Abstract
Just as in other neural systems, plasticity is a hallmark of the neural system controlling breathing. One spinal mechanism of respiratory plasticity is phrenic long-term facilitation (pLTF) following acute intermittent hypoxia. Although cellular mechanisms giving rise to pLTF occur within the phrenic motor nucleus, different signaling cascades elicit pLTF in different conditions. These cascades, referred to as “Q” and “S" pathways to phrenic motor facilitation (pMF), interact via cross-talk inhibition. Whereas the Q pathway dominates pLTF after mild to moderate hypoxic episodes, the S pathway dominates after severe hypoxic episodes. The biological significance of multiple pathways to pMF is not known. We discuss the possibility that interactions between pathways confer emergent properties to pLTF, including: 1) pattern sensitivity and 2) metaplasticity. Understanding these mechanisms and their interactions may enable us to optimize intermittent hypoxia induced plasticity as a treatment for patients that suffer from ventilatory impairment or other motor deficits.
Keywords: plasticity, motor neuron, intermittent hypoxia, phrenic nerve, pattern sensitivity, metaplasticity
1 Introduction
The respiratory control system has been historically viewed as fixed and immutable, controlled primarily via negative feedback from sensory receptors.1 This view was held in large part because breathing is an automatic, often subconscious motor behavior. However, control systems governed by negative feedback alone are frequently unstable due to inappropriate reflex gain.2 Systems that preserve homeostasis through strong negative feedback loops are vulnerable to insults; robust control systems optimize for worst-case scenarios (ie. ventilatory failure), and incorporate mechanisms to prevent such failure when the system is challenged. Plasticity is one key property of neural systems, such as the respiratory control system, that promote robust and effective homeostatic regulation.1 In this brief review, we use a general definition of respiratory plasticity, namely: a change in future system performance (ie. breathing or blood gas regulation) based on experience.1
Plasticity in the neural system controlling breathing has only been widely appreciated only for the past few decades .1,3–5 Recently, the field of respiratory neuroplasticity has grown considerably; plasticity has been discovered at the neuromuscular,6 chemoreceptor,7 spinal,8 and brainstem,9 levels of respiratory control, and our knowledge concerning these forms of plasticity is increasing at a rapid pace. Here we focus on a single, widely studied model of spinal respiratory plasticity, phrenic long-term facilitation (pLTF). pLTF is a persistent increase in phrenic motor output lasting hours after a few brief episodes of low oxygen, or acute intermittent hypoxia (AIH; see refs 10–13). Considerable progress has been made towards an understanding of cellular and network mechanisms giving rise to pLTF.10–11 One recent realization is that multiple distinct cellular cascades give rise to similar phenotypic plasticity.10 An important question is: why do these multiple pathways exist? In this brief review, we will consider the potential advantages conferred by this complexity. In specific, we develop the hypotheses that the existence of multiple interacting pathways confers two emergent properties of pLTF: pattern sensitivity and metaplasticity.
2 Phrenic long-term facilitation (pLTF)
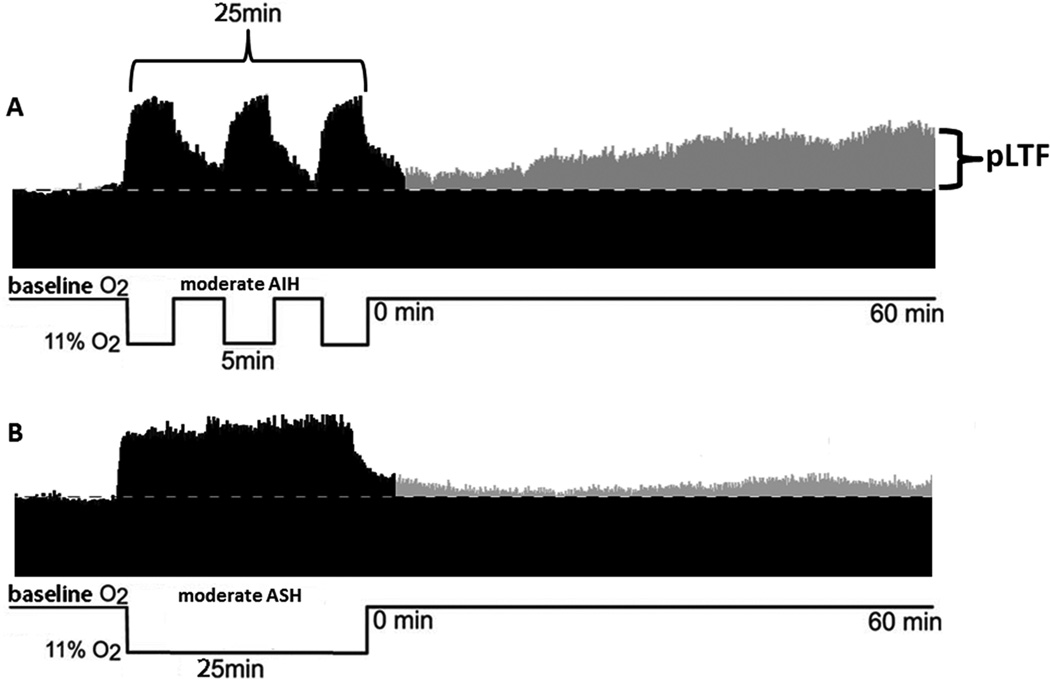
Millhorn and colleagues originally demonstrated that episodic carotid sinus nerve stimulation elicits a long lasting increase in phrenic motor output in anesthetized cats.3,4 We subsequently demonstrated a similar phenomenon in anesthetized rats following three brief hypoxic episodes, a phenomenon termed phrenic long-term facilitation (pLTF).12,13 Following acute intermittent hypoxia (AIH), pLTF is expressed as a prolonged increase in phrenic nerve burst amplitude lasting several hours after the final hypoxic episode (Figure 1A). pLTF is a form of serotonin- and protein synthesis-dependent spinal plasticity.12,14,15 pLTF induction is independent of increased phrenic nerve activity16–17 and represents a form of neuromodulator induced plasticity1 distinct from conventional forms of activity-dependent synaptic plasticity such as hippocampal long-term potentiation (LTP).18
Figure 1.
