Abstract
A silver-catalyzed vinylogous fluorination of vinyldiazoacetates to generate γ-fluoro-α,β-unsaturated carbonyls is presented. Application of this method to the fluorination of farnesol and steroid derivatives was achieved.
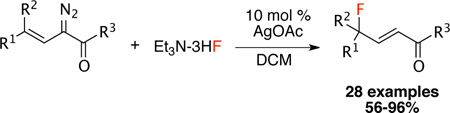
The development of new methods for achieving selective fluorination is a current research area of intense interest.1 Organofluorine compounds display broad utility as valuable pharmaceuticals, agrochemicals, materials and tracers for positron emission tomography.2 γ-Fluoro-α,β-unsaturated carbonyls represent a versatile class of intermediates in organic synthesis and are prevalent motifs in biologically relevant compounds such as steroids, amino acids and metalloprotease inhibitors.3 Traditional approaches for the synthesis of γ-fluoro-α,β-unsaturated carbonyls mainly rely on electrophilic fluorination of conjugated enol ethers4 and Wittig-type reaction of α-fluoro aldehydes or ketones.5 Recently, we6 and others7 have described that metal-stablized vinylcarbenes derived from vinyldiazoacetates can selectively display electrophilic reactivity at the vinylogous position instead of the carbene site. This type of behavior is especially favorable when silver catalysts are used.6c,6d,7h In this communication, we report a silver-catalyzed vinylogous fluorination to generate highly functionalized γ-fluoro-α,β-unsaturated carbonyls (eq. 1).8
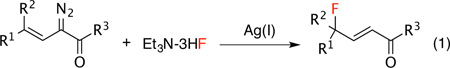 |
(1) |
Our fluorination study began with examination of different fluoride sources using the styryldiazoacetate 1 as the model substrate. Among fluoride sources examined, many of the standard nucleophilic sources of fluoride failed to give any fluorinated products (Table 1, entries 1–6), but Deoxo-Fluor and DAST9 can provide the desired product 2 in 44% and 55% yield, respectively (Table 1, entries 7 and 8). The use of triethylamine trihydrogen fluoride10 dramatically improved the yield to 90% (Table 1, entry 9). After determining the effect of different silver salts (Table 1, entries 9–11), we chose silver acetate and triethylamine trihydrogen fluoride in dichloromethane as our standard fluorination conditions. In all of these reactions, the ratio of vinylogous versus carbenoid fluorination is > 20/1.
Table 1.
Vinylogous Fluorination Optimizationa
 | |||
|---|---|---|---|
| entry | catalyst | fluoride | yield (%)b |
| 1 | AgOAc | TMAF | <5 |
| 2 | AgOAc | TBAFc | <5 |
| 3 | AgOAc | TBABF | <5 |
| 4 | AgOAc | KHF2d | <5 |
| 5 | AgOAc | Fluolead™ | <5 |
| 6 | AgOAc | TASF | <5 |
| 7 | AgOAc | Deoxo-Fluor | 44 |
| 8 | AgOAc | DAST | 55 |
| 9 | AgOAc | Et3N-3HF | 90 |
| 10 | AgSbF6 | Et3N-3HF | 88 |
| 11 | AgOTf | Et3N-3HF | 90 |
Vinyldiazoacetate (0.4 mmol, 1.0 equiv), silver catalyst (10 mol %), fluoride source (2.0 mmol, 5.0 equiv), under refluxing in dichloromethane.
Isolated yield, <5 refers to no observation of product 2 from 1H NMR analysis prior to chromatography.
1.0 M in THF.
Dry DMF as solvent at 90 °C.
