Abstract
Remediation of lead (Pb)-contaminated sites with phosphate amendments is one of the best studied and cost-effective methods for in situ immobilization. In this treatment, a very stable mineral, pyromorphite Pb5(PO4)3Cl, is formed. Several studies propose to improve this treatment method with the addition of phosphate-solubilizing bacteria (PSB). The effect of bacteria on solubilization of pyromorphite is unknown. In this study, the effect of the soil microorganisms on the stability of pyromorphite Pb5(PO4)3Cl has been investigated in a set of batch solution experiments. The mineral was reacted with Pseudomonas putida, a common soil microorganism. Dissolution of pyromorphite was enhanced by the presence of P. putida, resulting in an elevated Pb concentration in the solution. This occurred even when the bacteria were provided with an additional source of phosphate in the solution. Pyromorphite has been shown to be a potential source of nutrient phosphorus for common soil bacteria. Thus, the use of PSB in remediation treatments of Pb contaminated sites may have adverse long-term impacts on Pb immobilization. Conscious phosphate management is suggested for long-term sustainability of the in situ Pb immobilization by pyromorphite formation.
Keywords: Pb contamination, Pyromorphite, Pseudomonas, In situ immobilization, Bacteria mediated dissolution, Phosphate amendments
Introduction
Lead (Pb) contamination and its toxic effect on human and ecosystem health are well understood (Plumlee and Morman 2011). Elevated blood Pb levels are associated with numerous health problems including anemia, gastro-intestinal distress, neurodevelopment defects, blindness, and even death (Sullivan and Krieger 2001); children are particularly prone to suffer from Pb poisoning. The potential risk depends on Pb bioavailability and mobility in the environment. Although the term “bioavailability” has been defined differently in different contexts, one necessary component is the release of Pb from the solid phase, generally mineral, into a solution (Ehlers and Luthy 2003). Phosphate-rich fluids promote precipitation of Pb phosphates, which significantly decrease its bioavailability, especially under conditions of near neutral pH (∼7) (Plumlee and Morman 2011). To treat the problem of Pb-contaminated soils, the US-Environmental Protection Agency (USEPA) has declared phosphate application (generally as the Ca-phosphate mineral apatite and often as fish-bone meal) to be one of the Best Management Practice for Pb binding (USEPA 2005). In this treatment method, dissolution of apatite results in phosphate release. The PO4 3− ions combine with Pb and precipitate as nanoparticles of the mineral pyromorphite, Pb5(PO4)3Cl (e.g., Ma et al. 1993, 1994a, b, 1995; Ruby et al. 1994; Cotter-Howells 1996; Cotter-Howells and Caporn 1996).
Pyromorphite is a Pb apatite that in nature commonly occurs in the oxidation zones of Pb-bearing ore deposits (Nakamoto et al. 1969). The properties of this mineral have been extensively studied (Baker 1966; Dai and Hughes 1989; Xie and Giammar 2007; Manecki 2009). Pyromorphite forms a continuous solid solution with vanadinite (Pb5(VO4)3Cl) and mimetite (Pb5(AsO4)3Cl) (Flis et al. 2010). Considering their high thermodynamic stability, the minerals in the pyromorphite–mimetite series have gained considerable attention as metal sequestration agents in the areas contaminated by Pb and arsenic (e.g., Bajda 2010; Flis et al. 2011 and the literature therein). Pyromorphite (log K sp = −79 after Flis et al. 2011) is one of the most stable minerals in the environment, and its formation greatly decreases the availability of Pb to organisms (Manecki et al. 2006). However, recent studies have reported that several organic compounds and microbial metabolites may affect the stability of pyromorphite and cause Pb release by increased dissolution (Sayer et al. 1997; Fomina et al. 2004; Manecki and Maurice 2008; Manecki 2009; Debela et al. 2010). At the same time, it has been reported that the activity of phosphate-solubilizing bacteria (PSB) at Pb-contaminated sites that undergo phosphate-induced remediation might actually increase immobilization of Pb (Park et al. 2011a, b). The latter is based on the idea that PSB enhance solubilization of calcium phosphate amendments which further bind with Pb contaminant and form insoluble pyromorphite (Park et al. 2011b). The authors have proposed the addition of PSB to contaminated site along with phosphate amendments (Park et al. 2011a). The actual direct effect of PSB on pyromorphite stability is unknown. It is possible that the presence of PSB will not only induce solubilization of the phosphate amendments, but eventually may also solubilize the pyromorphite. Thus, knowledge of the potential role of soil bacteria in dissolution of pyromorphite is crucial for the long-term effectiveness of the P-induced Pb immobilization.
In this study, the microbial effect on stability of pyromorphite has been investigated. Several batch experiments on the dissolution of pyromorphite in the presence of Pseudomonas putida bacteria were carried out. The experiments were performed in soluble P-rich and P-deficient conditions. The experiments were designed specifically to test (1) if the presence of PSB in the pyromorphite milieu influences the stability of the mineral; (2) if the potential enhanced solubilization of pyromorphite by bacteria depends on availability of P in the solution. The P. putida bacteria was selected for this project because of its common occurrence in nature. The ubiquitous aerobic, gram-negative bacteria Pseudomonas can be found in unpolluted soils as well as in heavy metal-contaminated sites (Roane 1999; Leung et al. 2001; Rugierro et al. 2005; Matlakowska et al. 2008). P. putida is reported to exhibit a capability of solubilizing the mineral forms of phosphates via nutrient scavenging (e.g., Rosas et al. 2006), and it often serves as a model organism for environmental, genetic, and bioengineering experiments (Reva et al. 2006).
Experimental methods
Materials
Minerals
Two synthetic mineral samples were used in this study: pyromorphite Pb5(PO4)3Cl and chlorapatite Ca5(PO4)3Cl—the Ca isomorph of pyromorphite. The pyromorphite was synthesized as described by Flis et al. (2010) using a combination of 0.3 M Pb(NO3)2, 0.14 M K2HPO4, and 0.05 M NaCl solutions. Equal volumes of the solutions (500 mL) were simultaneously added with the use of a peristaltic pump (flow rate 1.5 mL min−1) to a glass beaker filled partly with 1 l of distilled deionized water (DDIW), while stirring with a magnetic stir bar. The resulting precipitate was aged in suspension for 24 h (Scheckel and Ryan 2002). After aging, the precipitate was filtered on paper filter (Whatman), washed with DDIW, air dried, and kept in a desiccator until use. To synthesize the chlorapatite, 0.5 g of synthetic hydroxylapatite Ca5(PO4)3OH (Merck) was mixed with 0.5 g of CaCl2 in a quartz crucible and roasted in an oven at 950 °C for 12 h. Both synthesis resulted in white homogeneous powders, which were confirmed to be pyromorphite and chlorapatite by means of X-ray diffraction (XRD) and scanning electron microscopy coupled with an energy–dispersive spectroscopy (SEM-EDS) analyzer. Additionally, a wet chemical analysis of the synthetic pyromorphite was conducted. The methodology of synthesis allowed for precipitation of particles similar in respect to size and morphology to those that are formed in rhizosphere at Pb-contaminated sites (Traina and Laperche 1999).
