Abstract
Antiretroviral drugs that inhibit viral replication were expected to reduce transmission of HIV by lowering the concentration of HIV in the genital tract. In 11 of 13 observational studies, antiretroviral therapy (ART) provided to an HIV-infected index case led to greatly reduced transmission of HIV to a sexual partner. In the HPTN 052 randomised controlled trial, ART used in combination with condoms and counselling reduced HIV transmission by 96·4%. Evidence is growing that wider, earlier initiation of ART could reduce population-level incidence of HIV. However, the full benefits of this strategy will probably need universal access to very early ART and excellent adherence to treatment. Challenges to this approach are substantial. First, not all HIV-infected individuals can be located, especially people with acute and early infection who are most contagious. Second, the ability of ART to prevent HIV transmission in men who have sex with men (MSM) and people who use intravenous drugs has not been shown. Indeed, the stable or increased incidence of HIV in MSM in some communities where widespread use of ART has been established emphasises the concern that not enough is known about treatment as prevention for this crucial population. Third, although US guidelines call for immediate use of ART, such guidelines have not been embraced worldwide. Some experts do not believe that immediate or early ART is justified by present evidence, or that health-care infrastructure for this approach is sufficient. These concerns are very difficult to resolve. Ongoing community-based prospective trials of early ART are likely to help to establish the population-level benefit of ART, and—if successful—to galvanise treatment as prevention.
Introduction
Development of many antiretroviral drugs has made HIV infection a treatable chronic disease.1 Initiation of antiretroviral therapy (ART) soon after infection offers near normal quality of life and lifespan.2 Early ART is also associated with a reduced latent viral reservoir,3 reduced viral DNA,4 and normalisation of some immune markers.5
Yet HIV prevention has been a constant struggle. Although the estimated incidence of HIV decreased by 50% in 25 countries between 2001 and 2011, 2·5 million people still became newly infected in 2011.6 Furthermore, encouraging reductions in the global incidence of HIV cannot be fully explained or ascribed to one intervention.
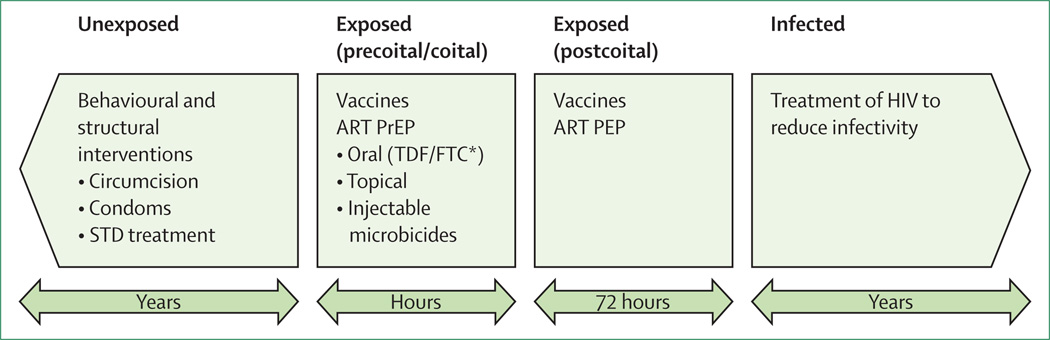
Figure 1 shows several strategies for HIV prevention. However, in the absence of a vaccine (which will probably be the case for the foreseeable future7), combinations of intervention strategies must be used.8,9 The combination prevention approach was put forward in the US Government’s new national HIV/AIDS strategy,10 and in the global President’s Emergency Plan for AIDS Relief (PEPFAR).11
Figure 1. Four opportunities for HIV prevention.
The four stages of infection risk are listed at the top of the figure. Potential interventions during each stage are listed within each box. The timeline for the intervention is listed in the arrows below the intervention boxes. STD = sexually transmitted diseases. ART = antiretroviral therapy. PrEP = pre-exposure prophylaxis. TDF/FTC = tenofovir disoproxil fumarate co-formulated with emtricitabine (Truvada; Gilead Sciences, Foster City, CA, USA). PEP=post-exposure prophylaxis. *TDF/FTC (Truvada) is the only ART intervention currently approved by the US Food and Drug Administration for PrEP.
Perhaps no part of combination HIV prevention has attracted more attention than the use of antiretroviral drugs. There are three ways in which these drugs can be deployed: as postexposure prophylaxis, as pre-exposure prophylaxis, and to reduce infectiousness of HIV-infected people to their sexual partners (treatment as prevention). First, suspected exposure to HIV can be followed by postexposure prophylaxis with antiretroviral drugs.12–14 This approach has been accepted as standard policy for both occupational exposure in health-care workers (eg, a needlestick injury)12,13 and non-occupational exposure (eg, an unprotected sexual encounter).14 Recommendations for postexposure prophylaxis are based on findings from experiments with macaques and an observational study in people who have been exposed to needlesticks.15,16 Second, gel-formulated and oral-based ART have been used successfully as pre-exposure prophylaxis for people at high risk for HIV infection. The combination of emtricitabine and tenofovir disoproxil fumarate (Truvada; Gilead Sciences, Foster City, CA, USA) has been approved by the US Food and Drug Administration as pre-exposure prophylaxis for particular high-risk groups. However, pre-exposure prophylaxis did not provide protection in all clinical trials, most likely because of poor adherence to study drugs.17–19 In this Review, we provide a comprehensive, timely, and critical assessment of the third use of ART, as treatment as prevention.
