Abstract
Obesity interventions can result in weight loss, but accurate prediction of the bodyweight time course requires properly accounting for dynamic energy imbalances. In this report, we describe a mathematical modelling approach to adult human metabolism that simulates energy expenditure adaptations during weight loss. We also present a web-based simulator for prediction of weight change dynamics. We show that the bodyweight response to a change of energy intake is slow, with half times of about 1 year. Furthermore, adults with greater adiposity have a larger expected weight loss for the same change of energy intake, and to reach their steady-state weight will take longer than it would for those with less initial body fat. Using a population-averaged model, we calculated the energy-balance dynamics corresponding to the development of the US adult obesity epidemic. A small persistent average daily energy imbalance gap between intake and expenditure of about 30 kJ per day underlies the observed average weight gain. However, energy intake must have risen to keep pace with increased expenditure associated with increased weight. The average increase of energy intake needed to sustain the increased weight (the maintenance energy gap) has amounted to about 0·9 MJ per day and quantifies the public health challenge to reverse the obesity epidemic.
Introduction
While the complexity of the obesity epidemic is graphically illustrated by the web of interacting variables in the Foresight Obesity Map,1 at the central core of the system map lies a fundamental principle of nutrition and metabolism: bodyweight change is associated with an imbalance between the energy content of food eaten and energy expended by the body to maintain life and perform physical work.2 Any successful intervention targeting obesity (eg, diet, exercise, drugs, bariatric surgery, etc) must tip the balance between energy intake and expenditure. Therefore, to assess the potential of an obesity intervention, its effect on both energy intake and energy expenditure over time needs to be quantified.
Despite the simplicity of this core energy balance principle, calculation of the dynamics of energy imbalance and translation of the imbalance to a change in bodyweight is not straightforward. Widespread official recommendations from the National Health Service in the UK, the National Institutes of Health and the American Dietetic Association in the USA erroneously state that reduction of energy intake by about 2 MJ per day will result in slow and steady weight loss of about 0·5 kg per week.3–6 This ubiquitous weight-loss rule (also known as the 3500 kcal per pound rule) was derived by estimation of the energy content of weight lost7 but it ignores dynamic physiological adaptations to altered body weight that lead to changes of both the resting metabolic rate as well as the energy cost of physical activity.8 Unfortunately, this static weight-loss rule continues to be used for weight-loss counselling and has been misapplied at the population level to predict the effect of policy interventions on obesity prevalence.9–12 While it is generally recognised that the static weight-loss rule is overly simplistic, there is a dearth of methods for accurate predictions of how changes of diet or physical activity will translate into weight changes over time.
We address this issue by using a dynamic mathematical modelling approach to human metabolism that integrates our knowledge about how the human body responds to changes of diet and physical activity. Although several mathematical models of human metabolism and weight change have been developed in the past,13–27 here, we describe some insights about weight loss resulting from our research group’s experience in developing and validating various mathematical models of human metabolism and body-composition change.7,28–37 Furthermore, we present a web-based implementation of one of our dynamic mathematical models of human metabolism with which readers can do their own simulations. We show how this dynamic simulation model of human metabolism can predict the time course of weight change at both the individual and population levels.
Quantitative physiology of weight change in adults
An imbalance between energy intake and energy expenditure is accounted for by a gain or loss of body fat and lean tissue which generally change in parallel.30,38 Thus, quantification of weight change requires both a dynamic assessment of how energy expenditure changes over time as well as how energy imbalances are partitioned between storage or mobilisation of body fat and lean tissue. The energy content per kg change of body fat is 39·5 MJ and 7·6 MJ for a kg of lean mass.7 Thus, to lose the same mass of fat as lean tissue requires about a 5-fold greater deficit in net energy. Changes of body fat and lean tissue are related by a non-linear function of the initial body fat mass such that people with higher initial adiposity partition a greater proportion of a net energy imbalance towards gain or loss of body fat versus lean tissue than do people with low initial adiposity.30,38 The physiological mechanisms underlying this non-linear relation are complex and involve the regulation of metabolic fuel selection,31,34 but the net result can be described by a simple equation first presented more than 30 years ago38 and subsequently updated and validated.30,37
Although less energy is stored in lean tissue than in body fat, lean tissue is more energetically expensive to maintain than is body fat and contributes more to the body’s overall energy expenditure rate.39 Thus, energy partitioning also contributes to changes in the energy expenditure rate of the body, particularly the resting metabolic rate and the energy cost of tissue deposition and turnover. These factors, along with the energy cost of physical activity, have recently been incorporated in validated mathematical models of human metabolism and body composition change for adults.28,29,31,33,36 The webappendix (pp 1–3) describes a non-linear dynamic model that captures the important physiology.
Modelling of the dynamics of weight change
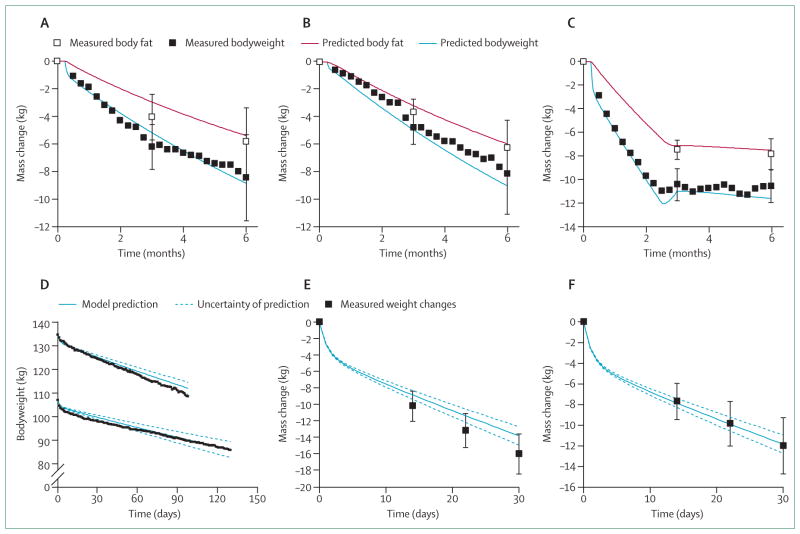
Figure 1A–C shows our model simulations of weight change and body fat change along with experimental data from the CALERIE study80 that investigated 6 months of 25% caloric restriction, 12·5% caloric restriction plus exercise, and 3 months of a liquid diet of 3·7 MJ per day followed by a period of weight maintenance. The close agreement between the model predictions and the data provides some validation of the mathematical model, since these data were not used for model development. Figure 1D shows another validation study, in which two obese women were provided with liquid diets of about 3·3 MJ per day in an inpatient setting.81 The model accurately reproduced the different rates of weight loss resulting from the differing predicted energy expenditure rates in these women. Similarly, figure 1E, F shows the simulated and measured weight changes over the course of a 30 day fast in obese men and women, respectively.83 Again, the simulated weight change dynamics agree reasonably well with the data thereby providing some confidence that the model accurately captures the quantitative physiology of weight loss.
Figure 1. Predicted bodyweight and fat-mass changes by use of a dynamic simulation model of human metabolism.
Error bars are ±1SD. (A) Predicted and measured average changes of bodyweight and fat mass during 25% caloric restriction in 12 overweight men and women.80 (B) Predicted and measured average changes of bodyweight and fat mass during 12·5% caloric restriction plus exercise in 12 overweight men and women.80 (C) Predicted and measured average changes of bodyweight and fat mass during a very low energy liquid diet followed by weight maintenance diet in 12 overweight men and women.80 (D) Daily weight changes in two obese women during a very low energy liquid diet.81 Model predictions are shown as solid blue curves and the dotted curves illustrate the uncertainty of the predictions based on the inherent imprecision of estimating baseline energy expenditure, assuming an uncertainty in the initial energy expenditure rate of ±1 MJ per day. (E) Average weight change during a 30-day fast in 18 obese men and (F) 58 obese women.82
Calculation of the energy deficit generated by a given diet requires knowledge of the energy needed to maintain the baseline bodyweight. Unfortunately, we cannot measure the initial energy requirements of a free-living individual with a precision better than about 5%.83 More typically, the initial uncertainty will be much greater than 5% unless the specialised and expensive doubly-labelled water method is used to measure energy expenditure before the intervention. The uncertainty of the baseline energy requirements translates to an expected inter-individual variability of weight loss even if adherence to the prescribed diet is perfect. This is a fundamental limitation on our ability to precisely calculate the predicted bodyweight time course of an individual.