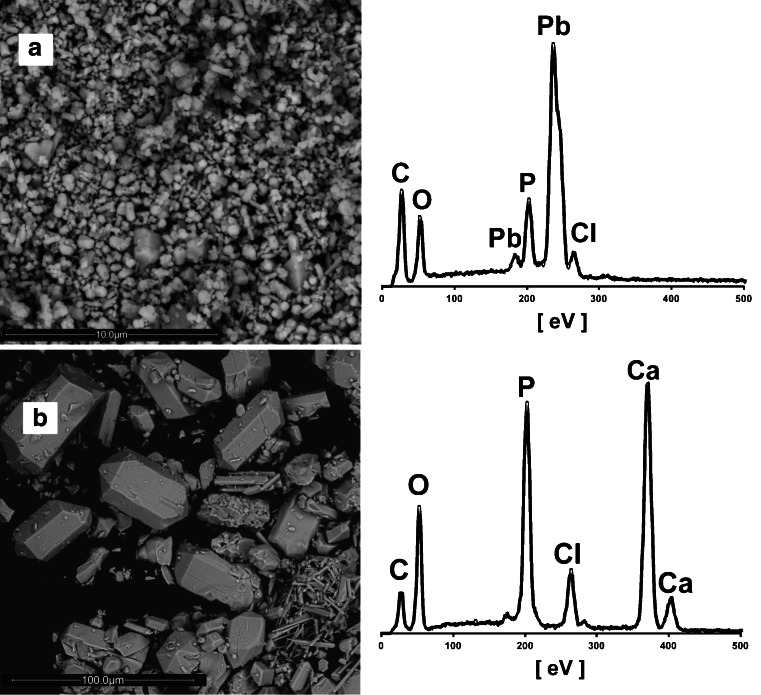
X-ray powder diffraction data from the synthetic mineral samples are presented in Table 1. The analyzed phases were identified as pure pyromorphite and chlorapatite. The SEM-EDS analysis of the precipitates confirmed this result (Fig. 1a, b). The particle size varied from 200 nm to 2 μm for pyromorphite and from 10 to 80 μm for chlorapatite The composition of the synthetic pyromorphite calculated on the basis of the wet chemical analysis was: Pb5.09(PO4)2.96Cl.
Table 1.
Diffraction data of minerals used in the experiments and of referenced minerals
| d-spacing | |||
|---|---|---|---|
| Pyromorphite (Dai and Hughes 1989) | Synthetic pyromorphite (this study) | Chlorapatite (Hughes et al. 1998) | Synthetic chlorapatite (this study) |
| 4.9882 | 4.992 | 8.3121 | 8.348 |
| 4.3199 | 4.325 | 5.2520 | 5.264 |
| 4.1276 | 4.127 | 3.9163 | 3.924 |
| 3.7244 | 3.726 | 3.5428 | 3.549 |
| 3.6755 | 3.663 | 3.3880 | 3.391 |
| 3.3822 | 3.373 | 3.1417 | 3.148 |
| 3.2655 | 3.269 | 2.8502 | 2.855 |
| 2.9843 | 2.986 | 2.7707 | 2.774 |
| 2.9590 | 2.954 | 2.7678 | – |
| 2.8799 | 2.883 | 2.6260 | 2.630 |
| 2.4941 | 2.496 | 2.5646 | 2.569 |
| 2.4412 | 2.440 | 2.3054 | 2.308 |
| 2.2669 | 2.266 | 2.3037 | – |
| 2.1993 | 2.195 | 2.1825 | 2.186 |
| 2.1600 | 2.162 | 2.1448 | 2.148 |
| 2.0638 | 2.064 | 2.0780 | 2.081 |
| 2.0073 | 2.007 | 2.0436 | 2.046 |
| 1.9821 | 1.985 | 1.9845 | 1.988 |
| 1.9599 | 1.957 | 1.9581 | 1.961 |
| 1.9138 | 1.916 | 1.9069 | 1.909 |
| 1.8854 | 1.888 | 1.9060 | – |
| 1.8622 | 1.863 | 1.8356 | 1.837 |
| 1.8378 | 1.833 | 1.8339 | 1.837 |
| 1.7245 | 1.721 | 1.8139 | 1.816 |
| 1.6911 | 1.699 | 1.7714 | 1.774 |
| 1.6775 | 1.678 | 1.7507 | 1.752 |
| 1.6627 | 1.664 | 1.6940 | 1.697 |
| 1.6328 | 1.635 | 1.6618 | 1.664 |
| 1.6218 | 1.624 | 1.6599 | – |
| 1.6016 | 1.599 | 1.6145 | 1.617 |
| 1.5638 | 1.565 | 1.6134 | – |
| 1.5518 | 1.554 | 1.5997 | 1.602 |
| 1.5492 | 1.547 | 1.5991 | – |
| 1.5411 | 1.541 | 1.5708 | 1.573 |
| 1.5183 | 1.519 | 1.5569 | 1.559 |
| 1.5149 | – | 1.5303 | 1.532 |
| 1.4102 | 1.407 | 1.4929 | 1.495 |
| 1.3997 | 1.398 | 1.4911 | – |
| 1.3835 | 1.386 | 1.4579 | 1.460 |
| 1.3759 | 1.376 | 1.4465 | 1.448 |
| 1.3596 | 1.361 | 1.4453 | – |
| 1.3476 | 1.347 | 1.4251 | 1.428 |
| 1.3661 | 1.369 | ||
| 1.3395 | 1.336 | ||
Fig. 1.
The SEM microphotographs and the EDS spectra of precipitates used in the experiments: a pyromorphite Pb5(PO4)3Cl and b chlorapatite Ca5(PO4)3Cl. Samples were carbon-coated prior to the analysis
Prior to the batch dissolution experiments, 0.05 g portions of pyromorphite and chlorapatite were sterilized in a heater at 180 °C for 3 h. The applied sterilization procedure did not alter the properties of the minerals, which was confirmed by SEM-EDS and XRD analysis (data not shown).
Bacteria
The P. putida strain used in this study, earlier identified by numerical taxonomy as P. putida strain IBPRS KKP 1136, was obtained from the collection of the Institute of Agricultural and Food Biotechnology in Warsaw, Poland. For the batch dissolution experiments, the bacteria were grown in the standard liquid medium (MP + solution) until an optical density at 600 nm (OD600) of 0.8 was reached (mid-logarithmic growth). The microbes were then pelleted by centrifugation, resuspended in the experimental solution and inoculated 1:100 into flasks containing the same growth solution.
Reaction solutions (growth media)
Two types of solutions: P-rich (MP+) and P-deficient solutions (MP−) were used in the dissolutions experiments. These were created with constituents necessary for bacterial growth. The solution contained the following ingredients, in units of liter: succinic acid disodium salt anhydrous, 5 g; K2HPO4, 0.5 g; NH4Cl, 1 g; MgSO4·7H2O, 0.2 g; CaCl2, 0.05 g; KCl, 0.5 g; FeEDTA, 30 mM; glycerol, 6.5 g; and 0.125 mL of trace elements (MnSO4·H2O, 0.005 g; CoSO4·7H2O, 0.0065 g; CuSO4, 0.0023 g; ZnSO4, 0.0033 g; and MoO3, 0.0024 g/100 mL of water). The pH of the solution was adjusted to ∼7.3 prior autoclaving. Reagent grade chemicals and ultra-pure, distilled 18 MΩcm−1 water (Milli-Q, Millipore) were used throughout. The MP- solution was identical to MP+ solution except for the absence of K2HPO4.