Pattern sensitivity of phrenic long-term facilitation (pLTF). pLTF is elicited by moderate, acute intermittent hypoxia (AIH), but not by moderate, acute sustained hypoxia (ASH). A: Representative tracing of phrenic nerve activity from an anesthestized, vagotomized, paralyzed and pump ventilated rat exposed to moderate AIH. Following AIH, there is a progressive increase in phrenic nerve burst amplitude, indicating pLTF. B: Representative tracing from a rat exposed to moderate ASH. Following ASH, there is little increase in phrenic nerve burst amplitude from baseline.
On hallmark of pLTF is pattern sensitivity; pLTF is elicited by intermittent, but not a single period of acute sustained hypoxia (ASH) with the same cumulative duration (Figure 1B).19 Although similar pattern sensitivity is shared by many forms of neuroplasticity,20–24 we have little understanding of how such pattern sensitivity arises in any system. Another property of pLTF is its ability to express metaplasticity;1 for example, following cervical dorsal rhizotomy25 or chronic intermittent hypoxia,26 subsequent responses to AIH (ie. pLTF) are amplified. We still do not fully understand mechanisms that confer metaplasticity.
Although our understanding of mechanisms giving rise to respiratory plasticity remains incomplete, considerable progress has been made.10,11,27,28 One realization is that multiple, distinct cellular cascades exist, each capable of eliciting long-lasting phrenic motor facilitation (pMF; a general term describing augmented phrenic burst amplitude that includes pLTF).10 These pathways interact in interesting and complex ways, possibly increasing flexibility as the respiratory control system responds to diverse challenges throughout life.
In this review, we make the argument that interactions between distinct cellular cascades to phrenic motor facilitation confer emergent properties to respiratory plasticity, including pLTF pattern sensitivity and metaplasticity. An understanding of the diverse mechanisms giving rise to pMF and their implications will help to understand pattern sensitivity and metaplasticity in other forms of neuroplasticity, and will be essential as we begin to harness the potential of AIH-induced spinal plasticity to treat severe clinical disorders that impair breathing, such as spinal cord injury29–31 or ALS.32
3 Multiple cellular mechanisms of pLTF: the Q and S pathways
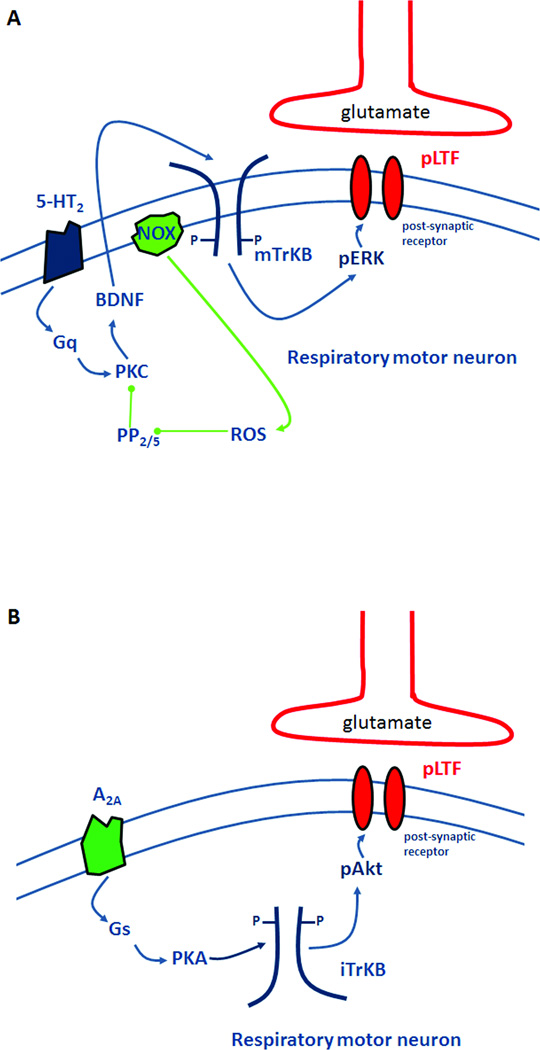
pLTF is frequently studied in anesthetized, paralyzed and ventilated rats administered a standardized AIH protocol (3, 5 min hypoxic episodes; PaO2 35–45 mmHg; 5 min intervals; see ref 25). We refer to this protocol as moderate AIH. Following moderate AIH, pLTF requires spinal serotonin type 2 (5-HT2) receptor activation,12,14,15 new synthesis of brain-derived neurotrophic factor (BDNF),33 activation of its high-affinity receptor (TrkB),33 and ERK MAP kinase signaling.34 Because 5-HT2 receptors are Gq protein-coupled receptors,35 we refer to this as the “Q pathway” to pMF (Figure 2A). Pharmacological activation of other Gq coupled receptors (such as α1 receptors) elicit similar pMF.10
Figure 2.
Working models of cellular pathways giving rise to phrenic motor facilitation following AIH. A:The “Q pathway” is initiated by activation of the Gq-protein coupled 5-HT2 receptor, leading to protein kinase C (PKC) activation and new synthesis of brain-derived neurotrophic factor (BDNF). BDNF then activates its high affinity receptor, TrkB. TrkB activation phosphorylates extracellular signal-related kinase (ERK) MAP kinase, which facilitates inputs to phrenic motor neurons by an unknown mechanism (possibly glutamate receptor trafficking). The Q pathway is regulated by protein phosphatases 2A and/or 5, which can inhibit PKC activation. NADPH oxidase-induced ROS formation inhibits these phosphatases, enabling pLTF expression. Thus, NADPH oxidase and PP2A/5 constitute a "regulatory cassette" for pLTF. B: The “S pathway” is induced by the Gs-protein coupled adenosine 2A (A2A) receptor, subsequently activating protein kinase A (PKA). PKA stimulates new synthesis of an immature TrkB isoform, which auto-activates and phosphorylates and activates Akt. Subsequent to Akt activation, synaptic inputs to phrenic motor neurons are facilitated by an unknown mechanism.