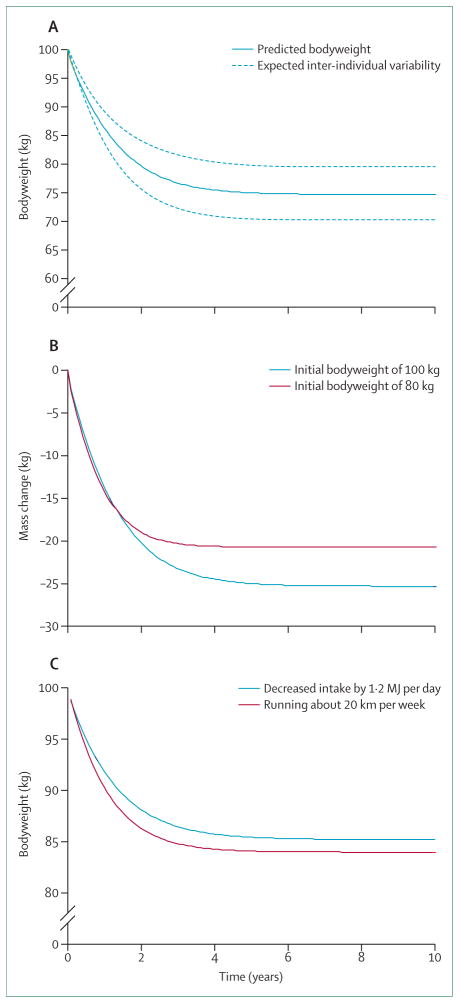
Figure 2A shows the predicted bodyweight time course of a 100 kg (220 lb) sedentary man following a step reduction of energy intake by 2 MJ per day (480 kcal per day). This constant diet perturbation was predicted to result in a bodyweight plateau at about 75 kg (165 lb) over a 10-year simulation taking roughly 1 year to reach half of the maximum weight loss and reaching 95% of this value after about 3 years. The dashed curves on figure 2A illustrate the ±4 kg weight-loss variability after several years and show that even seemingly small initial uncertainties can lead to large expected long-term inter-individual variability of weight change. This expected variability will be exacerbated by imperfect adherence to the intervention as well as any differences in physiological variables between individuals.
Figure 2. Predicted long-term bodyweight change trajectories.
(A) Bodyweight time course of a 100 kg man following a step decrease in dietary energy intake of 2 MJ per day. The dashed curves indicate the expected inter-individual variability of weight loss due to imprecise estimates of the initial state of energy balance (arising solely from an initial uncertainty in the energy expenditure rate of ±300 kJ per day or about 5%). (B) Differences of weight change between people with different initial body composition. People with a higher initial body fat mass lose more weight but the time to reach the plateau is longer. (C) Predicted effect of a step change of physical activity compared with an energy equivalent step change of dietary energy intake. Physical activity has an effect on both the magnitude and the timescale of bodyweight change.
By contrast with our dynamic model simulations, the popular dieting rule3–6 predicts that the same 2 MJ per day reduction of energy intake will result in a linear decrease of bodyweight over time with 22 kg lost in the first year (not shown), which is about 100% greater weight loss than our model prediction. This result shows the magnitude of the error introduced by ignoring dynamic changes of energy expenditure with weight loss. Moreover, it might help explain why even the most diligent followers of diet programmes often fail to reach weight loss goals that were set by use of the static weight-loss rule. Although practitioners of the erroneous dieting rule generally acknowledge that weight loss will slow over time, they had no way to estimate the weight-loss time course.
The timescale to reach a new bodyweight steady state is mathematically given by the effective energy density of the change in body tissue divided by the slope of the relation between the total energy expenditure rate and weight change (webappendix pp 3–4).28 Both of these factors are influenced by the initial body composition of the individual, and, therefore the bodyweight time course also depends on the initial body composition.33 Figure 2B shows the predicted change in bodyweight for the same step reduction of daily energy intake of 2 MJ per day in both a 100 kg man and an 80 kg man that differ in their initial body composition. Although the weight lost over the first year is similar, the greater initial fat mass of the 100 kg man results in a larger proportion of weight loss from body fat versus lean tissue than that in the 80 kg man. Because the energetically expensive lean tissue mass is preserved, the 100 kg man achieves a greater eventual weight loss than the initially lighter man because of the relative preservation of energy expenditure. However, to reach half of the maximum weight change takes longer for the 100 kg man than it does for the 80 kg man. Conversely, increased daily energy intake will result in greater weight gain in the 100 kg man than in the 80 kg man and a greater fraction of the weight change will be body fat (not shown).
These different bodyweight predictions at steady state result from the non-linear relation between initial mass of body fat and the fraction of weight change accounted for by change in lean tissue mass.30 Thus, the person with more initial body fat has a greater fraction of their weight change attributable to changes of body fat versus changes of lean tissue than does a person with less initial body fat. Since body fat contributes less than lean tissue to overall energy expenditure,39 the person with higher initial body fat will lose a greater amount of weight to achieve a new state of energy balance.37
Physical activity increases energy expenditure and can therefore cause weight loss, assuming no compensatory changes in energy intake. But does an increase of physical activity necessarily lead to the same weight loss as an energy-equivalent decrease of food intake? Figure 2C compares a step change of physical activity (ie, running roughly an additional 20 km per week at a moderate pace with an initial energy cost of about 1·2 MJ per day) with an energy-equivalent decrease of energy intake in the simulated 100 kg man. Such a relatively modest increase of physical activity expenditure results in slightly more rapid and greater predicted weight loss compared with an energy-equivalent reduction of food intake (figure 2C). However, as the magnitude of each intervention increases, there is a point when diet leads to greater weight loss than does physical activity. Increased physical activity versus an initially energy-equivalent reduction of food intake leads to differing predicted weight loss because the energy expenditure of added physical activity is proportional to bodyweight itself.71 Therefore, by contrast with the assumption that a calorie is a calorie with respect to physical activity versus diet, our model shows that energy-equivalent initial changes of physical activity versus food intake can lead to differences in weight change, but experimental confirmation of this result would be difficult.
We have not yet modelled the potential effect of exercise on change of body composition although this effect is likely to be modest for most aerobic exercises. More importantly, our model simulations assume that changing physical activity does not alter energy intake and vice versa. However, there is evidence that increased physical activity results in compensatory changes of energy intake that act to attenuate the energy imbalance84,85 and that the extent of compensation has high individual variability.86 Conversely, changes of energy intake might result in compensatory adaptation of non-exercise physical activity, which also has a high degree of individual variability.87 These compensatory feedback relations between energy intake and physical activity clearly warrant further investigation. Because our simulation model predicts the expected responses in the absence of these feedback mechanisms, we suggest that the difference between the measured and model-predicted weight change can be used to quantify the magnitude of compensation.
