Abstract
Background
The measurement of hemoglobin concentration ([Hb]) is performed routinely as a part of a complete blood cell count to evaluate the oxygen-carrying capacity of blood. Devices currently available to physicians and clinical laboratories for measuring [Hb] are accurate, operate on small samples and provide results rapidly, but may be prohibitively expensive for resource-limited settings. The unavailability of accurate but inexpensive diagnostic tools often precludes proper diagnosis of anemia in low-income developing countries. Therefore, we developed a simple paper-based assay for measuring [Hb].
Methods
A 20-μL droplet of a mixture of blood and Drabkin’s reagent was deposited onto patterned chromatography paper. The resulting blood stain was digitized with a portable scanner and analyzed. The mean color intensity of the blood stain was used to quantify [Hb]. We compared the performance of the paper-based Hb assay with a hematology analyzer (comparison method) using blood samples from 54 subjects.
Results
The values of [Hb] measured using the paper-based assay and the comparison method were highly correlated (R2 = 0.9598); the standard deviation of the difference between the two measurements was 0.62 g/dL. The assay was accurate within 1 g/dL 90.7% of the time, overestimating [Hb] by ≥1 g/dL in 1.9% and underestimating [Hb] by ≥1 g/dL in 7.4% of the subjects.
Conclusions
This study demonstrates the feasibility of the paper-based Hb assay. This simple, low-cost test should be useful for diagnosing anemia in resource-limited settings, particularly in the context of care for malaria, HIV and sickle cell disease patients in sub-Saharan Africa.
Introduction
Hemoglobin (Hb) is the oxygen transporting protein contained within red blood cells (RBCs). The mass concentration of Hb ([Hb]) is a measure of the oxygen-carrying capacity of blood. Normal [Hb] in blood of adults ranges 12–16 g/dL in females and 14–18 g/dL in males; values <5 g/dL or >20 g/dL are considered critical (1). A decreased [Hb], referred to as anemia, may be an indication of chronic blood loss, decreased RBC production, or abnormal destruction of RBCs (hemolysis). Hemolytic anemia may be associated with inherited blood disorders such as sickle cell disease, but can also be autoimmune in origin or result from exposure to certain drugs, toxins, infections, or transfusion of mismatched blood products (2). Other common causes of anemia include dietary deficiencies (3, 4), cirrhosis and renal disease (2). Anemia reduces the oxygen-carrying capacity of blood, straining the cardiopulmonary system, which responds by increasing the heart rate and stroke volume to maintain oxygen delivery (5). Acute onset anemia may be associated with fatigue, lethargy and/or pallor (4). Patients with chronic anemia and having [Hb] < 5 g/dL may experience more serious complications such as angina, congestive heart failure, heart attack and stroke (5). Depending on the clinical status of the patient, a [Hb] < 6–8 g/dL is currently recommended as the threshold trigger for initiating RBC transfusion (6). Increased [Hb] may indicate erythrocytosis due to smoking (7) or in response to hypoxia at high altitudes (8), polycythemia vera (9), congenital heart disease (10), severe chronic obstructive pulmonary disease (11) or severe dehydration (12). The increase in blood viscosity associated with an increased [Hb] may impair oxygen delivery by reducing the capillary perfusion, which may in turn increase the risk of hypoxia, as well as thrombotic and hemorrhagic complications (13).
Because of its importance, [Hb] is measured routinely as a part of a complete blood cell count. Clinical laboratories measure [Hb] using automated hematology analyzers as well as spectrophotometers, blood gas analyzers and stand-alone CO-oximeters (14). The need for rapid and accurate measurement of [Hb] at the bedside has driven the development and implementation of [Hb] testing on the point-of-care (POC) platform (15). Currently available POC devices are designed to operate in close proximity to the patient, provide accurate results (±1 g/dL of clinical laboratory analyzers), require small volumes of blood, and can be used by persons without expert training, making the nearly instantaneous measurement of [Hb] in operating room or emergency care settings a reality (16–18).
