., 2006; Ahern et al., 2009; Zheng et al., 2009). Additionally, human γδ T cells (Cipriani et al., 2000; Ebert et al., 2006; Zhou et al., 2012); human skin-resident memory CD4, CD8, and γδ T cells (Schaerli et al., 2004; Ebert et al., 2006); and a subset of mouse monocyte-derived dendritic cells are reported to express CCR8 (Qu et al., 2004; Jakubzick et al., 2006). CCR8 is also reported to be expressed by endothelial cells (Bernardini et al., 2000; Haque et al., 2001) and vascular smooth muscle cells (Haque et al., 2004).
CCR8 is notable for its association with Th2 lymphocytes and Th2 cell-mediated allergic diseases (D'Ambrosio et al., 1998; Zingoni et al., 1998; Panina-Bordignon et al., 2001). CCR8 is abundantly expressed on activated highly polarized in vitro differentiated Th2 cells (D'Ambrosio et al., 1998; Zingoni et al., 1998; Islam et al., 2011). CCR8 is also reported to be highly expressed on Th2 cells infiltrating the airway mucosa of humans with asthma (Panina-Bordignon et al., 2001), on T cells recovered from the BAL of patients with asthma (Mutalithas et al., 2010), and on lesional skin infiltrating leukocytes during atopic inflammation (Gombert et al., 2005). CCR8 expression on Th2 cells is restricted to more terminally differentiated Th2 cells previously exposed to antigen (D'Ambrosio et al., 1998; Iellem et al., 2001; Wei et al., 2010; Islam et al., 2011). Chromatin immunoprecipitation-sequencing analysis to study the epigenome during T helper lineage differentiation found that the CCR8 gene was in a rare category of genes containing both STAT4-repressive and STAT6-activating binding sites (Wei et al., 2010). STAT4 binding in the upstream region of the CCR8 gene was inhibitory and associated with strongly repressive epigenetic marks (Wei et al., 2010). Thus, successive rounds of polarization would enable removal of the inhibitory regulation of STAT4 on CCR8 expression, facilitating STAT6 induction of CCR8 expression in more terminally differentiated Th2 cells.
Both ex vivo human studies and in vivo mouse studies support an important role for CCR8-mediated leukocyte trafficking to skin in both the steady state and during inflammation (Schaerli et al., 2004; Gombert et al., 2005; Ebert et al., 2006; Islam et al., 2011). In the steady state, a large fraction of skin resident memory T cells, γδ T cells, and NK cells express CCR8, and the CCR8 pathway is thus purported to promote immune surveillance in the skin (Schaerli et al., 2004; Clark et al., 2006; Ebert et al., 2006). In vivo mouse studies also support a role for the CCR8 pathway in leukocyte migration to skin draining lymph nodes as well as to the mediastinal lymph nodes (Qu et al., 2004; Jakubzick et al., 2006; Islam et al., 2011).
Two skin-tropic viruses encode two high-affinity chemokine antagonists at CCR8 (Kledal et al., 1997; Dairaghi et al., 1999; Luttichau et al., 2000). MC148 (vMCC-I) from Molluscum contagiosum is specific for human but not mouse CCR8 (Luttichau et al., 2000, 2001). In contrast, HHV8-encoded vMIP-II is a broad-spectrum antagonist at CCR8 and several other chemokine receptors.
Multiple mouse studies support a role for the Ccr8 pathway in promoting chronic allergic inflammation, including a mast cell-mediated model of chronic asthma, a model of chronic fungal asthma, and a model of chronic atopic dermatitis (Buckland et al., 2007; Gonzalo et al., 2007; Islam et al., 2011). Recent studies have found that more terminally differentiated Th2 cells highly produce IL-5, express CCR8, and promote chronic eosinophilic inflammation through the recruitment of IL-5-enriched CCR8+ Th2 cells (Islam et al., 2011; Upadhyaya et al., 2011).
However, the role of CCR8 in Th2 cell migration has been controversial. Robust CCR8 levels permissive for agonist activity are only induced transiently after TCR activation of highly differentiated Th2 cells generated by multiple rounds of polarization. TCR activation also leads to autocrine production of CCL1, which accumulates in vitro, leading to receptor desensitization. In vivo models have also yielded conflicting results regarding the importance of Ccr8 in acute models of allergy and Th2 airway inflammation. In shorter acute models, only one study supported a role for Ccr8 in promoting allergic airway inflammation (Chensue et al., 2001), whereas other studies did not (Chung et al., 2003; Goya et al., 2003; Mikhak et al., 2009). However, in a 46-day mast cell-dependent model, Ccr8-expressing effector Th2 cells were shown to play a role in chronic allergic airway inflammation (Gonzalo et al., 2007). The relative importance of CCR8-regulated responses in different models of allergic airway inflammation may be dependent on the kinetics of in vivo antigen-specific memory T-cell generation. Shorter acute models may not capture the differentiation and reactivation of Ccr8+ highly differentiated Th2 cells, which may require multiple rounds of antigen rechallenge characteristic in models of chronic inflammation.
In addition to cell migration, studies also support a role for the CCR8 pathway in preventing apoptosis and promoting angiogenesis (Van Snick et al., 1996; Bernardini et al., 2000; Haque et al., 2001; Ruckes et al., 2001; Denis et al., 2012). Truncation of the C terminus of CCL1 by carboxypeptidase M is reported to enhance its CCR8-mediated calcium flux and antiapoptotic activity (Denis et al., 2012). In this regard, vMIP-1 and vMIP-II were reported to promote autocrine and paracrine cell survival (Choi and Nicholas, 2008). CCR8-dependent activation of the RAS/MAPK pathway mediates antiapoptotic activity of CCL1 and vMIP-I (Louahed et al., 2003). CCL1 has also been reported to induce the migration of human umbilical vein endothelial cells as well as the production of metalloproteinase-2, which allows these cells to remodel the vascular matrix (Haque et al., 2001, 2004). CCR8 and CCL1 are also induced under conditions associated with vascular smooth muscle cell proliferation and migration, suggesting that the CCR8 pathway may have a role in vessel wall pathology (Haque et al., 2000, 2004). It has also been reported that CCR8 can function as an HIV-1 coreceptor, although its activity in primary cells and in pathogenesis remains undefined (Horuk et al., 1998). However, a recent genome-wide association multivariate survival analysis study of a treatment naive cohort identified significant associations for CCR3, CCR8, and CCRL2 polymorphisms, in addition to the known CCR5 and CCR2 associations, with an increased risk of progression to AIDS (An et al., 2011).
Drug development.
Berlex has identified a potent nonpeptide agonist of CCR8, ZK-756326, that could competitively displace CCL1 (Haskell et al., 2006). Unlike mouse Ccl1, ZK-756326 bound to and activated a form of mouse Ccr8 that was mutated to eliminate O-linked sulfation at tyrosines 14 and 15. Therefore, ZK-756326 is most probably not binding in the same manner as CCL1 but can activate the switch mechanism involved in transducing signaling events (Haskell et al., 2006). Another nonpeptide agonist, LMD-009, was also described as a full CCR8 agonist and competitively displaced CCL1 (Jensen et al., 2007; Scholten et al., 2012). The naphthalene-based compounds from Millennium were among the first CCR8 antagonists to be presented (Jenkins et al., 2007) and recently their probe-dependent allosteric interactions with CCR8 were described (Rummel et al., 2012). AstraZeneca has developed CCR8 antagonists based on a diazospiroundecane scaffold that had nanomolar potencies and inhibited T cell, dendritic cell, and eosinophil migration to CCL1 (Connolly et al., 2012). The lead antagonist, AZ084, appears to act as an allosteric inhibitor with a Ki at 0.9 nM (Connolly et al., 2012). No clinical trials have been reported.
9. CCR9.
CCR9 is a homeostatic receptor specific for CCL25 (previously called thymus expressed chemokine) (Zaballos et al., 1999; Norment et al., 2000; Zlotnik et al., 2006), a chemokine strongly expressed in the thymus and to a lesser extent in the small intestine (Vicari et al., 1997). To understand the significance of CCR9, it is important to have a basic understanding of T-cell development in the thymus. This process commences when early T-cell progenitors, originating in the bone marrow, enter the thymus. These progenitor thymocytes do not yet express CD4 or CD8 markers and are therefore “double negative” (or DN). Four subsets of these progenitors have been identified based on the expression of CD44 and CD25 (Godfrey et al., 1993), now called DN1, DN2, DN3, and DN4. DN1 represents the earliest thymus immigrant progenitor cells, which have not yet committed to the T-cell lineage. DN2 thymocytes start to rearrange the β chain of the T-cell receptor and, after this step, become committed to the T-cell lineage (Godfrey et al., 1994). However, DN2 thymocytes have only ∼33% probability of rearranging this chain in frame (to express a functional β chain), but they have two chromosomes that can undergo this rearrangement. So, if they fail to rearrange in frame the β chain in one chromosome they can try to rearrange the other. Either way, many DN2 thymocytes will fail to rearrange their TCR β chain in frame and are destined to die within the thymus, because they will never be able to express a productive T-cell receptor. This requires a “quality control” step where developing DN3 thymocytes are selected on the basis of successful expression of TCR β chain that they express in combination with a “pre-T alpha” chain (because they have not yet rearranged the “real” TCR α chain in a process called “beta selection”). It was the nature of this process that Norment et al. (2000) were studying when they found that CCR9 was strongly upregulated during this process. CCR9 (then known as GPR-9-6) exhibited typical characteristics of other chemokine receptors, including a “DRY” box that allows the receptor to interact and signal through Gαi. It was likely that CCR9 would bind a thymus-specific chemokine. The main candidate was CCL25, originally described as thymus expressed chemokine, and this turned out to be the case.
The functional implication of this observation is that chemokines and their receptors are active participants in the intrathymic migration of developing thymocytes to the appropriate sites where they will receive signals to further their differentiation and selection into mature T cells, a process that terminates when the T cells are eventually exported from the thymus. Once DN3 cells “pass” β-selection and express CCR9, they can successfully migrate to another thymus region under the influence of CCL25 to become DN4 cells, start rearranging the TCR α chain, and eventually become CD4+CD8+ thymocytes that will undergo positive and negative selection to become the single positive CD4+ or CD8+ thymocytes, which are the precursors of mature T cells. In support of these important functions of CCR9 in T-cell development, a Ccr9−/− mouse exhibits altered T-cell development (Wurbel et al., 2001; Uehara et al., 2002).
CCR9 has also been implicated recently in the original homing of bone marrow hematopoietic precursor cells to the thymus. A triple knockout mouse (Ccr7, Ccr9, and Cxcr4) exhibits defective thymic colonization by these precursors (Krueger et al., 2010), and therefore it is likely that more than one chemokine mediates this step. In any case, the CCL25/CCR9 axis is part of this important process (Desanti et al., 2011).
The importance of the CCL25/CCR9 axis in T-cell development is likely to extend beyond original thymus colonization and beta selection, because some CD4+CD8+ and single positive CD4+ and CD8+ thymocytes also express CCR9 and migrate in response to CCL25 (Zaballos et al., 1999; Wurbel et al., 2000). However, the specific details on the role of CCR9 on these stages of thymocyte development require further study.
CCL25 expression was also originally observed in the small intestine (Vicari et al., 1997). It was soon realized that certain T cells that home to the intestine express CCR9 and respond to CCL25 (Papadakis et al., 2000). These cells also express the α4β7 integrin so they are known as CCR9+α4β7+ T cells, and they home specifically to the small intestine (Papadakis et al., 2000; Kunkel et al., 2003). These cells have been implicated in the pathogenesis of certain autoimmune gut inflammatory diseases such as Crohn’s disease or ulcerative colitis (Koenecke and Forster, 2009). For these reasons, several companies have become interested in developing CCR9 antagonists. A subset of dendritic cells that may represent precursors to mature dendritic cells also expresses CCR9 (Segura et al., 2009). CCR9 expression has also been found in plasmacytoid dendritic cells that home to the small intestine (Wendland et al., 2007).
CCR9 has also been shown to play an important role in the metastasis of certain melanomas. Chemokines have been implicated in the metastasis of a diverse number of cancers (Zlotnik et al., 2011). Several chemokine receptors have been implicated; these include CXCR4, which is the most widely expressed chemokine receptor in cancers and is likely to mediate metastasis to the lung, liver, and bone marrow; and CCR7, which likely mediates metastasis to the lymph nodes. CCR9 is not a widely expressed receptor in cancer, but it is expressed in a subset of melanomas (Letsch et al., 2004). Normally, melanoma metastasizes to lymph node, lung, liver, and other organs but not small intestine. In fact, small intestine is not a common metastatic destination for most cancers. However, in a small fraction of patients, melanoma expresses CCR9, and interestingly, these patients often exhibit metastasis to the small intestine. CCR9 may be the key determinant of metastasis to this site where CCL25 is normally expressed. The association between CCR9 expression and small intestine metastasis in melanoma is very significant clinically (Richmond, 2008).
Drug development.
ChemoCentryx has disclosed CCR9 antagonists for the treatment of Crohn’s disease. Their clinical candidate, CCX282 (Fig. 15), has been reported to have IC50 values of 5.4 nM for inhibition of Ca2+ mobilization and 3.4 nM for inhibition of chemotaxis of cells expressing CCR9; the IC50 was 33 nM in the presence of 100% human serum (Walters et al., 2010). The compound has clear selectivity against a panel of 25 other GPCRs tested. Oral administration was efficacious in murine models of ileal Crohn’s disease and ulcerative colitis (Walters et al., 2010). In a 4-week phase II clinical trial of patients with moderate-to-severe Crohn's disease, a single daily dose of 250 mg of the compound was well tolerated and displayed clear signs of clinical activity as recorded by a reduction in blood levels of C-reactive protein (Keshav, 2006). In 2006, a phase II/III clinical trial to further assess the safety and efficacy of the antagonist in patients with moderate-to-severe Crohn’s disease was initiated. The study revealed that the 500 mg once daily dose of CCX282 in patients with small bowel and/or colonic Crohn's disease was consistently superior to placebo across multiple efficacy endpoints. GlaxoSmithKline exercised its option to obtain an exclusive license to compound CCX282 and launched four pivotal phase III clinical trials. The company announced on its website in August, 2013 that the compound had failed to meet clinical endpoints in the first trial and that further development has been terminated.
Fig. 15.
Clinical candidate active at CCR9.
10. CCR10.
CCR10 is the receptor for two homeostatic chemokines: CCL27 (previously called CTACK) (Morales et al., 1999) and CCL28 (previously called MEC) (Wang et al., 2000). CCL27 and CCL28 are in different chromosomal locations, likely because of a recent translocation (Zlotnik et al., 2006), but may represent the offspring of an ancient chemokine gene that bound “ancient” CCR10 (Figs. 3–5). CCR10, through its interaction with its two ligands, mediates diverse processes, from homeostatic leukocyte trafficking to skin cancer. Additionally, CCR10 is arguably one of the most important chemokine receptors in the mucosal immune system.
In skin, there are several populations of cells that express CCR10, including dermal fibroblasts, dermal microvascular endothelial cells, melanocytes, and T cells (Homey et al., 2000). CCR10+ T cells also express cutaneous lymphocyte antigen (CLA), a surface marker that mediates homing to skin. It is widely believed that the combination of CCR10 responding to gradients of CCL27 and CLA surface expression are key factors that allow these T cells to take up residence within normal skin (Clark et al., 2006)). CCR10+ T cells have been identified in both psoriatic lesions and atopic dermatitis (Homey et al., 2000; Homey, 2005). Additionally, CCR10 is expressed on T cells in cutaneous T-cell lymphoma (Notohamiprodjo et al., 2005) and T cells in Sezary syndrome (Sokolowska-Wojdylo et al., 2005), a rare cutaneous T-cell lymphoma. It is important to note that these CCR10+CLA+ T cells do not express the gut homing receptor α4β7 and therefore only localize to CCL27 gradients in skin (Hudak et al., 2002). The interaction between CCL25 and CCR9 is responsible for the recruitment of α4β7 cells to the gut.
Under normal conditions, melanocytes robustly express CCR10, and melanoma cells continue to express high levels of CCR10. Because of the high level of CCL27 in skin, melanoma cells will likely continue residing within skin. However, if the chemokine receptor profile of melanoma cells changes, the cancer may metastasize. This is best exemplified by metastasis of melanoma to the small intestine associated with a shift in receptor expression from CCR10 to CCR9 (Amersi et al., 2008).
CCR10 has a unique interaction with its second ligand CCL28. IgA-secreting plasmablasts are CCR10+ and therefore respond to CCL28 gradients. CCL28 is predominantly expressed in the salivary gland, mammary gland, female reproductive tissues, and gut. Thus, it is not surprising to find populations of IgA-secreting plasmablasts in each of these sites. However, there are two interesting features to the CCL28/CCR10 axis that suggest it is critical in shaping the humoral immune response at mucosal sites.
The first begins at birth. Within the female mammary gland, constitutive CCL28 expression is very low under homeostatic conditions. However, CCL28 is sharply upregulated upon onset of lactation. This is associated with a large influx of CCR10+ IgA-secreting plasmablasts, which extravasate into the breast tissue and persist after birth (Bourges et al., 2008; Morteau et al., 2008). IgA produced by these cells translocates into milk and is passed to the infant (Bourges et al., 2008; Morteau et al., 2008). Therefore, the CCL28-CCR10 axis may be involved in the transfer of humoral immunity from mother to child during breast-feeding.
A recent study using a Ccr10−/− mouse suggests that CCL28 and CCR10 may not be the only chemokine/receptor pair involved in the recruitment of IgA plasmablasts, at least in the gut (Morteau et al., 2008). Although Ccr10−/− mice were unable to recruit IgA-secreting plasmablasts to the mammary gland upon onset of lactation, IgA responses within the gut were unaffected, suggesting that within the gut another chemokine/receptor axis may be responsible for IgA responses, or, alternatively, that Ccl28 may engage IgA-secreting plasmablasts through a second chemokine receptor that has yet to be identified.
Drug development.
No CCR10-blocking agents have been reported yet.
C. CX3C Chemokine Receptors
1. CX3CR1.
CX3CR1 was originally cloned as a 355-amino acid orphan receptor (Harrison et al., 1994; Combadiere et al., 1995; Raport et al., 1995) and its ligand CX3CL1 (previously called fractalkine), the only member of the class of chemokines characterized by 3 amino acids between the first two cysteines, was identified soon thereafter (Bazan et al., 1997; Imai et al., 1997b; Pan et al., 1997). CX3CR1 is the only known human receptor for CX3CL1, although CX3CL1 also binds to the human cytomegalovirus (HCMV) receptor US28. CX3CR1 has highest similarity to CCRs, which is consistent with its gene location on human chromosome 3p21 in the major CCR cluster (Combadiere et al., 1995). CX3CR1 is considered a “monogamous” inflammatory receptor for CX3CL1, although recently CCL26, a chemokine highly selective for CCR3, was found to be a 10-fold less potent agonist at CX3CR1 (Nakayama et al., 2010). This has not yet been confirmed by other groups. Two microbial products also bind CX3CR1 as follows: (1) vMIP-II, a broad-spectrum viral chemokine receptor antagonist encoded by HHV8, which blocks CX3CR1-dependent activities in vivo and in vitro (Chen et al., 1998), and (2) the G glycoprotein of respiratory syncytial virus, which binds to and triggers chemotaxis of CX3CR1+ cells (Tripp et al., 2001).
CX3CL1 exists in both soluble and membrane-anchored forms, providing a two-in-one leukocyte recruitment system: membrane-anchored CX3CL1 promotes strong selectin- and integrin-independent adhesion of leukocytes that express CX3CR1, whereas soluble CX3CL1, which is produced by the cleavage of native CX3CL1, is a potent chemoattractant acting at CX3CR1 (Imai et al., 1997b; Fong et al., 1998). Along with strong expression on monocytes (Geissmann et al., 2003) and NK cells (Imai et al., 1997b), CX3CR1 is present on lymphocytes, microglial cells (Nishiyori et al., 1998), and neurons (Meucci et al., 2000). CX3CR1 has HIV coreceptor activity (Reeves et al., 1997; Rucker et al., 1997), and its interaction with gp120 is blocked specifically by CX3CL1 (Combadiere et al., 1998). However, the pattern of HIV strains that can use CX3CR1 is restricted, suggesting that this receptor may not play a key role in HIV.
Antibodies have been useful to delineate CX3CR1 expression in humans; however, none is currently available for that purpose in mouse. Instead, transgenic mice expressing green fluorescent protein under control of the Cx3cr1 promoter have been the predominant tool to track expression in mice (Jung et al., 2000). This has revealed that Cx3cr1 is mainly expressed on monocytes, microglial cells, and NK cells. But interestingly, Cx3cr1 is hardly detected on murine T lymphocytes, whereas subpopulations of both CD4 and CD8 T lymphocytes are CX3CR1+ in human. Notably, it was shown to be expressed on activated and differentiated CCR7−CD45RA− memory T lymphocytes (Combadiere et al., 2003a) and to regulate the function of cytotoxic effector lymphocytes, including NK cells, γδ T cells, and terminally differentiated CD8+ T cells (Nishimura et al., 2002). CX3CR1 is highly expressed on a subset of terminally differentiated NK cells (Sciume et al., 2011). In addition, human and mouse platelets carry functional CX3CR1, and its expression is enhanced by inflammatory stimuli, allowing platelet recruitment to the lesion site (Postea et al., 2012).
Expression of CX3CR1 is a powerful tool to identify leukocyte subsets. For instance, distinct surface Cx3cr1 levels delineates two functional subsets of murine blood monocytes: “inflammatory” monocytes and “resident monocytes,” which express, respectively, low and high levels of Cx3cr1 (Geissmann et al., 2003). This dichotomy appears conserved in humans as CD14+CD16−, and CD14lowCD16+ monocytes may resemble “inflammatory” and “resident” monocytes. In combination with CD103 (αE integrin), Cx3cr1 was proposed to define different subsets of DCs in the lamina propria of the gut. However, although CD103 is a convenient marker to identify DCs producing retinoic acid and TGFβ in the lamina propria of the small intestine (Coombes et al., 2007), the expression of Cx3cr1 on DCs remains controversial. Initially described as a marker for myeloid-derived mucosal DCs (Hapfelmeier et al., 2008; Niess et al., 2005; Bogunovic et al., 2009; Schulz et al., 2009) that sample the lumen of the small intestine, its selectivity was highly debated (Medina-Contreras et al., 2011) because Cx3cr1+ cells are highly heterogeneous. Recently, five subpopulations were defined in the lamina propria (Rivollier et al., 2012). F4/80hi Cx3cr1hi macrophages positive or negative for CD11c produce large amounts of IL-10 and are poor APCs. In contrast, DCs express low to intermediate levels of Cx3cr1 and can be divided into three subsets: CD11c+CD103+Cx3cr1− CD11b− that cross-present antigens to CD8+ lymphocytes, CD11c+CD103+Cx3cr1−CD11b+ and CD11c+CD103−Cx3cr1+CD11b+, with both latter populations able to efficiently present antigens to CD4+ T cells. CX3CR1 is expressed in brain (Jung et al., 2000) and on retinal microglial cells (Combadiere et al., 2007). Among cutaneous DC populations, CD11clow F4/80+ Langerhans cells express high levels of Cx3cr1 (Jung et al., 2000). CX3CR1 is also expressed on plasmacytoid dendritic cells (Auffray et al., 2009).
Because of the preferential expression of CX3CR1 on cells of the innate immune system, it was anticipated that this receptor may play a key role in many inflammatory disorders. Cx3cr1-deficient mice are fertile and develop normally in a pathogen-free environment (Haskell et al., 2001; Combadiere et al., 2003b) but reveal altered inflammatory and immune functions in disease models. Cx3cr1−/− mice are partially protected from atherosclerosis (Combadiere et al., 2003b; Lesnik et al., 2003) and have reduced numbers of macrophages in the vessel wall. The mechanism appears to involve Cx3cr1 effects on both monocyte recruitment into atherosclerotic plaque and survival of monocytes, macrophages, and foam cells, favoring plaque progression (Landsman et al., 2009). Monocyte survival depends on membrane-tethered CX3CL1 and not on the shed soluble form, suggesting dedicated activities related to the structural form of the ligand (Kim et al., 2011; reviewed in White and Greaves, 2012).
Reduced macrophage number in the gut lamina propria of Cx3cr1−/− mice is associated with increased translocation of commensal bacteria to mesenteric lymph nodes and with exacerbated lesions in a model of inflammatory bowel disease (Medina-Contreras et al., 2011). On the other hand, in the setting of dysbiosis where the commensal microbiome is damaged, for example by oral antibiotics, Cx3cr1high DCs may transport pathogens from the lamina propria to draining lymph nodes (Diehl et al., 2013). Two recent reports have identified regulatory activities for a subset of CX3CR1+ macrophages that suppress T-cell responses (Zhang et al., 2012), leading to prevention of intestinal inflammation (Kayama et al., 2012).
CX3CL1 has been detected on diverse subpopulations of neurons and has been proposed to interact with CX3CR1-expressing resident macrophages in the brain to control neuroinflammation. Cx3cr1 deficiency was reported to promote microglial neurotoxicity in different neuropathologic murine models such as bacterial product-triggered inflammation, Parkinson’s disease, amyotrophic lateral sclerosis, and tau pathology (Cardona et al., 2006; Bhaskar et al., 2010). In contrast, Cx3cr1 deficiency was protective in a focal cerebral ischemia model with smaller infarcts and reduced inflammation (Denes et al., 2008) and in a mouse model of Alzheimer’s disease (Fuhrmann et al., 2010) where neuronal loss was prevented. These contradictory results suggest that the CX3CR1/CX3CL1 axis may have complex biologic effects depending on the host insult. The CX3CR1-CX3CL1 axis is a key regulator of neuron-microglial cell communication, and there is now strong evidence that it participates in pain modulation (reviewed (D'Haese et al., 2010; Clark et al., 2011). The effects of this interaction system are not clearly understood, because CX3CR1 may have central proalgesic (Milligan et al., 2004) and peripheral antialgesic (Holmes et al., 2008) activities.
The role of CX3CL1-CX3CR1 in inflammatory lung disorders such as chronic obstructive pulmonary diseases and pulmonary hypertension was recently reviewed (Zhang and Patel, 2010). This appears to involve recruitment of immune cells to lung vessel walls and parenchyma. Cx3cr1−/− mice displayed severe morbidity and mortality after vaccinia virus infection associated with a defect in lung DC recruitment and reduced T-cell effector functions (Bonduelle et al., 2012). These studies open up novel therapeutic strategies targeting myeloid cell recruitment. Aside from the prominent expression of CX3CR1 on cells of myeloid origin, recent work provided compelling evidence for functional expression of CX3CR1 on Th2 T cells in asthma (Mionnet et al., 2010). Cx3cr1−/− mice were protected, and transfer of control CD4+ T cells in these mice restored lung disease. It was proposed that CX3CR1 provides survival signals to Th2 cells in the lung, favoring their pathogenic functions. This is consistent with overexpression of CX3CR1 transcripts in patients with asthma (Laprise et al., 2004).
The first genetic tools to evaluate the biologic role of CX3CR1 in human disease were two common single nucleotide polymorphisms in the open reading frame of CX3CR1, each of which changed a single amino acid, V249I and T280M, in transmembrane domains 6 and 7 and may affect receptor function. They are in strong linkage disequilibrium and define common V249T280 and rare I249M280 haplotypes. The CX3CR1 M280 allele was associated with accelerated onset of AIDS among HIV+ individuals (Faure et al., 2000). CX3CR1 polymorphisms were associated with reduced risk of atherosclerosis independent of other established risk factors and uncorrelated with disease severity (McDermott et al., 2001, 2003; Moatti et al., 2001). The CX3CR1-I249 allele was associated with increased glioblastoma multiforme survival and with reduced tumor infiltration (Rodero et al., 2008). The first evidence in humans that chemokines have a genetic role in age-related macular degeneration (AMD) involved CX3CR1 (Tuo et al., 2004). A small-scale study (85 case patients and 105 controls) found CX3CR1-I249 and -M280 alleles at a higher frequency in people with AMD than in control subjects. Another study deciphered the role of retinal microglia cells, their relation to CX3CR1 polymorphisms, and their contribution to AMD (Combadiere et al., 2007). The authors showed first that microglial cells expressed the CX3CR1 gene and confirmed that the CX3CR1-M280 allele was associated with an increased risk of AMD. Interestingly, microglial cells accumulated in the subretinal space, specifically at sites of retinal degeneration and choroidal neovascularization in patients with AMD. The causal mechanism of these clinical effects may involve a delicate balance between excessive adhesion (Daoudi et al., 2004) and reduced chemotaxis (McDermott et al., 2003; Combadiere et al., 2007) of CX3CR1-expressing leukocytes. All these studies implicate CX3CR1 in human pathologies and more specifically in inflammatory conditions involving cells of the myeloid lineage.
Drug development.
Because of the pleiotropic role of the CX3CR1-CX3CL1 axis, it was not initially ranked as a priority target for drug development. However, a number of studies have evaluated whether CX3CR1-blocking agents may have effects in the treatment of inflammatory disorders in animal models. Treatment with CX3CR1-blocking antibodies significantly prolonged cardiac allograft survival (Robinson et al., 2000), reduced inflammation and fibrosis, and preserved renal function in a renal ischemia-reperfusion model (Furuichi et al., 2006) but delayed skin wound healing (Ishida et al., 2008) and decreased tumor cell clearance (Robinson et al., 2003). CX3CL1 analogs with antagonist properties were developed (Inoue et al., 2005; Dorgham et al., 2009) and provided efficient tools to block CX3CR1-mediated functions in vivo. They limited lupus nephritis progression (Inoue et al., 2005) and reduced epithelial ovarian tumor cell proliferation (Gaudin et al., 2011). Inhaled CX3CR1 antagonist or intraperitoneal injection of CX3CR1 antibody protects mice from asthma (Mionnet et al., 2010). These results underline the therapeutic potential of this chemokine axis. However, to date no CX3CR1-targeted therapeutics have been evaluated in humans.
D. XC Chemokine Receptors
1. XCR1.
Although XCR1 is encoded by a gene within the CCR cluster on human chromosome 3p21, it does not bind CC chemokines. Instead, it is the specific receptor for XCL1 and XCL2 (previously called lymphotactin α and β), which are encoded by a pair of genes located on human chromosome 1q24.2 and differ by only two amino acids at positions 7 and 8 of the mature proteins (Yoshida et al., 1996, 1998b). Because no significant functional difference has been shown between XCL1 and XCL2, XCL1 will be used to represent both proteins hereafter.
XCL1 is an exceptional chemokine because it has only two canonical cysteines instead of four and can form only one disulfide bond. Interestingly, XCL1 adopts two distinct structures in equilibrium in physiologic solution conditions: one has the canonical chemokine fold and functions as an XCR1 agonist but fails to bind heparin, whereas the second structure binds heparin with high affinity but fails to activate XCR1 (Tuinstra et al., 2008). Furthermore, in both human and mouse XCR1, the DRY motif (typically, DRYLAIV), which is commonly found in the second intracellular loop of other chemokine receptors and known to be crucial for G protein coupling, is altered to HRYLSVV, suggesting a possible difference in downstream signaling pathways of XCR1 from those of other chemokine receptors (Yoshida et al., 1998b, 1999).
XCL1 was originally reported to be produced by CD8+ T cells (Kelner et al., 1994; Kennedy et al., 1995; Muller et al., 1995; Yoshida et al., 1995). XCL1 is now known to be produced by activated CD8+ T cells, dendritic epidermal invariant Vγ3Vδ1 T cells, intestinal intraepithelial γδ T cells, and NK cells (Boismenu et al., 1996; Muller et al., 1995; Hedrick et al., 1997). Human CD8+ αβ T cells, especially a minor subpopulation lacking CD5 expression, were reported to be the major producer of XCL1 (Stievano et al., 2003). Activated CD8+ T cells and NK cells secrete XCL1 together with CCL3, CCL4, and CCL5, suggesting some functional cooperation among these chemokines (Dorner et al., 2002).
XCL1 was also originally described as a chemoattractant for lymphocytes but not for monocytes or neutrophils (Kelner et al., 1994; Kennedy et al., 1995). It was therefore considered to be the first bona fide lymphocyte-specific chemokine, hence the original naming of lymphotactin, and also to represent a new class of chemokine (now termed the XC subfamily). Independently, two other groups also reported the human counterpart of lymphotactin but explicitly mentioned that they were unable to demonstrate any chemotactic activity of the recombinant protein on lymphocytes or any other cell types examined (Muller et al., 1995; Yoshida et al., 1995). Later, it was further shown that natural XCL1 produced by activated CD8+ T cells only induced locomotion (chemokinesis), not chemotaxis, of purified CD4+ and CD8+ T cells (Dorner et al., 1997). Nevertheless, many researchers reported potent chemotactic activity of XCL1 on CD8+ T cells, NK cells, B cells, and neutrophils (Boismenu et al., 1996; Maghazachi et al., 1997; Huang et al., 2001).
As for XCR1, it was first reported to be strongly expressed in placenta but weakly in spleen and thymus, and XCL1 and XCL2 were equipotent agonists in chemotaxis and calcium flux assays in XCR1-transfectants (Yoshida et al., 1998b). Mouse Xcr1 was also shown to mediate chemotactic and calcium flux responses to Xcl1 (Yoshida et al., 1999). However, even after the discovery and initial characterization of XCR1, the precise cell types that express XCR1 long remained unknown because of the lack of good reagents for surface XCR1. Although XCR1 mRNA was reported to be expressed by CD8+ T cells, NK cells, and neutrophils, consistent with some but not all of the published results for the presumed target cells of XCL1 (Yoshida et al., 1999; Cairns et al., 2001; Huang et al., 2001; Stievano et al., 2003; Luttichau et al., 2007), the actual levels of expression were very low. Thus, these data may be due mostly to PCR amplification of contaminating DNA in the RNA samples. Recently, Dorner et al. (2009) discovered that, unlike most other chemokine receptor genes, which are encoded by one exon, the XCR1 gene has two exons. Thus, Dorner et al. devised a new set of primers spanning a large intron of mouse Xcr1 and finally demonstrated strong expression of Xcr1 exclusively in CD8+ dendritic cells (DCs). Other cell types examined, CD4+ T cells, CD8+ T cells, NK cells, plasmacytoid DCs, etc., were all negative, consistent with early microarray data (Robbins et al., 2008). Signals by other DC subsets were also very low (Dorner et al., 2009).
Resident DCs in mouse spleen and other lymphoid tissues commonly express CD11c and can be subdivided into three subsets: CD4+ DCs (∼70%), CD8+ DCs (∼20%), and double-negative DCs (∼10%) (Vremec et al., 2000). Among them, CD8+ DCs are involved in antigen cross-presentation, in which exogenous antigen is presented in the context of MHC class I instead of the classic MHC class II pathway. Cross-presentation is crucial for activation of antigen-specific CD8+ T cells in the host defense against intracellular pathogens, when DCs are not directly infected, and also in the recognition of tumor antigens (Kurts et al., 2010). In fact, Dorner et al. (2009) showed that, although XCR1 is exclusively expressed on murine CD8+ DCs, CD8+ T cells abundantly produce XCL1 after antigen recognition on CD8+ DCs, which might lead to stable T cell-DC interactions as well as recruitment of more CD8+ DCs to the site of antigen recognition for immune amplification. Indeed, absence of XCL1 impaired the development of cytotoxic CD8+ T cells to antigens cross-presented by CD8+ DCs (Dorner et al., 2009). Soon, the unique expression pattern of XCR1 observed in the mouse was shown to be conserved in humans, because human blood BDCA3+ DC, which had been previously reported to be the counterparts of mouse spleen CD8+ DCs (Robbins et al., 2008), exclusively express XCR1. Human DCs are subdivided into CD104+ plasmacytoid DCs and conventional (or myeloid) CD11c+ DCs, the latter consisting of a CD16+ subset (∼70%), a CD1c+ (also called BDCA-1 from blood dendritic cell antigen-1) subset (∼30%), and a CD141+ (thrombomodulin, also called BDCA-3) subset (<5%) (Ziegler-Heitbrock et al., 2010). Human CD141+/BDCA-3+ DCs are the only cells in human blood expressing XCR1 and have been proven to be superior to other DC subsets in cross-presentation of soluble or cell-associated antigen to CD8+ T cells (Bachem et al., 2010). Thus, CD141+ DCs are the human counterparts of mouse CD8+ DCs. Similarly, Crozat et al. (2010) have shown that XCR1 is a conserved discriminating marker for the CD8α+-type DCs in human, mouse, and sheep, whereas XCL1 mRNA is selectively expressed by NK cells and CD8+ T cells, particularly antiviral central memory CD8+ T cells. Furthermore, when challenged with an intracellular bacterial pathogen Lysteria monocytogenes, Xcr1−/− mice have decreased early CD8+ T-cell responses associated with higher bacterial loads early in infection (Crozat et al., 2010). Crozat et al. (2011) also have shown that dermal CD103+ DCs, which share with spleen CD8α+ DCs a high efficiency for antigen cross-presentation, also express XCR1 (Crozat et al., 2011).
