Abstract
Since hyper-homocysteinemia (HHcy) was recognized as a risk factor for Alzheimer’s disease (AD), many studies tried to induce HHcy in animal models to investigate its effect on amyloid-β protein precursor (AβPP) metabolism. Previous reports found that HHcy induced in AD transgenic mouse models, by either feeding a methionine-enriched diet or vitamin Bs deficient diet, is associated with elevation of amyloid-β (Aβ) levels. However, there is no data available on the effect of dietary intervention which combines both excessive methionine and low levels of vitamin Bs on amyloidogenesis in any of these models. In the current study, we investigated the effect of a combination diet, which was both enriched in methionine and deficient in folate, vitamin B6 and B12, in an AD mouse model, the Tg2576. We found that 7 months treatment of this diet induced severe HHcy in these mice with plasma homocysteine level higher than 150 μM. However, no difference was detected in brain Aβ levels or deposition between the diet-treated and control group. As shown by western blot, severe HHcy did not alter the steady state levels of proteins involved in AβPP metabolism, either. These results demonstrate that this combination diet-induced severe HHcy does not influence amyloidogenesis in vivo.
Keywords: Alzheimer’s disease, folate, methionine, plasma homocysteine, vitamin B
INTRODUCTION
High level of circulating homocysteine, also known as hyper-homocysteinemia (HHcy), has been found to be closely associated with many cardiovascular diseases such as atherosclerosis and stroke [1,2]. Recent epidemiological studies have found that mild to moderate HHcy is also a risk factor for sporadic Alzheimer’s disease (AD) [3–5]. Homocysteine is a non-protein amino acid and a metabolic precursor of both methionine and cysteine [6]. Dietary folate, vitamin B6, and vitamin B12 are required to maintain normal homocysteine levels in the body [7]. A diet with excessive methionine or deficient in vitamin Bs, or genetic alterations in certain enzymes such as cystathionine β-synthase (CBS), increase homocysteine levels in vivo [8,9].
Although our understanding of the link between HH-cy and AD is still limited, several mechanisms have been proposed to explain the deleterious effect of HH-cy in the central nervous system including oxidative stress [10], vascular damage [11], excitotoxicity [12], alterations in DNA methylation [13], and DNA repair [14]. Furthermore, there is an emerging line of evidence suggesting that HHcy may directly affect amyloidogensis and promote Aβ production, which is believed to be one of the main pathogenetic mechanisms underlying AD [15–19].
Since an imbalanced diet is the main cause of non-genetic human HHcy, several studies have used different dietary interventions to study the effect of HHcy in AD transgenic mouse models [14,15,19,20]. Among these dietary interventions, a diet either enriched with methionine or deficient in folate and vitamin Bs has been reported to successfully induce HHcy in the AD mouse models [15,19,21]. In these studies, diet-induced HHcy is generally associated with Aβ elevation and behavioral deficits. However, there is no report investigating the effect of the combination of these two kinds of diet on amyloidogenesis in AD mouse models.
In the present study, we investigated the effect of a diet which combines both excessive methionine and low level of folate, vitamin B6, and B12 on homocysteine level and amyloidogenesis in the Tg2576 mice, a well-established mouse model of AD-like amyloidosis [22]. After 7 months on this diet, we found that this diet induced a severe HHcy in Tg2576 mice but failed to cause any significant alterations in Aβ levels, deposition, or amyloid-β protein precursor (AβPP) metabolism.
MATERIALS AND METHODS
Tg2576 mice and diet treatments
Animal procedures were approved by the Institutional Animal Care and Usage Committee. Only female transgenic mice overexpressing the human AβPP with the Swedish mutation (K670N/M671L) [22] were used in this study. Polymerase chain reaction (PCR) analysis using tail DNA was used to confirm the genotype of all mice. All animals were kept in a pathogen-free environment, on a 12-hour light/dark cycle, and had access to food and water ad libitum.