Batch dissolution experiments
The experimental setup consisted of two basic, complementary experiments (marked as E.I: P. putida growth in presence of pyromorphite and E.II: dissolution of pyromorphite in the presence of P. putida), and several control experiments. A summary of the experimental conditions is presented in Table 2. The experiments were designed to test: (a) if pyromorphite can serve as a phosphate source for the microbes, (b) if the presence of P. putida in the environment affects the abiotic stability of pyromorphite in terms of dissolved Pb, and (c) if the potential microbial effect depends on the availability of PO4 3− aq ions in the solution. In the experiments, P. putida was grown in solutions, P-rich MP+ and P-deficient MP− in presence of pyromorphite. In the MP+ solution, aqueous PO4 3− was available for the microbes, whereas in MP− solution, pyromorphite was the sole source of phosphate. During the reactions, the solutions were periodically sampled and analyzed for the optical density of the bacteria culture (OD600, UV–vis absorbance at 600 nm). In addition, the time evolution of Pb and P concentrations (ICP-MS) and the pH of the suspensions were investigated. In control experiments, the culture was grown in MP+ and MP− solutions without any pyromorphite or with chlorapatite instead of pyromorphite. Finally, dissolution experiments of pyromorphite and of chlorapatite in MP+ and MP− solutions were conducted in the absence of bacteria. Both experiments and the controls were run in triplicate in 500 mL flasks containing 100 mL of the experimental solution. The reactors were incubated at room temperature (21 °C ± 1 °C) on a gyratory shaker (100 rpm) for 305 h until a full bacteria growth cycle was observed in the MP+ suspension. The aeration speed allowed the mineral particles to settle to the bottom of the flask so that they did not interfere with absorbance readings. Liquid growth cultures were monitored for contamination by streaking agar plates and examining the morphology of the resulting colonies. Any sign of contamination resulted in the termination of the experiment.
Table 2.
Experimental setup
| Experiment | Solution | Mineral | Bacteria | Time/Temperature | Analysis |
|---|---|---|---|---|---|
| E.I | MP+/MP− (100 mL in 500 mL flask) | Pyromorphite (0.05 g) | Pseudomonas putida | 305 h/21 ± 1 °C | OD |
| Control 1 | – | ||||
| Control 2 | Chlorapatite (0.05 g) | ||||
| E.II | MP+/MP− (100 mL in 500 mL flask) | Pyromorphite (0.05 g) | P. putida | 305 h/21 ± 1 °C | Concentration of Pb and P (pH) |
| Control 1 | Pyromorphite (0.05 g) | – | |||
| Control 2 | Chlorapatite (0.05 g) | - |
Analytical methods
Solid characterization
The characterization of the synthesized solids was performed by means of SEM-EDS. A FEI QUANTA 200 FEG was used for the SEM-EDS analysis at 15 kV. The X-ray powder diffraction (XRD) patterns were collected with a Philips PW 3020 X’Pert-APD Diffractometer system (with Cu anode and graphite monochromator) using a step scan mode at a step size of 0.02 2Θ and a rate of 1 s per step. To conduct the wet chemical analysis of the pyromorphite, a small amount of the mineral was digested in 0.02 M EDTA and analyzed for Pb, P, and Cl.
Bacterial growth
The optical density of the bacterial suspensions was determined by the absorbance measurement at 600 nm with a use of a Cary 50 Bio UV-visible spectrophotometer. A sterile solution was used as a blank.
Solution analysis
Concentration of Pb and P were analyzed with a Thermo Scientific X Series ICP-MS. Prior to the element analysis, the samples were centrifuged and filtered using 0.2 μm polycarbonate filters. Then, for the ICP-MS analysis, the supernatants were diluted in 0.01 M HCl and spiked with the internal standards of Ge and Re. A turbidimetric method with silver nitrate was used to determine the concentration of Cl in the solution for the wet chemical analysis of pyromorphite.
The amount of (Pb) bound to the bacterial cells
At 100 h of experimental time, six bacterial suspension samples were taken from the reactors with pyromorphite and MP+ solution (two samples from each of triplicate). The samples were taken after the mineral particles had settled to the bottom of the flask so that they neither interfere with absorbance readings nor Pb analysis. A half of each sample was centrifuged, filtered, and directly analyzed for Pb as described in the “Solution analysis.” The other half was first digested in 2 % HNO3 for 24 h and then centrifuged, filtered, and analyzed for Pb. Digestion resulted in destroying bacterial cells, thus releasing the bacterially bound Pb to the solution.
Statistics
The abiotic dissolution experiments were considered in equilibrium saturation state with respect to the dissolving mineral when the concentration of analyzed ions was statistically identical in four consecutive samples. A Student’s t test was used to verify that there is no trend in the data and that the slope of concentration pattern with time was not significantly different from zero, at the 95 % significance level.
Results
Experiment E1.I: P. putida growth in presence of pyromorphite
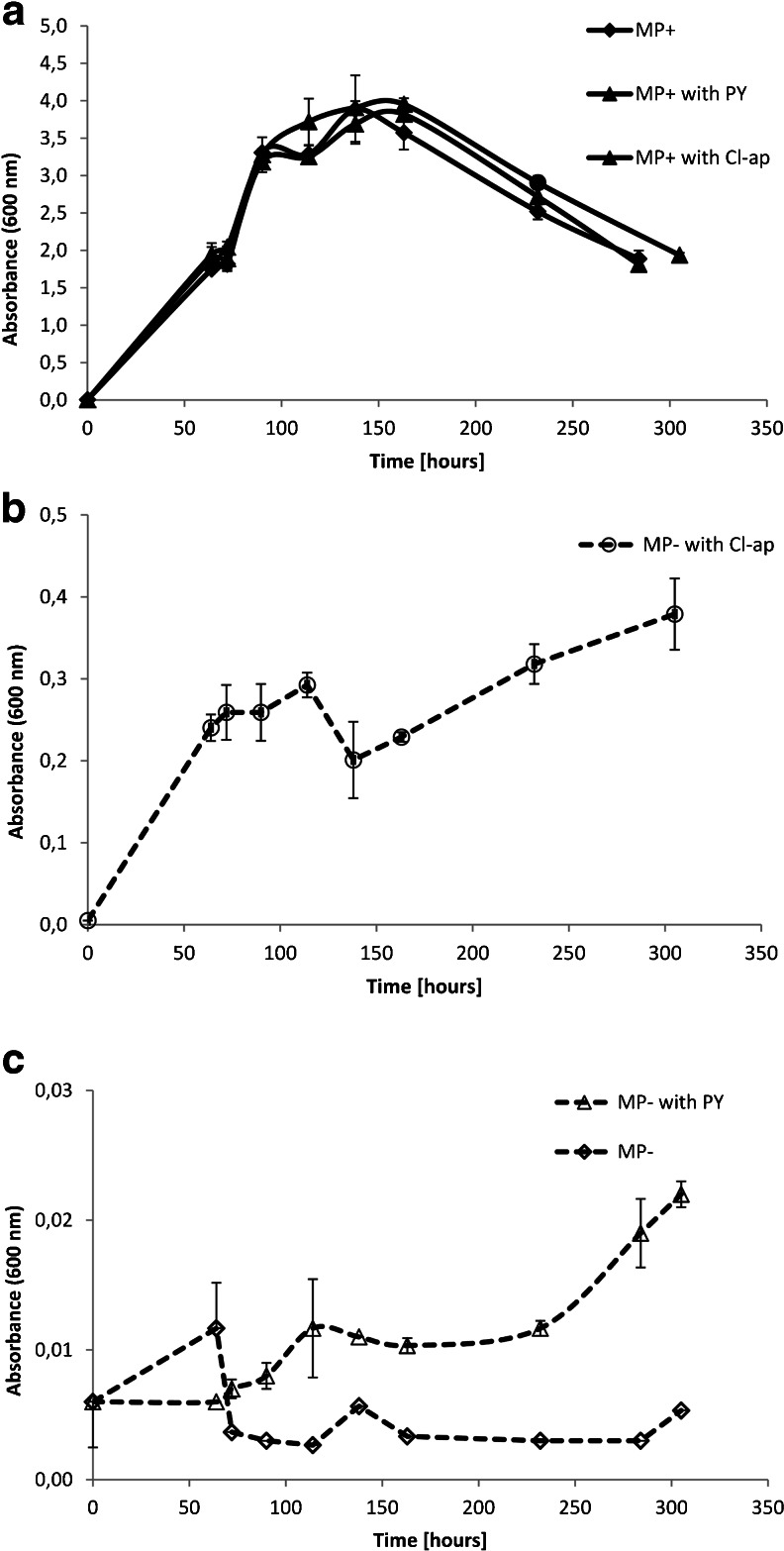
The culture growth in the E.I experimental series, expressed in terms of the OD600 readings, is presented in Fig. 2a–c. Fig. 2a shows the P. putida growth in the P-rich environment (all E.I reactors with the MP+ solution). In some of the reactors, besides the aqueous phosphate ions, also the mineral source of P was available for the bacteria. The experiment identified the effect of a mineral amendment in a solution on bacterial growth. The growth rate and the number of the bacteria in the suspensions with the MP+ solution were identical within experimental error, regardless if and what kind of the mineral was introduced to the solution. The stationary growth phase in MP+ suspensions occurred within approximately 110 h and lasted for another 50 h. The observations were terminated after 305 h when the OD600 of cultures declined by 50 %. The log curves of the bacterial growth cycle in the MP+ solution were used to determine the end of the batch experiments.
