Abstract
Many studies in recent years have investigated the effects of climate change on the future of biodiversity. In this review, we first examine the different possible effects of climate change that can operate at individual, population, species, community, ecosystem and biome scales, notably showing that species can respond to climate change challenges by shifting their climatic niche along three non-exclusive axes: time (e.g., phenology), space (e.g., range) and self (e.g., physiology). Then, we present the principal specificities and caveats of the most common approaches used to estimate future biodiversity at global and sub-continental scales and we synthesize their results. Finally, we highlight several challenges for future research both in theoretical and applied realms. Overall, our review shows that current estimates are very variable, depending on the method, taxonomic group, biodiversity loss metrics, spatial scales and time periods considered. Yet, the majority of models indicate alarming consequences for biodiversity, with the worst-case scenarios leading to extinction rates that would qualify as the sixth mass extinction in the history of the earth.
Keywords: Biodiversity, climate change, species extinctions
1. Introduction
Predicting the response of biodiversity to climate change has become an extremely active field of research (e.g., Dillon et al. 2010; Gilman et al. 2010; Pereira et al. 2010; Salamin et al. 2010; Beaumont et al. 2011; Dawson et al. 2011; McMahon et al. 2011). Predictions play an important role in alerting scientists and decision makers to potential future risks, provide a means to bolster attribution of biological changes to climate change and can support the development of proactive strategies to reduce climate change impacts on biodiversity (Pereira et al. 2010; Parmesan et al. 2011). Although there is relatively limited evidence of current extinctions caused by climate change, studies suggest that climate change could surpass habitat destruction as the greatest global threat to biodiversity over the next several decades (Leadley et al. 2010). However, the multiplicity of approaches and the resulting variability in projections make it difficult to get a clear picture of the future of biodiversity under different scenarios of global climatic change (Pereira et al. 2010). Hence, there is an urgent need to review our current understanding of the effects of climate change on biodiversity and our capacity to project future impacts using models. To this end, we have reviewed both the ranges of possible impacts of climate change that operate at individual, population, species, community, ecosystem and biome scales and the different responses that could occur at individual, population or species levels. We then present the principal specificities and caveats of the most common approaches used to model future biodiversity at global and sub-continental scales and we synthesize their results focusing on how model combinations are used to project the impacts of climate change on species loss. Finally, we highlight several challenges for future research, from theoretical (e.g., emerging models) and applied (e.g. population conservation and exploitation) realms.
2. Biodiversity and climate change: effects and responses
Climate change effects on biodiversity
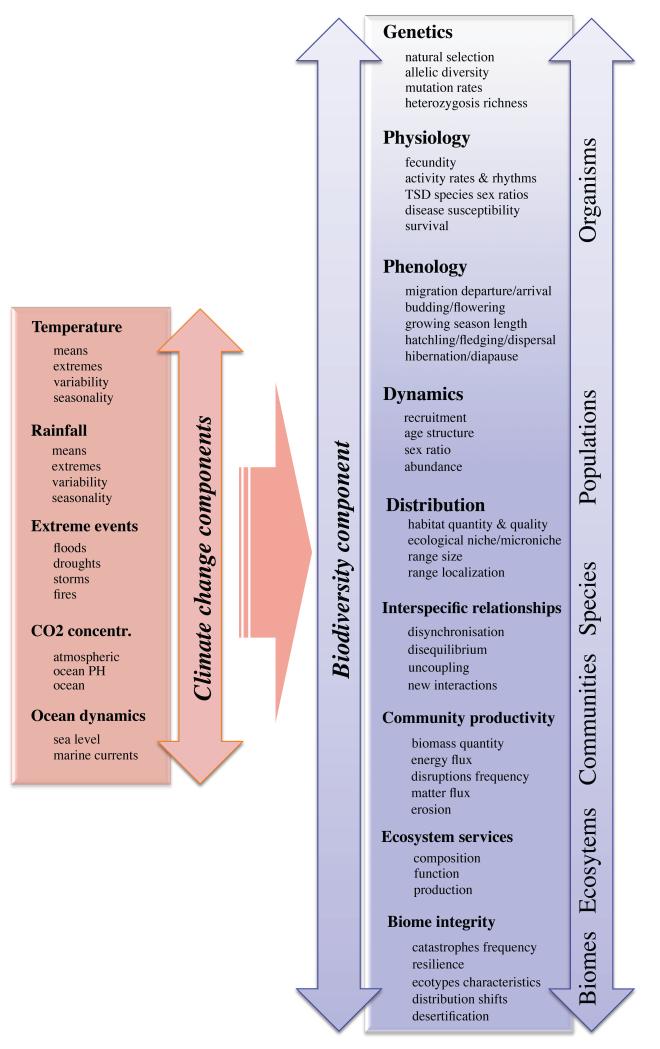
The multiple components of climate change are anticipated to affect all the levels of biodiversity, from organism to biome levels (Figure 1, and reviewed in detail in, e.g., Parmesan 2006). They primarily concern various strengths and forms of fitness decrease, which are expressed at different levels, and have effects on individuals, populations, species, ecological networks and ecosystems. At the most basic levels of biodiversity, climate change is able to decrease genetic diversity of populations due to directional selection and rapid migration, which could in turn affect ecosystem functioning and resilience (Botkin et al. 2007) (but, see Meyers & Bull 2002). However, most studies are centred on impacts at higher organizational levels, and genetic effects of climate change have been explored only for a very small number of species.
Figure 1.
Summary of some of the predicted aspects of climate change and some examples of their likely effects on different levels of biodiversity.
Beyond this, the various effects on populations are likely to modify the “web of interactions” at the community level (Gilman et al. 2010; Walther 2010). In essence, the response of some species to climate change may constitute an indirect impact on the species that depend on them. A study of 9,650 interspecific systems, including pollinators and parasites, suggested that around 6,300 species could disappear following the extinction of their associated species (Koh et al. 2004). In addition, for many species, the primary impact of climate change may be mediated through effects on synchrony with species’ food and habitat requirements (see below). Climate change has led to phenological shifts in flowering plants and insect pollinators, causing mismatches between plant and pollinator populations that lead to the extinctions of both the plant and the pollinator with expected consequences on the structure of plant-pollinator networks (Kiers et al. 2010; Rafferty & Ives 2010). Other modifications of interspecific relationships (with competitors, prey/predators, host/parasites or mutualists) also modify community structure and ecosystem functions (Lafferty 2009; Walther 2010; Yang & Rudolf 2010).
At a higher level of biodiversity, climate can induce changes in vegetation communities that are predicted to be large enough to affect biome integrity. The Millenium Ecosystem Assessment forecasts shifts for 5 to 20% of Earth’s terrestrial ecosystems, in particular cool conifer forests, tundra, scrubland, savannahs, and boreal forest (Sala et al. 2005). Of particular concern are “tipping points” where ecosystem thresholds can lead to irreversible shifts in biomes (Leadley et al. 2010).
A recent analysis of potential future biome distributions in tropical South America suggests that large portions of Amazonian rainforest could be replaced by tropical savannahs (Lapola et al. 2009). At higher altitudes and latitudes, alpine and boreal forests are expected to expand northwards and shift their tree lines upwards at the expense of low stature tundra and alpine communities (Alo & Wang 2008). Increased temperature and decreased rainfall mean that some lakes, especially in Africa, might dry out (Campbell et al. 2009). Oceans are predicted to warm and become more acid, resulting in widespread degradation of tropical coral reefs (Hoegh-Guldberg et al. 2007). The implications of climate change for genetic and specific diversity have potentially strong implications for ecosystem services. The most extreme and irreversible form of fitness decrease is obviously species extinction. To avoid or mitigate these effects, biodiversity can respond in several ways, through several types of mechanisms.
Biodiversity responses to climate change
Because of climate changes, species may no longer be adapted to the set of environmental conditions in a given region and could therefore fall outside its climatic niche. As other components of the ecological niche of species are not supposed to change directly, we will hereafter refer only to climatic niches of species (i.e., the climatic components of the n-dimensional hypervolume sensu Hutchinson). In order to persist, individuals, populations or species must produce adaptive responses, which can be of several types, and are provided by two categories of mechanisms.
Response mechanisms: plastic versus genetic
One of the crucial questions in the debate on ecological effects of climate change is whether species will be able to adapt fast enough to keep up with the rapid pace of changing climate (Lavergne et al. 2010; Salamin et al. 2010). Whatever the type of adaptive responses, underlying mechanisms are either due to micro-evolution (i.e., species can genetically adapt to new conditions through mutations or selection of existing genotypes Salamin et al. 2010; Olofsson et al. 2011) or plasticity, which provides a means of very short-term response (within individual’s lifetimes, Charmantier et al. 2008). It may involve intraspecific variation in morphological, physiological or behavioural traits, which can occur on different time scales within the populations’ spatial range (Botkin et al. 2007; Chevin et al. 2010). Empirical evidence suggests that plastic contribution is often more important than genetic contribution, as observed in birds and marmots (Hoffman & Sgro 2011). On the other hand, there is increasing empirical evidence that evolution can be very rapid (Lavergne et al. 2010). This is the case for many introduced species, for which selection-driven phenotypic changes have enhanced the invasive potential (e.g., Phillips 2009). Recent experiments on evolutionary rescue also confirm that rapid evolution through mutation and selection could allow species with rapid life cycles to adapt very severe and rapid environmental changes (Bell & Gonzalez 2009).