Starting at 8 months of age, Tg2576 mice were randomized to two different diets: standard rodent chow enriched in methionine (1.2%) and deficient in folate (< 0.2 mg/kg), vitamin B6 (< 0.1 mg/kg) and B12 (< 0.001mg/kg) (n = 6) or standard rodent chow with vehicle (n = 6). Diets were custom-made, prepared by a commercial vendor (Harlan Teklad, Madison, WI), and matched for kilocalories [8].
All the mice were sacrificed after 7 months of diet treatment. These 15-month-old animals were perfused with PBS with 10 mM EDTA. Brain was removed and dissected in two hemibrains by midsagittal dissection: the left hemibrain was used for biochemistry assays; the right one was fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.6) overnight for immunohistochemistry studies.
Immunohistochemistry
Immunostaining analyses were performed as previously described [23,24]. Briefly, brains were cut in serial 6-μm-thick coronal sections and mounted on Superfrost/plus microscope slides (Fisher scientific, Pittsburgh, PA, USA). The sections were pretreated with formic acid (88%) and incubated with 1% H2O2 in methanol for 30 min. After incubation with primary antibody 4G8 (Covance, Princeton, NJ, USA) overnight at 4°C, sections were incubated with a biotinylated anti-mouse secondary antibody (Vector laboratories, Burlingame, CA, USA). Finally, sections were developed with 3,3′-diaminobenzidine (DAB) by using the avidinbiotin complex method (Vector Laboratories, Burlingame, CA, USA).
Light microscopic images from the hippocampus and somatosensory cortex were used to calculate the area occupied by Aβ-immunoreactivity with the software Image-Pro Plus for Windows version 5.0 (Media Cybernetics, Inc., Silver Spring, MD, USA), as previously described [24]. The threshold of optical density that discriminated staining from background was determined and kept constant for all quantifications. The area occupied by Aβ-immunoreactivity was measured and divided by the total area of interest to calculate the percentage area occupied by Aβ-immunoreactivity. Analyses were always performed in a coded fashion.
Biochemical analyses
Both cortex and hippocampus were homogenized and sequentially extracted in RIPA and then formic acid (FA) as previously described [25]. Sensitive sandwich ELISA tests were performed to measure Aβ1–40 and Aβ1–42 levels (IBL America, Minneapolis, MN, USA). Analyses were always performed in duplicate and in a coded fashion.
The “Abbott Homocysteine assay”, a fluorescence polarization immunoassay, was used to determine the homocysteine levels on the IMx® analyzer (Abbott Laboratories, Abbott Park, IL, USA). Plasma cholesterol and triglycerides levels were determined enzymatically using Sigma reagents (Sigma, St. Louis, MO).
Western blot analyses
RIPA fractions of brain homogenates were used for immunoblot analyses. Samples were electrophoresed on both Trisglycine polyacrylamide gels made in-house, and precasted gels purchased from Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA) and transferred onto nitrocellulose membranes. Antibodies and dilution used in the present study were as followed: anti-AβPP N-terminal raised against amino acids 66-81 for total AβPP (22C11; 1:500; Chemicon International, Temecula, CA, USA), anti-sAβPPβ (6A1; 2.5 μg/ml, IBL America, Minneapolis, MN, USA), anti-sAβPPα (2B3; 2.5 μg/ml; IBL America, Minneapolis, MN, USA), anti-ADAM-10 (1:500, Chemicon International, Temecula, CA, USA), anti-AβPP C-terminal for CTFs (1:600; EMD Biosciences Inc, La Jolla, CA, USA), anti-BACE-1 (1:400; Prosci Incorporated, Poway, CA, USA), anti-PS1 (1:500; Cell Signaling Technology, Danvers, MA, USA), anti-Nicastrin (1:500; Cell Signaling Technology, Danvers, MA, USA), anti-IDE N-terminal (1:1000; EMD Biosciences Inc, La Jolla, CA, USA), anti-neprilysin (NEP) (1:150; Santa Cruz biotech. Santa Cruz, CA, USA), anti-apoE (1:100; Santa Cruz biotech.), and anti-β-actin (1:4000; Santa Cruz biotech). Odyssey infrared imager system (Li-COR, Lincoln, NE, USA) was used for detection.