In the thymus, DCs accumulate in the medulla and contribute to the establishment of self-tolerance. Thymic DCs are also shown to express XCR1, whereas medullary thymic epithelial cells express XCL1 (Lei et al., 2011). Xcl1−/− mice are defective in the medullary accumulation of thymic DCs and the thymic generation of naturally occurring regulatory T cells. Furthermore, the Xcl1 expression by medullary thymic epithelial cells, the medullary accumulation of thymic DCs, and the generation of naturally occurring regulatory cells are all diminished in Aire-deficient mice (Lei et al., 2011). Thus, in the thymus, the XCL1-XCR1 axis is involved in DC-epithelial cell interactions and development of naturally occurring regulatory cells.
Prior to the identification of the correct target cells of XCL1, and probably because of its attractive name “lymphotactin,” many researchers considered XCL1 as a suitable chemokine to recruit cytotoxic T cells for cancer immunotherapy and even succeeded to demonstrate its powerful adjuvant effect in cancer immunotherapy (Dilloo et al., 1996; Ju et al., 2000; Cairns et al., 2001). Later studies, in which XCL1 and other chemokines were carefully compared side by side, however, found no such adjuvant effect of XCL1 gene therapy (Gao et al., 2005b; Okada et al., 2006; Kanagawa et al., 2007). These discrepancies could be partly attributed to differences in the tumor models and delivery systems used. However, now that XCR1 expression in lymphocytes such as CD8+ T cells and NK cells has been shown to be negative, the efficient recruitment of these killer type cells to tumor tissues via a direct action of XCL1 is unlikely. Still, the XCL1-XCR1 axis may be most useful for the development of effective cancer immunotherapy after all, given that XCR1 would provide an excellent molecular tool for the efficient delivery of tumor antigens to CD8α+-type DCs for cross-presentation to CD8+ T cells in vivo (Kroczek and Henn, 2012).
Probably because of the importance of the XCL1-XCR1 axis in cross-presentation, XCR1 appears to be an important target for many viruses. The M3 gene of mouse γ-herpesvirus murine gamma herpesvirus-68 (MHV68) encodes a chemokine-binding protein that is expressed during latency and binds a broad-spectrum of chemokines including mouse Xcl1 (van Berkel et al., 2000). Thus, MHV68 may use M3 to suppress the host cross-presentation machinery to evade immune recognition. HHV8 encodes vCCL2 and vCCL3. vCCL2 is a broad spectrum antagonist of human chemokine receptors including XCR1, whereas vCCL3 is a highly selective agonist for XCR1, (Luttichau et al., 2007). Thus, HHV8 may have a sophisticated mechanism to exploit the XCR1 system during its life cycle by exerting both positive and negative effects on XCR1. HHV6A encodes a CC-chemokine receptor homolog named U51A, which is constitutively active in inositol phosphate turnover and also mediates chemotactic and calcium mobilization responses to various CC chemokines, and XCL1 (Catusse et al., 2008). U51A may promote virus dissemination within the host by enhancing migration of infected cells to sites of chemokine production and/or evasion of host immune recognition by chemokine diversion and downregulation.
Drug development.
Given the longtime confusion and the lack of understanding of the biologic functions of the XCL1-XCR1 axis, it is not surprising that there are still no reports on the development of small-molecule XCR1 antagonists. However, the recent identification of the correct cell types that express XCR1 has finally set research on the XCL1-XCR1 axis in the right track. Because of the importance of cross-presentation in immune responses to intracellular pathogens and tumors, XCR1 may eventually provide an excellent surface molecule to deliver antigens to CD8+-type DCs for cross-presentation to CD8+ cytotoxic T cells (Kroczek and Henn, 2012). The XCL1-XCR1 axis may also be involved in some diseases involving cytotoxic T cells such as organ-specific autoimmune diseases and could be a future target for drug development.
III. Host Atypical Chemokine Receptors
Several molecules are highly homologous to typical chemokine receptors and bind chemokines but do not signal through G proteins. Previously called decoys, interceptors, scavengers, or chemokine-binding proteins, the preferred term is now atypical chemokine receptors, which has been formalized in the new Nomenclature Committee of the International Union of Pharmacology- and Human Genome Organization Gene Nomenclature Committee-approved nomenclature stem ACKR# introduced below.
A. ACKR1 (Previously Duffy Antigen Receptor for Chemokines)
ACKR1 is a heptahelical cell membrane protein, structurally similar but with low homology (less than 25% amino acid identity) to classic chemokine receptors (Chaudhuri et al., 1993; Neote et al., 1994). Also, in sharp contrast to classic chemokine receptors, ACKR1 lacks within the second intracellular loop the entire DRYLAIV consensus motif (Neote et al., 1994), which is required for binding of G proteins. Accordingly, no conventional chemokine-induced intracellular signaling responses have been recorded downstream of ACKR1 (Horuk et al., 1993b; Neote et al., 1994), hence its designation as an ACKR or atypical chemokine receptor (Nibbs et al., 2003; Graham et al., 2012). However, complex cell responses take place after ACKR1 ligation by cognate chemokines (Pruenster et al., 2009), suggesting that it may activate alternatives to classic intracellular signaling pathways. ACKR1 is the most promiscuous among all chemokine receptors: it binds, albeit with a broad range of affinities, over 20 different chemokines from both the CC and CXC subfamilies, primarily from the inflammatory functional group (Chaudhuri et al., 1994; Horuk et al., 1994; Szabo et al., 1995; Gardner et al., 2004) (Fig. 6). ACKR1 is also the oldest known chemokine receptor. It was discovered in 1950 as a blood group antigen and named “Duffy” after the eponymic polytransfused patient with hemolysing antibodies against an allogeneic erythrocyte antigen, Fya (Cutbush and Mollison, 1950; Cutbush et al., 1950); the predicted antithetical Fyb antigen was described shortly afterward (Ikin et al., 1951). In addition to erythrocytes, ACKR1 is constitutively expressed by endothelial cells of venules and small veins, but not arteries or capillaries (Chaudhuri et al., 1994, 1997; Peiper et al., 1995; Rot, 2005), as well as on a subset of neurons, particularly cerebellar Purkinje cells (Chaudhuri et al., 1994; Horuk et al., 1997), but not by any leukocyte subsets. There is debate about whether it is expressed on epithelial cells (Chaudhuri et al., 1997). The two antithetical Duffy erythrocyte antigens, Fya and Fyb, are the products of two polymorphic alleles FYA and FYB present on chromosome 1. They differ only in a single nucleotide, G versus A within codon 42, encoding glycine and aspartic acid in Fya and Fyb, respectively (Chaudhuri et al., 1995; Iwamoto et al., 1995). ACKR1’s extracellular N-terminal domain, which bears the blood group determinants, is linked with the fourth extracellular domain via a disulfide bond. These domains together create an active chemokine binding pocket (Tournamille et al., 1997; Tournamille et al., 2003). The third major ACKR1 polymorphism results from a T to C substitution at nucleotide-67 within the promoter region of the FYB allele at the binding site of the erythroid transcription factor GATA-1 (Tournamille et al., 1995). This polymorphism, designated as erythroid silent, FYB(ES), effectively abolishes the transcription of ACKR1 in erythroid cells, leading to a “Duffy-negative” phenotype, but not in any other sites of ACKR1 expression (Peiper et al., 1995), The emergence and overwhelming prevalence of the FYB(ES) polymorphism in West Africa, in some regions reaching 100%, has been strongly associated with resistance of Duffy-negative individuals to Plasmodium vivax malaria (Miller et al., 1975, 1976). Indeed micronemes of the merozoite of this plasmodium strain, but not Plasmodium falciparum, express a specific Duffy-binding protein that binds ACKR1 and mediates parasite adhesion to erythrocytes required for their subsequent invasion (Horuk et al., 1993a). However, the role of Duffy-binding protein-ACKR1 interaction in parasite infectivity and the development of vivax malaria is not absolute. In regions of Africa populated by mixed Duffy-positive and -negative people, e.g., Madagascar, Duffy-negative individuals can carry parasites asymptomatically and develop fully symptomatic vivax malaria (Menard et al., 2010). This suggests that the resistance to vivax malaria in regions of West Africa may be conveyed by the FYB(ES) polymorphism at a population level by reduced parasite transmission from infected humans to mosquito vectors. Recently the relationship of ACKR1 to malaria has been extended to P. falciparum, at least in vitro. In this setting, platelets are toxic to the parasite and the toxic factor is CXCL4 (previously called platelet factor 4). CXCL4 binds to ACKR1 and is delivered by it to the internalized parasite (McMorran et al., 2012)
In related developments, P. falciparum-infected erythrocytes have been reported to bind endothelial cell CX3CL1 in vitro (Hatabu et al., 2003), suggesting the parasite expresses a CX3CR1 mimic. Conversely, the G glycoprotein of respiratory syncytial virus has been reported to function as a CX3CL1 mimic binding to CX3CR1 to induce chemotaxis and possibly to promote viral entry and pathogenesis (Amanatidou et al., 2006; Harcourt et al., 2006; Johnson et al., 2012), and human cytomegalovirus encodes US28, a chemokine receptor able to bind CX3CL1, possibly to mediate viral attachment to target cells (see below).
ACKR1 plays diverse pathophysiological roles in chemokine homeostasis, largely dependent on its cellular expression site. Erythrocyte ACKR1 was traditionally considered to act as a chemokine “sink,” dampening the levels of circulating inflammatory chemokines, thus suppressing systemic leukocyte activation (Darbonne et al., 1991; Neote et al., 1993; Dawson et al., 2000b; Reutershan et al., 2009). Curiously, erythrocyte ACKR1 also maintains chemokine levels in blood, not only on the erythrocyte surface, but also in plasma, both in normal conditions and during inflammation, although the latter finding was not observed by all investigators (Jilma-Stohlawetz et al., 2001; Fukuma et al., 2003; Lee et al., 2006; Mayr et al., 2008). Currently it is not clear what may be the teleologic role of ACKR1 as a chemokine blood reservoir. It was suggested that different chemokines compete for ACKR1 binding to erythrocytes and thus modify the equilibria of free and bound chemokines and influence specific responses to them (Mei et al., 2010). Such equilibria can also be shifted by other physiologic substances, e.g., heparin and serum serine proteases, able to displace chemokines from ACKR1 (Schnabel et al., 2010).
ACKR1 plays an entirely different role in venular endothelial cells. These cells can bind, internalize, and transport chemokines from the basolateral to apical membrane, leading to chemokine immobilization and presentation on the luminal microvilli (Rot, 1992; Middleton et al., 1997; Hub and Rot, 1998). ACKR1 was recently shown to mediate all these chemokine interactions (Pruenster et al., 2009), thus contributing inflammatory pathomechanisms in different organs (Zarbock et al., 2007; Pruenster et al., 2009). In contrast to other atypical chemokine receptors, ACKR2/D6 in particular (discussed below), chemokine internalization by ACKR1 does not lead to their lysosomal degradation (Pruenster et al., 2009). Nevertheless, chemokine internalization by ACKR1 may reduce their availability in pericellular microenvironments. This may be a potential mechanism for how ACKR1 downmodulates angiogenesis induced by ELR CXC chemokines (Du et al., 2002; Horton et al., 2007). Alternative mechanisms may involve heterodimerization of ACKR1 and classic signaling chemokine receptors, leading to attenuation of their signaling (Chakera et al., 2008).
Drug Development.
ACKR1 blocking agents have not been developed yet.
B. ACKR2 (Formerly D6 or CCBP2)
ACKR2, a close homolog of CCRs, is encoded within the CCR gene cluster and binds inflammatory chemokines promiscuously, but only ones from the CC subfamily (Bonini et al., 1997; Nibbs et al., 1997; Graham, 2009). However, ACKR2 lacks the canonical DRYLAIV motif in ICL2 (altered to DKYLEIV), possesses the atypical amino acid N instead of D in transmembrane domain 2, and is unable to signal, in the classic sense, in response to any of its known ligands. ACKR2 has therefore been classified as an “atypical” chemokine receptor (Graham et al., 2012). Interestingly, an ACKR2 homolog in chicken (XP_418499) lacks the DKYLEIV alteration, suggesting that the apparent loss of signaling competence may be peculiar to mammalian ACKR2.
Biochemically, ACKR2 is sulfated on N-terminal tyrosine residues and possesses a single N-terminal glycosylation site that is not required for function (Blackburn et al., 2004). In contrast to other chemokine receptors, which require ligand binding to induce phosphorylation, ACKR2 appears to be constitutively phosphorylated on the intracellular C-terminal domain, suggesting that it is in a permanently active configuration with regard to receptor internalization and recycling (McCulloch et al., 2008).
ACKR2 is expressed constitutively in the skin, gut, lung, and placenta, which are all tissue “barrier” sites. In placenta, it is strongly expressed on the syncytiotrophoblast layer, an expression pattern that is established early during pregnancy (Martinez de la Torre et al., 2007; Madigan et al., 2010). In adult tissues, ACKR2 is expressed on some but not all lymphatic endothelial cells, in both peripheral tissues and lymphoid organs (Nibbs et al., 2001; Lee et al., 2011). In addition, ACKR2 is expressed by a range of leukocyte subtypes, most notably dendritic cells and innate-like B cells (McKimmie et al., 2008; Hansell et al., 2011).
ACKR2 binds promiscuously all inflammatory CC-chemokines, but not homeostatic CC-chemokines or other classes of chemokines (Fig. 6) (Bonecchi et al., 2004; Savino et al., 2009). A number of analyses (Nibbs et al., 1999; Savino et al., 2009) have highlighted the importance of a proline residue in position 2 (P2) in chemokines for binding. This suggests that chemokine binding to ACKR2 can be manipulated by the protease CD26, which is capable of removing the two most amino-terminal amino acids from proteins bearing a P2 amino acid. Intriguingly, primates, including humans, have evolved a variant of the inflammatory CC-chemokine, CCL3, that lacks the P2 residue and which therefore is unable to bind with high affinity to ACKR2 (Menten et al., 1999; Nibbs et al., 1999). The importance of this variant for the regulation of the primate inflammatory response is currently unknown.
Promiscuous ligand binding and apparent lack of signaling suggest a novel role for ACKR2 in regulating inflammatory chemokine responses. A range of in vitro analyses have convincingly demonstrated that it is incapable of supporting chemokine-dependent directional migration of cells and that its primary role is to scavenge, and degrade, inflammatory CC-chemokines (Fra et al., 2003; Blackburn et al., 2004; Bonecchi et al., 2004; Weber et al., 2004). ACKR2 constitutively internalizes and recycles from the cell surface via intracellular vesicles using mechanisms that may be dependent upon arrestin recruitment (Galliera et al., 2004; Weber et al., 2004). ACKR2 recycles in a ligand-independent manner, but is able to bind and internalize ligand and target it for lysosomal degradation. Under resting conditions, the vast majority of ACKR2 is in intracellular vesicles, with only 3–5% on the cell surface (Blackburn et al., 2004). ACKR2 + vesicles correspond to Rab5+ and Rab11+ early and recycling endosomes (Galliera et al., 2004; Weber et al., 2004). Upon ligand engagement, there is upregulation of cell surface ACKR2 in response to exposure to ligand, resulting in more efficient internalization and scavenging (Bonecchi et al., 2008). This observation suggested that ACKR2 can signal in response to ligand. Consistent with this, ACKR2 redistribution to the cell surface and its chemokine-scavenging activity was recently shown to require activation of a β-arrestin1-dependent, G protein-independent signaling pathway, leading to the phosphorylation of the actin-binding protein cofilin through the Rac1-p21-activated kinase 1-LIM kinase 1 cascade (Borroni et al., 2013).
ACKR2−/− mice display no obvious resting phenotype, but marked phenotypes are revealed in models of inflammatory pathologies (Graham, 2009; Graham et al., 2012). In general, ACKR2−/− mice are unable to efficiently remove inflammatory CC-chemokines from inflamed sites and therefore to resolve inflammatory responses. Thus in models of skin inflammation (Graham, 2009; Graham et al., 2012; Jamieson et al., 2005; Martinez de la Torre et al., 2005), ACKR2−/− mice display markedly exaggerated inflammatory responses and indeed, in one model, develop a pathology reminiscent of human psoriasis (Jamieson et al., 2005). Exaggerated inflammatory responses are seen in all tissues of ACKR2−/− mice in which ACKR2 is normally expressed, including the gut (Bordon et al., 2009; Vetrano et al., 2010) and lung (Whitehead et al., 2007; Di Liberto et al., 2008). In addition, expression of ACKR2 on the syncytiotrophoblast layer in the placenta appears to be important for protecting the embryo during maternal inflammatory responses (Martinez de la Torre et al., 2007; Madigan et al., 2010). Thus, increased miscarriage rates have been reported in ACKR2−/− mice administered LPS or autoantibodies.
ACKR2 has also been shown to be aberrantly expressed in a range of human pathologic contexts. An ACKR2 polymorphism has been associated with extent of liver inflammation in HCV-infected patients (Wiederholt et al., 2008). This is consistent with animal models indicating exaggerated inflammatory responses in ACKR2−/− mice in models of liver inflammation (Berres et al., 2009). Strikingly, ACKR2 appears to be upregulated in peripheral blood leukocytes in a range of inflammatory pathologies including systemic sclerosis (Codullo et al., 2011) and psoriasis (Singh et al., 2012), suggesting an attempt by these patients to regulate circulating peripheral inflammatory chemokine levels. This is clearly evident in patients with systemic sclerosis who show an inverse correlation between leukocyte ACKR2 expression levels and circulating inflammatory chemokine levels (Codullo et al., 2011). In addition, in psoriasis, ACKR2 is prominently expressed by keratinocytes in apparently healthy looking uninvolved skin. Elevation of ACKR2 expression in keratinocytes is largely driven by interferon-γ, which, to date, is the strongest regulator of ACKR2 expression reported (Singh et al., 2012). In the context of cancer, animal models of skin (Nibbs et al., 2007) and gut (Vetrano et al., 2010) tumorigenesis have indicated an important role for ACKR2 as a tumor-suppressor gene, and, in this context, its major function is to limit tumor-promoting inflammatory responses. A number of clinical studies revealed an inverse correlation between ACKR2 expression and disease-free survival in a range of tumor contexts (Wu et al., 2008). Most recently, ACKR2 has been shown to be expressed in infarcted myocardium and to be important for preventing excessive inflammation and vascular remodeling in this context (Cochain et al., 2012). Finally, elevated expression of ACKR2 was reported in alveolar macrophages in patients with COPD (Bazzan et al., 2013). That elevated expression, positively correlated with markers of immune activation and negatively with lung function, suggests that ACKR2 may have roles in the pathogenesis of chronic inflammatory conditions such as COPD.
Drug Development.
Antagonists targeting ACKR2 have not yet been reported.
C. ACKR3 (Alias CXCR7)
ACKR3 is an atypical chemokine receptor first identified as the orphan receptor RDC1 in a dog cDNA library (Libert et al., 1990). Deorphanization was facilitated by similarity of its sequence to chemokine receptors and localization of human RDC1 to chromosome 2 in the vicinity of CXCR2 and CXCR4 (Fredriksson et al., 2003; Thelen and Thelen, 2008). Similar to CXCR4, ACKR3 binds CXCL12. Unlike CXCR4, it is not monogamous, but is also able to bind CXCL11, but not other CXCR3 ligands (Fig. 6) (Balabanian et al., 2005a; Burns et al., 2006). Of importance for its biologic function, ACKR3 has ~10-fold higher affinity than CXCR4 for CXCL12 (Crump et al., 1997; Balabanian et al., 2005a; Burns et al., 2006).
ACKR3 transcripts are detected in hematopoietic cells, mesenchymal cells, and neuronal tissue (Su et al., 2002). Among hematopoietic cells, ACKR3 mRNA is found in various primary leukocyte subsets including the human B-cell compartment and in mouse splenic marginal zone B cells and type 2 transitional marginal zone cells (Graham et al., 2012; Humpert et al., 2012; Wang et al., 2012).
Similar to Cxcr4, targeted disruption of Ackr3 in mice is perinatal lethal. However, mortality is due to stenotic defects in all four heart valves, a phenotype not observed in Cxcr4 knockout mice (Sierro et al., 2007; Gerrits et al., 2008; Yu et al., 2011). The same phenotype occurs when Ackr3 is deleted only in endothelium, suggesting that the function of the receptor is most critical in these cells (Sierro et al., 2007). It is noteworthy that C57BL/6 mice, which were used for Ackr3 deletion, are naturally null mutants for Cxcl11. This indicates that the lethal cardiac phenotype is not caused by a defective Cxcl11/Ackr3 interaction and that the critical ligand is Cxcl12 or another as yet undiscovered factor (Sierro et al., 2007). However, the stenotic cardiac valve phenotype in Ackr3−/− mice does not occur in Cxcl12−/− mice, which instead have the same phenotype as Cxcr4−/− mice, suggesting a functionally monogamous Cxcl12/Cxcr4 ligand/receptor pair (Thelen and Thelen, 2008).
Evidence for a functional role of Ackr3 in vivo also comes from studies in zebrafish, where the migration of lateral-line primordium is abrogated upon deletion of Cxcl12 or Ackr3 or of Cxcr4 together with Ackr3 but less affected upon deletion of Cxcr4 alone, compatible with the notion that Ackr3 activation may play a functional role in this process (Dambly-Chaudiere et al., 2007; Valentin et al., 2007). In the case of primordial germ cell migration, the mechanism of Ackr3 function appears to involve scavenging of Cxcl12 by stromal cells expressing Ackr3 behind the migrating front of primordial germ cells, which carves a gradient required for migration. Primordial germ cells arrive at sites where Cxcl12 is translated from mRNA but do not continue to migrate forward if the scavenger activity of Ackr3 does not create a guidance cue (Boldajipour et al., 2008).
Ackr3 continuously cycles between the plasma membrane and endosomal compartments where the receptor deploys the cargo for lysosomal degradation. The cycling of the receptor is ligand independent, but is also enhanced in the presence of cognate chemokine, a property originally identified for ACKR2 (Luker et al., 2010; Naumann et al., 2010). The enhanced cycling of Ackr3 in the presence of Cxcl12 can be seen as an agonist-driven response and thus fulfills the criteria of a signaling receptor. For this reason, it was given the name CXCR7. However, the signal transduction pathway involves stimulated arrestin recruitment and chemokine-enhanced coupling to the internalization machinery, for which activation of heterotrimeric G proteins by GPCRs is dispensable (Thelen, 2001) and the physiologic role of which remains unknown. Thus it behaves more like an atypical chemokine receptor and has therefore been renamed ACKR3.
The precise molecular pathway for ACKR3 signaling remains unclear (Zabel et al., 2009; Gravel et al., 2010). Several studies indicate that ACKR3 does not couple to or activate G proteins or trigger typical chemokine receptor signal transduction pathways (Graham et al., 2012). The conserved DRYLAIV motif located at the cytoplasmic extension of helix III, according to the structure of CXCR4 (Wu et al., 2010), is assumed to be essential for G protein coupling. ACKR3 displays a less pronounced divergence from the canonical sequence than other atypical chemokine receptors (Thelen and Thelen, 2008); however, if this motif is mutated to the canonical sequence the receptor still fails to trigger calcium flux in response to ligand binding (Graham et al., 2012), indicating that additional sequence elements are missing or the orientation of the helices is incompatible with effective G protein coupling. Because the receptor internalizes via arrestin, it is possible that ACKR3 to some extent and in a particular context may trigger arrestin-dependent signaling leading to ERK activation (Rajagopal et al., 2010) as observed in cortical GABAergic interneurons (Wang et al., 2011).
The physiologic importance of ACKR3-mediated scavenging of CXCL12 is not limited to zebrafish (Graham et al., 2012). Mammalian ACKR3 acts as a critical scavenger on brain microvessel endothelium. Under normal physiologic conditions perivascular CXCL12 acts as a kind of glue, preventing the few emigrating leukocytes to enter the parenchyma. During inflammation, IL-17- and IL-1-dependent upregulation of ACKR3 on the abluminal surface of the brain microvessel endothelium leads to marked depletion of perivascular CXCL12 and therefore to a loss of the localization signal for leukocytes. In EAE, inhibition of ACKR3 with the small molecule CCX771 sufficiently restores perivascular CXCL12 to prevent the disease (Cruz-Orengo et al., 2011).
The marked expression of ACKR3 in ganglia of the central nervous system suggests additional functional relevance (Schonemeier et al., 2008; Thelen and Thelen, 2008). Indeed, migration of interneurons requires expression of both CXCR4 and ACKR3 and is impaired in the absence of either receptor. On the one hand, signaling by both receptors has been suggested to contribute to directed migration by activation of G protein and MAPK pathways downstream CXCR4 and ACKR3, respectively (Wang et al., 2011). On the other hand, ACKR3 has also been shown to be essential for maintaining CXCL12 levels that are optimal for CXCR4-dependent migratory responses, its absence leading to excessive concentrations of CXCL12 that cause downregulation of CXCR4 and ultimately to a defect in migration (Sanchez-Alcaniz et al., 2011; Wang et al., 2011).
By analogy, growth and spreading of breast cancer cells depends on the expression of CXCR4 and ACKR3. Both receptors are mostly expressed on different tumor cells, where ACKR3, acting in trans, regulates the level of CXCL12 in the tumor, thereby controlling the activity of the growth-promoting and migration-inducing receptor CXCR4 (Luker et al., 2012). The propensity of CXCL12 to dimerize at higher concentrations, and the finding that dimers fail to trigger chemotaxis (Drury et al., 2011) further indicates a critical role for ACKR3 in tuning CXCR4 responses.
An alternative nonmutually exclusive principle of CXCR4 regulation by ACKR3 comes from investigations in which both receptors are ectopically expressed. The proposed heterodimerization of CXCR4 and ACKR3, which seems to depend on the expression levels of both receptors, indicates a negative cooperative effect of ACKR3 on CXCR4-G protein coupling (Levoye et al., 2009) and a shift in biased signaling toward the arrestin axis (Rajagopal et al., 2010; Decaillot et al., 2011). However, whether these data can be extrapolated to receptors expressed in native contexts remain to be demonstrated.
ACKR3 expression is enhanced during pathologic processes such as inflammation and cancer and is broadly expressed on cancers of hematopoietic origin, such as lymphomas, and of mesenchymal origin, such as sarcomas, prostate and breast cancer, and gliomas (Sun et al., 2010; Calatozzolo et al., 2011; Graham et al., 2012). However, the role of ACKR3 in the neoplasms remains unclear. Interestingly, ACKR3 expression on tumor cells is frequently accompanied by CXCR4. For tumor growth, survival, and metastasis, a functional CXCL12/CXCR4 axis is important (Balkwill, 2004). Given the potential regulatory properties of ACKR3 for this signaling axis it can be anticipated that ACKR3 is critical for tumor development and thus may represent a target for therapeutic intervention. The consequences of targeting ACKR3 with inhibitors are complex and potentially opposed, ranging from blocking metastasis due to local accumulation of CXCL12, which at elevated concentrations is not able to trigger cell migration and downregulates CXCR4 (Drury et al., 2011), to promoting dissemination by disrupting adhesion-mediating CXCL12/CXCR4 signaling. In an even more complex environment in which multiple chemokines and receptors can regulate tumor cell migration, additional outcomes are possible. For example, administration of CXCL12 to CXCR4+/ACKR3+ cancer cells potentiates their transendothelial migration toward the homeostatic chemokines CCL19 and CXCL13, which are both expressed in lymph nodes. CXCL12-mediated enhanced migration is abrogated when ACKR3 is inhibited but is markedly less sensitive to CXCR4 antagonists. Therefore, it is plausible that targeting ACKR3 could prevent lymph node entry of CXCR4+/ACKR3+-positive tumor cells (Zabel et al., 2011). The physiologic relevance of CXCL11 scavenging by ACKR3 is not known but could be relevant for the regulation of CXCR3-mediated immune responses.
Drug Development.
Small molecule inhibitors of ACKR3 have been manufactured by Chemocentryx, including CCX771 and its prototypes CCX754 and CCX733, which compete for CXCL12 binding to ACKR3 in the low nanomolar range (∼5 nM), but are agonists with respect to arrestin recruitment (Luker et al., 2009; Zabel et al., 2009). CCX662 is in preclinical development for treatment of glioblastoma multiforme. Although the CCX compounds show characteristics of competitive agonists, monoclonal antibodies can efficiently block CXCL12 binding and have minor effects on arrestin recruitment and could act as true inhibitors.
ACKR3 binds CXCL12 differently from CXCR4. Some high-affinity antagonists of CXCR4, such as AMD3100 and TC14012, may act as competitive agonists at ACKR3 but at very elevated concentrations (EC50 350 nM for TC14012 and 140 µM for AMD3100) (Gravel et al., 2010). Thus, although in this particular case cross-reactivity is unlikely, the specificity of drugs targeting either ACKR3 or CXCR4 has to be extensively tested. Ubiquitin, which does not share the binding site with CXCL12 at CXCR4, does not bind to ACKR3 (Saini et al., 2011).
D. ACKR4 (Formerly CCRL1 and CCX CKR)
ACKR4 is located within the CCR cluster on human chromosome 3p21 (Gosling et al., 2000). ACKR4 was first reported to bind CCL19, CCL21, and CCL25 and was therefore provisionally named “CCR10” (Gosling et al., 2000). It was then reported to bind and mediate cell migration in response to MCP-4, MCP-2, and MCP-1 and was provisionally named “CCR11” (Schweickart et al., 2000). Because subsequent studies failed to confirm signaling, it was renamed CCRL1 and finally ACKR4. It is now accepted that ACKR4 binds to the homeostatic chemokines CCL19, CCL21, and CCL25 and with lower affinity to CXCL13 (Fig. 6). In humans, a pseudogene is located on human chromosome 6q24.1. In both mouse and human, ACKR4 gives rise to alternative transcripts, either including or not an extended 5′-untranslated region derived from the neighboring acyl coenzyme A dehydrogenase gene (Townson and Nibbs, 2002).
After ligand engagement ACKR4 internalizes and drives its ligands to degradation (Comerford et al., 2006), thus representing the homeostatic counterpart to the inflammatory CC chemokine scavenger receptor ACKR2 and suggesting involvement in chemokine-mediated aspects of the adaptive immune response. Also in common with ACKR2 is the absence in ACKR4 of the conserved DRYLAIV motif in the second intracellular loop, here altered to DRYVAVT, which may be responsible for its inability to trigger cell migration (Mantovani et al., 2006).
In both human and mouse, ACKR4 transcripts are detected in heart, lung, and gut, but not leukocytes (Gosling et al., 2000; Townson and Nibbs, 2002). ACKR4 reporter mice revealed expression of the receptor in skin keratinocytes and a variety of secondary lymphoid organs, as well as in thymic epithelial cells, and also failed to demonstrate expression in leukocytes but did not confirm expression in heart tissues (Heinzel et al., 2007). Ackr4−/− mice are healthy and viable (Heinzel et al., 2007; Comerford et al., 2010) and confirmed a role for Ackr4 in in vivo scavenging of chemokines, because elevated levels of its ligands were detected in serum and lymph node (Comerford et al., 2010). Ackr4−/− mice also revealed a role for Ackr4 in adaptive immunity, because deficient mice displayed more rapid disease onset in the EAE model attributable to an increased Th17 response. This is consistent with a previously reported role for ACKR4 in basal trafficking of dendritic cells to lymph nodes and of embryonic thymic precursors to the thymus (Heinzel et al., 2007).
Limited information is available on the involvement of ACKR4 in human pathology. Ciliated bronchial epithelial cells from patients with pulmonary sarcoidosis express ACKR4, and its upregulation paralleled disease severity (Kriegova et al., 2006). ACKR4 expression also was reported in hepatic and breast cancer cell lines (Takatsuka et al., 2011; Zeng et al., 2011), and in breast cancer ACKR4 levels are associated with disease-free survival. Moreover, downregulation correlated with breast carcinoma invasiveness, lymph node metastasis, and poor survival rate (Feng et al., 2009; Zeng et al., 2011).
Drug Development.
ACKR4 blocking agents have not been developed yet.
E. CCRL2 (ACKR5, Reserved)
CCRL2 (ACKR5, reserved, pending confirmation; aliases CKRX, HCR, and CRAM) is located in the CCR cluster on human chromosome 3 (Fan et al., 1998), and its putative murine ortholog, L-CCR, is located in the syntenic location on chromosome 9 (Shimada et al., 1998). In humans, two variants have been reported, originally called CRAM-A and CRAM-B (chemokine receptor for activated macrophages A and B), that differ in the N-terminal region of the receptor. CCRL2 shares over 40% amino acid identity with CCR1, CCR2, CCR3, and CCR5. A study published in 2003 reported chemotaxis and calcium flux in cells expressing CCRL2 after stimulation with CCL2, CCL5, CCL7, and CCL8 (Biber et al., 2003); however, no evidence for direct binding was provided and the study has not been confirmed. More recently, CCL19 (Leick et al., 2010) and the chemoattractant protein chemerin (Zabel et al., 2008) were reported to bind CCRL2, but unlike the corresponding signaling receptors, CCR7 for CCL19 and ChemR23 and GPR1 for chemerin, these interactions did not induce cell migration or calcium transients. Similar to other atypical chemokine receptors, the apparent inability of CCRL2 to signal is in line with the nonconserved sequence in the canonical DRY motif in the second intracellular loop (Mantovani et al., 2006). Having considered these results and properties of CCRL2, the subcommittee on atypical chemokine receptor nomenclature has decided to reserve the name ACKR5 awaiting confirmatory reports of chemokine binding and lack of or atypical signaling.
Human CCRL2 is expressed by most leukocyte subtypes, including monocyte/macrophages, neutrophils, dendritic cells, T and B lymphocytes, NK cells, mast cells, and CD34+ bone marrow precursors in humans (Migeotte et al., 2002; Yoshimura and Oppenheim, 2011), whereas the mouse receptor is expressed by dendritic cells and macrophages but not eosinophils and T cells (Otero et al., 2010). Both in humans and mice, CCRL2 expression in dendritic cells and neutrophils is rapidly upregulated by LPS, TNF, and other inflammatory stimuli (Migeotte et al., 2002; Zuurman et al., 2003; Galligan et al., 2004; Otero et al., 2010). LPS also induced CCRL2 in astrocytes and microglia, both in vitro and in vivo (Zuurman et al., 2003), and its expression was also induced in EAE (Brouwer et al., 2004) and in bronchial epithelium and lung macrophages in ovalbumin-induced airway inflammation (Oostendorp et al., 2004).
Ccrl2−/− mice are healthy and viable and display enhanced tissue swelling and leukocyte infiltration in a model of passive immunoglobulin E-mediated cutaneous anaphylaxis (Zabel et al., 2008). They also show partial protection in a model of ovalbumin-induced airway inflammation (Otero et al., 2010). In this model, adoptive transfer experiments demonstrated a key role for dendritic cells, which failed to traffic to mediastinal lymph nodes to prime Th2 responses. In a different model based on in vivo challenge with LPS, induction of Ccrl2 in endothelial cells and enhanced ChemR23-dependent recruitment of NK cells in the airways was reported (Monnier et al., 2012). Whether these phenotypes relate to the role of CCRL2 as a chemerin receptor is unknown, but it is interesting to note that circulating levels of chemerin are significantly higher in Ccrl2−/− mice (Monnier et al., 2012). Because CCRL2 was shown to bind a portion of chemerin not involved in binding and activation of ChemR23 and CCRL2-expressing cells preloaded with chemerin induced functional responses in cells transfected with ChemR23, CCRL2 was suggested to regulate chemerin bioavailability, enriching its concentration at inflammatory sites and presenting it to the functional receptor ChemR23 (Zabel et al., 2008). CCRL2 is expressed in some stages of B-cell maturation, and CCL5 stimulation can modulate the level of its expression. CCL5 can induce ERK1/2 phosphorylation and actin polymerization in a G protein-independent manner on these cells that do not express known CCL5 receptors (Hartmann et al., 2008). Thus, this signaling pathway at the receptor level remains undefined. Finally, in B cells, CCRL2 was shown to bind CCL19 and to impair CCR7-dependent ERK1/2 phosphorylation and calcium flux in response to CCL19 but not CCL21 (Catusse et al., 2010). This resembles signaling interference previously reported for ACKR1 (Chakera et al., 2008) and ACKR2 (Bonecchi et al., 2004).
Drug Development.
Information on the role of CCRL2 in human pathologies is hampered by the absence of blocking reagents. However, CCRL2 expression was found in neutrophils and macrophages recovered from synovial fluids of patients with arthritis (Galligan et al., 2004), and genetic studies on CCRL2 nonconservative allelic variants suggest its involvement in pneumocystis pneumonia development in patients with AIDS (An et al., 2011).
F. PITPNM3 (Also Known as the CCL18/PARC Receptor; New Name: ACKR6, Reserved)
CCL18 was reported recently to signal through PITPNM3/Nir1, a member of the phosphatidyl-inositol transfer protein (PITP) family, not the GPCR superfamily (Chen et al., 2011). The PITP family is comprised of multimodular 6TM proteins, with an eponymous PITP domain, an acidic region that binds Ca+2, and a C-terminal PYK2-binding domain. A PITPNM3 homolog named RdgB has been implicated in vision in Drosophila, and mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in humans. PITPNM3 has now been implicated in breast cancer metastasis, acting in response to CCL18 release from tumor-associated macrophages (Chen et al., 2011). CCL18 is expressed by monocytes, macrophages, and dendritic cells; was originally named pulmonary and activation-regulated chemokine (PARC); and is thought to mediate T-cell attraction to DCs. As mentioned above, a typical 7TM G protein-coupled receptor has been recently identified for CCL18, namely CCR8 (Islam et al., 2013). The name ACKR6 has been reserved for PITPNM3 pending confirmation of atypical chemokine receptor properties.