Setting goals for weight loss and weight-loss maintenance
The long timescale for weight loss in obese and overweight individuals has important implications for clinical weight-loss interventions. For example, implementation of a weight-loss programme in various stages so that a goal weight can be achieved in an abbreviated timeframe might be desirable. The first stage might consist of a more aggressive temporary change in behaviour to achieve the weight loss goal in a specified time, after which the intervention can be relaxed to a permanent behavioural change to avoid the weight regain that typically occurs.88,89
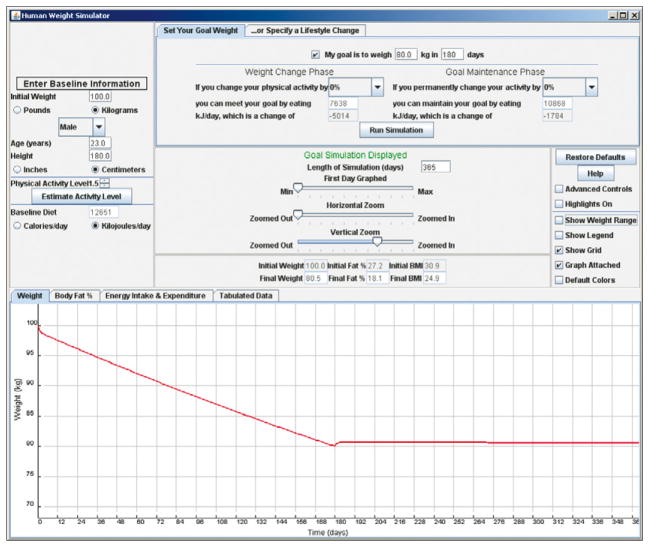
Figure 3 shows an example of such a two-phase programme of weight loss. A screen-shot of our web-based model simulating the required reduction of dietary energy intake of 5 MJ per day (1200 kcal per day) from a baseline of 12·7 MJ per day (3000 kcal per day) for a sedentary 100 kg (220 lb) man to lose 20 kg (44 lbs) in 6 months. This schedule was followed by a permanent reduction of energy intake to a value of 10·9 MJ per day (2600 kcal per day) to maintain the weight loss. Physical activity at baseline could be roughly estimated by answering two simple questions about work and leisure physical activities,90 and the simulations assumed no change in physical activity through the time course of the intervention. The effects of a weight-loss programme that includes modification of physical activity from its baseline value can also be simulated with the web-based model. By modifying the initial conditions of the model to represent an individual person, the simulator can be used for personalised goal setting and behavioural intervention planning.
Figure 3. Web-based simulation for setting goals for weight loss and weight-loss maintenance.
The panel located on the top-left part of the simulator window specifies the baseline characteristics of the individual person or population average values. This example selects a 100 kg, 180 cm tall, 23-year-old man. The top-middle panel specifies the goal weight (80 kg) and desired time interval to achieve the goal (180 days). Running the simulation displays the required changes of dietary energy intake to meet the goal and then maintain the weight change. The simulated bodyweight trajectory is graphically displayed in the lower panel. Users can also modify the physical activity to examine how combinations of diet and exercise interventions can achieve the same goal.
The simulation model can also display the range of expected individual weight-loss trajectories corresponding to the initial uncertainties in energy imbalance described before. If the bodyweight time course of a person falls substantially outside the expected trajectory range, then this could indicate non-compliance to the intervention. Alternatively, although the mathematical model variables seem to adequately represent the metabolic responses of an average person with a given initial body composition, an individual might have substantially different physiological variables that cause the bodyweight to fall well outside the expected trajectory range. For example, some individuals could have genetic differences causing them to respond to a reduced energy intake with a suppression of energy expenditure greater than average, which would result in less weight loss than expected.
Assessment of obesity interventions and patients’ adherence
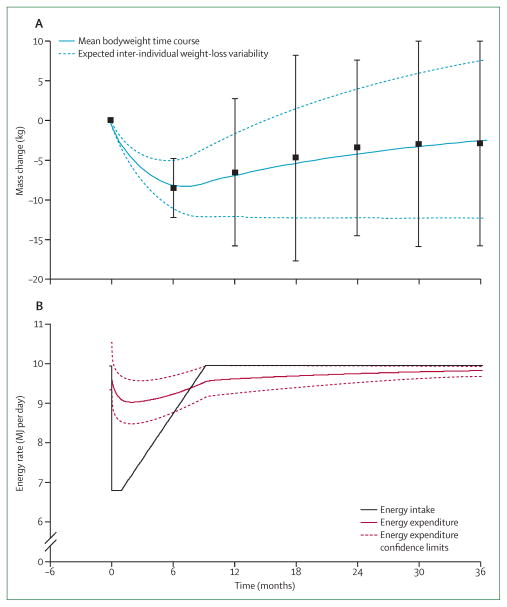
To assess the mechanisms and comparative effectiveness of various obesity treatments requires understanding their long-term effect on both energy intake and energy expenditure. However, a major difficulty in the field of obesity research is that current methods for assessment of free-living food intake (eg, 24 h recall, food frequency questionnaires, diet diaries, etc) are known to be inaccurate.91,92 Therefore, to interpret the results of various diet trials (panel) is difficult, as is to determine whether diet adherence is responsible for the typical failure to maintain substantial weight loss over extended time periods.93,94 For example, outpatient weight-loss interventions typically result in maximum weight loss after 6–8 months followed by gradual weight regain over subsequent years (figure 4A).89 A common explanation of the weight-loss plateau at 6–8 months is that a metabolic adaptation occurs such that energy expenditure decreases to match energy intake thereby halting further weight loss.95–98 Weight regain then occurs as people slowly relax their adherence to the diet that has stopped producing weight loss.
Figure 4. Energy balance dynamics underlying the typical out-patient weight loss, plateau, and regain trajectory.
(A) A typical range of bodyweight trajectories for patients engaged in an out-patient weight-loss programme. The solid blue curve is the mean bodyweight time course and the dashed curves indicate the expected inter-individual weight loss variability due to a ±0·65 MJ per day imprecision in the estimate of the initial energy expenditure rate. Datapoints are mean ±SD from Svetkey and colleagues.89 (B) The model predicted average energy intake (black) and energy expenditure (red) rates underlying the typical bodyweight loss and regain trajectory.
Although our mathematical model includes the physiology of metabolic adaptation to reduced energy diets, the simulated weight-loss plateau occurs on a much longer timescale. Therefore, assuming perfect adherence to a constant reduction of energy intake, the deviation between the model-simulated weight plateau that occurs after several years and the observed plateau at 6–8 months challenges the usual interpretation of the weight-loss, plateau, and regain trajectory described before. Perhaps the difference between the model simulation and the data is an indication that the model does not appropriately capture the energy metabolism dynamics on long timescales in free-living individuals. But because weight loss in controlled feeding studies is almost linear over 6 months (figures 1A and 1B in agreement with the model), a substantial slowing of metabolism shortly after 6 months would be needed to halt weight loss and thereby explain the typically observed plateau at 6–8 months. Rather, it is more likely that adherence to the prescribed diet was not constant over this timeframe—an interpretation supported by previous results from studies showing that total energy expenditure is much higher than self-reported energy intake at the weight-loss plateau.96
We investigated what change of energy intake would be needed to simulate a typically observed weight-loss and regain trajectory (figure 4A).31 The results show that the observed weight change could only have occurred if adherence to the initial reduction of energy intake by about 3·3 MJ per day (800 kcal per day) was rapidly and progressively relaxed, such that the patients returned to their original weight-maintenance diet within the first year, and kept this diet for the remainder of the 3-year simulation (figure 4B).
Panel. Does diet composition matter?
Clinicians are often asked to opine on a dizzying array of diets for weight loss. Theoretically, the energy balance principle would seem to suggest that all reduced energy diets must lead to equivalent weight loss. However, there have been several randomised clinical trials comparing diets that differ in macronutrient composition40–59 and many find that low carbohydrate diets lead to greater weight loss, at least in the short term. There are three possible reasons why some reduced energy diets may lead to more weight loss than others.