A distinct pathway to pMF relies on activation of Gs protein coupled metabotropic receptors, such as adenosine 2A (A2A)36 and 5-HT7 receptors.37 This form of pMF requires new synthesis of an immature TrkB isoform (not BDNF) and PI3 kinase/Akt signaling (not ERK).36 We refer to this as the “S pathway” to pMF since multiple Gs protein-coupled metabotropic receptors elicit the same mechanism (Figure 2B).10 Contrary to our initial expectation, the S pathway does not contribute to pLTF following moderate AIH,38 but dominates pLTF following severe AIH.39 In fact, the S pathway negatively regulates pLTF with moderate AIH, demonstrating inhibitory interactions between pathways;38 based on these findings, we proposed that the predominant interaction between pathways is “cross-talk inhibition.” Although the mechanistic basis of this cross-talk inhibition is not fully understood, S to Q inhibition may require PKA activity (Hoffman and Mitchell, unpublished).
During severe AIH, the S pathway is activated to a greater extent, and dominates pLTF.39 Rats exposed to a severe AIH exhibit pLTF phenotypically similar to moderate AIH, but via an A2A receptor-dependent (serotonin-independent) mechanism (i.e. S pathway).39 This finding suggests that cross-talk inhibition between pathways assures dominance of one or the other; the switch appears to be precipitous since PaO2 levels above 35 mmHg elicit pLTF via the Q pathway35 whereas PaO2 levels of 30 mmHg or below elicit pLTF via the S pathway.39 We suggest that this transition occurs because of relatively greater accumulation of extracellular adenosine during severe hypoxic episodes, shifting the balance towards the S pathway. Once the tipping-point is reached, we hypothesize that the now dominant S pathway suppresses the Q pathway. Although the subordinate pathway does not positively contribute to pLTF, it nevertheless modulates (inhibits) the dominant pathway.38
4 More pathways to phrenic motor facilitation
Three other stimuli elicit unique forms of pMF in anesthetized rats. Spinal injections of the growth/trophic factors vascular endothelial growth factor (VEGF) or erythropoietin (EPO) near the phrenic motor nucleus cause pMF via mechanisms that require both ERK and Akt activation for full expression.40,41 We suspect that these hypoxia-sensitive genes enable phrenic motor plasticity in longer time domains. For example, VEGF or EPO induced pMF might play a role in longer-term adjustments of phrenic motor activity during chronic intermittent or sustained hypoxia.40,41
A completely different cellular cascade gives rise to pMF after brief periods of phrenic inactivity (iPMF) induced by hypocapnia, vagal stimulation and/or isoflurane.42 Unlike pLTF, iPMF requires atypical PKC activation.43,44 iPMF might ensure that this critical motor pool is constantly active. For example, iPMF might contribute to the preservation of adequate phrenic activity when synaptic inputs are disrupted, such as by spinal injury.
5 Emergent properties of pLTF
Some properties of pLTF, such as pattern sensitivity and metaplasticity, may be determined by interactions between competing pathways to pMF.26,45 These emergent properties may determine whether pLTF is expressed or not after a given stimulus (eg. different patterns or severity of hypoxia), or its magnitude (eg. greater magnitude in response to the same AIH).
5.1 Pattern sensitivity
Pattern sensitivity is a common feature in many models of neuroplasticity, including models of serotonin-dependent synaptic facilitation.20,21 pLTF is pattern-sensitive since it is induced by moderate AIH, but not acute sustained hypoxia (ASH) of similar severity and cumulative duration (9–25 min; Figure 1B).12 Pattern sensitivity of respiratory plasticity was initially recognized in studies of ventilatory LTF (vLTF) in goats,46,47 and subsequently observed in rat48,49 and human vLTF.50–55 Interestingly, vLTF in humans requires slightly elevated CO2 during AIH.56 It was recently reported that elevated CO2 reveals vLTF in humans exposed to 32 minutes of ASH,57 although the investigators found that about half of this vLTF was due to drift in ventilation caused by sustained hypercapnia alone. Griffin et al.57 observed a persistent increase in ventilation for 20 min after AIH or ASH, but they did not measure ventilation at later time points necessary to clearly demonstrate LTF. Thus, further studies are needed to confirm that vLTF is pattern-sensitive in awake humans.
Although significant progress has been made, the mechanism of pLTF pattern sensitivity remains unknown. The optimal spacing interval has not been characterized, but is somewhere between 1 and 30 minutes.58 pLTF is relatively insensitive to other characteristics of AIH, such as the severity or duration of hypoxic episodes. For example, pLTF is unaffected by the level of PaO2 from 35 to 60 mmHg,59,60 or by episode durations between 15 sec to 5 min.61 Similar to AIH-induced pLTF, pMF induced by intraspinal 5-HT requires episodic injections, suggesting that pattern sensitivity occurs at or downstream from 5-HT receptor activation.16,17,62 Here, we propose three possible mechanisms that could contribute to pLTF pattern-sensitivity.
