Abstract
Background
Competing theories of adaptation and wear-and-tear describe psychological distress patterns among family caregivers.
Purpose
This study seeks to characterize psychological distress patterns in family caregivers and identify predictors.
Methods
One hundred three caregivers of care recipients with primary malignant brain tumors were interviewed within 1, 4, 8, and 12 months post-diagnosis regarding psychological distress; care recipients were interviewed regarding clinical/functional characteristics. Group-based trajectory modeling identified longitudinal distress patterns, and weighted logistic/multinomial regression models identified predictors of distress trajectories.
Results
Group-based trajectory modeling identified high-decreasing (51.1 % of caregivers) and consistently low (48.9 %) depressive symptom trajectories, high-decreasing (75.5 %) and low-decreasing (24.5 %) anxiety trajectories, and high (37.5 %), moderate (40.9 %), and low-decreasing (21.6 %) caregiver burden trajectories. High depressive symptoms were associated with high trajectories for both anxiety and burden, lower caregivers age, income, and social support, and lower care recipient functioning.
Conclusions
Our data support the adaptation hypothesis; interventions should target those at risk for persistent distress.
Keywords: Longitudinal data, Family caregivers, Primary malignant brain tumors, Depressive symptoms, Caregiver burden, Anxiety
Introduction
In the USA, 44.4 million caregivers provide care to family members with a chronic illness [1]. Taking on the role of family caregiver is associated with psychological distress (operationalized here as depressive symptoms, anxiety, and schedule burden, because they are the most common outcomes in both descriptive and intervention caregiver research) [2]. In addition to caring for their loved ones, family caregivers often assume primary responsibility for such tasks as managing household finances, ensuring employment and insurance coverage, and childcare. Associations between high psychological distress and poor physical health in family caregivers [3–6] may diminish caregivers’ ability to provide quality care, but may be ameliorated by early intervention.
Most studies of family caregiving, particularly in oncology, have focused on cross-sectional relationships between the provision of care and distress at specific points in time, with little attention given to the pattern of caregiver distress throughout the course of caregiving. Understanding how distress changes over time is vital to designing interventions that can provide timely and appropriate support for caregivers [7, 8]. Two hypotheses attempt to explain how caregivers cope with stress over time. According to the adaptation hypothesis [9], the caregiver must learn to cope with the devastating news of the care recipient’s diagnosis; initially high levels of psychological distress decrease over time, as the caregiving demands are assimilated and caregivers adjust to the situation and added role responsibilities. According to the wear-and-tear hypothesis [10], caregivers experience low levels of psychological distress at the time of diagnosis, as they employ coping strategies and resources. However, as the care situation progresses, the chronic stress, accumulating care demands, and progression of the care recipient’s illness begin to erode these coping strategies, as well as the caregiver’s psychological well-being. This drain on the caregiver may lead to increasing feelings of depression, anxiety, and burden. These hypotheses suggest that distinct subgroups of caregivers could experience different patterns of psychological distress throughout the course of care.
Few studies have characterized the individual patterns of depressive symptoms, anxiety, or burden among caregivers over time. Commonly, longitudinal data are summarized in terms of population averages at serial time points for predefined groups (i.e., repeated measures ANOVA [11–13]), correlation coefficients between mean outcomes at various time points [14], and mean population growth curves and individual variations about these means [15]. Analyses assuming that caregivers behave homogeneously over time can produce misleading results when the population contains distinct subgroups [16]. Northouse et al. [12] analyzed the effectiveness of a family intervention for prostate cancer patients and caregivers over 4 time points, using random effects regression models to estimate individual trajectories (i.e., estimated curves over time). However, only time-specific means were reported, and trajectory groups were not identified.
Trajectory analysis, or group-based trajectory modeling, simultaneously estimates patterns over time and identifies unobserved subgroups of individuals with similar trajectories [16]. Group-based trajectory modeling is based on finite mixture modeling of unobserved subpopulations, and hypotheses regarding trajectory shape and the number of trajectory groups can be tested using maximum likelihood. Although group-based trajectory modeling is widely used, to our knowledge this technique has not been used to characterize psychological distress in caregivers of patients with primary malignant brain tumors throughout the course of caregiving. Also, few studies relate caregiver psychological distress over time to either the adaptation or the wear-and-tear hypothesis.
How caregivers respond to the sudden and traumatic diagnosis of a primary malignant brain tumor in their loved ones depends on both the caregiver’s personal and social characteristics and their care recipient’s disease characteristics. The Adapted Pittsburgh Mind-Body Model has been used to describe the relationships between factors associated with the emotional and physical stress response in caring for someone with a primary malignant brain tumor [7].
According to this conceptual model, caregiver personal and social characteristics may indicate the type of attitudes caregivers will have towards the care situation, and the amount of outside support they will have. Female caregivers who are younger, have a lower income, or are married to the care recipient have been associated with greater risk of caregiver burden, depressive symptoms, anxiety, and sleep pattern changes [2, 17–21]. Mastery describes the level of control or ability the caregiver perceives to have in order to fulfill the role and challenge of the care situation. Caregivers with high levels of mastery generally feel more prepared and ready to face the care demands and challenges ahead and are less likely to have poor psychological response to the care situation [22–24]. Social support describes the perceived availability and willingness of friends and family to provide emotional support to the caregiver, and has been associated with caregiver burden and depressive symptoms in the presence of care recipient neurological status [25, 26]. Greater social support may provide the caregiver with relief when care demands are high.
The demands of the care situation will be dependent on the care recipient’s functional, neurologic, symptom, and tumor status. Tumor grade, location, and treatment options have been associated with aggressiveness of tumor recurrence, mortality and physical, cognitive, and functional changes in the care recipient. Care recipients with lower functionality and cognitive ability generally require more help with activities of daily living and make more demands on the caregiver [7]. Care recipient disease characteristics may describe the severity of the deterioration of the care recipient, which may lead to more caregiver distress. Care recipient tumor type and neuro-cognitive status can be used as proxies for the degree of caregiving required [27].
The purpose of this analysis is to (1) characterize patterns of change over time in caregiver psychological distress throughout the course of caregiving and (2) identify the caregiver and care recipient characteristics that are associated with various caregiver psychological distress trajectories over time.