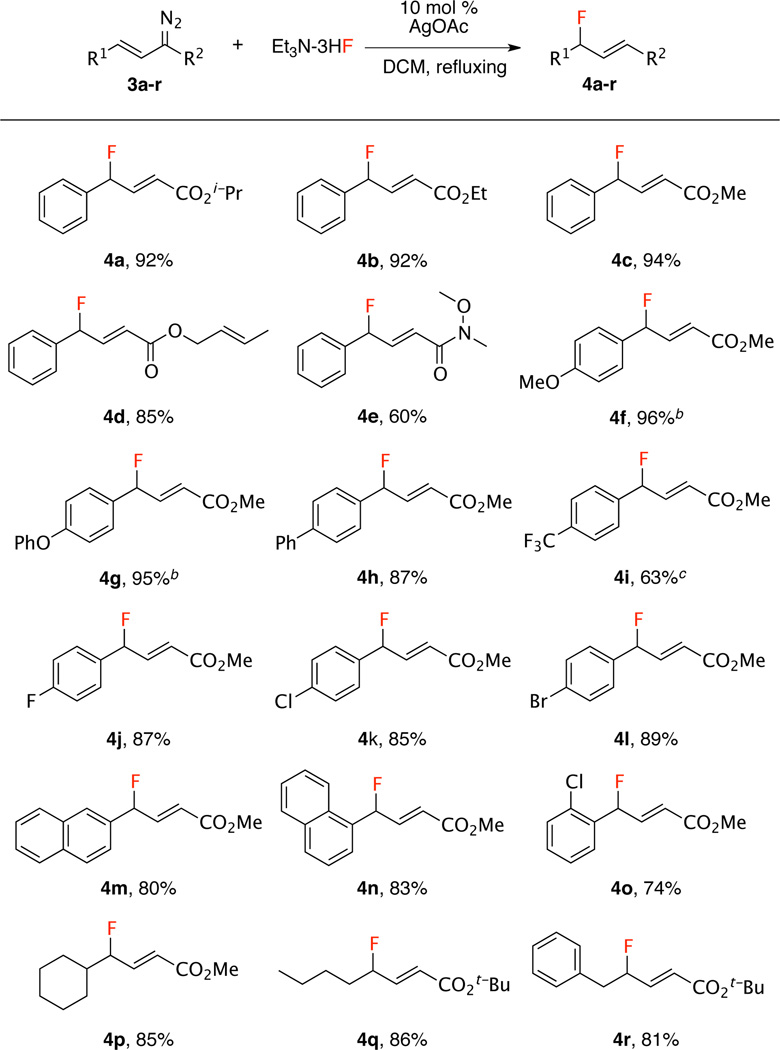
Having developed the optimized conditions, the scope of the vinylogous fluorination was examined with a variety of vinyldiazo derivatives. The reaction was found to be quite general as illustrated in Scheme 1. The size of ester group (tert-butyl to methyl) did not affect the efficiency of this reaction, affording the desired products 4a–c in high yields (92–94%). A particularly interesting example is the substrate 3d with a substituted allyl ester. The desired product 4d was isolated in 85% yield and no intramolecular cyclopropanation was observed. Moreover, when an amide was used as the acceptor group, the reaction can still afford the desired product 4e in 60% isolated yield. The reaction can tolerate a variety of functionality on the aryl group as illustrated by 4f–o (63–96%). Furthermore, the reaction can also be expanded to alkyl-substituted vinyldiazoacetates as seen from 4p–r (81–86%).
Scheme 1.
Synthesis of Secondary Allylic Fluoridesa
aVinyldiazoacetate (0.4 mmol, 1.0 equiv), silver catalyst (10 mol %), triethylamine trihydrogen fluoride (322 mg, 5.0 equiv), under refluxing in dichloromethane. bNMR yield using dibromomethane as internal standard due to product decomposition upon silica gel chromatography. c20 mol% AgOTf and 10 equiv. of Et3N-3HF.