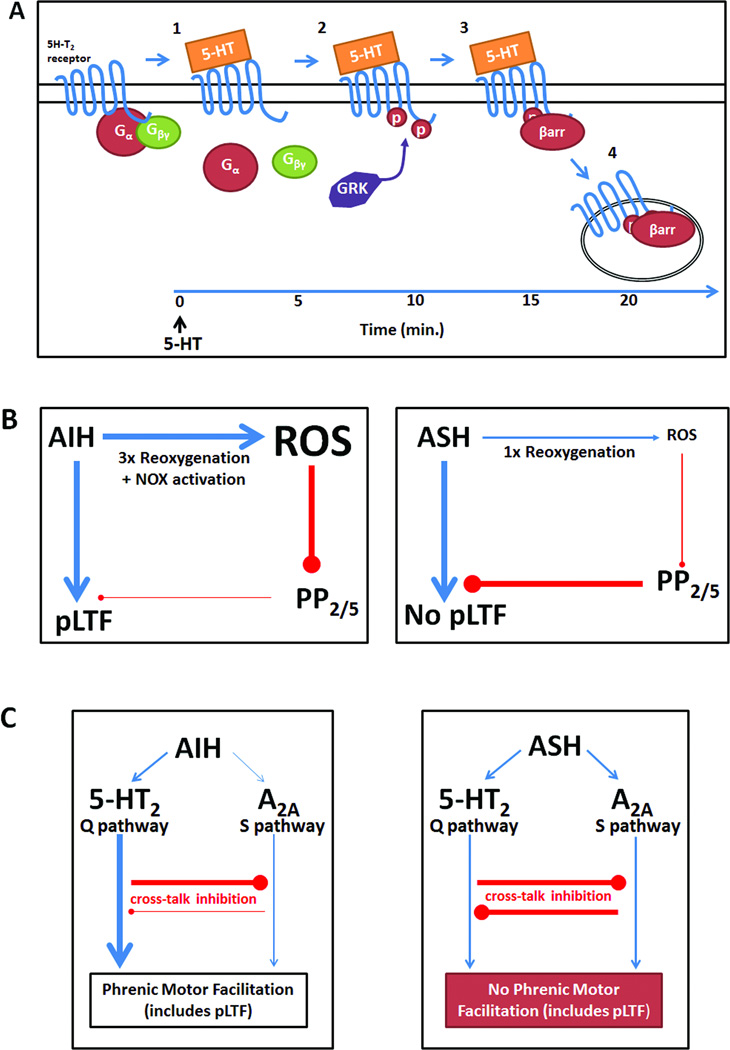
5.1a Serotonin receptor desensitization (Figure 3A)
Figure 3.
Possible mechanisms giving rise to pLTF pattern sensitivity. A: 5-HT2 receptor desensitization might occur with prolonged 5-HT release, such as during ASH: 1) 5-HT binds to its receptor, releasing Gα and Gβγ proteins, which activate downstream second messengers; 2) after prolonged agonist exposure (~10 min), 5-HT2 receptors are phosphorylated by G-protein coupled receptor kinase (GRK), preventing subsequent activation of G-proteins (ie. functional uncoupling) and promoting β-arrestin (βarr) binding to the 5-HT2 receptor; 3) β-arrestin binding prevents further 5-HT2 receptor activation while promoting receptor internalization; 4) receptors are internalized via a clathrin-dependent mechanism, decreasing the number of receptors available to elicit pLTF. B: Differential reactive oxygen species (ROS) formation during AIH vs. ASH may underlie pattern sensitivity. During AIH, ROS production during repeated reoxygenation events and NADPH oxidase (NOX) activation. Increased ROS would inhibit protein phosphatases 2 and 5, disinhibiting PKC (or other kinases) and enabling forward progression of pLTF. During ASH, insufficient ROS formation due to a single reoxygenation event may not inhibit the protein phosphatases sufficient to relieve their constraint to pLTF. C:Inhibitory interactions between the Q and S pathways to pMF may underlie pattern sensitivity of pLTF. 1) During AIH, the Q pathway is dominant because of 5-HT2 receptor activation with relatively little adenosine accumulation. 2) During ASH, greater adenosine release/accumulation may cause sufficient S pathway activation to offset Q pathway activation. Balanced activation of both pathways may prevent any pLTF expression due to balanced cross-talk inhibition.
Serotonin receptors are diverse, with complex signaling and activation requirements; the 5-HT2 class alone includes multiple isoforms with unconventional properties, such as g-protein independent signaling63 or constitutive activity.64 One pertinent feature of 5-HT2 receptors is desensitization, which is a decrease ligand response after prolonged ligand exposure. In the brain, 5-HT2 receptors desensitize rapidly with persistent elevations in extracellular serotonin via mechanisms that involve receptor internalization and/or functional uncoupling.65 Receptor internalization occurs when agonist bound receptors are internalized by clathrin coated pits into endosomes, where they are sequestered until reinserted or degraded. Functional uncoupling disrupts G-protein signaling and occurs via receptor phosphorylation, usually by G protein-coupled receptor kinases (GRKs); this phosphorylation causes the protein arrestin to bind the receptor, preventing further G protein activation.66 In vitro studies demonstrate 5-HT2 receptor desensitization after 5–10 min of agonist exposure,67,68 well within the time frame of a 25 minute ASH exposure. Although no studies have assessed 5-HT2 receptor desensitization in the spinal cord, it is a candidate to undermine pLTF during ASH.
5.1b Regulation of protein phosphatase activity (Figure 3B)
Protein phosphatases are inhibitory to many forms of CNS synaptic plasticity;69,70 the expression of plasticity is often regulated by protein phosphatase activity during the induction phase. pLTF is constrained by constitutive okadaic acid-sensitive protein phosphatases during ASH,45 Thus, in rats pretreated with spinal okadaic acid, ASH elicits pLTF.45 Reactive oxygen species (ROS) inhibit protein phosphatases involved in plasticity.71 Indeed, ROS produced by NADPH oxidase are necessary for pLTF expression,72 and this requirement is offset by spinal protein phosphatase inhibition with okadaic acid.73 Thus, NADPH oxidase and okadaic acid sensitive protein phosphatases (most likely PP2A) appear to constitute a "regulatory cassette" that determines if and how much pLTF will be expressed (Fig. 3B).37,64 One likely difference between ASH and AIH in their ability to elicit pLTF could be relative ROS production (and phosphatase inhibition). While no published studies have directly compared ROS production in response to AIH vs. ASH, there is evidence that hypoxia-induced superoxide production occurs predominantly during reoxygenation73–75 suggesting that AIH could generate greater ROS (vs. ASH) because of multiple reoxygenations. Further studies are needed to determine how AIH (vs. ASH) stimulates greater ROS production in the cervical spinal cord.