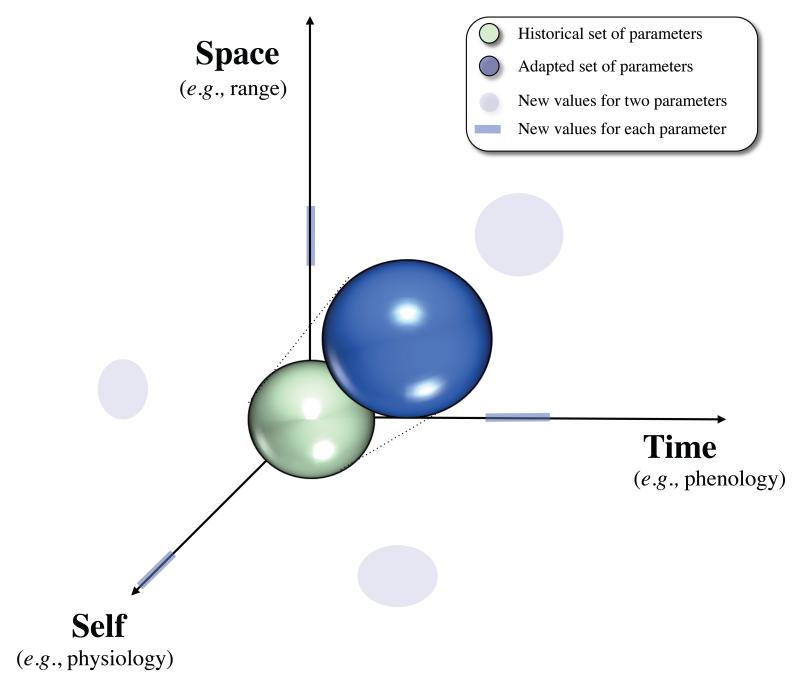
Responses: along three axes
Whatever the mechanisms involved in response to climate change, species can in theory change, and changes have already been observed, along three distinct but non-exclusive axes (Figure 2): spatial, temporal or self. The first two axes correspond to easily observable and well-documented responses to global warming (Parmesan 2006). “Self” corresponds to less visible physiological and behavioural changes that allow species to adapt to the new climatic conditions in the same spatial and temporal frame.
Figure 2.
The three directions of responses to climate change through phenotypic plasticity or evolutionary responses : moving in space (dispersing to areas with suitable habitat or changing location on a microhabitat scale), shifting life history traits in time (adjusting life cycle events to match the new climatic conditions, including phenology and diurnal rhythms), or changing life history traits in its physiology to cope with new climatic conditions. Species can cope with climate change by shifting along one or several of these three axes.
Spatial
First, species can track appropriate conditions in space and follow them. This is typically done through dispersion, but spatial changes are not limited to this: shifts to a different habitat at the local or micro-habitat levels are also relevant. One of the best-documented responses – from both paleontological records and recent observations - is a spatial shift of species tracking suitable climatic conditions at the regional scale. Latitudinal and altitudinal range shifts have already been observed in more than 1,000 species – especially those with high dispersal capacities like birds, insects and marine invertebrates (Parmesan 2006), leading to a reduction in range size particularly in polar and mountaintop species (Forero-Medina 2010). However, individuals shift their distribution in order to stay in quasi-equilibrium with the climatic conditions they are adapted to, but they may not be adapted to other abiotic variables such as photoperiod or novel biotic interactions (Visser 2008). In these cases, micro-evolution may be needed for them to persist (Visser 2008).
Temporal
In order to keep up with changing abiotic factors that show cyclic variation over time, such as temperature on a daily or yearly period, individuals can also respond to climate change through a shift in time (on a daily to seasonal basis). Phenology, i.e., the timing of life cycle events such as flowering, fruiting and seasonal migrations, is one of the most ubiquitous responses to 20th century climate warming. It has already been documented in many species (Parmesan 2006; Charmantier et al. 2008). In a meta-analysis of a wide range of species including animals and plants, the mean response across all species responding to climate change was a shift in key phenological events of 5.1 days earlier per decade over the last 50 years (Root et al. 2003). Flowering has advanced by more than 10 days per decade in some species (Parmesan 2006). These phenological changes can help species keep synchrony with cyclical abiotic factors. Yet, they can also be disruptive, by increasing asynchrony in predator-prey and insect-plant systems (Parmesan 2006), which may lead to species extinction. Temporal shifts may also occur at a small temporal scale, e.g. with activity patterns adjusted in daily activity rhythms, or behaviours adjusted in length to match changes to costs due to a different climatic condition.
Self
Last, species can cope with changing climatic conditions by adapting themselves to the new conditions in their local range, rather than by tracking their current optimal conditions in space or time. For lack of a better term, we refer to these in situ changes that are not related to spatial or temporal changes, as changes in “self”. Species can move along this third, “self” axis by physiological alterations that allow tolerance to warmer or drier conditions or by behavioural modifications of their diet, activity and energy budget, for example. Although they are often less obvious than changes in time or space, some physiological responses have already been reported (Johansen & Jones 2011) during the 20th century climate change, especially from many ectotherms, as their locomotion, growth, reproduction and sex determination are temperature sensitive (Tewksbury et al. 2008). However, for many traits, plastic phenotypic responses should reach a physiological limit and ‘saturate’ in extreme environments. For example, body size or metabolic rate cannot increase or decrease indefinitely under sustained environmental change (Chevin et al. 2010). In this case, strong selection is needed to cope with climate change. As they remain in the same spatial and temporal frame, thereby limiting alterations of interspecific relationships, changes in self also have different consequences for ecosystem responses than do changes in time and space.
Failing to adapt along one or several of these three axes, population or species will go extinct locally or globally. There is thus a multitude of possible responses for species to cope with climate change, and in fact relatively few taxa went extinct following climate change during the Quaternary period (Botkin et al. 2007). This should help temper catastrophist predictions regarding the global effects of the current climate change on biodiversity. However, the responses of many populations are likely to be inadequate to counter the speed and magnitude of the current climate change. In addition, unlike in past periods of climate change, species have now to cope with additional threats, some of which may act in synergy with climate change (Botkin et al. 2007). As we are already facing an irrefutable biodiversity crisis, the number of species that may go extinct following climate change has become a major concern over last few years.
3. Assessing the future of global biodiversity
Our understanding of the effects of global climate change on biodiversity and its different levels of response is still insufficiently well developed. Yet, it is enough to raise serious concern for the future of biodiversity. The most pressing issue is to quantitatively assess the prospects for biological diversity in the face of global climate change. Although several methods exist to draw inferences, starting with existing paleontological or recent data, experiments, observations, and meta-analyses (e.g., Lepetz et al. 2009), ecological modelling is the most commonly used tool for predictive studies. Progress in this field is characterised by both an extremely high pace and a plurality of approaches. In particular, there are three main approaches to projecting species loss, concentrating either on future changes in species range or species extinction or changes in species abundance. However, all three modelling approaches have so far largely focused on one axis of response (change in space), largely overlooking the importance of the other aspects. In addition, they seldom account for the mechanisms of these responses (plasticity and evolution). We briefly discuss here the basic principles and the weakness of the models that are the most widely used at global or at large regional scales in this context, focusing on representative examples of recent work. Table 1 summarizes the specific advantages and limitation of each model type.
Table 1.
Advantages and disadvantages of the two components of major modelling approaches used to estimate loss of biodiversity due to climate change. See Figure 3 for illustrations of how the two components can be combined to estimate biodiversity loss.