Notwithstanding their obvious advantages, the relatively high per-test and analyzer costs make the use of conventional POC devices for measuring [Hb] in resource-limited settings of low-income developing countries in sub-Saharan Africa, on the Indian subcontinent and throughout Southeast Asia prohibitively expensive (19, 20). Currently available low-cost alternatives including the “hematocrit/3” method (21) and the WHO hemoglobin color scale (HbCS) test (22) have several drawbacks that limit their use in the field. In addition to being somewhat inaccurate, the “hematocrit/3” method relies on knowledge of hematocrit and, therefore, requires a centrifuge (21, 23). The HbCS test is highly sensitive to the ambient lighting conditions, operator bias and deviations from the recommended readout time (22, 24). The lack of confidence in the quality of [Hb] measurements, may result in clinicians relying exclusively on their clinical judgment to prescribe transfusions in resource-limited settings (25). Therefore, our goal was to develop a simple, low-cost, paper-based Hb assay that addressed these limitations.
Methods
Blood samples
The study protocol was approved by Tulane University Biomedical IRB. After obtaining written informed consent, blood samples were collected into 4 mL or 9 mL Vacutainer tubes (K2EDTA, BD, Franklin Lakes, NJ) during routine blood draws (period January – April 2013) from patients of the Pediatric Hematology-Oncology Clinic (Tulane University Hospital) and the Sickle Cell Center of Southern Louisiana (New Orleans, LA). Standard hematological parameters including [Hb] were measured using a Medonic M-Series hematology analyzer (Medonic M16C/M20C, Boule Medical AB, Stockholm, Sweden). The Hb content of blood samples was adjusted artificially to prepare samples with [Hb] ranging from 2.5 g/dL to 25 g/dL by adding autologous plasma (for lower [Hb]) or pelleted RBCs (for higher [Hb]) to the original sample.
Fabrication of the microfluidic paper-based analytical devices
The microfluidic paper-based analytical devices (μPADs) were fabricated using a previously published method (26, 27). Briefly, the pattern of the μPADs was designed using illustration software (Canvas 11, ACD Systems International Inc, Seattle, WA). The devices were printed onto sheets of chromatography paper (No. 1, Whatman, Piscataway, NJ) using a solid-ink (wax) printer (Phaser 8560N, Xerox, Norwalk, CT), and then heated on a hot plate at 150°C for 3 min.
Quantification of the blood stain color
Blood samples were mixed at 1:10, 1:15 or 1:20 ratios (v:v) with Drabkin’s reagent (Ricca Chemical Company, Arlington, TX) and incubated at room temperature (20–25°C) for 10 min. The components of Drabkin’s reagent lysed red blood cells and reacted with all forms of Hb except sulfhemoglobin present in the sample, converting them to cyanmethemoglobin, a stable brownish-colored compound. A 20 μL drop of the mixture was placed onto the center of each μPAD. The resulting blood stain was allowed to dry for 25 min (28).
Quantification of [Hb] was accomplished by scanning the sheets of chromatography paper containing arrays of μPADs using a portable flatbed scanner (CanoScan LiDE110, Canon USA Inc, Lake Success, NY) and then analyzing the images automatically using a custom-coded algorithm (MATLAB, The Math Works Inc, Natick, MA). The quantitative analysis of blood stains was based on the red/green/blue (RGB) color model values of the digitized images. The color data in the red, green and blue channels were extracted from the RGB values of each pixel within a blood stain. We defined the color intensity of each channel as <color intensity> = 255 –<R, G or B> (e.g., for red channel, <color intensity> = 255 – <R>). The color values from all pixels within the blood stain, including the pixels corresponding to the darker ring on the periphery of the stain, were averaged to obtain the mean color intensity of the blood stain for each color channel. The correlation between the mean color intensity and the actual [Hb] was evaluated for all color channels; the green channel showed the best linear fit and was selected to quantify [Hb] in the blood samples.
Statistical analysis
Pearson’s correlation coefficient and regression were calculated to directly compare data obtained from the paper-based Hb assay and the Medonic M-Series hematology analyzer (comparison method). Three research associates performed both types of measurements on the same day. A Bland & Altman plot (29) was also constructed, with consideration for limitations, to visualize the comparison.