Drug Development.
Blocking agents for this receptor have not been developed yet.
G. C5L2
The C5L2 gene (C5a-like receptor 2, also called GPR77) is located on chromosome 19 in the human genome in the same cluster with C5aR1 and formyl peptide receptors (Ohno et al., 2000). At the structural level, C5L2 shares with C5aR 35% amino acid identity and an acidic ligand-binding N-terminal domain. C5L2 recognizes with high affinity the anaphylatoxins C5a and at lower affinity its desarginated form C5a des-arg (Cain and Monk, 2002), whereas its ability to bind C3a and C3a des-arg, also called acylation-stimulating protein, is still controversial (Kalant et al., 2003; Johswich et al., 2006). It has not been reported to bind chemokines, but appears to play a role for C5a that is similar to the atypical chemokine receptors and is therefore discussed here.
In contrast to C5aR, C5L2 is uncoupled from G protein signaling pathways. After binding to C5a, it is weakly phosphorylated and does not induce calcium flux, chemotaxis, or MAPK activation (Okinaga et al., 2003). Similar to ACKR2, C5L2 undergoes constitutive clathrin-dependent ligand-independent internalization, and after engagement by C5a it was shown to support its internalization and degradation (Scola et al., 2009). Interestingly, as for atypical chemokine receptors, in both human and murine C5L2, the DRY motif in the second intracellular domain is modified, but reconstituting this sequence only partially restored the receptor ability to induce calcium transients (Scola et al., 2009). Collectively, these results suggest a decoy function of C5L2 for C5a and C5a des-arg. Conversely, it was shown recently that in human neutrophils C5L2 is mostly stored in intracellular compartments, where as a consequence of C5aR activation, it associates to beta-arrestin 2 and negatively regulates C5aR signaling (Van Lith et al., 2009; Bamberg et al., 2010). C5L2 thus appears to belong to the emerging subfamily of beta arrestin-coupled atypical chemoattractant receptors whose function is to tune biologic activities of canonical G protein-coupled chemoattractant receptors.
C5L2 transcripts have been detected in human liver, lung, spleen, kidney, brain, heart, thyroid, skin, skeletal muscle, and adipose tissue, whereas in the mouse its expression is more restricted (Okinaga et al., 2003). In both species, the predominant expression is in bone marrow and mature leukocytes and in particular neutrophils, macrophages, and dendritic cells (Ohno et al., 2000; Chen et al., 2007), where it is negatively regulated by TLRs (Raby et al., 2011).
The biologic role of C5L2 has been investigated in different experimental models. In sepsis, increased expression of C5L2 on neutrophils, lung, and liver and a fourfold increase in IL-6 levels after C5L2 blockade was reported in a rat model, suggesting that this receptor limits the inflammatory response caused by C5aR (Gao et al., 2005a). Consistent with this, a negative correlation between C5L2 levels and clinical outcome in septic patients has been reported (Huber-Lang et al., 2005). Conversely, the same group reported that in the cecal ligation and puncture mouse model of sepsis, C5L2 and C5aR synergistically contribute to sepsis, with the contribution of C5L2-dependent release of HMGB1 because C5L2 blockade or absence in gene-targeted animals improved survival and attenuated production of proinflammatory mediators (Rittirsch et al., 2008), whereas a second group reported that C5L2-deficient animals are hypersensitive to LPS-induced septic shock (Chen et al., 2007). Conflicting results also were reported in a model of acute lung injury induced by immune complexes (Gerard et al., 2005; Chen et al., 2007). C5L2-deficient animals also showed an increased proinflammatory phenotype in a diet-induced insulin resistance model, with decreased insulin sensitivity and glucose uptake in adipose tissue and skeletal muscle (Fisette et al., 2013). Finally, C5L2 deficiency resulted in reduced airway hyperresponsiveness and airway inflammatory response in an ovalbumin-induced allergic asthma model (Chen et al., 2007; Zhang et al., 2010), but increased IL-17A production and airway neutrophil numbers in a house dust mite-induced allergic asthma model (Zhang et al., 2010). Taken collectively, results in vivo highlight a complex interplay between C5aR and C5L2, with C5L2 modulating the local inflammatory responses mediated by C5aR with different outcomes depending upon the experimental setting and the final outcome taken into consideration.
Drug Development.
C5L2 blocking agents have not been developed yet.
IV. Microbial Chemokine Receptors and Chemokine-Binding Proteins
A. Virus-Encoded Chemokine Receptors and Chemokine-Binding Proteins
Viruses have evolved numerous survival strategies, including acquiring host immunoregulatory genes and redesigning them to encode immune evasion factors. This is common for large DNA viruses, i.e., herpesviruses and poxviruses, which have a strong preference for chemokine and chemokine receptor genes (Rosenkilde et al., 2001; Ahuja et al., 1994; Wells and Schwartz, 1997). In addition, some of these viruses secrete soluble broad spectrum chemokine-binding proteins (so-called scavengers) for sequestration of host chemokines (Lalani and McFadden, 1997). Here we focus on virus-encoded 7TM receptors with confirmed chemokine-binding properties (Tables 6 and 7).
TABLE 6.
Properties of virus-encoded 7TM signaling chemokine receptors
| Family | Virus | Gene | Selectivity/Pharmacology | Cellular and Biologic Functions |
|---|---|---|---|---|
| β-Herpesvirus | HCMV | US28 | CC and CX3C constitutively active | Chemokine scavenger |
| Attachment and cell fusion | ||||
| Smooth muscle cell migration | ||||
| HHV6 | U12 | CC | Unknown | |
| U51 | CC | Downregulation of CCL5 expression | ||
| HHV7 | U12 | CC | Unknown | |
| U51 | CC | Unknown | ||
| γ2-Herpesvirus | EHV2 | E1 | CCL11 | Unknown |
| HHV8 | ORF74 | CXC | Lytic expression | |
| Constitutively active, but regulated by CXC agonists and inverse agonists | Possibly involved in Kaposi’s sarcoma | |||
| Antiapoptotic, angiogenic and tumorigenic | ||||
| HVS | ECRF3 or ORF74 | CXC Constitutively active | Unknown | |
| MHV68 | ORF74 | CXC | Lytic expression | |
| Reactivation from latency | ||||
| EHV2 | ORF74 | CXCL6 | Unknown | |
| Constitutively active | ||||
| Poxviruses | YLDV | 7L | CCL1 | Unknown |
HVS, Herpesvirus saimiri; ORF, open-reading frame; YLDV, Yaba-like disease virus.
TABLE 7.
Virus-encoded 7TM receptors with sequence homology to chemokine receptors, whose ligands remain undefined
| Homology | Family | Virus | Gene | Pharmacology | Important Functional Features |
|---|---|---|---|---|---|
| CCR-like | β-Herpesvirus | HCMV | US27 | Unknown | Located on viral envelope |
| UL33 | Constitutively active | Expressed in viral particle and on virus-infected cells | |||
| MCMV | M33 | Constitutively active | Virulence factor. Important for viral replication in salivary glands | ||
| RCMV | R33 | Constitutively active | Virulence factor. Important for viral replication in salivary glands | ||
| Poxvirus | YMTV | 7L | Unknown | Unknown | |
| 145R | Unknown | Unknown | |||
| CXCR-like | γ2-Herpesvirus | AtHV | ORF74 | Unknown | Unknown |
AtHV, Ateline herpesvirus; CMV, cytomegalovirus (H = human; M = mouse; R = rat); YMTV, Yaba monkey tumor virus.
1. US28 from Human Cytomegalovirus.
Human cytomegalovirus (HCMV) encodes four 7TM receptors as follows: US27, US28, UL33, and UL78. The first three are structurally most homologous to chemokine receptors (Rosenkilde et al., 2001). US27 and UL33 remain orphan receptors, whereas US28 was the first viral chemokine receptor to be identified. Its agonists include CX3CL1, CCL2, CCL3, CCL4, CCL5, and CCL7 (Gao and Murphy, 1994; Kuhn et al., 1995; Billstrom et al., 1998; Kledal et al., 1998; Vieira et al., 1998). CX3CL1 acts as both a partial inverse agonist (Casarosa et al., 2001; McLean et al., 2004) and an agonist, depending on the internalization properties of US28 (Waldhoer et al., 2003), whereas its CC chemokine ligands are agonists, inducing Gαq/11 activation, calcium flux, Gα12/13-mediated vascular smooth muscle cell migration, and MAPK signaling (Gao and Murphy, 1994; Billstrom et al., 1998; Vieira et al., 1998; Streblow et al., 1999; McLean et al., 2004; Melnychuk et al., 2004). US28 also exhibits ligand-independent constitutive signaling, e.g., through Gαq/11 and MAPK (Casarosa et al., 2001; Waldhoer et al., 2002; McLean et al., 2004). In model systems, US28 promotes HIV cell entry (Pleskoff et al., 1997), vascular smooth muscle cell migration (Streblow et al., 1999, 2001), and carcinogenesis (Maussang et al., 2006, 2009; Bongers et al., 2010; Slinger et al., 2010; Soroceanu et al., 2011). Allosteric inverse agonists have been discovered for US28 (Casarosa et al., 2003; Hulshof et al., 2003, 2005, 2006; Vischer et al., 2010; Kralj et al., 2011), but their ability to affect HCMV disease pathogenesis remains unknown.
2. U12 and U51 from HHV6.
HHV6 is a widespread β herpesvirus that causes persistent infection (Kondo et al., 1991; Isegawa et al., 1998) and encodes two chemokine receptor homologs, U12 and U51 (Gompels et al., 1995). U12 binds CCL2, CCL3, CCL4, and CCL5, with the latter being most potent in a calcium flux assay (Isegawa et al., 1998), whereas U51 binds CCL2, CCL5, CCL7, CCL11, CCL13, CCL19, XCL1, and the HHV8 encoded chemokine vMIP-II (Milne et al., 2000; Catusse et al., 2008). U51 displays constitutive activity through Gαq/11 pathways, whereas ligand binding induces chemotaxis and Gαi/o activation (Fitzsimons et al., 2006; Catusse et al., 2008). Expression of U51 leads to downregulation of CCL5 secretion and FOG-2, a repressor of the Th2 response (Milne et al., 2000; Catusse et al., 2008), in addition to being important for HHV6 replication and dissemination (Zhen et al., 2005).
3. U12 and U51 from HHV7.
HHV7 is also widespread and a cause of persistent infection (Wyatt et al., 1991; Latchney et al., 2004). The HHV7 genome encodes two chemokine receptor homologs, U12 and U51 (Nicholas, 1996). Some studies have demonstrated that in K562 cells both receptors elicit calcium flux induced by CCL17, CCL19, CCL21, or CCL22 (Nakano et al., 2003; Tadagaki et al., 2005, 2007), whereas in murine L1.2 cells little response was seen without the coexpression of CCR4 or CCR7 (Tadagaki et al., 2007). In line with the above, a chemotactic response was induced by U12 in response to CCL19 and CCL21 (Tadagaki et al., 2005); however, this was not observed in the murine L1.2 cell line (Tadagaki et al., 2007).
4. E1 from EHV2.
Equine herpesvirus 2 (EHV2) is a widespread γ herpesvirus that can be isolated from almost all apparently normal horses (Telford et al., 1995). The significance of EHV2 as a pathogen remains uncertain, although it has been associated with conjunctivitis, respiratory tract disease, and general malaise (Telford et al., 1995). EHV2 encodes at least two chemokine receptors, E1 and ORF74-EHV2, in addition to a 7TM receptor with no resemblance to the chemokine system (E6-EHV2). E1 has highest homology to CCRs (Telford et al., 1995), and its only identified ligand is CCL11, which induces calcium flux and chemotaxis (Camarda et al., 1999). ORF74-EHV2 is described below.
5. 7L from Yaba-like Disease Virus.
Yaba-like disease virus, which belongs to the Yatapoxvirus genus of the Chordopoxvirinae, infects primates and causes vesicular skin lesions (Knight et al., 1989; Najarro et al., 2003). The virus encodes two 7TM receptors, 7L and 145R, that have extraordinarily high homology to CCR8, 73 and 68%, respectively (Najarro et al., 2003). 7L-mediated G protein activation was observed in response to the CCR8 agonists human CCL1 and mouse Ccl1 as well as to human CCL4 and CCL7 and mouse Ccl3, Ccl4, Ccl7, and Ccl21. In addition, the HHV8-encoded chemokines vMIP-I and vMIP-II bind to 7L (Najarro et al., 2003, 2006). When the 7L gene was inserted into the genome of vaccinia, virulence was reduced (Najarro et al., 2006). The ligands for 145R are not known.
6. ECRF3 from Herpesvirus saimiri.
ECRF3 from Herpesvirus saimiri was characterized as the first γ-herpesvirus-encoded CXC chemokine receptor in 1993 (Ahuja and Murphy, 1993). H. saimiri is a T-cell lymphotropic virus giving rise to asymptomatic infection in the natural host, the squirrel monkey (Saimirii sciureus), and fatal lymphoproliferative disease upon transmission to certain other primates (Nicholas et al., 1992). The gene ECRF3 is located in ORF74, which is conserved among members of the rhadinovirus lineage (γ2-herpesvirus) (Albrecht, 2000; Rosenkilde et al., 2001). All ORF74s have highest structural homology to human CXCR2 (Ahuja and Murphy, 1993; Arvanitakis et al., 1997; Bais et al., 1998; Rosenkilde et al., 1999; Verzijl et al., 2004). Consistent with this, ECRF3 binds ELR+ CXC chemokines (Rosenkilde and Schwartz, 2004; Strieter et al., 1995), with the potency order CXCL6 > CXCL1 > CXCL8 > CXCL7. It signals constitutively through Gαi/o and Gα12/13, but activates Gαq/11, Gαi/o and the transcription factors NFAT, cAMP response element-binding protein, and nuclear factor-κB in a ligand-dependent manner. In addition to agonistic CXC-chemokines, the broad spectrum CC-chemokine antagonist vMIP-II encoded by HHV8 (Kledal et al., 1997) binds with high affinity and inhibits basal Gαi signaling as an inverse agonist. No other antagonists have yet been described for ECRF3.
7. ORF74 from Human Herpesvirus 8.
HHV8, or the Kaposi’s sarcoma-associated herpesvirus, is less widely distributed in the population than most other herpesviruses. Primary HHV8 infection is usually asymptomatic, but is associated with three neoplasms: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Similar to other γ2-herpesviruses, HHV8 encodes only one 7TM receptor, and it is located in ORF74. HHV8 ORF74 (first known as Kaposi’s sarcoma-associated herpesvirus-G protein-coupled receptor) binds multiple human CXC-chemokines (CXCL1-3, 5, 7–8, and 12) with high affinity, in addition to vMIP-II (Arvanitakis et al., 1997; Geras-Raaka et al., 1998; Gershengorn et al., 1998; Rosenkilde et al., 1999, 2000). Like US28, HHV8 ORF74 activates a broad spectrum of signaling pathways in a constitutive manner (G proteins, protein kinases, MAPKs, and transcription factors) (Arvanitakis et al., 1997; Bais et al., 1998; Geras-Raaka et al., 1998; Rosenkilde et al., 1999, 2000; Schwarz and Murphy, 2001; Smit et al., 2002). In addition, it is regulated by various chemokine ligands, with CXCL1-3 acting as agonists; CXCL5, -7, -8 being neutral antagonists; and CXCL10, -12, and vMIP-II acting as inverse agonists (Gershengorn et al., 1998; Rosenkilde et al., 1999). HHV8 ORF74 acts as a strong paracrine activator, because cells expressing this receptor secrete various growth, angiogenic, and proinflammatory cytokines (Montaner et al., 2001; Pati et al., 2001; Schwarz and Murphy, 2001; Shepard et al., 2001). These pharmacological features correlate with several in vivo studies placing HHV8 ORF74 centrally in relation to the oncogenic properties of HHV8 (Bais et al., 1998; Yang et al., 2000; Holst et al., 2001; Guo et al., 2003; Montaner et al., 2003; Jensen et al., 2005). No small-molecule ligands exist for native HHV8 ORF74, yet by site-directed mutagenesis and incorporation of metal-ion binding sites it has been shown that both its constitutive and ligand-mediated activity can be modulated by small molecule ligands, indicating that it may be a good drug target (Rosenkilde et al., 1999, 2000).
8. ORF74 from Murine Gammaherpesvirus-68.
Murine gammaherpesvirus-68 (MHV-68) is a natural pathogen of murid rodents and represents a well-established model for γ-herpesvirus infections in humans because of its close resemblance to HHV8 and Epstein-Barr virus (Blaskovic et al., 1980; Tripp et al., 1997). It encodes both a CXC-chemokine receptor in ORF74 (MHV68 ORF74) and a soluble chemokine-binding protein, M3. Similar to HHV8 ORF74, chemokine agonists activate MHV68 ORF74 to signal through multiple pathways, including Gαi/o, Gαq/11, and MAPKs (Verzijl et al., 2004). However, in contrast to HHV8 ORF74, no constitutive activity has been observed for this receptor. Interestingly, experiments done with MHV68 ORF74 knockout virus have shown that it is necessary for the reactivation of virus from latency (Lee et al., 2003; Moorman et al., 2003).
9. ORF74 from Equine Herpesvirus 2.
E1 and ORF74 are the two chemokine receptors encoded by the equine γ-herpesvirus 2 (EHV2) (Telford et al., 1995). EHV2 ORF74 is homologous to other ORF74 receptors from γ-herpesviruses in addition to being functionally similar, as it binds human CXCL6 (Rosenkilde et al., 2005) with subsequent activation of the Gαi/o pathway (Rosenkilde et al., 2004). EHV2 ORF74 also signals constitutively through this same pathway (Rosenkilde et al., 2004). EHV2 ORF74 is unusual in lacking a positive charge in the highly conserved DRY-motif in the bottom of TM-3 (Mirzadegan et al., 2003; Rosenkilde et al., 2005; Jensen and Rosenkilde, 2009; Jensen et al., 2012). This motif, and in particular the positive charge (position III:261, or 3.50), is important for 7TM receptor activation, as emphasized by direct interaction with G protein in the recent crystal structure of an active 7TM receptor, the β2-adrenergic receptor, in complex with the Gαs-subunit (Rasmussen et al., 2011). As recently described, there is a larger variation in the DRY motif among virus-encoded 7TM receptors compared with human class A 7TM receptors, yet still the positive charge in position III:26/3.50 seems largely conserved (Jensen et al., 2012). However, a DTW-motif is found in EHV2 ORF74 and reintroduction of the DRY-motif results in decreased signaling (Rosenkilde et al., 2005). Small molecule inverse agonists with micromolar potencies have been identified for EHV2 ORF74 (Rosenkilde et al., 2005).
10. Virus-Encoded Chemokines and Chemokine-Binding Proteins.
Agonists as well as antagonists are found among the virus-encoded chemokines, which range from highly selective to very broad-spectrum ligands. Thus, UL146 from HCMV, U83 from HHV6, and vMIP-I and -III from HHV8 are all agonists (Dairaghi et al., 1999; Endres et al., 1999; Penfold et al., 1999; Stine et al., 2000; Zou et al., 1999; Luttichau et al., 2003, 2007), whereas vMIP-II from HHV8 and MC148 from Molluscum contagiosum virus act as antagonists and inverse agonists (Kledal et al., 1997; Damon et al., 1998; Rosenkilde et al., 1999; DeBruyne et al., 2000; Luttichau et al., 2001; Rosenkilde and Schwartz, 2004; Luttichau, 2010; Rummel et al., 2012). The broad-spectrum antagonism by vMIP-II has been demonstrated in vivo in a rat model of experimental glomerulonephritis, where it significantly reduced leukocyte infiltration into the glomeruli (Chen et al., 1998). The virus-encoded chemokine-binding proteins (vCKBPs) have no known cellular homologs and their evolutionary sources therefore remain obscure. Yet, considering their origin and chemokine binding profile (and pattern of chemokine scavenging and subsequent immune-function modulation), a distinct pattern of four groups appear (vCKBPI-IV). These are all described in detail in Table 8.
TABLE 8.
Virus-encoded chemokine scavengers
| Name | Family | Virus | Gene | Chemokine Specificity | Biologic Functions |
|---|---|---|---|---|---|
| vCKBP-I | Leporipoxvirus | Myxoma virus | M-T7 S-T7 | XC, CC, and CXC | Viral immune evasion. Anti-inflammatory. Interferes with chemokine-GAG interaction and chemokine-receptor interaction. |
| Shope fibroma virus | |||||
| vCKBP-II | Orthopoxvirus and parapoxvirus | Myxoma virus, vaccinia virus, cowpoxvirus ectromelia virus, camel- and rabbitpox virus, shope fibroma virus, racoonpox virus, variola virus, myxoma virus. | M-TI 35kDaP35 vCCI B29R G5R | CC | Viral immune evasion. Anti-inflammatory. Blocks chemokine-receptor interaction. |
| vCKBP-III | γ2-Herpesvirus | MHV68 | M3 | XC, CC, CXC, and CX3C | Important for latency. Blocks chemokine-GAG interaction and chemokine-receptor interaction. |
| vCKBP-IV | α-Herpesvirus | Equine herpesvirus 1+3 bovine herpesvirus 1+5 rangiferine herpesvirus 1 caprine herpesvirus 1 cervine herpesvirus 1 | gG | XC, CC, and CXC | Interferes with chemokine-GAG interaction and chemokine-receptor interaction. |
MHV68, Murid herpesvirus 68.
In summary, the virus-encoded 7TM chemokine receptors are generally more promiscuous with respect to ligand binding and signaling pathways than their human counterparts. Moreover, many display constitutive signaling to levels not observed among classic host chemokine receptors. Importantly, chemokine and small-molecule antagonists and inverse agonists have been identified for several viral chemokine receptors, illustrating that they—like the endogenous pool of 7TM receptors— are generally easily targeted by drugs and thus suitable for the pharmaceutical industry provided proper target validation. In contrast, the virus-encoded chemokines and chemokine-binding proteins may serve as templates for development of novel anti-inflammatory drugs.
B. Protozoan Chemokine-Binding Proteins
S. mansoni is a nematode parasite that infects humans and causes schistosomiasis, a disease that is relatively common in third world countries. Using a cross-linking band shift assay with [125I]CXCL8 and [125I]CCL3, a chemokine-binding protein (CKBP) named smCKBP was identified in schistosome egg secretions but not from other stages of the worm’s life cycle (Smith et al., 2005). The molecular identity of smCKBP, achieved by a proteomic approach, revealed a novel sequence unrelated to chemokines, chemokine receptors, and other known proteins. smCKBP was shown to bind highly promiscuously to some CC chemokines, notably CCL3 and CCL5 but not CCL11; a CXC chemokine, CXCL8; and the CX3C chemokine, CX3CL1.
smCKBP also was tested in a number of animal disease models (Smith et al., 2005). Recombinant smCKBP was able to block ear swelling caused by neutrophil infiltration in a contact hypersensitivity model in mice and was also effective in blocking neutrophil infiltration in a CXCL8-induced model of pulmonary inflammation. In an experimental granulomatous inflammation model in which mice were injected intravenously with live schistosome eggs after treatment with anti-smCKBP rabbit sera, there was an approximately twofold increase in granuloma size relative to the granulomas in animals treated with nonreactive serum. Animals in which smCKBP was blocked had a significant increase in the proportion of neutrophils and macrophages and reduced number of eosinophils within the granuloma, suggesting that secretion of smCKBP by live eggs profoundly modulates the differential recruitment of cells and the size of the egg granuloma. On the other hand, recombinant smCKBP did not alter disease in EAE or collagen-induced arthritis models. Taken together these results suggest that smCKBP has specific in vivo activity in suppression of acute or local inflammation, although it cannot be ruled out that lack of effect in the chronic models could be caused by generation of a host anti-smCKBP antibody response.
V. Tick Chemokine-Binding Proteins
Ticks are bloodsucking ectoparasitic arthropods that feed on the blood of mammalian and nonmammalian terrestrial vertebrates. To survive, ticks need to remain undetected on their host for periods of up to 2 weeks. To do this, they secrete a plethora of pharmacologically active compounds in their saliva, including factors that block platelet aggregation, vasoconstriction, and blood clotting; molecules with analgesic properties; as well as anti-inflammatory and immunomodulatory molecules, including chemokine-binding proteins (Fig. 2; Table 9).
TABLE 9.
Characteristics of recombinant tick Evasins
| Evasin | Chemokine | Affinity by SPR | IC50c | Therapeutic Efficacy In Vivo | |
|---|---|---|---|---|---|
| nM | |||||
| Evasin-1 | CCL3 | 0.16a | 2.0 | Dermal inflammation in Ackr2−/− mice | |
| Bleomycin induced lung fibrosis | |||||
| Intestinal ischemia reperfusion | |||||
| GVHD | |||||
| CCL4 | 0.81 | 4.0 | Resistance to Leishmania major infection | ||
| CCL18 | 3.2 | nd | |||
| Evasin-3 | CXCL1 | 0.85a | 20 | mBSA induced arthritis | |
| CXCL3 | 0.97 | nd | Intestinal ischemia reperfusion | ||
| CXCL5 | 3.57 | nd | Myocardial ischemic reperfusion | ||
| CXCL6 | 2.00 | nd | Atherosclerotic ischemic stroke | ||
| CXCL8 | 0.43 | 3.35 | |||
| KC | 0.42 | nd | |||
| MIP-2 | 5.78 | nd | |||
| CINC | 0.64 | nd | |||
| Rat-Gro-β | 0.42 | nd | |||
| Evasin-4 | CCL2 | naa | nab | 540 | DSS colitis |
| CCL3 | 0.09 | 33 | 3.3 | Contact hypersensitivity | |
| CCL5 | 0.06 | 194 | 4.8 | ||
| CCL7 | 0.7 | nd | 5.0 | ||
| CCL8 | 0.25 | nd | 3.1 | ||
| CCL11 | 0.26 | 128 | 4.9 | ||
| CCL14 | 0.14 | nd | 2.4 | ||
| CCL15 | 2.31 | nd | 88 | ||
| CCL16 | 0.2 | nd | 304 | ||
| CCL17 | 0.61 | 126 | 69 | ||
| CCL18 | 0.03 | 32.0 | nd | ||
| CCL22 | 0.43 | 30.4 | 38 | ||
| CCL23 | 4.43 | 14 | 2.2 | ||
| CCL26 | 0.88 | 46.1 | 68 | ||
SPR, surface plasmon resonance; na, no binding; nd, not determined; GVHD, graft versus host disease; mBSA, modified bovine serum albumin.
In this column, SPR with Evasin immobilized.
In this column, SPR with chemokines immobilized.
Agonist concentrations for in vitro chemotaxis.
Tick salivary gland extracts (SGE) purified by fast-performance liquid chromatography from Dermocentor reticulatus, Rhiphicephalus appendiculatus, and Amblyomma variegatum fed for 3–5 days on mice, guinea-pigs, or rabbits, respectively, were shown to bind [125I]CXCL8 and inhibit CXCL8 activity in receptor binding and chemotaxis assays using human neutrophils (Hajnicka et al., 2001; Vancova et al., 2007). An analysis of D. reticulatus, A. variegatum, and Ixodes ricinus showed that antichemokine activities in SGE differ among tick species and between males and females (Vancova et al., 2010). In this study, D. reticulatus and I. ricinus SGE were collected after 5 days of feeding on mice, and A.variegatum SGE was collected after 12 days of feeding on rabbits, and extracts were tested for the presence of inhibitory activities against CXCL8, CCL2, CCL5, CCL3, and CCL11. In the case of D. reticulatus, male SGE inhibited all of these chemokines, whereas female SGE inhibited all chemokines tested except CCL5. Interestingly SGE from I. ricinus females were able to inhibit only CXCL8 with low potency, whereas male SGE showed inhibitory activity for all of the chemokines. This is consistent with the altruistic behavior of “mate guarding,” in which the male tick helps its mate to feed by secreting immunomodulators into the site where its mate is feeding–the size of the egg batch is determined by blood-meal size–thus ensuring survival.
The CKBPs present in saliva from Rhipicephalus sanguineus, the common brown dog tick, were identified by a cDNA expression cloning strategy (Frauenschuh et al., 2007) using a cross-linking assay to [125I]CCL3. Only one positive pool was identified, and after four rounds of sequential deconvolution and screening, a single cDNA clone responsible for the CCL3 binding activity was identified and named Evasin-1. During deconvolution, a second sequence was identified, named Evasin-2, that also was thought to be a CCL3-binding protein, but further study of the recombinant protein did not confirm this. To date Evasin-2 function and specificity for chemokines remains undefined (A. Proudfoot and C. Power, unpublished results). Subsequently, using the same cloning strategy, a CXCL8-binding protein was identified and named Evasin-3, and a CCL5 and CCL11-binding protein was identified and named Evasin-4.
Evasins bind their chemokine ligands with high affinity. Compared with viral CKBPs and smCKBP, Evasin-1 and Evasin-3 are more selective. Evasin-1 binds only CCL3, CCL4, and CCL18, whereas Evasin-3 binds only ELR+ CXC chemokines. Both bind and neutralize the rodent counterparts of their human chemokine ligands. In contrast, Evasin-4 bound to the majority of the proinflammatory CC chemokines tested (Deruaz, 2008).
The fact that Evasin-1 binds CCL18 is intriguing. CCL18 shows a high degree of homology to CCL3 and CCL4 and it has been postulated that it arose from duplication of the CCL3 gene (Hieshima et al., 1997; Atamas et al., 2003; Chang et al., 2010; Azzaoui et al., 2011; Chenivesse et al., 2012; Schraufstatter et al., 2012). The chemoattractant properties of CCL18 are not impressive, at least in vitro. Another peculiarity of CCL18 is that like CXCL8, it does not have a murine homolog. Nevertheless, both of these chemokines recruit cells when injected into mice (Luzina et al., 2006). So although the precise role of CCL18 in humans is unclear, the fact that ticks express a binding protein that specifically neutralizes its activity suggests that it may have an important role in host defense against pathogens. Evasin-1 may thus be a useful tool to elucidate the biologic role of CCL18.
The anti-inflammatory properties of the Evasins have been tested in a variety of animal models summarized in Table 9. Treatment of mice either prophylactically or therapeutically with Evasin-1 attenuated lung fibrosis, probably because of effects on neutrophil infiltration mediated by CCR1/CCL3, in a bleomycin model of pulmonary fibrosis (Russo et al., 2011). Evasin-1 treatment also reduced T lymphocyte recruitment and prevented mortality in an acute graft versus host disease model in mice (Castor et al., 2010). Evasin-3 antagonizes neutrophil recruitment mediated by CXCR2 ligands and has shown efficacy in CXCL1-dependent models, such as modified bovine serum albumin-induced arthritis (Déruaz et al., 2008). In addition, Evasin-3 also reduced infarct size and neutrophil recruitment in a mouse model of ischemia/reperfusion injury (Montecucco et al., 2010). Administration of Evasin-4 in a DSS colitis model greatly ameliorated clinical score, weight loss, and overall tissue destruction and mortality (Vieira et al., 2009). These effects are probably related to its ability to block several CC chemokines, including those responsible for eosinophil recruitment.
An important difference between mature Evasins and viral CKBPs is their small size (Evasin-1, 94 aa; Evasin-3, 66 aa; and Evasin-4, 104 aa). All appear to be heavily glycosylated, although small amounts of nonglycosylated proteins appear to also be present in saliva (Frauenschuh et al., 2007). Glycosylation does not appear to play a role in Evasin activity in vitro. It can therefore be postulated that glycosylation may serve as a means of extending the half-life of these proteins in vivo or rendering them invisible to the host’s immune system (Li and d'Anjou, 2009).
Evasin-1 and Evasin-3 are unrelated at the primary amino acid sequence level and have distinct crystal structures (Dias et al., 2009). In fact, both have completely novel folds lacking a structural homolog in the Protein Data Bank database. Evasin-4 shows the same disulfide topology as Evasin-1, suggesting that these two proteins are probably structurally related although the structure of Evasin-4 has not yet been elucidated. The structure of the Evasin-1:CCL3 complex is available. However, despite extensive analysis the exact protein-protein interactions that determine the strength of the CKBP-chemokine interaction have remained unclear (Dias et al., 2009).
At least five putative Evasin-1 and Evasin-3 homologs have been identified by in-depth sequence analysis of the R. sanguineus sialotranscriptome (Anatriello et al., 2010). Expressed sequence tags that are Evasin-3-like have been identified in I. scapularis, I. ricinus, and D. andersoni (Vancova et al., 2010), and potential Evasin homologs have been deposited in the Genome database for Boophilus microplus and I. scapularis (C. Power, unpublished results). At least 18 Evasin homologs have been identified for A. maculatum (Karim et al., 2011). Whether any of these homologs encodes a CKBP has not yet been determined.
The unique miniaturized scaffolds of the Evasins, which have evolved to bind specific chemokines, have the potential to be engineered to have specific chemokine binding profiles and to be developed as drugs for anti-inflammatory, autoimmune diseases, and cancer and are also proving to be useful tools for the elucidation of chemokine function in vitro and in vivo.
VI. Conclusions
A potential lesson to be learned from the study of atypical chemokine receptors and microbial and tick chemokine-binding proteins is that effectively interfering with inflammatory chemokine-mediated disease processes may require targeting of multiple chemokines or chemokine receptors simultaneously or sequentially. Biologic specificity of each chemokine receptor can often readily be demonstrated when an intervention precedes a challenge in animal models (e.g., in a gene knockout mouse), but once an inflammatory response is in full progress, as is the case for most human diseases subject to clinical trials, the process may evolve to one driven by chemokine redundancy. This may explain the difficulty in developing safe and effective medicines for immunologically mediated disease by targeting one chemokine or one chemokine receptor at a time. The successes of maraviroc and plerixafor for CCR5 and CXCR4, respectively, are atypical and not related to inflammation. The next phase of drug development may profit by testing blocking strategies that are robust in whole blood and tissue, by intervening as early as possible in the best-validated diseases in humans and by targeting where possible more than one chemokine or receptor.
Abbreviations
- 7TM
7-transmembrane
- aa
amino acid
- ACKR
atypical chemokine receptor
- AMD
age-related macular degeneration
- APCs
antigen presenting cells
- ATL
adult T-cell leukemia/lymphoma
- BM
bone marrow
- BMS
Bristol-Myers Squibb
- CKBP
chemokine-binding protein
- CLA
cutaneous lymphocyte antigen
- CMV
cytomegalovirus
- CNV
choroidal neovascularization
- COPD
chronic obstructive pulmonary disease
- CRAM
chemokine receptor for activated macrophages
- CTL
cytotoxic T lymphocytes
- DARC
Duffy antigen receptor for chemokines
- DC
dendritic cell
- DN
double negative
- DSS
dextran sodium sulfate
- EAE
experimental autoimmune encephalomyelitis
- EE
eosinophilic esophagitis
- EHV2
equine herpesvirus 2
- FDA
Food and Drug Administration
- GAG
glycosaminoglycan
- GM-CSF
granulocyte macrophage colony-stimulating factor
- GPCR
G protein-coupled receptors
- GSK
GlaxoSmithKline
- HCMV
human cytomegalovirus
- HEV
high endothelial venule
- HHV
human herpesvirus
- HIV
human immunodeficiency virus
- HMGB1
high mobility group protein B1
- ICL
intracellular loop
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinases
- MCP
monocyte chemotactic protein
- MHV-68
murine gamma herpesvirus-68
- MIP
macrophage inflammatory protein
- MS
multiple sclerosis
- NK
natural killer cell
- NKT
NK T cell
- PARC
pulmonary and activation-regulated chemokine
- PCR
polymerase chain reaction
- PI3K
phosphatidylinositol 3-kinase
- PITP
phosphatidyl-inositol transfer protein
- RA
rheumatoid arthritis
- RANTES
reduced upon activation, normal T expressed and secreted
- SGE
salivary gland extracts
- TCR
T-cell receptor
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- Tregs
regulatory T cells
- vCKBP
virus-derived chemokine-binding proteins
- vMIP
viral macrophage inflammatory protein
- WHIM
warts caused by human papillomavirus infection, hypogammaglobulinemia, infections, and myelokathexis
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Bachelerie, Ben-Baruch, Burkhardt, Combadiere, Farber, Graham, Horuk, Sparre-Ulrich, Locati, Luster, Mantovani, Matsushima, Murphy, Nibbs, Nomiyama, Power, Proudfoot, Rosenkilde, Rot, Sozzani, Thelen, Yoshie, Zlotnik.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health [National Institute of Allergy and Infectious Diseases].