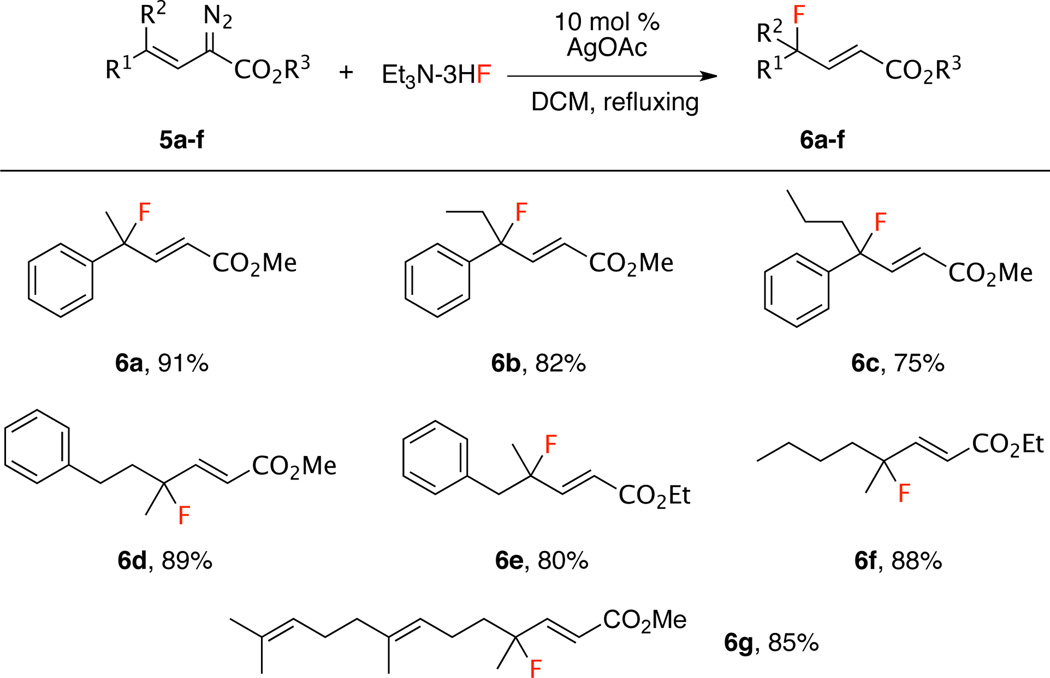
To further evaluate the fluorination method, we designed and synthesized di-substituted vinyldiazoacetates 5a–g. When these vinyldiazoacetates were subjected to the standard conditions, the fluorinated products 6a–g containing quaternary carbon-centers were readily formed in good to excellent yields (75–91%) with a variety of aryl- and alkyl-substituted vinyldiazoacetates (Scheme 2). A particularly interesting example is the synthesis of the fluorinated farnesol derivative 6g.
Scheme 2.
Synthesis of Tertiary Allylic Fluoridesa
aVinyldiazoacetate (0.4 mmol, 1.0 equiv), silver catalyst (10 mol %), triethylamine trihydrogen fluoride (322 mg, 5.0 equiv), under refluxing in dichloromethane.
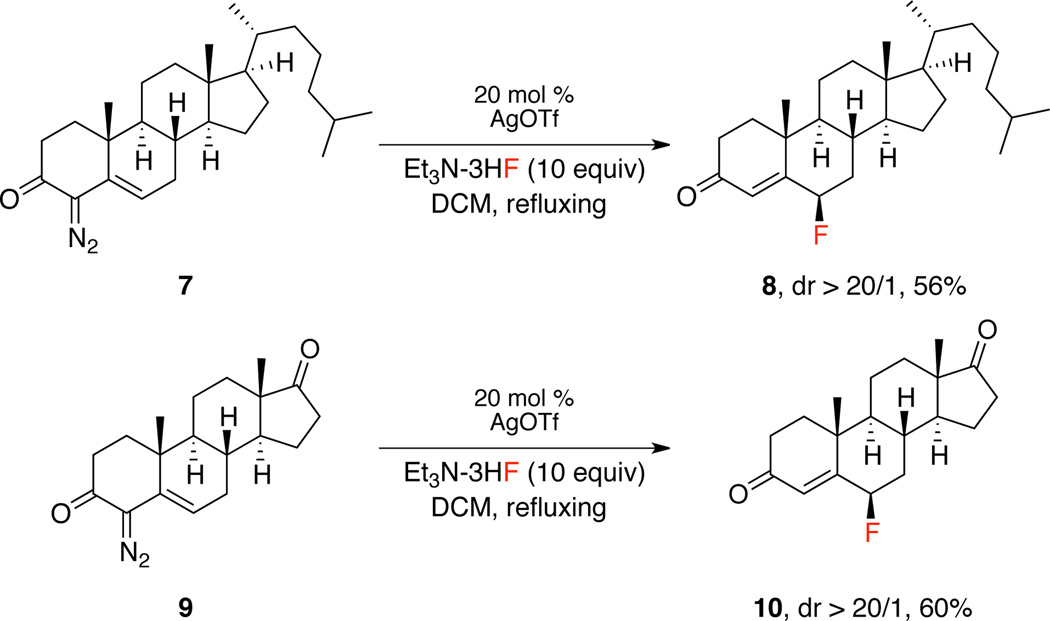
Fluorinated steroids constitute an important class of molecules with significant biological activity.11 Therefore, we sought to apply this method to late-stage fluorination of steroids (Scheme 3). The steroidal diazo derivatives 7 and 9 were readily formed by a diazo transfer reaction on the corresponding steroids. Under slightly modified reaction conditions using silver triflate, diazo 7 and 9 can be converted to the desired fluorinated steroids 8 and 10 in 56% and 60% yield, respectively. An intriguing feature of this fluorination process is the selective formation of the 6-β-fluoro isomer. A similar selectivity has been seen in vinylogous hydroxylation of steroidal diazo via silver catalysis and has been rationalized to be due to stereoelectronic effects from the conformation of the steroid used.6e
Scheme 3.