Data analysis
Data analyses were performed using SigmaStat. Statistical comparisons between the different treatment groups were performed by one way ANOVA and Fisher’s LSD post-hoc test. Values in all figures represent mean ± S.E.
RESULTS
Diet-induced severe HHcy in Tg2576 mice
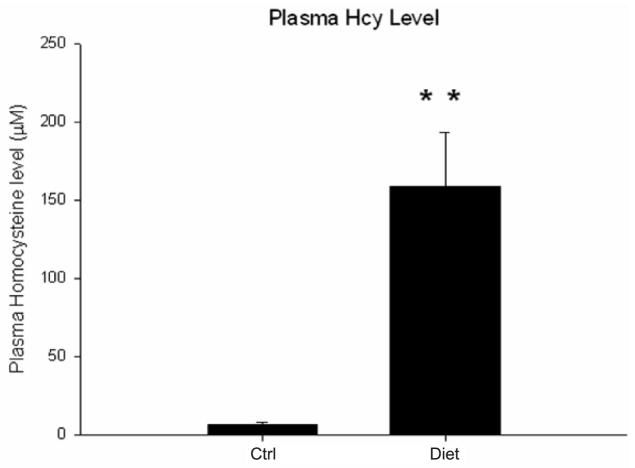
Starting at 8 month of age, Tg2576 mice were fed with a diet combining excessive methionine and low levels of folate, vitamin B6 and B12 (diet group) or vehicle diet (ctrl group) for 7 months. At the end of the diet treatment, body weight in the diet group was significantly lower than the control group (diet group: 23.7 ± 3.1 g; ctrl group: 31.2 ± 1.8 g; p < 0.05). By contrast, the diet group had a significant higher level of plasma homocysteine than the ctrl group, reaching a mean level of 150 μM (Fig. 1).
Fig. 1.
Plasma homocysteine levels in Tg2576 mice after receiving a combination diet. Mice were fed with a diet which combines excessive methionine and low levels of the folate, vitamin B6 and B12 (Diet group) or vehicle (Ctrl group) for 7 months. Values represent mean ± S.E.M. **P < 0.01.
Severe HHcy and Aβ levels
Sandwich ELISA quantification was performed to measure the Aβ1–40/42 levels in brain extractions from cortex and hippocampus of the two groups of mice. Mice with HHcy showed levels of Aβ40/42 which were similar to the ones observed in the control group. No difference was detected in either RIPA (for soluble Aβ) or FA (for insoluble Aβ) extracted fractions of both cortex and hippocampus between the two groups (Fig. 2).
Fig. 2.

Severe HHcy in Tg2576 mice and A β peptide levels. RIPA-soluble (RIPA) and formic acid (FA) extractable Aβ1-40 (A, B), and Aβ1-42 (C, D) levels in the cortex (Ctx) and hippocampus (Hippo) from Tg2576 on the special diet (Diet) or vehicle (Ctrl) were measured by sandwich ELISA. Values represent mean ± S.E.M.
Severe HHcy and Aβ deposition
Aβ deposition in the brain sections were examined by immunohistochemistry using 4G8, an anti-Aβ antibody reactive to amino acid residues 17–24. The percentage of area covered by positive immunoreactivity was calculated. Similar to the results of Aβ level, we found that both diet group and control group have same level of immunoreactivity in the hippocampus and the somatosensory cortex (Fig. 3).
Fig. 3.
Severe HHcy in Tg2576 mice and Aβ deposition. A) Representative sections of brains of Tg2576 receiving special diet (Diet), or vehicle (Ctrl) immunostained with 4G8 antibody. B) Quantification of the area occupied by Aβ immunoreactivity in hippocampus and somatosensory cortex (SSC) of Tg2576. Values represent mean ± S.E.M.