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. (2003) Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA 100:7947–7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, et al. (2004) The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol 172:6362–6372 [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646 [DOI] [PubMed] [Google Scholar]
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. (2000) The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol 165:5269–5277 [DOI] [PubMed] [Google Scholar]
- Ahern D, Lloyd CM, Robinson DS. (2009) Chemokine responsiveness of CD4+ CD25+ regulatory and CD4+ CD25- T cells from atopic and nonatopic donors. Allergy 64:1121–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja SK, Gao JL, Murphy PM. (1994) Chemokine receptors and molecular mimicry. Immunol Today 15:281–287 [DOI] [PubMed] [Google Scholar]
- Ahuja SK, Murphy PM. (1993) Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem 268:20691–20694 [PubMed] [Google Scholar]
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, et al. (1998) HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci USA 95:13153–13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht JC. (2000) Primary structure of the Herpesvirus ateles genome. J Virol 74:1033–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Nelson CA, van Berkel V, Lau EK, Studts JM, Brett TJ, Speck SH, Handel TM, Virgin HW, Fremont DH. (2002) Structural basis of chemokine sequestration by a herpesvirus decoy receptor. Cell 111:343–356 [DOI] [PubMed] [Google Scholar]
- Algood HM, Flynn JL. (2004) CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J Immunol 173:3287–3296 [DOI] [PubMed] [Google Scholar]
- Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Sousa C, Fairlamb A, Ribeiro JM, Sher A. (2003) Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol 4:485–490 [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. (1996) CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958 [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Liao F, Berger EA, Farber JM, Peden KW. (1997) A new SIV co-receptor, STRL33. Nature 388:238. [DOI] [PubMed] [Google Scholar]
- Allegretti M, Bertini R, Cesta MC, Bizzarri C, Di Bitondo R, Di Cioccio V, Galliera E, Berdini V, Topai A, Zampella G, et al. (2005) 2-Arylpropionic CXC chemokine receptor 1 (CXCR1) ligands as novel noncompetitive CXCL8 inhibitors. J Med Chem 48:4312–4331 [DOI] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. (2013) CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D, Arkinson JL, Sun J, Fattouh R, Walker T, Jordana M. (2007) Th2 differentiation in distinct lymph nodes influences the site of mucosal Th2 immune-inflammatory responses. J Immunol 179:3287–3296 [DOI] [PubMed] [Google Scholar]
- Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS, Gazzinelli RT, Teixeira MM, Ferreira SH, Cunha FQ. (2009) Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci USA 106:4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanatidou V, Sourvinos G, Apostolakis S, Tsilimigaki A, Spandidos DA. (2006) T280M variation of the CX3C receptor gene is associated with increased risk for severe respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 25:410–414 [DOI] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. (2003) An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 9:1390–1397 [DOI] [PubMed] [Google Scholar]
- Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS. (2008) Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res 14:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An P, Li R, Wang JM, Yoshimura T, Takahashi M, Samudralal R, O’Brien SJ, Phair J, Goedert JJ, Kirk GD, et al. (2011) Role of exonic variation in chemokine receptor genes on AIDS: CCRL2 F167Y association with pneumocystis pneumonia. PLoS Genet 7:e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anatriello E, Ribeiro JM, de Miranda-Santos IK, Brandão LG, Anderson JM, Valenzuela JG, Maruyama SR, Silva JS, Ferreira BR. (2010) An insight into the sialotranscriptome of the brown dog tick, Rhipicephalus sanguineus. BMC Genomics 11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic AV, Kerkovich D, Shanley J, Pulliam L, Pachter JS. (1999) Expression of binding sites for beta chemokines on human astrocytes. Glia 28:225–235 [PubMed] [Google Scholar]
- Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, et al. (2000) Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol 164:6303–6312 [DOI] [PubMed] [Google Scholar]
- Andrew DP, Ruffing N, Kim CH, Miao W, Heath H, Li Y, Murphy K, Campbell JJ, Butcher EC, Wu L. (2001) C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol 166:103–111 [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. (2002) Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med 196:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, et al. (2007) Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Harris RB, Cyster JG. (2002) CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16:67–76 [DOI] [PubMed] [Google Scholar]
- Antoniu SA. (2010) Mogamulizumab, a humanized mAb against C-C chemokine receptor 4 for the potential treatment of T-cell lymphomas and asthma. Curr Opin Mol Ther 12:770–779 [PubMed] [Google Scholar]
- Arias MA, Jaramillo G, López YP, Mejía N, Mejía C, Pantoja AE, Shattock RJ, García LF, Griffin GE. (2007) Mycobacterium tuberculosis antigens specifically modulate CCR2 and MCP-1/CCL2 on lymphoid cells from human pulmonary hilar lymph nodes. J Immunol 179:8381–8391 [DOI] [PubMed] [Google Scholar]
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. (1997) Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347–350 [DOI] [PubMed] [Google Scholar]
- Aslanian AM, Charo IF. (2006) Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation 114:583–590 [DOI] [PubMed] [Google Scholar]
- Atamas SP, Luzina IG, Choi J, Tsymbalyuk N, Carbonetti NH, Singh IS, Trojanowska M, Jimenez SA, White B. (2003) Pulmonary and activation-regulated chemokine stimulates collagen production in lung fibroblasts. Am J Respir Cell Mol Biol 29:743–749 [DOI] [PubMed] [Google Scholar]
- Atchison RE, Gosling J, Monteclaro FS, Franci C, Digilio L, Charo IF, Goldsmith MA. (1996) Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924–1926 [DOI] [PubMed] [Google Scholar]
- Attal H, Cohen-Hillel E, Meshel T, Wang JM, Gong W, Ben-Baruch A. (2008) Intracellular cross-talk between the GPCR CXCR1 and CXCR2: role of carboxyl terminus phosphorylation sites. Exp Cell Res 314:352–365 [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, et al. (2009) CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med 206:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, Gullestad L, Damås JK. (2008) Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol 28:1909–1919 [DOI] [PubMed] [Google Scholar]
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, et al. (2009) CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113:4341–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzaoui I, Yahia SA, Chang Y, Vorng H, Morales O, Fan Y, Delhem N, Ple C, Tonnel AB, Wallaert B, et al. (2011) CCL18 differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood 118:3549–3558 [DOI] [PubMed] [Google Scholar]
- Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. (2007) Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4- Tregs, regulate effector T cells using FasL. J Immunol 178:4891–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, Nomiyama H, Yoshie O. (1997) Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem 272:14893–14898 [DOI] [PubMed] [Google Scholar]
- Baba M, Takashima K, Miyake H, Kanzaki N, Teshima K, Wang X, Shiraishi M, Iizawa Y. (2005) TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob Agents Chemother 49:4584–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Farzan M, Sodroski J. (2003) Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem 278:3378–3385 [DOI] [PubMed] [Google Scholar]
- Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, et al. (2010) Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 207:1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Wurbel MA, Kivisäkk P, Wain CM, Power CA, Haraldsen G, Campbell JJ. (2005) A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med 201:1045–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. (1998) G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86–89 [DOI] [PubMed] [Google Scholar]
- Balabanian K, Brotin E, Biajoux V, Bouchet-Delbos L, Lainey E, Fenneteau O, Bonnet D, Fiette L, Emilie D, Bachelerie F. (2012) Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood 119:5722–5730 [DOI] [PubMed] [Google Scholar]
- Balabanian K, Harriague J, Décrion C, Lagane B, Shorte S, Baleux F, Virelizier JL, Arenzana-Seisdedos F, Chakrabarti LA. (2004) CXCR4-tropic HIV-1 envelope glycoprotein functions as a viral chemokine in unstimulated primary CD4+ T lymphocytes. J Immunol 173:7150–7160 [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. (2005a) The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280:35760–35766 [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, et al. (2005b) WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 105:2449–2457 [DOI] [PubMed] [Google Scholar]
- Balkwill F. (2004) The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol 14:171–179 [DOI] [PubMed] [Google Scholar]
- Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, et al. (2010) The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem 285:7633–7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannert N, Craig S, Farzan M, Sogah D, Santo NV, Choe H, Sodroski J. (2001) Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J Exp Med 194:1661–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlic J, Zhu W, Murphy PM. (2009) Atherogenic lipids induce high-density lipoprotein uptake and cholesterol efflux in human macrophages by up-regulating transmembrane chemokine CXCL16 without engaging CXCL16-dependent cell adhesion. J Immunol 182:7928–7936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugher PJ, Richmond A. (2008) The carboxyl-terminal PDZ ligand motif of chemokine receptor CXCR2 modulates post-endocytic sorting and cellular chemotaxis. J Biol Chem 283:30868–30878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640–644 [DOI] [PubMed] [Google Scholar]
- Bazzan E, Saetta M, Turato G, Borroni EM, Cancellieri C, Baraldo S, Savino B, Calabrese F, Ballarin A, Balestro E, et al. (2013) Expression of the atypical chemokine receptor D6 in human alveolar macrophages in COPD. Chest 143:98–106 [DOI] [PubMed] [Google Scholar]
- Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, Dupuy A, Kerob D, Beaupain B, Bordigoni P, et al. (2012) Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LA, Tancowny B, Brummet ME, Asaki SY, Curry SL, Penno MB, Foster M, Bahl A, Stellato C. (2006) Functional analysis of the chemokine receptor CCR3 on airway epithelial cells. J Immunol 177:3344–3354 [DOI] [PubMed] [Google Scholar]
- Beider K, Begin M, Abraham M, Wald H, Weiss ID, Wald O, Pikarsky E, Zeira E, Eizenberg O, Galun E, et al. (2011) CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol 39:282–292 [DOI] [PubMed] [Google Scholar]
- Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. (2006) T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem 281:11992–12000 [DOI] [PubMed] [Google Scholar]
- Ben-Baruch A, Xu L, Young PR, Bengali K, Oppenheim JJ, Wang JM. (1995) Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J Biol Chem 270:22123–22128 [DOI] [PubMed] [Google Scholar]
- Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ. (2005) Proteolytic activation of alternative CCR1 ligands in inflammation. J Immunol 174:7341–7351 [DOI] [PubMed] [Google Scholar]
- Berkman N, Ohnona S, Chung FK, Breuer R. (2001) Eotaxin-3 but not eotaxin gene expression is upregulated in asthmatics 24 hours after allergen challenge. Am J Respir Cell Mol Biol 24:682–687 [DOI] [PubMed] [Google Scholar]
- Bernardini G, Hedrick J, Sozzani S, Luini W, Spinetti G, Weiss M, Menon S, Zlotnik A, Mantovani A, Santoni A, et al. (1998) Identification of the CC chemokines TARC and macrophage inflammatory protein-1 beta as novel functional ligands for the CCR8 receptor. Eur J Immunol 28:582–588 [DOI] [PubMed] [Google Scholar]
- Bernardini G, Spinetti G, Ribatti D, Camarda G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. (2000) I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood 96:4039–4045 [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, et al. (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13:587–596 [DOI] [PubMed] [Google Scholar]
- Berres M-L, Trautwein C, Zaldivar MM, Schmitz P, Pauels K, Lira SA, Tacke F, Wasmuth HE. (2009) The chemokine scavenging receptor D6 limits acute toxic liver injury in vivo. Biol Chem 390:1039–1045 [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. (2010) Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya B, Carlsten J, Sabo E, Kethu S, Meitner P, Tavares R, Jakate S, Mangray S, Aswad B, Resnick MB. (2007) Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol 38:1744–1753 [DOI] [PubMed] [Google Scholar]
- Biber K, Zuurman MW, Homan H, Boddeke HW. (2003) Expression of L-CCR in HEK 293 cells reveals functional responses to CCL2, CCL5, CCL7, and CCL8. J Leukoc Biol 74:243–251 [DOI] [PubMed] [Google Scholar]
- Billstrom MA, Johnson GL, Avdi NJ, Worthen GS. (1998) Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol 72:5535–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K. (2009) Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med 15:417–424 [DOI] [PubMed] [Google Scholar]
- Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. (2006) ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther 112:139–149 [DOI] [PubMed] [Google Scholar]
- Blaak H, Boers PH, Gruters RA, Schuitemaker H, van der Ende ME, Osterhaus AD. (2005) CCR5, GPR15, and CXCR6 are major coreceptors of human immunodeficiency virus type 2 variants isolated from individuals with and without plasma viremia. J Virol 79:1686–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn PE, Simpson CV, Nibbs RJ, O’Hara M, Booth R, Poulos J, Isaacs NW, Graham GJ. (2004) Purification and biochemical characterization of the D6 chemokine receptor. Biochem J 379:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, et al. (2006) Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 116:536–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Migeotte I, Lee B, Vakili J, Doranz BJ, Govaerts C, Vassart G, Doms RW, Parmentier M. (1999) CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood 94:1899–1905 [PubMed] [Google Scholar]
- Blaskovic D, Stanceková M, Svobodová J, Mistríková J. (1980) Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol 24:468. [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. (1997) The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA 94:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodart V, Anastassov V, Darkes MC, Idzan SR, Labrecque J, Lau G, Mosi RM, Neff KS, Nelson KL, Ruzek MC, et al. (2009) Pharmacology of AMD3465: a small molecule antagonist of the chemokine receptor CXCR4. Biochem Pharmacol 78:993–1000 [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. (2009) Origin of the lamina propria dendritic cell network. Immunity 31:513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. (1996) Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol 157:985–992 [PubMed] [Google Scholar]
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. (2008) Control of chemokine-guided cell migration by ligand sequestration. Cell 132:463–473 [DOI] [PubMed] [Google Scholar]
- Bonduelle O, Duffy D, Verrier B, Combadière C, Combadière B. (2012) Cutting edge: Protective effect of CX3CR1+ dendritic cells in a vaccinia virus pulmonary infection model. J Immunol 188:952–956 [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, et al. (1998) Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 187:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecchi R, Borroni EM, Anselmo A, Doni A, Savino B, Mirolo M, Fabbri M, Jala VR, Haribabu B, Mantovani A, et al. (2008) Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood 112:493–503 [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, et al. (2004) Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol 172:4972–4976 [DOI] [PubMed] [Google Scholar]
- Bongers G, Maussang D, Muniz LR, Noriega VM, Fraile-Ramos A, Barker N, Marchesi F, Thirunarayanan N, Vischer HF, Qin L, et al. (2010) The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest 120:3969–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini JA, Martin SK, Dralyuk F, Roe MW, Philipson LH, Steiner DF. (1997) Cloning, expression, and chromosomal mapping of a novel human CC-chemokine receptor (CCR10) that displays high-affinity binding for MCP-1 and MCP-3. DNA Cell Biol 16:1249–1256 [DOI] [PubMed] [Google Scholar]
- Bordon Y, Hansell CA, Sester DP, Clarke M, Mowat AM, Nibbs RJ. (2009) The atypical chemokine receptor D6 contributes to the development of experimental colitis. J Immunol 182:5032–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. (1997) Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 100:2552–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. (1998) Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394:894–897 [DOI] [PubMed] [Google Scholar]
- Boring L, Gosling J, Monteclaro FS, Lusis AJ, Tsou CL, Charo IF. (1996) Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1alpha receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J Biol Chem 271:7551–7558 [DOI] [PubMed] [Google Scholar]
- Borroni EM, Cancellieri C, Vacchini A, Benureau Y, Lagane B, Bachelerie F, Arenzana-Seisdedos F, Mizuno K, Mantovani A, Bonecchi R, et al. (2013) β-Arrestin-dependent activation of the cofilin pathway is required for the scavenging activity of the atypical chemokine receptor D6. Sci Signal 6: S31–33 [DOI] [PubMed] [Google Scholar]
- Bosworth T. (2010) New biologics for IBD stalled at investigational stage. Gastroenterol Endoscopy News 61 Available from http://www.gastroendonews.com/Archive.aspx [Google Scholar]
- Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. (1983) Regulation of the macrophage content of neoplasms by chemoattractants. Science 220:210–212 [DOI] [PubMed] [Google Scholar]
- Bourges D, Meurens F, Berri M, Chevaleyre C, Zanello G, Levast B, Melo S, Gerdts V, Salmon H. (2008) New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol Immunol 45:3354–3362 [DOI] [PubMed] [Google Scholar]
- Bradley ME, Bond ME, Manini J, Brown Z, Charlton SJ. (2009) SB265610 is an allosteric, inverse agonist at the human CXCR2 receptor. Br J Pharmacol 158:328–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, et al. (2007) Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol 27:373–379 [DOI] [PubMed] [Google Scholar]
- Brenner D, Labreuche J, Touboul PJ, Schmidt-Petersen K, Poirier O, Perret C, Schönfelder J, Combadière C, Lathrop M, Cambien F, et al. GENIC Investigators (2006) Cytokine polymorphisms associated with carotid intima-media thickness in stroke patients. Stroke 37:1691–1696 [DOI] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD. (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 6:895–901 [DOI] [PubMed] [Google Scholar]
- Brouwer N, Zuurman MW, Wei T, Ransohoff RM, Boddeke HW, Biber K. (2004) Induction of glial L-CCR mRNA expression in spinal cord and brain in experimental autoimmune encephalomyelitis. Glia 46:84–94 [DOI] [PubMed] [Google Scholar]
- Brown MF, Bahnck KB, Blumberg LC, Brissette WH, Burrell SA, Driscoll JP, Fedeles F, Fisher MB, Foti RS, Gladue RP, et al. (2007) Piperazinyl CCR1 antagonists—optimization of human liver microsome stability. Bioorg Med Chem Lett 17:3109–3112 [DOI] [PubMed] [Google Scholar]
- Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F, Pellegrini L, Biordi L, Coletti G, Sorrentino C, et al. (2007) Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol 82:1239–1246 [DOI] [PubMed] [Google Scholar]
- Buckland KF, O’connor EC, Coleman EM, Lira SA, Lukacs NW, Hogaboam CM. (2007) Remission of chronic fungal asthma in the absence of CCR8. J Allergy Clin Immunol 119:997–1004 [DOI] [PubMed] [Google Scholar]
- Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, Risma KA, Akers RM, Kirby CL, Buckmeier BK, et al. (2007) Interplay of adaptive Th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 45:22–31 [DOI] [PubMed] [Google Scholar]
- Burger JA, Stewart DJ, Wald O, Peled A. (2011) Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev Anticancer Ther 11:621–630 [DOI] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. (2006) A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203:2201–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Benovic JL. (2007) Regulation of CXCR4 signaling. Biochim Biophys Acta 1768:952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. (1994) Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 265:682–684 [DOI] [PubMed] [Google Scholar]
- Cain SA, Monk PN. (2002) The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74). J Biol Chem 277:7165–7169 [DOI] [PubMed] [Google Scholar]
- Cairns CM, Gordon JR, Li F, Baca-Estrada ME, Moyana T, Xiang J. (2001) Lymphotactin expression by engineered myeloma cells drives tumor regression: mediation by CD4+ and CD8+ T cells and neutrophils expressing XCR1 receptor. J Immunol 167:57–65 [DOI] [PubMed] [Google Scholar]
- Calatozzolo C, Canazza A, Pollo B, Di Pierro E, Ciusani E, Maderna E, Salce E, Sponza V, Frigerio S, Di Meco F, et al. (2011) Expression of the new CXCL12 receptor, CXCR7, in gliomas. Cancer Biol Ther 11:242–253 [DOI] [PubMed] [Google Scholar]
- Camarda G, Spinetti G, Bernardini G, Mair C, Davis-Poynter N, Capogrossi MC, Napolitano M. (1999) The equine herpesvirus 2 E1 open reading frame encodes a functional chemokine receptor. J Virol 73:9843–9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella GS, Colvin RA, Luster AD. (2010) CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE 5:e12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella GS, Grimm J, Manice LA, Colvin RA, Medoff BD, Wojtkiewicz GR, Weissleder R, Luster AD. (2006) Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol 177:6991–6998 [DOI] [PubMed] [Google Scholar]
- Campanella GS, Lee EM, Sun J, Luster AD. (2003) CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10). J Biol Chem 278:17066–17074 [DOI] [PubMed] [Google Scholar]
- Campanella GS, Medoff BD, Manice LA, Colvin RA, Luster AD. (2008a) Development of a novel chemokine-mediated in vivo T cell recruitment assay. J Immunol Methods 331:127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. (2008b) Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci USA 105:4814–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW. (1999a) Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2-/- mice: the role of mast cells. J Immunol 163:2160–2167 [PubMed] [Google Scholar]
- Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. (1998) 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J Cell Biol 141:1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, et al. (1999b) The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400:776–780 [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. (2001) Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 166:6477–6482 [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9:917–924 [DOI] [PubMed] [Google Scholar]
- Carson KG, Harriman GCB, and Ghosh S (2011), inventors; Millennium Pharmaceuticals, Inc. (Cambridge, MA), assignee. CCR1 antagonists and methods of use therefore. U.S. patent application 13/151,701. 2 June 2011. [Google Scholar]
- Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. (2001) Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem 276:1133–1137 [DOI] [PubMed] [Google Scholar]
- Casarosa P, Menge WM, Minisini R, Otto C, van Heteren J, Jongejan A, Timmerman H, Moepps B, Kirchhoff F, Mertens T, et al. (2003) Identification of the first nonpeptidergic inverse agonist for a constitutively active viral-encoded G protein-coupled receptor. J Biol Chem 278:5172–5178 [DOI] [PubMed] [Google Scholar]
- Casrouge A, Decalf J, Ahloulay M, Lababidi C, Mansour H, Vallet-Pichard A, Mallet V, Mottez E, Mapes J, Fontanet A, et al. (2011) Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest 121:308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. (2006) Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440:890–895 [DOI] [PubMed] [Google Scholar]
- Castor MG, Rezende B, Resende CB, Alessandri AL, Fagundes CT, Sousa LP, Arantes RM, Souza DG, Silva TA, Proudfoot AE, et al. (2010) The CCL3/macrophage inflammatory protein-1alpha-binding protein evasin-1 protects from graft-versus-host disease but does not modify graft-versus-leukemia in mice. J Immunol 184:2646–2654 [DOI] [PubMed] [Google Scholar]
- Catley MC, Coote J, Bari M, Tomlinson KL. (2011) Monoclonal antibodies for the treatment of asthma. Pharmacol Ther 132:333–351 [DOI] [PubMed] [Google Scholar]
- Catusse J, Leick M, Groch M, Clark DJ, Buchner MV, Zirlik K, Burger M. (2010) Role of the atypical chemoattractant receptor CRAM in regulating CCL19 induced CCR7 responses in B-cell chronic lymphocytic leukemia. Mol Cancer 9:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catusse J, Spinks J, Mattick C, Dyer A, Laing K, Fitzsimons C, Smit MJ, Gompels UA. (2008) Immunomodulation by herpesvirus U51A chemokine receptor via CCL5 and FOG-2 down-regulation plus XCR1 and CCR7 mimicry in human leukocytes. Eur J Immunol 38:763–777 [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457:722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 5:919–923 [DOI] [PubMed] [Google Scholar]
- Chakera A, Seeber RM, John AE, Eidne KA, Greaves DR. (2008) The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Mol Pharmacol 73:1362–1370 [DOI] [PubMed] [Google Scholar]
- Chang Y, de Nadai P, Azzaoui I, Morales O, Delhem N, Vorng H, Tomavo S, Ait Yahia S, Zhang G, Wallaert B, et al. (2010) The chemokine CCL18 generates adaptive regulatory T cells from memory CD4+ T cells of healthy but not allergic subjects. FASEB J 24:5063–5072 [DOI] [PubMed] [Google Scholar]
- Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, Maurer D. (1999) Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells. J Exp Med 190:1755–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. (1994) Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA 91:2752–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvat TT, Hu C, Jin J, Li Y, Melikian A, Pennell AMK, Punna S, Ungashe S, and Zeng Y (2013), inventors; ChemoCentryx, Inc. (Mountain View, CA), assignee. Triazolyl pyridyl benzenesulfonamides, U.S. patent 8,445,518. 21 May 2013. [Google Scholar]
- Chaudhuri A, Nielsen S, Elkjaer ML, Zbrzezna V, Fang F, Pogo AO. (1997) Detection of Duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs. Blood 89:701–712 [PubMed] [Google Scholar]
- Chaudhuri A, Polyakova J, Zbrzezna V, Pogo AO. (1995) The coding sequence of Duffy blood group gene in humans and simians: restriction fragment length polymorphism, antibody and malarial parasite specificities, and expression in nonerythroid tissues in Duffy-negative individuals. Blood 85:615–621 [PubMed] [Google Scholar]
- Chaudhuri A, Polyakova J, Zbrzezna V, Pogo AO, Hesselgesser J, Horuk R. (1994) The major glycoprotein of the Duffy blood-group antigen (Gpd), which is the malarial Plasmodium-vivax erythrocyte receptor is also a novel class of chemokine receptor and is present in brain, kidney, lung, thymus, and spleen. FASEB J 8:A1386–A1386 [Google Scholar]
- Chaudhuri A, Polyakova J, Zbrzezna V, Williams K, Gulati S, Pogo AO. (1993) Cloning of glycoprotein D cDNA, which encodes the major subunit of the Duffy blood group system and the receptor for the Plasmodium vivax malaria parasite. Proc Natl Acad Sci USA 90:10793–10797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ding Y, Schröppel B, Zhang N, Fu S, Chen D, Zhang H, Bromberg JS. (2003) Differential chemokine and chemokine receptor gene induction by ischemia, alloantigen, and gene transfer in cardiac grafts. Am J Transplant 3:1216–1229 [DOI] [PubMed] [Google Scholar]
- Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, Liu B, Deng H, Wang F, Lin L, et al. (2011) CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 19:541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. (2007) C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446:203–207 [DOI] [PubMed] [Google Scholar]
- Chen S, Bacon KB, Li L, Garcia GE, Xia Y, Lo D, Thompson DA, Siani MA, Yamamoto T, Harrison JK, et al. (1998) In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med 188:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenivesse C, Chang Y, Azzaoui I, Ait Yahia S, Morales O, Plé C, Foussat A, Tonnel AB, Delhem N, Yssel H, et al. (2012) Pulmonary CCL18 recruits human regulatory T cells. J Immunol 189:128–137 [DOI] [PubMed] [Google Scholar]
- Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, et al. (2001) Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med 193:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ML, Yoon BY, Ju JH, Jung YO, Jhun JY, Park MK, Park SH, Cho CS, Kim HY. (2007) Expression of CCR2A, an isoform of MCP-1 receptor, is increased by MCP-1, CD40 ligand and TGF-beta in fibroblast like synoviocytes of patients with RA. Exp Mol Med 39:499–507 [DOI] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. (1996) The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135–1148 [DOI] [PubMed] [Google Scholar]
- Choi YB, Nicholas J. (2008) Autocrine and paracrine promotion of cell survival and virus replication by human herpesvirus 8 chemokines. J Virol 82:6501–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. (2010) Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33:266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KY, Brotin E, Ben Khalifa Y, Carthagena L, Teissier S, Danckaert A, Galzi JL, Arenzana-Seisdedos F, Thierry F, Bachelerie F. (2010) A pivotal role for CXCL12 signaling in HPV-mediated transformation of keratinocytes: clues to understanding HPV-pathogenesis in WHIM syndrome. Cell Host Microbe 8:523–533 [DOI] [PubMed] [Google Scholar]
- Christmann BS, Moran JM, McGraw JA, Buller RM, Corbett JA. (2011) Ccr5 regulates inflammatory gene expression in response to encephalomyocarditis virus infection. Am J Pathol 179:2941–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, Yuan GY, Li CG, Xue LQ, Shen M, et al. China Consortium for Genetics of Autoimmune Thyroid Disease (2011) A genome-wide association study identifies two new risk loci for Graves’ disease. Nat Genet 43:897–901 [DOI] [PubMed] [Google Scholar]
- Chung CD, Kuo F, Kumer J, Motani AS, Lawrence CE, Henderson WR, Jr, Venkataraman C. (2003) CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol 170:581–587 [DOI] [PubMed] [Google Scholar]
- Chuntharapai A, Kim KJ. (1995) Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol 155:2587–2594 [PubMed] [Google Scholar]
- Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, Paloscia L, Materazzo G, D’Annunzio E, Conti P, et al. (2001) Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol 21:327–334 [DOI] [PubMed] [Google Scholar]
- Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan CF. (2000) Activation of C-C beta-chemokines in human peripheral blood gammadelta T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood 95:39–47 [PubMed] [Google Scholar]
- Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, Dugnani E, Sordi V, Magistretti P, Daffonchio L, et al. (2012) CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest 122:3647–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. (1993) Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA 90:3574–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. (2003) Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem 278:289–295 [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Malcangio M. (2011) Fractalkine/CX3CR1 signalling in chronic pain and inflammation. Curr Pharm Biotechnol 12:1707–1714 [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. (2006) The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176:4431–4439 [DOI] [PubMed] [Google Scholar]
- Clarke C, Kuboki S, Sakai N, Kasten KR, Tevar AD, Schuster R, Blanchard J, Caldwell CC, Edwards MJ, Lentsch AB. (2011) CXC chemokine receptor-1 is expressed by hepatocytes and regulates liver recovery after hepatic ischemia/reperfusion injury. Hepatology 53:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson KJ, Clemetson JM, Proudfoot AE, Power CA, Baggiolini M, Wells TN. (2000) Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 96:4046–4054 [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. (1995) Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811–1815 [DOI] [PubMed] [Google Scholar]
- Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Récalde A, Zouggari Y, Yin KYHW, Bruneval P, et al. (2012) The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 32:2206–2213 [DOI] [PubMed] [Google Scholar]
- Codullo V, Baldwin HM, Singh MD, Fraser AR, Wilson C, Gilmour A, Hueber AJ, Bonino C, McInnes IB, Montecucco C, et al. (2011) An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann Rheum Dis 70:1115–1121 [DOI] [PubMed] [Google Scholar]
- Cohen C, DeJesus E, Mills A, Pierone G, Kumar P, Ruane P, Elion R, Fusco G, Levy R, and Solomon K (2007) Potent antiretroviral activity of the once daily antagonist INCB009471 over 14 days of monotherapy, in Fourth IAS Conference on HIV Pathogenesis, Treatment, and Prevention; 22–25 July 2007; Sydney, Australia. [Google Scholar]
- Cohen-Hillel E, Yron I, Meshel T, Soria G, Attal H, Ben-Baruch A. (2006) CXCL8-induced FAK phosphorylation via CXCR1 and CXCR2: cytoskeleton- and integrin-related mechanisms converge with FAK regulatory pathways in a receptor-specific manner. Cytokine 33:1–16 [DOI] [PubMed] [Google Scholar]
- Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, et al. (1998) Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 187:2009–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Manice LA, Luster AD. (2006) CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mol Cell Biol 26:5838–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Sun J, Luster AD. (2004) Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem 279:30219–30227 [DOI] [PubMed] [Google Scholar]
- Combadière B, Faure S, Autran B, Debré P, Combadière C. (2003a) The chemokine receptor CX3CR1 controls homing and anti-viral potencies of CD8 effector-memory T lymphocytes in HIV-infected patients. AIDS 17:1279–1290 [DOI] [PubMed] [Google Scholar]
- Combadiere C, Ahuja SK, Murphy PM. (1995) Cloning and functional expression of a human eosinophil CC chemokine receptor. J Biol Chem 270:16491–16494 [DOI] [PubMed] [Google Scholar]
- Combadiere C, Ahuja SK, Tiffany HL, Murphy PM. (1996) Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. J Leukoc Biol 60:147–152 [DOI] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, et al. (2007) CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 117:2920–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debré P, Tedgui A, Murphy PM, Mallat Z. (2003b) Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107:1009–1016 [DOI] [PubMed] [Google Scholar]
- Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117:1649–1657 [DOI] [PubMed] [Google Scholar]
- Combadiere C, Salzwedel K, Smith ED, Tiffany HL, Berger EA, Murphy PM. (1998) Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J Biol Chem 273:23799–23804 [DOI] [PubMed] [Google Scholar]
- Comerford I, Milasta S, Morrow V, Milligan G, Nibbs R. (2006) The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur J Immunol 36:1904–1916 [DOI] [PubMed] [Google Scholar]
- Comerford I, Nibbs RJ, Litchfield W, Bunting M, Harata-Lee Y, Haylock-Jacobs S, Forrow S, Korner H, McColl SR. (2010) The atypical chemokine receptor CCX-CKR scavenges homeostatic chemokines in circulation and tissues and suppresses Th17 responses. Blood 116:4130–4140 [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovère C. (2010) The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol 224:93–100 [DOI] [PubMed] [Google Scholar]
- Connolly S, Skrinjar M, Rosendahl A. (2012) Orally bioavailable allosteric CCR8 antagonists inhibit dendritic cell, T cell and eosinophil migration. Biochem Pharmacol 83:778–787 [DOI] [PubMed] [Google Scholar]
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, et al. (2000) CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495–503 [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier EG, Tran DN, Yukhayeva L, Olson WC, Dragic T. (2001) Mapping the determinants of the CCR5 amino-terminal sulfopeptide interaction with soluble human immunodeficiency virus type 1 gp120-CD4 complexes. J Virol 75:5541–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, Hipkin RW. (2001) Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol Pharmacol 59:707–715 [DOI] [PubMed] [Google Scholar]
- Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, et al. (2010) The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med 207:1283–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozat K, Tamoutounour S, Vu Manh TP, Fossum E, Luche H, Ardouin L, Guilliams M, Azukizawa H, Bogen B, Malissen B, et al. (2011) Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α+ type. J Immunol 187:4411–4415 [DOI] [PubMed] [Google Scholar]
- Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. (1997) Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J 16:6996–7007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, et al. (2011) CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med 208:327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutbush M, Mollison PL. (1950) The Duffy blood group system. Heredity (Edinb) 4:383–389 [DOI] [PubMed] [Google Scholar]
- Cutbush M, Mollison PL, Parkin DM. (1950) A new human blood group. Nature 165:188–189 [Google Scholar]
- Cyster JG. (1999) Chemokines and cell migration in secondary lymphoid organs. Science 286:2098–2102 [DOI] [PubMed] [Google Scholar]
- D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. (1998) Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol 161:5111–5115 [PubMed] [Google Scholar]
- D’Amico G, Frascaroli G, Bianchi G, Transidico P, Doni A, Vecchi A, Sozzani S, Allavena P, Mantovani A. (2000) Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat Immunol 1:387–391 [DOI] [PubMed] [Google Scholar]
- D’Haese JG, Demir IE, Friess H, Ceyhan GO. (2010) Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets 14:207–219 [DOI] [PubMed] [Google Scholar]
- Dairaghi DJ, Fan RA, McMaster BE, Hanley MR, Schall TJ. (1999) HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J Biol Chem 274:21569–21574 [DOI] [PubMed] [Google Scholar]
- Dairaghi DJ, Zhang P, Wang Y, Seitz LC, Johnson DA, Miao S, Ertl LS, Zeng Y, Powers JP, Pennell AM, et al. (2011) Pharmacokinetic and pharmacodynamic evaluation of the novel CCR1 antagonist CCX354 in healthy human subjects: implications for selection of clinical dose. Clin Pharmacol Ther 89:726–734 [DOI] [PubMed] [Google Scholar]
- Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, Wood B, Hsu FJ. (2011) The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood 118:4963–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj BB, McColl SR, Neote K, Songqing N, Ogborn KT, Hébert CA, Naccache PH. (1996) Identification of G-protein binding sites of the human interleukin-8 receptors by functional mapping of the intracellular loops. FASEB J 10:1426–1434 [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudière C, Cubedo N, Ghysen A. (2007) Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon I, Murphy PM, Moss B. (1998) Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc Natl Acad Sci USA 95:6403–6407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoudi M, Lavergne E, Garin A, Tarantino N, Debré P, Pincet F, Combadière C, Deterre P. (2004) Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J Biol Chem 279:19649–19657 [DOI] [PubMed] [Google Scholar]
- Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer DA, et al. (2009) The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS ONE 4:e6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbonne WC, Rice GC, Mohler MA, Apple T, Hébert CA, Valente AJ, Baker JB. (1991) Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest 88:1362–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. (1996) Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med 183:2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. (2000a) Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol 156:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC. (2000b) Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC). Blood 96:1681–1684 [PubMed] [Google Scholar]
- De Clercq E. (2009) The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem Pharmacol 77:1655–1664 [DOI] [PubMed] [Google Scholar]
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, et al. (1996) Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. see comments published erratum appears in Science 1996 Nov 15;274 5290:1069 Science 273:1856–1862 [DOI] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. (2005) Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 6:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne LA, Li K, Bishop DK, Bromberg JS. (2000) Gene transfer of virally encoded chemokine antagonists vMIP-II and MC148 prolongs cardiac allograft survival and inhibits donor-specific immunity. Gene Ther 7:575–582 [DOI] [PubMed] [Google Scholar]
- Décaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. (2011) CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem 286:32188–32197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco SJ, Henze H, Lederer A, Moehle K, Mukherjee R, Romagnoli B, Robinson JA, Brianza F, Gombert FO, Lociuro S, et al. (2006) Discovery of novel, highly potent and selective beta-hairpin mimetic CXCR4 inhibitors with excellent anti-HIV activity and pharmacokinetic profiles. Bioorg Med Chem 14:8396–8404 [DOI] [PubMed] [Google Scholar]
- Dénes A, Ferenczi S, Halász J, Környei Z, Kovács KJ. (2008) Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab 28:1707–1721 [DOI] [PubMed] [Google Scholar]