| Advantages | Disadvantages | Key references |
|
|---|---|---|---|
|
Biodiversity range
model component |
|||
| • Bioclimatic Envelope Models (BEM) |
-can be applied to a large number of species and a variety of taxonomic groups - implicitly capture many ecological processes in the relationship between occurrence data and spatial information - require few data |
- do not explicitly account for mechanisms that mediate species range - may handle novel climates poorly - lack temporal dynamics - assume that the current distribution of a species is a good indicator of favourable climate |
(3, 5, 6, 11, 12, 15, 16, 17) |
| • Dynamic Vegetation Models (DVMs) |
- include the dynamics of plant growth, competition and, in a few cases, migration - allow the identification of future trends in ecosystem function and structure - can be used to explore feedbacks between biosphere and atmospheric processes |
- require detailed physiological data - do not include plant interactions with other taxonomic groups - limit biodiversity to a very small number of plant functional types. - do not take into account fine scale spatial heterogeneity - are not adapted for predicting species extinctions at local scales |
(1, 4, 7, 17, 18, 19) |
|
Species loss model
component |
|||
| • Species Area Relationships (SAR) |
- are easy to couple with distribution models because they are based on range or habitat loss - can be applied to a variety of taxonomic groups - require few data |
- use values of key parameters that are not well constrained - lack empirical evidence concerning applicability of SAR for climate change or at species range level - lack temporal dynamics - don’t account for processes influencing extinction rates (e.g., population dynamics, adaptive responses) |
(3, 18, 20, 21) |
| • IUCN status methods |
- use a widely accepted measure of threat - are simple to couple with distribution models because partly based on criteria of range or habitat loss |
- depend on thresholds that are somewhat arbitrary - rely on sole criteria of declining range size in most studies - often don’t respect time frame for declines (i.e., 10 years or 3 generations in most cases) |
(3, 5, 22) |
| • Dose response relationships |
- are anchored in measured responses of biodiversity to global change drivers - can assess the impact of a wide range of global change factors alone or their cumulative effects - can include time lags |
- “undisturbed” ecosystems used as baseline are difficult to define - inadequately account for interactions between global change drivers. - lack validation at large regional or global scales - use metrics that are difficult to relate to common biodiversity indices |
(7, 8) |
Biodiversity range models components
Studies modelling species’ range shifts are generally based on the assumption that species niches are defined by a small set of environmental variables, i.e. the species’ “climate niche”, defines the suitable climatic habitat for that particular species. These Bioclimatic Envelope Models, or BEMs, relate current species ranges to multiple climatic variables and thereby define the climatic niche (envelope) for each species. It is then possible to project this niche for different future climate scenarios in order to determine the potential redistribution of the suitable climate space of the species. The extinction risks can then be calculated in different ways using species-area relationships (Thomas et al. 2004) or IUCN status (Thuiller et al. 2005). Despite a number of limitations (lack of biological process as well as methodological and theoretical issues, Soberon & Nakamura 2009), species distribution models still constitute the bulk of studies in this domain (Sinclair et al. 2010).
Shifts in distinct vegetation types, often referred to as biome or habitat shifts, are often simulated with Dynamic Vegetation Models (DVM). These models forecast shifts in vegetation and associated biogeochemical and hydrological cycles in response to climate change. DVMs use time series of climate data (e.g., temperature, precipitation, humidity, sunshine days) and take into account constraints of topography and soil characteristics in order to simulate monthly or daily dynamics of ecosystem processes. Plant species are represented as groups with similar physiological and structural properties, termed Plant Functional Types (PFTs), which are designed to represent all major types of plants (Sitch et al. 2008). PFT distributions can then be used to estimate changes in biome or habitat ranges. Currently, DVMs are of limited use for projecting responses in biodiversity directly (i.e., the absence of animals and the limitation to ca. 10 PFTs exclude direct utilization). However, coupled with extinction models, they allow extinction risk for species to be estimated at the regional or global scale (e.g., van Vuuren et al. 2006).
Species loss models components
The simplest method for calculating extinction risk is to assume that species go extinct when they no longer have any suitable habitat (Jetz et al. 2007). This may underestimate extinctions because species often enter an “extinction vortex” well before they loose their entire habitat. Yet, it could also substantially overestimate extinctions because many species have weak habitat specificity (Malcolm et al. 2006; Willis & Bhagwat 2009).
The Species Area Relationship (SAR), an empirical relationship between the number of species and the land area of a region, is often used to estimate extinction risk (Thomas et al. 2004). Species extinctions are calculated as a direct function of habitat loss or climate induced range contraction based on the observation that extinction risk increases with decreasing range and population size. SAR methods may over or underestimate species extinction risk depending on the capacity of species to persist in small populations or adapt to novel environments, and they also fail to provide a time frame in which extinctions are likely to occur (Chevin et al. 2010) because the extinction debt is not accounted for (Pereira et al. 2010). The relevance of using this relationship to project biodiversity loss is still hotly debated (He & Hubbell 2011).
The IUCN has developed criteria to assess species extinction risk for their Red List of the conservation status of plant and animal species (Baillie et al. 2004). These include 90 biological traits identified as enhancing species’ vulnerability (e.g., habitat specialisation, narrow environmental tolerance, dependence on specific environmental triggers, dependence on inter-specific interactions or poor ability to disperse to or colonize a new range, Foden et al. 2008). Recent attempts at projecting climate change impacts on biodiversity have used the IUCN status metric to obtain estimates of extinction rates based on projected range shifts (Thuiller et al. 2005), although the use of IUCN status for this purpose has also been questioned (Akcçakaya et al. 2006).
In Dose Response Relationship (DRR) models, observational data and experiments can be employed to generate empirical relationships between the relative importance of global drivers (dose) and changes in species loss (response).
Most models focus on species extinctions, which are only the last step of a decline in abundance and are a less immediate (although dramatic) impact of climate change. This fact has led to the development of new models that attempt to quantify the impact of human activities on species abundance (i.e., an equivalent of DRR for species abundance). Impacts on biodiversity are estimated using biodiversity indicators including Mean Species Abundance (MSA, Alkemade et al. 2009) and Biodiversity Intactness Index (Biggs et al. 2008). For example, the GLOBIO model uses a matrix of changes in mean local species abundances following conversion between two land use or land cover categories, derived from empirical studies (Leadley et al. 2010). These types of models use species abundances in pristine ecosystems as a baseline, and thereby provide a measure of the distance from “naturalness” of plant and animal communities following human disturbance. Species abundance models based on plant traits and abiotic characteristics can also provide evidence of changes in ecosystem services (Lavorel et al. 2011).
There are, however, several major limitations to these models, especially concerning the indices of biodiversity derived from DRR applied to species abundance. They depend largely on the quality of the input data (of which much more is needed than in most other models), uncertainty cannot be taken into account, and they are difficult to relate to commonly used biodiversity indices (Alkemade et al. 2009).
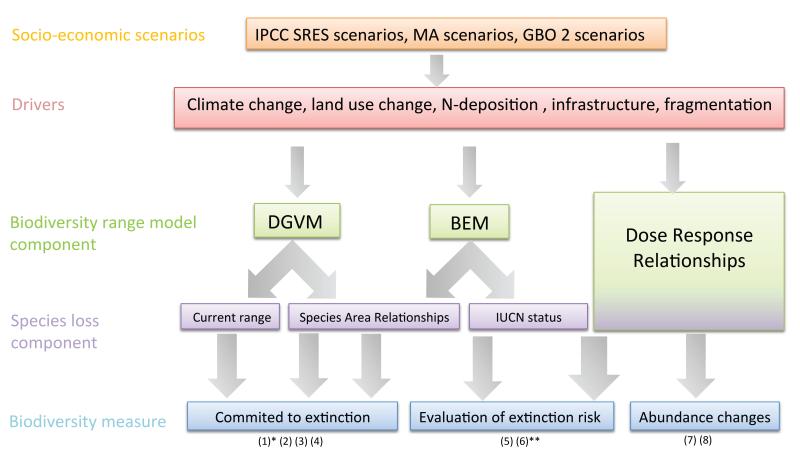
Model combinations
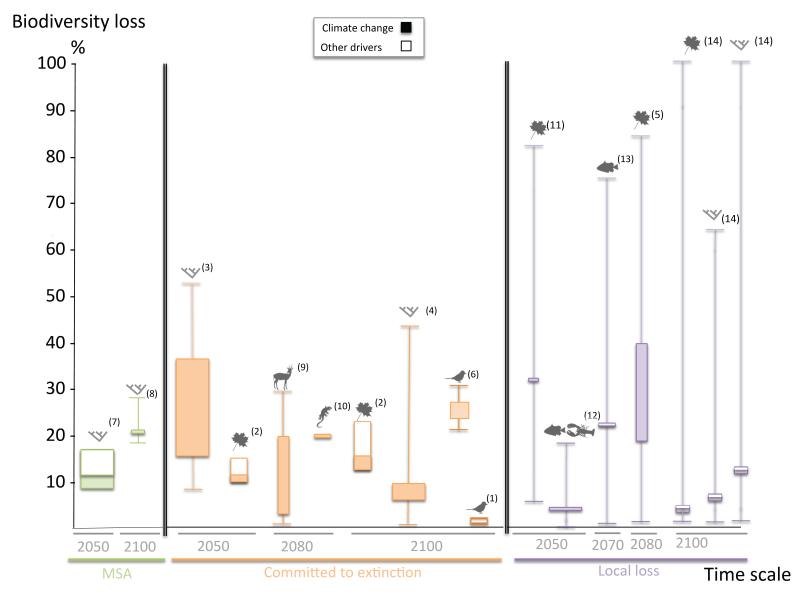
Current modelling techniques usually incorporate a succession of one (or several) future socio-economic scenario(s), one (or several) extinction driver(s), one habitat or species range model (Figure 3). They then express their projections in terms of various extinction metrics. This has generated a variety of “model combinations” leading to a wide range of projections that are not straightforward to compare, as the underlying assumptions differ greatly. We have synthesized in Figure 4 the results of all such projections made at a global scale either geographically or taxonomically. Despite important differences and some bias (e.g., marine biodiversity is still poorly represented), these models generally indicate that many species can be expected to decline rapidly at a global scale.
Figure 3.
