Abstract
Summary
The scorecard summarises key indicators of the burden of osteoporosis and its management in each of the member states of the European Union. The resulting scorecard elements were then assembled on a single sheet to provide a unique overview of osteoporosis in Europe.
Introduction
The scorecard for osteoporosis in Europe (SCOPE) is an independent project that seeks to raise awareness of osteoporosis care in Europe. The aim of this project was to develop a scorecard and background documents to draw attention to gaps and inequalities in the provision of primary and secondary prevention of fractures due to osteoporosis.
Methods
The SCOPE panel reviewed the information available on osteoporosis and the resulting fractures for each of the 27 countries of the European Union (EU27). The information researched covered four domains: background information (e.g. the burden of osteoporosis and fractures), policy framework, service provision and service uptake e.g. the proportion of men and women at high risk that do not receive treatment (the treatment gap).
Results
There was a marked difference in fracture risk among the EU27. Of concern was the marked heterogeneity in the policy framework, service provision and service uptake for osteoporotic fracture that bore little relation to the fracture burden. For example, despite the wide availability of treatments to prevent fractures, in the majority of the EU27, only a minority of patients at high risk receive treatment for osteoporosis even after their first fracture. The elements of each domain in each country were scored and coded using a traffic light system (red, orange, green) and used to synthesise a scorecard. The resulting scorecard elements were then assembled on a single sheet to provide a unique overview of osteoporosis in Europe.
Conclusions
The scorecard will enable healthcare professionals and policy makers to assess their country’s general approach to the disease and provide indicators to inform future provision of healthcare.
Keywords: SCOPE, Scorecard, Osteoporosis, Burden of disease, Cost, European Union, Treatment uptake, Treatment gap, Service provision, Service uptake, Policy framework
SCOPE
Scorecard for osteoporosis in Europe
About SCOPE
The ScoreCard for OsteoPorosis in Europe (SCOPE) is an independent project that seeks to raise awareness of osteoporosis care in Europe. SCOPE permits an in depth comparison of the quality of care of osteoporosis across the 27 member states of the European Union (EU27).
Osteoporosis is a complex disease that can be treated and managed in a number of ways. Improvements in medication and diagnostic techniques in the past 25 years have served to reduce the risk of osteoporotic fractures. In Europe, however, research has shown significant heterogeneity in the different national approaches to the management of the disease.
The scorecard summarises key indicators of the burden of osteoporosis and its management in each member state of the European Union to draw attention to the disparities in healthcare provision that can serve in the setting of benchmarks to inform patients, healthcare providers and policy makers in the EU.
The aim of this scorecard is to stimulate a balanced, common and optimal approach to the management of osteoporosis throughout the EU.
Table of contents
Letter to all Europeans 2
Introduction 3
1. Burden of disease 6
2. Policy framework 23
3. Service provision 33
4. Service uptake 48
Scorecard 59
Acknowledgments 61
Abbreviations and glossary 62
A letter to all Europeans
The statistics are startling.
One in three women and at least one in six men will suffer an osteoporotic fracture in their lifetime, and it is estimated that more than ten million men and women are at high risk of osteoporotic fractures in the European Union.
Osteoporosis and the 3.5 million fractures it causes cost the healthcare systems of Europe in excess of €39 billion each year based on data for 2010. But numbers don’t tell the full story. For the individuals who suffer fractures as a result of the disease, the stories are personal. Pain, disability, reduced mobility and long-term disability are all too frequent. Additionally, fractures related to osteoporosis result in early death. About 43,000 deaths occur each year in Europe as a direct consequence of hip or spine fractures.
The primary purpose of the scorecard for osteoporosis in Europe is to help individuals reduce their risk of osteoporosis and to ensure that all Europeans have access to the best diagnosis and treatment. Components that are critical to achieving this goal include government policy, access to assessment of risk and access to medications. This scorecard allows Europeans to measure how well their country is able to access these elements through the publicly funded healthcare systems. It also provides a benchmark to measure future progress.
Our research reveals that facilities and access to testing for osteoporosis are far from adequate. Access to drug treatment that can help prevent fractures varies markedly from country to country; in some member states, individuals with osteoporosis are restricted from accessing effective treatment options. Less than half of women at high risk of fracture are treated despite the high cost of fractures and the availability of affordable medications.
Action is required. The national osteoporosis societies of the International Osteoporosis Foundation are calling for a Europe-wide strategy and parallel national strategies to provide coordinated osteoporosis care and to reduce debilitating fractures and their impact on individual lives and the healthcare system. We welcome the opportunity to partner with governments at the national and European level to develop and implement these strategies. Together we can improve the bone health of all in Europe.
[Signatures to be invited from Scorecard panel]
Introduction
The basis for SCOPE
SCOPE comprises an independent panel of experts that have considered the information available on the burden of osteoporosis and healthcare provision and uptake in the EU27. SCOPE draws on independent research from two major sources. The first was a series of regional audits of the International Osteoporosis Foundation (IOF) [1–3]. This information base was broadened and updated by IOF to inform the SCOPE panel members through its outreach to over 30 national osteoporosis societies throughout Europe. The second major resource was a comprehensive report undertaken by the IOF and the Europian Federation of Pharmaceutical Industry Associations (EFPIA) on the burden of osteoporosis in the largest countries of the EU [4]. This was subsequently extended to all counties of the EU [5, 6] and made available to the panel.
From the information available, the panel developed indicators of osteoporosis that could be applied to each member state, categorised as:
Burden of disease—including the burden of osteoporosis, fractures and forecasts for the future
Policy framework—such as the availability of public health programmes
Service provision—including assessment and treatments of osteoporosis
Service uptake—e.g. the proportion of men and women at high risk that do not receive treatment (treatment gap).
Comparisons of indicators across countries are often limited by a lack of consistency of information retrieved across countries. One of the strengths of the resource documents considered by the panel is the consistency of the approach in documenting the burden of disease, wherever possible, by the use of country-specific information. The Scorecard Panel and the IOF invested substantial efforts to ensure that the European audits were updated by means of a structured questionnaire that was sent to all IOF national societies and key opinion leaders in each country. Discrepancies and ambiguities were resolved by correspondence. The panel recognised that consistency does not necessarily equal accuracy and, where information across countries is based only on opinion, this has been highlighted. The questionnaire is available on the web site of the IOF (http://www.iofbonehealth.org/).
For each domain, a synthesis was summarised and tabular information provided for each member state which appears in the body of the report. For key indicators, termed scorecard elements, the information was scored and the basis for the score allocation provided. For example, the remaining lifetime risk of a hip fracture at the age of 50 years ranged from 7.0 to 25.1 % in women from the different countries of the EU. Counties were categorised by tertile of risk. High risk countries were colour coded red, intermediate risk coded orange and low-risk countries coded green. A similar ‘traffic light’ approach was applied to each element in each domain. The resulting scorecard elements were then assembled on a single sheet to provide a unique overview of osteoporosis in Europe. It will enable healthcare professionals and policy makers to assess their country’s general approach to the disease and provide indicators to inform future provision of healthcare.
Some caveats are appropriate in the interpretation of scores. Green is not necessarily ‘good’ and red is not necessarily ‘bad’. An example of the former is the uptake of fracture liaison services. Whereas counties coded green have up to 10 % of hospitals with such a service, the panel would consider that 50 % or more hospitals would be an appropriate target. Coding all countries red would, however, not permit the comparative performance of one country against another. Other examples are highlighted in the text.
Osteoporosis
Osteoporosis is characterized by reduced bone mass and disruption of bone architecture, resulting in increased bone fragility and increased fracture risk [7]. The publication of a World Health Organization (WHO) report on the assessment of fracture risk and its application to screening for postmenopausal osteoporosis in 1994 provided diagnostic criteria for osteoporosis based on the measurement of bone mineral density (BMD) and recognised osteoporosis as an established and well-defined disease that affected more than 75 million people in the United States, Europe and Japan [8].
The diagnostic criterion for osteoporosis is based on the measurement of BMD [9]. Bone mineral density is most often described as a T score that describes the number of SDs by which the BMD in an individual differs from the mean value expected in young healthy women. The operational definition of is defined as a value for BMD 2.5 SD or more below the young female adult mean (T score less than or equal to −2.5 SD). BMD at the femoral neck is the international reference standard [10]. The consequences of low BMD reside in the fractures that arise. The relationship between BMD and fracture is continuous in that the lower the BMD, the higher the fracture risk [11].
Osteoporotic fractures
The most common fractures associated with osteoporosis are those at the hip, spine, forearm and humerus but many other fractures after the age of 50 years are associated with low BMD and should be regarded as osteoporotic [12]. These include fractures of the ribs, tibia, pelvis and other femoral fractures. The causation of fractures is not solely dependent on BMD but is multifactorial. Many factors such as liability to falling, age etc. contribute to the risk of fracture. Thus, not all fragility fractures occur in individuals with a BMD T score of −2.5 SD, and the terms osteoporosis, fragility fracture and osteoporotic fractures have inherent ambiguities. For the purpose of this report, the term osteoporosis is used in a generic sense rather than a specific sense unless otherwise specified. For example the ‘cost of osteoporosis’ refers to the cost of fractures at sites associated with osteoporosis irrespective of the T score.
The incidence of fragility fractures increases markedly with age, though the rate of rise with age differs for different fracture outcomes. For this reason, the proportion of fractures at any site also varies with age. For example, forearm fractures account for a greater proportion at younger ages than in the elderly. Conversely, hip fractures are rare at the age of 50 years but become the predominant osteoporosis fracture from the age of 75 years. In women, the median age for distal forearm fractures is around 65 years and for hip fracture, 80 years. Thus, both the number of fractures and the type of fracture are critically dependent on the age of the populations at risk.
Hip fracture is the most serious osteoporotic fracture. Hip fracture is painful and nearly always necessitates hospitalisation and surgical intervention. Up to 20 % of patients die in the first year following hip fracture, mostly as a result of serious underlying medical conditions [13], and less than half of survivors regain the level of function that they had prior to the hip fracture [14]. Thus, not all deaths associated with hip fracture are due to the hip fracture event and it is estimated that approximately 30 % of deaths are causally related. When this is taken into account, hip fracture causes more deaths than road traffic accidents in Sweden and about the same number as those caused by breast cancer [15].
References
1. International Osteoporosis Foundation (2001) Osteoporosis in the European Community: A call to action. An audit of policy developments since 1998. International Osteoporosis Foundation, Nyon, Switzerland.
2. International Osteoporosis Foundation (1998) Report on osteoporosis in the European Community: A call to action. Action for prevention. International Osteoporosis Foundation, Nyon, Switzerland
3. International Osteoporosis Foundation (2010) Osteoporosis in the European Union in 2008. Ten years of progress and ongoing challenges. Updated Aug 2010. International Osteoporosis Foundation, Nyon. Available at www.iofbonehealth.org. accessed 23rd Sept 2012
4. Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis; Burden, health care provision and opportunities in the EU. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155. DOI 10.1007/s11657-011-0060-1
5. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
6. Svedbom A, Hernlund E, Ivergård, M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA and the EU review panel of the IOF (2013) Osteoporosis in the European Union: A compendium of country-specific reports. Arch Osteoporos, in press
7. Anonymous (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94: 646–50
8. World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843, Geneva
9. Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9: 1137–41
10. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42: 467–75
11. Johnell O, Kanis JA, Oden A et al. (2005) Predictive value of bone mineral density for hip and other fractures. J Bone Miner Res 20:1185–1194
12. Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, Cummings SR (1991) Which fractures are associated with low appendicular bone mass in elderly women? The study of osteoporotic fractures research group. Ann Intern Med 115: 837–42
13. Keene GS, Parker MJ, Pryor GA (1993) Mortality and morbidity after hip fractures. BMJ 307: 1248–50
14. Melton LJ 3rd (2003) Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 18: 1139–41
15. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32: 468–73
Chapter 1. Burden of disease
1a Economic framework
Domain
Burden of disease—background information
Background and aims
Cost of illness studies provide no direct guidance on how resources should be allocated, but may provide relevant information concerning the consequences of a disease that may inform policy. Such data may aid decisions concerning societal resource allocation for research, development, and funding of new treatments. Results from cost-of-illness studies can also be utilised to assess the long-term consequences and value of medical progress.
The objective of this background section is to estimate the current cost of osteoporotic fracture in the countries of the European Union set against the wealth of the nation and the healthcare spend of that wealth. A more detailed consideration of the cost is given in Chapter 1b.
Methods
Direct costs of fractures in men and women from the EU27 aged 50 years or more were expressed as a proportion of total health care spending in the respective country [1] and as the cost per capita of the general population [2, 3].
Results
Health care spending varied markedly between countries, ranging from €500 million in Malta to €281 billion in Germany (Table 1). The total spend on healthcare in the European Union amounted to €1,260 billion, with the cost of osteoporotic fractures representing approximately 3 % of the healthcare spend (€37.4 billion in 2010). This clearly demonstrates a substantial impact on the present healthcare budget
Table 1.
Cost of osteoporotic fractures in relation to the population and health care spending (2010)
| Country | Population (000) | Health care spending (€000,000) | Health care spending (% GDP) | Health care spending (€/capita) | Fracture cost (% health care spending) |
|---|---|---|---|---|---|
| Austria | 8,392 | 31,000 | 10.2 | 3,741 | 2.5 |
| Belgium | 10,712 | 42,000 | 9.9 | 3,903 | 1.5 |
| Bulgaria | 7,493 | 2,700 | 7.2 | 354 | 1.6 |
| Cyprus | 1,103 | 1,000 | 6.2 | 937 | 5.2 |
| Czech Republic | 10,493 | 11,000 | 6.9 | 1,087 | 2.2 |
| Denmark | 5,551 | 26,000 | 10.8 | 4,759 | 4.0 |
| Estonia | 1,339 | 1,000 | 5.2 | 747 | 3.0 |
| Finland | 5,365 | 18,000 | 8.2 | 3,263 | 2.2 |
| France | 62,634 | 227,000 | 11.0 | 3,617 | 2.1 |
| Germany | 82,056 | 281,000 | 10.6 | 3,418 | 3.2 |
| Greece | 11,358 | 24,000 | 9.5 | 2,126 | 2.9 |
| Hungary | 9,985 | 7,000 | 8.3 | 709 | 3.6 |
| Ireland | 4,470 | 15,000 | 7.5 | 3,399 | 1.5 |
| Italy | 60,098 | 148,000 | 9.0 | 2,461 | 4.7 |
| Latvia | 2,252 | 1,000 | 6.6 | 520 | 3.2 |
| Lithuania | 3,325 | 2,000 | 6.2 | 546 | 2.6 |
| Luxembourg | 506 | 3,000 | 7.3 | 6,235 | 0.7 |
| Malta | 416 | 500 | 8.4 | 1,108 | 3.8 |
| Netherlands | 16,610 | 64,000 | 9.4 | 3,829 | 1.3 |
| Poland | 38,276 | 25,000 | 6.2 | 660 | 2.4 |
| Portugal | 10,676 | 19,000 | 10.2 | 1,826 | 3.0 |
| Romania | 21,486 | 7,000 | 4.5 | 309 | 2.0 |
| Slovakia | 5,463 | 3,000 | 7.1 | 1,092 | 3.6 |
| Slovenia | 2,028 | 6,000 | 8.4 | 1,485 | 0.9 |
| Spain | 45,317 | 102,000 | 8.4 | 2,247 | 2.8 |
| Sweden | 9,294 | 34,000 | 9.2 | 3,709 | 4.3 |
| UK | 61,899 | 159,000 | 8.2 | 2,564 | 3.4 |
| EU27 | 498,597 | 1,260,000 | 2,528 | 3.0 |
The share of health care spending allocated to osteoporosis varied across countries, ranging from 0.7 % in Luxembourg to 5.2 % in Cyprus (Fig. 1). As might be expected there was a significant but modest relationship between the amount spent on osteoporosis, GDP and the incidence of osteoporotic fractures.
Fig. 1.
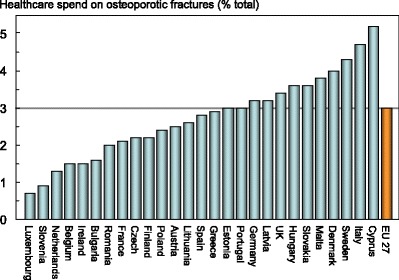
Proportion (%) of the total direct healthcare spend in the EU27 countries allocated to osteoporotic fractures [3]
The estimated cost of osteoporosis may be compared to the cost of other diseases. However, given that the EU27 is a relatively new construct, few directly comparable studies exist. Furthermore, methodological differences render some studies difficult to compare. However, a few studies are available conducted in a similar geographic area with comparable methodology.
In a report issued by the European Brain Council, the yearly societal costs for a number of brain disorders in the EU27 were estimated at €105 billion for dementia, €43.5 billion for headache, €14.6 billion for multiple sclerosis, and €13.9 billion for Parkinson’s disease [4].
The cost of coronary heart disease and cerebrovascular disease in the European Union (25 countries) has been estimated at approximately €45 billion and €34 billion, respectively, at 2003 prices [4]. The cost of epilepsy in the European Union (25 countries) has been estimated at €15.5 billion at 2004 prices. Healthcare costs comprised 18 % of costs, whereas direct medical costs and productivity losses represented 27 % and 55 %, respectively [5]. Thus, in relation to other common non-communicable diseases osteoporosis has major economic consequences for society.
Score allocation
None—not a score card element
Comment
It should be noted that not all fracture-related costs come from the countries’ healthcare budgets (e.g. long-term care and variable reimbursement policies). Data on healthcare spending are for 2006.
References
1. World Health Organization (2009) World Health Statistics. (2009) 107. Health expenditure www.who.int/whosis/whostat/EN_WHS09_Table7.pdf Accessed 21st December 2011
2. UN (2010) Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat (2010) World Population Prospects: The 2010 Revision, http://esa.un.org/unpd/wpp/index.htm Accessed 16th December 2011
3. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Archives of Osteoporosis, in press
4. Gustavsson A, Svensson M, Jacobi F, et al. (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:718–779
5. Pugliatti M, Beghi E, Forsgren L, Ekman M, Sobocki P (2007) Estimating the cost of epilepsy in Europe: a review with economic modeling. Epilepsia 48:2224–2233
1b Healthcare cost of osteoporotic fractures
Domain
Burden of disease—background information
Background and aims
Cost of illness studies can take on a societal perspective (includes all cost carried directly or indirectly by society) or a payer perspective (usually includes all costs carried by the healthcare and social system). Both play an important role in the understanding of disease implications and may aid decisions concerning societal resource allocation for research, development, and funding of new treatments. Results from cost of illness studies can also be utilised to assess the value of medical progress.
The main objective of this section is to provide detail on the current cost of osteoporotic fractures in the countries of the European Union.
Methods
The cost of osteoporotic fractures was first determined without intangible costs (i.e. the monetary value of QALYs lost due to death and disability) [1]. Costs of fracture-related productivity losses were not included because they are only incurred in patients below retirement age—median age 60 years in Europe [2]—and are less than 1 % of hip fracture cost in Sweden [3].
Empirical but incomplete cost estimates were available for Austria, Belgium, Czech Republic, Denmark, Finland, Germany, Ireland, Italy, Netherlands, Portugal Slovenia, Sweden and the UK. For countries where fracture costs were not found, the costs were imputed from the nearest country available by adjusting for differences in healthcare price levels between the relevant countries.
Costs were divided into the direct cost of fractures in 2010, the ongoing cost in 2010 of fractures occurring before 2010 (‘long-term disability’), and the cost of intervention for osteoporosis. It was conservatively assumed that fractures other than those at the hip did not incur any longer-term costs after the first year. Hip fracture costs in the second and following years after the event were based on the proportion of patients that become dependent in the long-term.
The health burden of osteoporosis was additionally measured in terms QALYs lost. The QALY is a multi-dimensional outcome measure that incorporates both the quality (health related) and quantity (length) of life. The value of a QALY was set at value of 2× GDP per capita [4].
Results
The direct cost of osteoporosis in the EU27 from the fractures that occurred in 2010 was €24.6 billion (Table 2). To this is added the ongoing cost in 2010 incurred by fractures that occurred before 2010 which amounted to €10.7 billion (long-term disability). The cost of pharmacological intervention (assessment and treatment) was €2.1 billion. Thus, the total direct cost in the EU27 (excluding the value of QALYs lost) amounted to €37.4 billion in 2010. First year, subsequent year, and pharmacological costs accounted for 66, 29 and 5 % of the costs respectively.
Table 2.
Cost of osteoporosis in the EU27 in 2010 (€ million, 2010) [1]
| Country | Incident fractures | Long-term disability | Intervention | Total | Cost per capita (€) | QALYs lost (€m) |
|---|---|---|---|---|---|---|
| Austria | 540 | 229 | 30 | 799 | 95 | 1 903 |
| Belgium | 419 | 157 | 29 | 606 | 57 | 1 734 |
| Bulgaria | 30 | 11 | 1 | 42 | 6 | 118 |
| Cyprus | 34 | 7 | 12 | 52 | 47 | 78 |
| Czech Republic | 165 | 56 | 53 | 273 | 26 | 630 |
| Denmark | 718 | 300 | 37 | 1,055 | 190 | 1 704 |
| Estonia | 22 | 7 | 1 | 30 | 22 | 59 |
| Finland | 269 | 104 | 10 | 383 | 71 | 829 |
| France | 3,179 | 1,329 | 346 | 4,853 | 77 | 8 309 |
| Germany | 6,617 | 2,055 | 336 | 9,008 | 110 | 14 927 |
| Greece | 488 | 102 | 91 | 680 | 60 | 1 263 |
| Hungary | 127 | 30 | 40 | 197 | 20 | 464 |
| Ireland | 125 | 62 | 35 | 223 | 50 | 426 |
| Italy | 4,269 | 2,402 | 361 | 7,032 | 117 | 8 771 |
| Latvia | 29 | 7 | 2 | 38 | 17 | 72 |
| Lithuania | 32 | 12 | 3 | 47 | 14 | 81 |
| Luxembourg | 15 | 4 | 2 | 22 | 43 | 148 |
| Malta | 11 | 4 | 2 | 17 | 41 | 24 |
| Netherlands | 360 | 434 | 29 | 824 | 50 | 1 863 |
| Poland | 355 | 162 | 76 | 593 | 16 | 991 |
| Portugal | 293 | 264 | 20 | 577 | 54 | 580 |
| Romania | 88 | 35 | 7 | 129 | 6 | 339 |
| Slovakia | 76 | 19 | 11 | 107 | 20 | 283 |
| Slovenia | 36 | 13 | 7 | 56 | 28 | 168 |
| Spain | 1,372 | 1,055 | 414 | 2,842 | 63 | 3 271 |
| Sweden | 927 | 529 | 29 | 1,486 | 160 | 2 666 |
| UK | 3,977 | 1,328 | 103 | 5,408 | 87 | 8 698 |
| EU27 | 24,574 | 10,718 | 2,087 | 37,378 | 75 | 57 243 |
Whilst the proportion of costs for pharmacological intervention to total costs was low on average, some inter-country variation was observed: the lowest proportion of costs attributable to intervention was observed in Sweden (2 %) and the highest costs in Hungary (4.7 %). Hip fractures were estimated to account for 54 % of the total costs, other fractures 39 %, vertebral fractures 5 %, and forearm fractures 2 %.
On average, the direct cost of osteoporotic fractures was €75 for each individual in the EU27. There was a large variation in the ‘osteoporosis tax’ (cost per capita) which was highest in Denmark (€188/person) and Sweden (€159) and lowest in Bulgaria (€6) and Romania (€6). The heterogeneity of this cost is in part related to the incidence of fracture (r = 0.67, p = 0.001) and the healthcare spend per capita (r = 0.63, p = 0.004).
The cost of QALYs lost in the EU27 was substantial amounting to €57.2 billion, giving a total cost of €94.6 billion in 2010. Intervention costs amounted to 2 % of the total cost (Fig. 2) and 5 % of the direct costs.