The HIV transmission event
The biology of HIV transmission has been best characterised in the rhesus macaque. Shortly after mucosal exposure, several foci of nascent HIV replication can be seen.20–22 Yet both in macaques exposed to a physiological dose of simian HIV23–25 and in people with acute infection25,26 a very small number of HIV variants (founder viruses) cause infection. These viruses use both CD4 and CCR5 receptors,25,27 and differ from other variants in envelope properties such as glycosylation and susceptibility to interferon α, suggesting a selective advantage of founder viruses for conditions at the mucosal surface.27,28
HIV transmission efficiency depends on the inoculum from the infected person, and the susceptibility of the exposed person.29 In 2000, Quinn and colleagues30 reported no heterosexual HIV transmission when the blood plasma viral load was less than 1500 copies per mL, and the greatest number of transmission events when viral load was greater than 37 500 copies per mL. The highest viral loads are noted immediately after infection (referred to as acute and early infection),4,31 and people with acute infection are probably the most contagious.31 However, mathematical modelling suggests that infectivity plateaus above a viral load of 80–100 000 copies of HIV RNA per mL blood plasma.32 Accordingly, the phenotype of the founder virus might also help to establish the probability of the HIV transmission event in addition to inoculum effects.27,28,33
The average risk for sexual HIV transmission in a serodiscordant heterosexual couple is approximately 0·0010–0·0019.34 However, this is probably an underestimate because discordant couples enrolled in longitudinal observational cohort studies inevitably receive more medical care and counselling than does a typical patient, and might generally be at lower risk for a transmission event (ie, due to selection bias since they have remained discordant despite past exposure).29 Additionally, inflammation in the genital tract can be expected to increase the probability of HIV transmission greatly by increasing the inoculum size in the HIV-infected individual and the number of receptive cells and state of cellular activation in the HIV-negative partner.35 Circumcision reduces acquisition efficiency36 by removing access to receptive cells in the foreskin.37 Anal intercourse with an HIV-infected insertive partner increases transmission probability,38 presumably because rectal mucosa is far more friable than vaginal or cervical mucosa, and is rich in cells that are susceptible to HIV.
Antiretroviral drugs and the genital tract
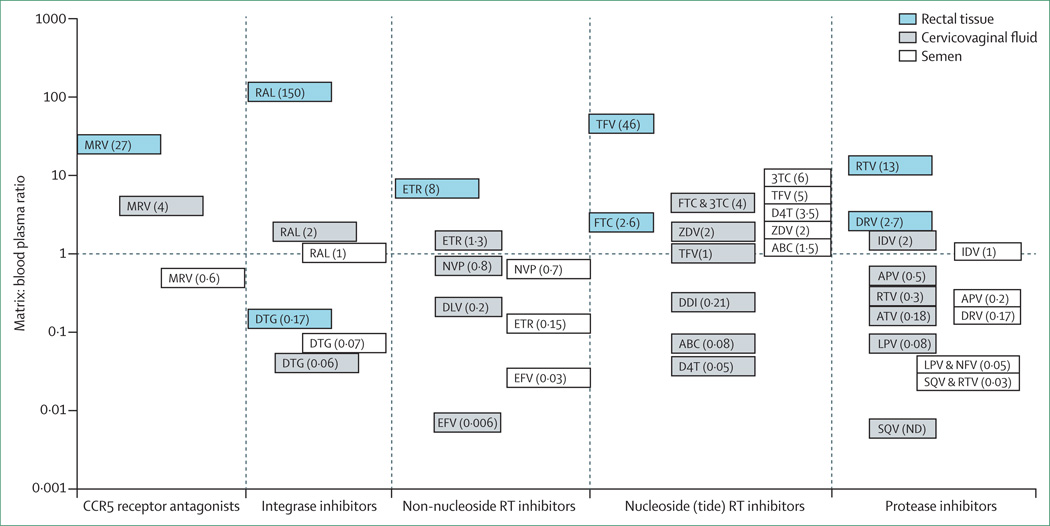
ART can be expected to reduce HIV transmission by reducing the concentration of virus in the blood and genital secretions of the person with HIV infection. Several groups have shown the ability of ART to penetrate the male and female genital tract39,40 and the ability of these drugs to suppress viral replication in the genital tract. Most (but not all) antiretroviral drugs can be expected to achieve similar or higher concentrations in the genital tract as in blood (figure 2). An important exception is among some of the protease inhibitors and non-nucleoside reverse transcriptase inhibitors. When protease inhibitors were first introduced, several investigators reported resistant variants in semen,41,42 most probably because the drugs did not reach sufficient concentration in this compartment and selected for viral resistance. A similar potential limitation for the non-nucleoside reverse transcriptase inhibitors in the female genital tract has been suggested.43
Figure 2. Comparison of antiretroviral exposure at mucosal surfaces.
Seminal plasma, cervicovaginal fluid, and colorectal tissue exposure is plotted as a ratio relative to matched blood plasma exposure. The Y axis is on a log scale. Y = 1 is the line of unity, at which mucosal surface exposure is similar to blood plasma. Total (protein free plus protein bound) drug concentrations were used to calculate these ratios. Ratios above the line of unity signify that drugs are concentrating at mucosal surfaces, whereas ratios below the line of unity suggest that drug concentrations are lower than blood plasma at mucosal surfaces. Semen concentration ratios are shown in white, cervicovaginal fluid in grey, and rectal tissue in blue. MRV = maraviroc. RAL = raltegravir. DTG = dolutegravir. ETR = etravirine. EFV = efavirenz. NVP = nevirapine. DLV = delavirdine. TFV = tenofovir. FTC = emtricitabine. 3TC = lamivudine. ZDV = zidovudine. ABC = abacavir. DDI = didanosine. D4T = stavudine. RTV = ritonavir. DRV = darunavir. IDV = indinavir. APV = amprenavir. ATV = atazanavir. LPV = lopinavir. NFV = nelfinavir. SQV = saquinavir. ND = not detected. Figure adapted from reference 40.
Patterson and colleagues44 have raised a different concern. In a study of the female genital tract and colorectum, the investigators noted that the concentrations of tenofovir and emtricitabine and their respective active metabolites (tenofovir diphosphate and emtricitabine triphosphate) varied according to mucosal tissue type. Tenofovir and its metabolite concentrations were 100-times greater in rectal tissue than in vaginal or cervical tissue, whereas emtricitabine and its metabolite concentrations were 10–15-times greater in vaginal or cervical tissue than in rectal tissue. Differential penetration or metabolism of antiretroviral drugs offers insight into the greatly variable level of protection conferred by antiretroviral drugs in some clinical trials of pre-exposure prophylaxis.39 These results suggest that selection of antiretrovirals for HIV prevention can be optimised by choosing drugs that preferentially penetrate sites of HIV acquisition or transmission, or by choosing those with long tissue half-life that might provide a so-called pharmacological buffer for imperfect drug adherence.