Examples on successive combinations of socio-economic scenarios, projections of extinction drivers, habitat or species range models and extinction models and biodiversity metrics, leading to projections of biodiversity losses following climate change. Numbers correspond to references (see Appendix S2 for details and reference list).
Figure 4.
Projections of loss of biodiversity due to climate change, for different taxonomic, temporal and spatial scales. The width of the box illustrates three levels of generality: global scale and several taxonomic groups ( ), global scale and only one taxonomic group, continental scale and only one taxonomic group. The box is delimited by the upper and lower boundaries of the intermediate scenario, while maximum and minimum values of the whiskers indicate the highest and lowest biodiversity losses across all projections. This figure illustrates that the different studies (i) generally predict significant biodiversity loss and (ii) use a combination of different biodiversity metrics, taxonomic groups and spatial scale and time horizon, making generalisations difficult. Numbers correspond to references (see Appendix S2 for details and references).
), global scale and only one taxonomic group, continental scale and only one taxonomic group. The box is delimited by the upper and lower boundaries of the intermediate scenario, while maximum and minimum values of the whiskers indicate the highest and lowest biodiversity losses across all projections. This figure illustrates that the different studies (i) generally predict significant biodiversity loss and (ii) use a combination of different biodiversity metrics, taxonomic groups and spatial scale and time horizon, making generalisations difficult. Numbers correspond to references (see Appendix S2 for details and references).
Projections of species loss
The field of climate change biology has thus followed several distinct and independent lines of model development (Figure 3). This is potentially advantageous, as the convergent predictions can be regarded as an indication of robustness. In essence, the results show that local species extinctions cover an extremely large range, with some areas experiencing virtually no losses and others facing nearly complete loss of current species. High local losses can concern relatively large areas; for example, Bakkenes et al. (Bakkenes et al. 2002) estimated the more than 16% of European landmass would have local species losses exceeding 50% by 2050. This must be distinguished from projections of species extinctions at the global level, which are unsurprisingly lower than at the local level, since local extinctions do not necessarily lead to global extinctions. Nevertheless, even these global estimates suggest major losses of biodiversity due to global climate change that are generally higher than current rates of loss and far higher than rates of species extinctions documented in the fossil record (Pereira et al. 2010; Barnosky et al. 2011). For example, one of the earliest global studies estimated that by 2050 15-37% of species are committed to extinction under intermediate climate warming (Thomas et al. 2004). Birds are projected to be particularly sensitive to climate change. Two studies have calculated losses by 2100 due to climate change: these range from less than 0.3% of the world’s 8,750 species of land birds would be committed to extinction (Jetz et al. 2007) and up to 30% of the 8,400 land bird species in the Western Hemisphere could go extinct (Sekercioglu et al. 2008). Regarding the vulnerability of 25 major biodiversity hotspots, Malcolm and colleagues suggested that the extinctions of endemic species could reach 39-43% under in worst-case scenarios, representing the potential loss of 56,000 endemic plant species and 3,700 endemic vertebrate species (Malcolm et al. 2006).
Studies based on changes in species abundance generally predict an erosion of biodiversity in the same order of magnitude as species extinction models do, i.e., losses of 11 to 17% of mean species abundance by the end of the century (Alkemade et al. 2009). Using data from 9,856 birds, 6,222 amphibians and 799 coral reefs, the IUCN forecasted that about 35% of the world’s birds, 52% of amphibians and 71% of warm-water reef-building corals are particularly susceptible to climate change (Foden et al. 2008). Variability in local extinctions is significantly higher than in global extinctions projections. Indeed, in some regions the species composition will not change at all and in others almost all species will be lost following climate change. Estimated losses of riverine fish richness at a local scale vary considerably between 0 and 75% according to the river considered (Xenopoulos et al. 2005). Similarly, estimated local losses of plant diversity in Europe range from 2 to 84% of species lost per pixel (Thuiller et al. 2005).
Overall, the diversity of taxonomic groups studied, of biodiversity loss metrics, of spatial scales and time periods render complex and uncertain comparisons between these studies. In addition, the large array of modelling techniques further complicates the comparisons. Moreover, several studies assess the combined effects on future biodiversity of both climate change and land use, making it difficult to distinguish the effect of each. Finally, there is growing concern that the underlying assumptions of all of these models could lead to large over- or underestimations of potential species losses (Pereira et al. 2010; Dawson et al. 2011). These are summarized Figure 5 (see also below). As an example, a recent study suggests that the realized effects of climate change might far exceed the current predictions (Maclean & Wilson 2011). Based on a global multi-taxon meta-analysis of recorded ecological responses, they estimated that the mean observed extinction risk by 2100 is systematically higher than the mean predicted extinction risk across the theoretical studies considered in the meta-analysis: 12.6% in plants (vs. 4% predicted), 9.4% in invertebrates (vs. 7,2 % predicted) and 17.7% in vertebrates (vs. 12.4 % predicted). Overall, these estimates of biodiversity loss are obviously a major concern.
Figure 5.
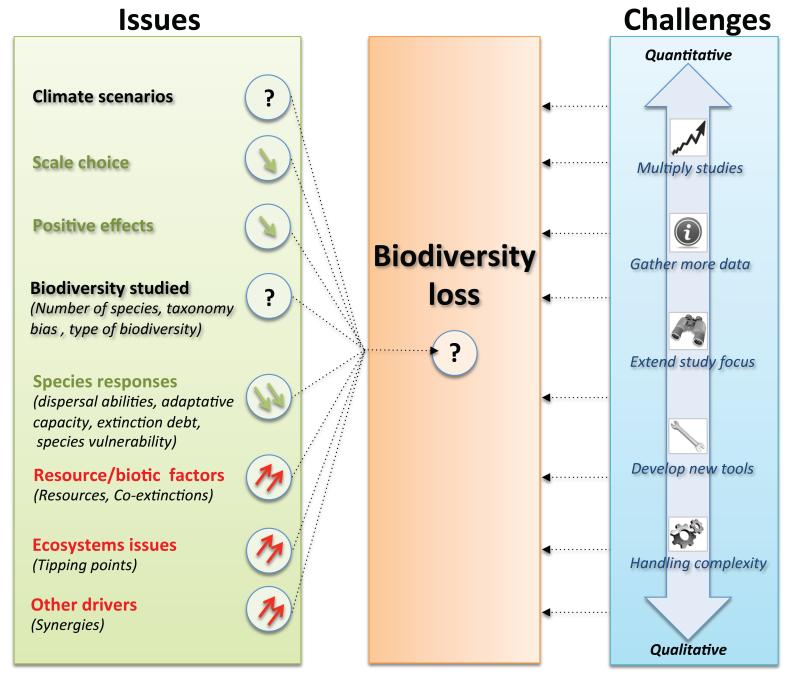
Issues and challenges for climate change driven biodiversity loss. Examples of each are presented and discussed in the main text. The issues are in the first box, which represents the main factors of uncertainty and the direction of the likely current bias (green factors and arrow down is a likely overestimation, while red factors and arrow up is a likely underestimation; both can occur simultaneously, according to the approach taken in different studies; black factors and question marks are for unknown direction of mis-estimation; double arrow means very large expected effect). Challenge types range from quantitative (such as increasing the number of studies and the quantity of available data, or extend the focus of studies to include more factors) to more qualitative ones (developing new tools and progressing in resolving complexity).
4. What further improvements are needed?
Climate change ecology is still in its infancy, and tremendous improvements are made rapidly in virtually all aspects of this emerging field. Critical requirements to be able to predict future trends include the need to study a much larger part of biodiversity, to overcome several major model limitations, to account for co-extinctions and other major drivers of biodiversity loss and to validate models by comparing projections with observations. These limitations are detailed below. Figure 5 provides a summary of the directions in which each of these weaknesses is likely to affect the current projections on biodiversity loss, as well as the major types of challenges that have to be faced to overcome these uncertainties.
Climate scenarios
Climate scenarios depend on a wide range of socio-economic storylines for greenhouse gas emissions in the future, including the Special Report on Emissions Scenarios (SRES) Millenium Assessment and Global Biodiversity Outlook scenarios (Pereira et al. 2010), and on a broad suite of General Circulation Models used to calculate climate change for given trajectories of greenhouse gas emissions. This means that the projections of species loss can yield highly contrasting results depending on the choice of combinations of emissions scenarios and climate models, independently of the model of biodiversity response that is used (Beaumont et al. 2008). In addition, internal climate model variability could result in greater differences in projected species’ distributions than variability between climate models (Beaumont et al. 2007). In addition, 4% to 39% of the world’s landmass will experience combinations of climate variables that do not currently have equivalent values anywhere on the globe (so called no-analog climates, Williams et al. 2007). One key challenge is to provide robust and credible uncertainty intervals for all model outcomes and, if possible, to reduce them.