Methods
Dyads of persons with primary malignant brain tumors and their family caregivers were recruited from suburban neuro-surgery and neuro-oncology clinics in Western Pennsylvania as part of a descriptive longitudinal study (R01 CA118711, PI Sherwood). Data collection began in 2005, with 15 dyads recruited for data collection at baseline and 4 months. After NIH funding was obtained in 2007, follow-up data collection at 8 and 12 months was added to the protocol. Subsequent dyads were recruited within a month of each patient’s diagnosis, and data collection occurred at baseline, 4, 8, and 12 months after diagnosis in either private clinic examination rooms or in the caregiver’s home. Each dyad received questionnaires specific to the caregiver regarding sociodemographic characteristics, personal characteristics, psychological responses, behavioral responses, biologic responses, and overall physical health; and questionnaires specific to the care recipient regarding tumor grade, functional and neurological ability, and symptom status. Approval from the institutional review board at the University of Pittsburgh and informed consent from participants were obtained prior to participant recruitment and data collection.
Participants
Caregivers were not required to be legally related to or live with care recipients, but were required to be nonprofessional, non-paid caregivers over 21 years of age, English-speaking, and not a primary caregiver for anyone else other than children under 21 years of age. Care recipients were required to be over 21 years of age and newly diagnosed (within 1 month) with a primary malignant brain tumor verified by pathology report. When a care recipient died, the corresponding caregiver could continue to participate in this study if they wished.
Measures
Caregiver
Baseline caregiver sociodemographic information included age, gender, relationship to care recipient, income, and years of education. Income was quantified as percentage above the poverty level using the 2011 Federal Poverty [28] guidelines accounting for family size (e.g., 200 % of poverty represents a family earning twice the poverty level). Personal attributes of the caregiver included mastery and social support. Mastery was measured using the 11-item Master scale [29], which rates the degree of caregiver perception of control over the care situation on a scale of 1=strongly disagree to 4=strongly agree. Social support was measured using the Interpersonal Evaluation List [30], which rates the degree of caregiver perception of emotional support and willingness available from friends and family on a scale of 1=definitely false to 4=definitely true.
Caregiver psychological responses were self-reported. Depressive symptoms were measured using the short form for the Center for Epidemiologic Studies-Depression scale, a 10-item questionnaire (CESD-10), rating the caregiver’s experience of such symptoms as feeling “lonely,” “fearful,” and “sad” on a 4-point scale [31]. Anxiety was measured using a shortened version of the anxiety subscale of the Profile of Moods States scale [32], a 3-item questionnaire that rates a caregiver’s report of being: “on edge,” “nervous,” and “tense”, on a 5-point scale. Caregiver burden was measured using the Caregiver Reaction Assessment scale, which measures the caregiver’s perception of the positive and negative effects of providing care on five areas of life: self-esteem, schedule, finances, feelings of abandonment, and health on a 5-point scale [33]. This analysis focuses on the schedule subscale, which measures the perception of burden on the caregiver’s daily activities as a result of providing care. For each of these scales/subscales, a higher score indicates a greater level of the corresponding attribute/response.
Care Recipient
Care recipients’ sociological, demographic, and clinical characteristics were collected at baseline; disease characteristics and functional, neurological, and symptom status were collected at every follow-up time. Disease characteristics were ascertained from the care recipient’s medical records and pathology reports. Care recipient cognitive functions were assessed using the Neurobehavioral Cognitive Status Examination [34], which captures a profile of cognitive ability in the domains of level of consciousness, orientation, attention, language (comprehension, naming, repetition), constructional ability, memory, calculations, and reasoning (similarities and judgment). Lower scores indicate neurobehavioral dysfunction.
Descriptive Analysis
For each caregiver, time-specific depression, anxiety, and schedule burden scores were plotted from the time of diagnosis to 12 months, using Stata version 9.0. Because follow-up visits were conducted within 2 weeks of each caregiver’s scheduled follow-up dates, time was defined as the number of months between the baseline measurement and each follow-up visit.
Trajectory Model Selection
A separate censored normal trajectory model was estimated for each measure of psychological distress using SAS Proc Traj [35]. Caregivers with only single time point measurements were excluded from the model to preserve the longitudinal aspect of the analyses. Model selection involved the iterative estimation of (1) the number of trajectory groups and (2) the shape/order of each trajectory group using both statistical [36] and non-statistical considerations [37].
Statistical criteria for ascertaining the best fitting model included four log-likelihood statistics (Akaike’s Information Criterion [AIC] [38], Bayesian Information Criterion [BIC] [39], the sample-size adjusted BIC [ssBIC] [40], and the consistent AIC [CAIC] [41]) which include a penalty for model complexity, and three classification statistics (classification likelihood criterion (CLC), integrated classification likelihood adjusting the BIC (ICL-BIC) [42], and entropy [43]). Smaller values of AIC, BIC, ssBIC, and CAIC denote better models. Entropy is an index used in classification accuracy based on posterior probabilities, with higher values denoting better classification. Currently, there is no commonly accepted single gold standard model fit statistic, but suggestions from simulation studies exist [36, 44].
Other criteria included non-overlapping confidence intervals, reasonable sample sizes in each identified trajectory group, and distinct average posterior probabilities across groups. Subjective judgment is important in trajectory model selection because the aim is to identify a useful and parsimonious model [37]. Depending on the outcome measure, either a clinically relevant cutoff or previous literature guided the number of trajectory groups chosen.
Associations Between Trajectory Groups
For each outcome, associations between trajectory groups were assessed using chi-square tests. To account for uncertainty in the assignment to trajectory groups, cross-tabulations were weighted by their average posterior probabilities. p values≤0.05 were considered to be statistically significant throughout.
Predictors of Trajectory Group Membership
Predictors of trajectory group membership were identified using weighted binary logistic regression for outcomes with two trajectory groups and weighted multinomial logistic regression for outcomes with more than two trajectory groups. Caregiver risk factors included age, gender, relationship to care recipient (spouse vs. other), years of education, income, and baseline mastery and social support scores. Care recipient risk factors included tumor type (astrocytoma I–II, oligodendroglioma, other versus astrocytoma III–IV) and cognitive domains. To reduce collinearity between predictors, continuous predictors were centered at their respective means.
The logistic and multinomial regression models were estimated including all caregiver and care recipient characteristics listed in Table 1, while treating caregiver age, gender, years of education, and care recipient tumor type as covariates. If needed, continuous predictors were log-transformed to correct for non-normal distributions. Missing baseline covariate data were multiply imputed using Markov Chain Monte Carlo. Model selection was conducted using all possible subsets regressions [45] selecting the lowest Mallow’s Cp criterion [46]. Multicollinearity was evaluated using variance inflation factor scores. In a sensitivity analysis, the final models were refit excluding influential cases (defined as deviance deletion >3.84). These models were fit using SAS version 9.2.
Table 1.