Fig. 2.
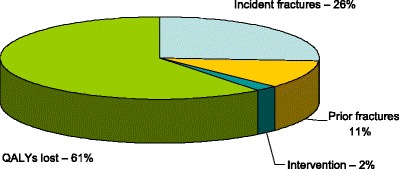
Components (%) of the cost of osteoporosis and fractures [1]
Score allocation
None—not a score card element
Comment
There are few directly comparable studies in other non-communicable diseases that exist.
For coronary heart disease, healthcare costs, productivity losses, and informal care comprised 51, 34 and 15 %, respectively. Costs for pharmacological treatment accounted for 12 % of the total cost, substantially higher than that for osteoporosis. For cerebrovascular disease, healthcare costs, productivity losses, and informal care comprised 61, 18 and 21 %, respectively. The cost for pharmacological treatment accounted for 3 % of the total cost for cerebrovascular disease [5], somewhat lower than that for osteoporosis.
References
1. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
2. European Commission (2012) Eurostat. Statistics database. Data retreived in November, 2011; http://epp.eurostat.ec.europa.eu
3. Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D (1997) Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int 7: 390–406
4. Borgstrom F, Johnell O, Kanis JA, Jonsson B, Rehnberg C. (2006) At what hip fracture risk is it cost-effective to treat? International intervention thresholds for the treatment of osteoporosis. Osteoporos Int 17:1459–71.
5. Leal J, Luengo-Fernandez R, Gray A, Petersen S, Rayner M (2006) Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J 27:1610–1619
1c Men and women with osteoporosis
Domain
Burden of disease—background information
Background and aims
Osteoporosis is diagnosed using dual-energy X-ray absorptiometry (DXA) to measure bone mineral density (BMD). The diagnostic reference site is the femoral neck using the NHANES III reference data [1]. Osteoporosis is diagnosed when the BMD measured at the femoral neck is more than 2.5 standard deviations below the average value of the young white female population [2]. The aim of this background information was to document the burden of osteoporosis as judged by densitometric criteria.
Methods
Accurate estimates of the prevalence of osteoporosis require country-specific data on the distribution of femoral neck BMD. However, large population-based reference data are lacking in the EU27 countries. For the purposes of this report, it is assumed that the mean femoral neck BMD is similar across EU countries at the age of 50 years as is the rate of bone loss at the femoral neck with age. The same assumptions have been used elsewhere [3–8]. On this basis, the prevalence of osteoporosis was calculated from the age- and sex-specific BMD in the NHANES III study. These prevalence estimates were then applied to the population demography in each EU country [9].
Results
In 2010, there were approximately 27.6 million men and women with osteoporosis in the EU27, of which 5,500,000 were men and 22,100,000 were women, i.e. there were four times as many women with osteoporosis as there were men. Of all member states, Germany was estimated to have the highest number of individuals with osteoporosis with approximately 1 million osteoporotic men and 4 million osteoporotic women. Overall, the prevalence of osteoporosis was 6.6 and 22.1 % in men and women aged 50 years or more (Table 3). In men over the age of 50 years, the prevalence of osteoporosis varied from 5.9 (Poland) to 7.2 % (Luxembourg). In women, the prevalence ranged from 19.1 (Cyprus) to 23.5 % (France).
Table 3.
Estimated number of men and women with osteoporosis, prevalence in population over 50 years, and prevalence in the total population, 2010 [9]
| Country | Men with osteoporosis | Women with osteoporosis | Men and women with osteoporosis | Prevalence in male population aged 50 or more (%) | Prevalence in female population aged 50 or more (%) | Prevalence in total population (%) |
|---|---|---|---|---|---|---|
| Austria | 89,862 | 368,685 | 458,547 | 6.5 | 22.2 | 5.5 |
| Belgium | 120,695 | 476,875 | 597,570 | 6.6 | 22.4 | 5.6 |
| Bulgaria | 81,482 | 336,425 | 417,907 | 6.4 | 20.9 | 5.6 |
| Cyprus | 9,263 | 31,032 | 40,295 | 6.2 | 19.3 | 3.7 |
| Czech Republic | 103,114 | 425,944 | 529,058 | 6.0 | 20.4 | 5.0 |
| Denmark | 61,456 | 221,912 | 283,368 | 6.5 | 21.1 | 5.1 |
| Estonia | 11,642 | 65,789 | 77,431 | 6.2 | 22.2 | 5.8 |
| Finland | 61,054 | 243,399 | 304,453 | 6.4 | 21.5 | 5.7 |
| France | 691,112 | 2,784,198 | 3,475,310 | 6.7 | 22.5 | 5.5 |
| Germany | 1,006,652 | 4,017,260 | 5,023,912 | 6.6 | 22.6 | 6.1 |
| Greece | 135,202 | 507,505 | 642,707 | 6.9 | 22.3 | 5.7 |
| Hungary | 94,949 | 452,158 | 547,107 | 6.2 | 21.1 | 5.5 |
| Ireland | 37,127 | 129,309 | 166,436 | 6.2 | 20.0 | 3.7 |
| Italy | 749,237 | 3,042,794 | 3,792,031 | 6.9 | 23.4 | 6.3 |
| Latvia | 19,210 | 111,236 | 130,446 | 6.1 | 22.3 | 5.8 |
| Lithuania | 27,136 | 148,375 | 175,511 | 6.1 | 21.7 | 5.3 |
| Luxembourg | 4,541 | 17,422 | 21,963 | 6.1 | 21.0 | 4.3 |
| Malta | 4,190 | 16,074 | 20,264 | 5.9 | 19.8 | 4.9 |
| Netherlands | 175,244 | 643,258 | 818,502 | 6.3 | 20.8 | 4.9 |
| Poland | 338,756 | 1,509,772 | 1,848,528 | 5.8 | 20.1 | 4.8 |
| Portugal | 117,738 | 475,882 | 593,620 | 6.7 | 22.0 | 5.6 |
| Romania | 198,065 | 835,885 | 1,033,950 | 6.2 | 20.5 | 4.8 |
| Slovakia | 42,726 | 188,911 | 231,637 | 5.7 | 19.4 | 4.2 |
| Slovenia | 20,543 | 89,489 | 110,032 | 6.0 | 21.5 | 5.4 |
| Spain | 496,368 | 1,952,987 | 2,449,355 | 6.8 | 22.6 | 5.4 |
| Sweden | 113,722 | 409,373 | 523,095 | 6.9 | 22.4 | 5.6 |
| UK | 679,424 | 2,527,331 | 3,206,755 | 6.7 | 21.9 | 5.2 |
| EU27 | 5,490,510 | 22,029,280 | 27,519,790 | 6.6 | 22.1 | 5.5 |
The prevalence of osteoporosis in the entire EU27 population (i.e. all ages) was 5.5 % and ranged from 3.7 % in Cyprus and Ireland to 6.3 % in Italy (Fig. 3).
Fig. 3.
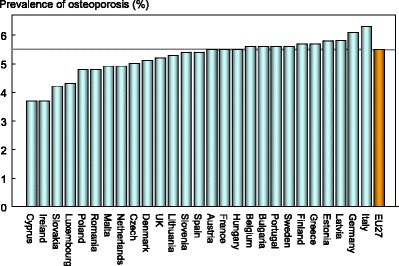
Components (%) of the cost of osteoporosis and fractures [1]
Score allocation
None—not a score card element
Comment
Although BMD is a strong predictor of fracture risk [10, 11], the prevalence of osteoporosis is not used as a score card element because the relationship of osteoporosis to fracture risk varies by age and between countries [12]. For this reason, fracture risk is the preferred metric.
References
1. Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr., Lindsay R (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8: 468–89
2. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, III, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42: 467–75
3. Gauthier A, Kanis JA, Jiang Y, Dreinhöfer K, Martin M, Compston J, Borgström F, Cooper C, McCloskey E (2012) Burden of postmenopausal osteoporosis in Germany: estimations from a disease model. Arch Osteoporos 7:209–18
4. Gauthier A, Kanis JA, Jiang Y, Martin M, Compston J, Borgström F, Cooper C, McCloskey E (2011) Epidemiological burden of postmenopausal osteoporosis in the UK from 2010 to 2021: Estimations from a Disease Model. Arch Osteoporos 6: 179–188
5. Gauthier A, Kanis JA, Martin M, Compston J, Borgström F, Cooper C, McCloskey E; Committee of Scientific Advisors, International Osteoporosis Foundation (2011) Development and validation of a disease model for postmenopausal osteoporosis. Osteoporos Int 22: 771–80
6. Cawston H, Maravic M, Fardellone P, Gauthier A, Kanis JA, Compston J, Borgström F, Cooper C, McCloskey E (2012) Epidemiological burden of postmenopausal osteoporosis in France from 2010 to 2020: estimations from a disease model. Arch Osteoporos. 7: 237–46
7. Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis; Burden, health care provision and opportunities in the EU. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
8. Odén A, McCloskey EV, Johansson H, Kanis JA (2013) The worldwide impact of osteoporosis on the burden of hip fractures. Calcif Tiss Int 92: 42–49
9. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
10. Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J 312: 1254–1259
11. Johnell O, Kanis JA, Oden A et al. (2005) Predictive value of bone mineral density for hip and other fractures. J Bone Miner Res 20:1185–1194
12. Kanis JA on behalf of the World Health Organization Scientific Group (2008) Assessment of osteoporosis at the primary healthcare level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK
1d Epidemiology of hip fracture
Domain
Burden of disease—scorecard element
Background and aims
Fracture incidence is poorly documented in the EU. The fracture incidence that has been best evaluated is hip fracture. Hip fractures account for the majority of health care expenditure, mortality and morbidity and can be used as a proxy for osteoporosis. There is a marked difference in the incidence of hip fracture worldwide and probably in other osteoporotic fractures [1]. Indeed, the difference in incidence between countries within Europe is greater than the differences in incidence between sexes within a country [2, 3]. The EU comprises countries with some of the highest hip fracture rates, but the documentation of the size of the problem and the quality of data vary between countries.
The aim of this scorecard element was to summarise the information base available for the incidence of hip fracture.
Methods
Studies on hip fracture risk were identified from 1950 to November 2011 by a Medline OVID search. Evaluable studies in each country were reviewed for quality and representativeness and a study (studies) chosen to represent that country. Age-specific incidence rates were age-standardised to the world population in 2010 in men and in women [1].
Results
National data on hip fracture rates were identified in 17 member states (Table 4). No data were available for four countries (Bulgaria, Cyprus, Latvia, and Luxembourg). In the remaining six countries, regional estimates were identified. For Estonia and Slovenia data were available in women only.
Table 4.
Information available on age-standardised (2010) hip fracture rates (/100,000/year) in countries of the European Union [1]
| Year | Sample | Incidence | F/M | ||
|---|---|---|---|---|---|
| Women | Men | ||||
| Austria | 2001–5 | National | 501 | 246 | 2.0 |
| Belgium | 2005–7 | National | 356 | 169 | 2.1 |
| Bulgaria | |||||
| Cyprus | |||||
| Czech Republic | 2008–9 | National | 374 | 211 | 1.8 |
| Denmark | 2004 | National | 574 | 290 | 2.0 |
| Estonia | 1991–4 | Regional | 225 | – | – |
| Finland | 2000–5 | National | 293 | 180 | 1.6 |
| France | 2004 | National | 291 | 126 | 2.3 |
| Germany | 2003–4 | National | 346 | 166 | 2.1 |
| Greece | 1986–92 | Regional | 326 | 158 | 2.1 |
| Hungary | 1999–03 | National | 367 | 206 | 1.8 |
| Ireland | 2008–10 | National | 406 | 191 | 2.1 |
| Italy | 2007 | National | 334 | 140 | 2.4 |
| Latvia | |||||
| Lithuania | 2010 | National | 270 | 156 | 1.7 |
| Luxembourg | |||||
| Malta | 2003–7 | National | 355 | 160 | 2.2 |
| Netherlands | 2005 | National | 249 | 121 | 2.1 |
| Poland | 2008 | Regional | 224 | 133 | 1.7 |
| Portugal | 2000–2 | National | 268 | 98 | 2.7 |
| Romania | 2005–9 | National | 198 | 142 | 1.4 |
| Slovakia | 2007 | National | 401 | 263 | 1.5 |
| Slovenia | 2003 | National | 349 | – | – |
| Spain | 1984–91 | Regional | 228 | 92 | 2.5 |
| Sweden | 1991 | Regional | 539 | 247 | 2.2 |
| UK | 1992–3 | Regional | 349 | 140 | 2.5 |
As expected, hip fracture rates were higher in women than in men with a female/male ratio that ranged from 1.4 (Romania) to 2.7 (Portugal). There was a nearly three-fold range of hip fracture rates throughout the EU from 198/100,000 (Romania) to 574/100,000 (Denmark). Thus, the international variation between countries was greater than the differences between men and women within countries.
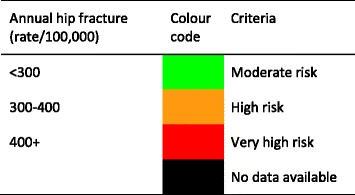
Score criteria
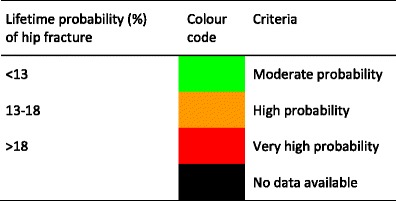
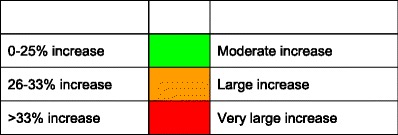
The age-standardised incidence was ranked. Women were chosen since fracture rates are more robust and it permitted the inclusion of Estonia and Slovenia for which no data were available in men. The criteria for categorisation were chosen as described in Table 5.
Table 5.
Criteria for allocating scores
Score allocation
The ranked incidence is shown in Fig. 4 and colour coded by category.
Fig. 4.
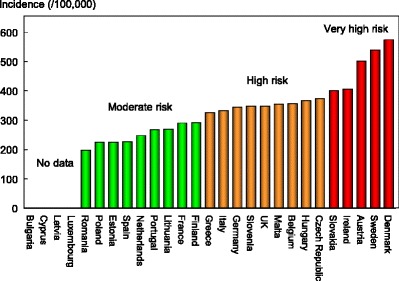
Annual incidence of hip fracture in women from countries of the EU age-standardised to the world population for 2010 [1]
Comment
On an international scale, all countries were at moderate or high risk (150–250/100,000 and >250/100,000, respectively) [1].
Reasons for the large variation in fracture risk between countries are speculative, but, ecological studies have shown a weak but significant relationship between hip fracture risk and latitude and socio-economic prosperity [4].
References
1. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C on behalf of the IOF Working Group on Epidemiology and Quality of Life (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23: 2239–2256.
2. Johnell, O, Gullberg, B, Allender, A, Kanis, JA, and the MEDOS Study Group (1992) The apparent incidence of hip fracture in Europe. Osteoporos Int 2, 298–302.
3. Elffors L, Allander E, Kanis JA, Gullberg B, Johnell O, Dequeker J, Dilzen G, Gennari C, Lopez-Vaz AA, Lyritis G, Mazzuoli GF, Miravet L, Passeri M, Perez Cano R, Rapado A, Ribot C (1994) The variable incidence of hip fracture in Southern Europe. The MEDOS Study. Osteoporos Int 4, 253–263.
4. Johnell O, Borgström F, Jonsson B, Kanis J (2007) Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int 18:333–337
1e Number of fragility fractures
Domain
Burden of disease—scorecard element
Background and aims
The most obvious and serious effect of osteoporosis is the fractures that occur as a consequence of increased bone fragility. This section determines the number of fractures associated with bone fragility in the EU27.
Methods
The fractures of interest include those at the hip, spine and forearm as well as osteoporotic fractures at other vulnerable sites (humerus, ribs, tibia, pelvis and other femoral fractures) grouped as other fractures. Information on the incidence of osteoporotic fractures varies between the countries of the EU27. In general, reports on hip fracture incidence are more complete than for fractures at other sites (see Chapter 1d).
The risk of hip fracture was taken from a systematic review of hip fracture incidence [1]. For the EU27 countries with incomplete information, incidence was taken from the nearest country where hip fracture incidence was available [2]. Where the incidence of fractures other than the hip was not available, the incidence was imputed from the hip fracture incidence in the relevant country, using the relationship between hip fracture incidence and incidence of fracture in other sites in Sweden [3].
The number of fractures in each country for each fracture site was computed from the age- and sex-specific estimates of incidence and population demography for 2010 [4]. Crude incidence in each country was expressed as the number of fragility fractures per 1000 of the population aged 50 years or more.
Results
There were estimated to be 3.5 million new fragility fractures in the EU in 2010—equivalent to 9,556 fractures/day (or 390/h) (Table 6). Almost twice as many fractures occurred in women compared to men. Hip, vertebral, forearm and other fractures accounted for 18, 15, 16 and 51 % of all fractures, respectively.
Table 16.
Characteristics of information available on fracture rates in the European Union
| Incidence of hip fracture | Established National Fracture Registries | Score | |||
|---|---|---|---|---|---|
| Qualitya | Sampleb | Present | Datac | ||
| Austria | G | N | Nod | 3 | |
| Belgium | G | N | Yes | Hip | 3 |
| Bulgaria | No | 0 | |||
| Cyprus | No | 0 | |||
| Czech Republic | G | N | No | 2 | |
| Denmark | G | N | Yes | Hip+ | 3 |
| Estonia | P | R | No | 1 | |
| Finland | G | N | Yes | Hip+ | 3 |
| France | G | N | Nod | 2 | |
| Germany | G | N | Yes | Hip+ | 3 |
| Greece | P/F/G | R | No | 1 | |
| Hungary | G | N | Yes | Hip+ | 3 |
| Ireland | G | N | Yes | Hip | 3 |
| Italy | G | N | Nod | Hip+ | 2 |
| Latvia | R | Yes | Hip+ | 3 | |
| Lithuania | F | R | No | 1 | |
| Luxembourg | No | 0 | |||
| Malta | G | N | No | 2 | |
| Netherlands | G | N | Yes | Hip+ | 3 |
| Poland | F | R | No | 1 | |
| Portugal | G | N | Yes | Hip+ | 3 |
| Romania | G | N | No | 2 | |
| Slovakia | G | N | Yes | Hip+ | 3 |
| Slovenia | F | N | No | 2 | |
| Spain | F/G | R | Nod | Hip+ | 1 |
| Sweden | G | R | Yes | Hip | 3 |
| UK | G | R | Yes | Hip | 3 |
Responses derived from questionnaire to National Societies
aQuality: G good; F fair; P poor [2]
bCatchment: N national; R regional
c Hip Registration of hip fracture only. Hip+ Registration of hip and other fracture outcomes
dRegional registers available
The number of incident fractures per country is shown in Table 7. Germany had the highest number of fractures for all fracture types in both men and women—approximately 724 000 incident fractures in total—predominately reflecting a large population size and comparatively high fracture incidence. Malta and Luxembourg had the lowest number of fractures for all types—(less than 3 000 incident fractures in each country), reflecting small population sizes.
Table 7.
The number of new fragility fractures in 2010 in men and women by country, the population at risk (men and women aged 50 years or more) and the crude incidence (/1000 of the population) [2]
| Country | New fractures | Population at risk (000) | Rate/1,000 |
|---|---|---|---|
| Austria | 86,536 | 3,041 | 28.5 |
| Belgium | 79,893 | 3,959 | 20.2 |
| Bulgaria | 38,198 | 2,876 | 13.3 |
| Cyprus | 5,129 | 311 | 16.5 |
| Czech Republic | 72,195 | 3,802 | 19.0 |
| Denmark | 66,358 | 2,003 | 33.1 |
| Estonia | 8,688 | 485 | 17.9 |
| Finland | 36,405 | 2,090 | 17.4 |
| France | 376,774 | 22,645 | 16.6 |
| Germany | 724,774 | 33,010 | 22.0 |
| Greece | 85,518 | 4,236 | 20.2 |
| Hungary | 102,457 | 3,683 | 27.8 |
| Ireland | 18,085 | 1,246 | 14.5 |
| Italy | 465,400 | 23,788 | 19.6 |
| Latvia | 14,305 | 812 | 17.6 |
| Lithuania | 15,074 | 1,127 | 13.4 |
| Luxembourg | 2700 | 158 | 17.1 |
| Malta | 2641 | 152 | 17.4 |
| Netherlands | 75,947 | 5,893 | 12.9 |
| Poland | 167,664 | 13,350 | 12.6 |
| Portugal | 51,821 | 3,922 | 13.2 |
| Romania | 94,282 | 7,289 | 12.9 |
| Slovakia | 38,634 | 1,730 | 22.3 |
| Slovenia | 15,510 | 759 | 20.4 |
| Spain | 204,151 | 15,905 | 12.8 |
| Sweden | 107,046 | 3,489 | 30.7 |
| United Kingdom | 535,873 | 21,636 | 24.8 |
| EU27 | 3,492,058 | 183,397 | 19.0 |
When fracture numbers were expressed as a rate of the population at risk, there was a greater than two-fold range in risk that varied from 12.6/1000 in Poland to 33.1/1000 in Denmark.
In addition to pain and disability, some osteoporotic fractures are associated with premature mortality. About 30 % of deaths after a hip or clinical spine fracture can be attributed to the fracture event [5–7]. In the EU, there were estimated to be 43,000 deaths causally related to in 2010. Approximately 50 % of fracture-related deaths in women were due to hip fractures, 28 % to clinical vertebral and 22 % to other fractures. Corresponding proportions for men were 47, 39 and 14 %, respectively. Fracture-related deaths by country are shown in Fig. 5. Note that the variability in death rates is more a reflection of the variable incidence of fractures rather than in standards of care.
Fig. 5.
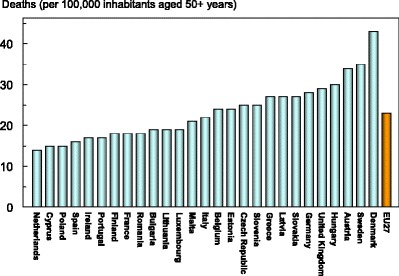
The number of deaths associated with fracture events expressed per 100,000 of the population added 50 years or more in the EU27 [2]
Score criteria
The number of fragility fractures in men and women combined in 2010 expressed/1,000 of the population aged 50 years or more was categorised approximately by tertiles as given in Table 8.
Table 8.
Criteria for allocating scores

Score allocation
Countries, ranked and categorised by risk, are shown in Fig. 6. The variation between countries reflects both the fracture risk and the distribution of age and sex in each country.
Fig. 6.
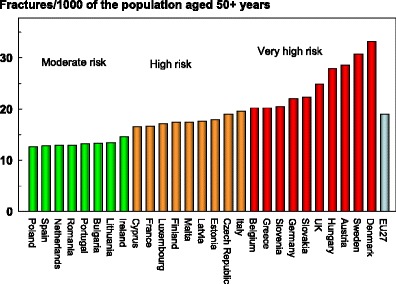
The annual number of fragility fractures in men and women combined expressed/1,000 of the population aged 50 years or more
Comment
The calculation of fracture numbers from hip fracture rates assumes that the ratios between age- and sex-specific incidence of hip fracture and fractures of other sites found in Sweden are similar in other countries. This assumption has been shown to hold true for the countries where this has been tested [3, 8].
These estimates do not include individuals who in 2010 were suffering the consequences of fractures sustained in previous years.
There are important data gaps in the documentation of the fracture burden between member states which form the component of a further scorecard element (Chapter 2a).
References
1. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C on behalf of the IOF Working Group on Epidemiology and Quality of Life (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Intl 23: 2239–2256.
2. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
3. Kanis JA, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12: 417–27
4. UN (2010) Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat (2010) World Population Prospects: The 2010 Revision, http://esa.un.org/unpd/wpp/index.htm Accessed 16th December 2012
5. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32; 468–473
6. Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jönsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15: 38–42
7. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15:108–12.