For unknown reasons, combination ART does not eliminate recovery of HIV from the male or female genital tract. Even when HIV replication is suppressed in the blood by ART, copies of HIV RNA can still routinely be recovered from the male45 and female46 genital tract, and the rectal mucosa.47 Whether copies of HIV recovered during treatment are replication competent and capable of causing infection, or are in fact defective and innocuous, is unclear.
Preventive use of ART
Findings from ecological studies, observational cohort studies, and one randomised control trial have shown the ability of ART to prevent sexual HIV transmission. Investigators of ecological studies have analysed changes in regional spread of HIV relative to use of ART to assess whether a policy of treatment as prevention (eg, frequent widespread testing coupled with ART initiation at diagnosis48,49) has slowed population-level HIV transmission. These studies take advantage of natural experiments in settings such as San Francisco (CA, USA)50 or British Columbia (Canada),51 where existing HIV control strategies have already achieved high testing and treatment coverage. The most encouraging results are from KwaZulu-Natal, South Africa, where investigators used geospatial techniques to assess the relation between ART use and HIV incidence. HIV seroconversion was monitored over several years in an observational cohort of more than 16 000 participants living in different communities.52 After adjustment for sexual behaviour and prevalent HIV cases, the investigators reported that each percentage point increase in ART coverage of HIV-infected people lowered the HIV-infection risk in a community by 1·7%. However, not all studies have reported a fall in HIV with increased availability of ART.53 Additionally, recent studies focused on MSM have not shown a population prevention benefit from more widespread use of ART. Importantly, ecological studies have methodological limitations,49,53 including insufficient person-level details that are needed to establish the causal effects,54 and inability to exclude all the potential confounding factors from biological mechanisms and behavioural risk.49,53,55 Associations inferred from ecological observations can almost never draw definitive conclusions of causality, and this limitation must be recognised by researchers and policy makers.
Serodiscordant couples
Findings from observational studies of serodiscordant, sexually engaged couples have informed individual-level investigations into the protective effects of ART. By comparing the experiences of serodiscordant couples in which infected partners were either receiving or not receiving ART, results of these studies strengthened the hypothesis that ART could reduce the risk of HIV transmission.56 On the basis of these results, in 2008, Swiss experts recommended that suppressive ART, when properly used, could provide sufficient protection to allow unprotected sexual intercourse.57 However, not every observational study has shown transmission prevention in couples by ART.58,59
Accordingly, to better define the role of ART for prevention, the US National Institutes for Health (NIH) supported a randomised clinical trial, HPTN 052, that was designed to quantify the magnitude and durability of benefit of early ART initiation for prevention of transmission in serodiscordant couples.60 The study included measurement of individual-level clinical consequences of earlier ART.61 1763 HIV discordant couples (97% heterosexual) were enrolled at 13 sites in nine countries. Enrolment required having a stable sexual partnership (>3 months) that led to three or more episodes of vaginal or anal intercourse during this time, no previous exposure to ART, and a CD4 count at enrolment between 350 and 550 cells per µL.
Participants in the HPTN 052 study were fully enrolled by April, 2009, and the trial will continue until mid-2015. However, in April, 2011, the trial’s independent data safety and monitoring board asked that interim results be made public, and at that time all HIV-infected participants were offered ART irrespective of CD4 cell count. The interim results showed that counselling and earlier initiation of ART reduced linked HIV transmissions by 96·4%. Linked transmissions are designated as those in which the viral sequence in the HIV-infected index case and newly infected partner are nearly identical, and different from other unrelated viral strains in the community.62 The results of the HPTN 052 trial also showed individual-level clinical benefits; earlier treatment significantly reduced tuberculosis and other less serious infections including candida and recurrent herpes zoster (shingles).61
In view of these findings, PEPFAR,11 the US Department of Health and Human Services,63 and WHO64,65 responded by amending their respective treatment guidelines to recommend immediate ART for people in HIV discordant relationships, irrespective of CD4 cell count. Three systematic reviews of sexual HIV transmission in heterosexual serodiscordant couples have shown significant reductions in transmission for people receiving ART versus those not.66–68
Moving from evidence to application
Translation of research findings into public health practice represents an exciting prospect but with many challenges. Efficacy shown in a randomised controlled trial might not lead to an effective intervention in the general population. Accordingly, the population-level benefits of treatment as prevention remain unproven. Although treatment of discordant couples is now standard, the effects of this approach on the overall epidemic are debated for several reasons.69,70
For example, the generalisability of results of treatment as prevention from studies of heterosexual couples is unknown. Will treatment reduce transmission by similar magnitudes in other high-risk heterosexual people (eg, sex workers and their clients), MSM, and intravenous drug users? HIV transmission through unsafe injection of drugs71 or anal sex38,72 has considerable transmission probability, which could limit the prevention benefits of ART. Similarly, the differential concentration and metabolism of antiviral drugs in the genital tract and rectum,39,40,44 and specific behavioural practices in high-risk groups,73 might compromise the efficacy of ART for prevention. Widespread use of ART in MSM populations in London (UK),74 Australia,75 and the Netherlands76 has not led to reductions in HIV incidence. This disappointing finding could have several explanations. First, imperfect use of ART by treated men might limit the transmission prevention benefit. Second, untreated men probably represent the source of continued spread of HIV in MSM, and men with acute and early HIV might represent the greatest risk for spread.74,77 Third, an increased number of HIV-infected cells and varying pharmacology of some antiretroviral drugs in rectal tissues (figure 2) could reduce the ability of ART to prevent HIV transmission associated with unprotected receptive anal intercourse. Similarly, vulnerable rectal mucosa might remain susceptible to a relatively small viral inoculum during unprotected insertive anal intercourse.74–76
Rapid ART rollout for preventive purposes raises the possibility of other substantial negative consequences. Increased risk behaviours in MSM associated with widespread availability of ART in some wealthier countries74,76,78,79 could presage similar patterns in other populations. A 2004 meta-analysis (most studies included MSM) showed no association between being on ART and increased sexual risk behaviour. Yet, beliefs about the protective and preventive benefits of ART were significantly associated with increased unprotected sex irrespective of HIV serostatus.79 An updated systematic review of studies published from 2009 to 2012 reported continuity in these findings, with associations noted between optimistic ART-related beliefs and increased risk of HIV transmission.80 Additionally, findings from some mathematical models have suggested that wider use of ART could lead to greater drug resistance, compromising both treatment and prevention.81
To address these and other concerns, more than 50 empirical studies of treatment as prevention are planned or ongoing.82 Several community randomised trials have already been launched.83 PEPFAR, NIH, the US Centers for Disease Control and Prevention, and the Bill & Melinda Gates Foundation are supporting very large-scale trials. In Zambia and South Africa, communities participating in the HPTN 071 trial (NCT01900977) have been randomly assigned to one of three groups so that HIV-infected people receive: standard of care, enhanced standard of care when CD4 count falls to 350 cells per µL, or immediate ART irrespective of CD4 count, depending on their community. In Botswana, the Mochudi Prevention Project (NCT01583439)84 is a targeted strategy being tested in which earlier treatment of HIV-infected patients will be directed towards those with the highest viral loads, who are arguably most contagious. The SEARCH (Sustainable East Africa Research in Community Health) study (NCT01864603) is designed to assess the health, economic, and educational effects of early HIV diagnosis and immediate initiation of ART with a streamlined care delivery system in rural communities in east Africa. The Agence Nationale de Recherche sur le Sida (ANRS) has designed a community randomised trial of 34 clusters in rural KwaZulu Natal; individuals in the intervention clusters will be started on ART irrespective of CD4 cell count when untreated HIV is detected in a screening campaign.85 All these studies are expected to use combination prevention with counselling, condoms, and treatment for sexually transmitted diseases, in addition to wider and earlier use of ART.