Baseline summary of caregivers and care recipients (N=103)
| Characteristics | M | SD |
|---|---|---|
| Caregiver | ||
| Age | 51.4 | 11.4 |
| Years of education | 14.3 | 2.5 |
| Income | 312.5 | 193.7 |
| Social support | 35.5 | 4.5 |
| Mastery | 20.9 | 2.8 |
| Care recipient | ||
| Tumor type (N, %) | ||
| Astrocytoma I–II, ligodendroglioma, other | 34 | 33.0 |
| Astrocytoma III–IV | 69 | 67.0 |
| Cognitive domains | ||
| Orientation | 11.6 | 1.1 |
| Attention | 6.9 | 1.6 |
| Language | 11.4 | 1.1 |
| Comprehension | 5.6 | 0.7 |
| Repetition | 11.5 | 1.2 |
| Naming | 7.7 | 0.8 |
| Judgment | 4.5 | 1.3 |
| Reasoning | 6.4 | 1.3 |
| Similarities | 6.8 | 1.6 |
| Constructional ability | 4.5 | 1.7 |
| Memory | 7.6 | 3.4 |
| Calculations | 3.6 | 0.9 |
Caregiver bereavement was evaluated as a potential covariate by (1) treating bereavement status as a time-varying covariate using Proc Traj and by (2) comparing the association between bereavement status and group membership at each follow-up time using weighted chi-square tests. A dropout analysis also was conducted comparing trajectory group membership between those who completed and those who dropped out of the study using weighted chi-square tests.
Results
Sample
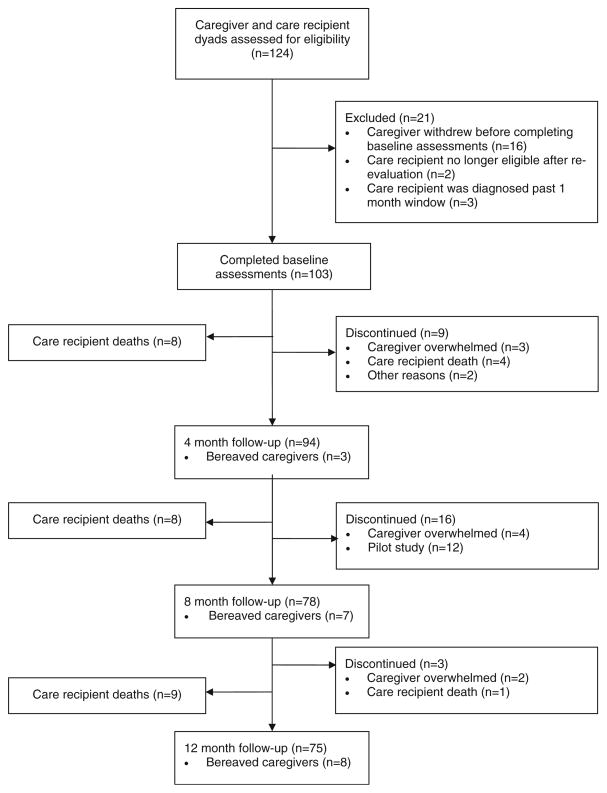
This analysis includes a total of 124 caregiver-care recipient dyads (See Fig. 1 for flow chart of study design). Of these, 16 dyads withdrew before completion of their baseline interview, 2 were no longer eligible once the care recipient’s tumor diagnosis was re-evaluated by a secondary pathology laboratory, and 3 were diagnosed outside of the 1-month window.
Fig. 1.
Flow diagram of study design
The remaining 103 caregivers completed baseline assessments. Among these dyads, 25 care recipients died during the study period (8 by 4 months, 8 by 8 months, and 9 by 12 months). At 4 months, 94 caregivers completed follow-up assessments (including 3 bereaved caregivers) and 9 caregivers dropped out (3 due to feeling overwhelmed, 4 due to bereavement, and 2 for other reasons). At 8 months, 78 caregivers completed follow-up assessments (including 7 bereaved caregivers), 4 caregivers dropped out due to feeling overwhelmed, and 12 caregivers who participated in the pilot study were not followed past 4 months. At 12 months, 75 caregivers completed assessments (including 8 bereaved caregivers) and 3 caregivers dropped out (2 due to feeling overwhelmed and 1 due to bereavement).
The trajectory models for both the depressive symptoms and anxiety outcomes included a total of 94 caregivers who provided baseline and at least one follow-up measurement. For the caregiver reaction assessment outcome, a total of 88 caregivers were included in the trajectory model: 6 caregivers with measurements at a single time point were excluded from the final model (3 were bereaved after baseline and did not receive the schedule burden subscale, and 3 did not complete their 4-month follow-up and were bereaved by 8 months).
Socio-Demographic and Clinical Characteristics
The majority of caregivers was female (n=76, 73.8 %) and caring for spouses (n=77, 74.8 %). As shown in Table 1, the average age of caregivers was 51.4 years (SD=11.4; range, 21–77), and caregivers completed 14.3 years of education on average (SD=2.7, range, 5–23). The majority of care recipients was diagnosed with an astrocytoma grade III–IV (n=69, 67.0 %).
Descriptive Analysis
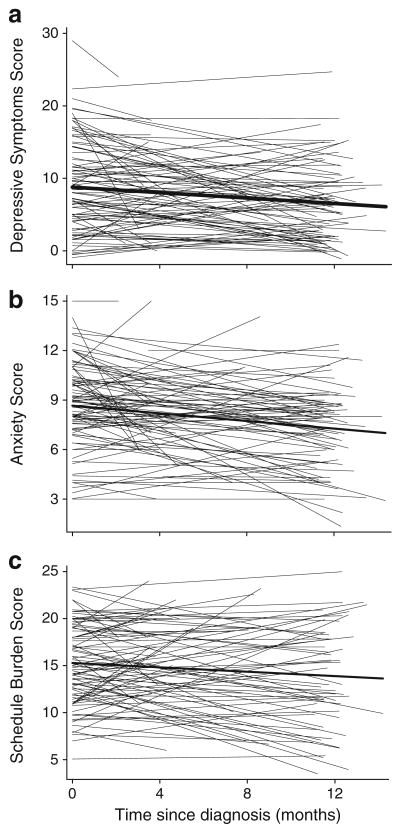
Cronbach’s alpha reliability coefficients for depressive symptoms, anxiety, and schedule burden scores were .85, .91 and .76, respectively. “Spaghetti” plots of the predicted slopes for each psychological outcome scale from baseline to 12 months revealed diverse patterns of individual change, i.e., increasing, decreasing, and flat trajectories over time (Fig. 2). For each scale, the thick line represents the population average decreases over time.
Fig. 2.
