Abstract
Sleep loss places caregivers at risk for poor health. Understanding correlates of sleep loss and relationships to health may enable improvement of health of caregivers of individuals with primary malignant brain tumors (PMBT). In this cross-sectional, descriptive study of 133 caregivers, relationships were examined between sleep loss and physical, mental, emotional, and social health at time of patient diagnosis. Sleep loss was not related to physical health. Shorter total sleep time was associated with greater fatigue and social support. Sleep quality was positively associated with quality of life. Further study is needed of the role of sleep loss in the PMBT caregiving trajectory and its long-term relationship with health outcomes.
Keywords: sleep, caregivers, brain tumor, cancer, cytokines, depression
Caring for those with chronic illness may interfere with important processes, like sleep, that have long-term physical, social, and emotional consequences for both patients and their caregivers. Sleep loss is prevalent in caregivers (Castro et al., 2009; Fonareva, Amen, Zajdel, Ellingson, & Oken, 2011; McCurry, Logsdon, Teri, & Vitiello, 2007). Sleep disturbances place persons at higher risk for cardiovascular disease (Javaheri, 2011), metabolic syndrome (Javaheri, 2011), endocrine disorders (Grunstein, 2011), kidney disease (Unruh & Sanders, 2011), altered immune functioning (Motivala & Irwin, 2007), and depression (Thompson, Fan, Unutzer, & Katon, 2008). Caregivers have more morbidities (Kunz & Herrmann, 2000) and a higher incidence of mortality (Schulz & Beach, 1999) than matched non-caregivers.
Both stress and sleep loss increase the levels of cortisol, lipids, and insulin resistance, which have been linked to increased cardiovascular disease and Type II diabetes (Akerstedt, Perski, & Kecklund, 2011). In addition, both stress and sleep loss have been associated with increased fatigue severity, cognitive impairments, and negative mood (Akerstedt et al., 2011).
There is strong evidence that impaired sleep is associated with depressive symptoms, which include subjective complaints of longer sleep latencies, more frequent and longer awakenings, shorter total sleep time (TST), and early morning awakenings (Mayers, Grabau, Campbell, & Baldwin, 2009; Peterson & Benca, 2011), all of which have been confirmed by polysomnography during depression (Sculthorpe & Douglass, 2010). However, it is unclear whether sleep disturbances are only present during depressive episodes or sleep abnormalities predate the depressive episode and reflect a vulnerability to depression (Sculthorpe & Douglass, 2010). Cognitive impairments seen in sleep deprivation mimic other symptoms of depression, including fatigue and a diminished ability to think, concentrate, and/or make decisions (Peterson & Benca, 2011). Like sleep loss, depression is associated with cardiovascular disease (Jiang et al., 2001) and with diabetes (Anderson, Freedland, Clouse, & Lustman, 2001).
Furthermore, sleep loss has been associated with altered immune functioning through its actions on pro-inflammatory cytokines, particularly interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (Krueger, 2008; Motivala & Irwin, 2007). Results from studies suggest that IL-6 promotes sleep because its circadian secretion correlates with sleep–wake rhythms. In normal sleep, IL-1 and IL-6 levels peak in the night, with progressive decline to nadir in the morning (Motivala & Irwin, 2007). Elevation of these cytokines in sleep loss, and the subsequent effect on inflammatory responses, may contribute to a range of health consequences, such as cardiovascular disease, metabolic syndrome, endocrine disorders like diabetes and thyroid disease, gastrointestinal disorders, and kidney disease (Aouizerat et al., 2009; Ovaskainen et al., 2009).
Like other caregivers, caregivers of individuals with primary malignant brain tumor (PMBT) experience stress, depressive symptoms, and sleep impairments (Pawl, Lee, Clark, & Sherwood, 2013). Although PMBT occurs less frequently than other cancers, it is associated with disproportionate morbidity and mortality. Dealing with cognitive impairments and care linked to the rapid progression of the tumor in the care recipient may contribute to sleep disturbances in this caregiver population and ultimately affect caregiver health. To date, little is known about PMBT caregivers’ health circumstances related to impaired sleep, other than that their sleep is likely to be negatively affected by the tasks of providing care.
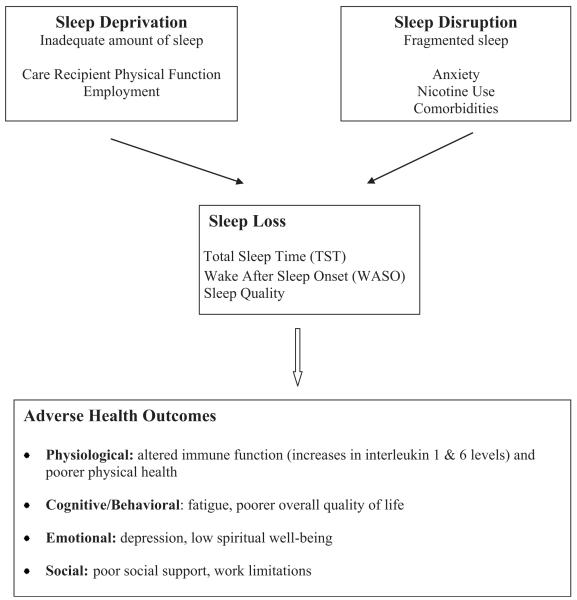
Using a conceptual model of impaired sleep (Lee, 2003) as a guide for this study, sleep loss and its relationship to health outcomes in PMBT caregivers were examined at 4–8 weeks after the diagnosis of the PMBT (see Fig. 1). The model posits that inadequate amount of sleep (sleep deprivation) and fragmented sleep (sleep disruption) result in sleep losses that adversely affect health. Factors that contribute to sleep deprivation include age, gender, caregiving tasks, and employment, whereas factors that contribute to sleep disruption include anxiety, nicotine use, and comorbidities. Age and gender have been treated as covariates of sleep in other sleep studies (e.g., McCurry et al., 2007; Ohayon & Vecchierini, 2005). Health in the model is defined as four dimensions: physical, cognitive/behavioral, emotional, and social health.
FIGURE 1.
Conceptual model of impaired sleep. From “Impaired Sleep” by K. A. Lee, 2003, In V. Carrieri-Kohlman, A. M. Lindsey, & C. M. West (Eds.), Pathophysiological phenomena in nursing: Human responses to illness (p. 364). St. Louis, MO: Saunders. Copyright 2003 by Elsevier. Reprinted with permission.