Modelling of the effect of ART on pandemics
Early modelling work of the effects of ART to reduce transmission led to very conservative estimates of benefit.86 However, newer model analyses suggest that the costs and attendant risk of expanding ART programmes will be justified by their benefits, especially in the long term.87
There are three possible ART expansion routes.88 The first is a low-cost, low-impact strategy of expanding treatment eligibility for those who are already attending clinic. A medium-cost, medium-impact strategy would use resources to reach groups who could benefit most from early initiation of ART. These groups vary between settings, but in generalised epidemics might include people with long-term uninfected partners, infected pregnant women, and sex workers. In concentrated epidemics, target groups might include sex workers, intravenous drug users, and MSM. A high-cost, high-impact strategy would launch massive outreach campaigns to connect all HIV-infected people with the clinic and start treatment. Importantly, cost differences between the scenarios are strongly related to the resources needed for outreach programmes, testing and linkage to care, and the cost of ART drugs themselves. Optimised spending across the HIV continuum of care thus represents the next frontier to control cost in response to HIV epidemics worldwide.
Despite the increasing amount of modelling evidence for a net benefit for expanded ART, the cost–benefit equations are more uncertain than commonly acknowledged. Three main areas of limitations in present model analyses stem from insufficient data and reliance on credible but unproven assumptions. First, most of the presumed population-level health benefits of expanded ART come from a reduction in HIV transmission. These gains are calculated from a complicated set of processes determined by the network of sexual contacts, and a set of assumptions about the biology of HIV infection and the transmission event.87–89 Indeed, the effect of ART in reducing transmission through routes other than heterosexual contact is not definitively known.90
Second, all model analyses must extrapolate from past performance of ART programmes to make future projections. This approach often works well, but the use of treatment to prevent HIV is unprecedented and data may be too scant for mathematical models to reliably and accurately reflect all the relevant contributing factors. For example, the assumptions for the adherence and retention of patients initiated on ART while still healthy are made from cohorts of patients who were started on ART after having had serious disease. Furthermore, most models have assumed that risk behaviour patterns would not change after ART initiation aimed at reduction of transmission. If, in fact, people starting ART much earlier have poorer adherence91 or increased risk behaviours,73,74,76,78,92 then models will overestimate the benefits of expanded treatment.
A closely related problem is the difficulty in estimation of the cost of a new intervention, particularly when the new intervention differs in scale to what has come before. For example, the costs of treating the most peripherally located HIV-infected people (both geographically and socially) are unlikely to equal the costs of treating typical residents in urban areas near medical facilities.93 Guides to estimate these cost increases are inadequate. Costing models also make assumptions about how programmes adapt to changing circumstances. For example, as programmes expand, the number of HIV-infected people not in care will decrease, but how efficiently programmes will be able to adapt their approach to find those remaining cases is unknown. Furthermore, cost-effectiveness research has yet to adequately quantify estimates for the costs of staff time, new infrastructure development, and expansion of the drug supply chain. Model analyses have used all data available to account for these factors, but the fundamental little experience with such programmes means that costs are a key source of uncertainty in discussions about the cost-effectiveness of treatment expansion.
The final limitation in present model analyses is in the unknown operationalisation of treatment as prevention. For example, how will clinics prioritise patients with CD4 counts less than 350 cells per µL, as recommended by the new WHO guidelines?65 In settings with low coverage of ART, implementation of new guidelines designed to start ART sooner for HIV infection could (for many reasons) actually reduce the opportunities for treatment of HIV-infected people with very low CD4 cell counts or more advanced disease, especially in resource-constrained settings. The extent to which this scenario might happen is unknown, but such an unintended consequence would be deleterious from both an equity and epidemiological perspective. Similarly, if a programme prioritised treatment for those in stable discordant relationships, how would such people be identified and how would the programme define stable? Model projections will not be useful or relevant for programmes if they have not correctly anticipated such operational issues.
Implementation challenges
The movement towards treatment as prevention has unmasked a massive gap in the strategy—namely, the difficulty to find and treat people at greatest risk for transmission, who may be hardest to reach. This limitation has both scientific and social underpinnings. Scientifically, routine HIV testing will not identify people with acute infection. In view of the potential importance of such people to the spread of HIV,31,94,95 we need to set an even higher scientific priority on finding means to identify early infections.96 Socially, those most likely to transmit HIV are often among the most stigmatised groups in society. Encouraging those at most risk to seek testing, and to adhere to ART if infected, will take political will and new resources to invest in evidence-based programmes for these marginalised key populations.