Results
Design and operation of the paper-based Hb assay
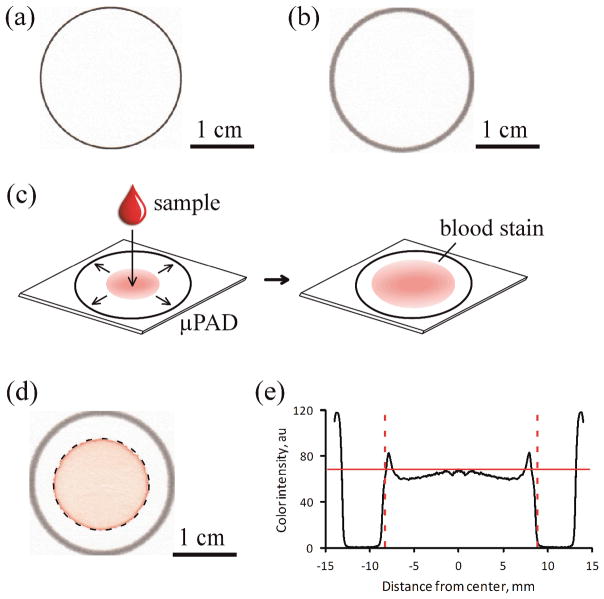
Figure 1 illustrates the design and operation of the paper-based Hb assay. Each individual μPAD comprised a 2.8 cm diameter circle (Fig. 1a) designed to create a constant area for quantification with an image analysis algorithm and to prevent potential cross-contamination of samples. Each sheet of chromatography paper contained a 5×4 array of μPADs and included an alignment mark to simplify automation of the subsequent image analysis. We fabricated the μPAD arrays by printing their pattern with a solid-ink (wax) printer (Fig. 1a). We then used a hot plate to heat the printed chromatography paper above the melting point of wax to allow the wax to reflow and form hydrophobic barriers through the full thickness of the paper (Fig. 1b). When designing the pattern of the μPAD (Fig. 1a) we accounted for the natural widening of the printed lines (to about 1 mm thick) during the melting process (Fig. 1b).
Figure 1.
Design and fabrication of the paper-based Hb assay. (a) The pattern of the μPAD was printed with a solid-ink (wax) printer on chromatography paper. (b) The printed pattern was melted to create a 1 mm-wide hydrophobic barrier spanning the entire thickness of the paper substrate. (c) To perform the assay, a drop of blood mixed with Drabkin’s reagent was placed onto the center of the device. The lysate wicked laterally towards the periphery, coloring the paper faint red (pink). (d) The image analysis algorithm automatically detected the blood stain within each μPAD (dashed circle) and extracted the RGB color information for all pixels contained within the blood stain. (e) The color intensity of the pixels (between dashed lines) was averaged to calculate the mean color intensity (solid red line) for each blood stain. The values of the mean color intensity were used to calculate the [Hb] in the blood samples.
We performed this paper-based Hb assay by first mixing a sample of whole blood with Drabkin’s reagent and then depositing a 20 μL droplet of the mixture onto the μPAD and letting the blood stain develop (Fig. 1c). The droplet spread radially from the center through the paper substrate towards the periphery of the μPAD due to wicking by capillary action (30, 31), forming the characteristic blood stain pattern (Fig. 1d) reminiscent of the stains produced by drying coffee (32). We selected the volume of the droplet (20 μL) and size of the μPAD (2.8 cm) so the outermost margin of the stain could not reach the periphery of the μPAD (Fig. 1d).
We used a portable flatbed scanner to digitize the sheets of chromatography paper carrying μPADs with developed blood stains. We analyzed the images to determine the mean color intensity for the blood stain contained within each μPAD using a simple image processing algorithm developed specifically for this purpose (Fig. 1d–e). The algorithm used the alignment mark on the sheets of paper to determine the location of each μPAD (Fig. 1d). The algorithm applied a circular binary mask to the image to select the area within the hydrophobic border of the μPAD, and then isolated the pixels of the blood stain within the circular region by removing pixels below a threshold value based on the difference in color intensity between the paper substrate and the blood stain (Fig. 1d, dashed circle). Finally, the algorithm averaged the color information from all pixels within the blood stain to calculate the mean color intensity of the blood stain (Fig. 1e, red solid line). We used the mean color intensity of the stain (Fig. 1e) and a calibration curve (Fig. 2b) to measure [Hb] in the blood sample.
Figure 2.
Dependence of the mean color intensity of the blood stain on the dilution ratio. (a) Whole blood samples were diluted or concentrated with plasma or pelleted RBCs to achieve [Hb] ranging 2.5 – 25 g/dL. A 20 μL drop of blood mixed with Drabkin’s reagent at 1:10, 1:15 and 1:20 ratio (v:v) was placed at the center of each μPAD. (b–d) The mean color intensities for the red (b), green (c) and blue (d) channels of the blood stain in a μPAD produced by each sample at each dilution ratio. Data shown as mean ± SD (n = 3).