Using objective and subjective measures of sleep loss and of health, the purpose of this study was to explore the relationships between PMBT caregivers’ sleep loss and dimensions of caregivers’ health, including physical, cognitive/ behavioral, emotional, and social, at time of diagnosis of the PMBT. Four hypotheses were tested in a sample of caregivers providing care for persons with PMBT: after controlling for age and gender, sleep deprivation, and disruptions (as measured by TST, awakenings after sleep onset [WASO], and sleep quality), will be significantly associated with: (1) physiological health outcomes (serum levels of IL-1ra and IL 6 and physical health); (2) cognitive/behavioral health outcomes (fatigue and overall quality of life); (3) emotional health outcomes (depression and spiritual well-being); and (4) social health outcomes (social interactions and work interactions for those caregivers who are employed).
Methods
Design
This cross-sectional, descriptive study was an analysis of baseline data collected within 1–2 months after the care recipient was diagnosed with a PMBT. The data were collected as part of a large, longitudinal study of mind–body interactions in caregivers of those with PMBT (RO1-CA118711; Sherwood, PI). The protocol for this secondary analysis was reviewed and approved by the Institutional Review Board at Georgia State University.
Sample and Setting
Between October 1, 2005 and April 30, 2011, 219 dyads four to eight weeks after the diagnosis of a PMBT were approached at an urban tertiary medical center in the eastern United States. Of the 219, 64 (29.2%) declined to participate. Reasons given for not participating included not being interested (51.6%), feeling too overwhelmed (32.8%), or other reasons (15.6%). Specifics of the eligibility criteria and sample characteristics have been reported elsewhere (Pawl et al., 2013). Briefly, caregivers were identified by care recipients as the person who would provide emotional and physical help on a regular basis. Caregivers were 21 years of age or older and neither a primary caregiver for other adults nor a professional caregiver. Care recipients had been diagnosed with a PMBT within 2 months of the start of data collection.
A total of 155 pairs of primary caregivers and care recipients were enrolled. Of the 155 dyads, 22 (14%) dyads did not complete the measures. Within the dyads not completing the measures, the care recipients were significantly older (M = 62.1, SD = 18.4) than those who did complete measures (M = 53.3, SD = 13.9, t (144) = 2.24, p = .03). No significant differences were found between the groups in the care recipients’ tumor type, race, or functional status.
Care recipients were mainly middle-aged (M = 53.3, SD = 13.9, range 22–85), white (98.5%) males (63.9%) with varying levels of cognitive impairment. Most care recipients had malignant gliomas (49.6%), 18 had low-grade astrocytomas (I–III), 19 had oligodendroglio-mas, and 12 had other tumor types. Care recipients were receiving treatment based on National Comprehensive Cancer Network (NCCN) guide-lines for radiation and chemotherapy or no treatment, based on tumor histology. Data about care recipient cognitive status and cancer treatment were not collected.
Most care recipients reported high levels of physical function (Karnofsky score, M = 82.6, SD = 12.6, range 50–100). Thirty-nine percent (n = 52) reported no complaints or minor symptoms related to the PMBT, 19% (n = 25) required minimal assistance with care, and 9% needed assistance with ADLs (n = 12). Fifteen care recipients (11.2%) required considerable assistance or were unable to carry on normal activity.
The majority of caregivers were middle-aged (M = 51.6, SD = 11.8, range 21–77), well-educated (94.5% ≥ completion of high school), white (94.0%) females (69.2%) who were mostly spouses or significant others (75.2%) of the care recipients. More than half of caregivers were overweight or obese (55.7%, – = 74), and most reported one or more comorbidity (64.8%, n = 70), such as high blood pressure, diabetes, cancer, and or stroke. Many were employed outside the home (55.6%). The dyads participating in this study reflect the demographics reported of others with PMBT (Armstrong, 2009; Schnell & Tonn, 2009) and in other studies of caregivers of persons with PMBT (Cashman et al., 2007; Schmer, Ward-Smith, Latham, & Salacz, 2008; Sherwood et al., 2006).
Measurements
Health measures
Caregiver health outcomes were separated into four dimensions (physiological, cognitive/behavioral, emotional, and social).
Physiological health
Physiological health was measured using serum markers of immune functioning and the physical health component of the Medical Outcomes Study short form (SF-36; McHorney, Ware, & Raczek, 1993). The physical health component (PHC) of the SF-36 is a valid and reliable measure of physical health (McHorney, Ware, Lu, & Sherbourne, 1994; McHorney et al., 1993). The 36-item self-reported survey includes a total of eight subscales that aggregate into two summary measures: a PHC (physical functioning, role-physical, bodily pain, and general health) and a mental health component (vitality, social functioning, role-emotional, and mental health). Higher total PHC scores reflect better physical health. Cronbach’s alpha for the physical health summary measure was .91 in this study.
Immune functioning was operationalized as levels of circulating IL-1 receptor antagonist (IL-1ra) and IL-6. Interleukin-1ra has been used as a proxy for IL-1β because both cytokines are secreted in response to physiological or psychological stress; however, IL-1ra levels remain elevated in the serum longer (Milaneschi et al., 2009). IL-1β is a pro-inflammatory cytokine that initiates an immune response to insult, whereas IL-1ra is an anti-inflammatory cytokine released to modulate IL-1β activity and return the immune system to homeostasis (Frey, Fleshner, & Wright, 2007; Lehto et al., 2010). Elevations of IL-1ra and IL-6 were presumed to indicate dysfunction of the immune/inflammatory system and high risk for poor physical health.
The average draw time for the ILs was 1400 hours (range 0830-2000). Specimens were centrifuged and stored at −80°C until analyzed in batch. IL-1ra levels were measured by enzyme-linked immunosorbent assay (ELISA; Quantikine Human kit, R & D Systems, Minne-apolis MN; RnDSystems.com) with a detectable limit of 18.3 pg/ml and interassay coefficient of variation of 4.6%. IL-6 serum levels were measured by high sensitivity ELISA (Quantikine HS Human, R & D Systems) with a detectable limit of 0.11 pg/ml and interassay coefficient of variation of 6.9%.
Cognitive/behavioral health
Caregiver fatigue was measured using the vitality subscale of the SF-36, which has four items, with higher scores reflecting feeling full of pep and energy all of the time during the last 4 weeks (Ware & Sherbourne, 1992). Lower scores represented lack of vitality or more fatigue. Cronbach’s alpha in this study was .89.
Caregiver QOL was measured using the Fox Simple Quality of Life Scale (FSQOL; Fox, 2004). The FSQOL is a 25-item instrument using a 5-point Likert type scale designed to measure the cognitive components of QOL, such as satisfaction and well-being, and affective components of QOL, such as health and functional status (total score range 25–125; Fox, 2004), with higher scores reflecting higher QOL. The Cronbach’s alpha in this study was .93.