Severe HHcy and AβPP metabolism
Finally, we checked A βPP metabolic pathways by western blot analysis. As shown in Fig. 4, we did not find any changes in AβPP, sA βPPα, sAβPPβ, or C-terminal fragments (CTFs) in the diet group, when compared to the control group (Fig. 4). Further, severe HH-cy did not alter protein levels of important secretases involved in AβPP metabolism (ADAM10, BACE1, PS1, Nicastrin), either (Fig. 5). This also remained true in the case of proteins involved in Aβ clearance (IDE and NEP) and transport (APOE) as shown in Fig. 6 [26].
Fig. 4.

AβPP metabolism in Tg2576 mice with severe HHcy A) Representative western blots of AβPP, sAβPPa, sAβPPβ, CTFs (C99 and C83), in brain homogenates from Diet group or Ctrl group. B) Densitometric analyses of the immunoreactivities to the antibodies shown in panel A (white bars: Ctrl group; black bars: Diet group). Values represent mean ± S.E.M.
Fig. 5.
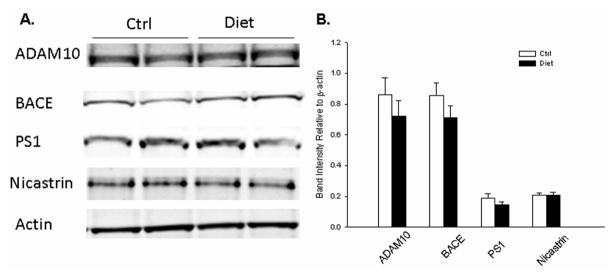
AβPP proteolytic pathways in Tg2576 mice with severe HHcy. A) Representative western blots of ADAM10, BACE1, PS1, and Nicastrin in brain homogenates from Diet group or Ctrl group. B) Densitometric analyses of the immunoreactivities to the antibodies shown in panel A (white bars: Ctrl group; black bars: Diet group). Values represent mean ± S.E.M.
Fig. 6.
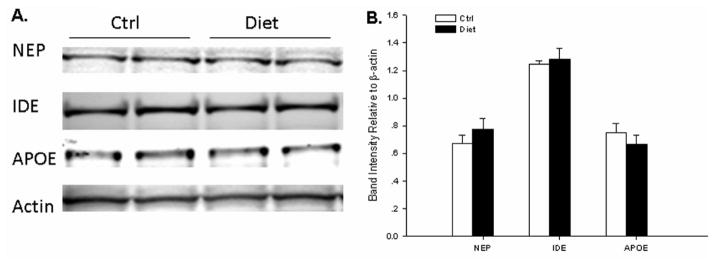
Aβ catabolic pathways in Tg2576 mice with severe HHcy. A) Representative western blots of NEP, IDE, and APOE in brain homogenates from Diet group or Ctrl group. B) Densitometric analyses of the immunoreactivities to the antibodies shown in panel A (white bars: Ctrl group; black bars: Diet group). Values represent mean ± S.E.M.
DISCUSSION
Previous studies have reported that feeding AD-like mouse models with either excessive methionine diet or B-vitamin deficient diet resulted in moderate HHcy and Aβ elevation [15,19,21]. However, to the best of our knowledge, no data are available on the effects of combination of these two diets in the same mouse models. In the current study, a diet combining excessive methionine with a deficiency in folate, vitamin B6, and vitamin B12 was fed to the Tg2576 mice for 7 months and the effects on homocysteine level and AβPP metabolism were then investigated.
Both excessive methionine and folate deficient diet can induce HHcy in animals [11,27]. However, they increase the homocysteine in the body through different metabolic mechanisms. High methionine intake promotes the synthesis of homocysteine and cysteine without limiting methionine production. In contrast, B-vitamin deficiency inhibits the conversion of homocysteine to methionine/cysteine, leading to its accumulation and methionine depletion [28]. Therefore, the combination of these two diets, as used in the present study, can theoretically increase homocysteine concentration to a level which is higher than using either diet alone. Either excessive methionine diet or B-vitamin deficiency diet can increase plasma homocysteine level from base level (~6 μM) to around 30 μM in Tg2576 mice or its background strain C57B6 mice [11,21]. By contrast, in the current study the combination diet feeding resulted in a much higher plasma homocysteine concentration, reaching more than 150 μM in Tg2576 mice. Compared to control group, this combination diet feeding also led to a lower body weight in diet group, which has been observed after similar combination diet feeding [27]. This highly elevated circulating homocysteine level alone could induce neuronal excitotoxicity in the brain through activating glutamate receptors, increasing calcium flux and oxidative stress [29,30].