First, energy is stored in the body as protein, fat, and glycogen, which is a form of carbohydrate. Any imbalance between the intake and use of these macronutrients will lead to an alteration of body composition since the stored protein, fat, or glycogen must change to compensate the imbalance. The energy stored per unit mass of carbohydrate, fat, and protein varies considerably, especially when accounting for the intracellular water associated with stored glycogen and protein.7 Furthermore, dietary carbohydrates have an effect on renal sodium excretion via insulin,60 which results in concomitant changes of extracellular fluid. Therefore, weight changes are expected when the macronutrient composition of the diet is altered, even when the energy content of the diets are held constant.
However, apart from a few notable exceptions,61–63 most results from inpatient studies with adequately controlled diets have shown little effect of diet composition on bodyweight and fat-mass changes.64–69 These results therefore show the exquisite regulation of metabolic fuel selection to adjust to the macronutrient content of the diet. Far from being an obvious consequence of the first law of thermodynamics (often expressed as “a calorie is a calorie”),70 the observation that bodyweight and composition seem to depend little on the macronutrient composition of the diet requires a robust physiological control system to adapt metabolic fuels to the diet composition.33 Understanding of the complex physiological mechanisms underlying metabolic fuel selection is a subject of active investigation.29,31,33,34
The second reason that diet composition might affect weight loss is that the body’s energy-expenditure rate might depend on the macronutrient mix of the diet. For example, consumption of dietary protein is known to elicit a substantially larger increment of energy expenditure for several hours after a meal than does dietary fat or carbohydrate.71 Furthermore, the fluxes through various energy-requiring metabolic pathways depend on the macronutrient composition of the diet. For instance, the breakdown and resynthesis of body fat requires eight molecules of ATP per molecule of triglyceride72 and the flux through this pathway is strongly influenced by dietary carbohydrate via insulin’s inhibition of lipolysis.73 Similarly, other metabolic processes such as gluconeogenesis, de novo lipogenesis, and protein turnover all require energy and their rates can be substantially influenced by diet composition.
Calculation of the net effect of diet changes on overall energy expenditure requires the use of mathematical models that simulate how the magnitudes of these various metabolic fluxes are altered by changing diet composition.29,31 Despite the attractive theoretical possibility of a substantial metabolic advantage of one diet over another,74–76 the overall calculated effect of diet composition on total energy expenditure seems to be relatively modest, especially when dietary protein is unchanged. Specifically, our computational model of macronutrient balance31 predicts that large isocaloric exchanges of dietary carbohydrate and fat result in changes of energy expenditure of less than 400 kJ per day, which corresponds to a difference in body fat of roughly 10 g per day. To detect such a difference in body fat with present body-composition methods would require a sustained diet change of more than 100 days. These model results agree with experimental findings70,77 and, therefore, the assumption that a “calorie is a calorie” is a reasonable first estimation as far as energy expenditure is concerned over short-time periods. Nevertheless, even small differences in energy expenditure and energy partitioning can theoretically lead to substantial differences of bodyweight and composition if the diets are maintained over long-time periods. This possibility requires further investigation.
Finally, some diets can lead to reduced hunger, improved satiety, and better overall diet adherence during a weight management intervention. While much work has examined the effect of diet composition on short term measures of hunger and satiety,78,79 the well-known difficulties in measurement of food intake in free-living people over extended time periods currently prohibits adequate comparison of different diets. This issue makes the interpretation of most diet trials40–59 particularly difficult since the reported energy and macronutrient intakes are almost certainly erroneous. Diet adherence is likely to be more transient than is generally believed and might underlie the typical pattern of weight-loss, plateau, and regain observed in outpatient diet interventions (see main text).
In summary, changes in the dietary macronutrient composition result in rapid physiological adaptations that strive to match metabolic fuel selection to the diet. These adaptations minimise changes of body composition and energy expenditure. As a result, all reduced energy diets have a similar effect on body-fat loss in the short run. However, little is known about the long-term effect of diets that vary in macronutrient composition since present methods prohibit quantitative assessment of food intake and diet adherence in an outpatient setting.
Our model’s predicted pattern of free-living energy intake raises several interesting issues. First, after the initial reduction in energy intake resulting from a dietary intervention, a progressive increase of energy intake occurs for many months before it finally meets the energy expenditure rate corresponding to the bodyweight plateau. In other words, weight loss continues for many months at the same time that the average energy intake is slowly increasing. The dieter might then incorrectly infer that adherence is not essential for continuing weight loss when, in fact, impending weight regain has already been set in motion. The slow timescale for weight change is responsible for the gradual weight regain over many years despite the fact that the original lifestyle was resumed within the first year. Interestingly, energy intake levels return to the original baseline diet only a few months after weight hits a minimum and starts increasing. We do not yet know the mechanisms that drive this predicted pattern of average energy intake during a weight-loss programme, but this clearly warrants further study.
The observed average weight regain at 3 years of all but about 3 kg of the lost weight contrasts with the expected weight loss of more than 12 kg that our model predicts would have resulted had the individuals eaten their self-reported energy intake (data not shown).89 These diverging results highlight the well-known deficiencies in the assessment of free-living energy intake,91 making the results of clinical trials comparing various diet interventions very difficult to interpret. To address this difficulty, mathematical models of human metabolism have recently been proposed to estimate changes of free-living energy intake by use of longitudinal measurements of bodyweight as model inputs.31,36,99,100
Modelling average weight change in a population
The obesity epidemic is measured at the population level, including the bodyweights of individuals within the population. Accordingly, it is important to develop models that can quantitatively relate individual changes in energy balance and bodyweight to population-level effects. Modelling of the dynamics of the entire population distribution over time is complex and beyond the scope of this report. However, by taking the average of the mathematical model over the US adult population, we managed to calculate the energy imbalance dynamics underlying the observed change of the average adult bodyweight.36 Fortunately, averaging over a large population reduces the previously described measurement uncertainty in the initial state of energy balance to a negligible value and results in a more precise estimate of the average weight change than can be obtained for an individual. Furthermore, whereas irregular jumps might characterise the energy intake or physical activity patterns in some individuals (eg, the step changes simulated previously), these transitions in behaviour will be smoothed out when averaged over the population.
An important caveat exists when populations are considered to be the sum of individuals. Adults within a population, on average, gain weight as they get older, at least up to the age of about 60 years. Thus, a longitudinal study of adults will inevitably show weight gain, whereas the population as a whole (as judged by serial cross-sectional surveys) might be weight stable, increasing in weight, or even decreasing in weight. This is typically a result of lean, young people entering the adult population at the same time as fatter, older people leave the population by dying. Nevertheless, averaging the mathematical model over a population can give important insights into the energy imbalances responsible for the rise of obesity.
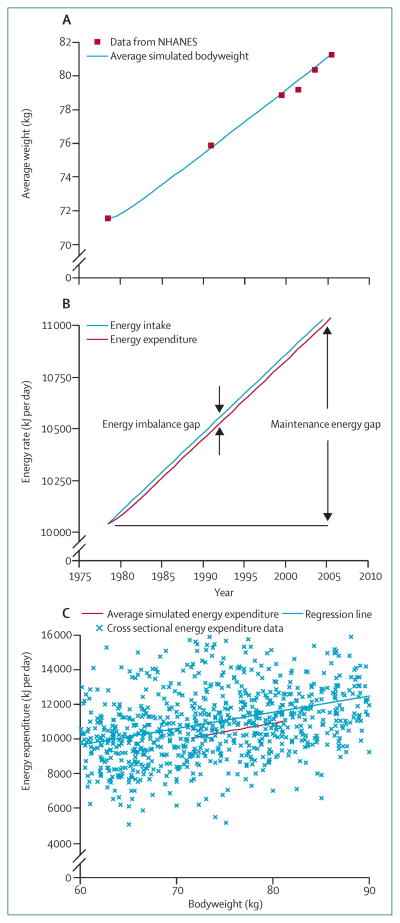
Over the past 30 years, the average adult bodyweight in the USA has linearly increased since 1978 (figure 5A).101–103 By using the average adult bodyweight in 1978 as the initial condition of our model, we quantified the energy balance dynamics underlying this observed average weight gain. Figure 5B plots the simulated progressive increase of overall daily energy intake and energy expenditure underlying the average bodyweight trajectory shown in figure 5A, assuming no change of average physical activity.