5.1c Cross-talk inhibition between Q and S pathways (Figure 3C)
Although pLTF shifts to S pathway-dependence with severe AIH,39 it is not known how this shift occurs. We proposed that “cross-talk inhibition” between pathways (Figure 3C) enables one pathway to gain an "upper hand" and, thus, dominate pMF. A key factor is the strength of the initiating stimulus (5-HT2 versus A2A receptor activation). For example, during severe AIH, greater adenosine formation/accumulation may increase A2A receptor activation, creating stronger S pathway activation and, subsequently, Q pathway suppression. The prevailing mechanism of pLTF may result from competition between pathways for dominance (Figure 3C).
We speculate that, at some levels of hypoxia (severity, duration, etc), inhibitory interactions could become balanced, creating an impasse where the S or Q pathways offset one another (ie. no pLTF). If so, this state represents an emergent property of competing pathways to pMF, and could underlie pLTF pattern-sensitivity. During longer hypoxic exposures, such as moderate ASH, greater adenosine formation/accumulation76 may cause balanced activation of the Q and S pathways (Figure 3C), thereby obscuring pLTF. This hypothesis is consistent with the study by Griffin et al., (2012) since hypercapnia could cause greater serotonin release, shifting the balance towards the Q pathway and vLTF expression. Further studies are needed to determine the role of pathway interactions in pLTF pattern sensitivity.
5.1d Are other forms of pMF pattern-sensitive?
While many studies have focused on pLTF pattern sensitivity following moderate AIH, no studies have examined pattern sensitivity in the other forms of pMF described above (ie. S pathway, VEGF- and EPO-induced pMF, or iPMF). Studies are needed to determine if these forms of pMF are also pattern-sensitive.
5.2 Metaplasticity
Metaplasticity is loosely defined as “plastic plasticity”, but more specifically as the ability of prior experience to alter subsequent plasticity.77 Metaplasticity can be expressed as enhanced pLTF triggered by pre-conditioning of adults with repetitive intermittent hypoxia26,78,79 or cervical sensory denervation.25 On the other hand, developmental exposure to chronic intermittent hypoxia suppresses pLTF in adult rats.80 pLTF enhancement from pre-conditioning is particularly interesting because it could increase the success of AIH protocols applied to improve breathing capacity or other motor functions after spinal injury.8,27–30 Thus, metaplasticity (and its mechanisms) are important considerations for clinical utilization of AIH-induced motor plasticity.
5.2a Enhanced pLTF after pre-conditioning with repetitive intermittent hypoxia
Rats exposed to chronic intermittent hypoxia (CIH; 10–12% O2/air, 2–5 min intervals, 8–12 hrs/night) exhibit enhanced serotonin-dependent pLTF.26 Other investigators have reproduced this effect with shorter hypoxic episodes (5–12% O2, 15s episodes with 5 min intervals, 8h/day) to more closely simulate episodic hypoxia during sleep disordered breathing.81 In this study, pLTF enhancement was blocked by antioxidant administration81 demonstrating ROS dependence. Thus, CIH enhances pLTF, presumably by enhanced Q pathway signaling (ie. serotonin and ROS dependent). Although CIH is a potent stimulus to metaplasticity, it also elicits morbidity, including hypertension, hippocampal apoptosis and cognitive deficits, among others.82–84 Thus, we developed more subtle protocols of repetitive intermittent hypoxia that elicit pLTF metaplasticity without detrimental effects elicited by CIH.30,85,86
Rats exposed to modest protocols of repetitive acute intermittent hypoxia (rAIH) exhibit enhanced pLTF78,79 and increased respiratory and non-respiratory somatic motor recovery following cervical spinal injury.30 Two different rAIH protocols were used in these studies. Daily AIH (10, 5 min episodes of 10.5% O2, 5 min intervals, 7 days) improved respiratory and forelimb function and increased phrenic burst amplitude in rats with C2 spinal hemisections.30 In Brown-Norway rats, a strain with low constitutive pLTF and no hypoglossal LTF, daily AIH enabled hypoglossal LTF, but an apparent doubling of pLTF was only marginally significant.85 These results suggest that daily AIH is a modest inducer of metaplasticity in the phrenic motor pool of otherwise normal rats. Rats exposed to AIH 3 times per week (3xwAIH; 10, 5min episodes/day, 3 days per week for 4 weeks) exhibit enhanced pLTF,78,79 and profound neurochemical plasticity in the phrenic motor nucleus,86 suggesting that longer, less frequent exposures are more effective at eliciting pLTF metaplasticity. Neither protocol caused hippocampal apoptosis, astrogliosis, or hypertension,30,85,86 suggesting the ability to elicit respiratory metaplasticity without detectable pathology. Further studies are needed to determine optimal protocols to elicit respiratory metaplasticity, so that AIH-induced plasticity can be harnessed as a means of restoring respiratory and non-respiratory motor function in clinical disorders that challenge ventilatory control.