8. Kanis JA, Hans D, Cooper C, et al. (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22: 395–411.
1f Lifetime hip fracture probability
Domain
Burden of disease—scorecard element
Background and aims
The most serious consequence of osteoporosis in terms of morbidity, mortality and health care expenditure is hip fracture. In the EU, for example, hip fractures comprise only 17 % of the total number of fragility fractures but account for 54 % of the direct costs and 49 % of deaths due to fracture [1]. The likelihood of hip fracture can be expressed as fracture probability from a given age over a given time interval (e.g. 10 years).
The aim of this element is to provide estimates of the remaining lifetime probability of hip fracture in men and women at the age of 50 and 70 years.
Methods
Hip fracture probability was computed taking both the risk of fracture and the risk of death into account [2]. The risk of hip fracture was taken from a systematic review of hip fracture incidence [3]. Where possible, the incidence of hip fracture was determined in men and women using 5-year age categories. Where 5-year age intervals were not available, 10 year intervals were used (intervals of greater than 10 years were an exclusion criterion). Mortality statistics of the WHO were used in 5 or 10 year age intervals for the year 2010 [4]. The remaining lifetime probabilities were calculated in men and women from the age of 50 and 70 years.
Results
Empirical data on hip fracture rates were available for 21 of the 27 EU member states in men and women. No data were available for men from Estonia and Slovenia. Hip fracture incidence is not documented in Bulgaria, Cyprus, Latvia or Luxembourg.
The average remaining lifetime probability of hip fracture in women at the age of 50 years ranged from 7.0 % (Romania) to 25.1 % (Sweden). Thus, there was approximately a three-fold range of lifetime probabilities between countries (Table 9).
Table 9.
Remaining lifetime probability of hip fracture (%) at the ages of 50 and 70 years in men and women by country, 2010 [3]
| Country | Lifetime probability (%) | |||
|---|---|---|---|---|
| At age 50 years | At age 70 years | |||
| Men | Women | Men | Women | |
| Austria | 8.3 | 19.7 | 8.8 | 20.7 |
| Belgium | 7.8 | 18.2 | 8.3 | 18.9 |
| Bulgaria | – | – | – | – |
| Cyprus | – | – | – | – |
| Czech Republic | 6.9 | 14.8 | 7.5 | 15.6 |
| Denmark | 10.6 | 22.1 | 11.1 | 23.6 |
| Estonia | – | 23.3 | – | 21.1 |
| Finland | 5.8 | 12.4 | 6.1 | 12.8 |
| France | 5.6 | 18.4 | 6.3 | 19.4 |
| Germany | 5.3 | 14.2 | 5.6 | 15.0 |
| Greece | 8.0 | 15.8 | 8.6 | 15.2 |
| Hungary | 4.1 | 10.6 | 5.2 | 12.0 |
| Ireland | 7.8 | 18.2 | 8.0 | 18.7 |
| Italy | 7.7 | 19.2 | 7.8 | 19.3 |
| Latvia | – | – | – | – |
| Lithuania | 4.4 | 11.3 | 5.3 | 11.9 |
| Luxembourg | – | – | – | – |
| Malta | 5.8 | 14.2 | 5.8 | 14.2 |
| Netherlands | 5.4 | 12.5 | 5.6 | 12.8 |
| Poland | 4.0 | 9.7 | 3.9 | 10.1 |
| Portugal | 4.8 | 14.4 | 5.3 | 14.9 |
| Romania | 3.8 | 7.0 | 3.7 | 7.2 |
| Slovakia | 9.5 | 20.3 | 9.9 | 20.3 |
| Slovenia | – | 11.6 | – | 12.0 |
| Spain | 4.0 | 12.1 | 4.3 | 12.6 |
| Sweden | 10.9 | 25.1 | 11.0 | 25.4 |
| United Kingdom | 4.8 | 13.8 | 5.0 | 14.6 |
– denotes no data
Probabilities of hip fracture were approximately two-fold lower in men than in women. In men, hip fracture probability at the age of 50 years ranged from 3.8 % (Romania) to 10.9 % (Sweden). There was a close correlation between hip fracture probability in men and women so that in those countries where fracture probability was high in women, so too was it high in men (Fig. 7). In Sweden, which had the highest hip fracture probabilities, the hip fracture risk in men (10.9 %) was higher than the hip fracture probability in women from Hungary, Poland or Romania.
Fig. 7.
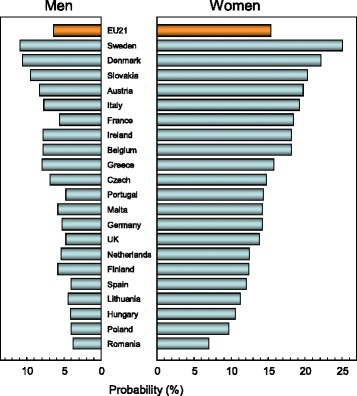
Remaining lifetime probability of hip fracture (%) in men and women from 21 countries in the EU from the age of 50 years [1]
Score criteria
The remaining lifetime probability of hip fracture at the age of 50 years was ranked. Women were chosen since it permitted the inclusion of Estonia and Slovenia for which no data were available in men. The criteria for categorisation are shown in Table 10.
Table 10.
Criteria for allocating scores
Score allocation
The ranked incidence is shown in Fig. 8 and colour coded by category.
Fig. 8.
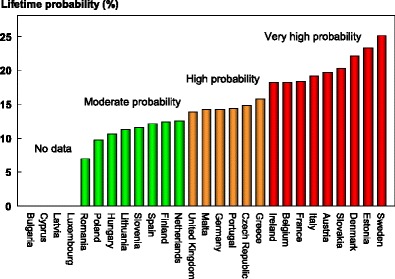
Remaining lifetime probability of hip fracture (%) in women in the EU from the age of 50 years [1]
Comment
Hip fracture probabilities from the age of 70 years were not markedly different from those from the age of 50 years. The reason for this is that increasing death and fracture hazards with age compete in the determination of probability.
References
1. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
2. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey EV (2008) FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385–397.
3. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C on behalf of the IOF Working Group on Epidemiology and Quality of Life (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23: 2239–2256.
4. United Nations (2010) World Population prospects, the 2010 revision. Department of Economic and Social Affairs, UN (http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm) accessed 2nd November 2011
1g Men and women at high fracture risk
Domain
Burden of disease—scorecard element
Background and aims
The advent of FRAX in 2008 [1] provided a clinical tool for the calculation of fracture probability. Probability-based assessment is increasingly being incorporated into clinical guidelines in Europe [2, 3] and elsewhere. Unlike fracture incidence, the probability of fracture at any given age depends upon the hazard of death as well as the hazard of fracture over a defined interval (e.g., 10 years or lifetime). A major advantage of using fracture probability is that it standardises the output from the multiple techniques and sites used for assessment and also permits the presence or absence of risk factors other than BMD to be incorporated as a single metric. FRAX models are also calibrated to country-specific epidemiology.
The ability to compute fracture probabilities in individuals permits an estimate of the prevalence of high risk individuals within a given population where the population demography and the distribution of FRAX-based probabilities are known.
The aim of this score card element was to present the burden of osteoporosis in men and women in the EU27 countries expressed as the proportion of the population that has a 10 year probability of a major fracture (hip, spine, forearm or humerus) above a given threshold.
Methods
There is no international standard for defining high risk based on probabilities. In Europe, intervention thresholds are commonly defined as the 10-year probability of a major fracture that equals or exceeds that of a woman with a prior fragility fracture [2, 3], termed the probability fracture threshold. In North America threshold risks have been set at probabilities of 10 and 20 % [4, 5] and these were used for this assessment.
The majority of EU member states have a country-specific FRAX model. Where unavailable, a surrogate model was used. The distribution of FRAX probabilities in men and women was simulated in 5-year age intervals for each member state between the ages of 50 to 89 years [6] and applied to the demography of each country for 2010 [7]. Burden of disease was expressed as the number of men and women with a probability of major fracture above a threshold of 10 or 20 %. For comparative purposes, the burden was expressed as the proportion of the population aged 50–89 years with probabilities above these thresholds.
Results
Approximately 12.9 million men and women in the EU27 have a 10-year fracture probability that is 20 % or more. When a 10 % threshold is used the population at high risk rises to 41.3 million, representing respectively 3 and 8 % of the total EU population for 2010.
The proportion of the population aged 50 years or more that in 2010 had a fracture probability of 20 % or more varied among member EU states, ranging from 2 % in Romania to 17 % in Sweden (Table 11). The proportion of the population aged 50 years or more that had a fracture probability of 10 % or more ranged from 12 % in Romania to 42 % in Sweden (Table 11). Figure 9 shows the rank order of population burden.
Table 11.
Number of men and women (000) and proportion of the population aged 50–89 years (%) with a 10-year probability of a major fracture that exceeds 10 %, 20 % or the fracture threshold for women
| Country | Number of men and women (000) | Proportion of population aged 50–89 years (%) | ||||
|---|---|---|---|---|---|---|
| >20 % | >10 % | >Fracture threshold | >20 % | >10 % | >Fracture threshold | |
| Austria | 407 | 1,101 | 325 | 14 | 37 | 11 |
| Belgium | 355 | 1,058 | 460 | 9 | 27 | 12 |
| Bulgaria | 51 | 308 | 330 | 2 | 11 | 12 |
| Cyprus | 20 | 68 | 36 | 6 | 22 | 12 |
| Czech | 293 | 926 | 431 | 8 | 25 | 11 |
| Denmark | 377 | 937 | 214 | 19 | 48 | 11 |
| Estonia | 24 | 92 | 60 | 5 | 19 | 13 |
| Finland | 109 | 402 | 222 | 5 | 20 | 11 |
| France | 1,667 | 4,638 | 2,717 | 8 | 21 | 12 |
| Germany | 2,434 | 7,840 | 3,773 | 7 | 24 | 12 |
| Greece | 333 | 1,110 | 524 | 8 | 27 | 13 |
| Hungary | 238 | 842 | 393 | 7 | 23 | 11 |
| Ireland | 110 | 339 | 141 | 9 | 28 | 11 |
| Italy | 2,093 | 6,592 | 2,864 | 9 | 28 | 12 |
| Latvia | 39 | 155 | 102 | 5 | 19 | 13 |
| Lithuania | 53 | 209 | 141 | 5 | 19 | 13 |
| Luxembourg | 13 | 39 | 18 | 8 | 25 | 12 |
| Malta | 10 | 35 | 18 | 7 | 23 | 12 |
| Netherlands | 221 | 881 | 681 | 4 | 15 | 12 |
| Poland | 375 | 1,567 | 1,540 | 3 | 12 | 12 |
| Portugal | 200 | 656 | 479 | 5 | 17 | 12 |
| Romania | 127 | 761 | 834 | 2 | 10 | 12 |
| Slovakia | 139 | 527 | 197 | 8 | 31 | 11 |
| Slovenia | 49 | 169 | 78 | 6 | 23 | 10 |
| Spain | 664 | 2,284 | 1,947 | 4 | 15 | 12 |
| Sweden | 567 | 1,437 | 398 | 17 | 42 | 12 |
| UK | 1,947 | 6,310 | 2,416 | 9 | 30 | 11 |
| EU27 | 12,915 | 41,283 | 21,339 | |||
Fig. 9.
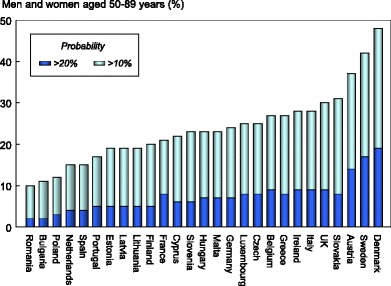
Proportion of men and women (%) aged 50–89 years with a 10-year probability of a major fracture that is 10 % or more and 20 % or more by member state
For completion, the table also shows the number of men and women that lie above a fracture threshold commonly used in assessment guidelines. This is considered later in relationship to the uptake of treatments in the EU27 (Chapter 4c).
Score criteria
Countries were ranked by tertiles of prevalence of the population aged 50–89 years above a 10 % probability threshold of a major osteoporotic fracture as given in Table 12.
Table 12.
Criteria for allocating scores
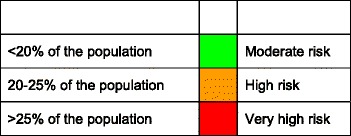
Score allocation
The proportion of the population (%) aged 50–89 years with a 10-year probability of a major fracture that is 10 % or more by member state is shown by category and rank in Fig. 10.
Fig. 10.
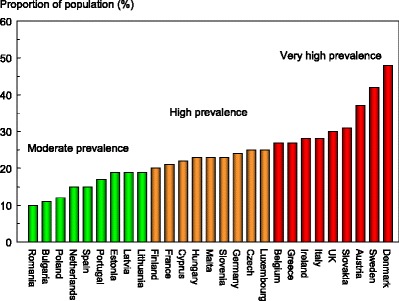
The proportion of the population (%) aged 50–89 years with a 10-year probability of a major fracture that is 10 % or more by member state
Comment
The majority of EU member states have a country-specific FRAX model. For those countries where a country-specific FRAX model was unavailable, a surrogate model was used, based on the estimate that the epidemiology of hip fracture was similar. For Bulgaria, the Romanian model was used; for Cyprus, the Maltese model was used; for Estonia and Latvia, the Lithuanian model was used; for Luxembourg, the Belgian model was used; and for Slovenia, the Hungarian model was used.
References
1. Kanis JA on behalf of the World Health Organization Scientific Group (2008) Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK. Accessed http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf
2. Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation ( IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24: 23–57
3. Lekawasam S, Adachi JD, Agnusdei D et al. for the Joint IOF-ECTS GIO Guidelines Working Group (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–76.
4. Dawson-Hughes B, Looker AC, Tosteson ANA, Johansson H, Kanis JA, Melton III LJ (2010) The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int 21: 41–52
5. Papaioannou A, Morin S, Cheung AM et al. (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182: 1864–73
6. Kanis JA, Johansson H, Odén A, McCloskey EV (2012) The distribution of FRAX® based probabilities in women from Japan. J Bone Miner Metab 30:700–5
7. UN (2010) Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat (2010) World Population Prospects: The 2010 Revision, http://esa.un.org/unpd/wpp/index.htm Accessed 16th December 2012
1h Population projections
Domain
Burden of disease—background information
Background and aims
Secular changes in life expectancy and birth rate are likely to increase the number of elderly individuals in the EU member states and thereby increase the need for resource allocation for diseases associated with ageing. The incidence of fragility fractures increases markedly with age, particularly in women. The aim of this background element is to estimate the increase in number of women aged 50 years or more in the EU member states.
Methods
The age and sex distribution of the EU member states was obtained from the UN for 2010 and 2025 using the medium variant [1].
Results
The population of women over 50 years is expected to increase by 22 % and in men by 17 % in the EU between 2010 and 2025. The number of men and women aged 50 years or more will increase in all countries except Bulgaria, Hungary and Latvia (Table 13). In the remaining countries, the increment in the population varies widely.
Table 13.
Projected percentage change in the male and female population between 2010 and 2025 according to category of age [1]
| Country | Men aged | Women aged | ||
|---|---|---|---|---|
| 50–74 years | 75+ years | 50–74 years | 75+ years | |
| Austria | 25 | 57 | 22 | 26 |
| Belgium | 18 | 27 | 17 | 13 |
| Bulgaria | −1 | −1 | −3 | 11 |
| Cyprus | 36 | 57 | 31 | 63 |
| Czech Republic | 9 | 57 | 4 | 37 |
| Denmark | 10 | 70 | 10 | 45 |
| Estonia | 6 | 21 | 0 | 14 |
| Finland | 2 | 81 | 1 | 41 |
| France | 17 | 40 | 17 | 22 |
| Germany | 13 | 52 | 10 | 23 |
| Greece | 23 | 18 | 17 | 18 |
| Hungary | 6 | 19 | −1 | 18 |
| Ireland | 37 | 73 | 38 | 53 |
| Italy | 25 | 35 | 18 | 21 |
| Latvia | 8 | 13 | −2 | 7 |
| Lithuania | 12 | 3 | 6 | 9 |
| Luxembourg | 36 | 64 | 43 | 25 |
| Malta | 6 | 100 | 5 | 50 |
| Netherlands | 19 | 75 | 19 | 41 |
| Poland | 8 | 24 | 7 | 21 |
| Portugal | 22 | 31 | 17 | 25 |
| Romania | 14 | 6 | 10 | 19 |
| Slovakia | 22 | 38 | 16 | 32 |
| Slovenia | 18 | 60 | 15 | 25 |
| Spain | 42 | 33 | 35 | 22 |
| Sweden | 10 | 61 | 9 | 35 |
| UK | 17 | 46 | 17 | 29 |
With some exceptions, the percentage increase in number of men and women aged 75 or more years is greater than that of the population aged 50–74 years. The exceptions include Belgium (women), Bulgaria (men), Greece (men), Lithuania (men), Luxembourg (women), Romania (men) and Spain (men and women).
For women over the age of 75 years, the change in the population ranged from less than 10 % in Latvia (7 %) and Lithuania (9 %) to more than 40 % in Cyprus, Denmark, Finland, Ireland, Malta and the Netherlands (Fig. 11).
Fig. 11.
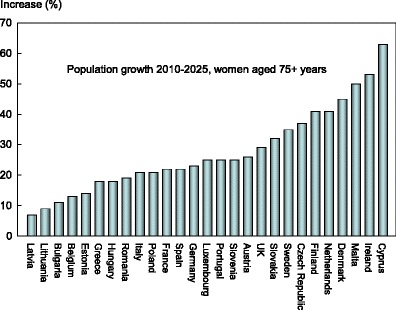
Projected increase by country in the female population aged 75 years or more (%) between 2010 and 2025 [1]
The increase in the male population aged over 75 years was generally more marked than in women. In men, the EU population aged 75 years or more is expected to increase by 33 %. In those countries with large expected changes in the proportion of the population aged 75 years or more, the increment is larger in men than in women (Fig. 12) since life expectancy, lower in men, is improving more rapidly in men than in women with time.
Fig. 12.
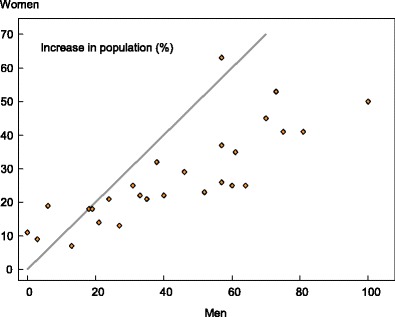
The relation between the percentage increase in the male and female population aged 75 years or more in EU member states. The diagonal shows the line of identity
Score criteria
None—not a score card element
Comment
UN population projections over 15 years are relatively robust in that all men and women in 2025 aged 50 years or more had already attained adulthood in 2010. The projections expressed in relative change for countries with very small populations are uncertain (e.g. Malta, Cyprus) since population numbers are given by the UN rounded to the nearest 1000.
References
1. United Nations (2010) Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm, accessed May2012;
1i Fracture projections
Domain
Burden of disease—scorecard element
Background and aims
As noted, the number of men and women aged 50 years or more is set to increase with time in the EU. The increase will be particularly marked in the elderly population. Since age is an important risk factor for fractures and the elderly population is projected to increase in the majority of member countries, the burden of fractures is also likely to increase.
The aim of this scorecard element was to estimate the increase in the annual number of fragility fractures from 2010 to 2025.
Methods
The incidence of hip fracture was determined from a systematic literature review [1, 2]. For other fractures, it was assumed that the age- and sex-specific incidence in relation to hip fracture followed that documented for Sweden [3] and other non-EU countries [4]. Outcomes included the three most common sites of osteoporotic fracture (hip, spine and forearm) as well as other fractures considered to be associated with osteoporosis (i.e. pelvis, rib, humerus, tibia, fibula, clavicle, scapula, sternum, and lower femur) [3]. For vertebral fractures, only those coming to clinical attention were included.
Fracture numbers were calculated from age- and sex-specific incidence and population sizes in 5-year age intervals for 2010 and 2025 [5]. It was assumed that the incidence of osteoporotic fractures did not change over time.
Results
The annual number of osteoporotic fractures in the EU27 will increase by 0.99 million from 3.49 million in 2010 to 4.48 million in 2025 (Table 14). The increase in the annual number of fractures is found in all countries (Fig. 13), ranging from a 53 % increase in Ireland to a modest 4 % increase in Bulgaria. In 2025, Germany is expected to have the largest number of fractures with almost 940,000 fractures, followed by the UK with 680,000.
Table 14.
Number of fractures in men and women in 2010 and number expected in 2025, and the percentage increase [2]
| Country | Number of fractures 2010 | Number of fractures 2025 | ∆ fractures 2010–2025 (number) | ∆ fractures 2010–2025 (%) | Share of EU27 increase |
|---|---|---|---|---|---|
| Austria | 86,031 | 115,686 | 29,655 | 34 | 3 |
| Belgium | 79,201 | 98,525 | 19,324 | 24 | 2 |
| Bulgaria | 38,184 | 39,612 | 1,429 | 4 | 0 |
| Cyprus | 5,022 | 7,536 | 2,514 | 50 | 0 |
| Czech Republic | 75,359 | 97,829 | 22,470 | 30 | 2 |
| Denmark | 66,066 | 86,094 | 20,028 | 30 | 2 |
| Estonia | 8,678 | 10,208 | 1,530 | 18 | 0 |
| Finland | 36,292 | 48,939 | 12,647 | 35 | 1 |
| France | 378,082 | 493,031 | 114,949 | 30 | 12 |
| Germany | 732,137 | 936,461 | 204,324 | 28 | 21 |
| Greece | 84,256 | 105,284 | 21,028 | 25 | 2 |
| Hungary | 90,011 | 101,544 | 11,533 | 13 | 1 |
| Ireland | 17,947 | 27,372 | 9,425 | 53 | 1 |
| Italy | 466,475 | 599,034 | 132,559 | 28 | 13 |
| Latvia | 14,284 | 16,204 | 1,920 | 13 | 0 |
| Lithuania | 15,084 | 17,484 | 2,400 | 16 | 0 |
| Luxembourg | 2,684 | 4,015 | 1,331 | 50 | 0 |
| Malta | 2,618 | 3,744 | 1,125 | 43 | 0 |
| Netherlands | 76,691 | 107,671 | 30,980 | 40 | 3 |
| Poland | 167,033 | 208,591 | 41,558 | 25 | 4 |
| Portugal | 51,329 | 68,448 | 17,119 | 33 | 2 |
| Romania | 94,240 | 110,099 | 15,858 | 17 | 2 |
| Slovakia | 38,363 | 49,508 | 11,145 | 29 | 1 |
| Slovenia | 15,471 | 21,795 | 6,323 | 41 | 1 |
| Spain | 203,794 | 285,453 | 81,659 | 40 | 8 |
| Sweden | 106,857 | 135,029 | 28,172 | 26 | 3 |
| UK | 535,724 | 681,956 | 146,231 | 27 | 15 |
| EU27 | 3,487,914 | 4,477,152 | 989,238 | 28 | 100 |
Fig. 13.
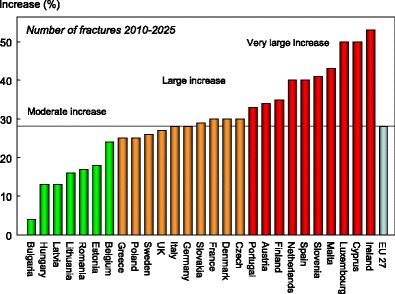
The percentage increase in the number of fragility fractures between 2010 and 2025 in the EU and its member states [2]
Score criteria
Countries were ranked by the percentage increase in the annual number of fractures in men and women between 2010 and 2025 as shown in Table 15.
Table 15.
Criteria for allocating scores
Score allocation
The percentage increase in the annual number of fractures in men and women between 2010 and 2025 is shown by category and rank in Fig. 13.