Figure 5. Simulating the increasing average adult bodyweight gain of the US population.
(A) Average simulated adult bodyweight along with data from the NHANES. (B) The simulated linear increase of average energy intake and energy expenditure underlying the observed increase in average bodyweight. The energy imbalance gap is the small difference between the energy intake and expenditure rates. The maintenance energy gap is the change of energy intake required to maintain the final bodyweight compared with the initial weight. (C) Plotting of the simulated average energy expenditure rate versus the average bodyweight gives the red curve with a slope very close to that of the regression line fit to cross-sectional energy expenditure data. The slope indicates that every 100 kJ per day increment of energy intake would eventually lead to about 1 kg change of average bodyweight. NHANES=National Health and Nutrition Examination Survey.
The small difference between the average daily energy intake and expenditure rates shown in figure 5B quantifies the persistent daily energy imbalance that amounts to about 30 kJ per day. This small average daily energy imbalance is equivalent to the average increase of energy stored in body fat and lean tissue divided by the time taken to store this energy. This small “energy imbalance gap” underlying the development of obesity at a population level was first identified by Hill and colleagues104 (although not referred to by this term). This observation led to the “small changes approach”104,105 to address the obesity epidemic based on the idea that only a small average daily energy imbalance underlies the population-wide increase of average bodyweight.105 Thus, it is argued that halting the rise of obesity prevalence requires only small changes.
However, reversal of obesity will require substantially larger changes, a fact fully acknowledged by Hill and colleagues.105 By contrast with the small energy imbalance gap, the “maintenance energy gap” (previously referred to as the energy flux gap)106 is defined as the increased average energy intake rate to maintain the final bodyweight compared with the initial bodyweight. The maintenance energy gap accounts for the changes of energy expenditure that occur with weight gain. Figure 5B shows that the maintenance energy gap underlying the obesity epidemic in adults in the USA is about 0·9 MJ per day (220 kcal per day) when 2005 data are compared with 1978 data.35,36 This estimate is similar to an analysis of US energy intake trends107 showing an increase of about 0·8 MJ per day (190 kcal per day). Measurement error in intake or a decrease of physical activity over the same period could contribute to the small differences in these estimates. Nevertheless, the large change of average energy intake characterises the magnitude of the public health challenge to reverse obesity rates back to 1970s values. Our updated estimate of the maintenance energy gap underlying the increased average US adult bodyweight is slightly less than our previous estimates106,108 as a result of some issues with linear regression methods35,109 that have now been corrected. It is important to note that much larger changes are needed for obese individuals to return to the average bodyweight of the 1970s. For example, an adult with a BMI higher than 35 kg/m2, representing 14% of the US population,102 needs a change greater than 2 MJ per day (500 kcal per day).
Figure 5C plots our model’s predicted average energy expenditure rate as a function of average bodyweight over the course of the 30-year weight gain along with energy expenditure data obtained in a cross-sectional sample with the doubly-labelled water method.106 The simple regression line relating the energy expenditure data to bodyweight has a remarkably similar slope compared with the dynamic model prediction (94±6 kJ/kg per day for linear regression versus the dynamic model slope of 100 kJ/kg per day). The magnitude of the slope determines the magnitude of average steady-state bodyweight change for a given dietary intervention. This result, along with the characteristic timescale described above, provides a simple approximate rule of thumb for prediction of the average impact of an intervention affecting energy intake: every permanent change of energy intake of 100 kJ per day will lead to an eventual weight change of about 1 kg (equivalently, 10 kcal per day per pound of weight change) and it will take about 1 year to achieve half of the total weight change and 95% of the total weight change will result in about 3 years.
Simulation of the potential effect of policy interventions to address obesity
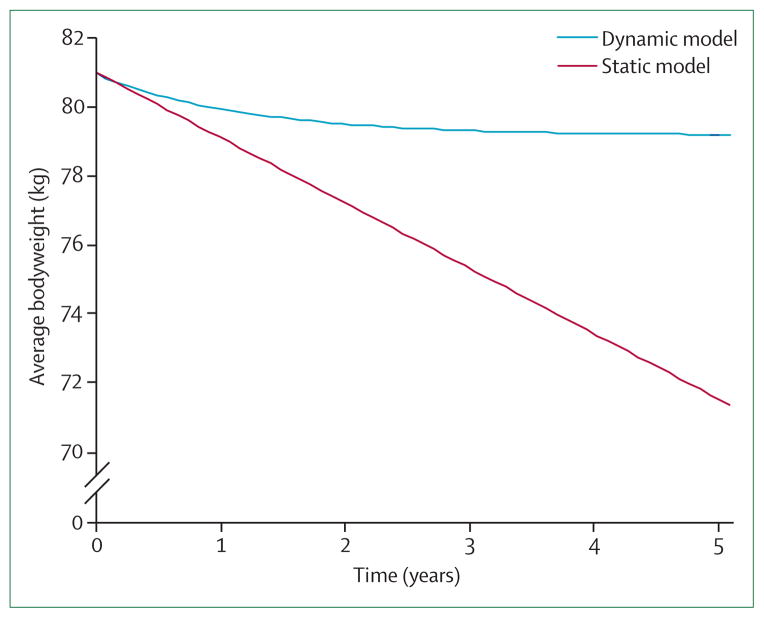
By modelling the magnitude of the maintenance energy gap we can begin to estimate the potential effect of population-wide policy interventions. For example, the US Department of Agriculture (USDA) recently issued a report12 describing the potential effect on obesity prevalence of taxing caloric sweetened beverages. The authors estimated that a 20% tax would result in a decrease of overall energy intake by about 170 kJ per day (40 kcal per day). Using the rule that every change of diet of 2 MJ per day will result in about 0·5 kg of weight loss per week, the authors incorrectly predicted an average weight loss of about 1·8 kg per year. Extrapolating this static model prediction over 5 years results in a weight loss of about 10 kg bringing the average adult bodyweight down to values characteristic of the 1970s (figure 6), suggesting that a tax on sugar-sweetened beverages alone might be sufficient to reverse the US obesity epidemic. By contrast, our dynamic simulation model predicts that the USDA estimated decrease of average energy intake from taxing caloric sweetened beverages would result in about 1·8 kg (4 lb) of average weight loss after 5 years and about 1 kg (2·2 lb) of weight loss after 1 year (figure 6). In collaboration with the USDA, we recently used our dynamic simulation model as part of an income-stratified analysis of taxing sugar sweetened beverages and its potential effect on overweight and obesity prevalence in US adults.110
Figure 6. Prediction of the effect of a policy intervention on the population average weight.
Simulated average weight change of a 20% tax on caloric sweetened beverages. The average energy-intake change was specified in a recent report by the US Department of Agriculture (USDA)12 and initial population average weight of 81 kg corresponded to the most recent measurement in the USA. Rather than produce the progressive weight loss predicted by the static model, the same decrease of energy intake led to a simulated modest weight-loss plateau.