While previous reports show that either excessive methionine diet or B-vitamin deficiency diet is associated with Aβ elevation [15,21], the combination diet failed to alter either Aβ level or Aβ deposition in the hippocampus or cortex of Tg2576 mice. Unsurprisingly, western blot analyses did not reveal any changes in protein levels of AβPP metabolites (sAβPPs, CTFs) or major secretases (ADAM10, BACE1, PS1, and Nicastrin). Neither did western blot find changes in APOE, IDE, and NEP, the proteins involved in Aβ clearance.
One explanation of these results is that the biological effect of severe HHcy (plasma homocysteine > 100 μM) is probably different from the effect of moderate HHcy (plasma homocysteine of 10 to 100 μM) [28, 31]. For example, Cbs−/& − homozygous mice with severe HHcy (>200 μM) have liver dysfunction resulting in premature death around 3–4 weeks old, while heterozygous Cbs+/− mice with moderate HHcy (~ 15 μM) grow normally and appear outwardly healthy [9]. Moreover, one study even found that liver gene expression profiles are drastically different between two transgenic mouse models with plasma homocysteine level at 169 μM or 296 μM, respectively [32]. These observations indicate that difference in circulating homocysteine levels can result in different regulation of genes, proteins, or functional pathways.
Severe HHcy in human is mostly due to rare genetic defects in the metabolism of methionine, folate, or vitamin B12 [33,34]. These individuals mainly suffer from arterial occlusions and venous thromboembolisms [35]. On the other hand, acquired moderate HHcy, rather than severe HHcy, is observed in aged population and associated with age-related central nervous system disorders such as AD [31]. In the Framingham study, plasma homocysteine level in normal seniors ranged from 3.5 μM to 66.9 μM and no single case of severe HHcy was detected. In addition, mild HHcy (plasma homocysteine level > 14 μM) in seniors is enough to double the risk of AD [3]. Despite some intrinsic limitations in our study due to the relative small sample size, our data clearly support the novel concept that a severe HHcy animal model may not be a good biological model for understanding the link between HHcy and AD related amyloidogenesis. Moreover, they suggest that the clinical treatment for HHcy patients may need to be adjusted and differentiated according to their more or less severe increase in homocysteine levels.
In summary, in the current study we found that a diet combining excessive methionine and low level of B-vitamin induced severe HHcy in Tg2576 mice without altering Aβ level, deposition or AβPP metabolism. These results suggest that the effect of HHcy on AβPP metabolism is complex and a moderate HHcy model, rather than severe HHcy model, could be a better choice to study mechanism underlying the association between HHcy and AD in seniors.
Acknowledgments
This study was supported by a grant from the National Institute of Health, AG-22512 (D.P.), and the Alzheimer’s Association (D.P.).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=349).