Late-Stage Fluorination of Steroids
Considerable interest has been shown in developing fast fluorination methods because they may be useful in developing positron emission tomography (PET tracers with 18F labeling, 18F half-life: 110 min).12 Metal-catalyzed reactions of diazo compounds can be extremely fast13 and accordingly we explored the possibility of achieving fast fluorination. Indeed, fluorination of vinyldiazoacetate 3c in 80% isolated yield was achieved in 5 min when 20 mol% of silver triflate was used as catalyst (Scheme 4).
Scheme 4.
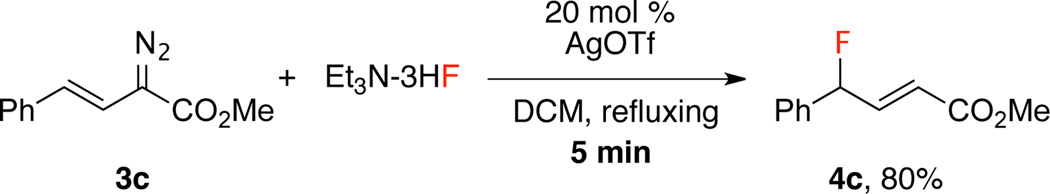
Rapid Fluorination Conditions
In summary, we have developed a silver catalyzed vinylogous fluorination of vinyldiazoacetates. This novel methodology is operationally simple and provides a diverse range of γ-fluoro-α,β-unsaturated carbonyl building blocks. The method offers a strategy for rapid late-stage generation of fluorinated compounds that may be used in the synthesis PET radioligands. Future work will be directed towards developing an enantioselective version of this fluorination methodology.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (GM099142).
Footnotes
Supporting Information Available: Experimental procedures and characterization and spectral data for new compounds. This material is available free of charge via the Internet at http://pubs.ac.org.
References
- 1.For recent leading reviews see: Liang T, Neumann C, Ritter T. Angew. Chem., Int. Ed. 2013;52:8214. doi: 10.1002/anie.201206566. Liu G. Org. Biomol. Chem. 2012;10:6243. doi: 10.1039/c2ob25702e. Hollingworth C, Gouverneur V. Chem. Commun. 2012;48:2929. doi: 10.1039/c2cc16158c. Furuya T, Kamlet AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108. Grushin VV. Acc. Chem. Res. 2010;43:160. doi: 10.1021/ar9001763. Furuya T, Klein JEMN, Ritter T. Synthesis. 2010;11:1804. doi: 10.1055/s-0029-1218742. For recent examples of fluorination, see: Mazzotti AR, Campbell MG, Tang P, Murphy JM, Ritter T. J. Am. Chem. Soc. 2013;135:14012. doi: 10.1021/ja405919z. Sladojevich F, Arlow SI, Tang P, Ritter T. J. Am. Chem. Soc. 2013;135:2470. doi: 10.1021/ja3125405. Braun MG, Doyle AG. J. Am. Chem. Soc. 2013;135:12990. doi: 10.1021/ja407223g. Braun MG, Katcher MH, Doyle AG. Chem. Sci. 2013;4:1216. Shunatona HP, Fruh N, Wang Y, Rauniyar V, Toste FD. Angew. Chem., Int. Ed. 2013;52:1. doi: 10.1002/anie.201302002. Li Z, Song L, Li C. J. Am. Chem. Soc. 2013;135:4640. doi: 10.1021/ja400124t. Truong T, Kilmovica k, Daugulis O. J. Am. Chem. Soc. 2013;135:9342. doi: 10.1021/ja4047125. Zhang Z, Wang F, Mu X, Chen P, Liu G. Angew. Chem., Int. Ed. 2013;52:7549. doi: 10.1002/anie.201301891. Liu W, Groves JT. Angew. Chem., Int. Ed. 2013;52:6024. doi: 10.1002/anie.201301097. Fier PS, Luo J, Hartwig JF. J. Am. Chem. Soc. 2013;135:2552. doi: 10.1021/ja310909q. Ye Y, Sanford MS. J. Am. Chem. Soc. 2013;135:4648. doi: 10.1021/ja400300g. Xue C, Jiang X, Fu C, Ma S. Chem. Commun. 