Scale choice
The choice of the spatial resolution scale is probably one of the most important factors generating variability. For example, a coarse, European-scale model (with 10′×10′ grid cells) predicted a loss of all suitable habitats during the 21st century, whereas a model using local-scale data (25×25 m grid cells) predicted persistence of suitable habitats in up to 100% of plant species (Randin et al. 2009). These differences are probably explained by the failure of coarser spatial scale models to capture both local topographic diversity and habitat heterogeneity (Luoto & Heikkinen 2008; Randin et al. 2009). On one hand, global models can be used for a large number of species but focus on one type of species’ response and lack therefore biological realism. On the other hand, population or species models provide insight into a very limited range of species, typically at regional scales (i.e., adaptation phenology, dynamic population) but cannot provide global scale trends. This is a classical trade-off between precise small-scale models and coarse large-scale models that lack biological realism (Thuiller 2003).
Positive effects
Climatic changes could also have positive effects on biodiversity. For instance, more clement temperatures and increased CO2 are likely to be beneficial to many plants, resulting in an acceleration of biomass production. Milder winters might increase survival of many currently threatened species in temperate regions. Increased precipitation may also benefit some plant communities and species depending on them. Moreover, several studies reported detrimental effects of climate change on biological invasions (e.g., Peterson et al. 2008). Although few studies report beneficial effects of global changes on biodiversity, they certainly exist and add to the difficulty of getting a clear overview of the effects of climate changes on the biodiversity of our planet.
Biodiversity measures
Even in the most ambitious studies, the range of species studied always represents a small percentage of known biodiversity. All studies are taxonomically biased, as they generally concentrate on a few conspicuous taxonomic groups such as plants, mammals and birds (Thuiller et al. 2011), with a particularly strong bias towards terrestrial vs. marine biodiversity. However, it is generally recognized that the vast majority of biodiversity in terms of species richness, evolutionary divergence, biomass and even ecosystem functioning is represented by “cryptic biodiversity”, especially micro-organisms and insects (Esteban & Finlay 2010). Similarly, there are important biases in data collection both across regions and ecosystems (McMahon et al. 2011). Furthermore, most studies focus on species richness, because it is thought to influence the resilience and resistance of ecosystems to environmental change. However, a few studies have explored the impact of climate change on functional (Thuiller et al. 2006) and phylogenetic diversity (Thuiller et al. 2011), and the effects on genetic diversity are only beginning to be explored. Moreover, it is likely that different levels of diversity are affected differently by climate change, so these should be evaluated in parallel in order to provide a broad picture of biodiversity response to climate change (Devictor et al. 2010).
In addition, there are different indicators of biodiversity change, such as the number of species “committed” to extinction (Thomas et al. 2004; Pereira et al. 2010), extinction risk (Thuiller et al. 2005), or change in abundances (Alkemade et al. 2009; Leadley et al. 2010). The number of species “committed” to extinction is probably not the most appropriate metric to forecast the future of biodiversity because the extinction debt could vary from decades to centuries (Kuussaari et al. 2009). A complementary metric of biodiversity can be changes in Mean Species Abundance, which is an index defined as the mean abundance of original species relative to their abundance in undisturbed ecosystems. As extinction is only the last step in the process of species decline, the MSA can provide a metric to evaluate ecosystem degradation. In addition, we need new approaches to predict the impacts of climate change at the community level (i.e., Mokany & Ferrier 2011). Currently, neither the estimates of species losses nor of the qualitative impacts on ecosystem functioning and services are satisfactory to give a global overview of the impacts of climate change (Dale et al. 2010).
Limitations of predictive tools
Each of the modelling approaches reviewed has methodological, spatial and temporal limitations that constrain their predictive power (e.g., McMahon et al. 2011). In general, models do not take into account the multiple responses of species to climate change, but rather focus mainly on one axis, spatial shifts of potential habitat (Figure 2). The temporal and the physiological responses are generally overlooked, as are the genetic and plastic capacity of species (Lavergne et al. 2010; Salamin et al. 2010). In addition, species are commonly considered as static and independent entities, although their dynamics and their role in ecological networks are both known to be essential. However a new generation of models is emerging that focuses on more realistic biological hypotheses and meets some of the challenges posed by each limitation (e.g., Thuiller et al. 2008 ; Brook et al. 2009; Gallien et al. 2010).
Species responses
Current global extinction models make very coarse assumptions about species responses. For example, the dispersal capability is a major issue for projections of future biodiversity. Until recently, models often addressed dispersal issues by using two extreme assumptions of either unlimited or no species dispersal (Thomas et al. 2004). This is clearly convenient for practical purposes, but most species are between these extremes. In addition, exceptional occurrences of long-distance dispersal are thought to have helped past species surmount prehistoric climate changes (Dawson et al. 2011 and references therein). Although rare, these events are of crucial significance in the current context, as they could in many cases make the difference between species survival and extinction, especially as human mediated long-distance dispersion is now common for many organisms.
Inherent differences in vulnerability are not always taken into account when making large-scale correlative predictions of the future of biodiversity. Indeed, most models work under the very strong hypotheses that species currently live in an optimal climatic niche and that they cannot survive if there is a change in the climatic conditions that contribute to define this niche. Such a hypothesis amounts to overlook the potential of adaptability of species. Numerous studies have shown that species are capable of fast responses through phenotypic plasticity or microevolution (Lavergne et al. 2010). Before being able to forecast species trajectories with some credibility, we therefore need to assess properly the vulnerability of species (including exposure, sensitivity, adaptive capacity and migration potential), habitats and regions to different components of climate change (Dawson et al. 2011; McMahon et al. 2011). Despite growing evidence for rapid adaptive evolution in response to climate change, the consequences of such evolution on species persistence remain to be explored (Lavergne et al. 2010). Currently, more flexible models of micro-evolutionary processes combine population based information with phylogenetic comparative methods to provide an estimation of the evolutionary potential (Salamin et al. 2010). Moreover, populations can be locally adapted to specific climatic conditions and therefore, models treating a species as a single homogeneous unit might be flawed. Consequently, studying under which circumstances losses in genetic and species diversity at local to regional scales occurred in the past could improve models outputs (Dawson et al. 2011; McMahon et al. 2011).
Population and metapopulation dynamics
An inherent property of current global extinction models is that they do not take into account population or metapopulation dynamics that determine species distributions, population structure and extinction risk at a local scale. Models are often based on habitat reduction and do not offer a mechanism by which the species’ fitness is reduced and ultimately led to extinction. Recently, new mechanistic niche models have endeavoured to estimate key fitness components such as survival, growth, development and reproduction as a function of species attributes (i.e., physiology, phenology, behaviour) that vary with climatic conditions (Kearney et al. 2010).
Currently the probability of extinction is based on a projection of suitable habitats entirely lost (species will be committed to extinction) or partially lost (species will increase their extinction risk), although it possible to combine niche models and abundance data (Iverson & Prasad 1998). In fact, on one side some species are more likely to go extinct because they consist of small and disconnected populations and on the other side, some species have shown compensatory changes in demographic rates following climate change (Doak & Morris 2010). The approach of Population Viability Analysis (PVA) in this context could provide a good alternative. It is used to estimate the likelihood of a population’s extinction within a given number of years through the species’ demographic characteristics and environmental variability, instead of species range shifts. Only a few studies have used PVA to predict the effects of climate change on extinction risks (Keith et al. 2008; Anderson et al. 2009), in part because this approach requires data on population dynamics that are not available for most species.
In addition, physio-demo genetic models could provide the most realistic method to forecast the future of key species, as they consider simultaneously demographic (e.g., growth, rate of survival and reproduction, migration processes, environmental stochasticity), physiological (e.g., metabolic rate) and genetics parameters (heritability and norm of reaction). However, studies using physio-demo genetic models to evaluate the impacts of climate changes remain scarce (Kramer et al. 2010).
Co-extinctions
Whether centred on a single species or taking into account large taxonomic groups, most studies and all models have disregarded interspecific relationships such as competition, facilitation or mutualism. Beyond single-species extinctions, both direct and indirect processes can lead to cascading and catastrophic co-extinctions, also called “chains of extinction” (Brook et al. 2008). Despite the importance of interspecific interactions, these relationships are exceptionally difficult to model; this is especially cogent in the context of lack of data on population dynamics and trophic webs (McMahon et al. 2011). As each species comes with its cortege of specific parasites and symbionts, as well as many trophic relationships, the consequences of global change on biodiversity might be substantially underestimated when focusing on species-specific extinction rates (Koh et al. 2004; Yang & Rudolf 2010). There is an urgent need not only to go beyond the single species approach, but also to get past the species richness approach and to consider interspecific interactions, trophic webs and ecological networks (Bascompte 2009).
Synergies between extrinsic drivers of extinction
Research on future species extinctions has so far mostly considered a limited set of the predicted changes. For example, sea level rise has seldom been considered, although the most recent scenarios projecting up to 2 meters of rise by 2100 are raising new concerns for coastal and insular biodiversity (Grinsted et al. 2009). In addition, studies have mainly focused on the sole impact of climate change, sometimes considering a single additional driver (e.g., land use change). These limitations may often lead to overly optimistic estimates. Indeed, other components of global change, such as habitat fragmentation, pollution, overexploitation and biological invasions have all been documented as major, additional threats for the future of biodiversity, with possible synergies, or reinforcing feedbacks between them (Sala et al. 2000). For instance climate-induced facilitation of invasions can occur at all stages of the invasions process (Walther et al. 2009; Bradley et al. 2010). In an experimental context, habitat fragmentation and overfishing combined with global warming have led to a decline in rotifer populations up to 50 times faster than when either threat acts alone (Mora et al. 2007). In this case, dose response relationship models could offer a powerful approach because they could simulate the impacts of many drivers of biodiversity.