Emotional health
Emotional health was conceptualized as caregiver depressive symptoms and spiritual wellbeing. Depressive symptoms were measured using the shortened 10-item form of the Center of Epidemiologic Studies-Depression scale (CES-D-SF; Andresen, Malmgren, Carter, & Patrick, 1994), derived from the original 20-item instrument designed to measure depressive symptoms in community populations (Radloff, 1977). Criterion validity for the CES-D-SF has been established using the full CES-D (R2 = .92; Cheung, Liu, Phil, & Yip, 2007). On the CES-D-SF, a cut-off score of ≥10 is an indicator of possible clinical depression (Andresen et al., 1994). Total CES-D-SF scores can range from 0 to 30, with higher scores indicating the presence of more depressive symptoms (Martens et al., 2006). The Cronbach’s alpha in this study was .87.
Spiritual well-being was measured using the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being Scale (FACIT-Sp; Peterman, Fitchett, Brady, Hernandez, & Cella, 2002). The FACIT-Sp is a 12-item scale designed to describe aspects of spirituality and/or faith that contribute to QOL of people experiencing chronic and life-threatening illnesses. Each item is assessed on a 5-point (0–4) scale; items measure truths about how the person has been feeling over the last 7 days with regard to his or her faith. Total scores range from 0 to 48, with higher scores reflecting a higher sense of spiritual wellbeing. The Cronbach’s alpha in this study was .90.
Social health
Social health was conceptualized as social support and, for those working caregivers, work-life interactions. Social support was measured using the Interpersonal Support Evaluation List (ISEL), a 40-item measure of the perceived quality and availability of social support (Cohen & Hoberman, 1983; Cohen, Mermelstein, Kamarck, & Hoberman, 1985). There are four 10-item subscales: (a) tangible; (b) appraisal; (c) self-esteem; and (d) belonging (Cohen et al., 1985). The overall score can range from 0 to 120, with higher scores indicating more perceived social support. The Cronbach’s alpha for the overall score in this study was .89.
For employed caregivers, work-life interactions were measured using the Work Limitations Questionnaire (WLQ), a 25-item instrument for measuring the impact of chronic health problems and/or treatments on job performance and work productivity (Lerner et al., 2001). Each item is assessed on a 5-point scale rating the frequency of difficulty in performing 25 specific job demands: 1 (able all of the time) to 5 (able none of the time). Higher scores reflect more productivity loss or limitations. The Cronbach’s alpha in this study was .92.
Sleep loss measures
Both objective and subjective measures of sleep loss were obtained. Sleep loss was defined as interrupted or shortened sleep time (less than the sleep time wanted by the caregiver). Objective sleep loss was measured as nocturnal TST and the percentage of awakening after sleep onset (WASO), as measured by Bodymedia® Sensewear™ Armbands (Bodymedia.com)—accelerometer devices containing 2-axis micro-electro-mechanical sensors that measure motion. The Bodymedia.® Sensewear™ Armbands, when compared with polysomnography, have been shown to predict TST in slow wave sleep and REM 100% of the time, with true positive predictions of 98.9% in Stage II sleep and 94% in Stage I sleep (Sunseri et al., 2009). However, the device is limited in detecting wake episodes <10 minutes (Sunseri et al., 2009), which could underestimate WASO. Participants were asked to wear the armbands for three nights. Caregiver sleep loss was calculated for each night the accelerometer was worn; only those with a minimum of two nights of data were included in final data analysis. Most caregivers wore their accelerometer for 3 nights (76%), but 15% either wore the accelerometer for only one night (n = 1) or had sleep graphs that were not readable (n = 19).
Subjective sleep loss in the month prior to data collection was measured using the sleep quality subscale, a single item of the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), on a 4-point scale ranging from 0 (very good) to 3 (very bad). Higher scores reflect poorer sleep quality (Buysse et al., 1989). The single-item sleep quality subscale had an overall correlation coefficient of .83 (p < .01) with the global score in the original PSQI psychometric testing (Buysse et al., 1989).
Sociodemographic variables
Self-reported caregiver sociodemographic data included age, gender, ethnicity, educational level, and employment. Comorbid conditions, smoking status, and weight may disrupt sleep (see Fig. 1) and were self-reported.
Caregiver anxiety was measured using the shortened Profile of Mood States-Anxiety scale (POMS-anxiety). The shortened POMS was developed to assess transient distinct mood states (McNair, Lorr, & Droppleman, 1971; Shacham, 1983). The tension-anxiety subscale is a 3-item scale in which items are rated on a 5-point scale (1 = never, 5 = always). The total subscale score can range from 3 to 15, with higher scores indicating more anxiety. The Cronbach’s alpha in this study was .90.
Care recipient demographic data included tumor status verified by pathology report and age collected from the medical record. The care recipient’s performance of activities of daily living (ADLs) was measured by trained research assistants (RAs) using the Karnofsky Performance Scale (KPS; Karnofsky, Abelmann, Craver, & Burchenal, 1948). The validity and reliability of the KPS have been supported in relation to other measures of physical performance (Simmonds, 2002; Yates, Chalmer, & McKegne, 1980).
Procedures
Individuals with PMBT were approached for identification of a primary caregiver and consent. Both members had to consent to participate. Data were collected by trained research assistants (RAs) from the medical record and by in-person interview of care recipients at time of consent using a standardized protocol. Inter-rater agreement for raters on data extraction was evaluated by independent extraction of sequential medical records between the RA and the principal investigator. Agreement >95% was achieved each time inter-rater reliability was tested.
Caregivers, after consent, were given instructions to wear the Bodymedia® Sensewear™ Armbands for three consecutive 24-hour days and then to return the armband to the study coordinator in a pre-paid mailed package. Within 72 hours of the care recipient’s interview, caregiver initial data were collected in a home visit. Blood samples and other measures were collected by RAs. Thirty minutes prior to blood sampling, caregivers were asked to refrain from smoking or ingesting caffeine. Referrals to primary care providers were made for those participants scoring ≥10 on the CES-D-SF. Those caregivers whose inter- and intraassay coefficients of variation for IL-1ra and IL-6 were <20% (n = 3) were excluded from analysis because the elevation may have indicated infection or other health conditions.
Data Analyses
Data were analyzed with SenseWear Professional 7.0 and PASW Statistics 18 for Windows. All interval/ratio level variables were assessed by examining skewness, kurtosis, and other assessments for normality of data as outlined by Field (2009). All variables were normally distributed except for IL-1ra and IL-6. A log transformation was done on both cytokine measures prior to data analysis.
Descriptive statistics were used to describe sample characteristics and major study variables. Bivariate correlation coefficients were used to examine relationships among sleep loss variables, caregiver health variables, and demographic variables. Independent t-tests were used to compare groups. Pearson’s correlation coefficients (r) were calculated for variables that were normally distributed, while Spearman’s rho correlation coefficient (rs) was calculated for those that were not.