Comment
The analysis assumes that the age- and sex-specific incidence of fractures did not change over the 15-year time interval. Secular trends in fracture risk are ill-documented with the exception of hip fracture [6] where limited information is available. In general, age- and sex-adjusted hip fracture incidence increased until the mid or end of the 20th century, with a subsequent plateau or even a small decrease [6]. In Europe, this tendency is best documented for Sweden, Finland, Spain, Germany, Netherlands and Hungary.
Countries with substantial increases in the number of fractures need to take this into account for future healthcare planning.
References
1. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C on behalf of the IOF Working Group on Epidemiology and Quality of Life (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporosis International 23: 2239–2256.
2. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
3. Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12; 417–427.
4. Kanis JA, Hans D, Cooper C et al. (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22: 395–411.
5. United Nations (2010) Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm, accessed May2012.
6. Cooper C, Cole ZA, Holroyd CR et al. (2011) Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 22: 1277–1288.
Chapter 2 Policy framework
2a Quality of existing information
Domain
Policy framework—scorecard element
Background and aims
Fracture incidence is poorly documented in the EU [1]. The fracture that has been best evaluated is hip fracture. Hip fractures account for the majority of health care expenditure, mortality and morbidity and can be used as a proxy for osteoporosis. The EU comprises countries with some of the highest hip fracture rates worldwide [2], but documentation of the size of the problem and the quality of data vary between countries.
Documentation of the burden of disease is an essential prerequisite to determine the resources that should be allocated to the diagnosis and treatment of the disorder. It also provides information concerning the priority a disease should be awarded by healthcare policy makers. A fracture registry is a centralised database collecting the number of individual fractures per person, per year within a population and is used for research and resource allocation. The data collected can also be used to identify high-risk patients in need of further prevention programs. The main objective of this scorecard element is to provide an integrated estimate of the quality of current documentation on the burden of osteoporosis fractures in the countries of the European Union.
Methods
Published information on hip fracture incidence was obtained by systematic review, in some cases through contact with Ministries of Health [2]. Available studies in each country were reviewed for quality and representativeness of the country. Epidemiology of other fractures was obtained by systematic review [1].
Data on national or regional fracture registers [3] were updated by an IOF questionnaire to the EU Osteoporosis Consultation Panel.
The quality of the available information was scored, with the presence of an established national fracture register as the highest grade. In the absence of a fracture register, an intermediate score was dependent on the presence of good quality national hip fracture rates.
Results
High quality national data on hip fracture rates were identified in 15 member states (Table 16). Fair to poor quality national estimates were found for Lithuania and Slovenia. No data were available for four countries (Bulgaria, Cyprus, Latvia, and Luxembourg). In the remaining six countries, regional estimates of variable quality were identified. Most index years included data from 2000 onwards.
Table 16.
Characteristics of information available on fracture rates in the European Union
| Incidence of hip fracture | Established National Fracture Registries | Score | |||
|---|---|---|---|---|---|
| Qualitya | Sampleb | Present | Datac | ||
| Austria | G | N | Nod | 3 | |
| Belgium | G | N | Yes | Hip | 3 |
| Bulgaria | No | 0 | |||
| Cyprus | No | 0 | |||
| Czech Republic | G | N | No | 2 | |
| Denmark | G | N | Yes | Hip+ | 3 |
| Estonia | P | R | No | 1 | |
| Finland | G | N | Yes | Hip+ | 3 |
| France | G | N | Nod | 2 | |
| Germany | G | N | Yes | Hip+ | 3 |
| Greece | P/F/G | R | No | 1 | |
| Hungary | G | N | Yes | Hip+ | 3 |
| Ireland | G | N | Yes | Hip | 3 |
| Italy | G | N | Nod | Hip+ | 2 |
| Latvia | R | Yes | Hip+ | 3 | |
| Lithuania | F | R | No | 1 | |
| Luxembourg | No | 0 | |||
| Malta | G | N | No | 2 | |
| Netherlands | G | N | Yes | Hip+ | 3 |
| Poland | F | R | No | 1 | |
| Portugal | G | N | Yes | Hip+ | 3 |
| Romania | G | N | No | 2 | |
| Slovakia | G | N | Yes | Hip+ | 3 |
| Slovenia | F | N | No | 2 | |
| Spain | F/G | R | Nod | Hip+ | 1 |
| Sweden | G | R | Yes | Hip | 3 |
| UK | G | R | Yes | Hip | 3 |
Responses derived from questionnaire to National Societies
aQuality: G good; F fair; P poor [2]
bCatchment: N national; R regional
c Hip Registration of hip fracture only. Hip+ Registration of hip and other fracture outcomes
dRegional registers available
Data on the incidence of clinical vertebral fractures are lacking in most of the countries in the EU, the exceptions being regional data for Sweden and the UK. In the UK, the incidence of clinically identified fractures has been studied within the General Practice Research Database (GPRD). The incidence is, however, very low and it is likely that the majority of fractures were not coded.
Information on forearm fracture is also scarce. Forearm fractures are treated in hospital outpatient departments. There are reports from EU27 countries on the incidence of forearm fractures that lead to hospitalisation, e.g. from France and Italy, but these are of limited value. There are also studies published from Slovenia and Italy which present incidence of forearm fractures treated both in inpatient and outpatient care. However, the Slovenian study only reports fractures occurring in women, and the Italian study lacks age stratification of data within the elderly population. Credible data are only available for Hungary, the UK and Sweden [1].
National fracture registries were in place in 12 of the EU countries (Table 16). The majority of these acquire information on all or several fracture outcomes (Austria, Denmark, Finland, Germany, Hungary, Ireland, Latvia, Netherlands, Portugal and Slovakia) and the remainder registered hip fracture alone (Belgium, Ireland, Sweden and UK). In several additional countries, local registers are available.
Score criteria
The presence of an established national fracture register was allocated the highest grade. In the absence of a fracture register, an intermediate score was given with the availability of good quality national hip fracture rates. Criteria for allocating scores are given in Table 17.
Table 17.
Criteria for allocating scores
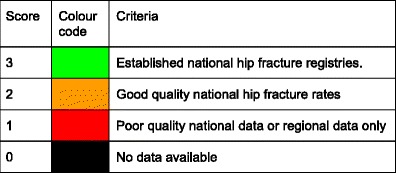
Score allocation
Countries, ranked and categorised by score, are shown in Fig. 14.
Fig. 14.
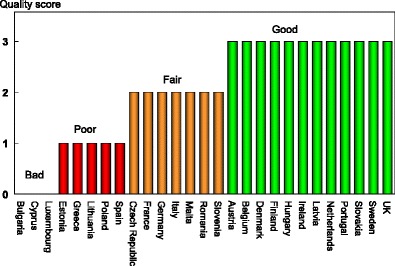
Quality of information available on the epidemiology of hip fractures in the EU [IOF audit]
Comments
The quality of this information is limited. Firstly, it is based on responses to a questionnaire to national societies and not to government agencies. Secondly, centralised data are not necessarily equivalent to a national registry.
References
1. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
2. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C on behalf of the IOF Working Group on Epidemiology and Quality of Life (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int23: 2239–2256
3. International Osteoporosis Foundation (2008) Osteoporosis in the European Union in 2008: Ten years of progress and ongoing challenges. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
2b National health priority
Domain
Policy framework—scorecard element
Background and aims
Data from the Global Burden of Disease 2010 Study indicate that musculoskeletal disorders are the second greatest cause of disability as measured by years lived with disability (YLD), worldwide and across most regions of the world [1]. In terms of both death and disability, musculoskeletal diseases are the non-communicable diseases that have the fourth greatest impact on the health of the world population (6.8 %). They closely follow cardiovascular and circulatory diseases (11.8 %), tumours (7.7 %), and mental/behavioural disorders (7.4 %) [2]. Disability due to musculoskeletal disorders has increased by 45 % from 1990 to 2010 compared to a 33 % average across all other disease areas. These data suggest that musculoskeletal disease merits a high priority in health care policy. Osteoporotic fractures in Europe accounted for more disability-adjusted life years lost (2,006,000 DALYs) than rheumatoid arthritis (1,048,000) but less than that for osteoarthritis (3,088,000) representing 33 % of the DALYs of these disorders [3].
When a disease becomes a National Health Priority (NHP), it is usually mandated by a government body/ministry of health or another official institution. Osteoporosis may be a designated NHP on its own, or it may be included as part of a musculoskeletal diseases NHP. The development of a national action plan, clear objectives and support for education and awareness programs also often result from a NHP mandate. The aim of this scorecard element was to determine the extent to which member states have recognised this need.
Methods
Information on NHP [4] was updated by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012.
Respondents were asked whether osteoporosis or musculoskeletal diseases were officially documented as a NHP in each member state and to provide the documentary evidence. Further questions related to action plans linked to the NHP and their implementation.
Results
The majority of member states (18/27) do not recognise osteoporosis or musculoskeletal diseases as a NHP (Table 18). Of those member states that have developed a NHP, the focus has been on nutrition (six countries), falls prevention (four countries), exercise (four countries), and the institution of fracture liaison services (two countries). Action plans have been implemented in Bulgaria, Luxembourg and Romania. There is scant evidence for the implementation of action plans in Finland, France, Italy and Portugal. In Sweden, osteoporosis has only recently become a NFP (2012). In the UK, implementation is indirect via the establishment of quality indicators in the audit of primary care practice (see Chapter 3h).
Table 18.
Countries in which osteoporosis or musculoskeletal diseases were officially documented as a NHP, its scope and action plans
| NHP and date | Government support | Scopea | Action plan | Score | |
|---|---|---|---|---|---|
| Austria | No | – | 1 | ||
| Belgium | No | – | 1 | ||
| Bulgaria | Yes 2006 | Yes | N, FLS | Yes | 3 |
| Cyprus | No | 1 | |||
| Czech Republic | No | 1 | |||
| Denmark | No | 1 | |||
| Estonia | No | 1 | |||
| Finland | Yes | Yes | N, E, F | No | 2 |
| France | Yes 2004 | Yes | N, E, F | No | 2 |
| Germany | No | 1 | |||
| Greece | No | 1 | |||
| Hungary | No | – | 1 | ||
| Ireland | No | 1 | |||
| Italy | Yes 2005 | Yes | N, F | Uncertain | 2 |
| Latvia | No | 1 | |||
| Lithuania | No | 1 | |||
| Luxembourg | Yes | Yes | N, E, F | Yes | 3 |
| Malta | No | 1 | |||
| Netherlands | No | 1 | |||
| Poland | No | 1 | |||
| Portugal | Yes 2004 | Yes | P | Rarely implemented | 2 |
| Romania | Yes | Yes | Case finding | Yes | 3 |
| Slovakia | No | 1 | |||
| Slovenia | No | 1 | |||
| Spain | No | 1 | |||
| Sweden | Yes 2012 | Yes | Not yet defined | No | 2 |
| UK | Yes 2009 | Yes | NE, FLS | Indirect | 3 |
Responses derived from questionnaire to National Societies
a N, E, F Nutrition, Exercise, Falls prevention; P professional education; FLS Fracture liaison services
Score criteria
The presence of government-backed NHP with an implemented action plan was allocated the highest grade. In the absence of an action plan, an intermediate score was given. Criteria for allocating scores are given in Table 19.
Table 19.
Criteria for allocating scores

Score allocation
Countries, ranked and categorised by score, are shown in Fig. 15.
Fig. 15.
Categorisation of EU countries according to the existence of government-backed NHP for osteoporosis or musculoskeletal diseases [IOF audit]
Comment
Unless osteoporosis prevention and treatment become a priority for governments and health care providers, the growing number of osteoporotic fractures will have a serious impact on society—not just in terms of people’s quality of life, but also because of increased costs incurred for acute healthcare, rehabilitation and nursing care.
References
1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–96
2. Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223.
3. Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
4. International Osteoporosis Foundation (2008) Osteoporosis in the European Union in 2008: Ten years of progress and ongoing challenges. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
2c Who manages osteoporosis?
Domain
Policy framework—scorecard element
Background and aims
In 2010, it is estimated that 22 million women and 5.5 million men in the EU had osteoporosis using the diagnostic criterion of the WHO [1]. In 2010, the number of new osteoporosis-related fractures in the EU was estimated at 3.5 million, comprising approximately 610,000 hip fractures, 520,000 vertebral fractures, 560,000 forearm fractures and 1,800,000 other fractures (i.e. pelvis, rib, humerus, tibia, fibula, clavicle, scapula, sternum, and other femoral fractures). Osteoporosis is a common disease and effective treatments are widely available. As such, in most health care systems the vast majority of patients with osteoporosis is preferably managed at the primary health care level by general practitioners with specialist referral reserved for difficult cases, for example men and individuals in whom a secondary cause of osteoporosis is suspected.
The aim of this element was to determine whether the care of osteoporosis was primarily devolved to primary care physicians (GPs, family doctors). If not, then the lead specialty was asked for. The training of specialists is considered in Chapter 2d.
Methods
Data were acquired by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012. Respondents were asked whether osteoporosis was primarily devolved to primary care physicians (GPs, family doctors). If not, the single specialty that looked after most cases of osteoporosis was asked. In the case where there was near equality between two or more specialties, they were each recorded.
Results
Primary care was the principal provider of the medical care of osteoporosis in 15 of the 27 EU member states (Table 20). In the remainder, the principal care was provided by hospital specialists. In Greece and Hungary, a single hospital specialty was the dominant provider (orthopaedics and rheumatology, respectively). In the remaining countries, the care of osteoporosis was split between disciplines. The number of disciplines was usually two (Bulgaria, Czech Republic, Denmark, Germany, Italy and Romania) but was three in the case of Ireland and Slovakia, and four or more for Malta, Poland and Sweden. The specialties involved comprised rheumatology (noted 10 times), endocrinology (9), orthopaedics (5), geriatrics (3), clinical osteology (2), internal medicine (2) gynaecology (1) and rehabilitation medicine (1). The panel were concerned by the multiplicity of specialists that had a primary role in the care pathway of patients in some countries and viewed this as an impediment to consistent care.
Table 20.
Care pathway for patients with osteoporosis by country
| Primarily devolved to primary care | Lead specialty | Score | |
|---|---|---|---|
| Austria | yes | 3 | |
| Belgium | yes | 3 | |
| Bulgaria | no | Rheumatology, endocrinology | 1 |
| Cyprus | yes | 3 | |
| Czech Republic | no | Clinical osteology, endocrinology | 1 |
| Denmark | no | Rheumatology, endocrinology | 1 |
| Estonia | yes | 3 | |
| Finland | yes | 3 | |
| France | yes | 3 | |
| Germany | no | Orthopaedics, clinical osteology | |
| Greece | no | Orthopaedics | 2 |
| Hungary | no | Rheumatology | 2 |
| Ireland | no | Rheumatology, endocrinology, geriatrics | 1 |
| Italy | no | Rheumatology, endocrinology | 1 |
| Latvia | yes | 3 | |
| Lithuania | yes | 3 | |
| Luxemburg | yes | 3 | |
| Malta | no | Rheumatology, gynaecology, endocrinology, geriatrics | 1 |
| Netherlands | yes | 3 | |
| Poland | no | Rheumatology, orthopaedics, rehabilitation medicine, internal medicine | 1 |
| Portugal | yes | 3 | |
| Romania | no | Rheumatology, endocrinology | 1 |
| Slovakia | no | Rheumatology, orthopaedics, endocrinology | 1 |
| Slovenia | yes | 3 | |
| Spain | yes | 3 | |
| Sweden | yes | Rheumatology, orthopaedics, endocrinology, internal medicine, geriatrics | 3 |
| UK | yes | 3 |
Responses derived from questionnaire to National Societies
Score criteria
Where the care of osteoporosis was primarily devolved to primary care physicians (GPs, family doctors), this was allocated the highest grade. If not, then an intermediate score was given where osteoporosis is mainly managed by a single specialty, as given in Table 21.
Table 21.
Criteria for allocating scores

Score allocation
Countries, ranked and categorised by score, are shown in Fig. 16.
Fig. 16.
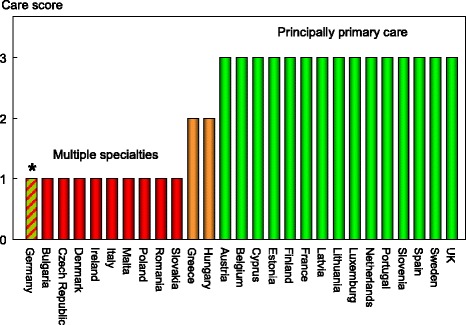
Patterns of principal care of patients with osteoporosis [IOF audit]. *See comment below
Comment
Care management pathways are not necessarily divided by primary care and specialty care. The panel supports the view that long-term management should preferably be undertaken by GPs, contingent on adequate training, but there is a specialist role in initial evaluation, particularly in the context of fracture liaison services (see Chapter 3g). In Germany, there is the opportunity for specialists in many disciplines to be specially trained and accredited in the primary care of patients with osteoporosis. These considerations should temper the interpretation of the scores allocated.
Reference
1. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
2d Is osteoporosis a component of specialty training?
Domain
Policy framework—scorecard element
Background and aims
The large number of men and women who suffer the consequences of osteoporosis raises the question of whether there is adequate training of medical practitioners in this specialty and, indeed, which specialty takes a leadership role.
The aim of this background element was to determine whether osteoporosis and metabolic bone disease are a recognised specialty or recognised component of specialty training.
Methods
Data were acquired by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012. Information requested included whether osteoporosis or metabolic bone disease is a recognised medical specialty in each country. Also asked was whether osteoporosis or metabolic bone disease is a recognised component of specialty medical training and, finally, which specialists took lead roles in the care of osteoporosis.
The available information was scored, with the presence of an established specialty as the highest grade. In the absence of osteoporosis or metabolic bone disease being a recognised medical specialty, an intermediate score was dependent on the disorder being a recognised component of specialty medical training.
Results
Osteoporosis and metabolic bone disease is a recognised specialty in only four of the EU member states (Czech Republic, Denmark, Estonia and Lithuania). In some countries, there are specialists that deal exclusively with metabolic bone diseases (e.g. the UK) most usually in an academic setting. The more usual finding is that the specialty care of osteoporosis is via another specialty (Table 22). The specialties involved include endocrinology, geriatrics, gynaecology, internal medicine, orthopaedic surgery, rehabilitation medicine and rheumatology. In the majority of countries, osteoporosis or metabolic bone disease is a recognised component of specialty medical training but there is no information on the extent to which this is taken advantage of. In Germany, a postgraduate training in clinical osteology is available to specialists from different disciplines to become certified. In two countries (Ireland and Poland) osteoporosis was neither an accepted medical specialty nor a component of specialty medical training. In the UK, experience in metabolic bone disease may form a component of specialist training but is not mandatory.
Table 22.
Specialists caring for osteoporosis (OP)
| OP recognised as a specialty | Lead specialistsa | OP recognised as a component of specialty training | Score | |
|---|---|---|---|---|
| Austria | No | Rh, Orth, Gyn, Endo, Int | Yes | 2 |
| Belgium | No | Rh, Gyn, Endo, Ger, Rehab, Int | Yes | 2 |
| Bulgaria | No | Orth, Gyn, Int | Yes | 2 |
| Cyprus | No | Rh, Orth, Gyn, Endo, Int | Yes | 2 |
| Czech Republic | Yes | Rh, Orth, Gyn, Endo, Int | Yes | 3 |
| Denmark | Yes | Ger | Yes | 3 |
| Estonia | Yes | Orth, Endo, Rh | Yes | 3 |
| Finland | No | Rh, Orth, Gyn, Endo, Ger, Rehab, Int | Yes | 2 |
| France | No | Rh, Gyn, Endo, Ger | Yes | 2 |
| Germany | No | Rh, Orth, Gyn, Endo, Ger, Rehab, Int | Yes | 2 |
| Greece | No | Rh, Gyn, Endo | Yes | 2 |
| Hungary | No | Rh, Orth, Gyn, Endo,Rehab, Int | Yes | 2 |
| Ireland | No | Rh, Gyn, Endo, Ger, Rehab | No | 1 |
| Italy | No | Rh, Endo, Ger, Rehab, Int | Yes | 2 |
| Latvia | No | Rh, Endo, Int | Yes | 2 |
| Lithuania | Yes | Rh, Orth, Endo, Ger, Rehab, Int | Yes | 3 |
| Luxembourg | No | Rh, Orth, Gyn, Endo, Ger, Rehab, Int | Yes | 2 |
| Malta | No | Rh Orth, Gyn, Endo,Rehab, Int | Yes | 2 |
| Netherlands | No | Rh, Orth, Endo, Ger, Int | Yes | 2 |
| Poland | No | Rh, Orth, Endo, | No | 1 |
| Portugal | No | Rh, Orth, Gyn, Endo, Ger, Rehab | Yes | 2 |
| Romania | No | Orth, Rehab | Yes | 2 |
| Slovakia | No | Gyn | Yes | 2 |
| Slovenia | No | Rh, Gyn, Endo,Rehab | Yes | 2 |
| Spain | No | Rh, Orth, Gyn, Endo, Ger, Rehab, Int | Yes | 2 |
| Sweden | No | Rh, Orth, Endo, Int | Yes | 2 |
| UK | No | Rh, Orth, Endo, Ger, Gyn | Yes | 2 |
Responses derived from questionnaire to National Societies
a Endo endocrinology, Ger geriatrics, Gyn gynaecology, Int internal medicine, Orth orthopaedic surgery, Rehab rehabilitation medicine, Rh rheumatology
With the exception of Slovakia, the lead specialties are multiple. In some countries, all seven specialties took what were considered lead roles in the management of osteoporosis. This clearly indicates that there is no dominant specialty that looks after osteoporosis in any one country and a great diversity between countries. The specialty representation is illustrated in Fig. 17.
Fig. 17.
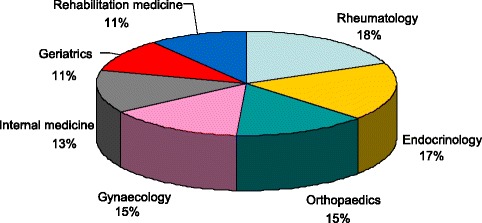
The specialty representation in the EU countries. Note that more than one specialty per country can be represented (see Table 22) [IOF audit]
Score criteria
The highest score was allocated to a country if osteoporosis or metabolic bone disease was an established specialty. In the absence of osteoporosis or metabolic bone disease being a recognised medical specialty, an intermediate score was dependent on the disorder being recognised component of specialty medical training (Table 23).
Table 23.
Criteria for allocating scores

Score allocation
The score allocation and grade for each country is shown in Fig. 18.
Fig. 18.
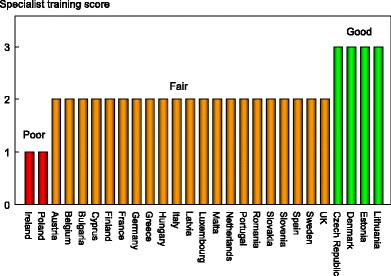
The score allocation and grade for specialist training in each country [IOF]
Comment
There is a wide variation in the specialties which cater for osteoporosis. Although it is possible that these specialties educate their trainees adequately, the wide variation may be reflected in inconsistent patient care, training of primary care physicians and a suboptimal voice to “defend” the interests of osteoporosis.
2e National Societies
Domain
Policy framework—scorecard element
Background and aims
The role of national patient societies is to improve the care of patients and increase awareness and prevention of osteoporosis and related fractures among the general public. In addition to their role in patient and public outreach, the societies provide practical and unbiased information for osteoporosis patients and their families through telephone help lines, local self-help groups and information events, media outreach, general educational activities and by distributing information via brochures and their websites. The patient societies often work closely with clinical and research associations to disseminate information about new treatments and patient guidelines. Finally, with their often large and active membership base, societies play an important role in advocacy by calling for access to timely and affordable diagnosis and treatment. This is particularly necessary for osteoporosis which, as a chronic ‘silent’ disease, is too often neglected by health authorities.