Overall, despite important uncertainties and much conflicting imprecision, both under- and overestimating species loss, the very large underestimations due to co-extinctions, synergies and tipping points are extremely worrisome for the future of biodiversity (Figure 5). Given the attractiveness of quantitative projections from mathematical models, it is tempting to underestimate their limits. To minimize this risk, it is crucial to use a variety of complementary approaches, ranging from observational studies (paleontological data, phenological responses, adaptive capacity) to field and laboratory experiments (on genetic, species and ecosystem levels) (Dawson et al. 2011).
5. Biodiversity management
The large variation of responses of different species necessitates the use and integration of multiple approaches in order to further our understanding of the impacts climate change can have on biodiversity (Dawson et al. 2011). Similarly, our responses in terms of biodiversity management ought to transcend disciplines. Beyond this, global climate change prompts several methodological issues and the implications for conservation and management of biodiversity and of ecosystem services.
Conservation of species and ecosystems
The large projected impacts of climate change on biodiversity at all levels mean that ecologists must quickly rise to the challenge of providing scientific guidance for the development of conservation strategies (Pressey et al. 2007; Araùjo et al. 2011; Dawson et al. 2011). A major role of conservation planning is to design reserve networks that protect biodiversity in situ. Currently few studies have attempted to use modelling for conservation purposes (Araùjo et al. 2011). It is increasingly important to protect the heterogeneity of habitats as well as genetic diversity within a species in order to sustain the capacity of a species to adapt. Also, the characteristics of protected areas, where planning has to be done decades in advance (Hansen et al. 2010), need to be reviewed under climate change. Climate modelling can help to re-evaluate the current set of protected areas, their places, size, layout and design (Araùjo et al. 2011). In particular, protection should be prioritized for places that minimize the effects of climate change – like forests, which contribute strongly to local climatic conditions – as well as for climate refuges for biodiversity (Carnaval et al. 2009). Finally, model predictions of where, when, and how future risks may affect species, biomes or ecosystems could assist in identifying the most appropriate conservation measures. For instance, species or ecosystem projected to be primarily affected by climate change may require adapted measures compared to species negatively affected by land-use change that could persist through protection of their remaining natural habitat (Hannah et al. 2007). In many cases there may no longer be any overlap between a species’ current range and its possible future range. As this situation will lead to extinction, climate change has been a major argument for the proponents of human-assisted colonisation (Loss et al. 2011). In this increasingly hot debate, case-by-case decisions have been advocated, based on the balance between threatened status of a species and threat of that species for the recipient ecosystem, as well as the socioeconomic context in which conservation is taking place (Richardson et al. 2009; Dawson et al. 2011). A widespread view is that an important strategy is to enhance landscape connectivity to enable species to move through a matrix of interconnected habitats in order to favour escapes from unsuitable climatic conditions (Hannah et al. 2007).
It is also essential to shift from a species centred focus to a holistic view encompassing species interaction networks, and other aspects of biodiversity such as functional and phylogenetic diversities (Devictor et al. 2010). Beyond these various strategies, there is a growing call to go past the predictive focus and start aiming for an integrated and unified framework to identify species vulnerability and adapt biodiversity management interventions (Dawson et al. 2011). In this regard, preventive actions are of foremost importance. For example, it should be remembered that the proportion of species extinction is a power function of the expected global warming (Hansen et al. 2010). Minimizing global warming could therefore have non-linear effects in the preservation of species from extinction, with each tenth of degree avoided saving an increasing number of species (Hansen et al. 2010). Acting to reduce global warming itself, and not only its effect on biodiversity, should remain a priority in conservation sciences. In addition, reducing other global change drivers could increase overall resilience of biodiversity in the face of climate change (Hughes et al. 2003).
Ecosystem services
Other aspects of biodiversity management will be affected by global change and will need adapting, including wildlife exploitation (e.g., forestry, (Dale et al. 2010) or fisheries, (Stram & Evans 2009)), agronomy (Howden et al. 2007), pest and invasive species control (Ziska et al. 2011) or human and wildlife disease management (Harvell et al. 2002). For example, major challenges in agronomy include the need to shift to species or varieties better adapted to particular components of climate change or to rethink strategies to control invasive and pest outbreaks, finding solutions in the increasing competition for water between the natural and the agricultural ecosystems, improving infrastructures and adapting cropping systems to meet future demands of a growing population living on poorer biodiversity resources (Howden et al. 2007).
Given the tremendous ecological impact of alien invasive species, and the expected exacerbation of invasion due to climate change (Walther et al. 2009), it is urgent to increase predictive power in this field. It is also crucial to move beyond predictions (Dawson et al. 2011) and to strengthen risk assessment, protocols of screening and of early detection, vector control and integrated management in area and/or of invasive species that will become at higher risk following climate change. Similar efforts are imperative for other drivers of biodiversity loss, such as overexploitation or habitat destruction.
6. Conclusion
Ecologists are developing a better understanding of the mechanisms by which species and ecosystems can be impacted by climate change. The timing of species’ life cycle events is expected to be further altered, species distributions will change radically, trophic networks will be affected and ecosystem functioning may be severely impaired, leading in the worst cases to countless species extinctions. Over the past decades, some of this understanding has been effectively translated into mathematical models that can be used to forecast climate change impacts on species distributions, abundance, and extinctions. These models are characterized by their high diversity of underlying structures and assumptions, with predictions differing greatly depending on the models used and species studied. Most of these models indicate alarming consequences for biodiversity with worst-case scenarios leading to extinction rates that would qualify as the sixth mass extinction in the history of the earth (Barnosky et al. 2011). However, all current approaches have serious weaknesses. An evaluation of known mechanisms of climate impacts on biodiversity suggests that the lack of several key mechanisms in models may lead to either very large underestimations or overestimations of risks for biodiversity. Improvements in existing models and, in particular, a new generation of models must address the shortcomings of current models in order to reduce uncertainties. It is also crucial to improve our understanding of the vulnerability of biodiversity to climate change, to develop other predictive approaches and to go beyond predictions.
Crucially, the diversity of approaches, methods, scales and underlying hypotheses used has led to an ensemble of global quantitative predictions that can seldom be compared. Consequently, we are left with a mosaic of information that cannot provide a quantitative, coherent picture of future biodiversity loss. Yet, standardization of future studies (of taxonomic groups, methods, time horizon, scale, etc…), which might help decrease uncertainty, would do so at the expense of the breath of knowledge and of a much needed innovation in this field. In this regard, a solution may come from a collective effort in conducting large meta-studies that would encompass many components of variability (biodiversity, time and space scale, models, …) in order to both infer similarities and assess sources of inconsistency. Given its scale, such an ambitious endeavour needs an enduring cooperative effort from coordinated research groups. The long awaited Intergovernmental Platform on Biodiversity and Ecosystem Services (Perrings et al. 2011) could provide the impetus for building such national and international efforts. A major near-term target to substantially improve our understanding, predictive capacity and reactive potential will be to contribute to this new IPCC-like assessment for biodiversity and ecosystem services.