All operations of the paper-based Hb assay, including sample preparation, placement of the sample onto the μPAD, drying of the blood stain, image scanning and the automated image analysis could be completed within 35 min.
Effect of dilution on the mean color intensity of the blood stain
Figure 2 illustrates the effect of dilution on the mean color intensity of the stains produced by blood on paper. To perform these experiments, we prepared a series of calibration samples with their [Hb] adjusted to 2.5, 5, 10, 15, 20 and 25 g/dL (as described in the Methods) using blood from 3 consenting volunteers. We then mixed a small volume of each calibration sample with Drabkin’s reagent at 1:10, 1:15 and 1:20 ratio (v:v), waited 10 minutes and then placed a 20 μL droplet of each mixture onto paper.
Droplets with markedly different Hb content, either due to a difference in [Hb] or due to dilution, produced blood stains with different color intensities that were easily distinguishable visually. As expected, the blood stain color intensity increased with increasing [Hb] for each mixing ratio (dilution), and decreased with increasing dilution for each [Hb] value (Fig. 2a). The mean color intensity of each blood stain correlated very strongly with its [Hb] (Fig. 2b). The combination of 1:15 mixing ratio and Green channel showed the best linear fit (mean color intensity = 2.0986 × [Hb] + 3.7226, R2=0.996). Therefore, we used the 1:15 mixing ratio to prepare samples in all further experiments, and used the mean color intensity in green channel and the linear equation produced by this graph (Fig. 2b) as the calibration curve for estimating the [Hb] of the sample based on the mean color intensity of its blood stain.
To determine the limit of detection (LOD) and the limit of quantification (LOQ) of the assay, we varied [Hb] of blood samples from 0.5 to 25 g/dL by diluting whole blood with autologous plasma or concentrating whole blood with packed RBCs (as described in the Methods). We defined the LOD as the lowest [Hb] at which the blood stain could be identified and measured using our image processing algorithm, and the LOQ as the lowest [Hb] at which the disagreement with the comparison method (the Medonic hematology analyzer) was less than ±1 g/dL. We found that the LOD for our paper-based Hb assay was 1 g/dL and the LOQ was 2.5 g/dL.
Relative accuracy and precision of the paper-based Hb assay
We tested the relative accuracy of the paper-based Hb assay by comparing the values of [Hb] measured using our assay and the Medonic M-Series hematology analyzer (Medonic M16C/M20C, Boule Medical AB, Stockholm, Sweden) for blood samples from n = 54 subjects. Subjects included individuals with Hb AA, Hb AS, Hb SC and Hb SS genotypes; the presence of Hb S in the blood samples did not affect the [Hb] measurements (data not shown). Figure 3 illustrates the results of this side-by-side comparison. The data points representing these measurements were distributed in close proximity to the diagonal line (Fig. 3a, red dashed line), indicating a good agreement between the [Hb] estimated from the mean color intensity of blood stains in our paper-based Hb assay and the actual [Hb] obtained from the standard analysis (Fig. 3a). The linear least-squares regression analysis (Fig. 3a, black solid line) showed a high degree of correlation between the two measurements (y = 1.05 x − 0.58, R2=0.9598).
Figure 3.
Comparison of the [Hb] measurements performed using the paper-based Hb assay and the Medonic hematology analyzer (n = 54 subjects). (a) The linear regression analysis of the correlation between the [Hb] values determined by a hematology analyzer (true [Hb]) and by the paper-based Hb assay (estimated [Hb]). The diagonal (red dashed line) represents a 1:1 correspondence between the true and the estimated [Hb] values. (b) Bland & Altman plots of the agreement between the paper-based Hb assay and the hematology analyzer. The solid red line is the average difference between the two methods (mean bias); the dashed red lines are limits of agreement (±2 SD). The mean [Hb] was 11.50 ± 2.86 g/dL for the paper-based Hb assay and 11.43 ± 3.05 g/dL for the Medonic hematology analyzer.