Hierarchical regression was used to examine the contribution of variation in demographical and sleep loss variables to levels of variables within each of the health dimensions. Age and gender were entered into the models first as covariates. The second step contained all other variables related to sleep deprivation and disruption, followed by the sleep loss variables (see Fig. 1). A separate hierarchical regression analysis was conducted for each of the nine measures of health outcomes.
Results
Sleep Loss
Descriptive statistics for the major study variables are shown in Table 1. In addition, more than half of the caregivers (59.4%) reported experiencing anxiety higher than the midpoint of 8 on the POMS Anxiety-Tension subscale scale (3–15).
Table 1. Description of Sleep Loss and Health Outcomes in Caregivers of Persons With PMBT.
| n | M | (SD) | Observed Range |
Possible Range |
||
|---|---|---|---|---|---|---|
| Sleep loss | Total sleep time (minutes) | 112 | 356.6 | (84.6) | 95–544 | |
| Wake after sleep onset | 112 | 15.1 | (9.2) | 0.5–47.7 | ||
| Sleep quality–Pittsburgh Sleep Quality Index |
123 | 1.3 | (0.9) | 0–3 | 0–3 | |
| Health outcomes: | Interleukin-lra (pg/ml) | 104 | 366.5 | (354.4) | 86.6–3349.2 | 31.2–2000 |
| Physiological dimension |
Interleukin-6 (pg/ml) | 105 | 1.8 | (1.3) | 0.5–8.3 | 0.2–10 |
| SF-36 Physical health summary | 103 | 81.2 | (17.2) | 14–100 | 0–100 | |
| Cognitive/behavioral dimension |
SF-36 Health survey-vitality subscale (fatigue) |
103 | 57.1 | (20.8) | 0–90 | 0–100 |
| Fox Simple Quality of Lifea | 89 | 63.4 | (9.1) | 33–80 | 25–125 | |
| Emotional dimension |
Centers for Epidemiological Studies-Depression Scale |
126 | 8.3 | (6.5) | 9–29 | 0–30 |
| Functional Assessment of Chronic Illness Therapy-Spiritual |
109 | 35.4 | (8.8) | 10–48 | 0–48 | |
| Social dimension | Interpersonal Support Evaluation List | 123 | 35.0 | (4.9) | 17–40 | 0–40 |
| Work Limitations Questionnaireb | 54 | 10.2 | (1.0) | 8–13 | 0–25 |
Note: PMBT, primary malignant brain tumor; n varied due to missing data.
Instrument added after first 30 participants.
Only 74 participants were employed.
PMBT caregivers experienced prolonged sleep latency (35 minutes on average) and short sleep times (5 hours and 57 minutes; reported elsewhere, Pawl et al., 2013). Self-reported sleep quality was on average fairly good to good (n = 81); however, 31.6% of the caregivers reported fairly bad or very bad sleep quality.
Correlations Among Personal Characteristics, Sleep Loss, and Health Outcomes
Relationships among the health outcome variables are in Table 2 and among predictors and outcomes in Table 3. Caregiver fatigue was positively correlated with QOL and social support and negatively correlated with depressive symptoms. Depressive symptoms were positively correlated with spirituality. Caregiver QOL was positively correlated with spirituality and social support. Spirituality was positively correlated with social support. For those caregivers who were employed either full-or part-time (n = 54), work limitations were positively correlated with depressive symptoms and IL-1ra levels.
Table 2. Correlations Among Caregiver Health Outcomes.
| Variable | Il-1ra | IL-6 | PHC | Fatigue | FSQOL | CES-D | FACIT-Sp | ISEL |
|---|---|---|---|---|---|---|---|---|
| Il-1ra | ||||||||
| IL-6 | .47** | |||||||
| PHC | .00 | −.06 | ||||||
| Fatigue | −.02 | −.19 | .32** | |||||
| FSQOL | .05 | .13 | .12 | .26* | ||||
| CES-D | −.05 | .04 | .15 | −.26** | −.11 | |||
| FACIT-Sp | .10 | −.08 | −.09 | .19 | .44** | .34** | ||
| ISEL | .01 | .06 | .06 | .37** | .52** | −.15 | .53** | |
| WLQ | .34* | −.08 | −.28 | .04 | −.22 | −.35** | .00 | −.15 |
Note: IL, interleukin; PHC, Medical Outcomes Study-Short Form 36 subscale: physical health component; FSQOL, Fox Simple Quality of Life; CES-D, Center for Epidemiologic Studies-Depression Scale; FACIT-Sp, functional assessment of chronic illness therapy-spiritual; ISEL, interpersonal support evaluation list; WLQ, work limitations questionnaire.
p < .05.
p < .01.
Table 3. Correlations Among Predictor Variables and Caregiver Health Outcomes.
| Variable | Il-1ra | IL-6 | PHealth | Fatigue | FSQOL | CES-D | FACIT-Sp | ISEL | WLQ |
|---|---|---|---|---|---|---|---|---|---|
| Demographic variables | |||||||||
| Age | 27** | .21* | .11 | −.07 | −.09 | −.01 | −.12 | −.06 | .21 |
| Gender | .17 | .19 | −.07 | −.03 | −.15 | −.05 | .01 | .02 | .04 |
| CR Karnofsky score | .04 | .06 | .23* | .15 | .01 | −.02 | .15 | .04 | .01 |
| Employment status | −.15 | −.06 | −.02 | −.02 | −.07 | .07 | .00 | −.01 | −.05 |
| POMS-Anxiety | .20 | .03 | .08 | −.22* | .39** | .30** | −.20* | −.17 | .01 |
| Smoking status | −.14 | −.04 | .02 | .21* | −.05 | .24* | −.11 | .00 | −.12 |
| Number of comorbidities | .25* | .14 | −.44** | −.12 | −.04 | −.05 | .00 | −.12 | .21 |
| Sleep loss variables | |||||||||
| TST | .18 | −.10 | −.06 | −.25* | −.33** | −.01 | −.05 | −.25** | .13 |
| WASO | −.11 | .15 | .03 | .02 | .33** | −.06 | .09 | .19 | −.18 |
| Sleep qualitya | −.05 | .01 | .05 | −.15 | .39** | .17 | −.21* | −.22* | −.04 |
Note: IL, Interleukin; Phealth, Medical Outcomes Study-Short Form 36 subscale: Physical Health; FSQOL, Fox Simple Quality of Life; CES-D, Center for Epidemiologic Studies-Depression Scale; FACIT-Sp, Functional Assessment of Chronic Illness Therapy- Spiritual; ISEL, Interpersonal Support Evaluation List; WLQ, Work Limitations Question naire; CR, Care Recipient; POMS, Profile of Mood States; TST, total sleep time; WASO, wake after sleep onset.