- Deng HK, Unutmaz D, KewalRamani VN, Littman DR. (1997) Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296–300 [DOI] [PubMed] [Google Scholar]
- Denis C, Deiteren K, Mortier A, Tounsi A, Fransen E, Proost P, Renauld JC, Lambeir AM. (2012) C-terminal clipping of chemokine CCL1/I-309 enhances CCR8-mediated intracellular calcium release and anti-apoptotic activity. PLoS ONE 7:e34199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruaz M (2008) Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. Ph.D. thesis, Universtiy of Geneva, Geneva, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, Ferreira BR, Graham GJ, Shaw JP, Wells TN, et al. (2008) Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med 205:2019–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desanti GE, Jenkinson WE, Parnell SM, Boudil A, Gautreau-Rolland L, Eksteen B, Ezine S, Lane PJ, Jenkinson EJ, Anderson G. (2011) Clonal analysis reveals uniformity in the molecular profile and lineage potential of CCR9(+) and CCR9(-) thymus-settling progenitors. J Immunol 186:5227–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detheux M, Ständker L, Vakili J, Münch J, Forssmann U, Adermann K, Pöhlmann S, Vassart G, Kirchhoff F, Parmentier M, et al. (2000) Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J Exp Med 192:1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. (2002) Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol 72:9–18 [PMC free article] [PubMed] [Google Scholar]
- Di Liberto D, Locati M, Caccamo N, Vecchi A, Meraviglia S, Salerno A, Sireci G, Nebuloni M, Caceres N, Cardona PJ, et al. (2008) Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J Exp Med 205:2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias JM, Losberger C, Déruaz M, Power CA, Proudfoot AE, Shaw JP. (2009) Structural basis of chemokine sequestration by a tick chemokine binding protein: the crystal structure of the complex between Evasin-1 and CCL3. PLoS ONE 4:e8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. (2013) Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. (1998) Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilloo D, Bacon K, Holden W, Zhong W, Burdach S, Zlotnik A, Brenner M. (1996) Combined chemokine and cytokine gene transfer enhances antitumor immunity. Nat Med 2:1090–1095 [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. (2007) Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38:1345–1353 [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. (2000) The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J Immunol 165:2677–2682 [DOI] [PubMed] [Google Scholar]
- Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. (1996) A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149–1158 [DOI] [PubMed] [Google Scholar]
- Dorgham K, Ghadiri A, Hermand P, Rodero M, Poupel L, Iga M, Hartley O, Gorochov G, Combadière C, Deterre P. (2009) An engineered CX3CR1 antagonist endowed with anti-inflammatory activity. J Leukoc Biol 86:903–911 [DOI] [PubMed] [Google Scholar]
- Dorner B, Müller S, Entschladen F, Schröder JM, Franke P, Kraft R, Friedl P, Clark-Lewis I, Kroczek RA. (1997) Purification, structural analysis, and function of natural ATAC, a cytokine secreted by CD8(+) T cells. J Biol Chem 272:8817–8823 [DOI] [PubMed] [Google Scholar]
- Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Güttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, et al. (2009) Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 31:823–833 [DOI] [PubMed] [Google Scholar]
- Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, Radbruch A, Flesch IE, Kroczek RA. (2002) MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci USA 99:6181–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, et al. (2005) Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49:4721–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, et al. (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. see comments Nature 381:667–673 [DOI] [PubMed] [Google Scholar]
- Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, Heveker N, Volkman BF, Dwinell MB. (2011) Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci USA 108:17655–17660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Luan J, Liu H, Daniel TO, Peiper S, Chen TS, Yu Y, Horton LW, Nanney LB, Strieter RM, et al. (2002) Potential role for Duffy antigen chemokine-binding protein in angiogenesis and maintenance of homeostasis in response to stress. J Leukoc Biol 71:141–153 [PMC free article] [PubMed] [Google Scholar]
- Duggal P, An P, Beaty TH, Strathdee SA, Farzadegan H, Markham RB, Johnson L, O’Brien SJ, Vlahov D, Winkler CA. (2003) Genetic influence of CXCR6 chemokine receptor alleles on PCP-mediated AIDS progression among African Americans. Genes Immun 4:245–250 [DOI] [PubMed] [Google Scholar]
- Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. (1999) Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 117:359–367 [DOI] [PubMed] [Google Scholar]
- Dwyer MP, Yu Y, Chao J, Aki C, Chao J, Biju P, Girijavallabhan V, Rindgen D, Bond R, Mayer-Ezel R, et al. (2006) Discovery of 2-hydroxy-N,N-dimethyl-3-2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylaminobenzamide (SCH 527123): a potent, orally bioavailable CXCR2/CXCR1 receptor antagonist. J Med Chem 49:7603–7606 [DOI] [PubMed] [Google Scholar]
- Ebert LM, Meuter S, Moser B. (2006) Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol 176:4331–4336 [DOI] [PubMed] [Google Scholar]
- Ebisawa M, Hase K, Takahashi D, Kitamura H, Knoop KA, Williams IR, Ohno H. (2011) CCR6hiCD11c(int) B cells promote M-cell differentiation in Peyer’s patch. Int Immunol 23:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Mankowski JL, Doranz BJ, Margulies BJ, Lee B, Rucker J, Sharron M, Hoffman TL, Berson JF, Zink MC, et al. (1997) CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA 94:14742–14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. (2004) Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol 173:6234–6240 [DOI] [PubMed] [Google Scholar]
- Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. (1999) The Kaposi’s sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J Exp Med 189:1993–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraltan H, Cacina C, Kahraman OT, Kurt O, Aydogan HY, Uyar M, Can A, Cakmakoğlu B. (2012) MCP-1 and CCR2 gene variants and the risk for osteoporosis and osteopenia. Genet Test Mol Biomarkers 16:229–233 [DOI] [PubMed] [Google Scholar]
- Esté JA. (2002) Sch-351125 and Sch-350634. Schering-Plough. Curr Opin Investig Drugs 3:379–383 [PubMed] [Google Scholar]
- Fan P, Kyaw H, Su K, Zeng Z, Augustus M, Carter KC, Li Y. (1998) Cloning and characterization of a novel human chemokine receptor. Biochem Biophys Res Commun 243:264–268 [DOI] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, et al. (2007) Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem 282:11658–11666 [DOI] [PubMed] [Google Scholar]
- Farber JM. (1990) A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA 87:5238–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, Choe H. (1999) Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96:667–676 [DOI] [PubMed] [Google Scholar]
- Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debré P, et al. (2000) Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science 287:2274–2277 [DOI] [PubMed] [Google Scholar]
- Feng LY, Ou ZL, Wu FY, Shen ZZ, Shao ZM. (2009) Involvement of a novel chemokine decoy receptor CCX-CKR in breast cancer growth, metastasis and patient survival. Clin Cancer Res 15:2962–2970 [DOI] [PubMed] [Google Scholar]
- Feng Z, Dubyak GR, Lederman MM, Weinberg A. (2006) Cutting edge: human beta defensin 3—a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol 177:782–786 [DOI] [PubMed] [Google Scholar]
- Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. (2002) Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol 119:1405–1410 [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. (2000) CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med 192:899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorina P, Jurewicz M, Vergani A, Petrelli A, Carvello M, D’Addio F, Godwin JG, Law K, Wu E, Tian Z, et al. (2011) Targeting the CXCR4-CXCL12 axis mobilizes autologous hematopoietic stem cells and prolongs islet allograft survival via programmed death ligand 1. J Immunol 186:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisette A, Munkonda MN, Oikonomopoulou K, Paglialunga S, Lambris JD, Cianflone K. (2013) C5L2 receptor disruption enhances the development of diet-induced insulin resistance in mice. Immunobiology 218:127–133 [DOI] [PubMed] [Google Scholar]
- Fitzsimons CP, Gompels UA, Verzijl D, Vischer HF, Mattick C, Leurs R, Smit MJ. (2006) Chemokine-directed trafficking of receptor stimulus to different g proteins: selective inducible and constitutive signaling by human herpesvirus 6-encoded chemokine receptor U51. Mol Pharmacol 69:888–898 [DOI] [PubMed] [Google Scholar]
- Floto RA, MacAry PA, Boname JM, Mien TS, Kampmann B, Hair JR, Huey OS, Houben EN, Pieters J, Day C, et al. (2006) Dendritic cell stimulation by mycobacterial Hsp70 is mediated through CCR5. Science 314:454–458 [DOI] [PubMed] [Google Scholar]
- Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. (1998) Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 188:1413–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R, Davalos-Misslitz AC, Rot A. (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8:362–371 [DOI] [PubMed] [Google Scholar]
- Förster R, Emrich T, Kremmer E, Lipp M. (1994) Expression of the G-protein—coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84:830–840 [PubMed] [Google Scholar]
- Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. (1996) A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87:1037–1047 [DOI] [PubMed] [Google Scholar]
- Fox JM, Najarro P, Smith GL, Struyf S, Proost P, Pease JE. (2006) Structure/function relationships of CCR8 agonists and antagonists. Amino-terminal extension of CCL1 by a single amino acid generates a partial agonist. J Biol Chem 281:36652–36661 [DOI] [PubMed] [Google Scholar]
- Fra AM, Locati M, Otero K, Sironi M, Signorelli P, Massardi ML, Gobbi M, Vecchi A, Sozzani S, Mantovani A. (2003) Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J Immunol 170:2279–2282 [DOI] [PubMed] [Google Scholar]
- Francis JN, Jacobson MR, Lloyd CM, Sabroe I, Durham SR, Till SJ. (2004) CXCR1+CD4+ T cells in human allergic disease. J Immunol 172:268–273 [DOI] [PubMed] [Google Scholar]
- Franciszkiewicz K, Le Floc’h A, Jalil A, Vigant F, Robert T, Vergnon I, Mackiewicz A, Benihoud K, Validire P, Chouaib S, et al. (2009) Intratumoral induction of CD103 triggers tumor-specific CTL function and CCR5-dependent T-cell retention. Cancer Res 69:6249–6255 [DOI] [PubMed] [Google Scholar]
- Frauenschuh A, Power CA, Déruaz M, Ferreira BR, Silva JS, Teixeira MM, Dias JM, Martin T, Wells TN, Proudfoot AE. (2007) Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus. J Biol Chem 282:27250–27258 [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272 [DOI] [PubMed] [Google Scholar]
- Freeman CM, Stolberg VR, Chiu BC, Lukacs NW, Kunkel SL, Chensue SW. (2006) CCR4 participation in Th type 1 (mycobacterial) and Th type 2 (schistosomal) anamnestic pulmonary granulomatous responses. J Immunol 177:4149–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendéus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C. (2000) Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J Exp Med 192:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Albelda SM. (2012) Tumor-associated neutrophils: friend or foe? Carcinogenesis 33:949–955 [DOI] [PubMed] [Google Scholar]
- Friedman RS, Jacobelli J, Krummel MF. (2006) Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol 7:1101–1108 [DOI] [PubMed] [Google Scholar]
- Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Holländer GA, Piali L. (2002) Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med 8:1414–1420 [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. (2010) Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci 13:411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Fujisawa R, Kato Y, Nakayama T, Morita A, Katsumata H, Nishimori H, Iguchi K, Kamiya H, Gray PW, et al. (2002) Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J Allergy Clin Immunol 110:139–146 [DOI] [PubMed] [Google Scholar]
- Fukuma N, Akimitsu N, Hamamoto H, Kusuhara H, Sugiyama Y, Sekimizu K. (2003) A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem Biophys Res Commun 303:137–139 [DOI] [PubMed] [Google Scholar]
- Fulkerson PC, Zimmermann N, Brandt EB, Muntel EE, Doepker MP, Kavanaugh JL, Mishra A, Witte DP, Zhang H, Farber JM, et al. (2004) Negative regulation of eosinophil recruitment to the lung by the chemokine monokine induced by IFN-gamma (Mig, CXCL9). Proc Natl Acad Sci USA 101:1987–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi K, Gao JL, Murphy PM. (2006) Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am J Pathol 169:372–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso JM, Harrison JK, Silverstein FS. (1998) Excitotoxic brain injury stimulates expression of the chemokine receptor CCR5 in neonatal rats. Am J Pathol 153:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. (2007) CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation 116:1801–1811 [DOI] [PubMed] [Google Scholar]
- Galliera E, Jala VR, Trent JO, Bonecchi R, Signorelli P, Lefkowitz RJ, Mantovani A, Locati M, Haribabu B. (2004) β-Arrestin-dependent constitutive internalization of the human chemokine decoy receptor D6. J Biol Chem 279:25590–25597 [DOI] [PubMed] [Google Scholar]
- Galligan CL, Matsuyama W, Matsukawa A, Mizuta H, Hodge DR, Howard OM, Yoshimura T. (2004) Up-regulated expression and activation of the orphan chemokine receptor, CCRL2, in rheumatoid arthritis. Arthritis Rheum 50:1806–1814 [DOI] [PubMed] [Google Scholar]
- Galvani AP, Slatkin M. (2003) Evaluating plague and smallpox as historical selective pressures for the CCR5-Delta 32 HIV-resistance allele. Proc Natl Acad Sci USA 100:15276–15279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangavarapu P, Rajagopalan L, Kolli D, Guerrero-Plata A, Garofalo RP, Rajarathnam K. (2012) The monomer-dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J Leukoc Biol 91:259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Neff TA, Guo RF, Speyer CL, Sarma JV, Tomlins S, Man Y, Riedemann NC, Hoesel LM, Younkin E, et al. (2005a) Evidence for a functional role of the second C5a receptor C5L2. FASEB J 19:1003–1005 [DOI] [PubMed] [Google Scholar]
- Gao JL, Murphy PM. (1994) Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J Biol Chem 269:28539–28542 [PubMed] [Google Scholar]
- Gao JQ, Sugita T, Kanagawa N, Iida K, Okada N, Mizuguchi H, Nakayama T, Hayakawa T, Yoshie O, Tsutsumi Y, et al. (2005b) Anti-tumor responses induced by chemokine CCL19 transfected into an ovarian carcinoma model via fiber-mutant adenovirus vector. Biol Pharm Bull 28:1066–1070 [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, Yi Y, Shi JY, Shi GM, Ding ZB, et al. (2012) CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res 72:3546–3556 [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J, Rueda P, Alcami J, Rognan D, Arenzana-Seisdedos F, Lagane B, Kellenberger E. (2011) Allosteric model of maraviroc binding to CC chemokine receptor 5 (CCR5). J Biol Chem 286:33409–33421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Zepeda EA, Combadiere C, Rothenberg ME, Sarafi MN, Lavigne F, Hamid Q, Murphy PM, Luster AD. (1996) Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol 157:5613–5626 [PubMed] [Google Scholar]
- Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J. (2004) The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem Biophys Res Commun 321:306–312 [DOI] [PubMed] [Google Scholar]
- Gatto D, Wood K, Brink R. (2011) EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct B cell migration and organization in follicles and the germinal center. J Immunol 187:4621–4628 [DOI] [PubMed] [Google Scholar]
- Gaudin F, Nasreddine S, Donnadieu AC, Emilie D, Combadière C, Prévot S, Machelon V, Balabanian K. (2011) Identification of the chemokine CX3CL1 as a new regulator of malignant cell proliferation in epithelial ovarian cancer. PLoS ONE 6:e21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau GM, Boulet LP, Cockcroft DW, Baatjes A, Cote J, Deschesnes F, Davis B, Strinich T, Howie K, Duong M, et al. (2008) Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am J Respir Crit Care Med 177:952–958 [DOI] [PubMed] [Google Scholar]
- Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, Young AJ, Hay JB, Debes GF. (2012) The skin, a novel niche for recirculating B cells. J Immunol 188:6027–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. (2005) Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 3:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. (2005) An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem 280:39677–39680 [DOI] [PubMed] [Google Scholar]
- Geras-Raaka E, Varma A, Clark-Lewis I, Gershengorn MC. (1998) Kaposi’s sarcoma-associated herpesvirus (KSHV) chemokine vMIP-II and human SDF-1alpha inhibit signaling by KSHV G protein-coupled receptor. Biochem Biophys Res Commun 253:725–727 [DOI] [PubMed] [Google Scholar]
- Gerlag DM, Hollis S, Layton M, Vencovský J, Szekanecz Z, Braddock M, Tak PP, ESCAPE Study Group (2010) Preclinical and clinical investigation of a CCR5 antagonist, AZD5672, in patients with rheumatoid arthritis receiving methotrexate. Arthritis Rheum 62:3154–3160 [DOI] [PubMed] [Google Scholar]
- Germanov E, Veinotte L, Cullen R, Chamberlain E, Butcher EC, Johnston B. (2008) Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J Immunol 181:81–91 [DOI] [PubMed] [Google Scholar]
- Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, Koenen TB, Krajnc-Franken MA, Gossen JA. (2008) Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis 46:235–245 [DOI] [PubMed] [Google Scholar]
- Gershengorn MC, Geras-Raaka E, Varma A, Clark-Lewis I. (1998) Chemokines activate Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Invest 102:1469–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. (1999) MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398:718–723 [DOI] [PubMed] [Google Scholar]
- Giagulli C, Magiera AK, Bugatti A, Caccuri F, Marsico S, Rusnati M, Vermi W, Fiorentini S, Caruso A. (2012) HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood 119:2274–2283 [DOI] [PubMed] [Google Scholar]
- Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, Wyant T, Davidson M, MLN1202 Study Group (2011) Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol 107:906–911 [DOI] [PubMed] [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, et al. (2010) CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 120:485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue RP, Cole SH, Roach ML, Tylaska LA, Nelson RT, Shepard RM, McNeish JD, Ogborne KT, Neote KS. (2006) The human specific CCR1 antagonist CP-481,715 inhibits cell infiltration and inflammatory responses in human CCR1 transgenic mice. J Immunol 176:3141–3148 [DOI] [PubMed] [Google Scholar]
- Gladue RP, Tylaska LA, Brissette WH, Lira PD, Kath JC, Poss CS, Brown MF, Paradis TJ, Conklyn MJ, Ogborne KT, et al. (2003) CP-481,715, a potent and selective CCR1 antagonist with potential therapeutic implications for inflammatory diseases. J Biol Chem 278:40473–40480 [DOI] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. (2005) Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 202:1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Liu MT, Kuziel WA, Lane TE. (2001) Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology 288:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. (2006) CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med 203:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D. (2002) Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J Immunol 168:4920–4929 [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. (1994) Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8- thymocyte differentiation. J Immunol 152:4783–4792 [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. (1993) A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol 150:4244–4252 [PubMed] [Google Scholar]
- Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. (1997) Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 185:1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding H, Aliberti J, King LR, Manischewitz J, Andersen J, Valenzuela J, Landau NR, Sher A. (2003) Inhibition of HIV-1 infection by a CCR5-binding cyclophilin from Toxoplasma gondii. Blood 102:3280–3286 [DOI] [PubMed] [Google Scholar]
- Gombert M, Dieu-Nosjean MC, Winterberg F, Bünemann E, Kubitza RC, Da Cunha L, Haahtela A, Lehtimäki S, Müller A, Rieker J, et al. (2005) CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol 174:5082–5091 [DOI] [PubMed] [Google Scholar]
- Gompels UA, Nicholas J, Lawrence G, Jones M, Thomson BJ, Martin ME, Efstathiou S, Craxton M, Macaulay HA. (1995) The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29–51 [DOI] [PubMed] [Google Scholar]
- Gong W, Howard OM, Turpin JA, Grimm MC, Ueda H, Gray PW, Raport CJ, Oppenheim JJ, Wang JM. (1998) Monocyte chemotactic protein-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J Biol Chem 273:4289–4292 [DOI] [PubMed] [Google Scholar]
- Gong X, Gong W, Kuhns DB, Ben-Baruch A, Howard OM, Wang JM. (1997) Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J Biol Chem 272:11682–11685 [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AE, Coyle AJ, Gearing D, et al. (1999) Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol 163:403–411 [PubMed] [Google Scholar]
- Gonzalo JA, Qiu Y, Lora JM, Al-Garawi A, Villeval JL, Boyce JA, Martinez-A C, Marquez G, Goya I, Hamid Q, et al. (2007) Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol 179:1740–1750 [DOI] [PubMed] [Google Scholar]
- Gosling J, Dairaghi DJ, Wang Y, Hanley M, Talbot D, Miao Z, Schall TJ. (2000) Cutting edge: identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J Immunol 164:2851–2856 [DOI] [PubMed] [Google Scholar]
- Goulding C, McManus R, Murphy A, MacDonald G, Barrett S, Crowe J, Hegarty J, McKiernan S, Kelleher D. (2005) The CCR5-D32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut 54:1157–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouwy M, Schiraldi M, Struyf S, Van Damme J, Uguccioni M. (2012) Possible mechanisms involved in chemokine synergy fine tuning the inflammatory response. Immunol Lett 145:10–14 [DOI] [PubMed] [Google Scholar]
- Goya I, Gutiérrez J, Varona R, Kremer L, Zaballos A, Márquez G. (1998) Identification of CCR8 as the specific receptor for the human beta-chemokine I-309: cloning and molecular characterization of murine CCR8 as the receptor for TCA-3. J Immunol 160:1975–1981 [PubMed] [Google Scholar]
- Goya I, Villares R, Zaballos A, Gutiérrez J, Kremer L, Gonzalo JA, Varona R, Carramolino L, Serrano A, Pallarés P, et al. (2003) Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J Immunol 170:2138–2146 [DOI] [PubMed] [Google Scholar]
- Graham GJ. (2009) D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur J Immunol 39:342–351 [DOI] [PubMed] [Google Scholar]
- Graham GJ, Locati M, Mantovani A, Rot A, Thelen M. (2012) The biochemistry and biology of the atypical chemokine receptors. Immunol Lett 145:30–38 [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, et al. (1996) Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med 184:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel S, Malouf C, Boulais PE, Berchiche YA, Oishi S, Fujii N, Leduc R, Sinnett D, Heveker N. (2010) The peptidomimetic CXCR4 antagonist TC14012 recruits beta-arrestin to CXCR7: roles of receptor domains. J Biol Chem 285:37939–37943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. (2011) Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol 186:6091–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, et al. (1997) CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med 186:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiff L, Ahlström-Emanuelsson C, Bahl A, Bengtsson T, Dahlström K, Erjefält J, Widegren H, Andersson M. (2010) Effects of a dual CCR3 and H1-antagonist on symptoms and eosinophilic inflammation in allergic rhinitis. Respir Res 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Luster AD. (2011) CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. (2012) CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. (1998) Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 2:275–281 [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. (2000) Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407–411 [DOI] [PubMed] [Google Scholar]
- Guan E, Wang J, Norcross MA. (2004) Amino-terminal processing of MIP-1beta/CCL4 by CD26/dipeptidyl-peptidase IV. J Cell Biochem 92:53–64 [DOI] [PubMed] [Google Scholar]
- Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martín-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. (2007) L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol 8:743–752 [DOI] [PubMed] [Google Scholar]
- Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, Imberti L, Pirovano S, Notarangelo LD, Soresina R, et al. (2004) Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood 104:444–452 [DOI] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. (1998) A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391:799–803 [DOI] [PubMed] [Google Scholar]
- Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. (2003) Kaposi’s sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol 77:2631–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. (1998) Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem 273:4282–4287 [DOI] [PubMed] [Google Scholar]
- Gutwein P, Schramme A, Sinke N, Abdel-Bakky MS, Voss B, Obermüller N, Doberstein K, Koziolek M, Fritzsche F, Johannsen M, et al. (2009) Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. Eur J Cancer 45:478–489 [DOI] [PubMed] [Google Scholar]
- Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, Chung MK. (2011) Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol Med Rep 4:419–424 [DOI] [PubMed] [Google Scholar]
- Hachet-Haas M, Balabanian K, Rohmer F, Pons F, Franchet C, Lecat S, Chow KY, Dagher R, Gizzi P, Didier B, et al. (2008) Small neutralizing molecules to inhibit actions of the chemokine CXCL12. J Biol Chem 283:23189–23199 [DOI] [PubMed] [Google Scholar]
- Hajnická V, Kocáková P, Sláviková M, Slovák M, Gasperík J, Fuchsberger N, Nuttall PA. (2001) Anti-interleukin-8 activity of tick salivary gland extracts. Parasite Immunol 23:483–489 [DOI] [PubMed] [Google Scholar]
- Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. (2001) Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med 193:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. (2005) Regulation of protein function by glycosaminoglycans–as exemplified by chemokines. Annu Rev Biochem 74:385–410 [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Schell E, Gouni-Berthold I, Melichar M, Vesela I, Sullivan T, Miao S, Johnson D, Jaen J, Bekker P, et al. (2011) Oral chemokine receptor 2 antagonist CCX140-B shows safety and efficacy in type 2 diabetes mellitus. Diabetes 60:A310 [Google Scholar]
- Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, et al. (2011) Oxysterols direct immune cell migration via EBI2. Nature 475:524–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansell CAH, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, Goodyear CS, Nibbs RJB. (2011) Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood 117:5413–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Müller AJ, Stecher B, Kaiser P, Barthel M, Endt K, Eberhard M, Robbiani R, Jacobi CA, Heikenwalder M, et al. (2008) Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med 205:437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque NS, Fallon JT, Pan JJ, Taubman MB, Harpel PC. (2004) Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion. Blood 103:1296–1304 [DOI] [PubMed] [Google Scholar]
- Haque NS, Fallon JT, Taubman MB, Harpel PC. (2001) The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood 97:39–45 [DOI] [PubMed] [Google Scholar]
- Haque NS, Zhang X, French DL, Li J, Poon M, Fallon JT, Gabel BR, Taubman MB, Koschinsky M, Harpel PC. (2000) CC chemokine I-309 is the principal monocyte chemoattractant induced by apolipoprotein(a) in human vascular endothelial cells. Circulation 102:786–792 [DOI] [PubMed] [Google Scholar]
- Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. (2006) Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 176:1600–1608 [DOI] [PubMed] [Google Scholar]
- Haringman JJ, Gerlag DM, Smeets TJ, Baeten D, van den Bosch F, Bresnihan B, Breedveld FC, Dinant HJ, Legay F, Gram H, et al. (2006) A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum 54:2387–2392 [DOI] [PubMed] [Google Scholar]
- Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, et al. (2012) Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486:545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Barber CM, Lynch KR. (1994) cDNA cloning of a G-protein-coupled receptor expressed in rat spinal cord and brain related to chemokine receptors. Neurosci Lett 169:85–89 [DOI] [PubMed] [Google Scholar]
- Hartl D, Griese M, Nicolai T, Zissel G, Prell C, Konstantopoulos N, Gruber R, Reinhardt D, Schendel DJ, Krauss-Etschmann S. (2005) Pulmonary chemokines and their receptors differentiate children with asthma and chronic cough. J Allergy Clin Immunol 115:728–736 [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Leick M, Ewers S, Diefenbacher A, Schraufstatter I, Honczarenko M, Burger M. (2008) Human B cells express the orphan chemokine receptor CRAM-A/B in a maturation-stage-dependent and CCL5-modulated manner. Immunology 125:252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell CA, Hancock WW, Salant DJ, Gao W, Csizmadia V, Peters W, Faia K, Fituri O, Rottman JB, Charo IF. (2001) Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection. J Clin Invest 108:679–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell CA, Horuk R, Liang M, Rosser M, Dunning L, Islam I, Kremer L, Gutiérrez J, Marquez G, Martinez-A C, et al. (2006) Identification and characterization of a potent, selective nonpeptide agonist of the CC chemokine receptor CCR8. Mol Pharmacol 69:309–316 [DOI] [PubMed] [Google Scholar]
- Hatabu T, Kawazu S, Aikawa M, Kano S. (2003) Binding of Plasmodium falciparum-infected erythrocytes to the membrane-bound form of Fractalkine/CX3CL1. Proc Natl Acad Sci USA 100:15942–15946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, et al. (1997) CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645–649 [DOI] [PubMed] [Google Scholar]
- Hedrick JA, Saylor V, Figueroa D, Mizoue L, Xu Y, Menon S, Abrams J, Handel T, Zlotnik A. (1997) Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol 158:1533–1540 [PubMed] [Google Scholar]
- Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, et al. (2009) CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest 119:2317–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman AS, Abonyo BO, Darling-Reed SF, Alexander MS. (2005) Cytokine-stimulated human lung alveolar epithelial cells release eotaxin-2 (CCL24) and eotaxin-3 (CCL26). J Interferon Cytokine Res 25:82–91 [DOI] [PubMed] [Google Scholar]
- Heinemann A, Hartnell A, Stubbs VE, Murakami K, Soler D, LaRosa G, Askenase PW, Williams TJ, Sabroe I. (2000) Basophil responses to chemokines are regulated by both sequential and cooperative receptor signaling. J Immunol 165:7224–7233 [DOI] [PubMed] [Google Scholar]
- Heinzel K, Benz C, Bleul CC. (2007) A silent chemokine receptor regulates steady-state leukocyte homing in vivo. Proc Natl Acad Sci USA 104:8421–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, Satsangi J, Wright M, Zhang L, Thomas HC, et al. (2003) Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology 38:1468–1476 [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. (2003) Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 34:70–74 [DOI] [PubMed] [Google Scholar]
- Heveker N, Tissot M, Thuret A, Schneider-Mergener J, Alizon M, Roch M, Marullo S. (2001) Pharmacological properties of peptides derived from stromal cell-derived factor 1: study on human polymorphonuclear cells. Mol Pharmacol 59:1418–1425 [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hübscher SG, Briskin M, Adams DH. (2005) CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol 174:1055–1062 [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Held KS, Baum E, Gao JL, Murphy PM, Lane TE. (2007) CCR1 deficiency increases susceptibility to fatal coronavirus infection of the central nervous system. Viral Immunol 20:599–608 [DOI] [PubMed] [Google Scholar]
- Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, et al. (1997) A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J Immunol 159:1140–1149 [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. (2007) Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med 204:1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. (2006) The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol 177:4488–4494 [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. (2007) Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 204:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H, et al. (2007) High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res 67:4725–4731 [DOI] [PubMed] [Google Scholar]
- Holmes FE, Arnott N, Vanderplank P, Kerr NC, Longbrake EE, Popovich PG, Imai T, Combadière C, Murphy PM, Wynick D. (2008) Intra-neural administration of fractalkine attenuates neuropathic pain-related behaviour. J Neurochem 106:640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. (1991) Structure and functional expression of a human interleukin-8 receptor. Science 253:1278–1280 [DOI] [PubMed] [Google Scholar]
- Holst PJ, Rosenkilde MM, Manfra D, Chen SC, Wiekowski MT, Holst B, Cifire F, Lipp M, Schwartz TW, Lira SA. (2001) Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J Clin Invest 108:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, Tsai M, Sadeh J, Magnussen H. (2010) SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J 35:564–570 [DOI] [PubMed] [Google Scholar]
- Homey B. (2005) Chemokines and inflammatory skin diseases. Adv Dermatol 21:251–277 [DOI] [PubMed] [Google Scholar]
- Homey B, Steinhoff M, Ruzicka T, Leung DY. (2006) Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol 118:178–189 [DOI] [PubMed] [Google Scholar]
- Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Müller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, et al. (2000) Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol 164:3465–3470 [DOI] [PubMed] [Google Scholar]
- Horikawa T, Nakayama T, Hikita I, Yamada H, Fujisawa R, Bito T, Harada S, Fukunaga A, Chantry D, Gray PW, et al. (2002) IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int Immunol 14:767–773 [DOI] [PubMed] [Google Scholar]
- Horton LW, Yu Y, Zaja-Milatovic S, Strieter RM, Richmond A. (2007) Opposing roles of murine Duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res 67:9791–9799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R. (2001) Chemokine receptors. Cytokine Growth Factor Rev 12:313–335 [DOI] [PubMed] [Google Scholar]
- Horuk R. (2009) Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov 8:23–33 [DOI] [PubMed] [Google Scholar]
- Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. (1993a) A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 261:1182–1184 [DOI] [PubMed] [Google Scholar]
- Horuk R, Colby TJ, Darbonne WC, Schall TJ, Neote K. (1993b) The human erythrocyte inflammatory peptide (chemokine) receptor. Biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry 32:5733–5738 [DOI] [PubMed] [Google Scholar]
- Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, et al. (1998) The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem 273:386–391 [DOI] [PubMed] [Google Scholar]
- Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, et al. (1997) Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol 158:2882–2890 [PubMed] [Google Scholar]
- Horuk R, Wang ZX, Peiper SC, Hesselgesser J. (1994) Identification and characterization of a promiscuous chemokine-binding protein in a human erythroleukemic cell line. J Biol Chem 269:17730–17733 [PubMed] [Google Scholar]
- Hoshino A, Kawamura YI, Yasuhara M, Toyama-Sorimachi N, Yamamoto K, Matsukawa A, Lira SA, Dohi T. (2007) Inhibition of CCL1-CCR8 interaction prevents aggregation of macrophages and development of peritoneal adhesions. J Immunol 178:5296–5304 [DOI] [PubMed] [Google Scholar]
- Hou C, Singer M, and Matheis M (2008) JNJ-17166864, a selective CCR2 antagonist with potential therapeutic implications for inflammatory diseases, in 104th International Conference American Thoracic Society; 16–21 May 2008; Toronto, ON, Canada. [Google Scholar]
- Howard OM, Dong HF, Shirakawa AK, Oppenheim JJ. (2000) LEC induces chemotaxis and adhesion by interacting with CCR1 and CCR8. Blood 96:840–845 [PubMed] [Google Scholar]
- Hu JK, Kagari T, Clingan JM, Matloubian M. (2011) Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc Natl Acad Sci USA 108:E118–E127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Li F, Cairns CM, Gordon JR, Xiang J. (2001) Neutrophils and B cells express XCR1 receptor and chemotactically respond to lymphotactin. Biochem Biophys Res Commun 281:378–382 [DOI] [PubMed] [Google Scholar]
- Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, et al. (1996) The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. see comments Nat Med 2:1240–1243 [DOI] [PubMed] [Google Scholar]
- Hub E, Rot A. (1998) Binding of RANTES, MCP-1, MCP-3, and MIP-1alpha to cells in human skin. Am J Pathol 152:749–757 [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Rittirsch D, Schreiber H, Weiss M, Flierl M, Younkin E, Schneider M, Suger-Wiedeck H, Gebhard F, et al. (2005) Changes in the novel orphan, C5a receptor (C5L2), during experimental sepsis and sepsis in humans. J Immunol 174:1104–1110 [DOI] [PubMed] [Google Scholar]
- Huber AR, Kunkel SL, Todd RF, 3rd, Weiss SJ. (1991) Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254:99–102 [DOI] [PubMed] [Google Scholar]
- Hudak S, Hagen M, Liu Y, Catron D, Oldham E, McEvoy LM, Bowman EP. (2002) Immune surveillance and effector functions of CCR10(+) skin homing T cells. J Immunol 169:1189–1196 [DOI] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. (2002) Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab 22:308–317 [DOI] [PubMed] [Google Scholar]
- Hughes RO, Rogier DJ, Devraj R, Zheng C, Cao G, Feng H, Xia M, Anand R, Xing L, Glenn J, et al. (2011) Discovery of ((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxy-tetrahydro-2H-pyran-4-ylamino)cyclopentyl)(4-(5-(trifluoromethyl)pyridazin-3-yl)piperazin-1-yl)methanone, PF-4254196, a CCR2 antagonist with an improved cardiovascular profile. Bioorg Med Chem Lett 21:2626–2630 [DOI] [PubMed] [Google Scholar]
- Hugues S, Scholer A, Boissonnas A, Nussbaum A, Combadière C, Amigorena S, Fetler L. (2007) Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol 8:921–930 [DOI] [PubMed] [Google Scholar]
- Huising MO, Stet RJ, Kruiswijk CP, Savelkoul HF, Lidy Verburg-van Kemenade BM. (2003) Molecular evolution of CXC chemokines: extant CXC chemokines originate from the CNS. Trends Immunol 24:307–313 [DOI] [PubMed] [Google Scholar]
- Hulshof JW, Casarosa P, Menge WM, Kuusisto LM, van der Goot H, Smit MJ, de Esch IJ, Leurs R. (2005) Synthesis and structure-activity relationship of the first nonpeptidergic inverse agonists for the human cytomegalovirus encoded chemokine receptor US28. J Med Chem 48:6461–6471 [DOI] [PubMed] [Google Scholar]
- Hulshof JW, Vischer HF, Verheij MH, Fratantoni SA, Smit MJ, de Esch IJ, Leurs R. (2006) Synthesis and pharmacological characterization of novel inverse agonists acting on the viral-encoded chemokine receptor US28. Bioorg Med Chem 14:7213–7230 [DOI] [PubMed] [Google Scholar]
- Hulshof S, van Haastert ES, Kuipers HF, van den Elsen PJ, De Groot CJ, van der Valk P, Ravid R, Biber K. (2003) CX3CL1 and CX3CR1 expression in human brain tissue: noninflammatory control versus multiple sclerosis. J Neuropathol Exp Neurol 62:899–907 [DOI] [PubMed] [Google Scholar]
- Humpert ML, Tzouros M, Thelen S, Bignon A, Levoye A, Arenzana-Seisdedos F, Balabanian K, Bachelerie F, Langen H, Thelen M. (2012) Complementary methods provide evidence for the expression of CXCR7 on human B cells. Proteomics 12:1938–1948 [DOI] [PubMed] [Google Scholar]
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, Schneider T, Hofmann J, Kücherer C, Blau O, et al. (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 360:692–698 [DOI] [PubMed] [Google Scholar]
- Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. (2001) Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 194:847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikin EW, Mourant AE, Pettenkofer HJ, Blumenthal G. (1951) Discovery of the expected haemagglutinin, anti-Fyb. Nature 168:1077–1078 [DOI] [PubMed] [Google Scholar]
- Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. (1997a) The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem 272:15036–15042 [DOI] [PubMed] [Google Scholar]
- Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. (1998) Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem 273:1764–1768 [DOI] [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, et al. (1997b) Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91:521–530 [DOI] [PubMed] [Google Scholar]
- Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. (1999) Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 11:81–88 [DOI] [PubMed] [Google Scholar]
- Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O. (1996) Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J Biol Chem 271:21514–21521 [DOI] [PubMed] [Google Scholar]
- Imaoka H, Campbell H, Babirad I, Watson RM, Mistry M, Sehmi R, Gauvreau GM. (2011) TPI ASM8 reduces eosinophil progenitors in sputum after allergen challenge. Clin Exp Allergy 41:1740–1746 [DOI] [PubMed] [Google Scholar]