Fig. 2.
The optical density (OD600) of the bacterial suspension as a function of time. Error bars represent the standard deviation of triplicate. a The absorbance readings for reactors with MP + solution with aqueous phosphates; b the absorbance readings for reactors with MP− solution with chlorapatite; c the absorbance readings for reactors with MP− solution with pyromorphite. In P-rich environment, the mineral presence in a solution had no effect on microbial growth. Nutrient shortage resulted in a limited bacterial growth. P. putida could grow if apatite was the only source of P in a solution. In reactors with P-deficient solution, only the beginning of the logarithmical growth was observed. The bacteria grown under nutrient-rich conditions exhibited the full-growth cycle for the same experimental time
Figure 2b, c shows the P. putida growth in the reactors from the E.I experiments with the P-deficient solution MP−. In some of the reactors, the mineral source of P was available for the bacteria. The experiment tested if the bacteria can utilize phosphate from pyromorphite. A P-deficient environment resulted in limited bacterial growth: the effect of P shortage on both the growth rate and the number of the bacteria in suspensions was observed. The culture growth was not observed in the reactor with the P-free solution in the absence of a mineral phase (absence of phosphate). A limited but apparent culture development was observed for the suspensions with apatite group minerals as a P source. The OD600 of the bacterial suspension in the reactor with pyromorphite as the sole source of phosphates (marked as MP− with PY) was up to 150 h, only slightly higher than the OD600 of the culture grown in the control without any P source. The apparent difference, however, constantly increased over time. The highest growth was observed in a suspension where chlorapatite was a nutrient source; For this suspension, at the end of the experiment, the OD(600) readings were 20 times higher than for the suspension with pyromorphite. The cultures grown in the P-deficient mineral-rich solution (marked as MP− with PY and MP− with Cl-ap) reached only the exponential phase of the growth cycle. They yielded the OD600 10 times lower in the chlorapatite suspension and 200 times lower in the pyromorphite suspension, in comparison with the maximum absorbance values measured for the experiments with the MP+ solution.
Experiment E.II: dissolution of pyromorphite in presence of P. putida
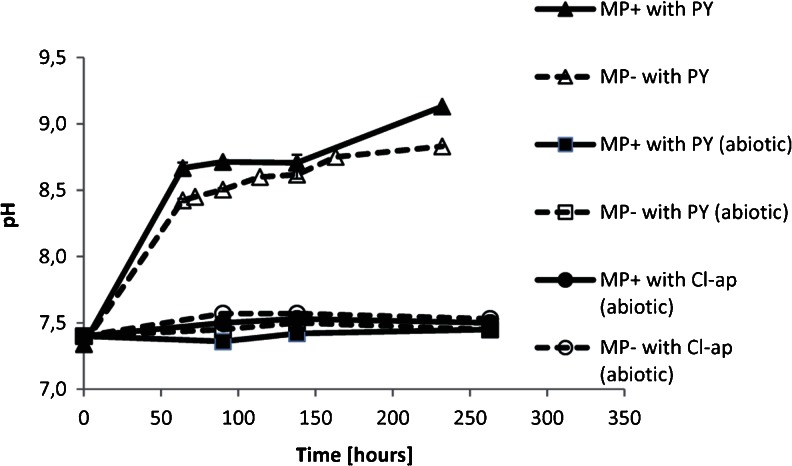
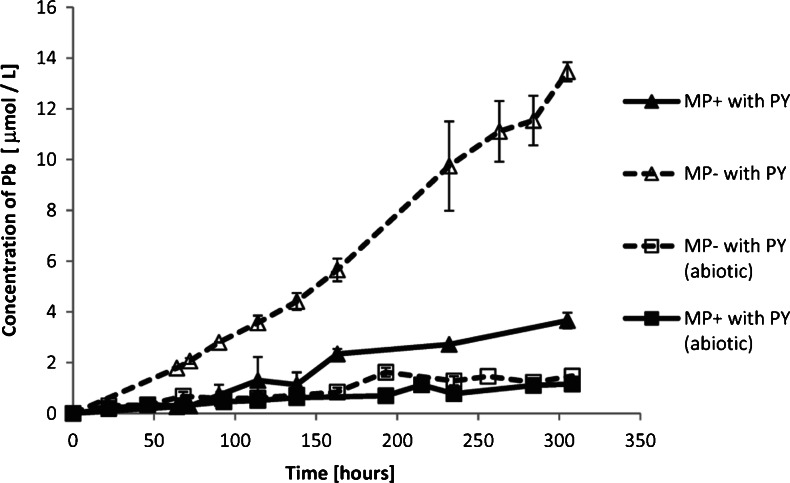
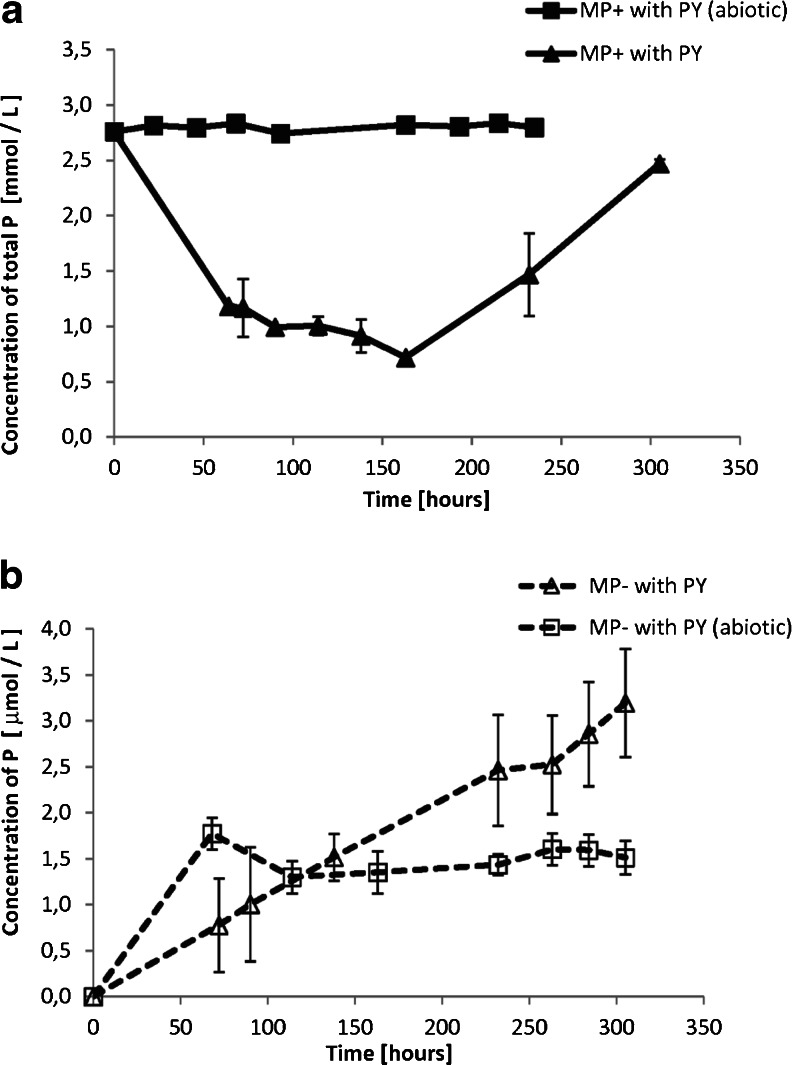
Figure 3 shows the evolution of pH in the reactors from the E.II series. The pH of the solutions inoculated with the bacteria increased over time regardless of the presence of aqueous phosphate. In the abiotic control solutions, the pH remained at the same initial level (7.3 ± 0.2 unit) for the whole experimental time (Fig. 3). Figure 4 shows the change of Pb concentration in the reactors from the E.II series with solutions amended with pyromorphite over time. In terms of the change in dissolved Pb, both abiotic controls (the P-rich and the P-deficient solution) were close to the steady state at the end of the experiment, as verified by the Student’s t test. The (Pb) evolution patterns for these solutions were almost identical within the experimental error. Both phosphate-rich and phosphate-deficient solutions inoculated with the bacteria (marked as MP+ with PY and MP− with PY, respectively) exhibited constant increase in the (Pb) with time. The highest Pb concentration was in the bacterial suspension in which pyromorphite served as the sole source of phosphate. In this experiment, the (Pb) was ten and four times higher than in the corresponding abiotic and bacterial P-rich controls, respectively. Digestion of the bacterial cells that were in the MP+ suspension with pyromorphite revealed that at 100 h of the experiment, the Pb concentration bounded to the cells was 13.6 ± 0.4 μM. This was 94 % of the total Pb dissolved in the reactor.
