Abstract
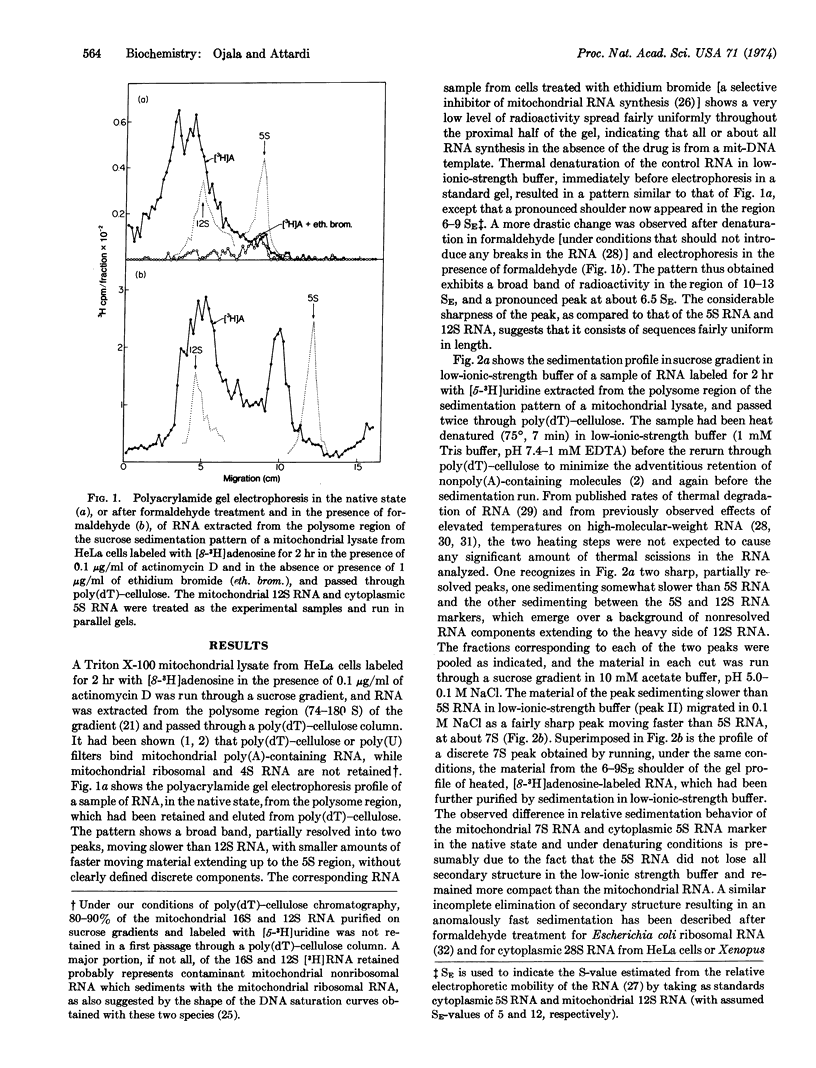
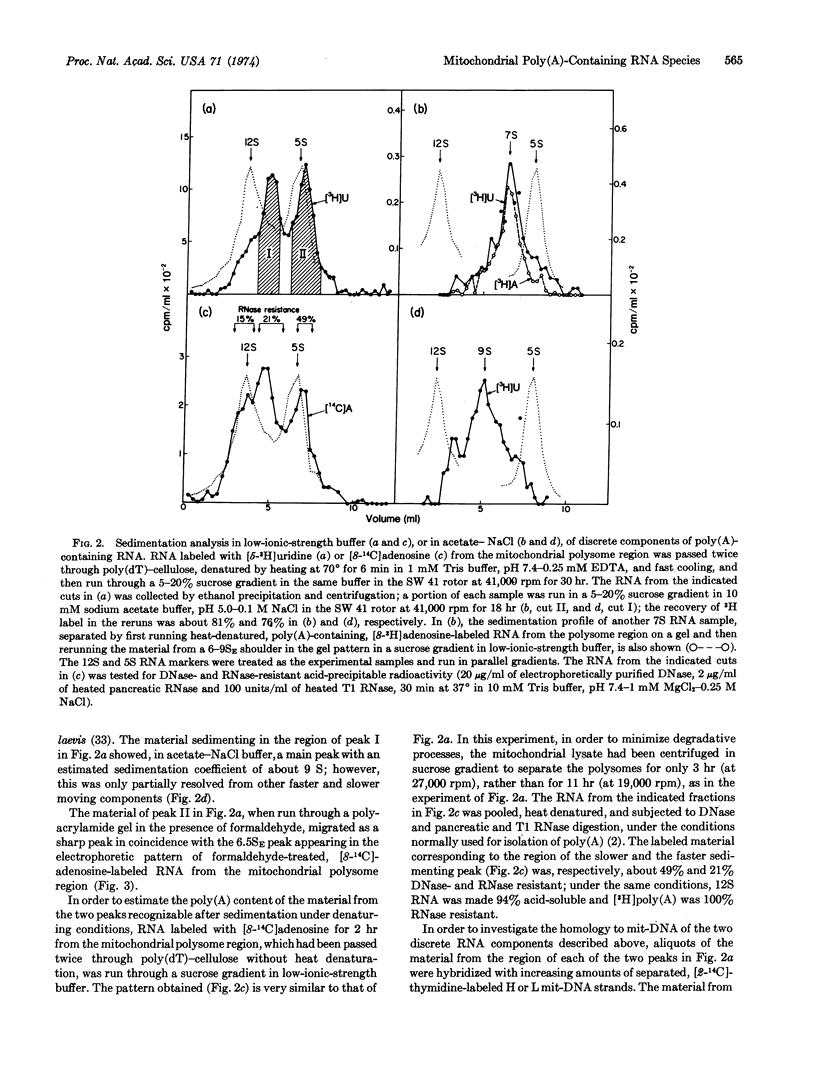
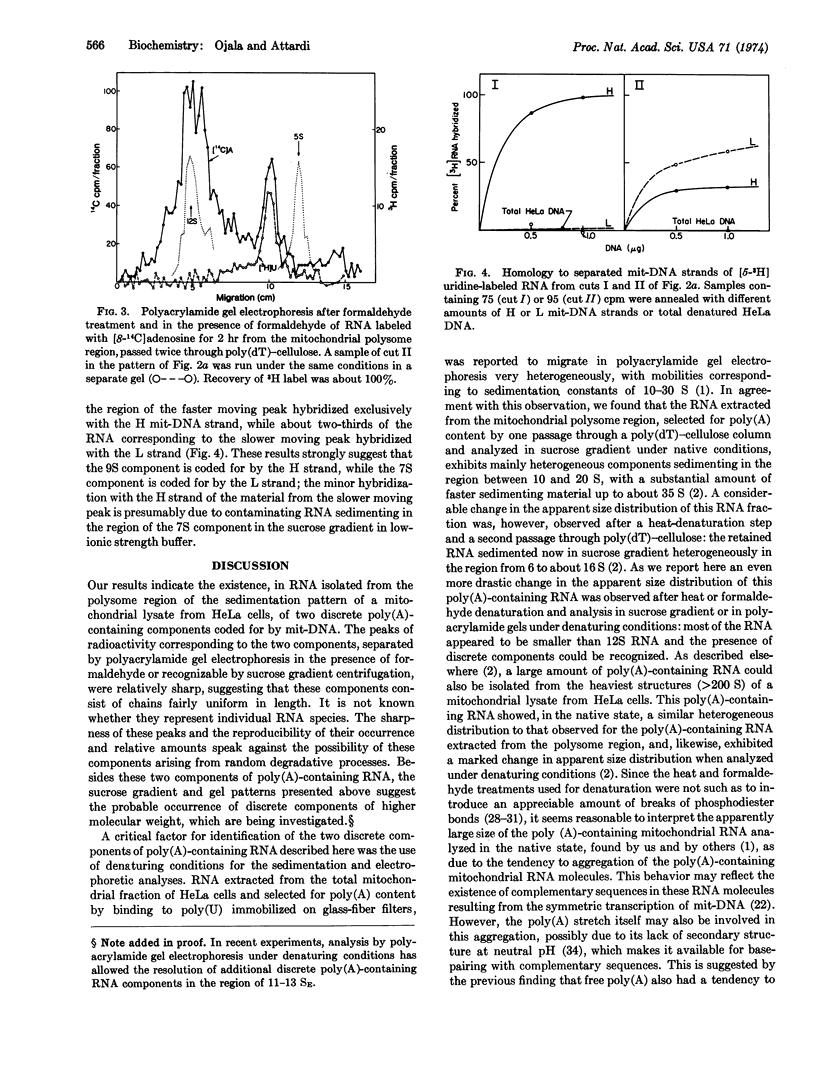
Polyacrylamide gel electrophoresis and sedimentation analysis under denaturing conditions of poly(A)-containing RNA from the polysome region of the sedimentation pattern of a HeLa-cell mitochondrial lysate has revealed the occurrence of a discrete RNA component, which sediments in the native state with a sedimentation constant of about 7 S. From the sedimentation behavior under native and denaturing conditions and the poly(A) content, a molecular weight of about 9 × 104 has been estimated for this component. RNA·DNA hybridization experiments have indicated that this component is coded for by the light strand of mitochondrial DNA. Evidence for the occurrence of a poly(A)-containing RNA component sedimenting at about 9 S and coded for by the heavy strand has also been obtained.