Additionally, the challenge of the HIV treatment cascade remains a major problem.97 Almost everywhere, large gaps exist between the number of people infected, those who know that they are infected, and those receiving reliable treatment. So-called leakage from the various stages of the cascade leads to programme inefficiencies and missed opportunities for both treatment and prevention. Furthermore, treatment providers can face logistical challenges in optimisation of therapy, and provision of HIV prevention services in treatment clinics has been difficult, whether such services are directed at infected people or their sexual partners, or HIV-negative people at risk for infection.
The treatment as prevention strategy also seems to be compromised by a lack of universal agreement about when to start ART for HIV infection, whether for individual health, to prevent HIV transmission, or for both benefits combined. Confusion among patients and providers about when to start ART must inevitably make cascade leakage worse, because of the de-facto message that treatment of HIV infection is not urgent. If early treatment is not perceived to be crucial, testing and linkage to care become optional, and retention becomes more difficult. Adakun and colleagues91 reported poor adherence to ART started at a CD4 count greater than 250 cells per µL, arguing that lack of recognised clinical benefit (since many participants were not symptomatic at baseline) and lack of social support otherwise available for patients with AIDS contributed to poor adherence among such individuals. At the same time, several investigators have reported that patients who seek care and are not offered ART have a remarkable rate of loss to follow-up.98,99
The debate about when to start ART has mixed the results of observational trials, randomised clinical trials, expert opinion, treatment guidelines, and logistical challenges, which makes a dispassionate evaluation of the topic confusing and difficult. Clearly, severe and fatal complications of HIV can be expected when CD4 count falls to less than 200 cells per µL. Findings from several observational studies,100–103 two randomised clinical trials (CIPRA Haiti104 and HPTN 05260), and one post-hoc analysis (SMART105) suggest a clinical benefit when ART is started between a CD4 count of 350 and 550 cells per µL. Investigators from the COHERE cohort, following more than 200 000 people for 1 154 803 person-years, reported measurable clinical benefit when ART was started at a CD4 count as high as 750 cells per µL, but not greater.106
On the basis of available evidence, WHO has recommended that all HIV-infected people in a discordant relationship64,65 and HIV-infected pregnant women107 start ART immediately. WHO now recommends that ART be started at a CD4 count of 500 cells per µL,65 and the International AIDS Society USA108 and US Department of Health and Human Services63 recommend immediate treatment for everyone, including people with acute HIV infection.
These results notwithstanding, some experts argue that there has not been sufficient evidence of meaningful clinical benefit to justify starting ART in asymptomatic people at CD4 counts greater than 350 cells per µL.109 And in fact, expansion of earlier ART in resource-constrained settings where older, more toxic regimens are still the standard of care could pose specific ethical and clinical challenges.110,111 Additionally, valid concerns exist about the degree of benefit of very early ART, which itself might be modest. First, although the inflammation associated with untreated HIV is assumed to be harmful, the exact degree to which earlier ART will reduce inflammation and the degree of resulting benefit—if any—are unknown. Second, early ART could add additional years of treatment, and the side-effects of ART over many years are unknown. Third, the logistical challenges of early ART in resource-constrained settings (eg, less toxic drugs unavailable, second-line drugs unavailable, no viral-load measurement, no resistance testing, drug stock outs) could compromise treatment, and lead to earlier treatment failure.111,112 Fourth, ART might lead to sexual disinhibition that might negate ART prevention benefits. Fifth, earlier initiation guidelines could make ART unavailable to people with more advanced disease.110,111 Finally, adherence to ART started early might not be as reliable as treatment offered to patients with more advanced disease.91
These important concerns lead to a counterpoint discussion. First, ART has unequivocal public health benefit in prevention of HIV transmission at all CD4 cell counts.60 A substantial proportion of people in Africa have shown high viral load throughout the course of HIV infection (irrespective of CD4 cell count),84 so the public health benefits of ART might be greatest in the epicentre of the pandemic. Second, although the extent to which people treated for HIV engage in risky behaviour is debated, HIV-infected people who are treated are probably far less contagious than are those who are untreated.52,60,66–68 Parenthetically, perhaps a greater concern is the reported increase in risk behaviour of people who are untested or untreated.74 Third, ongoing viral replication must be of some consequence.113 With ongoing replication, CD4 cell count will fall and might not recover quickly or completely.61,114 Increasing evidence suggests that ART reverses the T-cell activation that favours replication and some markers of inflammation.5,115
Two randomised controlled trials of early initiation of ART, TEMPERANO (NCT00495651) and START (NCT00867048), are in progress. However, randomised controlled trials designed to compare the costs and benefits of ART started at high CD4 cell counts (relative to a delay in therapy) are unlikely to last long enough to detect all the clinical events associated with delayed ART or long-term side-effects of earlier ART, either of which might not arise for decades.116 Finally, WHO guidelines suggest treatment of so many people irrespective of CD4 cell counts (eg, HIV-infected people in discordant sexual relationships, HIV-infected pregnant women, and perhaps people with acute HIV infection) that the number of people who might logically defer ART will continue to decrease, making the argument of the best time to start ART less relevant.
The logistical challenges needed to properly provide optimised treatment with earlier ART initiation are important and real.111 If safe, well tolerated antiretroviral drugs are not available, early treatment is far less likely to offer health benefit, and should be deferred. Similarly, if so little infrastructure is available that therapy cannot be monitored or sustained, early treatment makes little sense. But at the same time, logistical challenges must not prevent development of the best available medical care, or in this case the best use of medicine for public health. Logistical challenges will certainly slow ART rollout, but they should not be used as a reason to abandon the desire to treat more people, or to treat them sooner in the course of the disease.
Conclusion
ART, as a key component of combination prevention, has galvanised the call for an AIDS-free generation.11 In this Review we have provided the rationale for the development of treatment as prevention, described population-level evidence suggesting a chance for success with this approach, and outlined four community randomised trials designed to measure the population-level benefit from earlier or immediate ART. We have also stressed the many limitations and challenges of implementation of treatment as prevention to emphasise that sustained, population-level prevention benefit from earlier and wider use of ART is not guaranteed. Where do we go from here? The observational measurements tied to ART roll-out, and the large community trials in progress will inform estimates of HIV incidence under various circumstances for treatment as prevention, as well as HIV resistance, and population-level behaviour change. We hope to witness success in these studies, and to gain critical information to inform improved treatment as prevention and combination prevention approaches.