Predicted (a) depressive symptoms, (b) anxiety, and (c) schedule burden scores for each caregiver over time, based on random effects models. Each population average (thick line) decreases over time
Trajectory Analysis
Depressive Symptoms
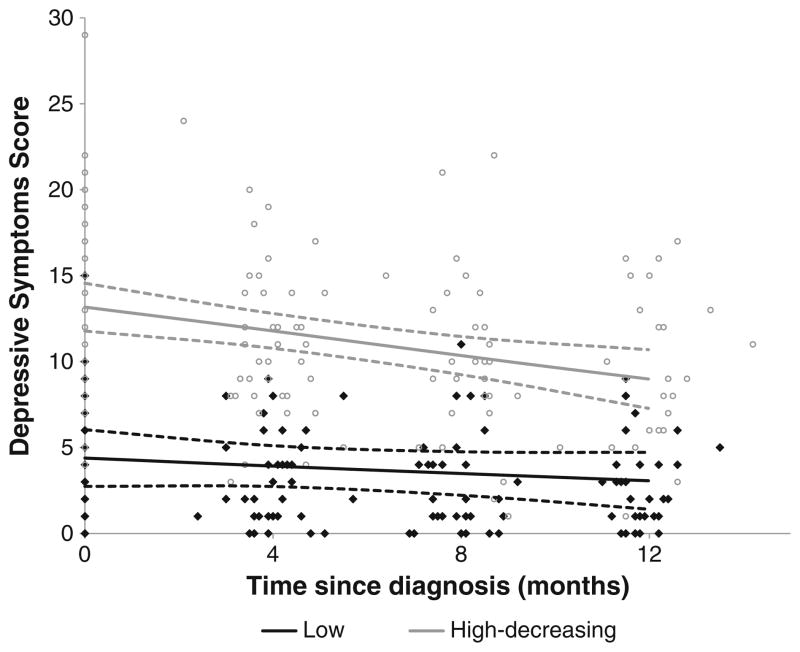
A two-group linear trajectory model best fit caregiver depressive symptoms (Fig. 3; Table 2). Figure 3 shows the scatter of depressive symptoms scores by time from diagnosis in months. About half (n=48, 5 l.1 %) of the caregivers were assigned to the high-decreasing depressive symptoms group. The “average” caregiver in the high-decreasing group has a depressive symptoms score of 13.25 at baseline that significantly decreases by approximately 0.28 points per month. The predicted depressive symptoms score of 9.96 at 1 year remains above the threshold for clinical depression (CESD-10 score ≥8). The other 48.9 % of the caregivers follow a trajectory that starts low at diagnosis and remains low, with no significant change over time.
Fig. 3.
Trajectory plots of depressive symptoms from diagnosis to 1 year. Dots and diamonds denote the individual scores. For each trajectory group, the solid line represents the predicted trajectory and the dashed lines represent the 95 % confidence interval
Table 2.
Summary of group-based trajectory analysis for each psychological distress outcome
| Trajectory group | Intercept B (SE) | Slope B (SE) | Predicted score at 12 months | Slope B p |
|---|---|---|---|---|
| Depressive symptoms (Range, 0–30) | ||||
| Low | 3.83 (0.68) | −0.08 (0.09) | 2.93 | .39 |
| High-decreasing | 13.25 (0.69) | −0.28 (0.09)** | 9.96 | .001 |
| Anxiety (Range, 3–15) | ||||
| Low-decreasing | 5.95 (0.44) | −0.16 (0.06)** | 4.02 | .004 |
| High-decreasing | 9.66 (0.23) | −0.01 (0.03)** | 8.47 | .002 |
| Schedule burden (Range, 5–25) | ||||
| Low-decreasing | 10.73 (0.61) | −0.34 (0.09)** | 6.71 | <.001 |
| Moderate | 13.88 (0.51) | −0.01 (0.07) | 13.7 | .86 |
| High | 18.91 (0.50) | 0.07 (0.07) | 19.7 | .35 |
The slope reflects the change per month
p<.05;
p<.005
Anxiety
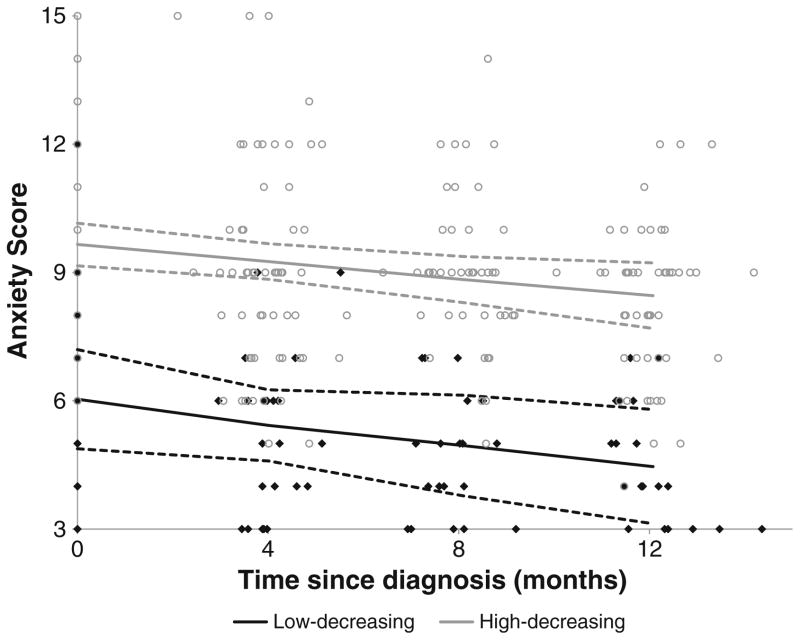
A two-group linear trajectory model best fit caregiver anxiety over time (Fig. 4; Table 2). The low-decreasing group represents the 24.5 % (n=23) of caregivers who expressed low levels of anxiety at baseline and improved significantly over time (slope B=−0.16, p=.001). The majority (n=71, 75.5 %) of caregivers were assigned to the high-decreasing group, who reported high anxiety at baseline and significantly improved by 12 months (slope B=−0.1; p=.003). Because no cutoff point has been established for being at risk for a clinical anxiety disorder, the clinical significance of these changes cannot be ascertained.
Fig. 4.