We also constructed the Bland & Altman plot (29) to evaluate the agreement between the paper-based Hb assay and the Medonic hematology analyzer (Fig. 3b). The standard deviation of the difference between the reference standard (hematology analyzer) and the paper-based Hb assay was 0.62 g/dL. The limits of agreement between the two methods were −1.30 and 1.18 g/dL. The paper-based Hb assay agreed with the hematology analyzer within 1 g/dL 90.7% of the time, overestimating [Hb] by ≥1 g/dL in 1.9% of the subjects, and underestimating [Hb] by ≥1 g/dL in 7.4% of the subjects (Fig. 3b).
In a separate experiment, we tested the precision of the paper-based Hb assay by measuring repeatedly (n = 5) the [Hb] for a series of blood samples obtained from the same subject with the actual [Hb] of each sample adjusted (as described in the Methods) to 2.5, 5, 10, 15, 20 and 25 g/dL. The standard deviation for the [Hb] measurements performed using different droplets from the same blood sample was consistently <0.5 g/dL for all values of the true [Hb] tested: 0.21 g/dL (CV=8.3%) for the sample with true [Hb] of 2.5 g/dL; 0.19 g/dL (CV=3.9%) for 5 g/dL; 0.11 g/dL (CV=1.1%) for 10 g/dL; 0.21 g/dL (CV=1.4%) for 15 g/dL; 0.34 g/dL (CV=1.7%) for 20 g/dL; and 0.78g/dL (CV=3.1%) for 25 g/dL. For comparison, the [Hb] measurement range for the Medonic hematology analyzer was 0 – 35 g/dL, with the system precision of CV<1.0% (values provided by the manufacturer).
Effect of droplet volume variation and long-term stability of the paper-based Hb assay
We measured the effect of variation in the volume of the sample droplet, which may occur during the use of the assay in the field, on the [Hb] measurement for samples with true [Hb] of 5, 10, 15 and 20 g/dL. A ±20% variation in the volume of the sample droplet (16 – 24 μL) resulted in a ≤0.21 g/dL standard deviation (CV=4.3%) of the estimated [Hb] for samples with true [Hb] = 5 g/dL, ≤0.66 g/dL (CV=5.6%) for 10 g/dL, ≤0.67 g/dL (CV=4.2%) for 15 g/dL, and ≤0.93 g/dL (CV=4.4%) for 20 g/dL (Supplemental Data Fig. 1a). As expected, the size of the blood stain depended on the droplet volume. For droplets of the same volume, but with different true [Hb] (5, 10, 15 and 20 g/dL) the normalized size of the blood stains produced by the droplets had a standard deviation of ≤0.03 (CV=4.7%) for 16 μL, ≤0.03 (CV=3.9%) for 18 μL, ≤0.03 (CV=4.5%) for 20 μL, ≤0.04 (CV=4.1%) for 22 μL, and ≤0.03 (CV=3.7%) for 24 μL droplet (Supplemental Data Fig. 1b).
Finally, we tested the long-term stability of the paper-based Hb assay measurements by scanning the sheets of paper containing the blood stains 4 times during the first 20 min, while the stain was still drying, and 13 times over the next 24 h, for samples with true [Hb] of 5, 10, 15 and 20 g/dL. The color of the dried blood stain remained stable after the initial drying period. We therefore were able to scan and analyze the blood stains at any point in time over the next 24 h without any significant change in the [Hb] measurement (Supplemental Data Fig. 2). During this study, the room temperature in our laboratory varied from 18 to 30°C, and humidity varied from 20 to 80%. We did not systematically test the effect of room temperature or humidity variations on the performance of the assay in this study.
Discussion
The measurement of blood [Hb] is one of the most frequently performed laboratory tests within a wide range of medical settings, from acute trauma care to chronic disease management (1). While the primary reason for initial [Hb] screening is to diagnose (or rule out) anemia, frequent monitoring of [Hb] is often required in the context of many conditions to quantify blood loss, track disease progression, assess treatment efficacy or monitor patients during procedures. When reliable diagnostic devices are available, they are often not used because they are considered to be prohibitively expensive for the resource-limited settings of developing countries (33). For example, in Kenyan district hospitals nearly 15% of children with clinical histories indicative of malaria or anemia did not have their [Hb] tested (20, 34). In this context, a reliable, low-cost assay for measuring [Hb] would improve the diagnosis of anemia and could help minimize wastage of limited resources on unneeded treatments and reduce the risk of complications associated with misdiagnosis (20, 25, 33). The urgent need for such an assay in sub-Saharan Africa (in areas endemic with sickle cell disease and malaria) was the primary motivation for this study.