Key messages.
Treatment of an HIV-infected person will greatly reduce the probability of an HIV transmission event
Treatment as prevention requires careful attention to the best drug combinations for clinical and public health benefit
For treatment to affect the epidemic, improved detection of infection at all stages, universal access to antiretroviral therapy (ART), and excellent adherence are essential
Treatment as prevention demands a robust health-care infrastructure
Ongoing community-based randomised controlled trials of early ART are measuring population-level benefit of treatment as prevention
Search strategy and selection criteria.
We searched PubMed and PsycInfo databases from Jan 1, 1990, to Aug 31, 2013, with the terms: (“HIV” OR “AIDS”) AND (“antiretroviral” OR “ART” OR “ARV”) AND (“treatment as prevention” OR “TasP” OR “prevent transmission”); and (“HIV” OR “AIDS”) AND (“treatment” OR “antiretroviral” OR “ART” OR “ARV”) AND (“discordant” OR “serodiscordant”) AND (“couples” OR “partners” OR “relationships”). We used no other inclusion or exclusion criteria.
Acknowledgments
We thank Joseph Eron and Ward Cates for review of this manuscript, and Christophe Fraser, Andrew Phillips, and Joel Gallant for helpful comments. This work was supported by University of North Carolina Center for AIDS Research and National Institute of Diabetes and Digestive and Kidney Diseases (grant DK R37 49381).
Footnotes
Contributors
MSC conceived of the primary idea and led the development of all parts of the Review. MKS and KEM completed literature reviews and syntheses of ecological, observational, and experimental studies. TBH and KAP reviewed and synthesised relevant modelling and cost-effectiveness studies. ADK served as primary pharmacological expert. All authors contributed substantively to the subsequent writing and development of the Review.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Ray M, Logan R, Sterne JA, et al. and the HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335–343. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 3.Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananworanich J, Schuetz A, Vandergeeten C, et al. and the RV254/SEARCH 010 Study Group. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taiwo B, Matining RM, Zheng L, et al. Associations of T cell activation and inflammatory biomarkers with virological response to darunavir/ritonavir plus raltegravir therapy. J Antimicrob Chemother. 2013;68:1857–1861. doi: 10.1093/jac/dkt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. UNAIDS Report on the Global AIDS Epidemic. [accessed Aug 5, 2013];2012 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf. [Google Scholar]
- 7.Koff WC, Russell ND, Walport M, et al. Accelerating the development of a safe and effective HIV vaccine: HIV vaccine case study for the Decade of Vaccines. Vaccine. 2013;31(suppl 2):B204–B208. doi: 10.1016/j.vaccine.2012.10.115. [DOI] [PubMed] [Google Scholar]
- 8.McNairy ML, Cohen M, El-Sadr WM. Antiretroviral therapy for prevention is a combination strategy. Curr HIV/AIDS Rep. 2013;10:152–158. doi: 10.1007/s11904-013-0152-1. [DOI] [PubMed] [Google Scholar]
- 9.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National HIV/AIDS Strategy for the United States. The White House. [accessed Aug 5, 2013];2010 Jul; http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 11.US Department of State. PEPFAR blueprint: creating an AIDS-free Generation. [accessed Aug 3, 2013];2013 http://www.pepfar.gov/documents/organization/201386.pdf.
- 12.Kuhar DT, Henderson DK, Struble KA, et al. Updated US public health service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875–892. doi: 10.1086/672271. [DOI] [PubMed] [Google Scholar]
- 13.Panlilio AL, Cardo DM, Grohskopf LA, Heneine W, Ross CS and the US Public Health Service. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1–17. [PubMed] [Google Scholar]
- 14.Smith DK, Grohskopf LA, Black RJ, et al. and the US Department of Health and Human Services. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep. 2005;54:1–20. [PubMed] [Google Scholar]
- 15.Cardo DM, Culver DH, Ciesielski CA, et al. and the Centers for Disease Control and Prevention Needlestick Surveillance Group. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 16.Gay CL, Cohen MS. Antiretrovirals to prevent HIV infection: pre- and postexposure prophylaxis. Curr Infect Dis Rep. 2008;10:323–331. doi: 10.1007/s11908-008-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krakower D, Mayer KH. Promising prevention approaches: tenofovir gel and prophylactic use of antiretroviral medications. Curr HIV/AIDS Rep. 2011;8:241–248. doi: 10.1007/s11904-011-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483–1493. doi: 10.3851/IMP2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankins CA, Dybul MR. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: a review. Curr Opin HIV AIDS. 2013;8:50–58. doi: 10.1097/COH.0b013e32835b809d. [DOI] [PubMed] [Google Scholar]
- 20.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 22.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 23.Stone M, Keele BF, Ma ZM, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Keele BF, Li H, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw GM, Hunter E. HIV transmission. Cold Spring Harb Perspect Med. 2012;2:1–23. doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ping LH, Joseph SB, Anderson JA, et al. and the CAPRISA Acute Infection Study and the Center for HIV-AIDS Vaccine Immunology Consortium. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol. 2013;87:7218–7233. doi: 10.1128/JVI.03577-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrish NF, Gao F, Li H, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn TC, Wawer MJ, Sewankambo N, et al. and the Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 31.Pilcher CD, Tien HC, Eron JJ, Jr, et al. and the Quest Study, and the Duke-UNC-Emory Acute HIV Consortium. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 32.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma ZM, Stone M, Piatak M, Jr, et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83:3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes JP, Baeten JM, Lingappa JR, et al. and the Partners in Prevention HSV/HIV Transmission Study Team. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 36.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26:609–615. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris BJ, Wamai RG. Biological basis for the protective effect conferred by male circumcision against HIV infection. Int J STD AIDS. 2012;23:153–159. doi: 10.1258/ijsa.2011.011228. [DOI] [PubMed] [Google Scholar]