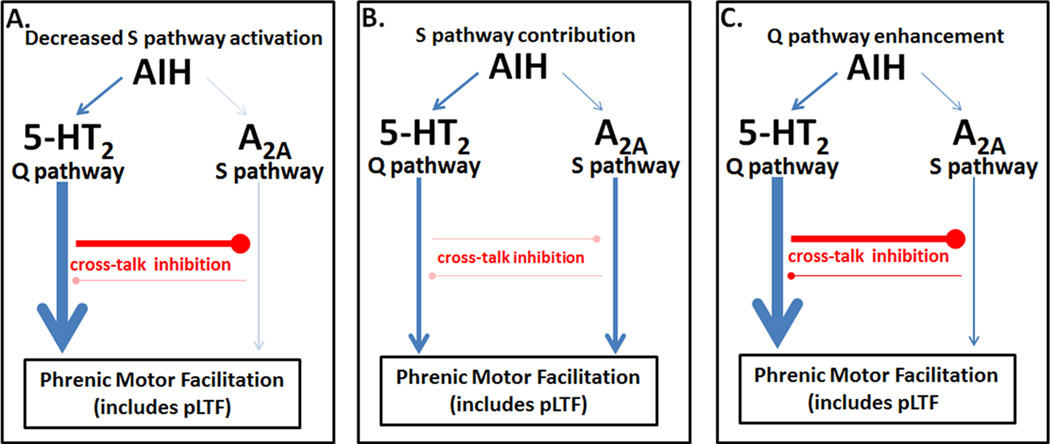
What mechanism underlies pLTF metaplasticity following rAIH? In animals exposed to daily AIH, increased spinal BDNF was observed,85 suggesting Q pathway enhancement. If the Q pathway is enhanced following rAIH, what mechanism underlies this enhancement? One possibility is decreased cross-talk inhibition. For example, decreased S pathway activation (Figure 4A) could increase Q pathway dependent pLTF and BDNF synthesis. Alternatively, decreased inhibitory interactions between pathways would enable direct S pathway contributions to pLTF, even with moderate AIH, since the pathways would be uncoupled (Figure 4B). Indeed, greater phospho-Akt levels were observed after 3xwAIH for 10 weeks,86 consistent with increased S pathway activation. Finally, pLTF metaplasticity may arise directly from a more robust Q pathway (Figure 4C); following 10 weeks of 3xwAIH, 5-HT2A receptor, BDNF, TrkB and phospho-ERK expression are all increased,86 suggesting "hypertrophy" of this cellular cascade. Further studies are needed to determine how rAIH causes pLTF metaplasticity.
Figure 4.
Possible mechanisms of metaplasticity, enhancing pLTF following repettive intermittent hypoxia. A: Decreased S pathway activation, thereby eliminating S to Q inhibition, could explain metaplasticity in pLTF following rAIH. B: Reduced cross-talk inhibition, enabling a positive S pathway contribution could enhance pLTF. C: Increased Q pathway signaling (“hypertrophy”), possibly involving increased 5-HT2 receptors, ROS production, or BDNF synthesis could explain greater pLTF following rAIH.
5.2b Other forms of metaplasticity in the phrenic motor system
This brief review was intended to pose rather than answer questions. It is not expansive enough to cover all potential forms of metaplasticity in respiratory motor pools. However, factors known to induce metaplasticity in other models of plasticity, such as hippocampal long-term potentiation include prior activation77,87,88 and stress.90 Hippocampal LTP is impaired by stress or glucocorticoids.90 In the phrenic motor pool, we postulate that stress has two separate effects. Initially, stress might induce pMF, due to norepinephrine release from locus coeruleus followed by activation of α1 receptors on phrenic motor neurons.10 On the other hand, if pLTF is similar to other models of plasticity, chronic exposure to stressful stimuli may impair pLTF, possibly through a glucocorticoid-dependent mechanism. Anecdotally, rats subject to stressful stimuli often fail to express pLTF; however, further studies are needed to determine the effects of stress on phrenic motor plasticity, and to confirm or refute these speculations.
6 Conclusion and Significance
Distinct pathways (ie. Q and S pathways) give rise to phenotypically similar phrenic motor facilitation. However, since the Q and S pathways interact via cross-talk inhibition, either pathway can dominate and produce pLTF; at a specific balance point, it is possible that these pathways neutralize one another, with equal and opposing inhibition of the other. Thus, cross-talk inhibition may have the ability to confer key emergent properties of pLTF, such as pattern-sensitivity. Another emergent property, enhanced pLTF (ie. metaplasticity) may arise from diminished inhibitory coupling of these pathways, so that both combine to produce a larger (enhanced) pLTF following AIH. The presence of multiple, competing pathways to pLTF confers flexibility, enabling different manifestations of plasticity as an animal responds to diverse conditions that vary in severity, pattern and/or duration of hypoxia. Greater understanding of multiple pathways giving rise to respiratory motor plasticity and their interactions could increase our understanding of physiological responses to environmental or pathological changes.
As one example, human subjects at high altitude experience chronic sustained hypoxia, giving rise to homeostatic increases in ventilation; this form of respiratory plasticity is often referred to as ventilatory acclimatization to high altitude.90,91 What role, if any, do the Q and S pathways and their interactions play in this process? Further studies are needed to determine how pathways interact to produce changes in respiratory control appropriate for the prevailing conditions. As another example, humans most frequently experience intermittent hypoxia during sleep, particularly in individuals suffering from obstructive sleep apnea. In such cases, does the severity and pattern of hypoxic episodes determine the type and extent of compensatory respiratory plasticity? These differences may also be of considerable relevance as we consider distinctions between ventilatory plasticity versus plasticity in respiratory-related motor output to the upper airways, which may stabilize or de-stabilize breathing depending on complex interactions between upper airway patency versus chemoreflex gain and apneic threshold. Further studies are needed to answer these questions.