Our paper-based Hb assay measures [Hb] by simply quantifying the appearance of a stain produced by the blood sample on paper. Perhaps the most widely used alternative low-cost method for estimating [Hb] in resource-limited settings is the WHO HbCS. To perform this test, the user must visually compare the color of a paper strip stained with blood to a standard at a certain time after the blood was applied to the strip. Unlike the HbCS, our paper-based Hb assay is not affected by changes in ambient light because the flatbed scanner itself provides consistent illumination, the color can be read at any time within a 24 h period following the collection of the blood stain, and the result is not affected by operator bias because the measurement is performed completely automatically.
Very little training is required to perform our paper-based Hb assay. All a user would need to do is to mix a finger-prick volume of blood with Drabkin’s reagent at the appropriate ratio, and deposit a droplet of this mixture on paper. The complexity of these operations is about the same as that of the sample preparation steps required from consumers for performing at-home rapid diagnostic tests for sperm count (35) or HbA1C (36)). All subsequent operations of the paper-based Hb assay are completely automated and user-independent, including the scanning and analysis of the stains. Notwithstanding its simplicity, our paper-based Hb assay demonstrated excellent comparison (SD = 0.62 g/dL, n = 54) with the Medonic hematology analyzer. The performance of our assay also compares favorably with conventional POC devices (14, 19, 37), particularly in the context of our target applications: (a) providing the measurement of [Hb] when no other methods are available, and (b) enabling public health screening in resource-limited settings where the lowest cost, rather than the highest accuracy, is most important.
We used a USB-powered flatbed scanner (Canon CanoScan LiDE110, $44 on Amazon.com as of 4/21/2013) and a laptop computer to digitize and analyze the blood stains. Any computer that has a functional USB port and complies with the system requirements of the scanner could in principle be used, including older salvaged or refurbished models. Importantly, the scanner and laptop could be used for many other purposes, not exclusively for performing our paper-based Hb assay. The primary reason for using these multi-purpose, consumer-grade electronic devices as the capital equipment part of a low-cost diagnostic assay is the ability to tap into the already well-established market of consumer electronics. These devices are much more available in resource-limited settings than specialized medical equipment such as a spectrophotometer and can be used for performing the [Hb] measurements without any modification of their original function. This approach represents an important advantage of our work with respect to recent studies that follow the conventional paradigm of POC development and propose to measure [Hb] using a new, potentially low-cost electronic device (38, 39). Such a device, designed specifically for measuring [Hb], would have to be separately developed, manufactured, distributed, calibrated, and maintained. The use of these POC devices, particularly the maintenance and/or replacement of broken devices, requires a level of medical and engineering infrastructure that is largely unavailable in resource-limited settings (40).
Another important advantage of our approach is that the use of existing scanners and laptops, which may already be present in even the most remote facilities because they were purchased by consumers for their personal use, could allow for an effectively “zero-cost” analyzer, compared with analyzer costs for conventional POC devices ranging from $800 to $15,000. In addition to the prohibitively high cost of the analyzers, conventional POC devices often require proprietary disposable supplies with per-test costs ranging from $0.02 to $3.67 (19). In contrast, the components required to perform our paper-based [Hb] assay are available generically at an estimated combined cost of <$0.007 (0.7 U.S. cents) per test.
In summary, we developed and validated a paper-based, point-of-care Hb assay for the low-cost assessment of [Hb]. This assay represents a major step towards effective intra-operative care as well as [Hb] testing at the bedside or in urgent care settings where traditional methods of the clinical laboratory are unavailable or prohibitively expensive. The paper-based Hb assay will be useful for diagnosing anemia in resource-limited settings of low-income developing countries (e.g. sub-Saharan Africa), particularly in the context of malaria, HIV and sickle cell disease.
Supplementary Material
Acknowledgments
This work was supported in part by an award from the National Blood Foundation (SSS), a subcontract from the President and Fellows of Harvard College under an award from the Bill & Melinda Gates Foundation (SSS), and a 2012 NIH Director’s Transformative Research Award (NHLBI R01HL117329, PI: SSS).