Methods
Data were acquired from the International Osteoporosis Foundation on the patient-contact societies operating in the European Union. Support societies fall into three categories: those primarily involved with direct patient contact (e.g. a help line), societies that are patient-orientated but without patient contact and scientific societies that have no outreach to patients. A high score was allocated to those countries with a patient-contact/support society. In its absence, an intermediate grade was allocated to patient-orientated societies and the lowest score to countries with scientific societies and no patient outreach.
Results
The individual societies are listed in the acknowledgements. The distribution of type of society and number by member state is shown in Table 24. Eight countries had a patient-contact society. For patient-orientated societies, there were 26 societies in 18 member states. There were 49 scientific societies in 26 member states (the exception was Ireland).
Table 24.
The number and type of osteoporosis societies in the EU member states [IOF Audit]
| Patient contact | Patient orientated | Scientific | Score | |
| Austria | 1 | 1 | 3 | 3 |
| Belgium | 1 | – | 1 | 3 |
| Bulgaria | – | 2 | 2 | 2 |
| Cyprus | – | 1 | 1 | 2 |
| Czech Republic | – | 1 | 2 | 2 |
| Denmark | – | 1 | 1 | 2 |
| Estonia | – | – | 1 | 1 |
| Finland | – | 1 | 1 | 2 |
| France | – | 2 | 3 | 2 |
| Germany | 4 | 2 | 3 | 3 |
| Greece | – | 1 | 3 | 2 |
| Hungary | – | 1 | 1 | 2 |
| Ireland | 1 | – | – | 3 |
| Italy | 1 | 4 | 4 | 3 |
| Latvia | 1 | – | 1 | 3 |
| Lithuania | – | 1 | 2 | 2 |
| Luxemburg | – | – | 1 | 1 |
| Malta | – | – | 1 | 1 |
| Netherlands | – | 1 | 1 | 2 |
| Poland | – | 1 | 3 | 2 |
| Portugal | – | 1 | 2 | 2 |
| Romania | – | 1 | 3 | 2 |
| Slovakia | – | – | 2 | 1 |
| Slovenia | 1 | – | 1 | 3 |
| Spain | – | 2 | 3 | 2 |
| Sweden | 1 | 1 | 2 | 3 |
| UK | 1 | – | 1 | 3 |
| EU27 | 8/27 | 18/27 | 26/27 |
Score criteria
Support societies was categorised by patient contact (Table 25). A high score was allocated to those countries with a patient-contact society. In its absence, an intermediate grade was allocated to patient-orientated societies and the lowest score to countries with scientific societies and no patient outreach.
Table 25.
Criteria for allocating scores

Score allocation
The score for each country by score and rank is shown in Fig. 19.
Fig. 19.
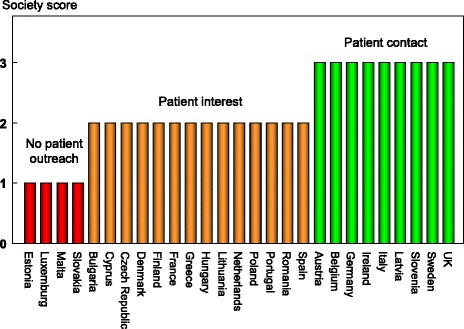
Society support to osteoporosis by score [IOF audit]
Comment
The score is based on the audit by the IOF of its affiliated societies. As such, it necessarily did not consider societies that are not members of the IOF Committee of National Societies. This consideration should temper the interpretation of this element.
Chapter 3. Service provision
3a Treatments for osteoporosis
Domain
Service provision—scorecard element
Background and aims
A wide variety of approved drug treatments is available for the management of osteoporosis. Potential limitations of their use in member states relate to reimbursement policies which may impair the delivery of health care.
The aim of this scorecard element was to review the provision of medical intervention in each member state and, in particular, to determine whether restricted reimbursement was considered an obstacle to the accessibility and long-term uptake of interventions.
Methods
Information on access to treatment [1] was updated by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012. Information requested included the treatments that are currently reimbursed, the level of reimbursement, the conditions on which reimbursement are offered and whether reimbursement policy interferes with what patients could accept or physicians in each country would wish to recommend to patients. We additionally asked whether there are designated first-line treatments in each country.
The following interventions were included: the bisphosphonates (alendronate, ibandronate, risedronate and zoledronic acid), raloxifene, denosumab, strontium ranelate, parathyroid hormone derivatives (PTH and teriparatide) and vitamin D analogues (alfacalcidol and calcitriol). We excluded gonadal steroids (prescribed for hypogonadal states rather than for osteoporosis) and calcium/vitamin D products (most usually available without prescription).
Costs for first- and second-line treatment per year (weighted on price and market share in each country) were taken from Hernlund [2].
The available information was scored on the basis of full or partial reimbursement. In those countries with restricted reimbursement, countries were identified where reimbursement policy interfered with what patients could accept or physicians would wish to recommend to patients.
Results
Most interventions were reimbursed in most countries. Full reimbursement was provided in only 7 of 26 EU member states (Table 26). In the remaining countries, the level of reimbursement ranged from 0 (Malta) to up to 100 % for selected treatments (Luxembourg and Spain). Restricted reimbursement was reported as a significant obstacle to accessibility and long-term uptake in several countries. Examples include unaffordable cost to the patient (Spain), age restrictions for some agents (Belgium, Italy, Poland), less reimbursement in the absence of a prior fracture (Estonia), and reimbursement for some or all agents conditional on a specialist referral (Czech Republic, Greece and Hungary).
In several countries, reimbursement was conditional on clinical criteria, which prevented health care professionals from prescribing some or all agents to individuals at high risk. Examples include reimbursement criteria based on BMD alone (i.e. irrespective of prior fractures in osteopenic cases) (Bulgaria, Lithuania, Romania), patients at high risk identified by FRAX (Belgium). In France, the intricacies of reimbursement are considered as too complicated by GPs so that many have lost interest in managing the disease. As might be expected, impedimenta were less frequent in those countries with full reimbursement (7/8) than in those with incomplete reimbursement (10/19).
First-line drugs were mandated in 18 of 29 countries. The majority comprised the oral bisphosphonates and, in particular generic alendronate.
As expected, the average cost of intervention (weighted on price and market share in each country) varied markedly and ranged from € 160 (Belgium) to € 1269 (Demark). There was similar price inequality for generic alendronate (Table 26).
Table 26.
Levels of reimbursement, reported barriers to care from reimbursement policies and costs of treatment [IOF audit]
| Reimbursed (%) | Patient or professional impediment | First-line drugs identified | Average cost (€/year) | Generic alendronate (€/year) | |
|---|---|---|---|---|---|
| Austria | 100 | No | Yes | 257 | 174 |
| Belgium | 10–20 | Yes | Yes | 160 | 123 |
| Bulgaria | 25 | Yes | No | 179 | 80 |
| Cyprus | 100a | No | No | 640 | 327 |
| Czech Republic | 50–90 | No | Yes | 359 | 187 |
| Denmark | 50–90 | No | Yes | 1269 | 126 |
| Estonia | 50–90 | Yes | No | 232 | 171 |
| Finland | 40 | No | Yes | 205 | 40 |
| France | 30–65 | Yes | Yes | 412 | 209 |
| Germany | 100 | No | Yes | 619 | 245 |
| Greece | Part | Yes | No | 391 | 239 |
| Hungary | 70–90 | Yes | Yes | 354 | 115 |
| Ireland | 100a | No | No | 570 | 240 |
| Italy | 100 | Yes | No | 619 | 294 |
| Latvia | 50 | No | nr | 308 | 85 |
| Lithuania | 50–80 | Yes | Yes | 409 | 146 |
| Luxembourg | 80–100 | No | No | 336 | 109 |
| Malta | 0 | No | Yes | 545 | 190 |
| Netherlands | 100 | No | Yes | 226 | 4 |
| Poland | 30 | Yes | No | 581 | 245 |
| Portugal | 69 | No | Yes | 313 | 16 |
| Romania | 50 | Yes | Yes | 173 | 53 |
| Slovakia | 90+ | No | Yes | 401 | 16 |
| Slovenia | 100 | No | Yes | 234 | 161 |
| Spain | 50–100 | Yes | Yes | 478 | 201 |
| Sweden | 100 | No | Yes | 667 | 27 |
| UK | 100 | No | Yes | 226 | 13 |
aDepending on income; nr not recorded;
In several countries, some registered treatments were not reimbursed which are listed by treatment in Table 27.
Table 27.
Registered treatments that are not reimbursed [2]
| Treatment | Countries where reimbursement is not offered for osteoporosis |
|---|---|
| Risedronate | Estonia, Malta |
| Alendronate | Malta |
| Ibandronate | Malta, Poland, Sweden |
| Zoledronic acid | Estonia, Malta, Poland, Romania |
| Raloxifene | Bulgaria, Latvia, Malta, Poland |
| Denosumab | Estonia, France, Malta, Portugal, Romania |
| Strontium Ranelate | Malta, Poland |
| Teriparatide and PTH (1–84) | Bulgaria, Estonia, Luxembourg, Malta, Netherlands, Poland, Romania |
| Alfacalcidol/calcitriol | Belgium, Denmark, France, Ireland, Poland |
Score criteria
The highest score was allocated for full reimbursement. In those countries with restricted reimbursement, countries were identified where reimbursement policy interfered with what patients could accept or physicians would wish to recommend to patients. Categories are shown in Table 28.
Table 28.
Criteria for allocating scores

Score allocation
Countries, ranked and categorised by score, are shown in Fig. 20.
Fig. 20.
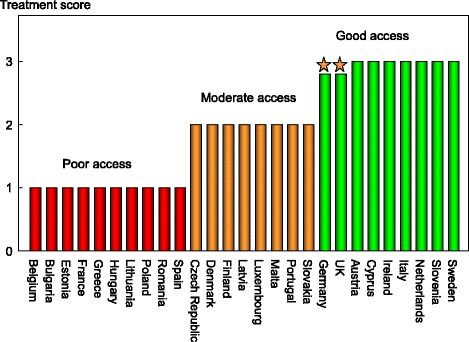
Ranking and score for access to medical intervention [IOF audit]. *See comment below [IOF audit]
Comment
Note that full reimbursement does not necessarily denote full access to treatment. For example, in Germany and the UK, the availability of drugs other than generic alendronate is restricted, sometimes severely so, by regional or local budgetary policies.
The large price range of generic alendronate (€4 to €294 per year) is remarkable as an index of inequality of provision within the EU.
References
1. International Osteoporosis Foundation (2008) Osteoporosis in the European Union in 2008: Ten years of progress and ongoing challenges. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
2. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
3b Availability of DXA
Domain
Service provision—scorecard element
Background and aims
The assessment of bone mass forms a cornerstone for the general management of osteoporosis being used for diagnosis, risk prediction, selection of patients for treatment and monitoring of patients on treatment. The appropriate sites and technology are measurement at the lumbar spine and hip with dual-energy X-ray absorptiometry (DXA). The capacity to service these needs depends therefore on the availability of equipment.
The aim of this score card element was to compare the availability of DXA in the EU member states.
Methods
An estimate of the number of operational DXA machines was determined from the combined sales information of the three major providers (GE Lunar, Hologic and Norland) provided in confidence to the IOF [1]. The metric for each country was the number of DXA units/million of the general population.
Results
The number of DXA units expressed per million of the general population varied markedly in the EU (Table 29). Belgium, Greece and France were the most well provided for and Bulgaria, Luxembourg and Romania the least. Previous surveys have indicated a marked heterogeneity in the availability of DXA in the EU [2–4] and the present survey, based on manufacturer sales, confirms this finding (Fig. 21).
Table 29.
The number of central DXA units available in the EU27 per million of the general population [1]
| Country | DXA units/million | Country | DXA units/million | Country | DXA units/million |
|---|---|---|---|---|---|
| Austria | 28.7 | Germany | 21.1 | Netherlands | 10.7 |
| Belgium | 53 | Greece | 37.5 | Poland | 4.3 |
| Bulgaria | 1.2 | Hungary | 6.0 | Portugal | 26.9 |
| Cyprus | 23.9 | Ireland | 10.0 | Romania | 2.4 |
| Czech Republic | 5.2 | Italy | 18.6 | Slovakia | 10.7 |
| Denmark | 14.6 | Latvia | 4.9 | Slovenia | 27.1 |
| Estonia | 8.9 | Lithuania | 3.4 | Spain | 8.4 |
| Finland | 16.8 | Luxemburg | 2.0 | Sweden | 10.0 |
| France | 29.1 | Malta | 9.7 | UK | 8.2 |
Fig. 21.
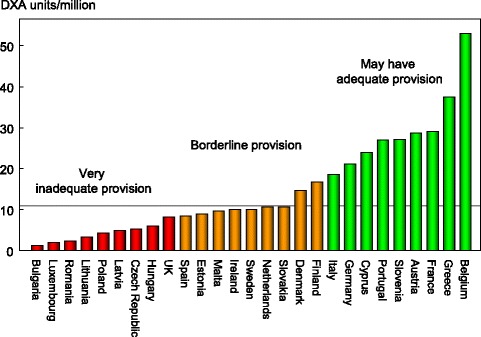
DXA units/million of the general population in 2010 based on sales of DXA in the EU supplied by manufacturers [1]. The horizontal line denotes a minimum service requirement [2]
Score criteria
The score was based on the number of DXA units/million of the general population categorised by tertiles given in Table 30.
Table 30.
Criteria for allocating scores
Score allocation
Countries, ranked and categorised by score, are shown in Fig. 21.
Comment
The requirement for assessing and monitoring the treatment of osteoporosis to implement practice guidelines has been estimated at approximately 11 DXA units per million of the general population [2]. The survey indicated that about 50 % of countries in the EU had the recommended number of DXA machines for their population. It is important to note that the figures provided do not distinguish between machines dedicated in part or in full to clinical research, and those that lie idle or are underutilised because of lack of funding. It is likely, therefore, that a majority of countries are under-resourced in the context of their practice guidelines.
The granularity of the data means that it is not possible to determine the use of DXA equipment (for research or service), the efficiency and quality of service or the extent of inequity of geographic distribution of DXA machines.
References
1. Kanis JA (2011) Personal communication, data on file.
2. Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16: 229–38
3. International Osteoporosis Foundation (2008) Osteoporosis in the European Union in 2008: Ten years of progress and ongoing challenges. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
4. International Osteoporosis Foundation (2010) The Eastern European and Central Asian regional audit. Epidemiology, cost and burden of osteoporosis in 2010. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
3c Access to DXA
Domain
Service provision—Scorecard element
Background and aims
The assessment of osteoporosis does not solely depend on the availability of bone mass measurements at the lumbar spine and hip with dual-energy X-ray absorptiometry (DXA) (see chapter 3b). Access also depends upon the efficiency with which the technology is used, the ease of patient access (e.g. travelling time), regulatory constraints and barriers to reimbursement.
The aim of this background element was to compare the access to DXA in the EU member states.
Methods
Data were acquired by means of an IOF questionnaire sent to the EU Osteoporosis Consultation Panel undertaken in December 2012. Respondents were asked to update previous estimates for the cost, waiting time and reimbursement for DXA. Where information was lacking, data for waiting times and reimbursement were taken from the 2008 IOF EU audit or the Eastern European and Central Asian Audit [1, 2]. Respondents were specifically invited to comment on whether the reimbursement policy (or lack of reimbursement) provided barriers to the physician’s assessment of patients.
Results
The average waiting time for DXA ranged from 0 (Bulgaria and Germany) to 140 days (Ireland) (Table 31 and Fig. 22). There was no clear relation between waiting times and the availability of DXA (see chapter 3b). For example, the average waiting time in Italy was reported to be 83 days, though the number of DXA machines is high (18.6 machines/million of the general population). This disparity arises because many of DXA units lie in research centres or the private sector and are unavailable to the majority of the population. Conversely, there is no waiting time in Bulgaria where the provision of DXA is low (1.2 DXA units/million). The latter observation reflects the fact that the few machines available are only used to service specialised departments and that BMD assessments are unavailable to the vast majority of the population at risk. Thus, a disparity between the availability of equipment and waiting time identifies a high heterogeneity in the use of BMD to assess osteoporosis. A further consideration is the uneven geographical location of equipment, which is known to be problematic in Italy, Spain and the UK.
Table 31.
Cost and reimbursement of DXA [IOF audit]
| Country | Waiting time (d) | Cost (€) | Reimbursement | Barriers to clinical practice |
|---|---|---|---|---|
| Austria | 14 | 35 | Yes | For some indications and <65 years |
| Belgium | 14 | 42.5a | Partial | Yes, in case of rather low-risk profile |
| Bulgaria | 0 | 37.5a | None | Depends on income |
| Cyprus | 20 | 70 | Yes (depending on income) | n.r. |
| Czech Republic | 40a | 25 | Yes | For some indications |
| Denmarkb | 30 | 35 | Yes | No |
| Estonia | 14 | 17 | Yes | n.r. |
| Finland | 1 | 105a | Yes | No |
| France | 14 | 40 | Yes (conditional) | Dependent on clinical risk factors. Algorithm is considered as too complicated for most GPs |
| Germany | 0 | 45 | Yes | Reimbursed only after fracture |
| Greeceb | 11a | 52 | Yes | No |
| Hungary | 15a | 30 | Yes | Reimbursed only for women |
| Ireland | 140a b | 100 | Yes (conditional) | Reimbursed if privately insured. Otherwise, depending on income |
| Italy | 83a | 50a | Yes (conditional) | For some indications |
| Latvia | 10a | 23.5a | Yes | No |
| Lithuania | 6a | 21.5a | No | No |
| Luxembourg | 30 | 35a | Yes | No |
| Malta | 105a | 184b | Yes | For some indications |
| Netherlands | 14a | 105 | Yes | No |
| Poland | 1 | 20a | Yes (conditional) | Reimbursement only if seen by specialists |
| Portugal | 8 | 50a | Yes | No |
| Romania | 7 | 22.5a | Yes | Yes |
| Slovakia | 18a | 30 | Yes | No |
| Slovenia | 11a | 40 | Yes (conditional) | Reimbursement only for secondary osteoporosis |
| Spain | 105a | 41a | Yes | No |
| Sweden | 60 | 90 | Yes | No |
| UK | 11a | 99 | Yes | Yes |
n.r. Data not recorded
aAverage of range
bData from [1]
Fig. 22.
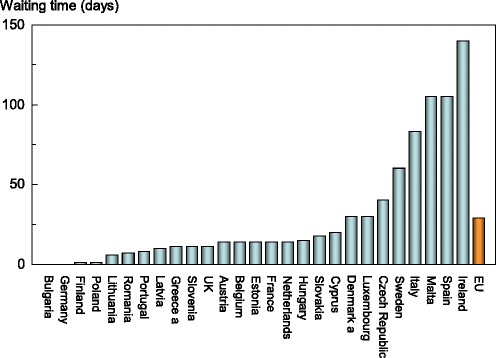
Reported average waiting time for a DXA assessment by EU country [IOF audit]
Reimbursement for DXA scans varied widely between member states both in terms of the criteria required and level of reimbursement awarded, and a majority of countries provided full reimbursement (Table 31). In others, reimbursement or partial reimbursement was limited and usually dependent on physician referral for approved indications, sometimes restricted to criteria that did not satisfy the requirements of good clinical practice. An example is seen in Bulgaria (and incidentally in Switzerland) where reimbursement is only offered if the BMD test turns out to be positive (i.e. shows osteoporosis). Other examples of restricted access included reimbursement only for limited indications (Austria, Czech Republic, Malta and Slovenia), only if seen by a specialist (Poland), only for women (Hungary) and only after fracture (Germany).
The cost of DXA also varied widely (Table 31) and bore little relation to the wealth of the nation or to the availability of DXA machines.
Score criteria
The highest score was allocated for unconditional reimbursement. In those countries with restricted reimbursement, countries were identified where reimbursement interfered with what patients could accept or physicians would wish to recommend to patients. Categories are shown in Table 32.
Table 32.
Criteria for allocating scores

Score allocation
Countries, ranked and categorised by score, are shown in Fig. 23.
Fig. 23.
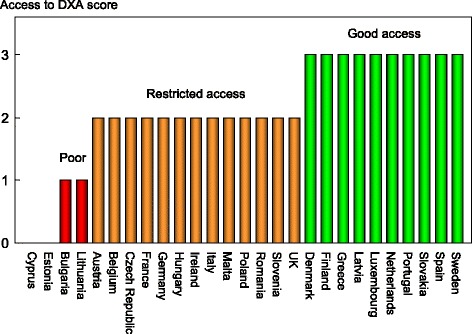
Categorisation of access to DXA by score in the EU27 [IOF audit]
Comment
There is a remarkable disparity between the availability of equipment and waiting time, which reflects a high heterogeneity in the use of BMD to assess osteoporosis. Unimpeded access to DXA is confined to a minority of member states.
References
1. International Osteoporosis Foundation (2008) Osteoporosis in the European Union in 2008: Ten years of progress and ongoing challenges. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
2. International Osteoporosis Foundation (2010b) The Eastern European and central asian regional audit. Epidemiology, cost and burden of osteoporosis in 2010. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
3d Access to risk assessment algorithms
Domain
Service provision—scorecard element
Background and aims
The effective targeting of treatment to those at highest risk of fracture requires an assessment of fracture risk. Historically, the targeting of treatment became feasible with the advent of bone mineral density measurements. The causation of fragility fractures is, however, heterogeneous and many additional factors have been identified that contribute to fracture risk. In turn, this has led to the development of risk algorithms that can enhance the assessment of fracture risk to better target interventions, particularly in primary care.
There are several assessment tools available in Europe [1–5]. The most widely used is FRAX®. FRAX is a computer-based algorithm (http://www.shef.ac.uk/FRAX) that calculates the 10-year probability of a major fracture (hip, clinical spine, humerus or wrist fracture) and the 10-year probability of hip fracture. Fracture risk is calculated from age, body mass index and well validated dichotomized risk factors. Femoral neck bone mineral density (BMD) can be optionally input to enhance fracture risk prediction. Fracture probability differs markedly in different regions of the world so that FRAX is calibrated to those countries where the epidemiology of fracture and death is known (currently 50 countries). In addition to the web site, FRAX has been incorporated into the software of densitometers and is available as an application for the iPhone/iPad.
The aim of this scorecard element was to document the availability of country-specific risk assessment models (their uptake is considered separately in Chapter 4b). The score was based on the availability of risk assessment models and specific guidance for their use.
Methods
The availability of country-specific FRAX models was provided at the FRAX web site. The availability of other risk engines was determined from an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012 together with a review of country-specific assessment guidelines. The metrics sought were the availability of country-specific risk models and whether national guidance was provided on how results from these assessments should be used in clinical practice.
Results
Risk assessment models were available in 21 of the member states (Table 33). The majority were based on FRAX. In Germany, probability-based fracture risk assessment comprises a component of national guidelines, but is not FRAX-based [1]. Alternative assessment algorithms are also available in the Netherlands [3]. In the UK both FRAX and QFracture has been approved [6]. No models are available for Bulgaria, Cyprus, Estonia, Latvia, Luxembourg and Slovenia due to the lack of appropriate epidemiology of fracture on which these could be based. In countries where a model is available, a small majority (12/21) provide guidance on its application to clinical practice.
Table 33.