An understanding of complex interactions between mechanisms of respiratory plasticity has considerable importance as we develop strategies to harness intermittent hypoxia induced motor plasticity to treat clinical disorders that impair breathing and other movements (eg. ref 31). While much research has been focused on characterizing new forms of plasticity, we are just beginning to understand the significance of interactions between them. These interactions, if understood and controlled, may enable us to optimize therapeutic protocols of rAIH in the treatment of spinal injury, neurodegenerative diseases and even sleep disordered breathing.27
Acknowledgements
Supported by HL080209, HL111598 and HL69064; NN supported by a fellowship from the Francis Family Foundation
Footnotes
Invited contribution to a special volume of Annals of the New York Academy of Sciences Conference on Cellular and Network Functions of the Spinal Cord, Madison, WI May, 2012
References
- 1.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J. Appl. Physiol. 2003;94(1):358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 2.Doyle JC, Csete M. Architecture, constraints, and behavior. Proc. Natl. Acad. Sci. U.S.A. 2011;108(Suppl 3):15624–15630. doi: 10.1073/pnas.1103557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41(1):87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- 4.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42(3):171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- 5.Eldridge FL, Millhorn DE. Oscillation, gating, and memory in the respiratory control system. In: Fishman AP, editor. Handbook of Physiology: Section 3, The Respiratory System II. Bethesda, MD: American Physiological Society; 1986. pp. 93–114. [Google Scholar]
- 6.Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respir Physiol Neurobiol. 2005;147(2–3):235–251. doi: 10.1016/j.resp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar NR. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir Physiol Neurobiol. 2011;178(3):375–380. doi: 10.1016/j.resp.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann. N. Y. Acad. Sci. 2010;1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG. Invited review: Neural network plasticity in respiratory control. J. Appl. Physiol. 2003;94(3):1242–1252. doi: 10.1152/japplphysiol.00715.2002. [DOI] [PubMed] [Google Scholar]
- 10.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv. Exp. Med. Biol. 2010;669:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB. Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001;90(6):2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 12.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104(2–3):251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am. J. Physiol. 1993;265(4 Pt 2):R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- 14.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J. Appl. Physiol. 2001;90(5):2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- 15.Baker-Herman TL, Mitchell GS. Phrenic Long-Term Facilitation Requires Spinal Serotonin Receptor Activation and Protein Synthesis. The Journal of Neuroscience. 2002;22(14):6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J. Physiol. (Lond.) 2009;587(Pt 22):5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc. Natl. Acad. Sci. U.S.A. 2004;101(12):4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neves G, Cooke SF, Bliss TVP. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 2008;9(1):65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 19.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J. Physiol. (Lond.) 2000;529(Pt 1):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn. Mem. 1998;5(3):246–256. [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton MA, Ide J, Masters SE, Carew TJ. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in aplysia. Learn. Mem. 2002;9(1):29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauer JA. Blockade of hippocampal long-term potentiation by sustained tetanic stimulation near the recording site. J. Neurophysiol. 1999;81(2):940–944. doi: 10.1152/jn.1999.81.2.940. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen PV, Duffy SN, Young JZ. Differential maintenance and frequency-dependent tuning of LTP at hippocampal synapses of specific strains of inbred mice. J. Neurophysiol. 2000;84(5):2484–2493. doi: 10.1152/jn.2000.84.5.2484. [DOI] [PubMed] [Google Scholar]
- 24.Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J. Neurophysiol. 2002;87(6):2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- 25.Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J. Neurosci. 1998;18(20):8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J. Neurosci. 2001;21(14):5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp. Physiol. 2007;92(1):27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell GS, Terada J. Should we standardize protocols and preparations used to study respiratory plasticity? Respir Physiol Neurobiol. 2011;177(2):93–97. doi: 10.1016/j.resp.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169(2):210–217. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JER, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci. 2012;32(11):3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26(2):163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- 32.Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, et al. Intermittent Hypoxia and Stem Cell Implants Preserve Breathing Capacity in a Rodent Model Of ALS. Am. J. Respir. Crit. Care Med. 2012;112(10):1678–1688. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci. 2004;7(1):48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation following acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J. Appl. Physiol. 2012;113(8):1184–1193. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard BE. Serotonin receptors—where are they going? Int Clin Psychophamacol. 1994;9(Suppl 1):7–17. doi: 10.1097/00004850-199403001-00003. [DOI] [PubMed] [Google Scholar]
- 36.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J. Neurosci. 2008;28(9):2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J. Physiol. (Lond.) 2011;589(Pt 6):1397–1407. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J. Physiol. (Lond.) 2010;588(Pt 1):255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J. Appl. Physiol. 2012;112(10):1678–1688. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J. Neurosci. 2011;31(21):7682–7690. doi: 10.1523/JNEUROSCI.0239-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J. Neurosci. 2012;32(17):5973–5983. doi: 10.1523/JNEUROSCI.3873-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahamed S, Strey KA, Mitchell GS, Baker-Herman TL. Reduced respiratory neural activity elicits phrenic motor facilitation. Respir Physiol Neurobiol. 2011;175(3):303–309. doi: 10.1016/j.resp.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strey KA, Nichols NL, Baertsch NA, Broytman O, Baker-Herman TL. Spinal atypical protein kinase C activity is necessary to stabilize inactivity-induced phrenic motor facilitation. J. Neurosci. 2012;32(46):16510–16520. doi: 10.1523/JNEUROSCI.2631-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol. 2011;179(1):48–56. doi: 10.1016/j.resp.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkerson JER, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J. Neurosci. 2008;28(11):2949–2958. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J. Physiol. (Lond.) 1997;499(Pt 2):543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dwinell MR, Janssen PL, Bisgard GE. Lack of long-term facilitation of ventilation after exposure to hypoxia in goats. Respir Physiol. 1997;108(1):1–9. doi: 10.1016/s0034-5687(96)02522-4. [DOI] [PubMed] [Google Scholar]