Footnotes
Conflict of Interest Disclosures
All authors declare no conflict of interest.
Disclaimer
“This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.”
Authorship Contributions
Shevkoplyas and Yang conceived and designed the study. Kanter and Benton designed the clinical protocol and obtained IRB approval. Kanter supervised the collection of clinical blood samples. Yang, Piety and Vignes performed the experiments and collected the data. Shevkoplyas, Yang, Piety, and Vignes analyzed and interpreted the data. Shevkoplyas, Yang and Kanter wrote the manuscript. Shevkoplyas obtained funding and supervised the study. All authors confirmed that they have contributed to the intellectual content of this paper and have met the following 3 requirements: (1) participated in conception, design, analysis, or interpretation; (2) drafted or critically revised the manuscript; and (3) read and approved the final submitted manuscript and revisions.
References
- 1.Pagana KD, Pagana TJ. Mosby’s manual of diagnostic and laboratory tests. 3. Philadelphia, PA: Mosby; 2010. [Google Scholar]
- 2.Shander A. Anemia in the critically ill. Crit Care Clin. 2004;20:159–78. doi: 10.1016/j.ccc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Aspuru K, Villa C, Bermejo F, Herrero P, Lopez SG. Optimal management of iron deficiency anemia due to poor dietary intake. Int J Gen Med. 2011;4:741–50. doi: 10.2147/IJGM.S17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, who vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12:444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 5.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–21. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Carless PA, Hebert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309:83–4. doi: 10.1001/jama.2012.50429. [DOI] [PubMed] [Google Scholar]
- 7.Aitchison R, Russell N. Smoking--a major cause of polycythemia. J R Soc Med. 1988;81:431. doi: 10.1177/014107688808100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horstman D, Weiskopf R, Jackson RE. Work capacity during 3-wk sojourn at 4,300 m: Effects of relative polycythemia. J Appl Physiol. 1980;49:311–8. doi: 10.1152/jappl.1980.49.2.311. [DOI] [PubMed] [Google Scholar]
- 9.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 10.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one Am J Respir Crit Care Med. 1994;150:833–52. doi: 10.1164/ajrccm.150.3.8087359. [DOI] [PubMed] [Google Scholar]
- 11.Vicari AM, Ponzoni M, Alberetto M, Martani C, Pontiroli AE, Folli F. Erythrocytosis in a patient with chronic obstructive pulmonary disease. Haematologica. 1998;83:183–6. [PubMed] [Google Scholar]
- 12.Tefferi A. Polycythemia vera: A comprehensive review and clinical recommendations. Mayo Clin Proc. 2003;78:174–94. doi: 10.4065/78.2.174. [DOI] [PubMed] [Google Scholar]
- 13.Lowe GD, Lee AJ, Rumley A, Price JF, Fowkes FG. Blood viscosity and risk of cardiovascular events: The edinburgh artery study. Br J Haematol. 1997;96:168–73. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- 14.Myers GJ, Browne J. Point of care hematocrit and hemoglobin in cardiac surgery: A review. Perfusion. 2007;22:179–83. doi: 10.1177/0267659107080826. [DOI] [PubMed] [Google Scholar]
- 15.Gehring H, Hornberger C, Dibbelt L, Rothsigkeit A, Gerlach K, Schumacher J, Schmucker P. Accuracy of point-of-care-testing (poct) for determining hemoglobin concentrations. Acta Anaesthesiol Scand. 2002;46:980–6. doi: 10.1034/j.1399-6576.2002.460809.x. [DOI] [PubMed] [Google Scholar]
- 16.Price CP, Kricka LJ. Improving healthcare accessibility through point-of-care technologies. Clin Chem. 2007;53:1665–75. doi: 10.1373/clinchem.2006.084707. [DOI] [PubMed] [Google Scholar]
- 17.Spielmann N, Mauch J, Madjdpour C, Schmugge M, Weiss M, Haas T. Accuracy and precision of hemoglobin point-of-care testing during major pediatric surgery. Int J Lab Hematol. 2012;34:86–90. doi: 10.1111/j.1751-553X.2011.01363.x. [DOI] [PubMed] [Google Scholar]
- 18.Tudos AJ, Besselink GJ, Schasfoort RB. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab on a chip. 2001;1:83–95. doi: 10.1039/b106958f. [DOI] [PubMed] [Google Scholar]