Provision of risk assessment models in the EU and guidance on their application to clinical practice
| FRAX model available | Other models | Guidance | Comments | |
|---|---|---|---|---|
| Austria | ✓ | – | ✓ | |
| Belgium | ✓ | – | ✓ | |
| Bulgaria | – | – | – | |
| Cyprusa | – | – | – | |
| Czech Republic | ✓ | – | No | |
| Denmark | ✓ | – | No | |
| Estonia | – | – | – | |
| Finland | ✓ | – | ✓ | |
| France | ✓ | – | ✓ | |
| Germany | ✓ | Yes | ✓ | DVO model [1] |
| Greece | ✓b | – | ✓ | |
| Hungary | ✓ | – | ✓ | |
| Ireland | ✓b | – | –c | |
| Italy | ✓ | – | –c | |
| Latvia | – | – | –c | |
| Lithuania | ✓b | – | No | |
| Luxemburg | – | – | –c | |
| Malta | ✓ | – | No | |
| Netherlands | ✓ | Yes | ✓ | CBO [3] |
| Poland | ✓ | – | ✓ | |
| Portugal | ✓b | – | ✓ | |
| Romania | ✓ | – | –c | |
| Slovakia | ✓ | – | Noa | |
| Slovenia | – | – | No | |
| Spain | ✓ | – | No | |
| Sweden | ✓ | – | ✓ | |
| UKd | ✓ | Yes | ✓ | QFracture [2] |
| Europe | – | – | ✓ |
aUses Greek FRAX tool as a surrogate model
bAvailable from June 2012 and September 2012 (Portugal);
cNoted in guidelines but without guidance; c to be implemented.
dGuidance not provided by NICE
European guidance that can be applied to member states has been recently published for postmenopausal and glucocorticoid-induced osteoporosis [7, 8].
Score allocation
The score was based availability of country-specific risk models and whether national guidance was provided on how results from these assessments should be used in clinical practice as given in Table 34.
Table 34.
Criteria for allocating scores

The score assigned to each country is shown in Fig. 24.
Fig. 24.
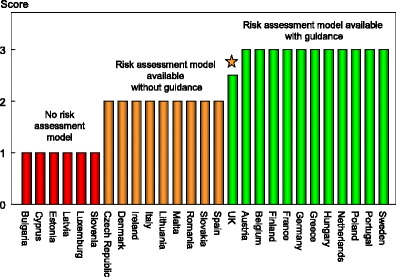
The score assigned to each country on the basis of its provision of fracture risk assessment algorithms. The star denotes that guidance given by the National Osteoporosis Guideline Group scores 3 but the score based on NICE is less
Comment
Risk assessment models for fractures based on FRAX were available in 21 out of 27 countries. In some countries (UK, Germany and the Netherlands) other models are also available. However, guidance on the use of risk assessment was available in only 12 out of 27 countries.
References
1. Dachverband Osteologie e.V (2011) DVO guideline 2009 for prevention, diagnosis and therapy of osteoporosis in adults. Osteologie 20: 55–74. Accessible at: http://www.schattauer.de/en/magazine/subject-areas/journals-a-z/osteology/contents/archive/issue/special (Accessed May 2012)
2. Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ. 2012 May 22;344:e3427.
3. Dutch Institute for Healthcare Improvement (CBO). Richtlijn Osteoporose en fractuurpreventie, derde herziening. Utrecht: CBO; 2011.
4. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey EV (2008) FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385–397.
5. Kanis JA on behalf of the World Health Organization Scientific Group (2008) Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK. Access at http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf
6. National Institute for Clinical Excellence (2012) Osteoporosis: assessing the risk of fragility fracture. Clinical guideline 146. www.nice.org.uk/CG146
7. Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24: 23–57.
8. Lekawasam S, Adachi JD, Agnusdei D et al. for the Joint IOF-ECTS GIO Guidelines Working Group (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–76.
3e Quality of guidelines for assessment and treatment
Domain
Service provision—scorecard element
Background and aims
The aim of guidelines is to provide an information platform for the assessment and treatment of osteoporosis so that appropriate treatment is directed to individuals at high fracture risk. Their scope most commonly includes postmenopausal osteoporosis, glucocorticoid-induced osteoporosis and osteoporosis in men. Less commonly, guidelines are available for the assessment of falls risk and its treatment. Ideally, guidelines should be based on systematic literature reviews and any recommendations supported by an adequate level of evidence.
The aim of this scorecard element was to determine the scope and quality of guidelines available in the EU27 countries.
Methods
Data were acquired by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012. Respondents were asked whether national guidelines were available for the assessment and/or treatment of osteoporosis. Responses were used to update an earlier audit of the IOF [1, 2]. Where guidelines were available, additional information was requested on their scope and quality.
Scope of guideline: Does it relate to postmenopausal women (PMW), men or glucocorticoid-induced osteoporosis (GIOP).
AGREE criteria: The quality of guidelines was judged according to the criteria developed the Appraisal of guidelines for research & evaluation (AGREE) next steps consortium [3] under 7 general domains (see footnote1).
Results
Guidelines for the management of osteoporosis were available in the majority of member states (unavailable in Cyprus and Malta). All of the remaining counties had guidelines available for postmenopausal women (Table 35). 17 of 25 countries had guidelines for osteoporosis in men and 19 had guidelines for glucocorticoid-induced osteoporosis. The availability of guidelines does not necessarily improve disease management and in some countries (e.g. Italy, Spain and UK) multiple guidelines were available from different sources that are likely to confuse rather than clarify clinical practice.
Table 35.
Availability and scope of guidelines for the assessment and treatment of osteoporosis in the EU [IOF audit]
| Developed (year) | Scopea | AGREE criteria | Score | |
|---|---|---|---|---|
| Austria | 2010 | PMW, men, GIOP | 5 | 8 |
| Belgium | 2005–11 | PMW, men, GIOP | 4 | 7 |
| Bulgaria | 2007 | PMW, men, GIOP | 6 | 9 |
| Cyprus | na | – | 0 | 0 |
| Czech Republic | 2003–10 | PMW, GIOP | 4 | 6 |
| Denmark | 2009 | PMW | 4 | 5 |
| Estonia | 2012 | PMW, men, GIOP | 5 | 8 |
| Finland | 2013 | PMW, men, GIOP | 7 | 10 |
| France | 2012 | PMW, men, GIOP | 5 | 6 |
| Germany | 2009 | PMW, men, GIOP | 7 | 10 |
| Greece | 2009–12 | PMW, men, GIOP | 5 | 8 |
| Hungary | 2003–11 | PMW, men, GIOP | 5 | 8 |
| Ireland | 2011 | PMW, men | 1 | 3 |
| Italy | −2011 | PMW, men, GIOP | 7 | 10 |
| Latvia | 2011 | PMW, men, GIOP | 7 | 10 |
| Lithuania | 2011 | PMW | 7 | 7 |
| Luxemburg | 2010 | PMW | 7 | 7 |
| Malta | na | – | 0 | 0 |
| Netherlands | 2011–12 | PMW, men, GIOP | 7 | 10 |
| Poland | 2011–12 | PMW, men, GIOP | 3 | 6 |
| Portugal | 2011 | PMW | 7 | 8 |
| Romania | 2011 | PMW, men, GIOP | 6 | 9 |
| Slovakia | 2006–10 | PMW, GIOP | 7 | 9 |
| Slovenia | 2002 | PMW | 2 | 3 |
| Spain | 2004–11 | PMW, men, GIOP | 4.5 | 6.5 |
| Sweden | 2008 | PMW, men, GIOP | 7 | 10 |
| UKb | 2008 | PMW, men, GIOP | 7 | 10 |
a PMW postmenopausal women, GIO glucocorticoid-induced osteoporosis
bRelates to guidance provided by the National Osteoporosis Guidelines Group [4]. National guidance with less scope also available from NICE [5–7]
Score criteria
A positive response in each AGREE category contributed a point to the score (maximum 7 points). Up to 3 additional points was given for the scope of the guideline (PMW, men, GIOP) to give a maximum score of 10. Where more than one guideline was available, a composite mean was used. Scores for each country were categorised as shown in Table 36.
Table 36.
Criteria for allocating scores
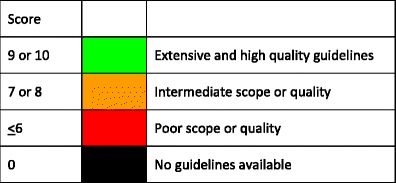
Score allocation
The score allocation and grade for each country is shown in Fig. 25.
Fig. 25.
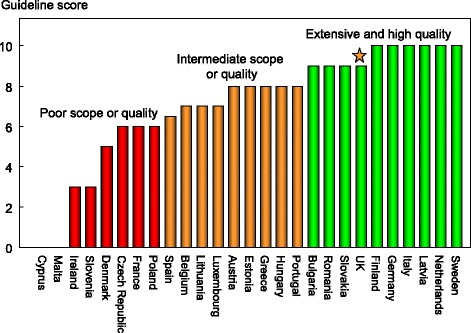
Score allocation based on the scope and quality of guidelines available for the assessment and treatment of osteoporosis. For the UK (star), the score for guidance provided by NICE is 8 and hat provided by the National Osteoporosis Guidelines Group has a score of 10 [IOF audit]
Comment
There was a large variation in the extent and quality of national guidelines according to the AGREE criteria. It should be noted that a high score reflects the quality of the process, but not necessarily the quality of the content.
References
1. International Osteoporosis Foundation (2010) Osteoporosis in the European Union in 2008. Ten years of progress and ongoing challenges. Updated Aug 2010. International Osteoporosis Foundation, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
2. International Osteoporosis Foundation (2010) The Eastern European and central Asian regional audit. Epidemiology, cost and burden of osteoporosis in 2010. IOF, Nyon. Available at www.iofbonehealth.org accessed 23rd Sept 2012
3. The AGREE next steps consortium (2009) Appraisal of guidelines for research & evaluation II. AGREE Research Trust, McMaster University, Hamilton, Ontario, Canada. Available at http://www.agreetrust.org Accessed December 2012
4. Compston J, Cooper A, Cooper Cet al, on behalf of the National Osteoporosis Guideline Group (NOGG) (2009) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas; 62:105–108.
5. National Institute for Clinical Excellence (2008) Technology Appraisal (TA) 160: Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal women (amended) http://www.nice.org.uk/nicemedia/live/11746/47176/47176.pdf
6. National Institute for Clinical Excellence (2008) Technology Appraisal (TA) 161: Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women http://www.nice.org.uk/nicemedia/live/11748/42508/42508.pdf
7. National Institute for Clinical Excellence (2010) Technology Appraisal (TA) 204: Osteoporotic fractures—denosumab http://www.nice.org.uk/nicemedia/live/13251/51293/51293.pdf
3f Guideline criteria for assessment and treatment
Domain
Service provision—supplementary information
Background and aims
The aim of this section was to summarise the differences in the application of guidelines to clinical practice and, in particular, to identify where guideline recommendations conflicted with reimbursement policy.
Methods
A review was undertaken of guidelines covering the assessment and treatment of osteoporosis in each EU member state that was available in English or French. Additionally, data were acquired from a structured IOF questionnaire administered to the EU Osteoporosis Consultation Panel undertaken in December 2012. Information requested included whether guidelines addressed population-based screening, the tools used for assessment, and the tools to decide eligibility for treatment. An enquiry was also made whether risk assessment or treatment recommendations were compatible with reimbursement policy.
Results
Guidelines were not available in Cyprus or Malta. In the remaining countries, guidelines were generally less than 5 years old, often related to updating, with one exception (Slovenia, 2002).
Population screening was considered in guidelines from 15 of 25 countries. Although reviewed, population based screening was not recommended. In Hungary, however BMD is offered free of charge to women aged 50 years or more, though the uptake is low.
Guidelines in 24 of the 25 countries covered the assessment of fracture risk (the exception was the Czech Republic). The most common tools used for fracture risk assessment were age in 22/25 countries (exceptions were Lithuania, Slovakia and Sweden) and bone mineral density (in all countries). The use of fracture risk assessment algorithms was less consistent and noted in 17 countries (Fig. 26). FRAX was the most widely used instrument though in Germany the DVO tool was recommended [1]. In the UK, both FRAX and QFracture has been approved [2].
Fig. 26.
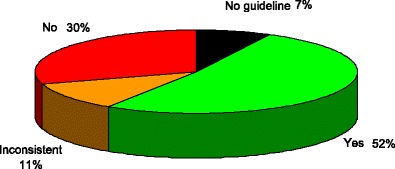
The distribution of the use of risk assessment models in assessment guidelines of the EU27 countries [IOF audit]
Guidelines in all 25 countries covered eligibility for treatment with a general commonality of approach. Eligibility for treatment depended on prior fracture (except Denmark and Sweden), age (except Lithuania, Slovakia and Sweden) and BMD (all countries). As was the case for assessment (see Chapter 3d), risk assessment tools provided criteria for intervention in fewer (17/25) countries (Fig. 26). In Italy, Spain and the UK, there are several guidelines from different sources that give different recommendations.
Several counties reported incompatibilities between recommendations for risk assessment or treatment with reimbursement policy (Table 37). For example, guidelines recommended the use of FRAX which was not provided for in reimbursement provision (Belgium, Poland, Romania and Slovakia), specific treatments were recommended but not reimbursed (Poland, Romania) and central DXA was recommended for risk assessment but other techniques (peripheral BMD and QCT) were reimbursed (Lithuania). In Luxembourg, the BMD thresholds for treatment differ from the criteria for reimbursement for DXA. In other instances, guidelines recommended the use of risk factors such as a prior fragility fractures but reimbursement was solely dependent on BMD (Lithuania, Romania). With regard to treatment, reimbursement was limited in time (18 months) but treatment recommended on a long-term basis (Lithuania). A problem inconsistently related to reimbursement was that multiple guidelines gave conflicting recommendations (Italy, Spain, and the UK). Another barrier to treatment is that reimbursement was only granted where the prescription was issued by a specialist (e.g. Czech Republic).
Table 37.
Scope of guidelines for patient assessment, treatment and consistency with reimbursement policy [IOF audit]
| Date | Assessment | Compatible/consistent | Treatment | Compatible/consistent | |
|---|---|---|---|---|---|
| Austria | 2010 | Yes | Yes | Yes | Yes |
| Belgium | 2005–11 | Yes | No | Yes | No |
| Bulgaria | 2007 | Yes | No | Yes | No |
| Cyprus | na | – | – | – | – |
| Czech Republic | 2003–10 | No | No | Yes | No |
| Denmark | 2009 | Yes | Yes | Yes | |
| Estonia | 2012 | Yes | Yes | Yes | Yes |
| Finland | 2013 | Yes | Yes | Yes | Yes |
| France | 2012 | Yes | Yes | Yes | Yes |
| Germany | 2009 | Yes | No | Yes | No |
| Greece | 2009 | Yes | Yes | Yes | Yes |
| Hungary | 2011 | Yes | Yes | Yes | Yes |
| Ireland | 2011 | Yes | No | Yes | Yes |
| Italy | −2011 | Yes | Yes/no | Yes | No |
| Latvia | 2011 | Yes | Yes | Yes | Yes |
| Lithuania | 2011 | Yes | No | Yes | No |
| Luxembourg | 2010 | Yes | No | Yes | Yes |
| Malta | na | – | – | – | – |
| Netherlands | 2011–12 | Yes | Yes | Yes | Yes |
| Poland | 2011–12 | Yes | No | Yes | No |
| Portugal | 2011 | Yes | Yes | Yes | Yes |
| Romania | 2011 | Yes | No | Yes | No |
| Slovakia | 2006/09/10 | Yes | No | Yes | Yes |
| Slovenia | 2002 | Yes | Yes | Yes | Yes |
| Spain | 2004–11 | Yes/no | Yes/no | Yes/no | Yes |
| Sweden | 2012 | Yes | Yes | Yes | Yes |
| UK | 2008–12 | Yes | Yes/no | Yes | Yes |
na not applicable, nr not recorded
Score allocation
Supplementary information, no score allocation
Comment
Risk assessment or treatment recommendations were compatible with reimbursement policy in approximately half of the EU member states.
References
1. Dachverband Osteologie e.V (2011) DVO guideline 2009 for prevention, diagnosis and therapy of osteoporosis in adults. Osteologie 20: 55–74. Accessible at: http://www.schattauer.de/en/magazine/subject-areas/journals-a-z/osteology/contents/archive/issue/special (Accessed May 2012)
2. National Institute for Clinical Excellence (2012) Osteoporosis: assessing the risk of fragility fracture. Clinical guideline 146. www.nice.org.uk/CG146
3g Fracture liaison services
Domain
Service provision—scorecard element
Background and aims
Fracture liaison services (FLS), also known as osteoporosis co-ordinator programmes and care manager programs, provide a system for the routine assessment and management of postmenopausal women and older men who have sustained a low trauma fracture [1–4]. Assessment includes DXA measurements, fall risk evaluation, and underlying secondary causes of osteoporosis. Although the importance of an incident fracture as a risk factor for further fracture is well recognised, the majority of patients presenting with a low trauma fracture do not receive appropriate assessment and treatment in the setting of standard hospital care. FLS address this need through a systematic approach to identifying such individuals and assessing their risk of further fractures and the need for treatment. Most FLS are based in secondary care although models in primary care have also been described. The clinical and cost-effectiveness of FLS has been demonstrated in several centres [5–7].
The aim of this scorecard element was to document the proportion of hospitals that have a fracture liaison service in the EU27 countries.
Methods
Information was acquired from a structured IOF questionnaire administered to the EU Osteoporosis Consultation Panel undertaken in December 2012. Correspondents were asked to estimate the proportion of hospitals in each member state that have a scheme in place that refers fracture patients over 50 years old to a fracture liaison service. Scoring was based on the distribution of the estimates.
Results
No estimates were provided from Malta. Of the remaining countries, no fracture liaison services were reported from Greece, Latvia, Portugal, Romania and Slovakia. The presence of FLS was acknowledged in the remaining member states, but for most countries, the proportion of hospitals that have a scheme in place was less than 10 %. Higher rates were reported from Austria, Cyprus, Czech Republic, Estonia, Finland, Hungary, Netherlands and Sweden (Fig. 27).
Fig. 27.
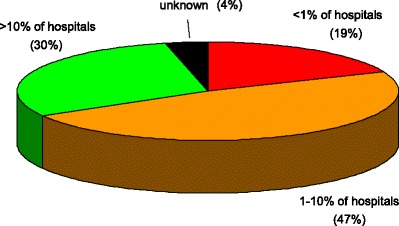
The proportion of hospitals (%) with FLS in the EU countries [IOF audit]
Score criteria
The proportion of hospitals in each member state that had fracture liaison services (FLS) in place were categorised as shown in Table 38.
Table 38.
Criteria for allocating scores
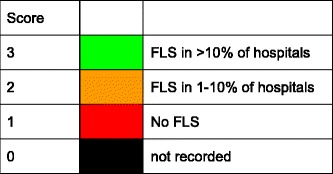
Score allocation
Figure 28 shows the scores allocated by country
Fig. 28.
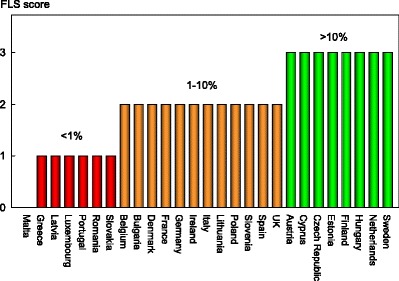
Scores allocated by country on the availability of fracture liaison services in hospitals by member state [IOF audit]
Comment
The information provided needs to be interpreted cautiously. It provides a perception of how many hospitals of a country has a fracture nurse working in a fracture liaison service, but is an expert opinion and not based on numerical evidence. Moreover, no account was taken of FLS in primary care. In addition, no information was available on the performance of the FLS. It is also notable that a colour code of green should not be interpreted as an endorsement since provision should, in the view of the panel, be expected in the majority of hospitals or care centres.
References
1. Cooper C, Mitchell P, Kanis JA (2011) Breaking the fragility fracture cycle. Osteoporos Int 22:2049–50.
2. Eisman JA, Bogoch ER, Dell R et al. (2012) Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 27: 2039–46
3. International Osteoporosis Foundation (2012) Capture the fracture: a global campaign to break the fragility fracture cycle. IOF, Nyon. Accessible at www.iofbonehealth.org
4. Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E (2011) Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 22: 2067–82.
5. Dell R (2011) Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int 22 (Suppl 3): 457–60
6. Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, McLellan AR, Mitchell PJ, Sale JE, Wahl DA; IOF CSA Fracture Working Group (2011) Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 22: 2051–65
7. McLellan AR, Wolowacz SE, Zimovetz EA, Beard SM, Lock S, McCrink L, Adekunle F, Roberts D (2011) Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011 22: 2083–98.
3h Use of quality indicators
Domain
Service provision—scorecard element
Background and aims
The use of indicators to systematically measure the quality of care provided to people with osteoporosis or associated fractures is a relatively new discipline, with the United States perhaps having the most developed system in place [1], as shown for use of healthcare quality indicators more broadly [2]. In the UK, the Department of Health Best Practice Tariff for hip fracture care has used financial incentives since April 2010 to drive adherence with the six core benchmarks, which include an assessment of bone health and risk of falling. In the 2 years following introduction of the tariff, the proportion of patients with fragility hip fracture for whom all six standards were met rose from 24 to 55 % [3].
The aim of this scorecard element was to document the systematic approaches to enhance the quality of osteoporosis care or secondary prevention of fragility fractures in the EU.
Methods
Data were acquired by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012. Respondents were asked whether national systems were in place that systematically collect data on the quality of care provided to people with osteoporosis or the secondary prevention of fragility fractures. Further questions were whether the systems use measures (quality indicators or standards) that are documented on a regular basis (e.g. annually) and use a set of explicit criteria to assess performance.
Results
Few countries had systems that include quality measures plus a regular audit for national health care agencies (Denmark, Germany, and UK). In the United Kingdom, osteoporosis/ secondary prevention of fragility fractures has been included in the Quality and Outcomes Framework as part of the general practitioner contract from April 2012 [4]. The Quality and Outcomes Framework is a pay for performance scheme for general practice in the UK, which awards ‘achievement points’ for adhering to procedural and treatment guidelines and meeting intermediate outcome targets for over 130 quality indicators. In the UK, there is also a system of clinical audits in place, seeking to improve patient care and outcomes through systematic review of care according to explicit criteria and the implementation of change. These include the National Audit of Falls and Bone Health in Older People [5] and the continuous National Hip Fracture Database [3]. In Germany, selected providers and health insurance funds have, in the framework of ‘integrated care contracts’ entered into agreements on coordinated osteoporosis care which may include the documentation of care standards to enable tracking of the quality of care provided. The nature and contents of these contracts vary across regions [6]. There is a systematic and nationwide collection of quality indicators for the inpatient care following hip fracture [7]; however a systematic collection of indicators that would permit assessment of care quality of those with osteoporosis and in the secondary prevention of fragility fractures is not in place.
Several other counties (Bulgaria, Finland, Greece) have systems that provide quality indicators or standards that are documented on a regular basis (Table 39) but it is unclear whether criteria are developed to assess performance. In Slovakia, quality measures are in place but no provision is made for regular audit.
Table 39.
Systems that provide quality indicators in the context of osteoporosis and fractures in the European Union [IOF audit]
| Systems in place | Targets | Score | |
|---|---|---|---|
| Austria | No | 1 | |
| Belgium | No | 1 | |
| Bulgaria | Yes | Hip fractures | 2 |
| Cyprus | No | 1 | |
| Czech Republic | No | 1 | |
| Denmark | Yes | Hip fractures | 3 |
| Estonia | No | 1 | |
| Finland | Yes | Hip fractures | 2 |
| France | No | 1 | |
| Germany | Yes | Hip fractures | 3 |
| Greece | Yes | Hip fractures | 2 |
| Hungary | Yes | Fragility fractures | 2 |
| Ireland | No | 1 | |
| Italy | No | 1 | |
| Latvia | No | 1 | |
| Lithuania | No | 1 | |
| Luxemburg | No | 1 | |
| Malta | No | 1 | |
| Netherlands | Yes | 3 | |
| Poland | No | 1 | |
| Portugal | No | 1 | |
| Romania | No | 1 | |
| Slovakia | Yes | Osteoporosis, fragility fracture, falls | 2 |
| Slovenia | No | 1 | |
| Spain | No | 1 | |
| Sweden | Yes | Fractures, treatment | 2 |
| UK | Yes | Hip fracture, falls, fragility fractures | 3 |
Score criteria
The score was based on the presence of systems and their use as quality indicators as given in Table 40
Table 40.