Fig. 3.
The variation of pH with time in all experimental reactors. Error bars represent the standard deviation of triplicate. In the abiotic experiments, pH remained constant for the whole experimental time, whereas in the microbial experiments, pH increased
Fig. 4.
Total concentration of Pb in the experimental reactors with solution amended with pyromorphite as a function of time. Error bars represent the standard deviation of triplicate. Microbially enhanced dissolution of pyromorphite is apparent. Pyromorphite dissolves significantly more in presence of the bacteria than in abiotic environment. The highest concentration of Pb is in the reactor with bacteria and P-deficient solution, whereas in abiotic solutions (P rich and P deficient), pyromorphite dissolution is almost identical within the experimental error
Figure 5a, b shows the evolution of total P concentration in the experimental reactors with pyromorphite. The triangles in Fig. 5a show time-resolved changes in the total P concentrations in the microbial P-rich suspension amended with pyromorphite. A fluctuation of the total P concentration in this suspension resembles the inversion of the culture growth cycle in the reactors with MP+ solution in E.I series (Fig. 2a); the initial drop caused by nutrient uptake is followed by P release due to culture extinction. The P concentration in the P-rich abiotic solution with pyromorphite was constant within the experimental error (squares in Fig. 5a). The time variation in the total (P) in the reactors with the solution initially devoid of the aqueous phosphorus is presented in Fig. 5b. In these experiments, a dissolving mineral was the only source of P in solution. The P concentration in the inoculated, P-limited, pyromorphite-rich solution constantly increased over the experimental time (triangles in Fig. 5b). Within first 200 h of the experiment, the (P) in this suspension was equal to the (P) in the corresponding control (abiotic) solution with pyromorphite. Later, a difference was apparent and at the end of the experiment the (P) in the solution with microbes was twice as high as in the abiotic control.
Fig. 5.
Total concentration of P in the experimental reactors from the E.II series as a function of time. Error bars represent the standard deviation of triplicate. a P-rich solution. A fluctuation of P concentration in this suspension resembles the inversion of the culture growth cycle; b P-deficient solution. The P concentration in the solution inoculated with bacteria constantly increased with time and was significantly higher than in the corresponding abiotic experiment. Note the scale difference
A summary of the final composition of the solutions of the E.II experimental series is presented in Table 3. The final P concentration in the P-rich abiotic solution with pyromorphite was equal to the P concentration in a solution with chlorapatite. In the P-deficient conditions, the (P) was almost two orders of magnitude higher in the solution with chlorapatite than with pyromorphite.
Table 3.
Final total concentrations of Pb and P in the E.II experimental series
| Pyromorphite (PY) | Chlorapatite (CAP) | |||||
|---|---|---|---|---|---|---|
| P deficient | P rich | P deficient | P rich | |||
| Bacterial MP− with PY | Abiotic MP− with PY | Bacterial MP+ with PY | Abiotic MP+ with PY | Abiotic MP− with CAP | Abiotic MP+ with CAP | |
| [P] μM | 3.19 ± 0.59 | 1.51 ± 0.18 | 2,470 ± 0.04 | 2,820 ± 20 | 119 ± 10.7 | 2,796 ± 9 |
| [Pb] μM | 13.5 ± 0.37 | 1.47 ± 0.16 | 3.66 ± 0.31 | 1.15 ± 0.07 | – | – |
| [Pb]/[P] | 4.22* | 0.97* | – | – | – | – |
“±” experimental error representing a standard deviation of triplicates
*Stoichiometric [Pb]/[P] ratio in pyromorphite is equal to 1.67
Discussion
P. putida growth in presence of pyromorphite as a phosphate source
Availability of P is one of the most crucial determinants of microbial growth. The presence of P-bearing minerals (pyromorphite or chlorapatite) in experimental suspensions that were replete with aqueous P did not affect the culture growth. The contribution of the dissolving minerals to a P supply in the solutions was negligible compared with the amount of P already in solution. The total P concentration in the abiotic P-rich controls was constant over the experimental time (Fig. 5a) and identical for pyromorphite- and chlorapatite-containing solution (Table 3). In addition, no toxic effect of the Pb released from pyromorphite on the microbial growth was observed; the culture growth cycles were identical in all MP+ suspensions whether the solution was amended with pyromorphite or chlorapatite. The resistance of P. putida to Pb has been investigated, e.g., by Rugierro et al. (2005). They concluded that the inhibition of the bacterial growth caused by the metal toxicity remains insignificant when the culture is exposed to up to 40 μM of the aqueous Pb. At the end of the experiments, the total dissolved (Pb) in P-rich suspension with pyromorphite was 3.66 μM.
In contrast to the experiments carried out in the MP+ solution, P. putida growth was limited when the bacteria were under P stress, even when the phosphate-bearing minerals were introduced to the solution (Fig. 2b, c). However, both minerals successfully served as a P source for the microbes promoting slow, but apparent culture growth in the suspensions. It seems that the chlorapatite was more accessible for the microbes than pyromorphite. The microbes grew better in the P-poor solution with chlorapatite than in the P-poor solution with pyromorphite (Fig. 2b, c). In general, the apatite group minerals exhibit high environmental stability (Smith et al. 1977). However, the results of the P concentration in abiotic control experiments indicate that, under the experimental conditions, pyromorphite was almost two orders of magnitude less soluble than chlorapatite (Table 3). This result suggests a reasonable explanation for the difference in the microbial growth observed for the reactors with P-deficient solution with pyromorphite and with chlorapatite. It should be mentioned here that the Pb concentration in all pyromorphite-containing reactors remained below its toxic level for the P. putida over the whole experimental time, thus it is not considered as a significant determinant of growth. Nevertheless, it appears that extremely stable and potentially toxic pyromorphite can serve as a phosphate source to the common, widespread bacteria.
Microbially enhanced Pb remobilization by P. putida
It has been shown that the P. putida bacteria utilize P from pyromorphite. The microbes acquired a limited nutrient from the Pb-apatite structure, by changing environmental conditions in a way that destabilized the mineral. To understand the microbial role in pyromorphite’s dissolution process, the results of the abiotic controls should be considered first.