Keywords: actinomycin D, denaturation, poly(dT)-cellulose, polyacrylamide gel, separated strands, RNA·DNA hybridization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Expression of the mitochondria genome in HeLa cells. IV. Titration of mitochondrial genes for 16 s, 12 s and 4 s RNA. J Mol Biol. 1971 Jan 28;55(2):271–276. doi: 10.1016/0022-2836(71)90197-5. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondria genome in HeLa cells. IV. Titration of mitochondrial genes for 16 s, 12 s and 4 s RNA. J Mol Biol. 1971 Jan 28;55(2):271–276. doi: 10.1016/0022-2836(71)90197-5. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondrial genome in HeLa cells. II. Evidence for complete transcription of mitochondrial DNA. J Mol Biol. 1971 Jan 28;55(2):251–267. doi: 10.1016/0022-2836(71)90195-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1757–1761. doi: 10.1073/pnas.68.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. I. Oligonucleotide pattern of 28 s and 18 s RNA after pancreatic ribonuclease digestion. J Mol Biol. 1968 May 14;33(3):737–755. doi: 10.1016/0022-2836(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Attardi B., Attardi G. Expression of the mitochondrial genome in HeLa cells. I. Properties of the discrete RNA components from the mitochondrial fraction. J Mol Biol. 1971 Jan 28;55(2):231–249. doi: 10.1016/0022-2836(71)90194-x. [DOI] [PubMed] [Google Scholar]
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtker H. Dependence of the sedimentation coefficient on molecular weight of RNA after reaction with formaldehyde. J Mol Biol. 1968 Jul 14;35(1):61–70. doi: 10.1016/s0022-2836(68)80036-1. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. Mitochondrial ribosomes. FEBS Lett. 1971 Feb 19;13(2):73–88. doi: 10.1016/0014-5793(71)80204-1. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Chase J. W. Mitochondrial RNA in Xenopus laevis. II. Molecular weights and other physical properties of mitochondrial ribosomal and 4 s RNA. J Mol Biol. 1972 Jan 28;63(2):217–231. doi: 10.1016/0022-2836(72)90371-3. [DOI] [PubMed] [Google Scholar]
- Dubin D. T. The effect of actinomycin on the synthesis of mitochondrial RNA in hamster cells. Biochem Biophys Res Commun. 1967 Dec 15;29(5):655–660. doi: 10.1016/0006-291x(67)90266-5. [DOI] [PubMed] [Google Scholar]
- EIGNER J., BOEDTKER H., MICHAELS G. The thermal degradation of nucleic acids. Biochim Biophys Acta. 1961 Jul 22;51:165–168. doi: 10.1016/0006-3002(61)91028-9. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell L. A., Reijnders L., Borst P. The effect of temperature and ionic strength of the electrophoretic mobility of yeast mitochondrial RNA. Eur J Biochem. 1971 Mar 1;19(1):64–72. doi: 10.1111/j.1432-1033.1971.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Schindler D. G., Morris H. P. Mitochondrial polyriboadenylate polymerase: relative lack of activity in hepatomas. Science. 1972 Nov 10;178(4061):639–640. doi: 10.1126/science.178.4061.639. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Schindler D. G. Polyriboadenylate polymerase solubilized from rat liver mitochondria. Biochem Biophys Res Commun. 1972 Jul 11;48(1):126–134. doi: 10.1016/0006-291x(72)90353-1. [DOI] [PubMed] [Google Scholar]
- Jeanteur P., Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. II. Evidence for sequences of non-ribosmal type in 45 and 32 s ribosomal RNA precursors. J Mol Biol. 1968 May 14;33(3):757–775. doi: 10.1016/0022-2836(68)90318-5. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Lederman M., Attardi G. Expression of the mitochondrial genome in HeLa cells. XVI. Electrophoretic properties of the products of in vivo and in vitro mitochondrial protein synthesis. J Mol Biol. 1973 Aug 5;78(2):275–283. doi: 10.1016/0022-2836(73)90116-2. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Expression of the mitochondrial genome in HeLa cells. X. Properties of mitochondrial polysomes. J Mol Biol. 1972 Mar 28;65(2):273–289. doi: 10.1016/0022-2836(72)90282-3. [DOI] [PubMed] [Google Scholar]
- PERRY R. P. ROLE OF THE NUCLEOLUS IN RIBONUCLEIC ACID METABOLISM AND OTHER CELLULAR PROCESSES. Natl Cancer Inst Monogr. 1964 May;14:73–89. [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Perlman S., Abelson H. T., Penman S. Mitochondrial protein synthesis: RNA with the properties of Eukaryotic messenger RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):350–353. doi: 10.1073/pnas.70.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICH A., DAVIES D. R., CRICK F. H., WATSON J. D. The molecular structure of polyadenylic acid. J Mol Biol. 1961 Feb;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- SPIRIN A. S. The "temperature effect" and macromolecular structure of high-polymer ribonucleic acids of various origin. Biokhimiia. 1961 Nov-Dec;26:454–463. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A. Assembly of the mitochondrial membrane system. 8. Properties of the products of mitochondrial protein synthesis in yeast. J Biol Chem. 1972 Oct 25;247(20):6517–6523. [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Poly A associated with SV40 messenger RNA. Nat New Biol. 1972 Jul 26;238(82):111–113. doi: 10.1038/newbio238111a0. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]