Supplementary Material
References
- Akcçakaya H, Butchart SHM, Mace GM, Stuart SN, Hilton-Taylor C. Use and misuse of the IUCN Red List Criteria in projecting climate change impacts on biodiversity. Global Change Biology. 2006;12:2037–2043. [Google Scholar]
- Alkemade R, van Oorschot M, Miles L, Nellemann C, Bakkenes M, ten Brink B. GLOBIO3: A Framework to Investigate Options for Reducing Global Terrestrial Biodiversity Loss. Ecosystems. 2009;12:374–390. [Google Scholar]
- Alo CA, Wang GL. Potential future changes of the terrestrial ecosystem based on climate projections by eight general circulation models. Journal of Geophysical Research-Biogeosciences. 2008;113:16. [Google Scholar]
- Anderson BJ, Akcakaya HR, Araujo MB, Fordham DA, Martinez-Meyer E, Thuiller W, Brook BW. Dynamics of range margins for metapopulations under climate change. Proceedings of the Royal Society B-Biological Sciences. 2009;276:1415–1420. doi: 10.1098/rspb.2008.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araùjo MB, Alagador D, Cabeza M, Nogues-Bravo D, Thuiller W. Climate change threatens European conservation areas. Ecology Letters. 2011;14:484–492. doi: 10.1111/j.1461-0248.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie JEM, Hilton-Taylor, C., Stuart SN. In: 2004 IUCN Red List of Threatened Species. Assessment. AGS, editor. IUCN; Gland, Switzerland: 2004. [Google Scholar]
- Bakkenes M, Alkemade JMR, Ihle F, Leemans R, Latour JB. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Global Change Biology. 2002;8:390–407. [Google Scholar]
- Barnosky AD, Matzke N, Tomiya S, Wogan GOU, Swartz B, Quental TB, Marshall C, McGuire JL, Lindsey EL, Maguire KC, Mersey B, Ferrer EA. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471:51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- Bascompte J. Disentangling the Web of Life. Science. 2009;325:416–419. doi: 10.1126/science.1170749. [DOI] [PubMed] [Google Scholar]
- Beaumont LJ, Hughes L, Pitman AJ. Why is the choice of future climate scenarios for species distribution modelling important? Ecology Letters. 2008;11:1135–1146. doi: 10.1111/j.1461-0248.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- Beaumont LJ, Pitman A, Perkins S, Zimmermann NE, Yoccoz NG, Thuiller W. Impacts of climate change on the world’s most exceptional ecoregions. Proceedings of the National Academy of Sciences. 2011;108:2306–2311. doi: 10.1073/pnas.1007217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont LJ, Pitman AJ, Poulsen M, Hughes L. Where will species go? Incorporating new advances in climate modelling into projections of species distributions. Global Change Biology. 2007;13:1368–1385. [Google Scholar]
- Bell G, Gonzalez A. Evolutionary rescue can prevent extinction following environmental change. Ecology Letters. 2009;12:942–948. doi: 10.1111/j.1461-0248.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- Biggs R, Simons H, Bakkenes M, Scholes RJ, Eickhout B, van Vuuren D, Alkemade R. Scenarios of biodiversity loss in southern Africa in the 21st century. Global Environmental Change-Human and Policy Dimensions. 2008;18:296–309. [Google Scholar]
- Botkin DB, Saxe H, Araujo MB, Betts R, Bradshaw RHW, Cedhagen T, Chesson P, Dawson TP, Etterson JR, Faith DP, Ferrier S, Guisan A, Hansen AS, Hilbert DW, Loehle C, Margules C, New M, Sobel MJ, Stockwell DRB. Forecasting the effects of global warming on biodiversity. Bioscience. 2007;57:227–236. [Google Scholar]
- Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH. Predicting plant invasions in an era of global change. Trends in Ecology & Evolution. 2010;25:310–318. doi: 10.1016/j.tree.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Brook BW, Akcakaya HR, Keith DA, Mace GM, Pearson RG, Araujo MB. Integrating bioclimate with population models to improve forecasts of species extinctions under climate change. Biology Letters. 2009;5:723–725. doi: 10.1098/rsbl.2009.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook BW, Sodhi NS, Bradshaw CJA. Synergies among extinction drivers under global change. Trends in Ecology & Evolution. 2008;23:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Campbell A, Kapos V, Scharlemann JPW, Bubb P, Chenery A, Coad L, Dickson B, Doswald N, Khan MSI, Kershaw F, Rashid M. Review of the Literature on the Links between Biodiversity and Climate Change: Impacts, Adaptation and Mitigation. In: Diversity SotCoB, editor. CBD Technical Series n°42. Secretariat of the Convention on Biological Diversity; Montreal: 2009. p. 124. [Google Scholar]
- Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C. Stability Predicts Genetic Diversity in the Brazilian Atlantic Forest Hotspot. Science. 2009;323:785–789. doi: 10.1126/science.1166955. [DOI] [PubMed] [Google Scholar]
- Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320:800–803. doi: 10.1126/science.1157174. [DOI] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM. Adaptation, Plasticity and Extinction in a Changing Environment: Towards a Predictive Theory. PLoS Biol. 2010;8:e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale VH, Tharp ML, Lannom KO, Hodges DG. Modeling transient response of forests to climate change. Science of The Total Environment. 2010;408:1888–1901. doi: 10.1016/j.scitotenv.2009.11.050. [DOI] [PubMed] [Google Scholar]
- Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. Beyond Predictions: Biodiversity Conservation in a Changing Climate. Science. 2011;332:53–58. doi: 10.1126/science.1200303. [DOI] [PubMed] [Google Scholar]
- Devictor V, Mouillot D, Meynard C, Jiguet F, Thuiller W, Mouquet N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecology Letters. 2010;13:1030–1040. doi: 10.1111/j.1461-0248.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- Dillon ME, Wang G, Huey RB. Global metabolic impacts of recent climate warming. Nature. 2010;467:704–706. doi: 10.1038/nature09407. [DOI] [PubMed] [Google Scholar]
- Doak DF, Morris WF. Demographic compensation and tipping points in climate-induced range shifts. Nature. 2010;467:959–962. doi: 10.1038/nature09439. [DOI] [PubMed] [Google Scholar]
- Esteban GF, Finlay BJ. Conservation work is incomplete without cryptic biodiversity. Nature. 2010;463:293–293. doi: 10.1038/463293c. [DOI] [PubMed] [Google Scholar]
- Foden W, Mace G, Vié J-C, Angulo A, Butchart SHM, DeVantier L, Dublin H, Gutsche A, Stuart S, Turak E. Species susceptibility to climate change impacts. In: Vié J-C, Hilton-Taylor C, S.N. S, editors. The 2008 Review of the IUCN Red List of Threatened Species. IUCN; Gland, Switzerland: 2008. pp. 1–12. [Google Scholar]
- Forero-Medina G, Joppa L, Pimm SL. Constraints to Species’ Elevational Range Shifts as Climate Changes. Conservation Biology. 2010;25:163–171. doi: 10.1111/j.1523-1739.2010.01572.x. [DOI] [PubMed] [Google Scholar]
- Gallien L, Munkemuller T, Albert CH, Boulangeat I, Thuiller W. Predicting potential distributions of invasive species: where to go from here? Diversity and Distributions. 2010;16:331–342. [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends in Ecology & Evolution. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejava S. Reconstructing sea level from paleo and projected temperatures 200 to 2100 AD. Climate dynamic. 2009 [Google Scholar]
- Hannah L, Midgley G, Andelman S, Araujo M, Hughes G, Martinez-Meyer E, Pearson R, Williams P. Protected area needs in a changing climate. Frontiers in Ecology and the Environment. 2007;5:131–138. [Google Scholar]
- Hansen L, Hoffman J, Drews C, Mielbrecht E. Designing Climate-Smart Conservation: Guidance and Case Studies. Conservation Biology. 2010;24:63–69. doi: 10.1111/j.1523-1739.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Ecology - Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- He F, Hubbell SP. Species–area relationships always overestimate extinction rates from habitat loss. Nature. 2011;473:368–371. doi: 10.1038/nature09985. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hoffman AA, Sgro CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- Howden SM, Soussana JF, Tubiello FN, Chhetri N, Dunlop M, Meinke H. Adapting agriculture to climate change. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19691–19696. doi: 10.1073/pnas.0701890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- Iverson LR, Prasad AM. Predicting abundance of 80 tree species following climate change in the eastern united states. Ecological Monographs. 1998;68:465–485. [Google Scholar]
- Jetz W, Wilcove DS, Dobson AP. Projected Impacts of Climate and Land-Use Change on the Global Diversity of Birds. Plos biology. 2007;5:1211–1219. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JL, Jones GP. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Global Change Biology. 2011;17:2971–2979. [Google Scholar]
- Kearney MR, Wintle BA, Porter WP. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conservation Letters. 2010;3:203–213. [Google Scholar]
- Keith DA, Akcakaya HR, Thuiller W, Midgley GF, Pearson RG, Phillips SJ, Regan HM, Araujo MB, Rebelo TG. Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biology Letters. 2008;4:560–563. doi: 10.1098/rsbl.2008.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL. Mutualisms in a changing world: an evolutionary perspective. Ecology Letters. 2010;13:1459–1474. doi: 10.1111/j.1461-0248.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. Species coextinctions and the biodiversity crisis. Science. 2004;305:1632–1634. doi: 10.1126/science.1101101. [DOI] [PubMed] [Google Scholar]
- Kramer K, Degen B, Buschbom J, Hickler T, Thuiller W, Sykes MT, de Winter W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change-Range, abundance, genetic diversity and adaptive response. Forest Ecology and Management. 2010;259:2213–2222. [Google Scholar]
- Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R, Ockinger E, Partel M, Pino J, Roda F, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I. Extinction debt: a challenge for biodiversity conservation. Trends in Ecology & Evolution. 2009;24:564–571. doi: 10.1016/j.tree.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Lapola DM, Oyama MD, Nobre CA. Exploring the range of climate biome projections for tropical South America: The role of CO2 fertilization and seasonality. Global Biogeochem. Cycles. 2009;23 [Google Scholar]