References
- 1.Huang T, Yuan G, Zhang Z, Zou Z, Li D. Cardiovascular pathogenesis in hyperhomocysteinemia. Asia Pac J Clin Nutr. 2008;17:8–16. [PubMed] [Google Scholar]
- 2.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 5.Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis. 2006;9:393–398. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- 6.Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7:813–822. doi: 10.1089/ars.2005.7.813. [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Evans DA, Schneider JA, Tangney CC, Bienias JL, Aggarwal NT. Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer’s disease. J Alzheimers Dis. 2006;9:435–443. doi: 10.3233/jad-2006-9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen DW. Hyperhomocysteinemia and oxidative stress: time for a reality check? Arterioscler Thromb Vasc Biol. 2000;20:1182–1184. doi: 10.1161/01.atv.20.5.1182. [DOI] [PubMed] [Google Scholar]
- 11.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 2008;105:12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuso A, Seminara L, Cavallaro RA, D’Anselmi F, Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28:195–204. doi: 10.1016/j.mcn.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Kruman, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D’Anselmi F, Coluccia P, Calamandrei G, Scarpa S. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci. 2008;37:731–746. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Pacheco-Quinto J, Rodriguez de Turco EB, DeRosa S, Howard A, Cruz-Sanchez F, Sambamurti K, Refolo L, Petanceska S, Pappolla MA. Hyperhomocysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid beta peptide levels. Neurobiol Dis. 2006;22:651–656. doi: 10.1016/j.nbd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, Zhang Y, Wang JZ. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 19.Fuso A, Nicolia V, Pasqualato A, Fiorenza MT, Cavallaro RA, Scarpa S. Changes in Presenilin 1 gene methylation pattern in diet-induced B vitamin deficiency. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.013. in press. [DOI] [PubMed] [Google Scholar]
- 20.Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2007;28:1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Zhuo JM, Portugal GS, Kruger WD, Wang H, Gould TJ, Praticò D. Diet-induced hyperhomocysteinemia increases Amyloid-β formation and deposition in a mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2010;7:140–149. doi: 10.2174/156720510790691326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 23.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 24.Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung S, Yang H, Uryu K, Lee EB, Zhao L, Shineman D, Trojanowski JQ, Lee VM, Pratico D. Modulation of nuclear factor-kappa B activity by indomethacin influences A beta levels but not A beta precursor protein metabolism in a model of Alzheimer’s disease. Am J Pathol. 2004;165:2197–2206. doi: 10.1016/s0002-9440(10)63269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leissring MA, Lu A, Condron MM, Teplow DB, Stein RL, Farris W, Selkoe DJ. Kinetics of amyloid beta-protein degradation determined by novel fluorescence- and fluorescence polarization-based assays. J Biol Chem. 2003;278:37314–37320. doi: 10.1074/jbc.M305627200. [DOI] [PubMed] [Google Scholar]
- 27.Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci U S A. 2003;100:15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dayal S, Lentz SR. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol. 2008;28:1596–1605. doi: 10.1161/ATVBAHA.108.166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boldyrev AA, Johnson P. Homocysteine and its derivatives as possible modulators of neuronal and non-neuronal cell glutamate receptors in Alzheimer’s disease. J Alzheimers Dis. 2007;11:219–228. doi: 10.3233/jad-2007-11209. [DOI] [PubMed] [Google Scholar]
- 30.Kuszczyk M, Gordon-Krajcer W, Lazarewicz JW. Homocysteine-induced acute excitotoxicity in cerebellar granule cells in vitro is accompanied by PP2A-mediated dephosphorylation of tau. Neurochem Int. 2009;55:174–180. doi: 10.1016/j.neuint.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Troen AM. The central nervous system in animal models of hyperhomocysteinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1140–1151. doi: 10.1016/j.pnpbp.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Kuhnisch J, Mustafa A, Lhotak S, Schlachterman A, Slifker MJ, Klein-Szanto A, High KA, Austin RC, Kruger WD. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009;23:883–893. doi: 10.1096/fj.08-120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mudd SH. Vascular disease and homocysteine metabolism. N Engl J Med. 1985;313:751–753. doi: 10.1056/NEJM198509193131210. [DOI] [PubMed] [Google Scholar]
- 34.Tonetti C, Saudubray JM, Echenne B, Landrieu P, Giraudier S, Zittoun J. Relations between molecular and biological abnormalities in 11 families from siblings affected with methylenetetrahydrofolate reductase deficiency. Eur J Pediatr. 2003;162:466–475. doi: 10.1007/s00431-003-1196-9. [DOI] [PubMed] [Google Scholar]
- 35.Edirisinghe SP. Homocysteine-induced thrombosis. Br J Biomed Sci. 2004;61:40–47. doi: 10.1080/09674845.2004.11732643. [DOI] [PubMed] [Google Scholar]