At the end of the abiotic experiments, equilibrium of the solutions was reached as indicated by the lack of change in dissolved Pb with time, and the concentration of dissolved Pb was almost identical in both P-rich and P-deficient abiotic solutions (Fig. 4). This indicates that under the experimental conditions, the initial presence of phosphate ions in the suspensions had a negligible effect on the dissolution of pyromorphite. In abiotic conditions, pyromorphite exhibited typical, extremely low solubility and dissolved incongruently. The Pb/P molar ratio in a solution initially devoid of the aqueous phosphorus was equal to 0.97 at the end of the experiment; the stoichiometric ratio of these elements in pyromorphite structure is equal to 1.67. Incongruent dissolution of pyromorphite was also observed by Scheckel and Ryan (2002) and Manecki (2009) and is likely to occur due to readsorption of Pb on the mineral crystals or precipitation of a small amount of Pb(OH)2. This phase was undetectable in the case of this study, however, by means of standard solid characterization methods e.g., SEM or XRD.
Among other well-known abiotic factors, e.g., thermodynamics of associated species, properties of the solvent, etc., the pH of a solution is often considered a main determinant of the mineral solubility. Because an apparent fluctuation of pH of the experimental solutions inoculated with bacteria was observed, its role in the dissolution phenomena described in this work should be considered. The solubility of pyromorphite varies parabolically with pH, reaching the minimum at pH ∼ 8 and increasing as pH increases or decreases from this value. As reported by Manecki and Maurice (2008), at pH from 7 to 9, the solubility of pyromorphite, in terms of Pb dissolved, is constant within the experimental error. Furthermore, the Pb concentration in a solution equilibrated with pyromorphite is calculated by PHREEQC program (Parkhurst and Appelo 1999) to vary only by 0.05 μmol/L within this pH range (data not shown). In this study, the pH of the abiotic solutions remained constant for the whole experimental time. The increase of the pH of solutions inoculated with bacteria from 7.3 to ∼9 was a typical result of the microbial activity (Fig. 3). This effect was also observed and described by Dehner et al. (2010) when they were working with the same growth medium. The time-resolved pH fluctuations were similar in all bacteria suspensions, yet the change of Pb concentration was significantly different (over 0.05 μmol/L) between the particular reactors (compare Figs. 3 and 4). This suggests that pH was not a major determinant in this study. The role of the pH variation observed for the bacterial experiments in pyromorphite dissolution is not completely excluded, however, its impact was considered insignificant in this case.
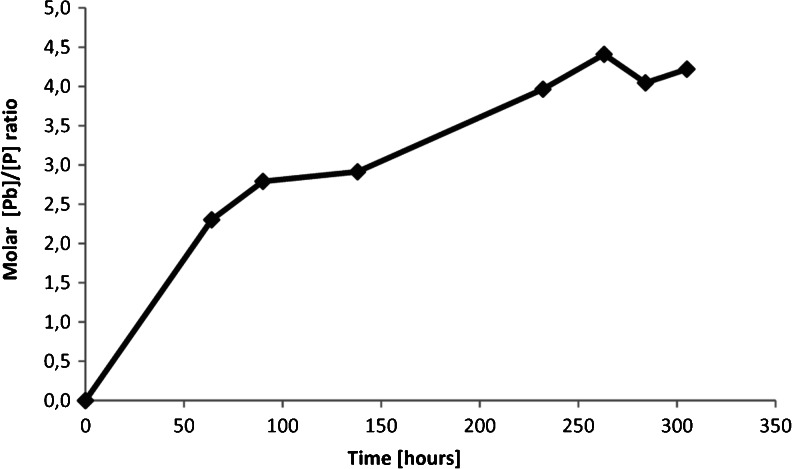
The presence and the activity of PSB affect the stability of pyromorphite, which can eventually lead to elevated Pb concentration in the environment. When compared with the results of the abiotic experiments, an apparent increase of the Pb concentration in the solutions inoculated with the bacteria was observed (Fig. 4). This indicates that the presence of PSB, in this case P. putida, not only affects stability of a wide range of calcium phosphates as reported in literature (Welch et al. 2002; Hutchens et al. 2006; Feng et al. 2011) but also intensify the weathering of extremely stable phases such as pyromorphite. It is interesting, that the process of enhanced dissolution of the mineral was observed, although soluble phosphate was available for the microbes in the solution. In this case, pyromorphite did not serve as the main nutrient source for P. putida and the bacteria did not have to actively scavenge for the phosphate. However, the milieu of a bacterial culture is full of microbial metabolites which change the speciation of the solution, thus might enhance dissolution processes. Furthermore, the cells of Pseudomonas can serve as an effective sorption surface for many elements, including Pb (Leung et al. 2001). We have calculated that at 100 h of the experimental time, in the MP(+) reactor with pyromorphite and the microbes, the amount of Pb bound to the P. putida cells was 3.39 ± 0.29 (*10−11) μmol/cell. This implies that the actual total [Pb] dissolved from pyromorphite in the bacterial suspension with MP+ solution could reach as high level as 14.5 μmol/L. The metal sorption on the bacterial surface can be a reversible process as described for example by Chang et al. (1997). In the paper on Pb, Cu and Cd adsorption on Pseudomonas aeruginosa, Chang et al. (1997) indicated that there are differences in adsorption capacities depending on the growth phase of the bacteria. The authors state that, the optimum of Pb adsorption on the bacteria falls on early stationary phase (∼100 h of our experiments with P. putida in the MP(+) solution). Hence, we assume that some part of the bacterially bound Pb could have been released to the solution when the bacteria reached a death phase of their growth (compare the patterns of full triangles in Figs. 2a and 4). It is worth mentioning here that the microbial biosorption/bioaccumulation processes should not be considered as a method for permanent immobilization and decrease of Pb bioavailability, in natural, uncontrolled ecosystems. Pyromorphite dissolved significantly more if it served as the sole source of phosphates for the microbes, although there were considerable less bacteria in the suspension. The P concentration (Fig. 5a) and the molar Pb/P ratio (Table 3) in the MP− solution with pyromorphite and inoculated with bacteria were significantly higher than in the corresponding abiotic experiment. Furthermore, the Pb/P molar ratio in the suspension constantly increased with time as presented in Fig. 6. Elevated Pb content and Pb to P ratio in the bacterial suspension along with increase of the culture growth imply intensified dissolution process associated with microbial uptake of P. The processes described here represent a positive feedback loop wherein P-stress results in microbial nutrient-scavenging; this in turn results in enhancement of the dissolution processes and release of phosphates and Pb; the more nutrient is available, the more the microbes grow and are active which dissolve the mineral. It is possible that the entire process will last until the bacteria reach satisfaction in terms of nutrient supply, which should be taken into account when the contamination treatment is designed.
Fig. 6.
Molar Pb/P ratio in the MP− suspension with pyromorphite as a sole source of phosphates for the microbes. Elevated Pb content in comparison to the P in the suspension was observed, indicating enhanced dissolution of PY associated with bacterial P uptake
It is worth mentioning here that the fish-bone meal, usually used as phosphate amendment in the Pb contaminated sites, is not a readily available but a mineral source of P for the microbes. The presence of a nutrient source in such a form promotes microbial active scavenging strategies, i.e., organic acids and chelates productions, formation of biofilm, etc. (Welch et al. 2002). Some of these mechanisms have been already reported as inhibiting the pyromorphite formation or as enhancing its dissolution (Manecki and Maurice 2008; Debela et al. 2010, 2013). The availability of dissolved phosphates is crucial in the processes discussed in this paper. The total deficiency of aqueous phosphate is unlikely to occur in nature, however, the opposite situation, in which the soil would be constantly saturated with phosphate in terms of the bacterial nutrient requirements, is also improbable, in a long run (Bardgett 2006). One of the reasons is that phosphorous is an essential nutrient for any form of life: plants, fungi, and other soil organisms compete with the bacteria in its acquisition. Low/medium dissolved phosphate concentration is the most probable long-run scenario at sites remediated by the P-induced method, especially with a use of the optimization by PSB. In such a situation, a relatively small amount of the nutrient promotes the bacterial growth, but does not meet the metabolic needs of the microbes. Consequently, a greater number of stressed bacteria is present in the environment which leads to even greater expression of scavenging strategies (Prieto et al. 1997; Dehner et al. 2010). In such a case, when the pyromorphite is in bacteria milieu, significant remobilization of Pb might occur.