2013;49:5651. doi: 10.1039/c3cc42014k. Liu W, Huang X, Cheng M, Nielsen RJ, Goddard WA, III, Groves JT. Science. 2012;337:1322. doi: 10.1126/science.1222327. Barker TJ, Boger DL. J. Am. Chem. Soc. 2012;134:13588. doi: 10.1021/ja3063716. Topczewski JJ, Tewson TJ, Nguyen HM. J. Am. Chem. Soc. 2011;133:19318. doi: 10.1021/ja2087213. Katcher MH, Sha A, Doyle AG. J. Am. Chem. Soc. 2011;133:15902. doi: 10.1021/ja206960k. Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, Choi DC, Hooker JM, Ritter T. Science. 2011;334:639. doi: 10.1126/science.1212625. Katcher MH, Doyle AG. J. Am. Chem. Soc. 2010;132:17402. doi: 10.1021/ja109120n. Tang P, Furuya T, Ritter T. J. Am. Chem. Soc. 2010;132:12150. doi: 10.1021/ja105834t. Watson DA, Su MJ, Teverovskiy G, Zhang Y, Garcia-Fortanet J, Kinzel T, Buchwald SL. Science. 2009;325:1661. doi: 10.1126/science.1178239.
- 2.(a) Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]; (b) Hagmann WK. J. Med. Chem. 2008;51:4359. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; (c) Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; (d) Jeschke P. ChemBioChem. 2004;5:570. doi: 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]; (e) Phelps ME. Proc. Natl. Acad. Sci. 2000;97:9226. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hougham GG, Cassidy PE, Johns K, Davidson T. Fluoropolymers: Synthesis and Properties. Kluwer Academic; 1999. [Google Scholar]; (g) Banks RE, Smart BE, Tatlow JC, editors. Organofluorine Chemistry: Principles and Commerical Applications. Plenum Press; New York: 1994. [Google Scholar]
- 3.(a) Chen J, Zheng F, Huang Y, Qing F. J. Org. Chem. 2011;76:6525. doi: 10.1021/jo200611w. [DOI] [PubMed] [Google Scholar]; (b) Fan S, He C, Zhang X. Tetrahedron. 2010;66:5218. [Google Scholar]; (c) Orvieto F, Koch U, Matassa VG, Muraglia E. Bioorg. Med. Chem. Lett. 2003;13:2745. doi: 10.1016/s0960-894x(03)00536-5. [DOI] [PubMed] [Google Scholar]; (d) Yoder NC, Kumar K. Chem. Soc. Rev. 2002;31:335. doi: 10.1039/b201097f. [DOI] [PubMed] [Google Scholar]; (e) Takeuchi Y, Shiragami T, Kimura K, Suzuki E, Shibata N. Org. Lett. 1999;1:1571. [Google Scholar]; (f) Poulter CD, Dolence JM. Tetrahedron. 1996;52:119. [Google Scholar]; (g) Pikul S, Mieling GE, Mieling KK, Solinsky KM, De B, Almstead NG, Natchus MG. 6,852,751B2. U. S. Patent. 2005 Feb.