- Lavergne S, Mouquet N, Thuiller W, Ronce O. Biodiversity and Climate Change: Integrating Evolutionary and Ecological Responses of Species and Communities. Annual Review of Ecology, Evolution, and Systematics, Vol 41. 2010;41:321–350. [Google Scholar]
- Lavorel S, Grigulis K, Lamarque P, Colace MP, Garden D, Girel J, Pellet G, Douzet R. Using plant functional traits to understand the landscape distribution of multiple ecosystem services. Journal of Ecology. 2011;99:135–147. [Google Scholar]
- Leadley P, Pereira HM, Alkemade R, Fernandez-Manjarrés JF, Proença V, Scharlemann JPW, Walpole MJ. Biodiversity Scenarios: Projections of 21st century change in biodiversity and associated ecosystem services. In: Diversity SotCoB, editor. Secretariat of the Convention on Biological Diversity. Montreal: 2010. p. 132. (Technical Series no. 50). 132 pages. [Google Scholar]
- Lepetz V, Massot M, Schmeller DS, Clobert J. Biodiversity monitoring some proposals to adequately study species’ responses to climate change. Biodiversity Conservation. 2009;18:3185–3203. [Google Scholar]
- Loss SR, Terwilliger LA, Peterson AC. Assisted colonization: Integrating conservation strategies in the face of climate change. Biological Conservation. 2011;144:92–100. [Google Scholar]
- Luoto M, Heikkinen RK. Disregarding topographical heterogeneity biases species turnover assessments based on bioclimatic models. Global Change Biology. 2008;14:483–494. [Google Scholar]
- Maclean IMD, Wilson RJ. Recent ecological responses to climate change support predictions of high extinction risk. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12337–12342. doi: 10.1073/pnas.1017352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm JR, Liu CR, Neilson RP, Hansen L, Hannah L. Global warming and extinctions of endemic species from biodiversity hotspots. Conservation Biology. 2006;20:538–548. doi: 10.1111/j.1523-1739.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- McMahon SM, Harrison SP, Armbruster WS, Bartlein PJ, Beale CM, Edwards ME, Kattge J, Midgley G, Morin X, Prentice IC. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends in Ecology & Evolution. 2011;26:249–259. doi: 10.1016/j.tree.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Meyers LA, Bull JJ. Fighting change with change: adaptive variation in an uncertain world. Trends in Ecology & Evolution. 2002;17:551–557. [Google Scholar]
- Mokany K, Ferrier S. Predicting impacts of climate change on biodiversity: a role for semi-mechanistic community-level modelling. Diversity and Distributions. 2011;17:374–380. [Google Scholar]
- Mora C, Metzger R, Rollo A, Myers RA. Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proceedings of the Royal Society B-Biological Sciences. 2007;274:1023–1028. doi: 10.1098/rspb.2006.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J, THickler T, Sykes MT, Araujo MB, Balletto E, Berry PM, Bonello S, Cabeza M, Dubuis A, Guisan A, Kujala H, Piper J, Rounsevell M, Settele J, Thuiller W, Kuhn I. Climate change impacts on European biodiversity - rates of recent observed responses broadly matches projected future rates. Biological Reviews. 2011 In press. [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Ecology Evolution. 2006;37:637–669. [Google Scholar]
- Parmesan C, Duarte CM, Poloczanska E, Richardson AJ, Singer MC. Overstretching attribution. Nature Climate Change. 2011;1 [Google Scholar]
- Pereira HM, Leadley PW, Proenca V, Alkemade R, Scharlemann JPW, Fernandez-Manjarres JF, Araujo MB, Balvanera P, Biggs R, Cheung WWL, Chini L, Cooper HD, Gilman EL, Guenette S, Hurtt GC, Huntington HP, Mace GM, Oberdorff T, Revenga C, Rodrigues P, Scholes RJ, Sumaila UR, Walpole M. Scenarios for Global Biodiversity in the 21st Century. Science. 2010;330:1496–1501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- Perrings C, Duraiappah A, Larigauderie A, Mooney H. The Biodiversity and Ecosystem Services Science-Policy Interface. Science. 2011;331:1139–1140. doi: 10.1126/science.1202400. [DOI] [PubMed] [Google Scholar]
- Peterson AT, Stewart A, Mohamed KI, Araujo MB. Shifting Global Invasive Potential of European Plants with Climate Change. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL. The evolution of growth rates on an expanding range edge. Biology Letters. 2009;5:802–804. doi: 10.1098/rsbl.2009.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey RL, Cabeza M, Watts ME, Cowling RM, Wilson KA. Conservation planning in a changing world. Trends in Ecology & Evolution. 2007;22:583–592. doi: 10.1016/j.tree.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Rafferty NE, Ives AR. Effects of experimental shifts in flowering phenology on plant– pollinator interactions. Ecology Letters. 2010;14:69–74. doi: 10.1111/j.1461-0248.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- Randin CF, Engler R, Normand S, Zappa M, Zimmermann NE, Pearman PB, Vittoz P, Thuiller W, Guisan A. Climate change and plant distribution: local models predict high-elevation persistence. Global Change Biology. 2009;15:1557–1569. [Google Scholar]
- Richardson DM, Hellmann JJ, McLachlan JS, Sax DF, Schwartz MW, Gonzalez P, Brennan EJ, Camacho A, Root TL, Sala OE, Schneider SH, Ashe DM, Clark JR, Early R, Etterson JR, Fielder ED, Gill JL, Minteer BA, Polasky S, Safford HD, Thompson AR, Vellend M. Multidimensional evaluation of managed relocation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9721–9724. doi: 10.1073/pnas.0902327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root BA, Price JT, Hall K. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:47–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Sala OE, van Vuuren D, Pereira HM, Lodge D, Alder J, Cumming G, Dobson A, Wolters V, MA X, Zaitsev A, Polo M, Gomes I, Queiroz C, JA R. Millenium Ecosystem Assesment. Millenium Ecosystem Assesment; 2005. Chap 10: Biodiversity across Scenarios; pp. 375–408. [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Salamin N, Wüest RO, Lavergne S, Thuiller W, Pearman PB. Assessing rapid evolution in a changing environment. Trends in Ecology & Evolution. 2010;25:692–698. doi: 10.1016/j.tree.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Sekercioglu CH, Schneider SH, Fay JP, Loarie SR. Climate Change, Elevational Range Shifts, and Bird Extinctions. Conservation biology. 2008;22:140–150. doi: 10.1111/j.1523-1739.2007.00852.x. [DOI] [PubMed] [Google Scholar]
- Sinclair SJ, White MD, Newell GR. How useful are species distribution models for managing biodiversity under future climates ? Ecology and Society. 2010;15:8. [Google Scholar]
- Sitch S, Huntingford C, Gedney N, Levy PE, Lomas M, Piao SL, Betts R, Ciais P, Cox P, Friedlingstein P, Jones CD, Prentice IC, Woodward FI. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs) Global Change Biology. 2008;14:2015–2039. [Google Scholar]
- Soberon J, Nakamura M. Niches and distributional areas: Concepts, methods, and assumptions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19644–19650. doi: 10.1073/pnas.0901637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stram DL, Evans DCK. Fishery management responses to climate change in the North Pacific. Ices Journal of Marine Science. 2009;66:1633–1639. [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA. Ecology - Putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, de Siqueira MF, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Townsend Peterson A, Phillips OL, Williams SE. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Thuiller W. BIOMOD - optimizing predictions of species distributions and projecting potential future shifts under global change. Global Change Biology. 2003;9:1353–1362. doi: 10.1111/gcb.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Albert C, Araújo MB, Berry PM, Cabeza M, Guisan A, Hickler T, Midgley GF, Paterson J, Schurr FM, Sykes MT, Zimmermann NE. Predicting global change impacts on plant species’ distributions: Future challenges. Perspectives in Plant Ecology, Evolution and Systematics. 2008;9:137–152. [Google Scholar]
- Thuiller W, Lavergne S, Roquet C, Boulangeat I, Lafourcade B, Araujo MB. Consequences of climate change on the tree of life in Europe. Nature. 2011;470:531–534. doi: 10.1038/nature09705. [DOI] [PubMed] [Google Scholar]
- Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8245–8250. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Lavorel S, Sykes MT, Araújo MB. Using niche-based modelling to assess the impact of climate change on tree functional diversity in Europe. Diversity and Distributions. 2006;12:49–60. [Google Scholar]
- van Vuuren DP, Sala OE, Pereira HM. The future of vascular plant diversity under four global scenarios. Ecology and Society. 2006;11 [Google Scholar]
- Visser ME. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proceedings of the Royal Society B-Biological Sciences. 2008;275:649–659. doi: 10.1098/rspb.2007.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G-R, Roques A, Hulme PE, Sykes MT, Pysek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarosík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J. Alien species in a warmer world: risks and opportunities. Trends in Ecology & Evolution. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Walther GR. Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:2019–2024. doi: 10.1098/rstb.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Jackson ST, Kutzbacht JE. Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5738–5742. doi: 10.1073/pnas.0606292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis KJ, Bhagwat SA. Biodiversity and Climate Change. Science. 2009;326:806–807. doi: 10.1126/science.1178838. [DOI] [PubMed] [Google Scholar]
- Xenopoulos MA, Lodge DM, Alcamo J, Marker M, Schulze K, Van Vuuren DP. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Global Change Biology. 2005;11:1557–1564. [Google Scholar]
- Yang LH, Rudolf VHW. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecology Letters. 2010;13:1–10. doi: 10.1111/j.1461-0248.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Ziska LH, Blumenthal DM, Runion GB, Hunt ER, Diaz-Soltero H. Invasive species and climate change: an agronomic perspective. Climatic Change. 2011;105:13–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.