Trajectory plots of anxiety from diagnosis to 1 year. Dots and diamonds denote the individual scores. For each trajectory group, the solid line represents the predicted trajectory and the dashed lines represent the 95 % confidence interval
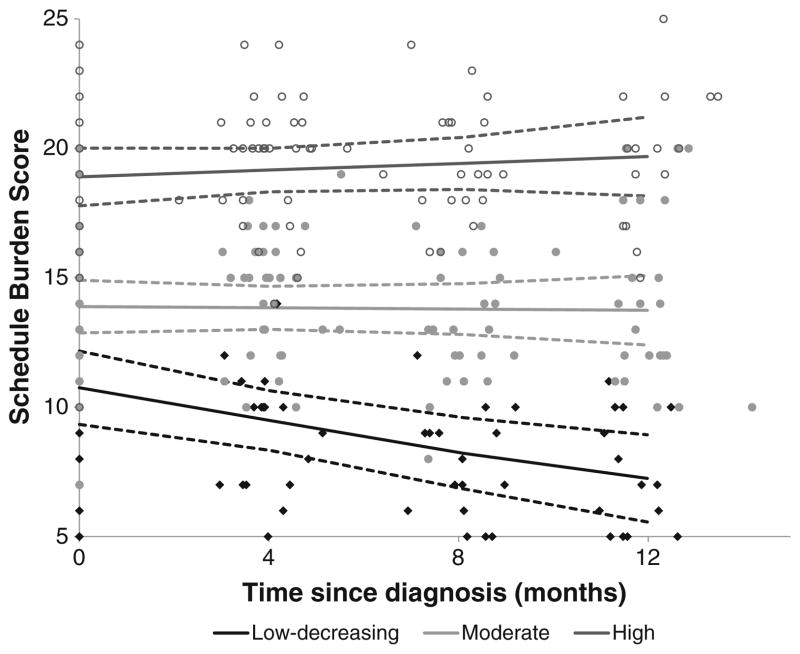
Schedule Burden
A three-group linear trajectory model best fit caregiver schedule burden scores over time (Fig. 5; Table 2). The low-decreasing burden group (n=19, 21.6 %) reported low burden at baseline with a significant decrease over time (slope B=−0.03; p=.002). Approximately 41 % (n=36) of caregivers reported moderate scores at baseline and 37.5 % (n=33) reported high scores; in both groups these scores did not change significantly over time (p=0.86 and p=0.35, respectively).
Fig. 5.
Trajectory plots of schedule burden scores from diagnosis to 1 year. Dots and diamonds denote the individual scores. For each trajectory group, the solid line represents the predicted trajectory and the dashed lines represent the 95 % confidence interval
Associations Between Trajectory Groups
A strong pair-wise association was observed between trajectory group membership for depressive symptoms and anxiety (χ2(1, N=94)=31.80, p<.001), with all caregivers in the high-decreasing depressive symptoms group being in the high-decreasing anxiety group (Table 3). Caregivers in the low depressive symptoms trajectory group were approximately evenly split between the high-decreasing and low-decreasing anxiety trajectory groups. There were no significant associations between trajectory group membership for schedule burden and depressive symptoms (χ2(2, N=88)= 3.93, p=.14) or anxiety (χ2(2, N=88)=4.62, p=.10).
Table 3.
Pair-wise associations between trajectory group memberships for depressive symptoms, anxiety and schedule burden
| Trajectory group | Schedule burden
|
Anxiety
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low-decreasing
|
Moderate
|
High
|
Low-decreasing
|
High-decreasing
|
||||||
| n | % | n | % | n | % | n | % | n | % | |
| Depressive symptoms | ||||||||||
| Low | 11 | 25.0 | 21 | 47.7 | 12 | 27.3 | 23 | 50.0 | 23 | 50.0 |
| 26.0 | 46.6 | 27.5 | 51.6 | 48.4 | ||||||
| High-decreasing | 8 | 18.2 | 15 | 34.1 | 21 | 47.7 | 0 | 0.0 | 48 | 100.0 |
| 17.6 | 33.4 | 49.1 | 0.0 | 100.0 | ||||||
| χ2(2, N=88)=3.93, p=.14 | χ2(1, N=94)=31.77, p<.001 | |||||||||
| χ2(2, N=82.04)=4.06, p=.13 | χ2(1, N=89.67)=31.84, p<.001 | |||||||||
| Anxiety | ||||||||||
| Low-decreasing | 8 | 34.8 | 10 | 43.5 | 5 | 21.7 | – | – | – | – |
| 34.9 | 43.3 | 21.8 | ||||||||
| High-decreasing | 11 | 16.9 | 26 | 40.0 | 28 | 43.1 | – | – | – | – |
| 16.9 | 38.6 | 44.5 | ||||||||
| χ2(2, N=88)=4.62, p=.10 | ||||||||||
| χ2(2, N=82.72)=4.57, p=.11 | ||||||||||
Un-weighted and weighted row percentages and χ2 statistics are shown, respectively
Predictors of Trajectory Group Membership
Depressive Symptoms
Relationship to care recipient and caregiver gender were significantly associated (Fisher’s exact test p=.02), caregivers of spouses were more likely to be female (data not shown). Relationship to care recipient was therefore excluded from all predictive analyses to reduce collinearity. The final logistic regression model included income, social support and care recipient calculations score, in addition to caregiver age, gender, education and care recipient tumor type. Caregivers were more likely to belong to the high depressive symptoms trajectory group with lower income (odds ratio (OR)=0.34, p=.02), and lower social support (OR=0.85, p=.02). A borderline association was found between younger age and belonging to the high trajectory group (OR=0.96, p=.06, see Table 4).
Table 4.
Predictors of high depressive symptoms (vs. low) and high anxiety (vs. low) based on logistic regression models, and high (vs. low) and moderate (vs. low) schedule burden based on a multinomial regression model
| Characteristics | Depressive symptoms | Anxiety | Schedule burden
|
|||||
|---|---|---|---|---|---|---|---|---|
| High vs. low | Moderate vs. low | |||||||
|
|
|
|
|
|||||
| OR | p | OR | p | RR | p | RR | p | |
| Age | 0.96 | .06 | 0.95 | .08 | 1.00 | .95 | 0.98 | .42 |
| Gender (male) | 0.85 | .79 | 0.40 | .12 | 0.33 | .21 | 1.09 | .92 |
| Education (years) | 0.84 | .11 | 0.90 | .37 | 1.22 | .18 | 0.94 | .69 |
| Tumor type (astrocytoma III–IV)a | 1.84 | .25 | 0.89 | .84 | 4.09* | .04 | 2.47 | .16 |
| Incomeb | 0.34* | .02 | ||||||
| Social support | 0.85* | .02 | 0.88 | .09 | ||||
| Care recipient cognitive domains | ||||||||
| Orientation (0–12) | 0.46 | .26 | ||||||
| Attention (0–8) | 0.91 | .67 | ||||||
| Language (0–12) | 0.60 | .28 | ||||||
| Constructional ability (0–6) | 0.48* | 0.03 | 0.53 | .05 | ||||
| Calculations (0–4) | 1.63 | .13 | 1.54 | .32 | ||||
Score ranges are listed in parentheses for neuropsychological tests. Memory, reasoning and mastery were not significant predictors in any model considered
OR odds ratio, RR risk ratio
p<.05
vs. astrocytoma I–II, oligodendroglioma, other
Income is log-transformed
Anxiety
The final logistic regression model included social support, and care recipient orientation, attention, language and calculations scores. There were no significant associations between caregiver and care recipient characteristics and belonging to the high anxiety group. Younger caregivers (OR=0.95, p=.08) and lower social support (OR=0.88, p=.09) were somewhat more likely to belong in the high anxiety group.