Our results indicate that the stability of pyromorphite in soil does not depend primarily on its thermodynamics and abiotic environmental parameters. This is in agreement with findings reported by Abbaspour and Arocena (2012) in their paper on stability of pyromorphite in presence of plant roots. In our studies, it appears that pyromorphite solubility, and thus the concentration of released Pb ions, strongly correlate with the amount of the soil bacteria and their nutrient needs. This was not considered in the proposed methodology of Park et al. (2011a).
The degree of Pb contamination varies from site to site. The scope of the problem is different for urban soils (from ∼10 to ∼300 ppm of Pb), at mining areas (from ∼300 to ∼80,000 ppm of Pb), and different at shooting ranges (∼400 to almost 100,000 ppm of Pb) (Rooney 2002). The average number of the bacterial cells in soil is estimated to be as many as 1010–1011/g (Horner-Devine et al. 2004). For comparison, at the end of the experiments with pyromorphite as a sole source of phosphate in the solution, the number of CFU was equal to ∼3 × 106/cm3. The diversity of contaminated ecosystems in terms of inhabiting organisms and concentration/bioavailability of Pb creates a countless number of environmental conditions to deal with during remediation treatment. Thus, it is crucial to test the bacterial activity in the contaminated soil prior to the remediation treatment and to proceed with caution when considering the introduction of PSB. At some places, the discussed adverse bacterial impact might be negligible, while at some others, it might undermine the whole concept of the P-induced method.
Summary
In this study, the effect of the soil microorganisms on the stability of pyromorphite has been investigated in a set of batch experiments. Pyromorphite, a Pb apatite, is an extremely stable product of a phosphate-induced, in-situ Pb immobilization in contaminated sites. The objective of this work was to determine: (1) if the presence of P. putida, a PSB in the environment, affects pyromorphite stability and may result in elevated Pb concentrations in the milieu; (2) if the process depends on the availability of P as a nutrient for the microbes.
The results of this study indicate the potential for PSB to increase the solubility of pyromorphite, thus decreasing the long-term effectiveness of in situ, phosphate induced Pb immobilization. The Pb concentration was almost three times higher in the P-rich experimental solution with bacteria than in the one without microbes. This has important implications for the recently proposed (Park et al. 2011a) modification of in-situ Pb immobilization by the addition of PSB. Such an addition might have adverse long-term impact on the stabilization of Pb.
The amount of P available for the bacteria in soils decreases naturally with time (Bardgett 2006). This study indicates that pyromorphite may help support the growth of common soil bacteria under P stress. In terms of Pb release, in P-deficient environment, the bacterial effect on pyromorphite solubility was greater than in the case when an alternative, readily available source of P was provided. This implies that conscious phosphate management might be crucial for the long term effectiveness of the in situ Pb immobilization method. Selection of the type and the amount of phosphate amendment should take into account not only a degree of contamination but also the soil ecosystem and its possible response to the applied treatment.
Acknowledgments
We would like to thank Dr Nicolai Kummer from the Institute of Geology at TU-Bergakademie in Freiberg as well as Mr. Janosch Gröning and Mrs. Beate Erler from the Institite of Biosciences at TU-Bergakademie in Freiberg for help and precious advises in laboratory works. This study was financed by MNiSW grant no. N N307 101535.
References
- Abbaspour A, Arocena JM. Evaluation of chloropyromorphite stability in the Rhizosphere of Brassica juncea and Medicago sativa in a sand culture. J Environ Qual. 2012;41:1525–1530. doi: 10.2134/jeq2012.0062. [DOI] [PubMed] [Google Scholar]
- Bajda T. Solubility of mimetite Pb5(AsO4)3Cl at 5–55 °C. Environ Chem. 2010;7:268–278. doi: 10.1071/EN10021. [DOI] [Google Scholar]
- Baker WE. An X-ray diffraction study of synthetic members of the pyromorphite series. Am Mineral. 1966;51:1712–1721. [Google Scholar]
- Bardgett RD (2006) The biology of soil: a community and ecosystem approach. The biology of habitats series. Oxford University Press, Oxford
- Chang JS, Law R, Chang CC. Biosorption of lead, copper and cadmium by biomass of Pseudomonas aeruginosa PU21. Water Res. 1997;31:1651–1658. doi: 10.1016/S0043-1354(97)00008-0. [DOI] [Google Scholar]
- Cotter-Howells J. Lead phosphate formation in soils. Environ Pollut. 1996;93:9–16. doi: 10.1016/0269-7491(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Cotter-Howells J, Caporn S. Remediation of contaminated land by formation of heavy metal phosphates. App Geochem. 1996;11:335–342. doi: 10.1016/0883-2927(95)00042-9. [DOI] [Google Scholar]
- Dai Y, Hughes JM. Crystal-structure refinements of vanadinite and pyromorphite. Can Mineral. 1989;27:189–192. [Google Scholar]
- Dehner CA, Barton L, Maurice PA, DuBois JL. Size-dependent bioavailability of hematite (α-Fe2O3) nanoparticles to a common aerobic bacterium. Environ Sci Tech. 2010;45:977–983. doi: 10.1021/es102922j. [DOI] [PubMed] [Google Scholar]
- Debela F, Arocena JM, Thring RW, Whithcombe T. Organic acid-induced release of lead from pyromorphite and its relevance to reclamation of Pb-contaminated soils. Chemosphere. 2010;80:450–456. doi: 10.1016/j.chemosphere.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Debela F, Arocena JM, Thring RW, Whithcombe T. Organic acids inhibit the formation of pyromorphite and Zn-phosphate in phosphorous amended Pb- and Zn-contaminated soil. J Environ Man. 2013;116:156–162. doi: 10.1016/j.jenvman.2012.11.037. [DOI] [PubMed] [Google Scholar]
- Ehlers LJ, Luthy RG. Contaminant bioavailability in soil and sediment. Environ Sci Tech. 2003;37:295A–302A. doi: 10.1021/es032524f. [DOI] [PubMed] [Google Scholar]
- Feng M, Ngwenya BT, Wang L, Li W, Olive V, Ellam RM. Bacterial dissolution of fluorapatite as a possible source of elevated dissolved phosphate in the environment. Geochim Cosmochim Acta. 2011;75:5785–5796. doi: 10.1016/j.gca.2011.07.019. [DOI] [Google Scholar]
- Flis J, Borkiewicz O, Bajda T, Manecki M, Klasa J. Synchrotron–based X-ray diffraction of the lead apatite series Pb10(PO4)6Cl2−Pb10(AsO4)6Cl2. J Synchrotron Radiat. 2010;17:207–214. doi: 10.1107/S0909049509048705. [DOI] [PubMed] [Google Scholar]