- 48.Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J. Appl. Physiol. 2001;91(2):709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura A, Olson EB, Terada J, Wenninger JM, Bisgard GE, Mitchell GS. Sleep state dependence of ventilatory long-term facilitation following acute intermittent hypoxia in Lewis rats. J. Appl. Physiol. 2010;109(2):323–331. doi: 10.1152/japplphysiol.90778.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J. Appl. Physiol. 1995;79(1):205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- 51.McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J. Appl. Physiol. 1996;81(2):866–875. doi: 10.1152/jappl.1996.81.2.866. [DOI] [PubMed] [Google Scholar]
- 52.McEvoy RD, Popovic RM, Saunders NA, White DP. Ventilatory responses to sustained eucapnic hypoxia in healthy males during wakefulness and NREM sleep. Sleep. 1997;20(11):1008–1011. doi: 10.1093/sleep/20.11.1008. [DOI] [PubMed] [Google Scholar]
- 53.Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J. Appl. Physiol. 2001;91(4):1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- 54.Tamisier R, Anand A, Nieto LM, Cunnington D, Weiss JW. Arterial pressure and muscle sympathetic nerve activity are increased after two hours of sustained but not cyclic hypoxia in healthy humans. J. Appl. Physiol. 2005;98(1):343–349. doi: 10.1152/japplphysiol.00495.2004. [DOI] [PubMed] [Google Scholar]
- 55.Querido JS, Kennedy PM, Sheel AW. Hyperoxia attenuates muscle sympathetic nerve activity following isocapnic hypoxia in humans. J. Appl. Physiol. 2010;108(4):906–912. doi: 10.1152/japplphysiol.01228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291(4):R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 57.Griffin HS, Kumar P, Pugh K, Balanos GM. Long-term facilitation of ventilation following acute continuous hypoxia in awake humans during sustained hypercapnia. The Journal of physiol. (Lond.) 2012;590(Pt 20):5151–5165. doi: 10.1113/jphysiol.2012.236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bach KB, Kinkead R, Mitchell GS. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and alpha2-adrenoreceptor antagonism. Brain Res. 1999;817(1–2):25–33. doi: 10.1016/s0006-8993(98)01181-0. [DOI] [PubMed] [Google Scholar]
- 59.Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121(2–3):135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- 60.Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol. 2008;162(1):8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J. Physiol. (Lond.) 2008;586(8):2171–2181. doi: 10.1113/jphysiol.2007.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006;142(3):885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 63.Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 1990;87(6):2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 2010;16(6):694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahman S, Neuman RS. Multiple mechanisms of serotonin 5-HT2 receptor desensitization. Eur. J. Pharmacol. 1993;238(2–3):173–180. doi: 10.1016/0014-2999(93)90845-9. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Recept. Channels. 1997;5(3–4):193–199. [PubMed] [Google Scholar]
- 67.Berry SA, Shah MC, Khan N, Roth BL. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol. Pharmacol. 1996;50(2):306–313. [PubMed] [Google Scholar]
- 68.Hanley NRS, Hensler JG. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J. Pharmacol. Exp. Ther. 2002;300(2):468–477. doi: 10.1124/jpet.300.2.468. [DOI] [PubMed] [Google Scholar]
- 69.Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat. Rev. Neurosci. 2001;2(7):461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- 70.Colbran RJ. Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J. Neurosci. 2004;24(39):8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011;14(10):2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J. Physiol. (Lond.) 2009;587(Pt 9):1931–1942. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacFarlane PM, Wilkerson JER, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008;164(1–2):263–271. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fabian RH, Perez-Polo JR, Kent TA. Extracellular superoxide concentration increases following cerebral hypoxia but does not affect cerebral blood flow. Int. J. Dev. Neurosci. 2004;22(4):225–230. doi: 10.1016/j.ijdevneu.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Wilkerson JER, Macfarlane PM, Hoffman MS, Mitchell GS. Respiratory plasticity following intermittent hypoxia: roles of protein phosphatases and reactive oxygen species. Biochem. Soc. Trans. 2007;35(Pt 5):1269–1272. doi: 10.1042/BST0351269. [DOI] [PubMed] [Google Scholar]
- 76.Conde SV, Monteiro EC. Hypoxia induces adenosine release from the rat carotid body. J. Neurochem. 2004;89(5):1148–1156. doi: 10.1111/j.1471-4159.2004.02380.x. [DOI] [PubMed] [Google Scholar]
- 77.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19(4):126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 78.MacFarlane PM, Vinit S, Roopra A, Mitchell GS. Enhanced Phrenic Long-Term Facilitation Following Repetitive Acute Intermittent Hypoxia: Role of Glycolytic Flux. FASEB J. 2010;24:799.15. (Meeting Abstract Supplement): [Google Scholar]
- 79.Vinit S, MacFarlane PM, Satriotomo I, Mitchell GS. Enhanced Phrenic Long-Term Facilitation (pLTF) Following Repetitive Acute Intermittent Hypoxia. FASEB J. 2010;24:799.14. (Meeting Abstract Supplement): [Google Scholar]
- 80.Reeves SR, Mitchell GS, Gozal D. Early postnatal chronic intermittent hypoxia modifies hypoxic respiratory responses and long-term phrenic facilitation in adult rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290(6):R1664–R1671. doi: 10.1152/ajpregu.00851.2005. [DOI] [PubMed] [Google Scholar]
- 81.Peng Y-J, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J. Appl. Physiol. 2003;94(6):2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- 82.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J. Hypertens. 1997;15(12 Pt 2):1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 83.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr. Res. 2002;52(3):449–453. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- 84.Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng ZJ. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguous of F344 rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296(2):R299–R308. doi: 10.1152/ajpregu.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilkerson JER, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp. Neurol. 2009;217(1):116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp. Neurol. 2012;237(1):103–115. doi: 10.1016/j.expneurol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews Neuroscience. 2008;9(5):387–387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 88.Tenorio G, Connor SA, Guévremont D, Abraham WC, Williams J, O’Dell TJ, Nguyen PV. “Silent” priming of translation-dependent LTP by β-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors. Learn Mem. 2010;17(12):627–638. doi: 10.1101/lm.1974510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21(12):505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 90.Powell FL, Huey KA, Dwinell MR. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir Physiol. 2000;121(2–3):223–236. doi: 10.1016/s0034-5687(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 91.Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J. Appl. Physiol. 1999;87(2):817–823. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]