Sleep quality item from Pittsburgh Sleep Quality Index. Lower score indicates better sleep quality.
p < .05.
p < .01.
Predictors of Physiological Health
Serum levels of IL-1ra were on average 366.5 pg/ml (SD = 354.4), and IL-6 levels averaged 1.8 pg/ml (SD = 1.3). The caregivers’ self-reported physical health (PHC) was high. Significant bivariate relationships of physiological health with other variables (see Table 3) included positive correlations of: (a) IL-1ra levels with age and comorbidities of the caregivers; and (b) IL-6 levels with caregiver age. Caregiver comorbidities and care recipient physical functioning were negatively correlated with caregiver physical health.
Predictors of the three measures of physiological health are shown in Table 4. The first regression model, predicting IL-1ra, was not significant. Age was an independent predictor of IL-1ra level (t = 2.7, p = .01). Similarly, the model, predicting IL-6, was not significant, although age was an independent predictor of Il-6 level (t = 2.1, p = .04). In the third model, the explained significant variance (34%) in physical health as measured by the SF-36. Comorbidities (t = −4.1, p < .001) and age (t = 2.3, p = .03) were the only independent of physical health.
Table 4. Predictors of Caregiver Physiological Health in Multiple Regression.
| Interleukin 1ra (n = 66) |
Interleukin 6 (n = 67) |
Physical Health (n = 78) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | B | SE B | β | Δ R 2 | B | SE B | β | Δ R 2 | B | SE B | β | Δ R 2 |
| Step 1 (covariates) | ||||||||||||
| Age | .02 | .01 | .36** | .02 | .01 | .30* | .40 | .18 | .26* | |||
| Gender | .20 | .14 | .18 | .16** | .32 | .17 | .24 | .12* | −5.42 | 4.20 | –.14 | .01 |
| Step 2 (deprive/disrupt) | ||||||||||||
| Karnofsky score | .01 | .01 | .16 | .01 | .01 | .21 | .42 | .16 | .30* | |||
| Employment | –.02 | .13 | –.02 | .09 | .17 | .08 | 2.81 | 4.22 | .08 | .10* | ||
| POMS-anxiety | .04 | .03 | .19 | .02 | .03 | .06 | 1.29 | .80 | .19 | |||
| Smoking status | –.03 | .17 | –.02 | .02 | .22 | .01 | .57 | 5.76 | .01 | |||
| Comorbidities | .14 | .08 | .21 | .09* | .04 | .10 | .05 | .02 | –9.86 | 2.38 | –.43** | .30** |
| Step 3 (sleep loss) | ||||||||||||
| TST | .00 | .00 | .08 | –.00 | .00 | –.18 | –.04 | .03 | –.17 | |||
| WASO | .00 | .01 | .05 | .01 | .01 | .21 | .02 | .25 | .01 | |||
| Sleep qualitya | –.01 | .09 | –.01 | .00 | –.01 | .11 | –.01 | .09 | –1.88 | 2.68 | –.08 | .03 |
| Total R2 | .26 | .22 | .34** | |||||||||
| Overall model | F (10,56) = 1.92, p = .06 | F (10, 57) = 1.63,p = .12 | F (10,. 68) = 3.48, p < .01 | |||||||||
Note: POMS, Profile of Mood States; TST, total sleep time; WASO, wake after sleep onset.
Item from Pittsburgh Sleep Quality Index. Lower score indicates better sleep quality.
p < .05.
p < .01.
Predictors of Cognitive/Behavioral Health
On average, caregivers showed high vitality and thus had low fatigue. Caregivers’ self-reported QOL was poor. Fatigue was positively correlated with smoking status and negatively correlated with anxiety and TST. QOL was positively correlated with WASO and sleep quality and negatively with anxiety and TST (see Table 3).
The variables together explained significant variance (25%) in fatigue (see Table 5). Two independent predictors of fatigue, care recipient Karnofsky score (t = 2.2, p = .03) and caregiver (t = −2.0, p = .05), were identified. The model predicting QOL also was significant, accounting for 37% of the variance in QOL. Self-reported sleep quality (t =−2.3, p = .02) and gender (t = −2.2, p = .03) were significant predictors of QOL.
Table 5. Predictors of Caregiver Cognitive/Behavioral Health in Multiple Regression.
| Fatigue (n = 78) |
Quality of Life (n = 63) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | B | SE B | β | β R 2 | B | SE B | β | Δ R 2 |
| Step 1 (covariates) | ||||||||
| Age | .08 | .22 | .04 | −.16 | .10 | −.22 | ||
| Gender | −6.60 | 5.32 − | .14 | .03 | −4.90 | 2.26 | −.25* | .08 |
| Step 2 (deprive/disrupt) | ||||||||
| Karnofsky score | .44 | .20 | .26* | .02 | .08 | .02 | ||
| Employment | 3.58 | 5.35 | .08 | −.73 | 2.38 | −.04 | ||
| POMS-Anxiety | −1.50 | 1.01 − | .18 | −.42 | .47 | −.12 | ||
| Smoking status | 12.51 | 7.30 | .19 | −4.43 | 3.26 | −.16 | ||
| Comorbidities | −4.36 | 3.02 − | .16 | .20* | −.53 | 1.34 | −.05 | .16 |
| Step 3 (sleep loss) | ||||||||
| TST | −.08 | .04 − | .28* | −.01 | .02 | −.06 | ||
| WASO | −.31 | .31 − | .14 | .25 | .14 | .27 | ||
| Sleep qualitya | −.68 | 3.39 − | .02 | .05 | −3.67 | 1.56 | −.30* | .13* |
| Total R2 | .25* | .37** | ||||||
| Overall model | F (10, 68) = 2.25, p = .03 | F (10, 53) = 3.07, p < .01 | ||||||
Note: POMS, Profile of Mood States; TST, total sleep time; WASO, wake after sleep onset.
Item from Pittsburgh Sleep Quality Index. Lower score indicates better sleep quality.
p < .05.
p < .01.
Predictors of Emotional Health
Caregivers reported high spiritual wellbeing. Twenty-eight percent scored ≥10, the commonly used clinical cut-off for the CES-D-SF, indicating high depressive symptoms and a need for referral for assessment of clinical depression. Depressive symptoms were positively correlated with caregiver anxiety and smoking status. Caregiver spirituality was positively correlated with sleep quality and negatively with anxiety (see Table 3). Neither model of predictors of depressive symptoms and spiritual health (see Table 6) was statistically significant.