Schedule Burden
The final multinomial logistic model selected included care recipient constructional ability score while adjusting for covariates to predict schedule burden trajectory group. Care recipients who perform poorly on the constructional ability test were more likely to belong to the high (risk ratio (RR)= 0.48, p=.03) or moderate (RR=0.53, p=.05) schedule burden trajectory group than the low trajectory group. Additionally, caregivers of care recipients diagnosed with an astrocytoma grade III–IV were significantly more likely to belong to the high schedule burden group than the low burden group (RR=4.09, p=.04). This association was not reflected in the moderate schedule burden trajectory group.
Bereavement
A total of 18 caregivers were bereaved in this analysis, 3 at 4 months, 7 at 8 months, and 8 at 12 months. The effect of bereavement as a time-varying covariate was significant in both trajectory groups for the depressive symptoms trajectory model but not for the anxiety trajectory model (data not shown). Bereavement was not tested in the schedule burden trajectory model since this questionnaire is not given to bereaved caregivers. Testing bereavement at each time point against the trajectory group using Fisher’s exact tests showed non-significant results. Trajectory models were also rerun after censoring bereaved caregivers. These trajectory models showed little change from the original models without censoring, since SAS Proc Traj imputes for missing values. Therefore, no adjustment for bereavement was included in the prediction models.
Sensitivity Analyses
Sensitivity analyses of potentially influential observations were conducted for depressive symptoms (n=2), anxiety (n=4), and schedule burden (n=11); a total of 16 caregivers. Similar results were obtained when these observations were excluded. Dropout analyses revealed no significant differences in trajectory group membership between those who completed versus those who dropped out of the study for depressive symptoms, anxiety, and schedule burden outcomes.
Discussion
Group-based trajectory modeling revealed patterns that have not been fully explored in family caregiving. There appear to be distinct subsets of caregivers at risk; some caregivers display persistent distress throughout the year following diagnosis, while another group of caregivers did not display high levels of distress as a result of providing care.
Approximately one half of the family caregivers experienced low levels of depressive symptoms upon care recipient diagnosis that remained low across the disease trajectory. The remaining half reported scores above the established cutoff for being at risk for clinical depression at the time of care recipient diagnosis and remained above the cutoff, despite some improvement over time. Anxiety scores showed a similar pattern, despite the lack of an established clinical cutoff. Caregivers reporting high levels of depressive symptoms also report high levels of anxiety. However, caregivers reporting low levels of depressive symptoms evenly report both low and high levels of anxiety.
Schedule burden scores behaved differently, with caregivers who scored low at baseline continuing to decrease significantly and caregivers who scored moderate to high at baseline experiencing no significant change over time. Our results lend support to the adaptation hypothesis, suggesting most caregivers learn to adjust and cope with the demands of the care situation over time. However, some caregivers do not adapt over time, but continue to experience levels of moderate to high feelings of schedule burden.
Contrary to some other research in the field [47–49], we did not find a strong positive association between caregiver burden with depressive symptoms and anxiety. Interestingly, Given et al. [50] reported only a modest correlation of .14 between schedule burden and depressive symptoms in cancer caregivers of patients at end of life. The lack of an association is likely due to the measures chosen for the study. A large number of tools measuring caregiver burden are uni-dimensional and focus on the care recipient’s cognitive dysfunction, which has shown a clear association with caregiver burden. Unlike other studies, we measured the care recipient’s cognitive status with neuropsychological tests. Our measure of caregiver burden focuses on the degree to which providing care disrupts the caregiver’s schedule. It may be that this dimension of burden has a lower association with depressive symptoms or precedes depressive symptom in some way. Further research is required to parse out the way in which varying dimensions of burden contribute to depressive symptoms and anxiety.
To our knowledge, no other caregiver study has used group-based trajectory modeling of longitudinal caregiving data to estimate distinct trajectories of depressive symptoms, anxiety scores, and caregiver burden scores over time in caregivers of persons with a primary malignant brain tumor. Recent studies have used growth curve modeling [51, 52] and generalized estimating equation models [53], both of which characterize patterns over time for subgroups defined a priori. Growth curve modeling may be appropriate for monotonic longitudinal data (i.e., language development) but may not be appropriate in situations where the pattern of change over time is non-monotonic or unknown [54].
Our findings suggest that caregivers with higher income and social support are more likely to report fewer depressive symptoms over time. These findings are consistent with previous literature [7]. Contrary to our expectations, our analysis failed to identify significant associations between risk factors that have been shown to be associated with depressive symptoms and anxiety in caregivers (e.g., gender, relationship to care recipient, and care recipient tumor type). A trend of association between caregiver age and anxiety trajectory group suggests that younger caregivers may be more likely to report high anxiety over time. Younger caregivers have been shown to be more likely to be highly anxious, regardless of care recipient health [55, 56].
Schedule burden was predicted only by care recipient characteristics, specifically tumor type and cognitive function. Care recipients with more aggressive tumor types will most likely have more frequent doctor’s visits, treatment appointments and shorter survival time, which may explain why caregivers feel burden on their schedules. In addition, poor performance on constructional ability (i.e., the inability to assemble shapes to copy a two-dimensional drawing) suggests that care recipients have both cognitive and functional limitations, specifically in the use of tools, and will be more likely to require help from the caregiver in performing activities of daily living, such as dressing, bathing, and eating. This places a greater burden on the caregiver since the care recipient requires constant attention and help. Clinicians and other health-related professionals commonly use the Neurobehavioral Cognitive Status Examination as a brief screening tool to capture a profile of specific abilities and disabilities of the patient. Therefore, these scores may not be equivalent to a formal neuro-cognitive functioning assessment.
Longitudinal data that describe the natural response to care demands are vital for designing and implementing interventions that target specific caregivers at risk for distress. Caregivers have been shown to benefit more from personalized and targeted interventions; however, current healthcare systems may lack the amount of time, personnel and financial resources to offer such interventions to help individuals adjust to the psychological demands of caregiving [52, 57, 58]. Our data suggests caregivers follow the adaptation hypothesis upon diagnosis of a primary malignant brain tumor in their loved one and identified a number of risk factors at baseline associated with trajectory group membership. Caregivers at risk of experiencing high distress can be identified early on, suggesting an early intervention facilitating caregiver ability to adapt to the caregiving situation would be preferable to a later-stage intervention.