Criteria for allocating scores

Score allocation
Score allocation for quality indicators by country are given in Fig. 29.
Fig. 29.
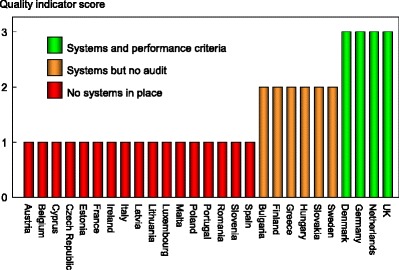
Score allocation for quality indicators by country [IOF audit]
Given the relative novelty of using QI for the tracking of quality of care provided to people with osteoporosis or associated fractures in the European region, it should be recognised that the score is a ‘proxy’ measure. Though audited quality measures have been introduced in some countries, the UK is far advanced in this regard.
References
1. Conklin A, Yaqub O, Celia C, Nolte E (2012) Postmenopausal osteoporosis management. A review of the evidence to inform the development of quality indicators. Santa Monica: RAND Corporation.
2. Nolte E, Roland M, Damberg CL, Mattke S, Cacace M, Goshev S, Brereton L, Conklin A, Hiatt L, Quigley DD, Lovejoy SL (2011) Informing the development of a resource allocation framework in the German healthcare system. Santa Monica: RAND Corporation.
3. National Hip Fracture Database. Available at http://www.nhfd.co.uk/.
4. NHS Employers and British Medical Association. Quality and Outcomes Framework for 2012/13. London: NHS Employers, 2012.
5. Treml J, Husk J, Lowe D, Vasilakis N (2011) Falling standards, broken promises. Report of the National Audit of Falls and Bone Health in Older People. London: Royal College of Physicians.
6. Grothaus F-J. Entwicklung der integrierten Versorgung in der Bundesrepublik Deutschland 2004 – 2008. Gemeinsame Registrierungsstelle zur Unterstützung der Umsetzung des § 140d SGB V, 2009.
7. AQUA. Beschreibung der Qualitätsindikatoren für das Erfassungsjahr 2011. Hüftgelenknahe Femurfraktur. Qualitätsindikatoren 2011. [Online] Available at http://www.sqg.de/downloads/QIDB/2011/AQUA_17n1_Indikatoren_2011.pdf (Accessed on 29 June 2012). [also: http://www.sqg.de/sqg/upload/CONTENT/EN/Quality-Report/AQUA-German-Hospital-Quality-Report-2010.pdf]
Chapter 4. Service uptake
4a Uptake of DXA
Domain
Service uptake—background information
Background and aims
The ability to assess osteoporosis depends in part on the availability of bone mass measurements at the lumbar spine and hip with dual energy X-ray absorptiometry (DXA). The requirement for the technology will depend on the assessment guidelines in each member state and the policy with respect to the use of DXA to diagnose osteoporosis and monitor treatment. The uptake of this technology depends upon the efficiency with which the technology is used, the ease of patient access (e.g. travelling time), regulatory constraints and barriers to reimbursement.
The aim of this element was to compare the access to DXA measured as a function of the requirements recommended in relevant assessment guidelines.
Methods
Ideally, uptake should be measured as the number of scans undertaken in relation to treatment guidelines in each member state. Such data are not available in the EU as a whole. Data are available by age and sex from the National Health Service of Denmark and are reported here [Abrahamsen, 2011].
Results
The Danish National Health Service release claims data for DXA and was available most recently for 2005. The uptake of BMD testing by age and sex for 2005 is shown in Fig. 30. Although the accuracy of the claims to tests is uncertain and tests in the private sector are not captured, the uptake was very low even accounting for these errors. Thus, guidelines based on BMD testing indicate that 173 women/1,000 women aged 50 years or more qualify for BMD testing [2] whereas the corresponding figure for Denmark was 28.6 or 16 % of the desired uptake. The use of probability-based guidelines reduces the number of scans needed to 81/1000 women [2] but is still considerably higher than that attained in Denmark. The uptake in men over the age of 50 years was 4 times lower (7/1000) than in women. In men and women combined the uptake of DXA was 18.5/1000.
Fig. 30.
The uptake of BMD testing in men and women by age and sex in Denmark in 2005 [Data kindly provided by Bo Abrahamsen, Gentofte Hospital Copenhagen, Denmark]
Score allocation
No score allocation
Comment
More information is required from all member states on the utilisation of DXA with regard to guidelines on the assessment and monitoring of treatment. The available evidence from Denmark, a country moderately provided with DXA machines, is that service uptake is less than optimal.
References
1. Abrahamsen B (2011) Personal communication to Kanis JA.
2. Johansson H, Kanis JA, Oden A, Compston J, McCloskey E (2012) A comparison of case-finding strategies in the UK for the management of hip fractures. Osteoporos Int 23: 907–915.
4b Uptake of risk assessment algorithms
Domain
Service uptake—scorecard element
Background and aims
FRAX is an algorithm that determines fracture probability in individuals by integrating the weight of important clinical risk factors for fracture and mortality risk, with or without information on BMD (see Chapter 1 g). Each tool is country-specific and calibrated to the national epidemiology of fracture. They were developed by the WHO Collaborating Centre for Metabolic Bone Diseases at Sheffield, UK and launched in 2008 [1, 2]. The FRAX tools (www.shef.ac.uk/FRAX) compute the 10-year probability of hip fracture or a major osteoporotic fracture. A major osteoporotic fracture is a clinical spine, hip, forearm and humerus fracture. The use of the tool improves risk assessment compared to the use of BMD alone.
FRAX is now a component of many national guidelines for the assessment of osteoporosis (see Chapter 3f) and European guidelines for postmenopausal osteoporosis and glucocorticoid-induced osteoporosis [3, 4]. The aim of this element was to determine the usage of FRAX in the EU27 countries.
Methods
Each FRAX model on the web counts the number of calculations performed for that particular country. A problem with these data is that some countries, particularly those without a country-specific FRAX model, may use a surrogate. For example, the UK model was adopted as a surrogate in Poland before the advent of a Polish model and the Greek model is presently used in Cyprus. For this reason, we assessed the number of calculations by the source of the calculation [Google Analytics]. FRAX usage was computed as the number of calculations originating from each country and expressed as calculations/million of the general population over a period of one year (November 2010 to December 2011).
Results
The web-based usage of the models is shown in Table 41 which shows considerable heterogeneity in uptake. Belgium, UK, Luxembourg, Sweden and Ireland have the highest usage of FRAX. Lithuania, Latvia, Germany and Bulgaria have the lowest uptake. The average uptake for the EU27 was 880 calculations/million of the general population.
Table 41.
Uptake of FRAX in EU counties expressed as the number of calculations per million of the general population
| Country | Calculations /million | Country | Calculations /million | Country | Calculations /million |
|---|---|---|---|---|---|
| Austria | 1534 | Germany | 83.5 | Netherlands | 526 |
| Belgium | 5003 | Greece | 502 | Poland | 338 |
| Bulgaria | 112 | Hungary | 1205 | Portugal | 1039 |
| Cyprus | 272 | Ireland | 1643 | Romania | 230 |
| Czech Republic | 175 | Italy | 518 | Slovakia | 372 |
| Denmark | 942 | Latvia | 57.7 | Slovenia | 1322 |
| Estonia | 207 | Lithuania | 28.5 | Spain | 1115 |
| Finland | 444 | Luxembourg | 2293 | Sweden | 1911 |
| France | 314 | Malta | 1541 | UK | 2293 |
Country-specific models are available in 21 member states (see Chapter 3d). FRAX models are not available for Bulgaria, Cyprus, Estonia, Latvia, Luxembourg and Slovenia. There is, however, no clear relationship between the availability of a country-specific model and the use of FRAX.
Score criteria
FRAX calculations/million of the general population/year was categorised by tertiles as given in Table 42
Table 42.
Criteria for allocating scores
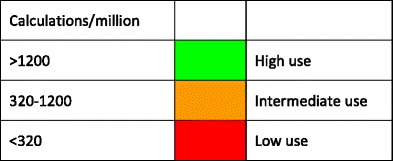
Score allocation
Countries, ranked and categorised by score, are shown in Fig. 31.
Fig. 31.
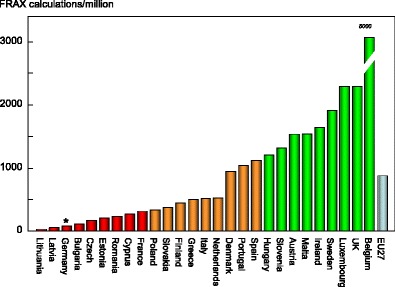
The uptake of fracture risk assessment tools as judged by the use of FRAX from each EU country by score category. *See comment below with regard to Germany
Comment
These data underestimate the use of FRAX by approximately 25 % due to the availability of FRAX on bone densitometers. FRAX calculations on densitometers are not performed through the web site. In addition, hand held calculators are used in several countries, particularly in Poland. FRAX is also available as an application on the iPhone.
These data underestimate the use of risk assessment in Germany. Fracture risk assessment comprises a component of the German national guidelines, but is not FRAX based. Alternative assessment algorithms are also available in the UK and the Netherlands.
The caveats above indicate that the figures are conservative. Even so, there are reasons to believe that FRAX is underutilised. For example, the use of FRAX in Denmark (942 calculations/million per year) is much lower than the number of BMD tests/year (18,500 /million per year; see Chapter 4a). Thus, a colour code of green should not be interpreted as an endorsement of appropriate uptake.
References
1. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey EV (2008) FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385–397.
2. Kanis JA on behalf of the World Health Organization Scientific Group (2008) Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK. Access at http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf
3. Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation ( IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24: 23–57
4. Lekawasam S, Adachi JD, Agnusdei D et al. for the Joint IOF-ECTS GIO Guidelines Working Group (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporosis International 23:2257–76.
4c Treatment uptake and treatment gap
Domain
Service uptake—scorecard element
Background and aims
Many surveys indicate that a minority of men and women at high fracture risk receive treatment [1, 2]. The aim of this section was to estimate the proportion of women at high risk that receives therapy for osteoporosis in the EU27.
Methods
The proportion of patients eligible for treatment depends on defining an intervention threshold, i.e. the risk of fracture above which treatment can be recommended. Though treatment guidelines are available in nearly all EU member states [3] (see Chapters 3e and 3f), there is no uniform approach to intervention thresholds across the EU27.
The advent of FRAX (www.shef.ac.uk/FRAX) in 2008 provided a clinical tool for the calculation of fracture probability that has been applied to the development of intervention thresholds [4]. For the purposes of this analysis, the intervention threshold set was at the 10-year fracture probability equivalent to women with a prior fragility fracture without knowledge of BMD as adopted in several European guidelines [5–7]. Thus, the intervention threshold can be likened to a ‘fracture threshold’ expressed in terms of fracture probability. The proportion of women who exceed the fracture threshold was computed by simulation [8] based on the distribution of the risk-score among the cohorts used by WHO to develop FRAX and the epidemiology of fracture and death in each EU country.
The number of patients treated in each country was computed from IMS Health sales data for 2010 and expressed as treatment years [3]. The use of hormone replacement treatment was excluded since the majority of women take hormone replacement treatment for menopausal symptoms rather than for osteoporosis. An adjustment factor (estimated from data from the Swedish Prescribed Drug Register) was used to correct for suboptimal adherence. The number of women potentially treated was subtracted from the number of women exceeding the intervention threshold and expressed as a percentage. No sales data were available for Cyprus or Malta and these two countries were therefore excluded from analyses.
Results
Table 43 indicates that there is wide inter-country variation in the treatment penetration of women at high risk for osteoporotic fractures. The treatment gap varied from 25 % in Spain to 95 % in Bulgaria. Large treatment gaps were identified in countries with populations at both high and low risk of fracture. In total in the EU, it is estimated that, out of the 18.4 million women that exceed the risk level, 10.6 million are untreated. These figures are conservative since an undetermined proportion of low-risk women will have received treatment (see comments, below).
Table 43.
Number of women eligible for treatment, treated and treatment gap in 2010 [3]
| Country | Number potentially treated (000s) | Number exceeding fracture risk threshold (000s) | Difference (000s) | Treatment gap (%) |
|---|---|---|---|---|
| Austria | 139 | 282 | 143 | 51 |
| Belgium | 214 | 402 | 188 | 47 |
| Bulgaria | 13 | 240 | 227 | 95 |
| Czech Republic | 79 | 330 | 251 | 76 |
| Denmark | 87 | 190 | 103 | 54 |
| Estonia | 7 | 48 | 41 | 86 |
| Finland | 53 | 172 | 119 | 69 |
| France | 1,390 | 2,437 | 1,047 | 43 |
| Germany | 730 | 3,231 | 2,501 | 77 |
| Greece | 333 | 482 | 149 | 31 |
| Hungary | 238 | 332 | 94 | 28 |
| Ireland | 91 | 124 | 33 | 26 |
| Italy | 1,069 | 2,635 | 1,566 | 59 |
| Latvia | 12 | 80 | 68 | 85 |
| Lithuania | 11 | 109 | 98 | 90 |
| Luxembourg | 9 | 16 | 7 | 43 |
| Netherlands | 242 | 605 | 363 | 60 |
| Poland | 245 | 1,127 | 882 | 78 |
| Portugal | 269 | 425 | 156 | 37 |
| Romania | 100 | 599 | 499 | 83 |
| Slovakia | 75 | 148 | 73 | 49 |
| Slovenia | 35 | 62 | 27 | 44 |
| Spain | 1,277 | 1,709 | 432 | 25 |
| Sweden | 100 | 358 | 258 | 72 |
| UK | 1,064 | 2,298 | 1,234 | 54 |
| EU27 | 7,881 | 18,441 | 10,560 | 57 |
Score criteria
Countries were categorised by approximate tertiles as shown in Table 44.
Table 44.
Criteria for allocating scores
Score allocation
The score allocation and the treatment gap for each country is shown in Fig. 32.
Fig. 32.
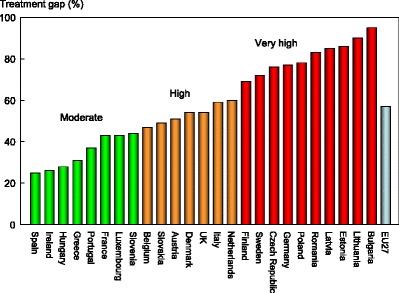
Proportion of women of women at high risk that are untreated (treatment gap) in 2010 ranked by country and score [3]
Comment
It is unlikely that 100 % of sales are captured in any country but it is difficult to define the magnitude of underestimation. IMS Health attempts to correct for under- and over-estimation, and in the absence of any additional information, there is no further adjustment of the available sales figures.
Another difficulty in interpretation may arise from parallel trade. IMS Health adjusts the data for parallel trade in some countries. The countries for which adjustments have been made are Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden and the UK.
The pattern of use within countries cannot be ascertained in this analysis, so that it is not possible to determine whether treatment is targeted appropriately to high risk individuals. There are several indicators that suggest that the targeting of treatment is heterogeneous in the EU27. Good evidence comes from the Global Longitudinal Study of Osteoporosis in Women (GLOW) which is a general practice based observational cohort study in women aged 55 years or more conducted in 10 countries, including several EU countries [1]. In the EU countries, there was heterogeneity of treatment uptake between countries with the lowest proportion of women aged 55 years or more treated in the Netherlands (7 %) and the highest in Spain (23 %) (Fig. 33). Although treatment uptake was higher in women at very high risk (a prior hip or spine fracture), a minority (45 %) were receiving treatment in these countries. Again, there was heterogeneity in treatment uptake that ranged from 36 % in the Netherlands to 57 % in Italy. Moreover, some low-risk women were targeted in all countries.
Fig. 33.
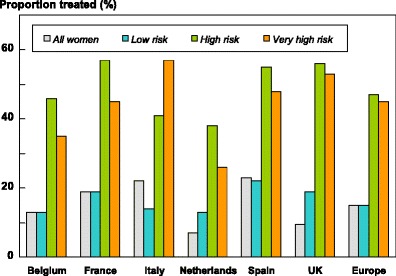
Proportion of women receiving treatment in six EU member states according to category of risk. All women refer to women aged 55 years or more (n = 24,249). Low risk comprises women aged less than 75 years with a T score for BMD in the range of osteopenia, no prior fracture, no maternal hip fracture or osteoporosis (n = 1166). High risk refers to women reported to have a BMD measurement in the range of osteoporosis (n = 5258). Very high risk comprises women with a previous hip or spine fracture (n = 913) [9]
These data demonstrate that a large number of women at high risk of fractures are not receiving treatment, that a substantial number of women at low risk are prescribed treatment (13–22 %) and confirm important differences in the uptake of treatment between countries. Thus, a colour code of green should not be interpreted as an optimum.
References
1. Díez-Pérez A, Hooven FH, Adachi JD et al. (2011) Regional differences in treatment for osteoporosis. The Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 49: 493–8.
2. Guggina P, Flahive J, Hooven FH et al. (2012) Characteristics associated with anti-osteoporosis medication use: Data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) USA cohort. Bone 51: 975–980
3. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
4. Kanis JA, Hans D, Cooper C, et al. (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22: 395–411
5. Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P, Wilkins M; on behalf of the National Osteoporosis Guideline Group (NOGG) (2009) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas; 62:105–108.
6. Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24: 23–57.
7. Lekawasam S, Adachi JD, Agnusdei D et al. for the Joint IOF-ECTS GIO Guidelines Working Group (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–76.
8. Kanis JA, Johansson H, Odén A, McCloskey EV (2012) The distribution of FRAX® based probabilities in women from Japan. J Bone Miner Metab 30: 700–5.
9. Hooven FH and the GLOW investigators (2012) Previously unpublished data derived from Díez-Pérez et al., 2011 [1], courtesy of F Hooven and the GLOW investigators
4d National prescription database
Domain
Service uptake—Background information
Background and aims
IMS Health provides sales data that can be examined by country but give no information on who receives the agent in question or the purpose for which it was given (see Chapter 4c). Such data are not available on an EU-wide basis. However, several EU countries have a National Prescription Database that can provide more detailed information.
Methods
The National Prescription Databases of Sweden was accessed to determine the number of individuals by age who had received a prescription for a bone-active medication in 2010. Exposure to oestrogens was not included. Treatment days (daily defined doses; DDD) were computed from the prescription refills in 2010 and converted to person-years of exposure to treatment (DYD). Treatment rates were expressed as a proportion of the Swedish population [1].
Results
The numbers of individuals by age who had received bone-active medication and the DYDs are shown in Table 45. Treatment uptake is expressed as the number of individuals who were taking a bone-active medication (i.e. filled a prescription) and the number of person-years of treatment in that year. For example, there were 18,931 men and women aged 80–84 years who had been prescribed a treatment in 2010. The total prescription base for that age range was equivalent to 13,888 person-years. Over all ages, 3 % of the Swedish population aged 50 years or more received treatment.
Table 45.
Population size, patients treated and patient-years of treatment by age in Sweden 2010
| Age (years) | Population (000) | Patients treated | Patient-years of treatment | DYD |
|---|---|---|---|---|
| 50–54 | 577 | 2,347 | 1,779 | 1,779 |
| 55–59 | 573 | 5,328 | 3,953 | 3,953 |
| 60–64 | 626 | 10,346 | 7,665 | 7,665 |
| 65–69 | 535 | 15,685 | 11,693 | 11,693 |
| 70–74 | 378 | 17,081 | 12,659 | 12,659 |
| 75–79 | 303 | 18,802 | 14,055 | 14,055 |
| 80–84 | 247 | 18,931 | 13,888 | 13,888 |
| 85+ | 249 | 16,883 | 12,394 | 12,394 |
| 50+ | 3,488 | 105,403 | 78,086 |
Figure 34 shows the uptake of bone-active agents (oestrogens excluded) for 2010. For example, there were 77 prescriptions filled per 1,000 of the population aged 80–85 years in 2010. The number of unique patients that received a prescription for that age range was 56 per 1,000 person-years. These figures are low when compared with the population at high risk. For example, the number of patients in this age range that received a treatment was approximately 13,500 representing 5.6 % of the population at this age. In contrast, the number of individuals in this age range with osteoporosis is estimated at 67,800 and those with a fracture probability above a fracture threshold is estimated at 32,000 [2]. Thus, although treatments are targeted by age, the majority of high risk individuals remain untreated.
Fig. 34.
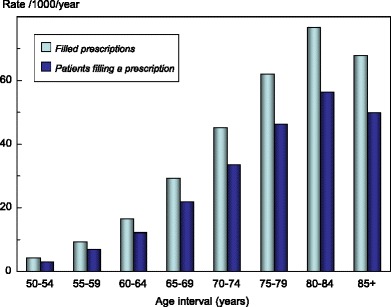
The number (rate/thousand) of prescriptions for bone-active medications and the number of patients filling a prescription for bone-active medications in 2010 [National Prescription Databases of Sweden]
Score allocation
None—supplementary information
Comment
Note that the data do not give information on persistence or compliance. Although treatments are targeted by age, the majority of high risk individuals remain untreated.
References
1. UN (2010) Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat (2010) World Population Prospects: The 2010 Revision, http://esa.un.org/unpd/wpp/index.htm Accessed 16th December 2011
2. Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis; Burden, health care provision and opportunities in the EU. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
4e Treatment gap and treatment need
Domain
Service uptake—Background information
Background and aims
Patients who sustain a prior fragility fracture are at great risk of a future fracture. The risk is increased approximately two-fold [1] and is largely independent of BMD [2]. The risk is sufficiently high that many treatment guidelines in the EU recommend that postmenopausal women with a prior fragility fracture should be offered treatment. However, the majority of such patients are untreated so that the prevalence of a prior fracture in the community provides an index of opportunity lost. This may be set against the treatment gap to provide an index of the relationship between service provision and service need. The aim of this element was to provide an index of the prevalence of a prior fracture in the EU member countries in relation to the treatment gap.
Methods
For the purposes of this report, a prior fracture was defined as a hip or clinical vertebral fracture in an individual who was alive in 2010 that had occurred after the age of 50 years before 2010. The unit was the individual so that multiple fractures at the same site in one individual were only counted as one prior fracture of that site. A micro-simulation model, programmed in TreeAge, was used to simulate the prevalence of prior hip and vertebral fractures from incidence data [3]. Note that the prevalence of a hip or clinical vertebral fracture will underestimate the prevalence of previous fragility fracture at other sites.
More complete information on prior fractures is available for six member states (France, Germany, Italy, Spain, Sweden and the UK) using a different approach [4] and is also considered.
For the treatment gap, the data from Chapter 4c were used [1].
Results
In 2010, approximately 6.7 million men and women in the EU had sustained a prior hip or clinical spine fracture before 2010 (Table 46). As would be expected, the prevalence increased progressively with age. At the age of 95 years or more, the prevalence of a prior hip fracture was 22.5 % and for a prior vertebral fracture was 14.5 % [1]. Overall, 1.8 % of the population at the age of 50 years or more had a prior hip fracture and 1.9 % a prior clinical spine fracture.
Table 46.