Table 6. Predictors of Caregiver Emotional Health in Multiple Regression.
| Depression (n = 83) |
Spiritual Health (n = 80) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | B | SE B | β | Δ R 2 | B | SE B | β | Δ R 2 |
| Step 1 (covariate) | ||||||||
| Age | .02 | .07 | .03 | −.13 | .10 | −.17 | ||
| Gender | −.48 | 1.61 | −.04 | .00 | −2.39 | 2.36 | −.12 | .04 |
| Step 2 (deprive/disrupt) | ||||||||
| Karnofsky score | −.03 | .06 | −.06 | .14 | .09 | .19 | ||
| Employment | 1.11 | 1.55 | .09 | −.62 | 2.24 | −.03 | ||
| POMS-Anxiety | .47 | .30 | .20 | −.65 | .44 | −.19 | ||
| Smoking status | 2.28 | 2.31 | .12 | −3.36 | 3.21 | −.12 | ||
| Comorbidities | −.88 | .92 | −.11 | .08 | .49 | 1.35 | .04 | .09 |
| Step 3 (sleep loss) | ||||||||
| TST | 1.09 | .01 | .00 | .01 | .02 | .05 | ||
| WASO | −.01 | .09 | −.01 | .19 | .13 | .20 | ||
| Sleep qualitya | .37 | .99 | .05 | .00 | −1.11 | 1.44 | −.09 | .03 |
| Total R2 | .09 | .16 | ||||||
| Overall model | F (10, 73) = .69, p = .74 | F (10, 70) = 1.37, p = .21 | ||||||
Note: POMS, Profile of Mood States; TST, total sleep time; WASO, wake after sleep onset.
Item from Pittsburgh Sleep Quality Index. Lower score indicates better sleep quality.
Predictors of Social Health
Caregivers reported high perceived social support. Those caregivers who were employed either full-or part-time (n = 74) reported few work limitations on average. There was no difference in work limitations between the caregivers employed full-time and those who were employed part-time. Social support was positively correlated with sleep quality and negatively correlated with TST. There were no significant relationships with work limitations (see Table 3).
The hierarchal regression model predicting social support was significant, accounting for 22% of the variance (see Table 6). TST (t = −2.1, p = .04) was an independent predictor of social support (Table 7). The regression model predicting work limitations was not significant.
Table 7. Predictors of Caregiver Social Health in Multiple Regression.
| Social Support (n = 82) |
Work Limitations (n = 37) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | B | SE B | β | Δ R 2 | B | SE B | β | Δ R 2 |
| Step 1(covariate) | ||||||||
| Age | −.07 | .05 | −.15 | .04 | .02 | .41 | ||
| Gender | 1.23 | 1.27 | −.11 | .05 | −.20 | .42 | −.09 | .09 |
| Step 2 (deprive/disrupt) | ||||||||
| Karnofsky score | .03 | .05 | .07 | .00 | .02 | .02 | ||
| Employment | .67 | 1.22 | .07 | .05 | .42 | .02 | ||
| POMS-anxiety | −.23 | .24 | −.12 | .11 | .09 | .27 | ||
| Smoking status | 2.13 | 1.74 | −.14 | .33 | .52 | .12 | ||
| Comorbidities | 1.00 | .72 | −.15 | .08 | .30 | .25 | .22 | .07 |
| Step 3 (sleep loss) | ||||||||
| TST | −.02 | .01 | −.29* | .00 | .00 | .24 | ||
| WASO | .04 | .07 | .08 | .01 | .02 | .05 | ||
| Sleep qualitya | −.41 | .77 | −.06 | .09* | −.29 | .27 | −.22 | .08 |
| Total R2 | .22* | .24 | ||||||
| Overall model | F (10,72) = 2.00, p = .05 | F (10, 27) = .83, p = .60 | ||||||
Note: POMS, profile of mood states; TST, total sleep time; WASO, wake after sleep onset.
Item from Pittsburgh Sleep Quality Index. Lower score indicates better sleep quality.
p < .05.
Discussion
At time of diagnosis of PMBT, TST, and sleep quality were associated with some adverse cognitive/behavioral and social health outcomes but not with any physical or emotional health outcomes. WASO was not associated with any of the health outcomes. A possible explanation for lack of relationship of many of the sleep measures to health outcomes is that these data were collected early in the trajectory of the disease process and very soon after diagnosis. However, the average TST was less than the recommended amount of sleep per night, and WASO was higher than population norms, indicating that caregivers were experiencing sleep loss. With the short duration in the caregiving role and the lack of accumulation of chronic sleep debt, the effects of this sleep loss may not be evident at this point in the caregiving trajectory.
It is unclear when untoward health effects of sleep loss may occur during the caregiving trajectory. At time of diagnosis of PMBT, care recipients often have substantial cognitive impairment, with rapid progression of symptoms. With the intense emotional demand on the caregiver, which may result in sleep disruption or deprivation, we thought that some effects might have been evident at this point in the illness trajectory. With the effect on physiological regulatory systems of chronic and acute exposures to stressors, it was reasonable to expect that this allostatic load (McEwen & Stellar, 1993) would reach health-impairing levels after the diagnosis of cancer in family members, particularly because the majority of the sample were middle-aged caregivers. Further analysis of longitudinal data in the parent study will provide an opportunity to identify when the health effects are seen.
Care recipients’ cognitive impairments place the burden of decision-making on caregivers, who are dealing with their own health issues. Given the relatively high depressive symptoms in this sample, the caregivers were at risk for further sleep losses, which would compound the effects on mental health. Persons with depression experience disruptions in their sleep (Mayers et al., 2009). In this study, 28% of caregivers of persons with PMBT reported depressive symptoms, indicating possible clinical depression, a rate three times greater than that reported in the general population (Centers for Disease Control and Prevention, 2010). The lack of association between the sleep variables and depressive symptoms in this study was unexpected. There is evidence that shorter sleep time increases the risk of developing major depressive disorder, particularly in females (Brooks Girgenti, & Mills, 2009; Swanson, Hoffman, & Armitage, 2010), but recent study also suggests that the association between depression and sleep disturbance may not be a linear pattern (Sbarra & Allen, 2009).
The immune biomarkers and sleep loss variables were not related. Others have reported increases in WASO with higher IL-6 levels in dementia caregivers (Von Kanel et al., 2006); however, this was not supported in this study. Prather et al. (2009) found that as sleep debt accumulated, there were elevations in IL-1β, of which IL-1ra is a proxy, and in IL-6. Our findings may have been influenced by inconsistent draw times for the immune biomarkers, which have a circadian secretion pattern, and the use of a sleep measurement device with limited sensitivity, which could have underestimated WASO. Interestingly, caregivers in this study with self-reported poor sleep quality had lower IL-1ra levels (298 pg/ml), which may place them at risk for infections (Prather et al., 2009).