- 4.(a) Poss AJ, Shia GA. Tetrahedron Lett. 1995;36:4721. [Google Scholar]; (b) Purrington ST, Woodard DL, Cale NC. J. Fluorine Chem. 1990;48:345. [Google Scholar]; (c) Fleming L, Goldhill J, Paterson L. Tetrahedron Lett. 1979;20:3205. [Google Scholar]
- 5.(a) Jiang H, Falcicchio A, Jensen KL, Paixão MW, Bertelsen S, Jørgensen KA. J. Am. Chem. Soc. 2009;131:7153. doi: 10.1021/ja901459z. [DOI] [PubMed] [Google Scholar]; (b) Oldendorf J, Haufe G. J. Prakt. Chem. 2000;342:52. [Google Scholar]; (c) Davis FA, Kasu PVN, Sundarababu G, Qi H. J. Org. Chem. 1997;62:7546. [Google Scholar]
- 6.(a) Davies HML, Saikali E, Clark TJ, Chee EH. Tetrahedron. Lett. 1990;31:6299. [Google Scholar]; (b) Davies HML, Hu B, Saikaki E, Bruzinski PR. J. Org. Chem. 1994;59:4535. [Google Scholar]; (c) Sevryugina Y, Weaver B, Hansen J, Thompson J, Davies HML, Petrukina MA. Organometallics. 2008;27:1750. [Google Scholar]; (d) Hansen J, Davies HML. Chem. Sci. 2011;2:457. [Google Scholar]; (e) Morton D, Dick AR, Ghosh D, Davies HML. Chem. Comm. 2012;48:5838. doi: 10.1039/c2cc31973j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Valette D, Lian Y, Haydek JP, Hardcastle KI, Davies HML. Angew. Chem., Int. Ed. 2012;51:8636. doi: 10.1002/anie.201204047. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Smith AG, Davies HML. J. Am. Chem. Soc. 2012;134:18241. doi: 10.1021/ja3092399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Qin C, Davies HML. J. Am. Chem. Soc. 2013;135:14516. doi: 10.1021/ja4069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wang X, Xu X, Zavalji PY, Doyle MP. J. Am. Chem. Soc. 2011;133:16402. doi: 10.1021/ja207664r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu X, Zavalij PY, Hu W, Doyle MP. J. Org. Chem. 2013;78:1583. doi: 10.1021/jo302696y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qian Y, Zavalij PJ, Hu W, Doyle MP. Org. Lett. 2013;15:1564. doi: 10.1021/ol400339c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang X, Abrahams QM, Zavalij PY, Doyle MP. Angew. Chem. Int. Ed. 2012;51:5907. doi: 10.1002/anie.201201917. [DOI] [PubMed] [Google Scholar]; (e) Qian Y, Xu X, Wang X, Zavalij PJ, Hu W, Doyle MP. Angew. Chem., Int. Ed. 2012;51:5900. doi: 10.1002/anie.201202525. [DOI] [PubMed] [Google Scholar]; (f) Barluenga J, Lonzi G, Riesgo L, Lopez LA, Tomas M. J. Am. Chem. Soc. 2010;132:13200. doi: 10.1021/ja106751t. [DOI] [PubMed] [Google Scholar]; (g) Pagar VV, Jadhav AM, Liu RS. J. Am. Chem. Soc. 2011;133:20728. doi: 10.1021/ja209980d. [DOI] [PubMed] [Google Scholar]; (h) Yue Y, Wang Y, Hu W. Tetrahedron Lett. 2007;48:3975. [Google Scholar]
- 8.Previous transformations on carbenoid fluorination of diazo compounds have been achieved under acidic conditions: Olah GA, Welch JT. Synthesis. 1974:896. Setti EL, Mascaretti OA. J. Chem. Soc. Perkin Trans I. 1988:2059. Pasceri R, Bartrum HE, Hayes CJ, Moody CJ. Chem. Commun. 2012;48:12077. doi: 10.1039/c2cc37284c.
- 9.(a) Singh RP, Shreeve JM. Synthesis. 2002;17:2561. [Google Scholar]; (b) Middleton WJ. J. Org. Chem. 1975;40:574. [Google Scholar]; (c) Lal GS, Pez GP, Pesaresi RJ, Prozonic FM. Chem. Commun. 1999:215. [Google Scholar]; (d) Lal GS, Pez GP, Pesaresi RJ, Prozonic FM, Cheng H. J. Org. Chem. 1999;64:7048. [Google Scholar]
- 10.(a) Haufe G. J. Prakt. Chem. 1996;338:99. [Google Scholar]; (b) Nelson TD, Crouch RD. Synthesis. 1996:1031. [Google Scholar]
- 11.(a) Begue JP, Bonnet-Delpon D. J. Fluorine Chem. 2006;127:992. [Google Scholar]; (b) Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr Science. 1995;270:283. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lee E, Hooker JM, Ritter T. J. Am. Chem. Soc. 2012;134:17456. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pimlott SL, Sutherland A. Chem. Soc. Rev. 2011;40:149. doi: 10.1039/b922628c. [DOI] [PubMed] [Google Scholar]; (c) Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]; (d) Adam MJ, Wilbur DS. Chem. Soc. Rev. 2005;34:153. doi: 10.1039/b313872k. [DOI] [PubMed] [Google Scholar]
- 13.Pelphrey P, Hansen J, Davies HML. Chem. Sci. 2010;1:254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.