- 19.Patel KP, Hay GW, Cheteri MK, Holt DW. Hemoglobin test result variability and cost analysis of eight different analyzers during open heart surgery. J Extra Corpor Technol. 2007;39:10–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in africa: A barrier to effective health care. Clin Infect Dis. 2006;42:377–82. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 21.Carneiro IA, Drakeley CJ, Owusu-Agyei S, Mmbando B, Chandramohan D. Haemoglobin and haematocrit: Is the threefold conversion valid for assessing anaemia in malaria-endemic settings? Malar J. 2007;6:67. doi: 10.1186/1475-2875-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stott GJ, Lewis SM. A simple and reliable method for estimating haemoglobin. Bull World Health Organ. 1995;73:369–73. [PMC free article] [PubMed] [Google Scholar]
- 23.Quinto L, Aponte JJ, Menendez C, Sacarlal J, Aide P, Espasa M, et al. Relationship between haemoglobin and haematocrit in the definition of anaemia. Trop Med Int Health. 2006;11:1295–302. doi: 10.1111/j.1365-3156.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 24.Paddle JJ. Evaluation of the haemoglobin colour scale and comparison with the hemocue haemoglobin assay. Bull World Health Organ. 2002;80:813–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Allain JP, Anokwa M, Casbard A, Owusu-Ofori S, Dennis-Antwi J. Sociology and behaviour of west african blood donors: The impact of religion on human immunodeficiency virus infection. Vox Sang. 2004;87:233–40. doi: 10.1111/j.1423-0410.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Forouzan O, Brown TP, Shevkoplyas SS. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab on a chip. 2012;12:274–80. doi: 10.1039/c1lc20803a. [DOI] [PubMed] [Google Scholar]
- 27.Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81:7091–5. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Kanter J, Piety NZ, Benton MS, Vignes SM, Shevkoplyas SS. A simple, rapid, low-cost diagnostic test for sickle cell disease. Lab on a chip. 2013;13:1464–7. doi: 10.1039/c3lc41302k. [DOI] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 30.Khan MS, Thouas G, Shen W, Whyte G, Garnier G. Paper diagnostic for instantaneous blood typing. Anal Chem. 2010;82:4158–64. doi: 10.1021/ac100341n. [DOI] [PubMed] [Google Scholar]
- 31.Fu E, Ramsey SA, Kauffman P, Lutz B, Yager P. Transport in two-dimensional paper networks. Microfluid Nanofluid. 2011;10:29–35. doi: 10.1007/s10404-010-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA. Capillary flow as the cause of ring stains from dried liquid drops. Nature. 1997;389:827–9. [Google Scholar]
- 33.Bates I, Maitland K. Are laboratory services coming of age in sub-saharan africa? Clin Infect Dis. 2006;42:383–4. doi: 10.1086/499368. [DOI] [PubMed] [Google Scholar]
- 34.English M, Esamai F, Wasunna A, Were F, Ogutu B, Wamae A, et al. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in kenya. Lancet. 2004;363:1948–53. doi: 10.1016/S0140-6736(04)16408-8. [DOI] [PubMed] [Google Scholar]
- 35.Coppola MA, Klotz KL, Kim KA, Cho HY, Kang J, Shetty J, et al. Spermcheck fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod. 2010;25:853–61. doi: 10.1093/humrep/dep413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin a1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56:44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Simon A, Navarro-Nunez L, Perez-Ceballos E, Lozano ML, Candela MJ, Cascales A, et al. Evaluation of four rapid methods for hemoglobin screening of whole blood donors in mobile collection settings. Transfus Apher Sci. 2007;36:235–42. doi: 10.1016/j.transci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Bond M, Elguea C, Yan JS, Pawlowski M, Williams J, Wahed A, et al. Chromatography paper as a low-cost medium for accurate spectrophotometric assessment of blood hemoglobin concentration. Lab on a chip. 2013 doi: 10.1039/c3lc40908b. [DOI] [PubMed] [Google Scholar]
- 39.Kim D-S, Choi J-H, Nam M-H, Yang J-W, Pak JJ, Seo S. Led and cmos image sensor based hemoglobin concentration measurement technique. Sensors and Actuators B: Chemical. 2011;157:103–9. [Google Scholar]
- 40.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–44. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.