- 38.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrix CW. The clinical pharmacology of antiretrovirals for HIV prevention. Curr Opin HIV AIDS. 2012;7:498–504. doi: 10.1097/COH.0b013e32835847ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013;63(suppl 2):S240–S247. doi: 10.1097/QAI.0b013e3182986ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imrie A, Beveridge A, Genn W. Transmission of human immunodeficiency virus type 1 resistant to nevirapine and zidovudine. J Infect Dis. 1997;175:1502–1506. doi: 10.1086/516487. [DOI] [PubMed] [Google Scholar]
- 42.Mayer K, Boswell S, Goldstein R, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis. 1999;28:1252–1259. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 43.Newstein M, Losikoff P, Caliendo A, et al. Prevalence and persistence of nonnucleoside reverse transcriptase inhibitor mutations in the female genital tract. J Acquir Immune Defic Syndr. 2005;38:364–366. [PubMed] [Google Scholar]
- 44.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:1–17. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith DM, Wong JK, Shao H, et al. Long-term persistence of transmitted HIV drug resistance in male genital tract secretions: implications for secondary transmission. J Infect Dis. 2007;196:356–360. doi: 10.1086/519164. [DOI] [PubMed] [Google Scholar]
- 46.Cu-Uvin S, Caliendo AM. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2011;25:880–881. doi: 10.1097/QAD.0b013e328344ccf8. [DOI] [PubMed] [Google Scholar]
- 47.Zuckerman RA, Whittington WL, Celum CL, et al. Higher concentration of HIV RNA in rectal mucosa secretions than in blood and seminal plasma, among men who have sex with men, independent of antiretroviral therapy. J Infect Dis. 2004;190:156–161. doi: 10.1086/421246. [DOI] [PubMed] [Google Scholar]
- 48.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 49.Wilson DP. HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med. 2012;9:e1001231. doi: 10.1371/journal.pmed.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith MK, Powers KA, Muessig KE, Miller WC, Cohen MS. HIV treatment as prevention: the utility and limitations of ecological observation. PLoS Med. 2012;9:e1001260. doi: 10.1371/journal.pmed.1001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]
- 55.Grulich AE, Wilson DP. Is antiretroviral therapy modifying the HIV epidemic? Lancet. 2010;376:1824–1825. doi: 10.1016/S0140-6736(10)62162-9. author reply 1825. [DOI] [PubMed] [Google Scholar]
- 56.Smith K, Powers KA, Kashuba AD, Cohen MS. HIV-1 treatment as prevention: the good, the bad, and the challenges. Curr Opin HIV AIDS. 2011;6:315–325. doi: 10.1097/COH.0b013e32834788e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vernazza P, Hirschel B, Bernasconi E, Flepp M. Les personnes seropositives ne souffrant d’aucune autre MST et suivant un traitment antiretroviral efficace ne transmettent pas le VIH par voie sexuelle. Bulletinde Medecins Suisses. 2008;89:165–169. [Google Scholar]
- 58.Birungi J, Wang H, Ngolobe M, Muldoon K, Khanakwa S, King R. Lack of effectiveness of antiretroviral therapy (ART) as an HIV prevention tool for serodiscordant couples in rural ART program without viral load monitoring in Uganda. 19th International AIDS Conference; July 22–27, 2012; Washington, DC, USA. TUAC0103 (abstr). [Google Scholar]
- 59.Wang L, Ge Z, Jing L, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–238. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen MS, Chen YQ, McCauley M, et al. and the HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grinsztejn B, Hosseinipour MC, Ribaudo H, et al. Effects of early versus delayed initiation of anitretroviral therapy on HIV clinical outcomes: results from the HPTN 052 trial. 19th International AIDS Conference; July 22–27, 2012; Washington, DC, USA. THLBB05 (abstr). [Google Scholar]
- 62.Eshleman SH, Hudelson SE, Redd AD, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. J Infect Dis. 2011;204:1918–1926. doi: 10.1093/infdis/jir651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.US Department of Health and Human Services. Panel on antiretroviral guidelines for adults and adolescents (2012): guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. [accessed Aug 3, 2013];2012 http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 64.World Health Organization. Guidance on couples HIV testing and counselling, including antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach. [accessed April 24, 2012];2012 http://www.who.int/hiv/pub/guidelines/9789241501972/en/index.html. [PubMed]
- 65.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [accessed Aug 19, 2013];2013 http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed]
- 66.Loutfy MR, Wu W, Letchumanan M, et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PLoS One. 2013;8:e55747. doi: 10.1371/journal.pone.0055747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baggaley RF, White RG, Hollingsworth TD, Boily MC. Heterosexual HIV-1 infectiousness and antiretroviral use: systematic review of prospective studies of discordant couples. Epidemiology. 2013;24:110–121. doi: 10.1097/EDE.0b013e318276cad7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2013;4 doi: 10.1002/14651858.CD009153.pub3. CD009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Sadr WM, Coburn BJ, Blower S. Modeling the impact on the HIV epidemic of treating discordant couples with antiretrovirals to prevent transmission. AIDS. 2011;25:2295–2299. doi: 10.1097/QAD.0b013e32834c4c22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 71.Marmor M, Des Jarlais DC, Cohen H, et al. Risk factors for infection with human immunodeficiency virus among intravenous drug abusers in New York City. AIDS. 1987;1:39–44. [PubMed] [Google Scholar]
- 72.Jin F, Jansson J, Law M, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24:907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fallon SJ, Forrest DW. Unexamined challenges to applying the treatment as prevention model among men who have sex with men in the United States: a community public health perspective. AIDS Behav. 2012;16:1739–1742. doi: 10.1007/s10461-012-0258-2. [DOI] [PubMed] [Google Scholar]
- 74.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS One. 2013;8:e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wand H, Yan P, Wilson D, et al. Increasing HIV transmission through male homosexual and heterosexual contact in Australia: results from an extended back-projection approach. HIV Med. 2010;11:395–403. doi: 10.1111/j.1468-1293.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 76.van Sighem A, Jansen I, Bezemer D, et al. Increasing sexual risk behaviour among Dutch men who have sex with men: mathematical models versus prospective cohort data. AIDS. 2012;26:1840–1843. doi: 10.1097/QAD.0b013e3283574df9. [DOI] [PubMed] [Google Scholar]