In our population of caregivers of persons with a primary malignant brain tumor, early intervention could be targeted to the caregivers upon diagnosis of their care recipient. Group-based trajectory analyses and plots revealed caregivers who report high levels of depressive symptoms (51.1 %), anxiety (75.5 %), and/or schedule burden (37.5 %) at baseline may not clinically improve by 1 year. Without having to follow these caregivers for a year, clinicians can assess caregivers for psychological distress upon diagnosis and identify those in need of intervention if they perform within the 95 % confidence range of scores in the high trajectory groups. These distinct trajectory groups can be used as a screening tool to identify caregivers who may be at increased risk of psychological distress over time.
Our analysis also indicates that some caregivers do not display negative psychological responses as a result of providing care. While these caregivers still may have needs related to the care situation, they may be able to employ adequate coping skills without outside intervention. These findings could help to explain the inconsistent effect sizes across prior intervention studies.
Since depressive symptoms and anxiety tend to be associated with certain personality types, such as neuroticism, these patterns of distress may also be present in other caregiving situations. However, since the onset of illness and length of the care situation differ greatly among caregiving populations (e.g., dementia, stroke, traumatic brain injury, and cancer) the analysis should be replicated in those caregiving situations to determine whether they follow similar trajectory patterns.
Limitations
One limitation of this analysis is that the small number of measurement time points affects trajectory shape, group membership, and peaks. Because follow-up assessments in this study spanned 1 year, only linear trajectory shapes were considered. In future research, a prospective validation component could test whether trajectory group membership can be extrapolated to new caregiver and care recipient dyads, and assess whether the patterns observed within 12 months persist over longer periods of time.
Potential selection bias may have also occurred in this analysis. Sixteen caregivers dropped out of the study after consenting but before baseline measurements could be assessed. These caregivers could be more highly stressed within the first month of their care recipient diagnosis than others. If these caregivers tend to be those who are experiencing more caregiver distress, then our sample would be biased in favor of caregivers who are more able to handle the stress of caregiving and participating in a longitudinal study. However, we observed no difference in psychological distress by whether caregivers stayed or left the study throughout the follow-up period.
Conclusions
Caregivers in this study typically followed a trajectory of decreasing psychological distress over 12 months following diagnosis, lending support to the adaptation hypothesis. Our findings indicate group-based trajectory modeling is an effective technique to estimate distinct trajectories of longitudinal caregiver psychological distress, and when coupled with predictive models to examine associated risk factors can lead to the development of targeted interventions and screening tools customized to caregivers most in need.
Acknowledgments
Support for this research was provided by the National Cancer Institute (R01 CA118711-01A1) as part of the Mind-Body Interactions in Neuro-Oncology Family Caregivers study, Principal Investigator Paula R. Sherwood. The authors wish to thank the Innovations in Cancer Care team for data collection and management, and our participants who donated their time and insight to this project.
Footnotes
Conflict of Interest Statement The authors have no conflict of interest to disclose.
Contributor Information
Chien-Wen J. Choi, Email: cjk28@pitt.edu, Department of Acute and Tertiary Care School of Nursing, University of Pittsburgh, Pittsburgh, PA, USA. School of Nursing, University of Pittsburgh, 3500 Victoria Street, Suite 336, Pittsburgh, PA 15217, USA.
Roslyn A. Stone, Department of Biostatistics Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
Kevin H. Kim, Department of Psychology in Education School of Education, University of Pittsburgh, Pittsburgh, PA, USA.
Dianxu Ren, Department of Health and Community Systems School of Nursing Department of Biostatistics Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
Richard Schulz, University Center for Social and Urban Research, University of Pittsburgh, Pittsburgh, PA, USA.
Charles W. Given, Department of Family Medicine, College of Human Medicine, Michigan State University, East Lansing, MI, USA.
Barbara A. Given, College of Nursing, Michigan State University, East Lansing, MI, USA.
Paula R. Sherwood, Department of Acute and Tertiary Care, School of Nursing Department of Neurosurgery, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.The National Alliance for Caregiving. AARP: Caregiving in the US. Washington, DC: NAC and AARP; 2005. [Google Scholar]
- 2.Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2003;58:112–128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- 3.Carter PA. Caregivers’ descriptions of sleep changes and depressive symptoms. Oncol Nurs Forum. 2002;29:1277–1283. doi: 10.1188/02.ONF.1277-1283. [DOI] [PubMed] [Google Scholar]
- 4.Picot SJ, Genet CA. Behavior upset in hypertensive and normotensive black female caregivers. Clin Excell Nurse Pract. 1998;2:23–29. [PubMed] [Google Scholar]
- 5.Schulz R, Beach SR. Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the caregiver health effects study. Psychology & Aging. 2000;15:607–616. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood PR, Given BA, Donovan H, et al. Guiding research in family care: A new approach to oncology caregiving. Psychooncology. 2008;17:986–996. doi: 10.1002/pon.1314. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors: Across the trajectory of the illness. Cancer. 2008;112:2556–2568. doi: 10.1002/cncr.23449. [DOI] [PubMed] [Google Scholar]
- 9.Helson H. Adaptation-level theory: An Experimental and Systematic Approach to Behavior. Harper & Row; 1964. [Google Scholar]
- 10.Townsend A, Noelker L, Deimling G, Bass D. Longitudinal impact of interhousehold caregiving on adult children’s mental health. Psychological Aging. 1989;4:393–401. doi: 10.1037//0882-7974.4.4.393. [DOI] [PubMed] [Google Scholar]
- 11.Hudson P, Quinn K, Kristjanson L, et al. Evaluation of a psycho-educational group programme for family caregivers in home-based palliative care. Palliat Med. 2008;22:270–280. doi: 10.1177/0269216307088187. [DOI] [PubMed] [Google Scholar]
- 12.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 13.Collinge W, Kahn J, Yarnold P, Bauer-Wu S, McCorkle R. Couples and cancer: Feasibility of brief instruction in massage and touch therapy to build caregiver efficacy. J Soc Integr Oncol. 2007;5:147–154. doi: 10.2310/7200.2007.013. [DOI] [PubMed] [Google Scholar]