These results have important implications for gauging the ability of a policy change to mitigate the obesity epidemic. To better inform policy decisions, a dynamic model should be used to estimate both the magnitude and time course of expected weight change resulting from a given policy’s predicted effect on energy balance rather than the inappropriate use of the 3500 kcal per pound rule.9,11,12 Alternatively, our proposed rule of thumb that 100 kJ per day per kg of weight change can be applied to estimate the steady-state weight differences between the presence and absence of an intervention—as was recently done to assess front-of-pack traffic-light nutrition labelling and a “junk food” tax.111 While even modest weight loss can be associated with substantial health benefits,112,113 we suggest that unrealistic weight loss expectations obtained by erroneous use of the static dieting rule should be replaced by our methods to assess other population-wide and more targeted obesity prevention interventions. Of course, our methodology only addresses the energy balance core of the obesity systems map1 and does not account for the dynamic complex web of interacting variables that might respond and adapt to policy level interventions.
Conclusions
This report describes how mathematical modelling of human metabolism has resulted in several important insights about weight change in adults. We have shown how inter-individual weight-loss variability resulting from the same intervention can be caused by differences in the initial body composition between individuals as well as the uncertainty about the baseline energy expenditure. We also showed that the timescale of human weight change is long and depends on the initial body composition. Furthermore, changes of energy intake can theoretically result in different weight changes compared with initially energy-equivalent changes of physical activity expenditure.
Although our mathematical models have been validated in various situations,7,29–32,34,36,37 dynamic simulation models have not yet been widely adopted for clinical weight management or informing policy discussions. Thus, we have created a web-based dynamic model to allow users to easily perform simulations. Our web-based instrument represents a substantial step forward from previously available predictions of weight change that rely on the erroneous use of common dieting rules.3–6 The bodyweight simulation tool will be updated to reflect continuing improvements in the model.
An important limitation of our simulations is that our model requires estimation of how an intervention affects both energy intake and physical activity. Once these parameters are specified, the model assumes perfect adherence to the intervention and does not automatically simulate any compensatory effects (eg, increased energy intake at the start of an exercise programme84,85). Although limited adherence and compensatory effects are likely to have a prominent role in the determination of overall weight change, their potential effect can be simulated by explicitly testing various hypotheses in the model.
We previously showed good agreement between the model’s prediction of the relation between weight change and energy expenditure obtained with 24-h indirect calorimetry in Pima Indians after a 3-year follow-up.36,114 However, additional longitudinal validation studies over extended periods are needed, ideally with the doubly-labelled water method to assess longitudinal changes of free-living total energy expenditure.
Our mathematical model was developed to accurately simulate the physiology of weight change in adults. A similar model for children and adolescents is not yet available. The same notions of the energy imbalance gap and the maintenance energy gap can also apply to children, and previous mathematical models have focused on the quantification of the energy imbalance underlying the development of childhood obesity in various populations.17,115,116 However, these models are limited because they cannot accurately predict how a child’s body weight trajectory will change in response to an intervention, because they do not account for the dynamic energy partitioning between fat and lean tissue during various phases of growth.117,118 Future work must address this issue.
In summary, accurate mathematical models of human metabolism are needed to properly assess the quantitative effect of interventions at both the individual and population levels. Widespread past use of erroneous rules for estimation of human weight change have led to unrealistic expectations about the potential effect of both behavioural and policy interventions. By modelling the quantitative physiology of human weight change and providing easy access to a web-based simulation tool, we believe that health-care and health-policy practitioners will be in a position to make better informed decisions.
Key messages.
Health and nutrition organisations have perpetuated the myth that a reduction of food intake of 2 MJ per day will lead to a steady rate of weight loss of 0·5 kg per week. Because this static weight-loss rule does not account for dynamic physiological adaptations that occur with decreased bodyweight, its widespread use at both the individual and population levels has led to drastically overestimated expectations for weight loss.
We introduce a validated web-based dynamic simulation model of adult human metabolism that predicts the time course of individual weight change in response to behavioural interventions. Model simulations can be clinically useful to help set personalised weight-loss goals and track adherence to an intervention.
On the basis of our model, we propose an approximate rule of thumb for an average overweight adult: every change of energy intake of 100 kJ per day will lead to an eventual bodyweight change of about 1 kg (equivalently, 10 kcal per day per pound of weight change) with half of the weight change being achieved in about 1 year and 95% of the weight change in about 3 years.
Our model simulations show that present limitations on the precision of measuring energy expenditure before a diet intervention result in a substantial expected inter-individual variability of weight loss, since a given diet results in an uncertain degree of energy deficit.
Applications of dynamic simulation models include: prediction of individual weight changes resulting from energy balance interventions; assessment of effects of policy interventions targeting energy intake or physical activity; estimation of the magnitude of the maintenance energy gap that determines the increased energy intake needed to maintain higher average bodyweights as a result of the obesity epidemic.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health, NIDDK, and grants from the Robert Wood Johnson Foundation (#260639, #61468, and #66284), the US Centers for Disease Control and Prevention (U48/DP00064-00S1 and 1U48DP001946) and the Office of Behavioral and Social Sciences Research of the National Institutes for Health (#HHSN276200700356P). The funders had no role in the formulation of the major concepts, data analysis, data interpretation, or writing of the report. The authors received no payment for writing the report.
Footnotes
For more on our web-based model see http://bwsimulator.niddk.nih.gov
Contributors
All authors jointly formulated the major concepts, and read and approved the final version of the manuscript. KDH led the writing of the report with the assistance of GS and CCC. YCW, SLG, and BAS provided comments on the report. KDH and CCC developed the mathematical model fully described in the webappendix and DC implemented the model in the web-based simulation tool under the direction of KDH.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Butland B, Jebb SA, Kopelman PG, et al. Foresight tackling obesities: future choices—project report. 2. London: Government Office for Science; 2007. [DOI] [PubMed] [Google Scholar]
- 2.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 3.Duyff RL. American dietetic association complete food and nutrition guide. 3. Hoboken: John Wiley & Sons Inc; 2006. [Google Scholar]
- 4.NHLBI. Aim for a healthy weight: National Institutes of Health, National Heart, Lung, and Blood Institute. 2005. Report No: 05-5213. [Google Scholar]
- 5.The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health; National Heart, Lung, and Blood Institute; North American Association for the study of obesity; 2000. NHLBI obesity education initiative expert panel on the identification evaluation, and treatment of overweight and obesity in adults. [Google Scholar]
- 6.NHS. Your Weight Your Health: how to take control of your weight. London: National Health Service, Department of Health; 2006. Report no: 274537. [Google Scholar]