- Inoue A, Hasegawa H, Kohno M, Ito MR, Terada M, Imai T, Yoshie O, Nose M, Fujita S. (2005) Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum 52:1522–1533 [DOI] [PubMed] [Google Scholar]
- Isaacman-Beck J, Hermann EA, Yi Y, Ratcliffe SJ, Mulenga J, Allen S, Hunter E, Derdeyn CA, Collman RG. (2009) Heterosexual transmission of human immunodeficiency virus type 1 subtype C: Macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol 83:8208–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. (1998) Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol 72:6104–6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, et al. (2012a) Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 30:837–842 [DOI] [PubMed] [Google Scholar]
- Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, Takeuchi G, Shimizu S, Ito M, Komatsu H, et al. (2003) Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 9:3625–3634 [PubMed] [Google Scholar]
- Ishida Y, Gao JL, Murphy PM. (2008) Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol 180:569–579 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kimura A, Kuninaka Y, Inui M, Matsushima K, Mukaida N, Kondo T. (2012b) Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest 122:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. (2011) Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat Immunol 12:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SA, Ling MF, Leung J, Luster AD. (2013) Identification of human CCR8 as a CCL18 receptor. J Exp Med 210:1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto S, Omi T, Kajii E, Ikemoto S. (1995) Genomic organization of the glycoprotein D gene: Duffy blood group Fya/Fyb alloantigen system is associated with a polymorphism at the 44-amino acid residue. Blood 85:622–626 [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. (2000) Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med 192:1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J, Thompson M, and Fischl M (2009) Phase 2a study of PRO 140 in HIV-infected adults, in 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2009), Abstract H-1229; 12--15 September 2009; San Francisco, CA [Google Scholar]
- Jähnichen S, Blanchetot C, Maussang D, Gonzalez-Pajuelo M, Chow KY, Bosch L, De Vrieze S, Serruys B, Ulrichts H, Vandevelde W, et al. (2010) CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc Natl Acad Sci USA 107:20565–20570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. (2006) Modulation of dendritic cell trafficking to and from the airways. J Immunol 176:3578–3584 [DOI] [PubMed] [Google Scholar]
- Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. (2005) The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol 6:403–411 [DOI] [PubMed] [Google Scholar]
- Jenh CH, Cox MA, Cui L, Reich EP, Sullivan L, Chen SC, Kinsley D, Qian S, Kim SH, Rosenblum S, et al. (2012) A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AS, Sparre-Ulrich AH, Davis-Poynter N, Rosenkilde MM. (2012) Structural diversity in conserved regions like the DRY-motif among viral 7TM receptors— a consequence of evolutionary pressure? Adv Virol 2012:231813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TJ, Guan B, Dai M, Li G, Lightburn TE, Huang S, Freeze BS, Burdi DF, Jacutin-Porte S, Bennett R, et al. (2007) Design, synthesis, and evaluation of naphthalene-sulfonamide antagonists of human CCR8. J Med Chem 50:566–584 [DOI] [PubMed] [Google Scholar]
- Jensen KK, Manfra DJ, Grisotto MG, Martin AP, Vassileva G, Kelley K, Schwartz TW, Lira SA. (2005) The human herpes virus 8-encoded chemokine receptor is required for angioproliferation in a murine model of Kaposi’s sarcoma. J Immunol 174:3686–3694 [DOI] [PubMed] [Google Scholar]
- Jensen PC, Nygaard R, Thiele S, Elder A, Zhu G, Kolbeck R, Ghosh S, Schwartz TW, Rosenkilde MM. (2007) Molecular interaction of a potent nonpeptide agonist with the chemokine receptor CCR8. Mol Pharmacol 72:327–340 [DOI] [PubMed] [Google Scholar]
- Jensen PC, Rosenkilde MM. (2009) Chapter 8. Activation mechanisms of chemokine receptors. Methods Enzymol 461:171–190 [DOI] [PubMed] [Google Scholar]
- Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. (2008) Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol 180:6846–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. (2010) Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 120:2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilma-Stohlawetz P, Homoncik M, Drucker C, Marsik C, Rot A, Mayr WR, Seibold B, Jilma B. (2001) Fy phenotype and gender determine plasma levels of monocyte chemotactic protein. Transfusion 41:378–381 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Miao C, Blanchard EG, Caidi H, Radu GU, Harcourt JL, Haynes LM. (2012) Effect of chemokine receptor CX3CR1 deficiency in a murine model of respiratory syncytial virus infection. Comp Med 62:14–20 [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Li AR, Liu J, Fu Z, Zhu L, Miao S, Wang X, Xu Q, Huang A, Marcus A, et al. (2007) Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett 17:3339–3343 [DOI] [PubMed] [Google Scholar]
- Johnson Z, Kosco-Vilbois MH, Herren S, Cirillo R, Muzio V, Zaratin P, Carbonatto M, Mack M, Smailbegovic A, Rose M, et al. (2004) Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J Immunol 173:5776–5785 [DOI] [PubMed] [Google Scholar]
- Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. (2003) Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol 171:2960–2969 [DOI] [PubMed] [Google Scholar]
- Johswich K, Martin M, Thalmann J, Rheinheimer C, Monk PN, Klos A. (2006) Ligand specificity of the anaphylatoxin C5L2 receptor and its regulation on myeloid and epithelial cell lines. J Biol Chem 281:39088–39095 [DOI] [PubMed] [Google Scholar]
- Jones CP, Pitchford SC, Lloyd CM, Rankin SM. (2009) CXCR2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling. Stem Cells 27:3074–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. (1996) Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci USA 93:6682–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph PR, Sarmiento JM, Mishra AK, Das ST, Garofalo RP, Navarro J, Rajarathnam K. (2010) Probing the role of CXC motif in chemokine CXCL8 for high affinity binding and activation of CXCR1 and CXCR2 receptors. J Biol Chem 285:29262–29269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert P, Lajoie-Kadoch S, Labonté I, Gounni AS, Maghni K, Wellemans V, Chakir J, Laviolette M, Hamid Q, Lamkhioued B. (2005) CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol 175:2702–2708 [DOI] [PubMed] [Google Scholar]
- Ju DW, Tao Q, Cheng DS, Zhang W, Zhang M, Hamada H, Cao X. (2000) Adenovirus-mediated lymphotactin gene transfer improves therapeutic efficacy of cytosine deaminase suicide gene therapy in established murine colon carcinoma. Gene Ther 7:329–338 [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20:4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Förster R, Wolf I, Ebensperger C, Kuehl WM, Lipp M. (1993) The G protein-coupled receptor BLR1 is involved in murine B cell differentiation and is also expressed in neuronal tissues. Eur J Immunol 23:2532–2539 [DOI] [PubMed] [Google Scholar]
- Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K, et al. (2001) Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol 107:535–541 [DOI] [PubMed] [Google Scholar]
- Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Komine M, Asahina A, Tamaki K. (2002) Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin Exp Immunol 127:270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. (2003) The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem 278:11123–11129 [DOI] [PubMed] [Google Scholar]
- Kanagawa N, Niwa M, Hatanaka Y, Tani Y, Nakagawa S, Fujita T, Yamamoto A, Okada N. (2007) CC-chemokine ligand 17 gene therapy induces tumor regression through augmentation of tumor-infiltrating immune cells in a murine model of preexisting CT26 colon carcinoma. Int J Cancer 121:2013–2022 [DOI] [PubMed] [Google Scholar]
- Kang YS, Cha JJ, Hyun YY, Cha DR. (2011) Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin Investig Drugs 20:745–756 [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. (2011) A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS ONE 6:e28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. (1995) An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 155:5003–5010 [PubMed] [Google Scholar]
- Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. (2013) Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 38:502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Choi U, Cardwell L, DeRavin SS, Naumann N, Whiting-Theobald NL, Linton GF, Moon J, Murphy PM, Malech HL. (2007) WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood 109:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, et al. (2001) Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol 166:2055–2062 [DOI] [PubMed] [Google Scholar]
- Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, et al. (2012) Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci USA 109:5010–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. (2010) CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res 106:91–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl JH. (2006) Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res 34:211–227 [DOI] [PubMed] [Google Scholar]
- Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. (1999) The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol 162:3859–3864 [PubMed] [Google Scholar]
- Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ, et al. (1994) Lymphotactin: a cytokine that represents a new class of chemokine. Science 266:1395–1399 [DOI] [PubMed] [Google Scholar]
- Kennedy J, Kelner GS, Kleyensteuber S, Schall TJ, Weiss MC, Yssel H, Schneider PV, Cocks BG, Bacon KB, Zlotnik A. (1995) Molecular cloning and functional characterization of human lymphotactin. J Immunol 155:203–209 [PubMed] [Google Scholar]
- Kerstjens HA, Bjermer L, Eriksson L, Dahlström K, Vestbo J. (2010) Tolerability and efficacy of inhaled AZD4818, a CCR1 antagonist, in moderate to severe COPD patients. Respir Med 104:1297–1303 [DOI] [PubMed] [Google Scholar]
- Keshav S (2006) CCX282-B, an orally active inhibitor of chemokine receptor CCR9, in a randomized, double-blind, placebo-controlled phase 2 study in moderate to severe Crohn's disease, in 14th United European Gastroenterology Week (UEGW 2006); 21–25 October 2006; Berlin, Germany. [Google Scholar]
- Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, Luster AD. (2000) IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483–494 [DOI] [PubMed] [Google Scholar]
- Khandaker MH, Mitchell G, Xu L, Andrews JD, Singh R, Leung H, Madrenas J, Ferguson SS, Feldman RD, Kelvin DJ. (1999) Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood 93:2173–2185 [PubMed] [Google Scholar]
- Kim BS, Uhm TG, Lee SK, Lee SH, Kang JH, Park CS, Chung IY. (2010) The crucial role of GATA-1 in CCR3 gene transcription: modulated balance by multiple GATA elements in the CCR3 regulatory region. J Immunol 185:6866–6875 [DOI] [PubMed] [Google Scholar]
- Kim CH, Johnston B, Butcher EC. (2002) Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood 100:11–16 [DOI] [PubMed] [Google Scholar]
- Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, Greenberg HB, Butcher EC. (2001a) Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest 107:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Nagata K, Butcher EC. (2003) Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J Immunol 171:152–158 [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. (2001b) Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest 108:1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Jang SW, Sung HJ, Lee JS, Ko J. (2005) Differential CCR1-mediated chemotaxis signaling induced by human CC chemokine HCC-4/CCL16 in HOS cells. FEBS Lett 579:6044–6048 [DOI] [PubMed] [Google Scholar]
- Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. (2011) In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 118:e156–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung Ss, Kuziel WA, Feldman S, Fu SM, Rose CE., Jr (2001c) Enhanced airway Th2 response after allergen challenge in mice deficient in CC chemokine receptor-2 (CCR2). J Immunol 166:5183–5192 [DOI] [PubMed] [Google Scholar]
- Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, Takamura T, Yamamoto H, Miyamoto KI, Ginsberg HN, et al. (2012) CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 61:1680–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Farber JM, Kelsall BL. (2010) CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J Immunol 185:3295–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O. (1996) Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem 271:7725–7730 [DOI] [PubMed] [Google Scholar]
- Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Lüttichau HR, Gerstoft J, Clapham PR, et al. (1997) A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science 277: 1656–1659 [DOI] [PubMed] [Google Scholar]
- Kledal TN, Rosenkilde MM, Schwartz TW. (1998) Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett 441:209–214 [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. (2005) CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood 105:2877–2886 [DOI] [PubMed] [Google Scholar]
- Knight JC, Novembre FJ, Brown DR, Goldsmith CS, Esposito JJ. (1989) Studies on Tanapox virus. Virology 172:116–124 [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi Y, Okada Y, Suzuki A, Ikari K, Terao C, Takahashi A, Yamazaki K, Hosono N, Myouzen K, Tsunoda T, et al. (2010) A regulatory variant in CCR6 is associated with rheumatoid arthritis susceptibility. Nat Genet 42:515–519 [DOI] [PubMed] [Google Scholar]
- Koelink PJ, Overbeek SA, Braber S, de Kruijf P, Folkerts G, Smit MJ, Kraneveld AD. (2012) Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol Ther 133:1–18 [DOI] [PubMed] [Google Scholar]
- Koenecke C, Förster R. (2009) CCR9 and inflammatory bowel disease. Expert Opin Ther Targets 13:297–306 [DOI] [PubMed] [Google Scholar]
- Köhler A, De Filippo K, Hasenberg M, van den Brandt C, Nye E, Hosking MP, Lane TE, Männ L, Ransohoff RM, Hauser AE, et al. (2011) G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117:4349–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. (2008) The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 29:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. (1991) Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol 72:1401–1408 [DOI] [PubMed] [Google Scholar]
- Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG. (2011) Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology 132:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska MA, Ratajczak MZ, Majka M, Jin J, Kunapuli S, Brass L, Poncz M. (2000) Stromal cell-derived factor-1 and macrophage-derived chemokine: 2 chemokines that activate platelets. Blood 96:50–57 [PubMed] [Google Scholar]
- Kralj A, Wetzel A, Mahmoudian S, Stamminger T, Tschammer N, Heinrich MR. (2011) Identification of novel allosteric modulators for the G-protein coupled US28 receptor of human cytomegalovirus. Bioorg Med Chem Lett 21:5446–5450 [DOI] [PubMed] [Google Scholar]
- Krasinski A, Punna S, Ungashe S, Wang Q, and Zeng Y (2011), inventors; Chemocentryx, assignee. Fused heteroaryl pyridyl and phenyl benzenesulfonamides as CCR2 modulators for the treatment of inflammation. US patent 7,884,110. 2008 Jul 11. [Google Scholar]
- Kremer L, Carramolino L, Goya I, Zaballos A, Gutiérrez J, Moreno-Ortiz M del C, Martínez-A C, Márquez G. (2001) The transient expression of C-C chemokine receptor 8 in thymus identifies a thymocyte subset committed to become CD4+ single-positive T cells. J Immunol 166:218–225 [DOI] [PubMed] [Google Scholar]
- Kriegova E, Tsyrulnyk A, Arakelyan A, Mrazek F, Ordeltova M, Petzmann S, Zatloukal J, Kolek V, du Bois RM, Popper H, et al. (2006) Expression of CCX CKR in pulmonary sarcoidosis. Inflamm Res 55:441–445 [DOI] [PubMed] [Google Scholar]
- Kristensen NN, Gad M, Thomsen AR, Lu B, Gerard C, Claesson MH. (2006) CXC chemokine receptor 3 expression increases the disease-inducing potential of CD4+ CD25- T cells in adoptive transfer colitis. Inflamm Bowel Dis 12:374–381 [DOI] [PubMed] [Google Scholar]
- Kroczek RA, Henn V. (2012) The role of XCR1 and its ligand XCL1 in antigen cross-presentation by murine and human dendritic cells. Front Immunol 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Förster R. (2010) CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood 115:1906–1912 [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Hudson JT, 3rd, Waikel RL, Martin WD, Williams IR. (2002) CCR6 expression distinguishes mouse myeloid and lymphoid dendritic cell subsets: demonstration using a CCR6 EGFP knock-in mouse. Eur J Immunol 32:104–112 [DOI] [PubMed] [Google Scholar]
- Kuhn DE, Beall CJ, Kolattukudy PE. (1995) The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun 211:325–330 [DOI] [PubMed] [Google Scholar]
- Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, et al. (2013) BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res 19:357–366 [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, et al. (2002) Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol 160:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Campbell DJ, Butcher EC. (2003) Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation 10:313–323 [DOI] [PubMed] [Google Scholar]
- Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, Tomura M, Sugihara K, Takamura S, Kakimi K, et al. (2011) Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med 208:1605–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Bravo R. (1996) Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem 271:11603–11607 [DOI] [PubMed] [Google Scholar]
- Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, et al. (2006) CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity 24:165–177 [DOI] [PubMed] [Google Scholar]
- Kurts C, Robinson BW, Knolle PA. (2010) Cross-priming in health and disease. Nat Rev Immunol 10:403–414 [DOI] [PubMed] [Google Scholar]
- Kuschert GS, Hoogewerf AJ, Proudfoot AE, Chung CW, Cooke RM, Hubbard RE, Wells TN, Sanderson PN. (1998) Identification of a glycosaminoglycan binding surface on human interleukin-8. Biochemistry 37:11193–11201 [DOI] [PubMed] [Google Scholar]
- Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. (2008) CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood 112:34–44 [DOI] [PubMed] [Google Scholar]
- Lagu B, Gerchak C, Pan M, Hou C, Singer M, Malaviya R, Matheis M, Olini G, Cavender D, Wachter M. (2007) Potent and selective CC-chemokine receptor-2 (CCR2) antagonists as a potential treatment for asthma. Bioorg Med Chem Lett 17:4382–4386 [DOI] [PubMed] [Google Scholar]
- Lalani AS, Masters J, Zeng W, Barrett J, Pannu R, Everett H, Arendt CW, McFadden G. (1999) Use of chemokine receptors by poxviruses. Science 286:1968–1971 [DOI] [PubMed] [Google Scholar]
- Lalani AS, McFadden G. (1997) Secreted poxvirus chemokine binding proteins. J Leukoc Biol 62:570–576 [DOI] [PubMed] [Google Scholar]
- Lalezari J, Gathe J, Brinson C, Thompson M, Cohen C, Dejesus E, Galindez J, Ernst JA, Martin DE, Palleja SM. (2011) Safety, efficacy, and pharmacokinetics of TBR-652, a CCR5/CCR2 antagonist, in HIV-1-infected, treatment-experienced, CCR5 antagonist-naive subjects. J Acquir Immune Defic Syndr 57:118–125 [DOI] [PubMed] [Google Scholar]
- Lalezari J, Yadavalli GK, Para M, Richmond G, Dejesus E, Brown SJ, Cai W, Chen C, Zhong J, Novello LA, et al. (2008) Safety, pharmacokinetics, and antiviral activity of HGS004, a novel fully human IgG4 monoclonal antibody against CCR5, in HIV-1-infected patients. J Infect Dis 197:721–727 [DOI] [PubMed] [Google Scholar]
- Lamkhioued B, Abdelilah SG, Hamid Q, Mansour N, Delespesse G, Renzi PM. (2003) The CCR3 receptor is involved in eosinophil differentiation and is up-regulated by Th2 cytokines in CD34+ progenitor cells. J Immunol 170:537–547 [DOI] [PubMed] [Google Scholar]
- Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. (2009) CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113:963–972 [DOI] [PubMed] [Google Scholar]
- Laprise C, Sladek R, Ponton A, Bernier MC, Hudson TJ, Laviolette M. (2004) Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genomics 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, et al. (2003) An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197:1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchney LR, Fallon MA, Culp DJ, Gelbard HA, Dewhurst S. (2004) Immunohistochemical assessment of fractalkine, inflammatory cells, and human herpesvirus 7 in human salivary glands. J Histochem Cytochem 52:671–681 [DOI] [PubMed] [Google Scholar]
- Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, Tal-Singer R. (2011) SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol 72:282–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Richmond A. (2010) Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med 16:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Aït-Yahia S, Vicari A, Kaiserlian D, et al. (2006) Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24:191–201 [DOI] [PubMed] [Google Scholar]
- Lebre MC, Vergunst CE, Choi IY, Aarrass S, Oliveira AS, Wyant T, Horuk R, Reedquist KA, Tak PP. (2011) Why CCR2 and CCR5 blockade failed and why CCR1 blockade might still be effective in the treatment of rheumatoid arthritis. PLoS ONE 6:e21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Koszinowski UH, Sarawar SR, Adler H. (2003) A gammaherpesvirus G protein-coupled receptor homologue is required for increased viral replication in response to chemokines and efficient reactivation from latency. J Immunol 170:243–251 [DOI] [PubMed] [Google Scholar]
- Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. (2005) Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med 201:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Wurfel MM, Matute-Bello G, Frevert CW, Rosengart MR, Ranganathan M, Wong VW, Holden T, Sutlief S, Richmond A, et al. (2006) The Duffy antigen modifies systemic and local tissue chemokine responses following lipopolysaccharide stimulation. J Immunol 177:8086–8094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, McKimmie CS, Gilchrist DS, Pallas KJ, Nibbs RJ, Garside P, McDonald V, Jenkins C, Ransohoff R, Liu L, et al. (2011) D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood 118:6220–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. (1998) B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med 187:655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bösl MR, Holländer GA, Hayashi Y, Malefyt RdeW, et al. (2011) Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med 208:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leick M, Catusse J, Follo M, Nibbs RJ, Hartmann TN, Veelken H, Burger M. (2010) CCL19 is a specific ligand of the constitutively recycling atypical human chemokine receptor CRAM-B. Immunology 129:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik P, Haskell CA, Charo IF. (2003) Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J Clin Invest 111:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsch A, Keilholz U, Schadendorf D, Assfalg G, Asemissen AM, Thiel E, Scheibenbogen C. (2004) Functional CCR9 expression is associated with small intestinal metastasis. J Invest Dermatol 122:685–690 [DOI] [PubMed] [Google Scholar]
- Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. (2009) CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 113:6085–6093 [DOI] [PubMed] [Google Scholar]
- L’Heureux GP, Bourgoin S, Jean N, McColl SR, Naccache PH. (1995) Diverging signal transduction pathways activated by interleukin-8 and related chemokines in human neutrophils: interleukin-8, but not NAP-2 or GRO alpha, stimulates phospholipase D activity. Blood 85:522–531 [PubMed] [Google Scholar]
- Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. (2005) Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis 8:63–71 [DOI] [PubMed] [Google Scholar]
- Li F, Gordon JR. (2001) Il-8((3-73))K11R is a high affinity agonist of the neutrophil CXCR1 and CXCR2. Biochem Biophys Res Commun 286:595–600 [DOI] [PubMed] [Google Scholar]
- Li H, d’Anjou M. (2009) Pharmacological significance of glycosylation in therapeutic proteins. Curr Opin Biotechnol 20:678–684 [DOI] [PubMed] [Google Scholar]
- Li Y, Huang D, Xia X, Wang Z, Luo L, Wen R. (2011) CCR3 and choroidal neovascularization. PLoS ONE 6:e17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Mallari C, Rosser M, Ng HP, May K, Monahan S, Bauman JG, Islam I, Ghannam A, Buckman B, et al. (2000) Identification and characterization of a potent, selective, and orally active antagonist of the CC chemokine receptor-1. J Biol Chem 275:19000–19008 [DOI] [PubMed] [Google Scholar]
- Liang X. (2008) CXCR4, inhibitors and mechanisms of action. Chem Biol Drug Des 72:97–110 [DOI] [PubMed] [Google Scholar]
- Liao F, Alderson R, Su J, Ullrich SJ, Kreider BL, Farber JM. (1997a) STRL22 is a receptor for the CC chemokine MIP-3alpha. Biochem Biophys Res Commun 236:212–217 [DOI] [PubMed] [Google Scholar]
- Liao F, Alkhatib G, Peden KW, Sharma G, Berger EA, Farber JM. (1997b) STRL33, A novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med 185:2015–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Lee HH, Farber JM. (1997c) Cloning of STRL22, a new human gene encoding a G-protein-coupled receptor related to chemokine receptors and located on chromosome 6q27. Genomics 40:175–180 [DOI] [PubMed] [Google Scholar]
- Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. (1999) CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol 162:186–194 [PubMed] [Google Scholar]
- Liao F, Shirakawa AK, Foley JF, Rabin RL, Farber JM. (2002) Human B cells become highly responsive to macrophage-inflammatory protein-3 alpha/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J Immunol 168:4871–4880 [DOI] [PubMed] [Google Scholar]
- Libert F, Parmentier M, Lefort A, Dumont JE, Vassart G. (1990) Complete nucleotide sequence of a putative G protein coupled receptor: RDC1. Nucleic Acids Res 18:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehn EA, Merx MW, Postea O, Becher S, Djalali-Talab Y, Shagdarsuren E, Kelm M, Zernecke A, Weber C. (2008) Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. J Cell Mol Med 12:496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JK, Louie CY, Glaser C, Jean C, Johnson B, Johnson H, McDermott DH, Murphy PM. (2008) Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis 197:262–265 [DOI] [PubMed] [Google Scholar]
- Limou S, Coulonges C, Herbeck JT, van Manen D, An P, Le Clerc S, Delaneau O, Diop G, Taing L, Montes M, et al. (2010) Multiple-cohort genetic association study reveals CXCR6 as a new chemokine receptor involved in long-term nonprogression to AIDS. J Infect Dis 202:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liò P, Vannucci M. (2003) Investigating the evolution and structure of chemokine receptors. Gene 317:29–37 [DOI] [PubMed] [Google Scholar]
- Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, et al. (2011) Oxysterols direct B-cell migration through EBI2. Nature 475:519–523 [DOI] [PubMed] [Google Scholar]
- Liu J, Fu Z, Li AR, Johnson M, Zhu L, Marcus A, Danao J, Sullivan T, Tonn G, Collins T, et al. (2009) Optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett 19:5114–5118 [DOI] [PubMed] [Google Scholar]
- Liu L, Darnall L, Hu T, Choi K, Lane TE, Ransohoff RM. (2010) Myelin repair is accelerated by inactivating CXCR2 on nonhematopoietic cells. J Neurosci 30:9074–9083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. (1996) Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367–377 [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez-A C, Coyle AJ, Gutierrez-Ramos JC. (2000) CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med 191:265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher M, Amara A, Oberlin E, Brass N, Legler D, Loetscher P, D’Apuzzo M, Meese E, Rousset D, Virelizier JL, et al. (1997) TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol 7:652–660 [DOI] [PubMed] [Google Scholar]
- Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. (1996a) Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med 184:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher M, Loetscher P, Brass N, Meese E, Moser B. (1998a) Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol 28:3696–3705 [DOI] [PubMed] [Google Scholar]
- Loetscher P, Gong JH, Dewald B, Baggiolini M, Clark-Lewis I. (1998b) N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem 273:22279–22283 [DOI] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Baggiolini M, Moser B. (1996b) Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med 184:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. (2005) T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 106:3432–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louahed J, Struyf S, Demoulin JB, Parmentier M, Van Snick J, Van Damme J, Renauld JC. (2003) CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/ CCL1 and vMIP-I. Eur J Immunol 33:494–501 [DOI] [PubMed] [Google Scholar]
- Lu B, Humbles A, Bota D, Gerard C, Moser B, Soler D, Luster AD, Gerard NP. (1999) Structure and function of the murine chemokine receptor CXCR3. Eur J Immunol 29:3804–3812 [DOI] [PubMed] [Google Scholar]
- Lu P, Li L, Liu G, van Rooijen N, Mukaida N, Zhang X. (2009) Opposite roles of CCR2 and CX3CR1 macrophages in alkali-induced corneal neovascularization. Cornea 28:562–569 [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen X, Galson DL, Taichman RS, Zhang J. (2008) CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res 6:546–554 [DOI] [PubMed] [Google Scholar]
- Lucendo AJ, De Rezende L, Comas C, Caballero T, Bellón T. (2008) Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. Am J Gastroenterol 103:2184–2193 [DOI] [PubMed] [Google Scholar]
- Lucotte G, Mercier G. (1998) Distribution of the CCR5 gene 32-bp deletion in Europe. J Acquir Immune Defic Syndr Hum Retrovirol 19:174–177 [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. (2001) Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J Exp Med 194:551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker KE, Gupta M, Luker GD. (2009) Imaging chemokine receptor dimerization with firefly luciferase complementation. FASEB J 23:823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, Thomas DG, Luker GD. (2012) Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene 31:4750–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker KE, Steele JM, Mihalko LA, Ray P, Luker GD. (2010) Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands. Oncogene 29:4599–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy SK, Lira SA, Smit JJ, Cook DN, Berlin AA, Lukacs NW. (2005) Attenuation of allergen-induced responses in CCR6-/- mice is dependent upon altered pulmonary T lymphocyte activation. J Immunol 174:2054–2060 [DOI] [PubMed] [Google Scholar]
- Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. (1998) Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians 110:183–196 [PubMed] [Google Scholar]
- Luster AD, Greenberg SM, Leder P. (1995) The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med 182:219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Unkeless JC, Ravetch JV. (1985) Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315:672–676 [DOI] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. (2000) Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA 97:12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttichau HR. (2010) The cytomegalovirus UL146 gene product vCXCL1 targets both CXCR1 and CXCR2 as an agonist. J Biol Chem 285:9137–9146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttichau HR, Clark-Lewis I, Jensen PO, Moser C, Gerstoft J, Schwartz TW. (2003) A highly selective CCR2 chemokine agonist encoded by human herpesvirus 6. J Biol Chem 278:10928–10933 [DOI] [PubMed] [Google Scholar]
- Lüttichau HR, Gerstoft J, Schwartz TW. (2001) MC148 encoded by human molluscum contagiosum poxvirus is an antagonist for human but not murine CCR8. J Leukoc Biol 70:277–282 [PubMed] [Google Scholar]
- Lüttichau HR, Johnsen AH, Jurlander J, Rosenkilde MM, Schwartz TW. (2007) Kaposi sarcoma-associated herpes virus targets the lymphotactin receptor with both a broad spectrum antagonist vCCL2 and a highly selective and potent agonist vCCL3. J Biol Chem 282:17794–17805 [DOI] [PubMed] [Google Scholar]
- Lüttichau HR, Stine J, Boesen TP, Johnsen AH, Chantry D, Gerstoft J, Schwartz TW. (2000) A highly selective CC chemokine receptor (CCR)8 antagonist encoded by the poxvirus molluscum contagiosum. J Exp Med 191:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP. (2006) Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis Rheum 54:2643–2655 [DOI] [PubMed] [Google Scholar]
- Madigan J, Freeman DJ, Menzies F, Forrow S, Nelson SM, Young A, Sharkey A, Moffett A, Graham GJ, Greer IA, et al. (2010) Chemokine scavenger D6 is expressed by trophoblasts and aids the survival of mouse embryos transferred into allogeneic recipients. J Immunol 184:3202–3212 [DOI] [PubMed] [Google Scholar]
- Maeda K, Nakata H, Koh Y, Miyakawa T, Ogata H, Takaoka Y, Shibayama S, Sagawa K, Fukushima D, Moravek J, et al. (2004) Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J Virol 78:8654–8662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghazachi AA, Skalhegg BS, Rolstad B, Al-Aoukaty A. (1997) Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J 11:765–774 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bonecchi R, Locati M. (2006) Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol 6:907–918 [DOI] [PubMed] [Google Scholar]
- Mariani M, Lang R, Binda E, Panina-Bordignon P, D’Ambrosio D. (2004) Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur J Immunol 34:231–240 [DOI] [PubMed] [Google Scholar]
- Martin MP, Dean M, Smith MW, Winkler C, Gerrard B, Michael NL, Lee B, Doms RW, Margolick J, Buchbinder S, et al. (1998) Genetic acceleration of AIDS progression by a promoter variant of CCR5. Science 282:1907–1911 [DOI] [PubMed] [Google Scholar]
- Martinez CO, McHale MJ, Wells JT, Ochoa O, Michalek JE, McManus LM, Shireman PK. (2010) Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol Regul Integr Comp Physiol 299:R832–R842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. (2008) Macrophage activation and polarization. Front Biosci 13:453–461 [DOI] [PubMed] [Google Scholar]
- Martínez MA, Gutiérrez A, Armand-Ugón M, Blanco J, Parera M, Gómez J, Clotet B, Esté JA. (2002) Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. AIDS 16:2385–2390 [DOI] [PubMed] [Google Scholar]
- Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, Pasqualini F, Doni A, Lauri E, Agostinis C, et al. (2007) Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci USA 104:2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, et al. (2005) Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol 35:1342–1346 [DOI] [PubMed] [Google Scholar]
- Martínez Muñoz L, Lucas P, Navarro G, Checa AI, Franco R, Martínez-A C, Rodríguez-Frade JM, Mellado M. (2009) Dynamic regulation of CXCR1 and CXCR2 homo- and heterodimers. J Immunol 183:7337–7346 [DOI] [PubMed] [Google Scholar]
- Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. (2001) 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res 65:529–542 [DOI] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. (2000) A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 1:298–304 [DOI] [PubMed] [Google Scholar]
- Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, et al. (2008) Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 181:3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, Slinger E, Schreiber A, Michel D, Tensen CP, et al. (2009) The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res 69:2861–2869 [DOI] [PubMed] [Google Scholar]
- Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. (2006) Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA 103:13068–13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma-Stohlawetz P, Derendorf H, Jilma B. (2008) Duffy antigen modifies the chemokine response in human endotoxemia. Crit Care Med 36:159–165 [DOI] [PubMed] [Google Scholar]
- McCulloch CV, Morrow V, Milasta S, Comerford I, Milligan G, Graham GJ, Isaacs NW, Nibbs RJ. (2008) Multiple roles for the C-terminal tail of the chemokine scavenger D6. J Biol Chem 283:7972–7982 [DOI] [PubMed] [Google Scholar]
- McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D’Agostino RB, O’Donnell CJ, Patel DD, et al. (2003) Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest 111:1241–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Halcox JP, Schenke WH, Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA, Murphy PM. (2001) Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res 89:401–407 [DOI] [PubMed] [Google Scholar]
- McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O’Brien S, Penzak SR, Filho JO, Priel DA, Kelly C, Garofalo M, et al. (2011) The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood 118:4957–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Yang Q, Kathiresan S, Cupples LA, Massaro JM, Keaney JF, Jr, Larson MG, Vasan RS, Hirschhorn JN, O’Donnell CJ, et al. (2005) CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation 112:1113–1120 [DOI] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366 [DOI] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. (2007) CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol 170:1229–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimmie CS, Fraser AR, Hansell C, Gutiérrez L, Philipsen S, Connell L, Rot A, Kurowska-Stolarska M, Carreno P, Pruenster M, et al. (2008) Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. J Immunol 181:3353–3363 [DOI] [PubMed] [Google Scholar]
- McLean KA, Holst PJ, Martini L, Schwartz TW, Rosenkilde MM. (2004) Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology 325:241–251 [DOI] [PubMed] [Google Scholar]
- McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ. (2012) Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 338:1348–1351 [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. (2011) CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest 121:4787–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, Farber JM, Luster AD. (2006) CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol 176:7087–7095 [DOI] [PubMed] [Google Scholar]
- Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. (2010) CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 33:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melar M, Ott DE, Hope TJ. (2007) Physiological levels of virion-associated human immunodeficiency virus type 1 envelope induce coreceptor-dependent calcium flux. J Virol 81:1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado M, Martín de Ana A, Gómez L, Martínez C, Rodríguez-Frade JM. (2008) Chemokine receptor 2 blockade prevents asthma in a cynomolgus monkey model. J Pharmacol Exp Ther 324:769–775 [DOI] [PubMed] [Google Scholar]
- Mellado M, Rodríguez-Frade JM, Vila-Coro AJ, de Ana AM, Martínez-A C. (1999) Chemokine control of HIV-1 infection. Nature 400:723–724 [DOI] [PubMed] [Google Scholar]
- Mellado M, Rodríguez-Frade JM, Vila-Coro AJ, Fernández S, Martín de Ana A, Jones DR, Torán JL, Martínez-A C. (2001) Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J 20:2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. (2004) Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Galpha12. J Virol 78:8382–8391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, et al. (2010) Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci USA 107:5967–5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, Lu B, Farber J, Luster AD, Kelley VR. (2008) CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol 19:1177–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten P, Struyf S, Schutyser E, Wuyts A, De Clercq E, Schols D, Proost P, Van Damme J. (1999) The LD78beta isoform of MIP-1alpha is the most potent CCR5 agonist and HIV-1-inhibiting chemokine. J Clin Invest 104:R1–R5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. (2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA 97:8075–8080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Coyle AJ, Proudfoot AE, Wells TN, Power CA. (1996) Cloning and characterization of a novel murine macrophage inflammatory protein-1 alpha receptor. J Biol Chem 271:14445–14451 [DOI] [PubMed] [Google Scholar]
- Meyer M, Hensbergen PJ, van der Raaij-Helmer EM, Brandacher G, Margreiter R, Heufler C, Koch F, Narumi S, Werner ER, Colvin R, et al. (2001) Cross-reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur J Immunol 31:2521–2527 [DOI] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. (1997) Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91:385–395 [DOI] [PubMed] [Google Scholar]
- Migeotte I, Franssen JD, Goriely S, Willems F, Parmentier M. (2002) Distribution and regulation of expression of the putative human chemokine receptor HCR in leukocyte populations. Eur J Immunol 32:494–501 [DOI] [PubMed] [Google Scholar]
- Mikhak Z, Fukui M, Farsidjani A, Medoff BD, Tager AM, Luster AD. (2009) Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol 123:67–73, e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. (1976) The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med 295:302–304 [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. (1975) Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 189:561–563 [DOI] [PubMed] [Google Scholar]
- Miller MD, Hata S, De Waal Malefyt R, Krangel MS. (1989) A novel polypeptide secreted by activated human T lymphocytes. J Immunol 143:2907–2916 [PubMed] [Google Scholar]
- Miller MD, Wilson SD, Dorf ME, Seuanez HN, O’Brien SJ, Krangel MS. (1990) Sequence and chromosomal location of the I-309 gene. Relationship to genes encoding a family of inflammatory cytokines. J Immunol 145:2737–2744 [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, et al. (2004) Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci 20:2294–2302 [DOI] [PubMed] [Google Scholar]
- Milne RS, Mattick C, Nicholson L, Devaraj P, Alcami A, Gompels UA. (2000) RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J Immunol 164:2396–2404 [DOI] [PubMed] [Google Scholar]