- 77.Alam SJ, Zhang X, Romero-Severson EO, et al. Detectable signals of episodic risk effects on acute HIV transmission: strategies for analyzing transmission systems using genetic data. Epidemics. 2013;5:44–55. doi: 10.1016/j.epidem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jansen IA, Geskus RB, Davidovich U, et al. Ongoing HIV-1 transmission among men who have sex with men in Amsterdam: a 25-year prospective cohort study. AIDS. 2011;25:493–501. doi: 10.1097/QAD.0b013e328342fbe9. [DOI] [PubMed] [Google Scholar]
- 79.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004;292:224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y. Treatment-related optimistic beliefs and risk of HIV transmission: a review of recent findings (2009–2012) in an era of treatment as prevention. Curr HIV/AIDS Rep. 2013;10:79–88. doi: 10.1007/s11904-012-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagner BG, Blower S. Universal access to HIV treatment versus universal ‘test and treat’: transmission, drug resistance & treatment costs. PLoS One. 2012;7:e41212. doi: 10.1371/journal.pone.0041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Granich R, Gupta S, Suthar AB, et al. and the ART in Prevention of HIV and TB Research Writing Group. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res. 2011;9:446–469. doi: 10.2174/157016211798038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boily MC, Masse B, Alsallaq R, et al. HIV treatment as prevention: considerations in the design, conduct, and analysis of cluster randomized controlled trials of combination HIV prevention. PLoS Med. 2012;9:e1001250. doi: 10.1371/journal.pmed.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novitsky V, Wang R, Bussmann H, et al. HIV-1 subtype C-infected individuals maintaining high viral load as potential targets for the “test-and-treat” approach to reduce HIV transmission. PLoS One. 2010;5:e10148. doi: 10.1371/journal.pone.0010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iwuji CC, Orne-Gliemann J, Tanser F, et al. and the ANRS 12249 TasP Study Group. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14:230. doi: 10.1186/1745-6215-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baggaley RF, Ferguson NM, Garnett GP. The epidemiological impact of antiretroviral use predicted by mathematical models: a review. Emerg Themes Epidemiol. 2005;2:9. doi: 10.1186/1742-7622-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.HIV Modelling Consortium Treatment as Prevention Editorial Writing Group. HIV treatment as prevention: models, data, and questions--towards evidence-based decision-making. PLoS Med. 2012;9:e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.HIV Modelling Consortium. Modelling for the WHO 2013 guidelines for use of antiretrovirals. [accessed Sept 30, 2013]; http://www.hivmodelling.org/projects/modelling-who-2013-guidelines-use-antiretrovirals. [Google Scholar]
- 89.Eaton JW, Johnson LF, Salomon JA, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muessig KE, Smith MK, Powers KA, et al. Does ART prevent HIV transmission among MSM? AIDS. 2012;26:2267–2273. doi: 10.1097/QAD.0b013e328355713d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adakun SA, Siedner MJ, Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62:317–321. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior. JAMA. 2004;292:224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 93.Meyer-Rath G, Over M. HIV treatment as prevention: modelling the cost of antiretroviral treatment--state of the art and future directions. PLoS Med. 2012;9:e1001247. doi: 10.1371/journal.pmed.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis. 2010;202(suppl 2):S270–S277. doi: 10.1086/655651. [DOI] [PubMed] [Google Scholar]
- 97.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geng EH, Bwana MB, Muyindike W, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63:e64–e71. doi: 10.1097/QAI.0b013e31828af5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pati R, Lahuerta M, Elul B, et al. and the Identifying Optimal Models of HIV Care in Mozambique Study Group. Factors associated with loss to clinic among HIV patients not yet known to be eligible for antiretroviral therapy (ART) in Mozambique. J Int AIDS Soc. 2013;16:18490. doi: 10.7448/IAS.16.1.18490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cain LE, Logan R, Robins JM, et al. and the HIV-CAUSAL Collaboration. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitahata MM, Gange SJ, Abraham AG, et al. and the NA-ACCORD Investigators. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sterne JA, May M, Costagliola D, et al. and the When To Start Consortium. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Emery S, Neuhaus JA, Phillips AN, et al. and the Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 106.Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥200 cells/µL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57:1038–1047. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- 107.World Health Organization. Programmatic update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. [accessed Sept 12, 2013];2012 http://www.who.int/hiv/PMTCT_update.pdf. [PubMed]
- 108.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 109.Sabin C, Cooper DA, Collins S, Schechter M. Rating evidence in treatment guidelines: a case example of when to initiate combination antiretroviral therapy (cART) in HIV-positive asymptomatic persons. AIDS. 2013;27:1839–1846. doi: 10.1097/qad.0b013e328360d546. [DOI] [PubMed] [Google Scholar]
- 110.Lundgren JD, Wood R. Editorial commentary: universal antiretroviral therapy for HIV infection? Clin Infect Dis. 2013;57:888–890. doi: 10.1093/cid/cit381. [DOI] [PubMed] [Google Scholar]
- 111.Gallant JE, Mehta SH, Sugarman J. Universal Antiretroviral Therapy for HIV Infection: Should US Treatment Guidelines Be Applied to Resource-Limited Settings? Clin Infect Dis. 2013;57:884–887. doi: 10.1093/cid/cit382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Granich R, Williams B, Montaner J. Fifteen million people on antiretroviral treatment by 2015: treatment as prevention. Curr Opin HIV AIDS. 2013;8:41–49. doi: 10.1097/COH.0b013e32835b80dd. [DOI] [PubMed] [Google Scholar]
- 113.Mugavero MJ, Napravnik S, Cole SR, et al. and the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort Study. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–935. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.START Protocol Team. Letter of amendment #2 for Strategic Timing of AntiRetroviral Treatment (START) [accessed Aug 29, 2013];2013 Feb; http://insight.ccbr.umn.edu/official_documents/START/protocol_documents/START_v2_LoA2_SampleSizeRe-estimation_14Feb2013.pdf. [Google Scholar]