- Flis J, Manecki M, Bajda T. Solubility of pyromorphite Pb5(PO4)3Cl–mimetite Pb5(AsO4)3Cl solid solution series. Geochim Cosmochim Acta. 2011;75:1858–1868. doi: 10.1016/j.gca.2011.01.021. [DOI] [Google Scholar]
- Fomina M, Alexander IJ, Hillier S, Gadd GM. Zinc Phosphate and pyromorphite solubilization by soil plant-symbiotic fungi. Geomicrobiol J. 2004;21:351–366. doi: 10.1080/01490450490462066. [DOI] [Google Scholar]
- Horner-Devine MC, Carney KM, Bohnnan BJM. An ecological perspective on bacterial diversity. Proc Royal Soc London (B) 2004;271:113–122. doi: 10.1098/rspb.2003.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Cameron M, Crowley KD. Structural variations in natural F, OH, and Cl apatites. Am Mineral. 1998;74:870–876. [Google Scholar]
- Hutchens E, Valsami-Jones E, Narouiya N, Vhairat C, Oelkers EH, McEldoney S. An experimental investigation of the effect of Bacillus megaterium on apatite dissolution. Geomicrobiol J. 2006;23:177–182. doi: 10.1080/01490450600599239. [DOI] [Google Scholar]
- Leung WC, Chua H, Lo W. Biosorption of heavy metals by bacteria isolated from activated sludge. App Biochem Biotech. 2001;91–93:171–184. doi: 10.1385/ABAB:91-93:1-9:171. [DOI] [PubMed] [Google Scholar]
- Ma QI, Logan TJ, Traina SJ. Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ Sci Tech. 1995;29:1118–1126. doi: 10.1021/es00004a034. [DOI] [PubMed] [Google Scholar]
- Ma QI, Logan TJ, Traina SJ, Ryan JA. Effects of NO3−, Cl−, F−, SO42−, and CO32− on Pb2+ immobilization by hydroxyapatite. Environ Sci Tech. 1994;28:408–418. doi: 10.1021/es00052a011. [DOI] [PubMed] [Google Scholar]
- Ma QI, Traina SJ, Logan TJ, Ryan JA. Effects of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb immobilization by hydroxyapatite. Environ Sci Tech. 1994;28:1219–1228. doi: 10.1021/es00056a007. [DOI] [PubMed] [Google Scholar]
- Ma QY, Traina SJ, Logan TJ, Ryan JA. In-situ lead immobilization by apatite. Environ Sci Tech. 1993;27:1803–1810. doi: 10.1021/es00046a007. [DOI] [Google Scholar]
- Manecki M. Rola i dynamika przemian piromorfitu Pb5(PO4)3Cl w środowisku. Kraków: Rozprawy i Monografie 196. UWND AGH; 2009. [Google Scholar]
- Manecki M, Maurice PA. Siderophore promoted dissolution of pyromorphite. Soil Sci. 2008;173:82–830. doi: 10.1097/SS.0b013e31818e8968. [DOI] [Google Scholar]
- Manecki M, Bogucka A, Bajda T, Borkiewicz O. Decrease of Pb bioavailability in soils by addition of phosphate ions. Environ Chem Lett. 2006;3:178–181. doi: 10.1007/s10311-005-0030-1. [DOI] [Google Scholar]
- Matlakowska R, Drewniak L, Sklodowska A. Arsenic-hypertolerant pseudomonas isolated from ancient gold and copper-bearing black shale deposits. Geomicrobiol J. 2008;25:357–362. doi: 10.1080/01490450802402810. [DOI] [Google Scholar]
- Nakamoto A, Urasima Z, Sugiura S, Nakano H, Yachi T, Tadokoro K. Pyromorphite-mimetite minerals from the Otaru–Matsukura barite mine in Hokkaido, Japan. Mineralogical J. 1969;6:85–101. [Google Scholar]
- Park JH, Bolan N, Megharaj M, Naidu R. Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J Haz Mat. 2011;185:829–836. doi: 10.1016/j.jhazmat.2010.09.095. [DOI] [PubMed] [Google Scholar]
- Park JH, Bolan N, Merharaj M, Naidu R. Comparative value of phosphate sources on the immobilization of lead and leaching of lead and P in lead contaminated soils. Sci Tot Environ. 2011;409:853–860. doi: 10.1016/j.scitotenv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water-Resources Investigation Rep No. 99-4259
- Plumlee GS, Morman SA. Mine Wastes and human Health. Elements. 2011;7:399–404. doi: 10.2113/gselements.7.6.399. [DOI] [Google Scholar]
- Prieto B, Pardo MA, Garbisu C, Llama MJ, Serra JL. Phosphate uptake by phosphorus-starved cells of the cyanobacterium Phormidium laminosum. World J Microb Biot. 1997;13:699–705. doi: 10.1023/A:1018583224294. [DOI] [Google Scholar]
- Reva ON, Weinel C, Weinel M, Böhm K, Stjepandic D, Hoheisel JD, Burkhard T. Functional genomics of stress response in Pseudomonas putida KT2440. J Bacteriol. 2006;188:4079–4092. doi: 10.1128/JB.00101-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roane TM. Lead resistance in two bacterial isolates from heavy metal-contaminated soils. Microb Ecol. 1999;37:218–224. doi: 10.1007/s002489900145. [DOI] [PubMed] [Google Scholar]
- Rooney C (2002) Contamination of shooting ranges. The lead group incorporated. http://www.lead.org.au/fs/shootingranges.pdf
- Rosas SB, Andres JA, Rovera M, Correa NS. Phosphate-solubilizing Pseudomonas putida can influence the rhizobia-legume symbiosis. Soil Biol Biochem. 2006;38:3502–3505. doi: 10.1016/j.soilbio.2006.05.008. [DOI] [Google Scholar]
- Ruby MV, Davis A, Nicholson A. In situ formation of lead phosphates in soil as a method to immobilize lead. Environ Sci Tech. 1994;28:646–654. doi: 10.1021/es00053a018. [DOI] [PubMed] [Google Scholar]
- Rugierro CE, Boukhalfa H, Forsythe JH, Lack JG, Hersman LE, Neu MP. Actinide and metal toxicity to prospective bioremediation bacteria. Environ Microbiol. 2005;7(1):88–97. doi: 10.1111/j.1462-2920.2004.00666.x. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Kierans M, Geoffrey MG. Solubilization of some naturally occurring metal-bearing minerals lime scale and lead phosphate by Asperigillus niger. FEMS Microbiol Lett. 1997;154:29–35. doi: 10.1111/j.1574-6968.1997.tb12620.x. [DOI] [PubMed] [Google Scholar]
- Scheckel KG, Ryan JA. Effect of aging and pH on dissolution kinetics and stability of chloropyromorphite. Environ Sci Tech. 2002;36:2198–2204. doi: 10.1021/es015803g. [DOI] [PubMed] [Google Scholar]
- Smith EA, Mayfield CI, Wong PTS. Physical and chemical characterization of selected natural apatites in synthetic and natural aqueous solutions. Water Air Soil Poll. 1977;8:401–415. [Google Scholar]
- Sullivan JB, Krieger GR. Clinical and environmental health and toxic expousures. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Traina SJ, Laperche V (1999) Contaminant bioavailability in soils, sediments and aquatic environments. In: Smith JV (ed) Colloquium on geology, mineralogy, and human welfare, vol. 96. National Academies Press, Washington, DC. pp. 3365–3371 [DOI] [PMC free article] [PubMed]
- United States Environmental Protection Agency (2005) Best management practices for lead at outdoor shooting ranges. EPA-902-B-01-00.
- Welch SA, Taunton AE, Banfield JF. Effect of microorganisms and microbial metabolites on apatite dissolution. Geomicrobiol J. 2002;19:343–367. doi: 10.1080/01490450290098414. [DOI] [Google Scholar]
- Xie L, Giammar D. Equilibrium solubility and dissolution rate of the lead phosphate chloropyromorphite. Environ Sci Tech. 2007;41:8050–8055. doi: 10.1021/es071517e. [DOI] [PubMed] [Google Scholar]