- 7.Hall KD. What is the required energy deficit per unit weight loss? Int J Obes (Lond) 2008;32:573–76. doi: 10.1038/sj.ijo.0803720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–28. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 9.Gustavsen GW, Rickertsen K. The effects of taxes on purchases of sugar-sweetened carbonated soft drinks: a quantile regression approach. Appl Econ. 2011;43:707–16. [Google Scholar]
- 10.Finkelstein EA, Zhen C, Nonnemaker J, Todd JE. Impact of targeted beverage taxes on higher- and lower-income households. Arch Intern Med. 2010;170:2028–34. doi: 10.1001/archinternmed.2010.449. [DOI] [PubMed] [Google Scholar]
- 11.Simon P, Jarosz CJ, Kuo T, Fielding JE. Menu labeling as a potential strategy for combating the obesity epidemic: a health impact assessment. Los Angeles: County of Los Angeles Department of Public Health; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TA, Bing-Hwan L, Jong-Ying L. Taxing caloric sweetened beverages: potential effects on beverage consumption, calorie intake, and obesity. Washington DC: Economic Research Service, US Department of Agriculture; 2010. [Google Scholar]
- 13.Alpert SS. A two-reservoir energy model of the human body. Am J Clin Nutr. 1979;32:1710–18. doi: 10.1093/ajcn/32.8.1710. [DOI] [PubMed] [Google Scholar]
- 14.Alpert SS. Growth, thermogenesis, and hyperphagia. Am J Clin Nutr. 1990;52:784–92. doi: 10.1093/ajcn/52.5.784. [DOI] [PubMed] [Google Scholar]
- 15.Alpert SS. A limit on the energy transfer rate from the human fat store in hypophagia. J Theor Biol. 2005;233:1–13. doi: 10.1016/j.jtbi.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Antonetti VW. The equations governing weight change in human beings. Am J Clin Nutr. 1973;26:64–71. doi: 10.1093/ajcn/26.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Butte NF, Christiansen E, Sorensen TI. Energy imbalance underlying the development of childhood obesity. Obesity (Silver Spring) 2007;15:3056–66. doi: 10.1038/oby.2007.364. [DOI] [PubMed] [Google Scholar]
- 18.Christiansen E, Garby L. Prediction of body weight changes caused by changes in energy balance. Eur J Clin Invest. 2002;32:826–30. doi: 10.1046/j.1365-2362.2002.01036.x. [DOI] [PubMed] [Google Scholar]
- 19.Christiansen E, Garby L, Sorensen TI. Quantitative analysis of the energy requirements for development of obesity. J Theor Biol. 2005;234:99–106. doi: 10.1016/j.jtbi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Flatt JP. Carbohydrate-fat interactions and obesity examined by a two-compartment computer model. Obes Res. 2004;12:2013–22. doi: 10.1038/oby.2004.252. [DOI] [PubMed] [Google Scholar]
- 21.Kozusko FP. Body weight setpoint, metabolic adaption and human starvation. Bull Math Biol. 2001;63:393–403. doi: 10.1006/bulm.2001.0229. [DOI] [PubMed] [Google Scholar]
- 22.Kozusko FP. The effects of body composition on setpoint based weight loss. Math Comput Model. 2002;35:973–82. [Google Scholar]
- 23.Payne PR, Dugdale AE. A model for the prediction of energy balance and body weight. Ann Hum Biol. 1977;4:525–35. doi: 10.1080/03014467700002521. [DOI] [PubMed] [Google Scholar]
- 24.Song B, Thomas DM. Dynamics of starvation in humans. J Math Biol. 2007;54:27–43. doi: 10.1007/s00285-006-0037-7. [DOI] [PubMed] [Google Scholar]
- 25.Thomas DM, Ciesla A, Levine JA, Stevens JG, Martin CK. A mathematical model of weight change with adaptation. Math Biosci Eng. 2009;6:873–87. doi: 10.3934/mbe.2009.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinsier RL, Bracco D, Schutz Y. Predicted effects of small decreases in energy expenditure on weight gain in adult women. Int J Obes Relat Metab Disord. 1993;17:693–700. [PubMed] [Google Scholar]
- 27.Westerterp KR, Donkers JH, Fredrix EW, Boekhoudt P. Energy intake, physical activity and body weight: a simulation model. Br J Nutr. 1995;73:337–47. doi: 10.1079/bjn19950037. [DOI] [PubMed] [Google Scholar]
- 28.Chow CC, Hall KD. The dynamics of human body weight change. PLoS Comput Biol. 2008;4:e1000045. doi: 10.1371/journal.pcbi.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall KD. Computational model of in vivo human energy metabolism during semistarvation and refeeding. Am J Physiol Endocrinol Metab. 2006;291:E23–37. doi: 10.1152/ajpendo.00523.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall KD. Body fat and fat-free mass inter-relationships: Forbes’s theory revisited. Br J Nutr. 2007;97:1059–63. doi: 10.1017/S0007114507691946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab. 2010;298:E449–66. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall KD. Mathematical modelling of energy expenditure during tissue deposition. Br J Nutr. 2010;104:4–7. doi: 10.1017/S0007114510000206. [DOI] [PubMed] [Google Scholar]
- 33.Hall KD. Mechanisms of metabolic fuel selection: modeling human metabolism and body-weight change. IEEE Eng Med Biol Mag. 2010;29:36–41. doi: 10.1109/MEMB.2009.935465. [DOI] [PubMed] [Google Scholar]
- 34.Hall KD, Bain HL, Chow CC. How adaptations of substrate utilization regulate body composition. Int J Obes (Lond) 2007;31:1378–83. doi: 10.1038/sj.ijo.0803608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall KD, Chow CC. Estimating the quantitative relation between food energy intake and changes in body weight. Am J Clin Nutr. 2010;91:816. doi: 10.3945/ajcn.2009.28922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall KD, Guo J, Dore M, Chow CC. The progressive increase of food waste in America and its environmental impact. PLoS One. 2009;4:e7940. doi: 10.1371/journal.pone.0007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall KD, Jordan PN. Modeling weight-loss maintenance to help prevent body weight regain. Am J Clin Nutr. 2008;88:1495–503. doi: 10.3945/ajcn.2008.26333. [DOI] [PubMed] [Google Scholar]
- 38.Forbes GB. Lean body mass-body fat interrelationships in humans. Nutr Rev. 1987;45:225–31. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 39.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley U, Spence M, Courtney CH, et al. Low-fat versus low-carbohydrate weight reduction diets: effects on weight loss, insulin resistance, and cardiovascular risk: a randomized control trial. Diabetes. 2009;58:2741–48. doi: 10.2337/db09-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 42.Brehm BJ, Spang SE, Lattin BL, Seeley RJ, Daniels SR, D’Alessio DA. The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J Clin Endocrinol Metab. 2005;90:1475–82. doi: 10.1210/jc.2004-1540. [DOI] [PubMed] [Google Scholar]
- 43.Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90:23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- 44.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–57. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 46.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z weight loss study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT) J Am Coll Nutr. 2009;28:159–68. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- 48.Johnston CS, Tjonn SL, Swan PD, White A, Hutchins H, Sears B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am J Clin Nutr. 2006;83:1055–61. doi: 10.1093/ajcn/83.5.1055. [DOI] [PubMed] [Google Scholar]
- 49.McAuley KA, Hopkins CM, Smith KJ, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- 50.Noakes M, Foster PR, Keogh JB, James AP, Mamo JC, Clifton PM. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr Metab (Lond) 2006;3:7. doi: 10.1186/1743-7075-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 52.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–90. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 53.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 55.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 56.Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol. 2008;51:59–67. doi: 10.1016/j.jacc.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 57.Volek J, Sharman M, Gomez A, et al. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Metab (Lond) 2004;1:13. doi: 10.1186/1743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wycherley TP, Brinkworth GD, Keogh JB, Noakes M, Buckley JD, Clifton PM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. 2010;267:452–61. doi: 10.1111/j.1365-2796.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 59.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 60.DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21:165–71. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- 61.Kekwick A, Pawan GL. Calorie intake in relation to body-weight changes in the obese. Lancet. 1956;271:155–61. doi: 10.1016/s0140-6736(56)91691-9. [DOI] [PubMed] [Google Scholar]
- 62.Rabast U, Schonborn J, Kasper H. Dietetic treatment of obesity with low and high-carbohydrate diets: comparative studies and clinical results. Int J Obes. 1979;3:201–11. [PubMed] [Google Scholar]
- 63.Rabast U, Vornberger KH, Ehl M. Loss of weight, sodium and water in obese persons consuming a high- or low-carbohydrate diet. Ann Nutr Metab. 1981;25:341–49. doi: 10.1159/000176515. [DOI] [PubMed] [Google Scholar]