Estimated number and percentage of men and women aged above 50 years with a prior hip or vertebral fracture by country in 2010 [3]
| Hip fracture | Vertebral fracture | A + B | |||
|---|---|---|---|---|---|
| Number | % Population (A) | Number | % Population (B) | ||
| Austria | 74,270 | 2.4 | 83,623 | 2.7 | 5.1 |
| Belgium | 74,485 | 1.9 | 80,706 | 2.0 | 3.9 |
| Bulgaria | 31,370 | 1.1 | 32,780 | 1.1 | 2.2 |
| Cyprus | 4,921 | 1.6 | 6,150 | 2.0 | 3.6 |
| Czech | 58,979 | 1.6 | 64,340 | 1.7 | 3.3 |
| Denmark | 49,746 | 2.5 | 58,573 | 2.9 | 5.4 |
| Estonia | 7,350 | 1.5 | 7,486 | 1.5 | 3.0 |
| Finland | 34,181 | 1.6 | 37,192 | 1.8 | 3.4 |
| France | 434,674 | 1.9 | 435,507 | 1.9 | 3.8 |
| Germany | 669,799 | 2.0 | 775,529 | 2.3 | 4.3 |
| Greece | 87,413 | 2.1 | 101,760 | 2.4 | 4.5 |
| Hungary | 57,225 | 1.6 | 60,594 | 1.6 | 3.2 |
| Ireland | 17,247 | 1.4 | 18,742 | 1.5 | 2.9 |
| Italy | 517,126 | 2.2 | 539,036 | 2.3 | 4.5 |
| Latvia | 11,862 | 1.5 | 11,575 | 1.4 | 2.9 |
| Lithuania | 13,046 | 1.2 | 12,782 | 1.1 | 2.3 |
| Luxemburg | 2,446 | 1.5 | 2,790 | 1.8 | 3.3 |
| Malta | 1,974 | 1.3 | 2,316 | 1.5 | 2.8 |
| Netherlands | 74,594 | 1.3 | 82,206 | 1.4 | 2.7 |
| Poland | 139,212 | 1.0 | 144,863 | 1.1 | 2.1 |
| Portugal | 52,106 | 1.3 | 53,653 | 1.4 | 2.7 |
| Romania | 72,024 | 1.0 | 82,829 | 1.1 | 2.1 |
| Slovakia | 28,065 | 1.6 | 32,488 | 1.9 | 3.5 |
| Slovenia | 12,429 | 1.6 | 14,306 | 1.9 | 3.5 |
| Spain | 210,560 | 1.3 | 212,428 | 1.3 | 2.6 |
| Sweden | 98,952 | 2.8 | 111,348 | 3.2 | 6.0 |
| UK | 418,881 | 1.9 | 437,499 | 2.0 | 3.9 |
| EU27 | 3,254,939 | 1.8 | 3,503,101 | 1.9 | 3.7 |
The ranked prevalences by country are shown in Fig. 35. As would be expected, there was a close relationship between fracture risk (see Chapter 1b) and the proportion of the population with a prior hip or clinical vertebral fracture.
Fig. 35.
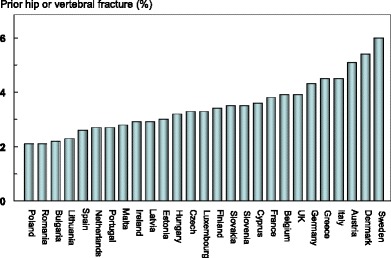
The proportion (%) of the population aged 50 years or more with a prior hip or vertebral fracture in 2010 [3]
Table 47 summarises data for the EU5+Sweden which shows the prevalence of all prior fractures in 2010 in comparator studies. The estimation of prior vertebral + prior hip fracture, shown in Fig. 35, appears to capture approximately 30 % of prior fractures. This suggests that the prevalence of a prior clinical spine or hip fracture is a reasonable surrogate for the service needs of each member state.
Table 47.
The prevalence (%) of a prior fracture at sites associated with osteoporosis in men and women aged 50 years or more compared with the prevalence of a prior vertebral or hip fracture as given in Table 46. The last column shows the proportion (%) of prior fractures accounted for by hip or clinical vertebral fracture [3]
| Country | Author | Prevalence (%) | EU/C | |
|---|---|---|---|---|
| Comparator | EU27 | |||
| France | Cawston 2012 [5] | 11.2 | 3.8 | 34 |
| Germany | Gauthier 2012 [6] | 14.1 | 4.3 | 30 |
| Italy | Piscitelli 2012 [7] | 16.2 | 4.5 | 28 |
| Spain | Gauthier 2012 [8] | 8.9 | 2.6 | 29 |
| Sweden | Gauthier 2011 [4] | 22.6 | 6.0 | 27 |
| UK | Gauthier 2011[9] | 10.3 | 3.9 | 39 |
| Average | 30 | |||
The relationship between this service need and the treatment gap is shown in Fig. 36 for each of the EU member states and for the EU countries combined. The top right quadrant can be considered to represent countries of high need but poor provision. These included Denmark and Sweden. The other extreme (lower left quadrant) represents countries of lower need but better provision. These included Estonia, Ireland Hungary and Spain.
Fig. 36.
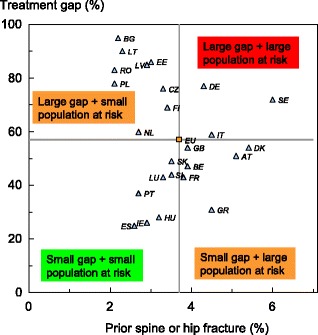
The relationship between the prevalence of a prior spine or hip fracture (service need) and the treatment gap (service provision) in the EU27 countries. The horizontal and vertical lines intersect at the EU average (weighted for population size) [3]. Country codes (ISO 3166–1 alpha-2); AT Austria; BE Belgium; BG Bulgaria; CY Cyprus; CZ Czech Republic; DE Germany; DK Denmark; EE Estonia; ES Spain; FI Finland; FR France; GB United Kingdom; GR Greece; HU Hungary; IE Ireland; IT Italy; LT Lithuania; LU Luxembourg; LV Latvia; MT Malta; NL Netherlands; PL Poland; PT Portugal; RO Romania; SE Sweden; SI Slovenia; SK Slovakia
Score allocation
Supplementary information, no score allocation
Comment
There is a wide variation in both (hip and spine) fractures and treatment gap between countries. This is of particular concern in countries with a high fracture burden and a high treatment gap.
References
1. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15: 721–39
2. Kanis JA, Johnell O, De Laet C et al. (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35: 375–82
3. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
4. Gauthier A, Kanis JA, Martin M, Compston J, Borgström F, Cooper C, McCloskey E; Committee of Scientific Advisors, International Osteoporosis Foundation (2011) Development and validation of a disease model for postmenopausal osteoporosis. Osteoporos Int 22: 771–80.
5. Cawston H, Maravic M, Fardellone P et al. (2013) Epidemiological burden of postmenopausal osteoporosis in France from 2010 to 2020: estimations from a disease model Arch Osteoporos. 7: 237–46
6. Gauthier A, Kanis JA, Jiang Y, Dreinhöfer K, Martin M, Compston J, Borgström F, Cooper C, McCloskey E (2013) Burden of postmenopausal osteoporosis in Germany: estimations from a disease model. Arch Osteoporos 7:209–18
7. Piscitelli P, Brandi M, Cawston H, Gauthier A, Kanis JA, Compston J, Borgström F, Cooper C, McCloskey E (2012) Epidemiological burden of postmenopausal osteoporosis in Italy from 2010 to 2020: Estimations from a disease model. Unpublished.
8. Gauthier A, Kanis JA, Jiang Y, Cannata J, Compston J, Borgström F, Cooper C, McCloskey E (2012) Burden of post-menopausal osteoporosis in Spain: Estimations from a disease model. Unpublished
9. Gauthier A, Kanis JA, Jiang Y, Martin M, Compston J, Borgström F, Cooper C, McCloskey E (2011) Epidemiological burden of postmenopausal osteoporosis in the UK from 2010 to 2021: Estimations from a Disease Model. Arch Osteoporos 6: 179–188
4f Waiting time for hip surgery
Domain
Service uptake—scorecard element
Background and aims
About 5 % of people with a hip fracture die within 1 month and about one quarter within 12 months. Most deaths are due to associated conditions and not to the fracture itself [1], reflecting the high prevalence of co-morbidity. In the EU27, hip fractures were estimated to result in approximately 11,000 premature deaths in women that were directly attributable to the fracture event in 2010. The corresponding numbers for men were estimated at approximately 9,000 [2]. A determinant of peri-operative morbidity and mortality is the time a patient takes to get to surgery which, in turn, is an early marker of a patient’s progress following a hip fracture. Early surgery (<48 h) is associated with a statistically and clinically significant reduction in mortality at 1 year and an increase in the proportion of patients returning to their original residence [3].
The aim of this scorecard element was to determine average waiting times for hip surgery in the EU member states.
Methods
Data were acquired by an IOF questionnaire to the EU Osteoporosis Consultation Panel undertaken in December 2012. Respondents were asked to provide information on the average waiting time for hip surgery after hip fracture. Countries were categorised according to average waiting times between hospital admission and surgical intervention. An additional indicator of management that was sought was the proportion of hip fracture cases that were managed surgically.
Results
Waiting times between admission to hospital and surgical intervention were on average 1 day or less in 7 countries, 1–2 days in 13 countries and greater than 2 days in 6 countries (Table 48). Information was not recorded for Malta. More than 90 % of hip fracture cases received surgery in the majority of countries. Exceptions included Bulgaria, Czech Republic, Hungary and Italy where 75–90 % of cases received a surgical intervention.
Table 48.
Average waiting times between hospital admission and surgical intervention and the proportion of hip fracture cases managed surgically [IOF audit]
| Waiting time (days) | Surgical management (%) | Score | |
|---|---|---|---|
| Austria | 1–2 | >90 | 2 |
| Belgium | <1 | >90 | 3 |
| Bulgaria | 1–2 | 75–90 | 2 |
| Cyprus | 2–3 | >90 | 1 |
| Czech Republic | 1–2 | 75–90 | 2 |
| Denmark | 1–2 | >90 | 2 |
| Estonia | <1 | >90 | 3 |
| Finland | 1–2 | >90 | 2 |
| France | 1–2 | >90 | 2 |
| Germany | <1 | >90 | 3 |
| Greece | 2–3 | >90 | 1 |
| Hungary | <1 | 75–90 | 3 |
| Ireland | 2–3 | >90 | 1 |
| Italy | 2–3 | 75–90 | 1 |
| Latvia | <1 | >90 | 3 |
| Lithuania | <1 | >90 | 3 |
| Luxembourg | 1–2 | >90 | 2 |
| Malta | nr | nr | |
| Netherlands | 1–2 | >90 | 2 |
| Poland | 1–2 | >90 | 2 |
| Portugal | 2–3 | >90 | 1 |
| Romania | 1–2 | >90 | 2 |
| Slovakia | 1–2 | >90 | 2 |
| Slovenia | 1–2 | >90 | 2 |
| Spain | 2–3 | >90 | 1 |
| Sweden | <1 | >90 | 3 |
| UK | 1–2 | >90 | 2 |
nr, not recorded
Score criteria and allocation
Uptake was categorised by average waiting time for hip surgery (Table 49)
Table 49.
Criteria for allocating scores
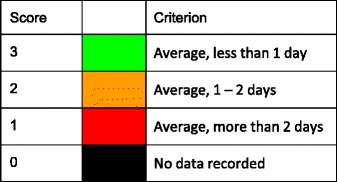
Score allocation
Score results are given in Table 48 and colour coded in Fig. 37.
Fig. 37.
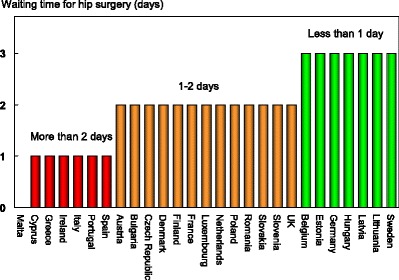
Countries categorised by the average waiting time for surgical intervention for hip fracture [IOF audit]
Comment
Note that average waiting times give no index of the dispersion around the mean
References
1. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32; 468–473.
2. Hernlund E, Svedbom A, Ivergård M Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos, in press
3. National Clinical Guideline Centre, (2011) The Management of Hip Fracture in Adults. London: National Clinical Guideline Centre. Available from: www.ncgc.ac.uk Accessed Jan 2013
The scorecard
SCOPE. The Scorecard for Osteoporosis in Europe, is an innovative tool that allows health and policy professionals to assess key indicators on the healthcare provision for osteoporosis in countries and between counties within the EU.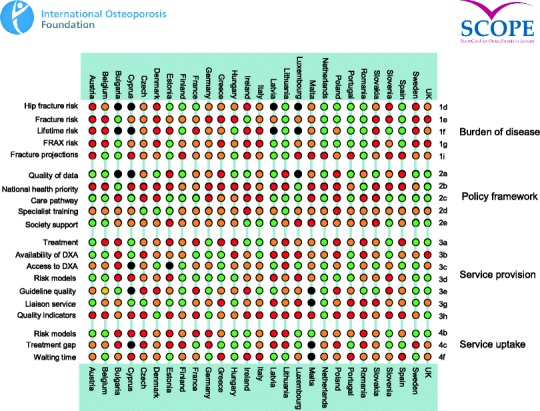
Scorecard key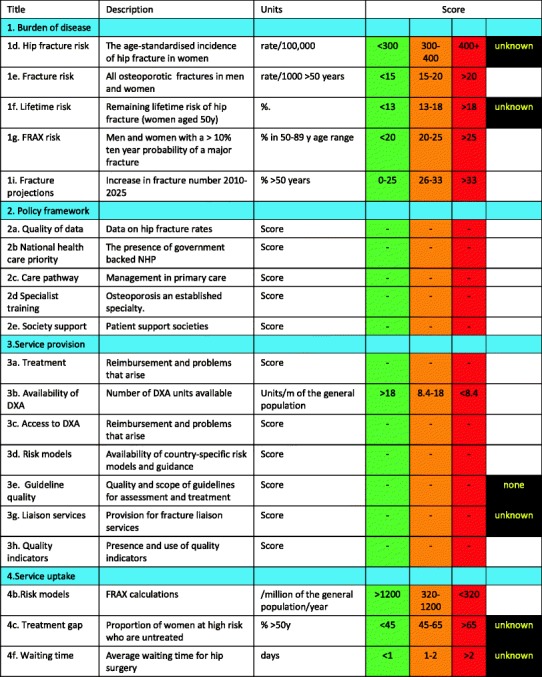
Competing interests
The panel has received speaker fees, advisory board fees and/or unrestricted research grants from governmental and non-governmental sources. A full list of disclosures is available from the International Osteoporosis Foundation upon request.
Acknowledgments
SCOPE was supported by an unrestricted grant from Amgen and GlaxoSmithKline (GSK). Neither company was involved in the design or writing of the report. We are grateful to Carey Kyer of the International Osteoporosis Foundation for the administration of the IOF questionnaire for her help with formatting and editing the report and to Gilberto Lontro of the International Osteoporosis Foundation for designing the scorecard. We thank Robert McGruer, Simone Boselli of Hill & Knowlton for their support services. Prof F Hooven, University of Massachusetts Medical School and B Abrahamsen, University of Southern Denmark kindly supplied previously unpublished data for use in this report. We are grateful to H Sundseth (European Institute of Women's Health) for her help in the early phase of this project. We are grateful for the help provided by the National Societies of the IOF based in the EU, listed below for updating the information base of the European audit ‘Osteoporosis in the European Union in 2008: Ten years of progress and ongoing challenges’. Finally, we thank the Committee of Scientific Advisors of the IOF their review and endorsement of this report.
| Austria | Austrian Society for Bone and Mineral Research (AUSBMR) |
| Action for Healthy Bones | |
| Austrian Menopause Society | |
| National Osteoporosis Patient Society Austria | |
| Belgium | The Royal Belgian Rheumatology Society |
| Belgian Bone Club | |
| Bulgaria | Bulgarian Society for Clinical Densitometry |
| Association Women without Osteoporosis | |
| Bulgarian League for the Prevention of Osteoporosis-blpo | |
| Bulgarian Medical Society of Osteoporosis and Osteoathrosis | |
| Cyprus | Cyprus Society for Osteoporosis |
| Cyprus Society against Osteoporosis and Myoskeletal Diseases | |
| Czech Republic | Osteologic Academy zlin |
| Czech Osteoporosis League | |
| Czech Society for Metabolic Skeletal Diseases (SMOS) | |
| Denmark | National Osteoporosis Foundation Denmark |
| Danish Bone Society | |
| Estonia | Estonian Osteoporosis Society |
| Finland | Finnish Bone Society |
| Finnish Osteoporosis Association | |
| France | French League Against Rheumatism (AFLAR) |
| French Society of Orthopaedic And Trauma Surgery (SOFCOT) | |
| French Society For Clinical Densitometry (SO.F.O.C.) | |
| Research And Information Group On Osteoporosis (GRIO) | |
| Germany | German Osteoporosis Patient Society (BFO) |
| Osteoporose Selbsthilfegruppen Dachverband E.V (OSD) | |
| Netzwerk-osteoporose e.v | |
| German Society For Endocrinology (DGE) | |
| Committee For Healthy Bones– Kuratorium Knochengesundheit | |
| Orthopädische Gesellschaft Für Osteologie (OGO) | |
| Greece | Hellenic Society For The Study Of Bone Metabolism |
| Hellenic Foundation Of Osteoporosis | |
| Hellenic Society Of Osteoporosis Patient Support | |
| Hellenic Endocrine Society - Panhellenic Association Of Endocrinologists | |
| Hungary | Hungarian Osteoporosis Patients Association (HOPA) |
| Hungarian Society For Osteoporosis And Osteoarthrology | |
| Ireland | Irish Osteoporosis Society (IOS) |
| Italy | Italian Association Of Osteoporosis Patients |
| Italian Foundation For Research On Osteoporosis And Musculoskeletal Diseases - FIROMMS | |
| Italian COPD Patient Association | |
| Osteoporosis Italian Association - Osteo Stop | |
| Italian Society For Osteoporosis Mineral Metabolism And Skeletal Diseases (SIOMMMS) | |
| Italian Federation Of Osteoporosis And Diseases Of The Skeleton (FEDIOS) | |
| Italian Osteoporosis League (LIOS) | |
| Italian Society Of Rheumatology | |
| Latvia | Latvia Osteoporosis Patient And Invalid Association |
| Latvian Osteoporosis And Bone Metabolism Diseases Association | |
| Lithuania | Lithuanian Osteoporosis Foundation |
| Lithuanian Association Of Metabolic Bone Diseases Incorporated In Lithuanian Endocrine Society | |
| Luxembourg | Association luxembourgeoise d’étude du métabolisme osseux et de l’ostéoporose (ALEMO) |
| Malta | Malta Osteoporosis Society |
| Netherlands | Osteoporose Vereniging |
| Osteoporose Stichting - Dutch Osteoporosis Foundation | |
| Poland | Healthy Bone Enthusiasts Society (STENKO) |
| Polish Foundation of Osteoporosis | |
| Polish Osteoarthrology Society | |
| Multidisciplinary Osteoporotic Forum | |
| Portugal | Portuguese Osteoporosis Association (APO) |
| Portuguese Society Of Osteoporosis And Other Metabolic Bone Diseases (SPODOM) | |
| National Association Against Osteoporosis (APOROS) | |
| Romania | Romanian Society Of Rheumatology |
| Association For Prevention Of Osteoporosis In Romania (ASPOR) | |
| Romanian Society Of Osteoporosis And Musculoskeletal Diseases | |
| Romanian Foundation Of Osteoarthrology (OSART) | |
| Slovakia | Slovak Union Against Osteoporosis |
| Slovak Society Osteoporosis & Metabolic Bone Diseases | |
| Slovenia | Slovene Bone Society |
| Slovene Osteoporosis Patients Society | |
| Spain | Spanish Society for Rheumatology |
| Spanish Society for Research on Bone and Mineral Metabolism (SEIOMM) | |
| Hispanic Foundation Of Osteoporosis And Metabolic Bone Diseases (FHOEMO) | |
| Spanish Association Against Osteoporosis (AECOS) | |
| Spanish Society of Osteoporotic Fractures | |
| Sweden | 1.6 million club |
| Swedish Osteoporosis Patient Society (ROP) | |
| Swedish Rheumatism Association | |
| Swedish Osteoporosis Society | |
| UK | The Bone Research Society |
| National Osteoporosis Society (NOS) | |
| Multinational | International Osteoporosis Foundation (IOF) |
| Umbrella organisation of German speaking osteoporosis patient societies (DOP) | |
| Umbrella organisation of German speaking societies of osteology (DVO) | |
| European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) | |
| Deustche Gesellschaft fur Osteologie | |
| International Society For Fracture Repair (ISFR) | |
| Mediterranean Society For Osteoporosis And Other Skeletal Diseases (MSOSD) | |
| Ibero American Society of Osteology and Mineral Metabolism (SIBOMM) | |
| European Union Geriatrics Medicine (EUGMS) | |
| European Calcified Tissue Society (ECTS) |
Abbreviations and glossary
- BMD
Bone mineral density, usually measured as the amount of calcium in bone per unit area.
- BMI
Body mass index, an index of leanness or obesity measured from height and weight
- CI
Confidence interval
- CRF
Clinical risk factor, in this context for fracture
- DALY
Disability-adjusted life year, a product of years of life lost and the remaining years of life disabled (i.e., disutility).
- DDD
Defined daily dosage
- Direct costs
Used in health technology assessment to describe direct healthcare costs (e.g., hospital admissions, medical examinations, drug therapy, etc.), the indirect costs (e.g., losses in productivity resulting from absence to work) and intangible costs (e.g. pain and suffering, poor quality of life).
- DXA
Dual-energy X-ray absorptiometry, a method for measuring BMD
- EFPIA
European Federation of Pharmaceutical Industries Association
- EU27
The 27 member states of the European Union
- FRAX
Fracture risk assessment tool developed by the WHO Collaborating Centre, University of Sheffield Medical School, UK. FRAX calculates the 10-year probability of a major osteoporotic fracture and hip fracture in individuals from clinical risk factors and BMD
- GDP
Gross domestic product, the total value of goods produced and services provided in a country in 1 year
- GLOW
Global Longitudinal Study of Osteoporosis in Women
- ICD
International classification of diseases
- IMS
Intercontinental Marketing Services
- Incidence
The frequency of an event, usually expressed as a rate e.g. 10 per 1000 of the population/year.
- IOF
International Osteoporosis Foundation
- mg
Milligram
- MPR
Medication possession ratio
- NHANES
National Health and Nutrition Examination Survey
- NICE
National Institute of Health and Clinical Excellence
- NOGG
National Osteoporosis Guideline Group
- Prevalence
The number of cases of disease (e.g. osteoporosis) for a given area or at a given time
- Probability
The likelihood of an event, e.g. fracture. Fracture probability depends on two hazards—the incidence of fracture and the incidence of death
- QALY
The QALY is a multi-dimensional outcome measure that incorporates both the quality (health related) and quantity (length) of life. The value of a QALY was set at value of 2× GDP per capita
- QCT
Quantitative computed tomography
- QoL
Quality of life
- QUS
Quantitative ultrasound
- SCOPE
Scorecard for osteoporosis in Europe
- SD
Standard deviation
- T score
Describes the number of standard deviations (SD) by which the BMD in an individual differs from the mean value expected in young healthy women. The operational definition of osteoporosis is defined as a value for BMD 2.5 SD or more below the young female adult mean (T score less than or equal to −2.5 SD).
- WHO
World Health Organization
- WTP
Willingness to pay, used in Health Technology assessment to describe the value that society or a health care payer is prepared to pay to gain a QALY. The value of a QALY was set at value of 2× GDP per capita.
Footnotes
AGREE criteria
Systematic search. How thorough was the evidence base? Were the guidelines based on a systematic literature review conducted at the time of the guideline development (or on a previously conducted review that was updated).
Recommendations: Were recommendations graded (e.g. A, B, C) according to the levels of evidence provided by the systematic review?
Stakeholder involvement: Was there involvement from patient organisations, primary care physicians, national/EU societies in the consultation process for the guidelines?
External review: Were the guidelines reviewed by independent experts? i.e. have they undergone a rigorous external review in addition to consultation.
Procedure for update: Were the guidelines updated as and when necessary or was there explicit mention of a provision to update the guidelines in the future?
Economic analysis: Were the recommendations underpinned by an economic analysis?
Editorial independence: Did the guidelines explicitly state that there was editorial independence of the writing group from any funding body?