- Miltz W, Loetscher P, Janser P, Hersperger R, Hiestand P, and Meingassner J (2008) Dual CCR2/CCR5 antagonists: a new approach for the treatment of autoimmune diseases, in ACS Meeting; 6–10 April 2008; New Orleans, LA Available from http://oasys2.confex.com/acs/235nm/techprogram/
- Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, et al. (2010) CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med 16:1305–1312 [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Benkö G, Filipek S, Palczewski K. (2003) Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry 42:2759–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Rothenberg ME. (2003) Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 125:1419–1427 [DOI] [PubMed] [Google Scholar]
- Miu J, Mitchell AJ, Müller M, Carter SL, Manders PM, McQuillan JA, Saunders BM, Ball HJ, Lu B, Campbell IL, et al. (2008) Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol 180:1217–1230 [DOI] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, Amara Mel-W, Seknadji P, McDermott DH, Debré P, Aumont MC, Murphy PM, de Prost D, et al. (2001) Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood 97:1925–1928 [DOI] [PubMed] [Google Scholar]
- Mohan K, Issekutz TB. (2007) Blockade of chemokine receptor CXCR3 inhibits T cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol 179:8463–8469 [DOI] [PubMed] [Google Scholar]
- Molon B, Gri G, Bettella M, Gómez-Moutón C, Lanzavecchia A, Martínez-A C, Mañes S, Viola A. (2005) T cell costimulation by chemokine receptors. Nat Immunol 6:465–471 [DOI] [PubMed] [Google Scholar]
- Molteni R, Crespo CL, Feigelson S, Moser C, Fabbri M, Grabovsky V, Krombach F, Laudanna C, Alon R, Pardi R. (2009) Beta-arrestin 2 is required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation. Blood 114:1073–1082 [DOI] [PubMed] [Google Scholar]
- Monnier J, Lewén S, O’Hara E, Huang K, Tu H, Butcher EC, Zabel BA. (2012) Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol 189:956–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. (2003) Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23–36 [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. (2001) The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res 61:2641–2648 [PubMed] [Google Scholar]
- Montecucco F, Lenglet S, Braunersreuther V, Pelli G, Pellieux C, Montessuit C, Lerch R, Deruaz M, Proudfoot AE, Mach F. (2010) Single administration of the CXC chemokine-binding protein Evasin-3 during ischemia prevents myocardial reperfusion injury in mice. Arterioscler Thromb Vasc Biol 30:1371–1377 [DOI] [PubMed] [Google Scholar]
- Moorman NJ, Virgin HW, 4th, Speck SH. (2003) Disruption of the gene encoding the gammaHV68 v-GPCR leads to decreased efficiency of reactivation from latency. Virology 307:179–190 [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, et al. (1999) CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA 96:14470–14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Bian Y, Gao P, Yashiro-Ohtani Y, Zhou XY, Ono S, Nakahara H, Kogo M, Hamaoka T, Fujiwara H. (2005) Induction of surface CCR4 and its functionality in mouse Th2 cells is regulated differently during Th2 development. J Leukoc Biol 78:753–761 [DOI] [PubMed] [Google Scholar]
- Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, Law Y, Distelhorst K, Nielsen EM, Hill ED, et al. (2008) An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol 181:6309–6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle G (2006) The last word on aplaviroc: a CCR5 antagonist with poor efficacy. The Body http://www.thebody.com/content/confs/hiv8/art39205.html
- Moyle G, DeJesus E, Boffito M, Wong RS, Gibney C, Badel K, MacFarland R, Calandra G, Bridger G, Becker S, X4 Antagonist Concept Trial Study Team (2009) Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis 48:798–805 [DOI] [PubMed] [Google Scholar]
- Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, Williams TJ, Pease JE. (2008) CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol 83:875–882 [DOI] [PubMed] [Google Scholar]
- Mukaida N. (2003) Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 284:L566–L577 [DOI] [PubMed] [Google Scholar]
- Müller S, Dorner B, Korthaüer U, Mages HW, D’Apuzzo M, Senger G, Kroczek RA. (1995) Cloning of ATAC, an activation-induced, chemokine-related molecule exclusively expressed in CD8+ T lymphocytes. Eur J Immunol 25:1744–1748 [DOI] [PubMed] [Google Scholar]
- Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. (1999) Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest 104:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. (2000) International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52:145–176 [PubMed] [Google Scholar]
- Murphy PM, Tiffany HL. (1991) Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 253:1280–1283 [DOI] [PubMed] [Google Scholar]
- Mutalithas K, Guillen C, Raport C, Kolbeck R, Soler D, Brightling CE, Pavord ID, Wardlaw AJ. (2010) Expression of CCR8 is increased in asthma. Clin Exp Allergy 40:1175–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najarro P, Gubser C, Hollinshead M, Fox J, Pease J, Smith GL. (2006) Yaba-like disease virus chemokine receptor 7L, a CCR8 orthologue. J Gen Virol 87:809–816 [DOI] [PubMed] [Google Scholar]
- Najarro P, Lee HJ, Fox J, Pease J, Smith GL. (2003) Yaba-like disease virus protein 7L is a cell-surface receptor for chemokine CCL1. J Gen Virol 84:3325–3336 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. (2009) CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 462:510–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Gunn MD. (2001) Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol 166:361–369 [DOI] [PubMed] [Google Scholar]
- Nakano K, Tadagaki K, Isegawa Y, Aye MM, Zou P, Yamanishi K. (2003) Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol 77:8108–8115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T, Kaburagi Y, Shimada Y, Inaoki M, Takehara K, Mukaida N, Sato S. (2001) CCR4 memory CD4+ T lymphocytes are increased in peripheral blood and lesional skin from patients with atopic dermatitis. J Allergy Clin Immunol 107:353–358 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Arao T, Jin Z, Nagakubo D, Shirakawa AK, Yamada Y, Fujii M, Oiso N, Kawada A, et al. (2008) Aberrant expression of Fra-2 promotes CCR4 expression and cell proliferation in adult T-cell leukemia. Oncogene 27:3221–3232 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. (2003) Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol 170:1136–1140 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Watanabe Y, Oiso N, Higuchi T, Shigeta A, Mizuguchi N, Katou F, Hashimoto K, Kawada A, Yoshie O. (2010) Eotaxin-3/CC chemokine ligand 26 is a functional ligand for CX3CR1. J Immunol 185:6472–6479 [DOI] [PubMed] [Google Scholar]
- Nanki T, Takada K, Komano Y, Morio T, Kanegane H, Nakajima A, Lipsky PE, Miyasaka N. (2009) Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 11:R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansen A, Marker O, Bartholdy C, Thomsen AR. (2000) CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur J Immunol 30:1797–1806 [DOI] [PubMed] [Google Scholar]
- Napolitano M, Zingoni A, Bernardini G, Spinetti G, Nista A, Storlazzi CT, Rocchi M, Santoni A. (1996) Molecular cloning of TER1, a chemokine receptor-like gene expressed by lymphoid tissues. J Immunol 157:2759–2763 [PubMed] [Google Scholar]
- Nardelli B, Tiffany HL, Bong GW, Yourey PA, Morahan DK, Li Y, Murphy PM, Alderson RF. (1999) Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J Immunol 162:435–444 [PubMed] [Google Scholar]
- Nasser MW, Marjoram RJ, Brown SL, Richardson RM. (2005) Cross-desensitization among CXCR1, CXCR2, and CCR5: role of protein kinase C-epsilon. J Immunol 174:6927–6933 [DOI] [PubMed] [Google Scholar]
- Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, Rot A, Thelen M. (2010) CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE 5:e9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya A, Ishikawa M, Matsuda K, Ohwaki K, Saeki T, Noguchi K, Ohtake N. (2003) Structure-activity relationships of xanthene carboxamides, novel CCR1 receptor antagonists. Bioorg Med Chem 11:875–884 [DOI] [PubMed] [Google Scholar]
- Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. (1993) Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem 268:12247–12249 [PubMed] [Google Scholar]
- Neote K, Mak JY, Kolakowski LF, Jr, Schall TJ. (1994) Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood 84:44–52 [PubMed] [Google Scholar]
- Neptune ER, Iiri T, Bourne HR. (1999) Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem 274:2824–2828 [DOI] [PubMed] [Google Scholar]
- Nibbs R, Graham G, Rot A. (2003) Chemokines on the move: control by the chemokine “interceptors” Duffy blood group antigen and D6. Semin Immunol 15:287–294 [DOI] [PubMed] [Google Scholar]
- Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. (2007) The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest 117:1884–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, et al. (2001) The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol 158:867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Wylie SM, Pragnell IB, Graham GJ. (1997) Cloning and characterization of a novel murine beta chemokine receptor, D6. Comparison to three other related macrophage inflammatory protein-1alpha receptors, CCR-1, CCR-3, and CCR-5. J Biol Chem 272:12495–12504 [DOI] [PubMed] [Google Scholar]
- Nibbs RJ, Yang J, Landau NR, Mao JH, Graham GJ. (1999) LD78beta, a non-allelic variant of human MIP-1alpha (LD78alpha), has enhanced receptor interactions and potent HIV suppressive activity. J Biol Chem 274:17478–17483 [DOI] [PubMed] [Google Scholar]
- Nicholas J. (1996) Determination and analysis of the complete nucleotide sequence of human herpesvirus. J Virol 70:5975–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J, Cameron KR, Honess RW. (1992) Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature 355:362–365 [DOI] [PubMed] [Google Scholar]
- Nichols WG, Steel HM, Bonny T, Adkison K, Curtis L, Millard J, Kabeya K, Clumeck N. (2008) Hepatotoxicity observed in clinical trials of aplaviroc (GW873140). Antimicrob Agents Chemother 52:858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. (2005) CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254–258 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. (2002) Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol 168:6173–6180 [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. (1998) Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett 429:167–172 [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Hieshima K, Nakayama T, Sakaguchi T, Fujisawa R, Tanase S, Nishiura H, Matsuno K, Takamori H, Tabira Y, et al. (2001) Human CC chemokine liver-expressed chemokine/CCL16 is a functional ligand for CCR1, CCR2 and CCR5, and constitutively expressed by hepatocytes. Int Immunol 13:1021–1029 [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O. (1998) Human chemokines fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) are clustered on chromosome 16q13. Cytogenet Cell Genet 81:10–11 [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. (2010) The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev 21:253–262 [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. (2011) A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev Comp Immunol 35:705–715 [DOI] [PubMed] [Google Scholar]
- Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. (2000) Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol 164:639–648 [DOI] [PubMed] [Google Scholar]
- Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, Buck W, Nelson PJ, von Luettichau I. (2005) CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer 115:641–647 [DOI] [PubMed] [Google Scholar]
- Nouri-Aria KT, Wilson D, Francis JN, Jopling LA, Jacobson MR, Hodge MR, Andrew DP, Till SJ, Varga EM, Williams TJ, et al. (2002) CCR4 in human allergen-induced late responses in the skin and lung. Eur J Immunol 32:1933–1938 [DOI] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, et al. (1996) The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382:833–835 [DOI] [PubMed] [Google Scholar]
- Obmolova G, Teplyakov A, Malia TJ, Grygiel TL, Sweet R, Snyder LA, Gilliland GL. (2012) Structural basis for high selectivity of anti-CCL2 neutralizing antibody CNTO 888. Mol Immunol 51:227–233 [DOI] [PubMed] [Google Scholar]
- Ockinger J, Stridh P, Beyeen AD, Lundmark F, Seddighzadeh M, Oturai A, Sørensen PS, Lorentzen AR, Celius EG, Leppä V, et al. (2010) Genetic variants of CC chemokine genes in experimental autoimmune encephalomyelitis, multiple sclerosis and rheumatoid arthritis. Genes Immun 11:142–154 [DOI] [PubMed] [Google Scholar]
- Ogilvie P, Thelen S, Moepps B, Gierschik P, da Silva Campos AC, Baggiolini M, Thelen M. (2004) Unusual chemokine receptor antagonism involving a mitogen-activated protein kinase pathway. J Immunol 172:6715–6722 [DOI] [PubMed] [Google Scholar]
- Ohno M, Hirata T, Enomoto M, Araki T, Ishimaru H, Takahashi TA. (2000) A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol Immunol 37:407–412 [DOI] [PubMed] [Google Scholar]
- Okada N, Sasaki A, Niwa M, Okada Y, Hatanaka Y, Tani Y, Mizuguchi H, Nakagawa S, Fujita T, Yamamoto A. (2006) Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther 13:393–405 [DOI] [PubMed] [Google Scholar]
- Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. (2005) Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol 3:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, Littman DR, Cyster JG. (2002) Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med 196:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, et al. (2003) C5L2, a nonsignaling C5A binding protein. Biochemistry 42:9406–9415 [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, et al. (2012) Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336:489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszyna DP, Florquin S, Sewnath M, Branger J, Speelman P, van Deventer SJ, Strieter RM, van der Poll T. (2001) CXC chemokine receptor 2 contributes to host defense in murine urinary tract infection. J Infect Dis 184:301–307 [DOI] [PubMed] [Google Scholar]
- Oostendorp J, Hylkema MN, Luinge M, Geerlings M, Meurs H, Timens W, Zaagsma J, Postma DS, Boddeke HW, Biber K. (2004) Localization and enhanced mRNA expression of the orphan chemokine receptor L-CCR in the lung in a murine model of ovalbumin-induced airway inflammation. J Histochem Cytochem 52:401–410 [DOI] [PubMed] [Google Scholar]
- Otero K, Vecchi A, Hirsch E, Kearley J, Vermi W, Del Prete A, Gonzalvo-Feo S, Garlanda C, Azzolino O, Salogni L, et al. (2010) Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood 116:2942–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, et al. (1997) Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 387:611–617 [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. (2001) The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest 107:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. (2000) The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol 165:5069–5076 [DOI] [PubMed] [Google Scholar]
- Park SH, Casagrande F, Cho L, Albrecht L, Opella SJ. (2011) Interactions of interleukin-8 with the human chemokine receptor CXCR1 in phospholipid bilayers by NMR spectroscopy. J Mol Biol 414:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Das BB, Casagrande F, Tian Y, Nothnagel HJ, Chu M, Kiefer H, Maier K, De Angelis AA, Marassi FM, et al. (2012) Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 491:779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C. (2010) A novel 3-D mineralized tumor model to study breast cancer bone metastasis. PLoS ONE 5:e8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S, Cavrois M, Guo HG, Foulke JS, Jr, Kim J, Feldman RA, Reitz M. (2001) Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J Virol 75:8660–8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, et al. (2010) Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11:1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease J, Horuk R. (2012) Chemokine receptor antagonists. J Med Chem 55:9363–9392 [DOI] [PubMed] [Google Scholar]
- Pease JE. (2006) Asthma, allergy and chemokines. Curr Drug Targets 7:3–12 [DOI] [PubMed] [Google Scholar]
- Pease JE. (2011) Targeting chemokine receptors in allergic disease. Biochem J 434:11–24 [DOI] [PubMed] [Google Scholar]
- Pease JE, Horuk R. (2009) Chemokine receptor antagonists: Part 1. Expert Opin Ther Pat 19:39–58 [DOI] [PubMed] [Google Scholar]
- Peiper SC, Wang ZX, Neote K, Martin AW, Showell HJ, Conklyn MJ, Ogborne K, Hadley TJ, Lu ZH, Hesselgesser J, et al. (1995) The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med 181:1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold ME, Dairaghi DJ, Duke GM, Saederup N, Mocarski ES, Kemble GW, Schall TJ. (1999) Cytomegalovirus encodes a potent alpha chemokine. Proc Natl Acad Sci USA 96:9839–9844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton-Rol G, Cota M, Polentarutti N, Luini W, Bernasconi S, Borsatti A, Sica A, LaRosa GJ, Sozzani S, Poli G, et al. (1999) Up-regulation of CCR2 chemokine receptor expression and increased susceptibility to the multitropic HIV strain 89.6 in monocytes exposed to glucocorticoid hormones. J Immunol 163:3524–3529 [PubMed] [Google Scholar]
- Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A, Sozzani S, Mantovani A. (1998) Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-gamma. J Immunol 160:3869–3873 [PubMed] [Google Scholar]
- Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, et al. (2011) A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 118:4853–4862 [DOI] [PubMed] [Google Scholar]
- Pereira JP, Kelly LM, Xu Y, Cyster JG. (2009) EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460:1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W, Cyster JG, Mack M, Schlöndorff D, Wolf AJ, Ernst JD, Charo IF. (2004) CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol 172:7647–7653 [DOI] [PubMed] [Google Scholar]
- Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. (2001) Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA 98:7958–7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. (1998) The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol 28:961–972 [DOI] [PubMed] [Google Scholar]
- Pleskoff O, Tréboute C, Brelot A, Heveker N, Seman M, Alizon M. (1997) Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science 276:1874–1878 [DOI] [PubMed] [Google Scholar]
- Polentarutti N, Allavena P, Bianchi G, Giardina G, Basile A, Sozzani S, Mantovani A, Introna M. (1997) IL-2-regulated expression of the monocyte chemotactic protein-1 receptor (CCR2) in human NK cells: characterization of a predominant 3.4-kilobase transcript containing CCR2B and CCR2A sequences. J Immunol 158:2689–2694 [PubMed] [Google Scholar]
- Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. (1996) Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med 183:2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. (2005a) Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem 280:13952–13961 [DOI] [PubMed] [Google Scholar]
- Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. (2005b) The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 175:5341–5350 [DOI] [PubMed] [Google Scholar]
- Postea O, Vasina EM, Cauwenberghs S, Projahn D, Liehn EA, Lievens D, Theelen W, Kramp BK, Butoi ED, Soehnlein O, et al. (2012) Contribution of platelet CX(3)CR1 to platelet-monocyte complex formation and vascular recruitment during hyperlipidemia. Arterioscler Thromb Vasc Biol 32:1186–1193 [DOI] [PubMed] [Google Scholar]
- Potteaux S, Combadière C, Esposito B, Lecureuil C, Ait-Oufella H, Merval R, Ardouin P, Tedgui A, Mallat Z. (2006) Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 26:1858–1863 [DOI] [PubMed] [Google Scholar]
- Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, Sozzani S, Mantovani A, Wells TN. (1997) Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med 186:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AE, Wells TN. (1995) Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem 270:19495–19500 [DOI] [PubMed] [Google Scholar]
- Procopiou PA, Ford AJ, Graves RH, Hall DA, Hodgson ST, Lacroix YM, Needham D, Slack RJ. (2012) Lead optimisation of the N1 substituent of a novel series of indazole arylsulfonamides as CCR4 antagonists and identification of a candidate for clinical investigation. Bioorg Med Chem Lett 22:2730–2733 [DOI] [PubMed] [Google Scholar]
- Proost P, Loos T, Mortier A, Schutyser E, Gouwy M, Noppen S, Dillen C, Ronsse I, Conings R, Struyf S, et al. (2008) Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J Exp Med 205:2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, Detheux M, Parmentier M, Durinx C, Lambeir AM, et al. (2001) Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 98:3554–3561 [DOI] [PubMed] [Google Scholar]
- Proudfoot AE, Power CA, Hoogewerf AJ, Montjovent MO, Borlat F, Offord RE, Wells TN. (1996) Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem 271:2599–2603 [DOI] [PubMed] [Google Scholar]
- Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, et al. (2009) The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol 10:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic I, DiPersio JF. (2010) Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol 17:319–326 [DOI] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. (1998) The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodrá J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. (2004) Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med 200:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C, Ren YQ, Xiang LH, Sun LD, Xu AE, Gao XH, Chen HD, Pu XM, Wu RN, Liang CZ, et al. (2010) Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet 42:614–618 [DOI] [PubMed] [Google Scholar]
- Quinones MP, Estrada CA, Kalkonde Y, Ahuja SK, Kuziel WA, Mack M, Ahuja SS. (2005) The complex role of the chemokine receptor CCR2 in collagen-induced arthritis: implications for therapeutic targeting of CCR2 in rheumatoid arthritis. J Mol Med (Berl) 83:672–681 [DOI] [PubMed] [Google Scholar]
- Raby AC, Holst B, Davies J, Colmont C, Laumonnier Y, Coles B, Shah S, Hall J, Topley N, Köhl J, et al. (2011) TLR activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur J Immunol 41:2741–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. (2006) Monocyte chemoattractant protein-1 correlates with subcortical brain injury in HIV infection. Neurology 66:1255–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar R, Murooka TT, Fish EN. (2009) Role for CCR5 in dissemination of vaccinia virus in vivo. J Virol 83:2226–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. (2010) Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA 107:628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Neel NF, Sai J, Mernaugh RL, Ham AJ, Richmond AJ. (2009) Characterization of chemokine receptor CXCR2 interacting proteins using a proteomics approach to define the CXCR2 “chemosynapse”. Methods Enzymol 460:315–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul W, Auvynet C, Camelo S, Guillonneau X, Feumi C, Combadière C, Sennlaub F. (2010) CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J Neuroinflammation 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. (1996) Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem 271:17161–17166 [DOI] [PubMed] [Google Scholar]
- Raport CJ, Schweickart VL, Eddy RL, Jr, Shows TB, Gray PW. (1995) The orphan G-protein-coupled receptor-encoding gene V28 is closely related to genes for chemokine receptors and is expressed in lymphoid and neural tissues. Gene 163:295–299 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011) Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 469:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravensberg AJ, Ricciardolo FL, van Schadewijk A, Rabe KF, Sterk PJ, Hiemstra PS, Mauad T. (2005) Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J Allergy Clin Immunol 115:779–785 [DOI] [PubMed] [Google Scholar]
- Ravindran R, Rusch L, Itano A, Jenkins MK, McSorley SJ. (2007) CCR6-dependent recruitment of blood phagocytes is necessary for rapid CD4 T cell responses to local bacterial infection. Proc Natl Acad Sci USA 104:12075–12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10:514–523 [DOI] [PubMed] [Google Scholar]
- Reeves JD, McKnight A, Potempa S, Simmons G, Gray PW, Power CA, Wells T, Weiss RA, Talbot SJ. (1997) CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR-3, and V28 for entry. Virology 231:130–134 [DOI] [PubMed] [Google Scholar]
- Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Förster R, Cyster JG. (2002) Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416:94–99 [DOI] [PubMed] [Google Scholar]
- Reutershan J, Harry B, Chang D, Bagby GJ, Ley K. (2009) DARC on RBC limits lung injury by balancing compartmental distribution of CXC chemokines. Eur J Immunol 39:1597–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A. (2008) CCR9 homes metastatic melanoma cells to the small bowel. Clin Cancer Res 14:621–623 [DOI] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Köhl J, et al. (2008) Functional roles for C5a receptors in sepsis. Nat Med 14:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner HL, Brack A, Stein C. (2008) Pain and the immune system. Br J Anaesth 101:40–44 [DOI] [PubMed] [Google Scholar]
- Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. (2004) Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med 200:725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL. (2012) Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209:139–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, et al. (2008) Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol 9:R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MJ. (2002) Role of chemokines in the biology of natural killer cells. J Leukoc Biol 71:173–183 [PubMed] [Google Scholar]
- Robinson LA, Nataraj C, Thomas DW, Cosby JM, Griffiths R, Bautch VL, Patel DD, Coffman TM. (2003) The chemokine CX3CL1 regulates NK cell activity in vivo. Cell Immunol 225:122–130 [DOI] [PubMed] [Google Scholar]
- Robinson LA, Nataraj C, Thomas DW, Howell DN, Griffiths R, Bautch V, Patel DD, Feng L, Coffman TM. (2000) A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. J Immunol 165:6067–6072 [DOI] [PubMed] [Google Scholar]
- Rodero M, Marie Y, Coudert M, Blondet E, Mokhtari K, Rousseau A, Raoul W, Carpentier C, Sennlaub F, Deterre P, et al. (2008) Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol 26:5957–5964 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Frade JM, Vila-Coro AJ, de Ana AM, Albar JP, Martínez-A C, Mellado M. (1999) The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc Natl Acad Sci USA 96:3628–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos RS, Loetscher M, Legler DF, Clark-Lewis I, Baggiolini M, Moser B. (1997) Identification of CCR8, the receptor for the human CC chemokine I-309. J Biol Chem 272:17251–17254 [DOI] [PubMed] [Google Scholar]
- Rosenblum JM, Zhang QW, Siu G, Collins TL, Sullivan T, Dairaghi DJ, Medina JC, Fairchild RL. (2009) CXCR3 antagonism impairs the development of donor-reactive, IFN-gamma-producing effectors and prolongs allograft survival. Transplantation 87:360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde MM, Andersen MB, Nygaard R, Frimurer TM, Schwartz TW. (2007) Activation of the CXCR3 chemokine receptor through anchoring of a small molecule chelator ligand between TM-III, -IV, and -VI. Mol Pharmacol 71:930–941 [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Bräuner-Osborne H, Schwartz TW. (1999) Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J Biol Chem 274:956–961 [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Holst PJ, Schwartz TW. (2000) Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded seven transmembrane oncogene ORF74. J Biol Chem 275:26309–26315 [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Schwartz TW. (2005) High constitutive activity of a virus-encoded 7TM receptor in the absence of the conserved DRY-motif (Asp-Arg-Tyr) in transmembrane helix 3. Mol Pharmacol 66:11–19 [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, McLean KA, Holst PJ, Schwartz TW. (2004) The CXC chemokine receptor encoded by herpesvirus saimiri, ECRF3, shows ligand-regulated signaling through Gi, Gq, and G12/13 proteins but constitutive signaling only through Gi and G12/13 proteins. J Biol Chem 279:32524–32533 [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. (2004) The chemokine system — a major regulator of angiogenesis in health and disease. APMIS 112:481–495 [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Waldhoer M, Lüttichau HR, Schwartz TW. (2001) Virally encoded 7TM receptors. Oncogene 20:1582–1593 [DOI] [PubMed] [Google Scholar]
- Rot A. (1992) Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today 13:291–294 [DOI] [PubMed] [Google Scholar]
- Rot A. (2005) Contribution of Duffy antigen to chemokine function. Cytokine Growth Factor Rev 16:687–694 [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. (2006) The eosinophil. Annu Rev Immunol 24:147–174 [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. (1997) Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med 185:785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman JB, Slavin AJ, Silva R, Weiner HL, Gerard CG, Hancock WW. (2000) Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur J Immunol 30:2372–2377 [DOI] [PubMed] [Google Scholar]
- Rubbert A, Combadiere C, Ostrowski M, Arthos J, Dybul M, Machado E, Cohn MA, Hoxie JA, Murphy PM, Fauci AS, et al. (1998) Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. J Immunol 160:3933–3941 [PubMed] [Google Scholar]
- Rucker J, Edinger AL, Sharron M, Samson M, Lee B, Berson JF, Yi Y, Margulies B, Collman RG, Doranz BJ, et al. (1997) Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol 71:8999–9007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker J, Samson M, Doranz BJ, Libert F, Berson JF, Yi Y, Smyth RJ, Collman RG, Broder CC, Vassart G, et al. (1996) Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell 87:437–446 [DOI] [PubMed] [Google Scholar]
- Ruckes T, Saul D, Van Snick J, Hermine O, Grassmann R. (2001) Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 98:1150–1159 [DOI] [PubMed] [Google Scholar]
- Rueda P, Balabanian K, Lagane B, Staropoli I, Chow K, Levoye A, Laguri C, Sadir R, Delaunay T, Izquierdo E, et al. (2008) The CXCL12gamma chemokine displays unprecedented structural and functional properties that make it a paradigm of chemoattractant proteins. PLoS ONE 3:e2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda P, Richart A, Récalde A, Gasse P, Vilar J, Guérin C, Lortat-Jacob H, Vieira P, Baleux F, Chretien F, et al. (2012) Homeostatic and tissue reparation defaults in mice carrying selective genetic invalidation of CXCL12/proteoglycan interactions. Circulation 126:1882–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz ME, Cicala C, Arthos J, Kinter A, Catanzaro AT, Adelsberger J, Holmes KL, Cohen OJ, Fauci AS. (1998) Peripheral blood-derived CD34+ progenitor cells: CXC chemokine receptor 4 and CC chemokine receptor 5 expression and infection by HIV. J Immunol 161:4169–4176 [PubMed] [Google Scholar]
- Ruiz-Argüello MB, Smith VP, Campanella GS, Baleux F, Arenzana-Seisdedos F, Luster AD, Alcami A. (2008) An ectromelia virus protein that interacts with chemokines through their glycosaminoglycan binding domain. J Virol 82:917–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel PC, Arfelt KN, Baumann L, Jenkins TJ, Thiele S, Lüttichau HR, Johnsen A, Pease J, Ghosh S, Kolbeck R, et al. (2012) Molecular requirements for inhibition of the chemokine receptor CCR8—probe-dependent allosteric interactions. Br J Pharmacol 167:1206–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RC, Alessandri AL, Garcia CC, Cordeiro BF, Pinho V, Cassali GD, Proudfoot AE, Teixeira MM. (2011) Therapeutic effects of evasin-1, a chemokine binding protein, in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 45:72–80 [DOI] [PubMed] [Google Scholar]
- Saccani A, Saccani S, Orlando S, Sironi M, Bernasconi S, Ghezzi P, Mantovani A, Sica A. (2000) Redox regulation of chemokine receptor expression. Proc Natl Acad Sci USA 97:2761–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadik CD, Kim ND, Iwakura Y, Luster AD. (2012) Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc Natl Acad Sci USA 109:E3177–E3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H, Moore AM, Brown MJ, Hwang ST. (1999) Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol 162:2472–2475 [PubMed] [Google Scholar]
- Sai J, Walker G, Wikswo J, Richmond A. (2006) The IL sequence in the LLKIL motif in CXCR2 is required for full ligand-induced activation of Erk, Akt, and chemotaxis in HL60 cells. J Biol Chem 281:35931–35941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini V, Staren DM, Ziarek JJ, Nashaat ZN, Campbell EM, Volkman BF, Marchese A, Majetschak M. (2011) The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1α function through distinct receptor interactions. J Biol Chem 286:33466–33477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Kuboki S, Van Sweringen HL, Tevar AD, Schuster R, Blanchard J, Edwards MJ, Lentsch AB. (2011) CXCR1 deficiency does not alter liver regeneration after partial hepatectomy in mice. Transplant Proc 43:1967–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, et al. (2006) CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity 24:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, Oppenheim JJ. (2001) Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol 166:7571–7578 [DOI] [PubMed] [Google Scholar]
- Salchow K, Bond ME, Evans SC, Press NJ, Charlton SJ, Hunt PA, Bradley ME. (2010) A common intracellular allosteric binding site for antagonists of the CXCR2 receptor. Br J Pharmacol 159:1429–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. (2000) Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev 177:134–140 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Mackay CR. (1998a) Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today 19:568–574 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. (1998b) Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 187:875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. (1998c) Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 28:2760–2769 [DOI] [PubMed] [Google Scholar]
- Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N. (2002) Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol 169:2685–2693 [DOI] [PubMed] [Google Scholar]
- Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. (1996a) Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 35:3362–3367 [DOI] [PubMed] [Google Scholar]
- Samson M, LaRosa G, Libert F, Paindavoine P, Detheux M, Vassart G, Parmentier M. (1997) The second extracellular loop of CCR5 is the major determinant of ligand specificity. J Biol Chem 272:24934–24941 [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. (1996b) Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. see comments Nature 382:722–725 [DOI] [PubMed] [Google Scholar]
- Samson M, Stordeur P, Labbé O, Soularue P, Vassart G, Parmentier M. (1996c) Molecular cloning and chromosomal mapping of a novel human gene, ChemR1, expressed in T lymphocytes and polymorphonuclear cells and encoding a putative chemokine receptor. Eur J Immunol 26:3021–3028 [DOI] [PubMed] [Google Scholar]
- Sánchez-Alcañiz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, López-Bendito G, Stumm R, Marín O. (2011) Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron 69:77–90 [DOI] [PubMed] [Google Scholar]
- Sanders SK, Crean SM, Boxer PA, Kellner D, LaRosa GJ, Hunt SW., 3rd (2000) Functional differences between monocyte chemotactic protein-1 receptor A and monocyte chemotactic protein-1 receptor B expressed in a Jurkat T cell. J Immunol 165:4877–4883 [DOI] [PubMed] [Google Scholar]
- Santella JB, 3rd, Gardner DS, Yao W, Shi C, Reddy P, Tebben AJ, DeLucca GV, Wacker DA, Watson PS, Welch PK, et al. (2008) From rigid cyclic templates to conformationally stabilized acyclic scaffolds. Part I: the discovery of CCR3 antagonist development candidate BMS-639623 with picomolar inhibition potency against eosinophil chemotaxis. Bioorg Med Chem Lett 18:576–585 [DOI] [PubMed] [Google Scholar]
- Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. (1997) Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med 185:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. (2007) Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med 204:1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. (2000) CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J Exp Med 192:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Aranami T, Yamamura T. (2007) Cutting edge: Human Th17 cells are identified as bearing CCR2+CCR5- phenotype. J Immunol 178:7525–7529 [DOI] [PubMed] [Google Scholar]
- Savino B, Borroni EM, Torres NM, Proost P, Struyf S, Mortier A, Mantovani A, Locati M, Bonecchi R. (2009) Recognition versus adaptive up-regulation and degradation of CC chemokines by the chemokine decoy receptor D6 are determined by their N-terminal sequence. J Biol Chem 284:26207–26215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, Moser B. (2004) A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med 199:1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall TJ, Proudfoot AE. (2011) Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol 11:355–363 [DOI] [PubMed] [Google Scholar]
- Schecter AD, Calderon TM, Berman AB, McManus CM, Fallon JT, Rossikhina M, Zhao W, Christ G, Berman JW, Taubman MB. (2000) Human vascular smooth muscle cells possess functional CCR5. J Biol Chem 275:5466–5471 [DOI] [PubMed] [Google Scholar]
- Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, et al. (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel RB, Baumert J, Barbalic M, Dupuis J, Ellinor PT, Durda P, Dehghan A, Bis JC, Illig T, Morrison AC, et al. (2010) Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood 115:5289–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. (2007) CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med 204:735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten DJ, Canals M, Maussang D, Roumen L, Smit MJ, Wijtmans M, de Graaf C, Vischer HF, Leurs R. (2012) Pharmacological modulation of chemokine receptor function. Br J Pharmacol 165:1617–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. (2008) Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol 510:207–220 [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Zhao M, Khaldoyanidi SK, Discipio RG. (2012) The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 135:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh JM, Blease K, Hogaboam CM. (2002) The role of CC chemokine receptor 5 (CCR5) and RANTES/CCL5 during chronic fungal asthma in mice. FASEB J 16:228–230 [DOI] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. (2009) Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206:3101–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann K, Lämmermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Förster R, Lutz MB, Sorokin L, et al. (2010) Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32:703–713 [DOI] [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. (2003) The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev 14:409–426 [DOI] [PubMed] [Google Scholar]
- Schwarz M, Murphy PM. (2001) Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol 167:505–513 [DOI] [PubMed] [Google Scholar]
- Schweickart VL, Epp A, Raport CJ, Gray PW. (2000) CCR11 is a functional receptor for the monocyte chemoattractant protein family of chemokines. J Biol Chem 275:9550–9556 [DOI] [PubMed] [Google Scholar]
- Sciumè G, De Angelis G, Benigni G, Ponzetta A, Morrone S, Santoni A, Bernardini G. (2011) CX3CR1 expression defines 2 KLRG1+ mouse NK-cell subsets with distinct functional properties and positioning in the bone marrow. Blood 117:4467–4475 [DOI] [PubMed] [Google Scholar]
- Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. (2009) The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol 46:1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Wong J, Villadangos JA. (2009) Cutting edge: B220+CCR9- dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol 183:1514–1517 [DOI] [PubMed] [Google Scholar]
- Seibert C, Veldkamp CT, Peterson FC, Chait BT, Volkman BF, Sakmar TP. (2008) Sequential tyrosine sulfation of CXCR4 by tyrosylprotein sulfotransferases. Biochemistry 47:11251–11262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Kossmann T, Morganti-Kossmann MC. (2010) Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab 30:459–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7:311–317 [DOI] [PubMed] [Google Scholar]
- Severin IC, Gaudry JP, Johnson Z, Kungl A, Jansma A, Gesslbauer B, Mulloy B, Power C, Proudfoot AE, Handel T. (2010) Characterization of the chemokine CXCL11-heparin interaction suggests two different affinities for glycosaminoglycans. J Biol Chem 285:17713–17724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, et al. (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17:51–62 [DOI] [PubMed] [Google Scholar]
- Sharma B, Singh S, Varney ML, Singh RK. (2010) Targeting CXCR1/CXCR2 receptor antagonism in malignant melanoma. Expert Opin Ther Targets 14:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrack B, Leach T, Jacobson E, Donaldson DD, Xu X, Hu M. (2007) Frequent MRI study of a novel CCR2 antagonist in relapsing-remitting multiple sclerosis. Ann Neurol 62 (Suppl. 11):S74 [Google Scholar]
- Sharron M, Pöhlmann S, Price K, Lolis E, Tsang M, Kirchhoff F, Doms RW, Lee B. (2000) Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 96:41–49 [PubMed] [Google Scholar]
- Shea-Donohue T, Thomas K, Cody MJ, Aiping Zhao, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. (2008) Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun 14:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard LW, Yang M, Xie P, Browning DD, Voyno-Yasenetskaya T, Kozasa T, Ye RD. (2001) Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J Biol Chem 276:45979–45987 [DOI] [PubMed] [Google Scholar]
- Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. (1999) Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol 163:6236–6243 [PubMed] [Google Scholar]
- Shimada T, Matsumoto M, Tatsumi Y, Kanamaru A, Akira S. (1998) A novel lipopolysaccharide inducible C-C chemokine receptor related gene in murine macrophages. FEBS Lett 425:490–494 [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, Yonehara S. (2000) Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem 275:40663–40666 [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Fukumoto N, Kume N, Takahashi S, Yamaguchi J, Minami M, Hayashida K, Kita T, Ohsumi J, et al. (2004) Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol 75:267–274 [DOI] [PubMed] [Google Scholar]
- Shin N, Solomon K, Zhou N, Wang KH, Garlapati V, Thomas B, Li Y, Covington M, Baribaud F, Erickson-Viitanen S, et al. (2011) Identification and characterization of INCB9471, an allosteric noncompetitive small-molecule antagonist of C-C chemokine receptor 5 with potent inhibitory activity against monocyte migration and HIV-1 infection. J Pharmacol Exp Ther 338:228–239 [DOI] [PubMed] [Google Scholar]
- Sica A, Saccani A, Borsatti A, Power CA, Wells TN, Luini W, Polentarutti N, Sozzani S, Mantovani A. (1997) Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med 185:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierro F, Biben C, Martínez-Muñoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, et al. (2007) Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA 104:14759–14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TN, Proudfoot AE. (1997) Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276–279 [DOI] [PubMed] [Google Scholar]
- Singh MD, King V, Baldwin H, Burden D, Thorrat A, Holmes S, McInnes IB, Nicoll R, Shams K, Pallas K, et al. (2012) Elevated expression of the chemokine-scavenging receptor D6 is associated with impaired lesion development in psoriasis. Am J Pathol 181:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. (2008a) Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 180:214–221 [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW., Jr (2008b) CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(-/-) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res 28:31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, Lira SA, Söderberg-Nauclér C, Smit MJ. (2010) HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal 3:ra58. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, Tensen CP. (2003) CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 102:1959–1965 [DOI] [PubMed] [Google Scholar]
- Smit MJ, Verzijl D, Casarosa P, Navis M, Timmerman H, Leurs R. (2002) Kaposi’s sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways. J Virol 76:1744–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MW, Carrington M, Winkler C, Lomb D, Dean M, Huttley G, O’Brien SJ. (1997) CCR2 chemokine receptor and AIDS progression. Nat Med 3:1052–1053 [DOI] [PubMed] [Google Scholar]
- Smith P, Fallon RE, Mangan NE, Walsh CM, Saraiva M, Sayers JR, McKenzie AN, Alcami A, Fallon PG. (2005) Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med 202:1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove RJ. (2011) Targeting of a common receptor shared by CXCL8 and N-Ac-PGP as a therapeutic strategy to alleviate chronic neutrophilic lung diseases. Eur J Pharmacol 667:1–5 [DOI] [PubMed] [Google Scholar]
- Sogawa Y, Ohyama T, Maeda H, Hirahara K. (2011) Inhibition of neutrophil migration in mice by mouse formyl peptide receptors 1 and 2 dual agonist: indication of cross-desensitization in vivo. Immunology 132:441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohy D, Parmentier M, Springael JY. (2007) Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem 282:30062–30069 [DOI] [PubMed] [Google Scholar]
- Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, Parmentier M, Springael JY. (2009) Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem 284:31270–31279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, Abuzahra F, Bowman E, Roszkiewicz J, Bieber T, et al. (2005) Circulating clonal CLA(+) and CD4(+) T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol 152:258–264 [DOI] [PubMed] [Google Scholar]
- Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. (2003) Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol 171:6267–6274 [DOI] [PubMed] [Google Scholar]
- Soler D, Chapman TR, Poisson LR, Wang L, Cote-Sierra J, Ryan M, McDonald A, Badola S, Fedyk E, Coyle AJ, et al. (2006) CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol 177:6940–6951 [DOI] [PubMed] [Google Scholar]
- Soler D, Humphreys TL, Spinola SM, Campbell JJ. (2003) CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood 101:1677–1682 [DOI] [PubMed] [Google Scholar]
- Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, Greene M, Soteropoulos P, Cobbs CS. (2011) Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res 71:6643–6653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soruri A, Grigat J, Forssmann U, Riggert J, Zwirner J. (2007) beta-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol 37:2474–2486 [DOI] [PubMed] [Google Scholar]
- Soto H, Wang W, Strieter RM, Copeland NG, Gilbert DJ, Jenkins NA, Hedrick J, Zlotnik A. (1998) The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci USA 95:8205–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Allavena P, Vecchi A, Mantovani A. (1999) The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol 66:1–9 [DOI] [PubMed] [Google Scholar]
- Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells TN, et al. (1998) Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med 187:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmann A, Zarbock A. (2012) CXCR2: from bench to bedside. Front Immunol 3:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al. (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, Velden J, Hopfer H, Fehr S, Krieger T, et al. (2009) CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol 183:4693–4704 [DOI] [PubMed] [Google Scholar]
- Stievano L, Tosello V, Marcato N, Rosato A, Sebelin A, Chieco-Bianchi L, Amadori A. (2003) CD8+ alpha beta+ T cells that lack surface CD5 antigen expression are a major lymphotactin (XCL1) source in peripheral blood lymphocytes. J Immunol 171:4528–4538 [DOI] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. (2009) The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol 86:529–543 [DOI] [PubMed] [Google Scholar]
- Stine JT, Wood C, Hill M, Epp A, Raport CJ, Schweickart VL, Endo Y, Sasaki T, Simmons G, Boshoff C, et al. (2000) KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood 95:1151–1157 [PubMed] [Google Scholar]
- Streblow DN, Orloff SL, Nelson JA. (2001) Do pathogens accelerate atherosclerosis? J Nutr 131:2798S–2804S [DOI] [PubMed] [Google Scholar]
- Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, Mattison K, Altschuler Y, Nelson JA. (1999) The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511–520 [DOI] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. (1995) The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270:27348–27357 [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, et al. (2002) Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99:4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. (2005) Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol 175:5396–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. (2010) CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 29:709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. (2012) Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 150:1249–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Sawa H, Kobayashi Y, Nakata Y, Nakagawa Ki, Uzawa A, Sakiyama H, Kakinuma S, Iwabuchi K, Nagashima K. (1999) Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J Immunol 162:5981–5985 [PubMed] [Google Scholar]
- Swartzendruber JA, Byrne AJ, Bryce PJ. (2012) Cutting edge: histamine is required for IL-4-driven eosinophilic allergic responses. J Immunol 188:536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. (2007) Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo MC, Soo KS, Zlotnik A, Schall TJ. (1995) Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J Biol Chem 270:25348–25351 [DOI] [PubMed] [Google Scholar]
- Szalai C, Duba J, Prohászka Z, Kalina A, Szabó T, Nagy B, Horváth L, Császár A. (2001) Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1 -2518 G/G genotype in CAD patients. Atherosclerosis 158:233–239 [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. (1998) The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393:591–594 [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. (2007) Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagaki K, Nakano K, Yamanishi K. (2005) Human herpesvirus 7 open reading frames U12 and U51 encode functional beta-chemokine receptors. J Virol 79:7068–7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagaki K, Yamanishi K, Mori Y. (2007) Reciprocal roles of cellular chemokine receptors and human herpesvirus 7-encoded chemokine receptors, U12 and U51. J Gen Virol 88:1423–1428 [DOI] [PubMed] [Google Scholar]
- Tagat JR, McCombie SW, Nazareno D, Labroli MA, Xiao Y, Steensma RW, Strizki JM, Baroudy BM, Cox K, Lachowicz J, et al. (2004) Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl]ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J Med Chem 47:2405–2408 [DOI] [PubMed] [Google Scholar]
- Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, et al. (2004) Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol 31:395–404 [DOI] [PubMed] [Google Scholar]
- Takata H, Tomiyama H, Fujiwara M, Kobayashi N, Takiguchi M. (2004) Cutting edge: expression of chemokine receptor CXCR1 on human effector CD8+ T cells. J Immunol 173:2231–2235 [DOI] [PubMed] [Google Scholar]
- Takatsuka S, Sekiguchi A, Tokunaga M, Fujimoto A, Chiba J. (2011) Generation of a panel of monoclonal antibodies against atypical chemokine receptor CCX-CKR by DNA immunization. J Pharmacol Toxicol Methods 63:250–257 [DOI] [PubMed] [Google Scholar]
- Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, et al. (2009) CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 460:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Zhu Y, Li J, Chen Z, Han GW, Kufareva I, Li T, Ma L, Fenalti G, Li J, et al. (2013) Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 341:1387–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HL, Cyster JG. (1999) Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science 284:819–822 [DOI] [PubMed] [Google Scholar]
- Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, et al. (2010) Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol 184:2677–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone L, Moratto D, Vermi W, De Francesco M, Notarangelo LD, Porta F, Lougaris V, Facchetti F, Plebani A, Badolato R. (2010) Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood 116:4870–4873 [DOI] [PubMed] [Google Scholar]
- Telford EA, Watson MS, Aird HC, Perry J, Davison AJ. (1995) The DNA sequence of equine herpesvirus 2. J Mol Biol 249:520–528 [DOI] [PubMed] [Google Scholar]
- Terashima Y, Onai N, Murai M, Enomoto M, Poonpiriya V, Hamada T, Motomura K, Suwa M, Ezaki T, Haga T, et al. (2005) Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat Immunol 6:827–835 [DOI] [PubMed] [Google Scholar]
- Thapa M, Carr DJ. (2009) CXCR3 deficiency increases susceptibility to genital herpes simplex virus type 2 infection: Uncoupling of CD8+ T-cell effector function but not migration. J Virol 83:9486–9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa M, Kuziel WA, Carr DJ. (2007) Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J Virol 81:3704–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa M, Welner RS, Pelayo R, Carr DJ. (2008) CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol 180:1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M. (2001) Dancing to the tune of chemokines. Nat Immunol 2:129–134 [DOI] [PubMed] [Google Scholar]
- Thelen M, Muñoz LM, Rodríguez-Frade JM, Mellado M. (2010) Chemokine receptor oligomerization: functional considerations. Curr Opin Pharmacol 10:38–43 [DOI] [PubMed] [Google Scholar]
- Thelen M, Stein JV. (2008) How chemokines invite leukocytes to dance. Nat Immunol 9:953–959 [DOI] [PubMed] [Google Scholar]
- Thelen M, Thelen S. (2008) CXCR7, CXCR4 and CXCL12: an eccentric trio? J Neuroimmunol 198:9–13 [DOI] [PubMed] [Google Scholar]
- Thoma G, Streiff MB, Kovarik J, Glickman F, Wagner T, Beerli C, Zerwes HG. (2008) Orally bioavailable isothioureas block function of the chemokine receptor CXCR4 in vitro and in vivo. J Med Chem 51:7915–7920 [DOI] [PubMed] [Google Scholar]
- Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, Strominger JL, Brenner MB, Gumperz JE, Wilson SB, et al. (2003) CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol 171:2571–2580 [DOI] [PubMed] [Google Scholar]
- Tian Y, New DC, Yung LY, Allen RA, Slocombe PM, Twomey BM, Lee MM, Wong YH. (2004) Differential chemokine activation of CC chemokine receptor 1-regulated pathways: ligand selective activation of Galpha 14-coupled pathways. Eur J Immunol 34:785–795 [DOI] [PubMed] [Google Scholar]
- Tiffany HL, Lautens LL, Gao JL, Pease J, Locati M, Combadiere C, Modi W, Bonner TI, Murphy PM. (1997) Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J Exp Med 186:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournamille C, Colin Y, Cartron JP, Le Van Kim C. (1995) Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet 10:224–228 [DOI] [PubMed] [Google Scholar]
- Tournamille C, Filipe A, Wasniowska K, Gane P, Lisowska E, Cartron JP, Colin Y, Le Van Kim C. (2003) Structure-function analysis of the extracellular domains of the Duffy antigen/receptor for chemokines: characterization of antibody and chemokine binding sites. Br J Haematol 122:1014–1023 [DOI] [PubMed] [Google Scholar]
- Tournamille C, Le Van Kim C, Gane P, Blanchard D, Proudfoot AE, Cartron JP, Colin Y. (1997) Close association of the first and fourth extracellular domains of the Duffy antigen/receptor for chemokines by a disulfide bond is required for ligand binding. J Biol Chem 272:16274–16280 [DOI] [PubMed] [Google Scholar]
- Townson JR, Nibbs RJ. (2002) Characterization of mouse CCX-CKR, a receptor for the lymphocyte-attracting chemokines TECK/mCCL25, SLC/mCCL21 and MIP-3beta/mCCL19: comparison to human CCX-CKR. Eur J Immunol 32:1230–1241 [DOI] [PubMed] [Google Scholar]
- Tran EH, Kuziel WA, Owens T. (2000) Induction of experimental autoimmune encephalomyelitis in C57BL/6 mice deficient in either the chemokine macrophage inflammatory protein-1alpha or its CCR5 receptor. Eur J Immunol 30:1410–1415 [DOI] [PubMed] [Google Scholar]
- Traves SL, Smith SJ, Barnes PJ, Donnelly LE. (2004) Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. J Leukoc Biol 76:441–450 [DOI] [PubMed] [Google Scholar]
- Trebst C, Sørensen TL, Kivisäkk P, Cathcart MK, Hesselgesser J, Horuk R, Sellebjerg F, Lassmann H, Ransohoff RM. (2001) CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am J Pathol 159:1701–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. (2009) Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 10:864–871 [DOI] [PubMed] [Google Scholar]
- Tripp RA, Hamilton-Easton AM, Cardin RD, Nguyen P, Behm FG, Woodland DL, Doherty PC, Blackman MA. (1997) Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J Exp Med 185:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. (2001) CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738 [DOI] [PubMed] [Google Scholar]
- Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. (1996) CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187 [DOI] [PubMed] [Google Scholar]
- Tuinstra RL, Peterson FC, Kutlesa S, Elgin ES, Kron MA, Volkman BF. (2008) Interconversion between two unrelated protein folds in the lymphotactin native state. Proc Natl Acad Sci USA 105:5057–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. (2004) The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J 18:1297–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, et al. (2005) CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med 11:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Suto H, Ra C, Ogawa H, Kobata T, Okumura K. (2002) Preferential expression of T(h)2-type chemokine and its receptor in atopic dermatitis. Int Immunol 14:1431–1438 [DOI] [PubMed] [Google Scholar]
- Uehara S, Grinberg A, Farber JM, Love PE. (2002) A role for CCR9 in T lymphocyte development and migration. J Immunol 168:2811–2819 [DOI] [PubMed] [Google Scholar]
- Ueland JM, Gwira J, Liu ZX, Cantley LG. (2004) The chemokine KC regulates HGF-stimulated epithelial cell morphogenesis. Am J Physiol Renal Physiol 286:F581–F589 [DOI] [PubMed] [Google Scholar]
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 6:3282–3289 [PubMed] [Google Scholar]
- Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, Rao P, Ponath PD, Baggiolini M, Dahinden CA. (1997) High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest 100:1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutmaz D, Xiang W, Sunshine MJ, Campbell J, Butcher E, Littman DR. (2000) The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol 165:3284–3292 [DOI] [PubMed] [Google Scholar]
- Upadhyaya B, Yin Y, Hill BJ, Douek DC, Prussin C. (2011) Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J Immunol 187:3111–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. (2010) Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11:636–646 [DOI] [PubMed] [Google Scholar]
- Valentin G, Haas P, Gilmour D. (2007) The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol 17:1026–1031 [DOI] [PubMed] [Google Scholar]
- Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, Pozzi S, Breitkreutz I, Kiziltepe T, Yasui H, et al. (2007) MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood 110:3744–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkel V, Barrett J, Tiffany HL, Fremont DH, Murphy PM, McFadden G, Speck SH, Virgin HW IV. (2000) Identification of a gammaherpesvirus selective chemokine binding protein that inhibits chemokine action. J Virol 74:6741–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancová I, Hajnická V, Slovák M, Kocáková P, Paesen GC, Nuttall PA. (2010) Evasin-3-like anti-chemokine activity in salivary gland extracts of ixodid ticks during blood-feeding: a new target for tick control. Parasite Immunol 32:460–463 [DOI] [PubMed] [Google Scholar]
- Vancová I, Slovák M, Hajnická V, Labuda M, Simo L, Peterková K, Hails RS, Nuttall PA. (2007) Differential anti-chemokine activity of Amblyomma variegatum adult ticks during blood-feeding. Parasite Immunol 29:169–177 [DOI] [PubMed] [Google Scholar]
- van der Voort R, Kramer M, Lindhout E, Torensma R, Eleveld D, van Lieshout AW, Looman M, Ruers T, Radstake TR, Figdor CG, et al. (2005) Novel monoclonal antibodies detect elevated levels of the chemokine CCL18/DC-CK1 in serum and body fluids in pathological conditions. J Leukoc Biol 77:739–747 [DOI] [PubMed] [Google Scholar]
- Van Lith LH, Oosterom J, Van Elsas A, Zaman GJ. (2009) C5a-stimulated recruitment of beta-arrestin2 to the nonsignaling 7-transmembrane decoy receptor C5L2. J Biomol Screen 14:1067–1075 [DOI] [PubMed] [Google Scholar]
- Van Snick J, Houssiau F, Proost P, Van Damme J, Renauld JC. (1996) I-309/T cell activation gene-3 chemokine protects murine T cell lymphomas against dexamethasone-induced apoptosis. J Immunol 157:2570–2576 [PubMed] [Google Scholar]
- Varona R, Cadenas V, Gómez L, Martínez-A C, Márquez G. (2005) CCR6 regulates CD4+ T-cell-mediated acute graft-versus-host disease responses. Blood 106:18–26 [DOI] [PubMed] [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutiérrez J, Torres M, Martínez-A C, Márquez G. (2001) CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest 107:R37–R45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillard NR, Braunersreuther V, Arnaud C, Burger F, Pelli G, Steffens S, Mach F. (2006) Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis 188:51–58 [DOI] [PubMed] [Google Scholar]
- Vergunst CE, Gerlag DM, Lopatinskaya L, Klareskog L, Smith MD, van den Bosch F, Dinant HJ, Lee Y, Wyant T, Jacobson EW, et al. (2008) Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 58:1931–1939 [DOI] [PubMed] [Google Scholar]
- Vergunst CE, Gerlag DM, von Moltke L, Karol M, Wyant T, Chi X, Matzkin E, Leach T, Tak PP. (2009) MLN3897 plus methotrexate in patients with rheumatoid arthritis: safety, efficacy, pharmacokinetics, and pharmacodynamics of an oral CCR1 antagonist in a phase IIa, double-blind, placebo-controlled, randomized, proof-of-concept study. Arthritis Rheum 60:3572–3581 [DOI] [PubMed] [Google Scholar]
- Verzijl D, Fitzsimons CP, Van Dijk M, Stewart JP, Timmerman H, Smit MJ, Leurs R. (2004) Differential activation of murine herpesvirus 68- and Kaposi’s sarcoma-associated herpesvirus-encoded ORF74 G protein-coupled receptors by human and murine chemokines. J Virol 78:3343–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard C, Yoneyama H, Murai M, Nakamura K, Tamaki K, Terashima Y, Imai T, Yoshie O, Irimura T, Mizutani H, et al. (1999) Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J Clin Invest 104:1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, et al. (2010) The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut 59:197–206 [DOI] [PubMed] [Google Scholar]
- Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, et al. (1997) TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity 7:291–301 [DOI] [PubMed] [Google Scholar]
- Vieira AT, Fagundes CT, Alessandri AL, Castor MG, Guabiraba R, Borges VO, Silveira KD, Vieira EL, Gonçalves JL, Silva TA, et al. (2009) Treatment with a novel chemokine-binding protein or eosinophil lineage-ablation protects mice from experimental colitis. Am J Pathol 175:2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, Schall TJ, Corey L, Geballe AP. (1998) Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J Virol 72:8158–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo-Borbolla A, Martinez-Martín N, Nel HJ, Rueda P, Martín R, Blanco S, Arenzana-Seisdedos F, Thelen M, Fallon PG, Alcamí A. (2012) Enhancement of chemokine function as an immunomodulatory strategy employed by human herpesviruses. PLoS Pathog 8:e1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayanand P, Durkin K, Hartmann G, Morjaria J, Seumois G, Staples KJ, Hall D, Bessant C, Bartholomew M, Howarth PH, et al. (2010) Chemokine receptor 4 plays a key role in T cell recruitment into the airways of asthmatic patients. J Immunol 184:4568–4574 [DOI] [PubMed] [Google Scholar]
- Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martínez-A C, Varona R. (2009) CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol 39:1671–1681 [DOI] [PubMed] [Google Scholar]
- Virtala R, Ekman AK, Jansson L, Westin U, Cardell LO. (2012) Airway inflammation evaluated in a human nasal lipopolysaccharide challenge model by investigating the effect of a CXCR2 inhibitor. Clin Exp Allergy 42:590–596 [DOI] [PubMed] [Google Scholar]
- Vischer HF, Hulshof JW, Hulscher S, Fratantoni SA, Verheij MH, Victorina J, Smit MJ, de Esch IJ, Leurs R. (2010) Identification of novel allosteric nonpeptidergic inhibitors of the human cytomegalovirus-encoded chemokine receptor US28. Bioorg Med Chem 18:675–688 [DOI] [PubMed] [Google Scholar]
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. (2000) CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol 164:2978–2986 [DOI] [PubMed] [Google Scholar]
- Wakugawa M, Nakamura K, Kakinuma T, Onai N, Matsushima K, Tamaki K. (2001) CC chemokine receptor 4 expression on peripheral blood CD4+ T cells reflects disease activity of atopic dermatitis. J Invest Dermatol 117:188–196 [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, Schwartz TW. (2003) The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem 278:19473–19482 [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Kledal TN, Farrell H, Schwartz TW. (2002) Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol 76:8161–8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MJ, Wang Y, Lai N, Baumgart T, Zhao BN, Dairaghi DJ, Bekker P, Ertl LS, Penfold ME, Jaen JC, et al. (2010) Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther 335:61–69 [DOI] [PubMed] [Google Scholar]
- Wang H, Beaty N, Chen S, Qi CF, Masiuk M, Shin DM, Morse HC., 3rd (2012) The CXCR7 chemokine receptor promotes B-cell retention in the splenic marginal zone and serves as a sink for CXCL12. Blood 119:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lu Y, Wang J, Koch AE, Zhang J, Taichman RS. (2008) CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Res 68:10367–10376 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang K, Zhang H, Kugathasan S, Annese V, Bradfield JP, Russell RK, Sleiman PM, Imielinski M, Glessner J, Hou C, et al. (2009) Diverse genome-wide association studies associate the IL12/IL23 pathway with Crohn Disease. Am J Hum Genet 84:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, et al. (2000) Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J Biol Chem 275:22313–22323 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. (2011) CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron 69:61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14:6735–6741 [DOI] [PubMed] [Google Scholar]
- Weber M, Blair E, Simpson CV, O’Hara M, Blackburn PE, Rot A, Graham GJ, Nibbs RJ. (2004) The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol Biol Cell 15:2492–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. (2013) Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339:328–332 [DOI] [PubMed] [Google Scholar]
- Wehrli N, Legler DF, Finke D, Toellner KM, Loetscher P, Baggiolini M, MacLennan IC, Acha-Orbea H. (2001) Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur J Immunol 31:609–616 [DOI] [PubMed] [Google Scholar]
- Wei L, Vahedi G, Sun HW, Watford WT, Takatori H, Ramos HL, Takahashi H, Liang J, Gutierrez-Cruz G, Zang C, et al. (2010) Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation. Immunity 32:840–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TN, Schwartz TW. (1997) Plagiarism of the host immune system: lessons about chemokine immunology from viruses. Curr Opin Biotechnol 8:741–748 [DOI] [PubMed] [Google Scholar]
- Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Förster R. (2007) CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA 104:6347–6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K, Kremmer E, Sixt M, Hoffmeyer A, Pabst O, et al. (2011) Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 35:945–957 [DOI] [PubMed] [Google Scholar]
- Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, Gould SL, Springer MS, DeMartino JA. (1998) Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem 273:18288–18291 [DOI] [PubMed] [Google Scholar]
- Werry TD, Wilkinson GF, Willars GB. (2003) Cross-talk between P2Y2 nucleotide receptors and CXC chemokine receptor 2 resulting in enhanced Ca2+ signaling involves enhancement of phospholipase C activity and is enabled by incremental Ca2+ release in human embryonic kidney cells. J Pharmacol Exp Ther 307:661–669 [DOI] [PubMed] [Google Scholar]
- White GE, Greaves DR. (2012) Fractalkine: a survivor’s guide: chemokines as antiapoptotic mediators. Arterioscler Thromb Vasc Biol 32:589–594 [DOI] [PubMed] [Google Scholar]
- White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, et al. (1998) Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem 273:10095–10098 [DOI] [PubMed] [Google Scholar]
- Whitehead GS, Wang T, DeGraff LM, Card JW, Lira SA, Graham GJ, Cook DN. (2007) The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am J Respir Crit Care Med 175:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson KL, Elliott JD, Veber DF, Nie H, Rutledge MC, McCleland BW, Xiang JN, Jurewicz AJ, Hertzberg RP, Foley JJ, et al. (2004) Evaluation of potent and selective small-molecule antagonists for the CXCR2 chemokine receptor. J Med Chem 47:1319–1321 [DOI] [PubMed] [Google Scholar]
- Wiederholt T, von Westernhagen M, Zaldivar MM, Berres M-L, Schmitz P, Hellerbrand C, Müller T, Berg T, Trautwein C, Wasmuth HE. (2008) Genetic variations of the chemokine scavenger receptor D6 are associated with liver inflammation in chronic hepatitis C. Hum Immunol 69:861–866 [DOI] [PubMed] [Google Scholar]
- Wiekowski MT, Chen SC, Zalamea P, Wilburn BP, Kinsley DJ, Sharif WW, Jensen KK, Hedrick JA, Manfra D, Lira SA. (2001) Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol 167:7102–7110 [DOI] [PubMed] [Google Scholar]
- Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. (2001) Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol 166:5145–5154 [DOI] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW. (2012) Molecular mechanisms of HIV entry. Adv Exp Med Biol 726:223–242 [DOI] [PubMed] [Google Scholar]
- Willems LI, Ijzerman AP. (2010) Small molecule antagonists for chemokine CCR3 receptors. Med Res Rev 30:778–817 [DOI] [PubMed] [Google Scholar]
- Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. (1998) The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol 28:2025–2034 [DOI] [PubMed] [Google Scholar]
- Wong LM, Myers SJ, Tsou CL, Gosling J, Arai H, Charo IF. (1997) Organization and differential expression of the human monocyte chemoattractant protein 1 receptor gene. Evidence for the role of the carboxyl-terminal tail in receptor trafficking. J Biol Chem 272:1038–1045 [DOI] [PubMed] [Google Scholar]
- Wood A, Armour D. (2005) The discovery of the CCR5 receptor antagonist, UK-427,857, a new agent for the treatment of HIV infection and AIDS. Prog Med Chem 43:239–271 [DOI] [PubMed] [Google Scholar]
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, et al. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP, Shen ZZ, Shao ZM. (2008) Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol Cancer Res 6:1276–1288 [DOI] [PubMed] [Google Scholar]
- Wu Y, Yoder A. (2009) Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog 5:e1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. (2001) Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood 98:2626–2632 [DOI] [PubMed] [Google Scholar]
- Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, et al. (2000) The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol 30:262–271 [DOI] [PubMed] [Google Scholar]
- Wyatt LS, Rodriguez WJ, Balachandran N, Frenkel N. (1991) Human herpesvirus 7: antigenic properties and prevalence in children and adults. J Virol 65:6260–6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. (2005) Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood 106:3300–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, Rosa R, Di Salvo J, Mudgett J, Peterson LB, et al. (2003) Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol 73:771–780 [DOI] [PubMed] [Google Scholar]
- Xu L, Khandaker MH, Barlic J, Ran L, Borja ML, Madrenas J, Rahimpour R, Chen K, Mitchell G, Tan CM, et al. (2000) Identification of a novel mechanism for endotoxin-mediated down-modulation of CC chemokine receptor expression. Eur J Immunol 30:227–235 [DOI] [PubMed] [Google Scholar]
- Xu L, Rahimpour R, Ran L, Kong C, Biragyn A, Andrews J, Devries M, Wang JM, Kelvin DJ. (1997) Regulation of CCR2 chemokine receptor mRNA stability. J Leukoc Biol 62:653–660 [DOI] [PubMed] [Google Scholar]
- Xue C, Feng H, Cao G, Huang T, Glenn J, Anand R, Meloni D, Zhang K, Kong L, Wang A, et al. (2011) Discovery of INCB3284, a potent, selective, and orally bioavailable hCCR2 antagonist. ACS Med Chem Lett 2:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, et al. (2000) Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol 68:568–574 [PubMed] [Google Scholar]
- Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, Yamaguchi K, Yamada Y, Hanada S, Tamura K, et al. (2010) Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol 28:1591–1598 [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. (1998) EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol 161:3096–3102 [PubMed] [Google Scholar]
- Yang AG, Bai X, Huang XF, Yao C, Chen S. (1997) Phenotypic knockout of HIV type 1 chemokine coreceptor CCR-5 by intrakines as potential therapeutic approach for HIV-1 infection. Proc Natl Acad Sci USA 94:11567–11572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, et al. (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525–528 [DOI] [PubMed] [Google Scholar]
- Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, et al. (2000) Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med 191:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Walton W, Cook DN, Hua X, Tilley S, Haskell CA, Horuk R, Blackstock AW, Kirby SL. (2011) The chemokine, CCL3, and its receptor, CCR1, mediate thoracic radiation-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 45:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Loy J, Ryseck RP, Carrasco D, Bravo R. (1998) Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood 92:3912–3923 [PubMed] [Google Scholar]
- Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, Tao X, Cardarelli PM, Leblanc H, Nichol G, et al. (2012) A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 64:1730–1739 [DOI] [PubMed] [Google Scholar]
- Yi T, Wang X, Kelly LM, An J, Xu Y, Sailer AW, Gustafsson JA, Russell DW, Cyster JG. (2012) Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity 37:535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. (2005) Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 174:8183–8190 [DOI] [PubMed] [Google Scholar]
- Ying S, Robinson DS, Meng Q, Barata LT, McEuen AR, Buckley MG, Walls AF, Askenase PW, Kay AB. (1999) C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES). J Immunol 163:3976–3984 [PubMed] [Google Scholar]
- Ying S, Zhang G, Gu S, Zhao J. (2006) How much do we know about atopic asthma: where are we now? Cell Mol Immunol 3:321–332 [PubMed] [Google Scholar]
- Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. (1997) Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem 272:13803–13809 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Nagira M, Kitaura M, Imagawa N, Imai T, Yoshie O. (1998a) Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem 273:7118–7122 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Imai T, Kakizaki M, Nishimura M, Takagi S, Yoshie O. (1998b) Identification of single C motif-1/lymphotactin receptor XCR1. J Biol Chem 273:16551–16554 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Imai T, Kakizaki M, Nishimura M, Yoshie O. (1995) Molecular cloning of a novel C or gamma type chemokine, SCM-1. FEBS Lett 360:155–159 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Imai T, Takagi S, Nishimura M, Ishikawa I, Yaoi T, Yoshie O. (1996) Structure and expression of two highly related genes encoding SCM-1/human lymphotactin. FEBS Lett 395:82–88 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Izawa D, Nakayama T, Nakahara K, Kakizaki M, Imai T, Suzuki R, Miyasaka M, Yoshie O. (1999) Molecular cloning of mXCR1, the murine SCM-1/lymphotactin receptor. FEBS Lett 458:37–40 [DOI] [PubMed] [Google Scholar]
- Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, Hieshima K, Tatsumi Y, Matsushima K, Hasegawa H, et al. (2002) Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 99:1505–1511 [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Oppenheim JJ. (2011) Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp Cell Res 317:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Crawford D, Tsuchihashi T, Behrens TW, Srivastava D. (2011) The chemokine receptor CXCR7 functions to regulate cardiac valve remodeling. Dev Dyn 240:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD. (2007) CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med 204:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. (2006) CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med 203:2451–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaballos A, Gutiérrez J, Varona R, Ardavín C, Márquez G. (1999) Cutting edge: identification of the orphan chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine TECK. J Immunol 162:5671–5675 [PubMed] [Google Scholar]
- Zaballos A, Varona R, Gutiérrez J, Lind P, Márquez G. (1996) Molecular cloning and RNA expression of two new human chemokine receptor-like genes. Biochem Biophys Res Commun 227:846–853 [DOI] [PubMed] [Google Scholar]
- Zabel BA, Lewén S, Berahovich RD, Jaén JC, Schall TJ. (2011) The novel chemokine receptor CXCR7 regulates trans-endothelial migration of cancer cells. Mol Cancer 10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Nakae S, Zúñiga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, et al. (2008) Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med 205:2207–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Wang Y, Lewén S, Berahovich RD, Penfold ME, Zhang P, Powers J, Summers BC, Miao Z, Zhao B, et al. (2009) Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol 183:3204–3211 [DOI] [PubMed] [Google Scholar]
- Zamilpa R, Kanakia R, Cigarroa J, 4th, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML. (2011) CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 300:H1418–H1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Allegretti M, Ley K. (2008) Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br J Pharmacol 155:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Schmolke M, Bockhorn SG, Scharte M, Buschmann K, Ley K, Singbartl K. (2007) The Duffy antigen receptor for chemokines in acute renal failure: A facilitator of renal chemokine presentation. Crit Care Med 35:2156–2163 [DOI] [PubMed] [Google Scholar]
- Zeng XH, Ou ZL, Yu KD, Feng LY, Yin WJ, Li J, Shen ZZ, Shao ZM. (2011) Coexpression of atypical chemokine binders (ACBs) in breast cancer predicts better outcomes. Breast Cancer Res Treat 125:715–727 [DOI] [PubMed] [Google Scholar]
- Zernecke A, Shagdarsuren E, Weber C. (2008) Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol 28:1897–1908 [DOI] [PubMed] [Google Scholar]
- Zhang J, Patel JM. (2010) Role of the CX3CL1-CX3CR1 axis in chronic inflammatory lung diseases. Int J Clin Exp Med 3:233–244 [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. (2007) Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 27:12396–12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, König P, Gerard NP, Gerard C, Wills-Karp M, Köhl J. (2010) A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol 185:6741–6752 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yu S, Hoffmann K, Yu K, Förster R. (2012) Neonatal lymph node stromal cells drive myelodendritic lineage cells into a distinct population of CX3CR1+CD11b+F4/80+ regulatory macrophages in mice. Blood 119:3975–3986 [DOI] [PubMed] [Google Scholar]
- Zhang XW, Liu Q, Wang Y, Thorlacius H. (2001a) CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br J Pharmacol 133:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Zhang L, Ketas T, Korber BT, Moore JP. (2001b) HIV type 1 molecular clones able to use the Bonzo/STRL-33 coreceptor for virus entry. AIDS Res Hum Retroviruses 17:217–227 [DOI] [PubMed] [Google Scholar]
- Zhen Z, Bradel-Tretheway B, Sumagin S, Bidlack JM, Dewhurst S. (2005) The human herpesvirus 6 G protein-coupled receptor homolog U51 positively regulates virus replication and enhances cell-cell fusion in vitro. J Virol 79:11914–11924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Cao G, Xia M, Feng H, Glenn J, Anand R, Zhang K, Huang T, Wang A, Kong L, et al. (2011) Discovery of INCB10820/PF-4178903, a potent, selective, and orally bioavailable dual CCR2 and CCR5 antagonist. Bioorg Med Chem Lett 21:1442–1446 [DOI] [PubMed] [Google Scholar]
- Zheng X, Nakamura K, Furukawa H, Nishibu A, Takahashi M, Tojo M, Kaneko F, Kakinuma T, Tamaki K. (2003) Demonstration of TARC and CCR4 mRNA expression and distribution using in situ RT-PCR in the lesional skin of atopic dermatitis. J Dermatol 30:26–32 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. (2009) Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458:351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kang N, Cui L, Ba D, He W. (2012) Anti-γδ TCR antibody-expanded γδ T cells: a better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol Immunol 9:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Luo Z, Hall JW, Luo J, Han X, Huang Z. (2000) Molecular modeling and site-directed mutagenesis of CCR5 reveal residues critical for chemokine binding and signal transduction. Eur J Immunol 30:164–173 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Kurihara T, Ryseck RP, Yang Y, Ryan C, Loy J, Warr G, Bravo R. (1998) Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J Immunol 160:4018–4025 [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80 [DOI] [PubMed] [Google Scholar]
- Zimmerman PA, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, Weissman D, Cohen O, Rubbert A, Lam G, et al. (1997) Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol Med 3:23–36 [PMC free article] [PubMed] [Google Scholar]
- Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, et al. (1998) The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol 161:547–551 [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, Homey B. (2011) Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11:597–606 [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. (2012) The chemokine superfamily revisited. Immunity 36:705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. (2006) The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P, Isegawa Y, Nakano K, Haque M, Horiguchi Y, Yamanishi K. (1999) Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J Virol 73:5926–5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. (1998) Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393:595–599 [DOI] [PubMed] [Google Scholar]
- Zuurman MW, Heeroma J, Brouwer N, Boddeke HW, Biber K. (2003) LPS-induced expression of a novel chemokine receptor (L-CCR) in mouse glial cells in vitro and in vivo. Glia 41:327–336 [DOI] [PubMed] [Google Scholar]