- 14.Carter PA. A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer Nursing. 2006;29:95–103. doi: 10.1097/00002820-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 16.Nagin DS. Group-based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 17.Bookwala J, Schulz R. A comparison of primary stressors, secondary stressors, and depressive symptoms between elderly caregiving husbands and wives: The Caregiver Health Effects Study. Psychol Aging. 2000;15:607–616. doi: 10.1037//0882-7974.15.4.607. [DOI] [PubMed] [Google Scholar]
- 18.Gaugler JE, Hanna N, Linder J, et al. Cancer caregiving and subjective stress: A multi-site, multi-dimensional analysis. Psychooncology. 2005;14:771–785. doi: 10.1002/pon.916. [DOI] [PubMed] [Google Scholar]
- 19.Nijboer C, Tempelaar R, Triemstra M, van den Bos GA, Sanderman R. The role of social and psychologic resources in caregiving of cancer patients. Cancer. 2001;91:1029–1039. [PubMed] [Google Scholar]
- 20.Pinquart M, Sorensen S. Differences between caregivers and non-caregivers in psychological health and physical health: A meta-analysis. Psychol Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 21.Pinquart M, Sorensen S. Gender differences in caregiver stressors, social resources, and health: An updated meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2006;61:33–45. doi: 10.1093/geronb/61.1.p33. [DOI] [PubMed] [Google Scholar]
- 22.Bookwala J, Schulz R. The role of neuroticism and mastery in spouse caregivers’ assessment of and response to a contextual stressor. Journal of Gerontology Series B, Psychological sciences and social sciences. 1998;53:155–164. doi: 10.1093/geronb/53b.3.p155. [DOI] [PubMed] [Google Scholar]
- 23.Li LW, Seltzer MM, Greenberg JS. Change in depressive symptoms among daughter caregivers: An 18-month longitudinal study. Psychological Aging. 1999;14:206–219. doi: 10.1037//0882-7974.14.2.206. [DOI] [PubMed] [Google Scholar]
- 24.Skaff M, Pearlin L, Mullan J. Transitions in the caregiving career: Effects on the sense mastery. Psychological Aging. 1996;11:247–257. doi: 10.1037//0882-7974.11.2.247. [DOI] [PubMed] [Google Scholar]
- 25.Pinquart M, Sorensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: A meta-analysis. Gerontologist. 2005;45:90–106. doi: 10.1093/geront/45.1.90. [DOI] [PubMed] [Google Scholar]
- 26.Ergh TC, Hanks RA, Rapport LJ, Coleman RD. Social Support Moderates Caregiver Life Satisfaction Following Traumatic Brain Injury. Journal of Clinical and Experimental Neuropsychology. 2003;25:1090–1101. doi: 10.1076/jcen.25.8.1090.16735. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood P, Given B, Given C, Schiffman R, Murman D, Lovely M. Caregivers of persons with a brain tumor: A conceptual model. Nurs Inq. 2004;11:43–53. doi: 10.1111/j.1440-1800.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Annual Update of the HHS Poverty Guidelines. Jan 20, 2011. [Google Scholar]
- 29.Pearlin L, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- 30.Sarason IG, Sarason BR North Atlantic Treaty Organization, Scientific Affairs Division. Social Support: Theory, Research, and Applications. Springer; 1985. [Google Scholar]
- 31.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 32.Usala PD, Hertzog C. Measurement of affective states in adults. Evaluation of an adjective rating scale instrument. Res Aging. 1989;11:403–426. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]
- 33.Given CW, Given B, Stommel M, Collins C, King S, Franklin S. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 34.Kiernan RJ, Mueller J, Langston JW, Van Dyke C. The Neurobehavioral Cognitive Status Examination: A brief but quantitative approach to cognitive assessment. Ann Intern Med. 1987;107:481–485. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 35.Jones BL, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- 36.Henson JM, Reise SP, Kim KH. Detecting mixtures from structural model differences using latent variable mixture modeling: A comparison of relative model fit statistics. Structural Equation Modeling. 2007;14:202–226. [Google Scholar]
- 37.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 38.Akaike H. A new look at statistical model identification. IEEE transactions on automatic control. 1974;6:716–723. [Google Scholar]
- 39.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 40.Sclove LS. Application of model-selection criteria to some problems in multivariate analysis. Psychometrics. 1987;52:333–343. [Google Scholar]
- 41.Bozdogan H. Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- 42.McLachlan G, Peel D. Finite Mixture Models. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 43.Ramaswarmy V, DeSarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Science. 1997;12:103–124. [Google Scholar]
- 44.Nylund KL, Tihomir A, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- 45.Miller A. Subset Selection in Regression. Boca Raton, FL: Chapman and Hall; 2002. [Google Scholar]
- 46.Mallows C. Some comments on Cp. Technometrics. 2000;42:87–94. [Google Scholar]
- 47.Caap-Ahlgren M, Dehlin O. Factors of importance to the caregiver burden experienced by family caregivers of Parkinson’s disease patients. Aging Clin Exp Res. 2002;14:371–377. doi: 10.1007/BF03324464. [DOI] [PubMed] [Google Scholar]
- 48.Grunfeld E, Coyle D, Whelan T, et al. Family caregiver burden: Results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ. 2004;170:1795–1801. doi: 10.1503/cmaj.1031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morimoto T, Schreiner AS, Asano H. Caregiver burden and health-related quality of life among Japanese stroke caregivers. Age Ageing. 2003;32:218–223. doi: 10.1093/ageing/32.2.218. [DOI] [PubMed] [Google Scholar]
- 50.Given B, Wyatt G, Given C, et al. Burden and depression among caregivers of patients with cancer at the end of life. Oncol Nurs Forum. 2004;31:1105–1117. doi: 10.1188/04.ONF.1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaugler JE, Davey A, Pearlin LI, Zarit SH. Modeling caregiver adaptation over time: The longitudinal impact of behavior problems. Psychol Aging. 2000;15:437–450. doi: 10.1037//0882-7974.15.3.437. [DOI] [PubMed] [Google Scholar]
- 52.Jang Y, Clay OJ, Roth DL, Haley WE, Mittelman MS. Neuroticism and longitudinal change in caregiver depression: Impact of a spouse-caregiver intervention program. Gerontologist. 2004;44:311–317. doi: 10.1093/geront/44.3.311. [DOI] [PubMed] [Google Scholar]
- 53.Tang ST, Li CY, Chen CC. Trajectory and determinants of the quality of life of family caregivers of terminally ill cancer patients in Taiwan. Qual Life Res. 2008;17:387–395. doi: 10.1007/s11136-008-9316-7. [DOI] [PubMed] [Google Scholar]
- 54.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 55.Blood GW, Simpson KC, Dineen M, Kauffman SM, Raimondi SC. Spouses of individuals with laryngeal cancer: Caregiver strain and burden. J Commun Disord. 1994;27:19–35. doi: 10.1016/0021-9924(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 56.Gilbar O. Gender as a predictor of burden and psychological distress of elderly husbands and wives of cancer patients. Psychooncology. 1999;8:287–294. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<287::AID-PON385>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 57.Knight BG, Lutzky SM, Macofsky-Urban F. A meta-analytic review of interventions for caregiver distress: Recommendations for future research. Gerontologist. 1993;33:240–248. doi: 10.1093/geront/33.2.240. [DOI] [PubMed] [Google Scholar]
- 58.Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]