- 64.Kinsell LW, Gunning B, Michaels GD, Richardson J, Cox SE, Lemon C. Calories do count. Metabolism. 1964;13:195–204. doi: 10.1016/0026-0495(64)90098-8. [DOI] [PubMed] [Google Scholar]
- 65.Leibel RL, Hirsch J, Appel BE, Checani GC. Energy intake required to maintain body weight is not affected by wide variation in diet composition. Am J Clin Nutr. 1992;55:350–55. doi: 10.1093/ajcn/55.2.350. [DOI] [PubMed] [Google Scholar]
- 66.Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992;41:406–14. doi: 10.1016/0026-0495(92)90076-m. [DOI] [PubMed] [Google Scholar]
- 67.Werner SC. Comparison between weight reduction on a high-calorie, highfat diet and on an isocaloric regimen high in carbohydrate. N Engl J Med. 1955;252:661–65. doi: 10.1056/NEJM195504212521604. [DOI] [PubMed] [Google Scholar]
- 68.Yang MU, Barbosa-Saldivar JL, Pi-Sunyer FX, Van Itallie TB. Metabolic effects of substituting carbohydrate for protein in a low-calorie diet: a prolonged study in obese patients. Int J Obes. 1981;5:231–36. [PubMed] [Google Scholar]
- 69.Yang MU, Van Itallie TB. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J Clin Invest. 1976;58:722–30. doi: 10.1172/JCI108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buchholz AC, Schoeller DA. Is a calorie a calorie? Am J Clin Nutr. 2004;79:899S–906S. doi: 10.1093/ajcn/79.5.899S. [DOI] [PubMed] [Google Scholar]
- 71.Blaxter K. Energy metabolism in animals and man. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- 72.Elia M, Zed C, Neale G, Livesey G. The energy cost of triglyceride-fatty acid recycling in nonobese subjects after an overnight fast and four days of starvation. Metabolism. 1987;36:251–55. doi: 10.1016/0026-0495(87)90184-3. [DOI] [PubMed] [Google Scholar]
- 73.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism. 2007;56:68–76. doi: 10.1016/j.metabol.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Feinman RD, Fine EJ. Thermodynamics and metabolic advantage of weight loss diets. Metab Syndr Relat Disord. 2003;1:209–19. doi: 10.1089/154041903322716688. [DOI] [PubMed] [Google Scholar]
- 75.Fine EJ, Feinman RD. Thermodynamics of weight loss diets. Nutr Metab (Lond) 2004;1:15. doi: 10.1186/1743-7075-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manninen AH. Is a calorie really a calorie? Metabolic advantage of low-carbohydrate diets. J Int Soc Sports Nutr. 2004;1:21–26. doi: 10.1186/1550-2783-1-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schoeller DA, Buchholz AC. Energetics of obesity and weight control: does diet composition matter? J Am Diet Assoc. 2005;105 (5 suppl 1):S24–28. doi: 10.1016/j.jada.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 78.Gerstein DE, Woodward-Lopez G, Evans AE, Kelsey K, Drewnowski A. Clarifying concepts about macronutrients’ effects on satiation and satiety. J Am Diet Assoc. 2004;104:1151–53. doi: 10.1016/j.jada.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 79.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 80.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berlin NI, Watkin DM, Gevirtz NR. Measurement of changes in gross body composition during controlled weight reduction in obesity by metabolic balance and body density—body water technics. Metabolism. 1962;11:302–14. [PubMed] [Google Scholar]
- 82.Runcie J, Hilditch TE. Energy provision, tissue utilization, and weight loss in prolonged starvation. BMJ. 1974;2:352–56. doi: 10.1136/bmj.2.5915.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schoeller DA, Fjeld CR. Human energy metabolism: what have we learned from the doubly labeled water method? Annu Rev Nutr. 1991;11:355–73. doi: 10.1146/annurev.nu.11.070191.002035. [DOI] [PubMed] [Google Scholar]
- 84.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc. 2003;62:651–61. doi: 10.1079/PNS2003286. [DOI] [PubMed] [Google Scholar]
- 85.King NA, Caudwell P, Hopkins M, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring) 2007;15:1373–83. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 86.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008;32:177–84. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 87.Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab. 2004;286:E675–85. doi: 10.1152/ajpendo.00562.2003. [DOI] [PubMed] [Google Scholar]
- 88.Stunkard A, Mc L-HM. The results of treatment for obesity: a review of the literature and report of a series. JAMA. 1959;103:79–85. doi: 10.1001/archinte.1959.00270010085011. [DOI] [PubMed] [Google Scholar]
- 89.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 90.Johansson G, Westerterp KR. Assessment of the physical activity level with two questions: validation with doubly labeled water. Int J Obes (Lond) 2008;32:1031–33. doi: 10.1038/ijo.2008.42. [DOI] [PubMed] [Google Scholar]
- 91.Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48:373–79. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 92.Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6:199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 93.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 94.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82 (suppl 1):222S–25S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 95.Dulloo AG. Suppressed thermogenesis as a cause for resistance to slimming and obesity rebound: adaptation or illusion? Int J Obes (Lond) 2007;31:201–03. doi: 10.1038/sj.ijo.0803537. [DOI] [PubMed] [Google Scholar]
- 96.Heymsfield SB, Harp JB, Reitman ML, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85:346–54. doi: 10.1093/ajcn/85.2.346. [DOI] [PubMed] [Google Scholar]
- 97.Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes (Lond) 2007;31:204–12. doi: 10.1038/sj.ijo.0803523. [DOI] [PubMed] [Google Scholar]
- 98.Tremblay A, Chaput JP. Adaptive reduction in thermogenesis and resistance to lose fat in obese men. Br J Nutr. 2009;102:488–92. doi: 10.1017/S0007114508207245. [DOI] [PubMed] [Google Scholar]
- 99.Hall KD, Chow CC. Estimating changes of free-living energy intake and its confidence interval. Am J Clin Nutr. 2011;94:66–74. doi: 10.3945/ajcn.111.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92:1326–31. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 102.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 103.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–11. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 104.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–55. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 105.Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am J Clin Nutr. 2009;89:477–84. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- 106.Swinburn BA, Sacks G, Lo SK, et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr. 2009;89:1723–28. doi: 10.3945/ajcn.2008.27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Austin GL, Ogden CL, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011;93:836–43. doi: 10.3945/ajcn.110.000141. [DOI] [PubMed] [Google Scholar]
- 108.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–56. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 109.Swinburn BA, Sacks G. Reply to KD Hall and CC Chow. Am J Clin Nutr. 2010;91:817. doi: 10.3945/ajcn.2009.28922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin BH, Smith TA, Lee JY, Hall KD. Measuring weight outcomes for obesity intervention strategies: the case of a sugar-sweetened beverage Tax. Econ Hum Biol. 2011 doi: 10.1016/j.ehb.2011.08.007. under revision. [DOI] [PubMed] [Google Scholar]
- 111.Sacks G, veerman JL, Moodie M, Swinburn B. ‘Traffic-light’ nutrition labelling and ‘junk-food’ tax: a modelled comparison of cost-effectiveness for obesity prevention. Int J Obes. 2010 doi: 10.1038/ijo.2010.228. published online Nov 16. [DOI] [PubMed] [Google Scholar]
- 112.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3 (suppl 2):211s–16s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 113.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401–06. [PubMed] [Google Scholar]
- 114.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85:1087–94. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- 115.Swinburn BA, Jolley D, Kremer PJ, Salbe AD, Ravussin E. Estimating the effects of energy imbalance on changes in body weight in children. Am J Clin Nutr. 2006;83:859–63. doi: 10.1093/ajcn/83.4.859. [DOI] [PubMed] [Google Scholar]
- 116.Wang YC, Gortmaker SL, Sobol AM, Kuntz KM. Estimating the energy gap among US children: a counterfactual approach. Pediatrics. 2006;118:e1721–33. doi: 10.1542/peds.2006-0682. [DOI] [PubMed] [Google Scholar]
- 117.Ellis KJ, Shypailo RJ, Abrams SA, Wong WW. The reference child and adolescent models of body composition. A contemporary comparison. Ann NY Acad Sci. 2000;904:374–82. doi: 10.1111/j.1749-6632.2000.tb06486.x. [DOI] [PubMed] [Google Scholar]
- 118.Jordan PN, Hall KD. Dynamic coordination of macronutrient balance during infant growth: insights from a mathematical model. Am J Clin Nutr. 2008;87:692–703. doi: 10.1093/ajcn/87.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]