Limitations of the study included instrumental threats to internal validity. The sleep quality subscale of the PSQI was a single-item scale, one of seven components of the total PSQI global score, but this single-item is highly correlated with the PSQI global score (Buysse et al., 1989; Grutsch et al., 2011). In this study, sleep quality was positively correlated with TST (r = .21, p = .031), indicating that individuals who slept less reported poorer sleep quality, but future studies should adopt the whole PSQI to increase internal validity and glean further information from the other components about sleep. In addition, not every study participant wore the accelerometer for a minimum of 72 consecutive hours, and the monitor is unable to detect wake time less than 10 minutes. These are other limitations that should be addressed in further study.
The varying specimen collection times may have influenced the levels of IL-1ra and IL-6. As the nocturnal period approaches, IL levels begin to rise, helping to initiate sleep. Although blood draws were completed during daylight hours, some were in the morning and others in the afternoon, and resulting variations in levels could affect the detection of relationships between the immune biomarkers and sleep. However, caregivers were instructed not to smoke or ingest caffeine within 30 minutes prior to blood draw, and in a sample of 104 caregivers, the effect of diurnal variation should have been minimized statistically.
The majority of caregivers were white, well-educated, and older, reflecting the population with PMBT. Furthermore, the study caregivers also had at least one comorbidity and may have been on medications that interfered with sleep, a factor that should be measured in future studies. Data on care recipient cognitive status and treatment also would have provided a clearer understanding of caregiver demands.
Strengths of the study included the use of objective measures of sleep and physiological markers of immune function. The large sample of caregivers of a relatively rare cancer helped to provide insight into the needs of this unique population.
Conclusions
Health care providers (HCPs) are in an ideal situation to preserve or even improve PMBT caregiver health. At time of diagnosis, the caregiver is assimilating the reality of the diagnosis and the functions of caregiving. Because the downward trajectory of persons with PMBT is typically rapid, with increasingly poor cognitive and physical function, caregiving demands quickly escalate. Sleep loss and high anxiety during this recognition and learning stage have the potential to become patterns, with detrimental effects throughout the PMBT caregiving trajectory. Through systematic assessments of caregiver sleep and anxiety, HCPs may improve caregiver mental health and prevent potential deterioration in physical health, decision-making, and ability to continue to provide care across the illness trajectory. Sleep interventions for caregivers of individuals with PMBT have been suggested previously (Pawl et al., 2013). With heightened awareness of the relationship of chronic sleep loss to certain aspects of caregiver health, HCPs can provide early recognition and intervention to improve caregiver sleep, health, and quality of life.
Acknowledgments
The study was supported in part by the following grants: Mind-Body Interactions in Neuro-Oncology Caregivers, National Cancer Institute R01 (CA118711-02) to Paula R. Sherwood, PI, 2007 and STEPS: Supporting Technologically Enhanced Ph.D. Studies, Georgia State University, 2011.
Footnotes
At the time of the work, the first author was a doctoral student at Georgia State University.
References
- Akerstedt T, Perski A, Kecklund G. Sleep, stress, and burnout. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Elsevier Saunders; St. Louis, MO: 2011. pp. 814–821. [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. DOI: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American Journal of Preventive Medicine. 1994;10:77–84. Retrieved from http://www.ajpmonline.org. [PubMed] [Google Scholar]
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research in Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. DOI: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Armstrong TS. Head’s up on the treatment of malignant glioma patients. Oncology Nursing Forum. 2009;36:E232–E240. doi: 10.1188/09.ONF.E232-E240. DOI: 10.1188/09.ONF. E232-E240. [DOI] [PubMed] [Google Scholar]
- Brooks PR, Girgenti AA, Mills MJ. Sleep patterns and symptoms of depression in college students. College Student Journal. 2009;43:464–472. Retrieved from http://www.projectinnovation. biz/index.html. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. DOI: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cashman R, Bernstein LJ, Bilodeau D, Bovett G, Jackson B, Yousefi M, Perry J. Evaluation of an educational program for the caregivers of persons diagnosed with a malignant glioma. Canadian Oncology Nursing Journal. 2007;17:6–10. doi: 10.5737/1181912x171610. Retrieved from http://www.cano-acio.ca/journal/ [DOI] [PubMed] [Google Scholar]
- Castro CM, Lee KA, Bliwise DL, Urizar GG, Woodward SH, King AC. Sleep patterns and sleep-related factors between caregiving and non-caregiving women. Behavioral Sleep Medicine. 2009;7:164–179. doi: 10.1080/15402000902976713. DOI: 10.1080/15402000902976713. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Current depression among adults—United States, 2006 and 2008. Morbidity and Mortality Weekly Report (MMWR) 2010;59(38):1229–1235. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5938a2.htm?s_cid mm5938a2_w. [PubMed] [Google Scholar]
- Cheung YB, Liu KY, Phil M, Yip PS. Performance of the CES-D and its short forms in screening suicidality and hopelessness in the community. Suicide and Life-Threatening Behavior. 2007;37:79–88. doi: 10.1521/suli.2007.37.1.79. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology. 1983;13:99–125. DOI: 10.1111/j.1559-1816.1983.tb02325.x. [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: Theory, research and applications. Martinus Nijhoff; Hague, Holland: 1985. pp. 73–94. [Google Scholar]
- Field A. Discovering statistics using SPSS. 3rd ed. Sage; Los Angeles, CA: 2009. [Google Scholar]
- Fonareva I, Amen AM, Zajdel DP, Ellingson RM, Oken BS. Assessing sleep architecture in dementia caregivers at home using a ambulatory polysomnographic system. Journal of Geriatric Psychiatry and Neurology. 2011;24:50–59. doi: 10.1177/0891988710397548. DOI: 10.1177/0891988710397548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S. Preliminary psychometric testing of the Fox simple quality-of-life scale. Journal of Neuroscience Nursing. 2004;36:157–166. Retrieved from http://www.aann.org/journal/content/index.html. [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain, Behavior, and Immunity. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. DOI: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Grunstein R. Endocrine disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Elsevier Saunders; St. Louis, MO: 2011. pp. 1435–1441. [Google Scholar]
- Grutsch JF, Wood PA, Du-Quiton J, Reynolds JL, Lis CG, Levin RD, Hrushesky WJ. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. Journal of Circadian Rhythms. 2011;9:1–12. doi: 10.1186/1740-3391-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S. Sleep and cardiovascular disease: Present and past. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Elsevier Saunders; St. Louis, MO: 2011. pp. 1349–1352. [Google Scholar]
- Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, O’Connor CM. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Archives of Internal Medicine. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. Retrieved from http://www.archinternmed.com. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. DOI: 10.1002/1097.-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L. [Google Scholar]
- Krueger JM. The role of cytokines in sleep regulation. Current Pharmaceutical Design. 2008;14:3408–3416. doi: 10.2174/138161208786549281. Retrieved from http://www.benthams-cience.com/cpd/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D, Herrmann WM. Sleep-wake cycle, sleep-related disturbances, and sleep disorders: A chronobiological approach. Comprehensive Psychiatry. 2000;41:104–115. doi: 10.1016/s0010-440x(00)80016-4. DOI: 10.1016/S0010-440X(00)80016-4. [DOI] [PubMed] [Google Scholar]
- Lee KA. Impaired sleep. In: Carrieri-Kohlman V, Lindsey AM, West CM, editors. Pathophysiological phenomena in nursing: Human responses to illness. Saunders; St. Louis, MO: 2003. pp. 363–385. [Google Scholar]
- Lehto SM, Niskanen L, Miettola J, Tolmunen T, Viinamaki H, Mantyselka P. Serum anti-inflammatory markers in general population subjects with elevated depressive symptoms. Neuroscience Letters. 2010;484:201–205. doi: 10.1016/j.neulet.2010.08.054. DOI: 10.1016/j.neulet.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Lerner D, Amick BC, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Medical Care. 2001;39:72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Martens MP, Parker JC, Smarr KL, Hewett JE, Ge B, Slaughter JR, Walker SE. Development of a shortened Center of Epidemiological Studies Depression scale for assessment of depression in rheumatoid arthritis. Rehabilitation Psychology. 2006;51:135–139. DOI: 10.1037/0090-5550.51.2.135. [Google Scholar]
- Mayers AG, Grabau EA, Campbell C, Baldwin DS. Subjective sleep, depression and anxiety: Inter-relationships in a non-clinical sample. Human Psychopharmacology: Clinical & Experimental. 2009;24:495–501. doi: 10.1002/hup.1041. DOI: 10.1002/hup.1041. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: Contributing factors and treatment implications. Sleep Medicine Reviews. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. DOI: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Stellar E. Stress and the individual: Mechanisms leading to disease. Achives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Lu R, Sherbourne CD. The MOS 36-item Short Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-item Short-form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EITS manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Milaneschi Y, Corsi A, Pinninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: The InCHIANTI study. Biological Psychiatry. 2009;65:973–978. doi: 10.1016/j.biopsych.2008.11.011. DOI: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Irwin MR. Sleep and immunity. Current Directions in Psychological Science. 2007;16:21–25. DOI: 10.1111/j.1467-8721.2007.00468.x. [Google Scholar]
- Ohayon MM, Vecchierini M. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–989. [PubMed] [Google Scholar]
- Ovaskainen Y, Koponen H, Jokelainen J, Keinanen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Depressive symptomatology is associated with decreased interleukin-1 beta and increased interleukin-1 receptor antagonist levels in males. Psychiatry Research. 2009;167:73–79. doi: 10.1016/j.psychres.2007.12.004. DOI: 10.1016/j.psychres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Pawl J, Lee S, Clark P, Sherwood P. Sleep characteristics of family caregivers of persons with a primary malignant brain tumor. Oncology Nursing Forum. 2013;40:171–179. doi: 10.1188/13.ONF.171-179. DOI: 10.1188/13.ONF.171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: The Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being scale (FACIT-Sp) Annals of Behavioral Medicine. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. DOI: 10.1207/S15324796 ABM2401_06. [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Mood disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 5th ed. Elsevier Saunders; St. Louis, MO: 2011. pp. 1488–1500. [Google Scholar]
- Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biological Psychology. 2009;82:12–17. doi: 10.1016/j.biopsycho.2009.04.008. DOI: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. DOI: 10.1177/014662167700100306. [Google Scholar]
- Sbarra DA, Allen JJ. Decomposing depression: on the prospective and reciprocal dynamics of mood and sleep disturbances. Journalof Abnormal Psychology. 2009;118:171–182. doi: 10.1037/a0014375. DOI: 10.1037/a0014375. [DOI] [PubMed] [Google Scholar]
- Schmer C, Ward-Smith P, Latham S, Salacz M. When a family member has a malignant brain tumor: The caregiver perspective. Journal of Neuroscience Nursing. 2008;40:78–84. doi: 10.1097/01376517-200804000-00006. [DOI] [PubMed] [Google Scholar]
- Schnell O, Tonn J. Unmet medical needs in glioblastoma management and optimal sequencing of the emerging treatment options. European Journal of Clinical and Medical Oncology. 2009;1:41–49. [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. DOI: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Sculthorpe LD, Douglass AB. Sleep pathologies in depression and the clinical utility of polysomnography. The Canadian Journal of Psychiatry. 2010;55:413–421. doi: 10.1177/070674371005500704. [DOI] [PubMed] [Google Scholar]
- Shacham S. A shortened version of the Profile of Mood States. Journal of Personality Assessment. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- Sherwood PR, Given BA, Given CW, Schiff-man RE, Murman DL, Lovely M, Remer S. Predictors of distress in caregivers of persons with a primary malignant brain tumor. Research in Nursing & Health. 2006;29:105–120. doi: 10.1002/nur.20116. DOI: 10.1002/nur.20116. [DOI] [PubMed] [Google Scholar]
- Simmonds MJ. Physical function in patients with cancer: Psychometric characteristics and clinical usefulness of a physical performance test battery. Journal of Pain and Symptom Management. 2002;24:404–414. doi: 10.1016/s0885-3924(02)00502-x. DOI: 10.1016/S0885-3924(02)00502-X. [DOI] [PubMed] [Google Scholar]
- Sunseri M, Liden C, Farringdon J, Pelletier R, Safier S, Stovoris J, Vishnubhatla S. The Sensewear Armband as a sleep detection device. 2009 Retrieved from http://www.bodymedia.com/Professionals/Whitepapers.
- Swanson LM, Hoffmann R, Armitage R. Sleep macroarchitecture in depression: Sex differences. The Open Sleep Journal. 2010;3:12–18. DOI: 10.2174/1874620901003010012. [Google Scholar]
- Thompson A, Fan M, Unutzer J, Katon W. One extra month of depression: The effects of caregiving on depression outcomes in the IMPACT trial. International Journal of Geriatric Psychiatry. 2008;23:511–516. doi: 10.1002/gps.1929. DOI:10.1002/gps.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh ML, Sanders MH. Sleep in chronic kidney disease. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 5th ed. Elsevier Saunders; St. Louis, MO: 2011. pp. 1462–1472. [Google Scholar]
- Von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. The Journal of the American Geriatrics Society. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. DOI: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item Short Form Health Survey (SF-36): Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Yates JW, Chalmer B, McKegney P. Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. DOI: 10.1002/1097.-0142(19800415) 45:8<2220::AID-CNCR2820450835>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]