Abstract
The anti-NeuN antibody has been widely used for over 15 years to unambiguously identify post-mitotic neurons in the central nervous system of a wide variety of vertebrates including mice, rats and humans. In contrast to its widely reported nuclear localization, we found significantly higher NeuN reactivity in the cytoplasm of neurons in brain sections from HIV-infected individuals with cognitive impairment compared to controls. The protein target of anti-NeuN antisera was recently identified as the neuron-specific RNA splicing factor, Rbfox3, but its significance in diseases affecting the brain has not been previously reported. RNA splicing occurs in the nucleus hence, the altered localization of RbFox3 to the cytoplasm may lead to the downregulation of neuronal gene expression.
Keywords: NeuN, Rbfox3, HIV-associated neurocognitive disorders, splicing, neurodegeneration, gene expression
INTRODUCTION
While many neurodegenerative disorders arise as a result of either inherited or sporadic genetic alterations, in the case of human immunodeficiency virus type I (HIV-1) associated neurocognitive disorders (HAND), neuronal dysfunction arises as a sequelae of HIV-1 infection of macrophages and microglia in the brain which induces, by mechanisms yet incompletely understood, synaptodendritic damage leading to the impairment of neuronal function [9]. Over 34 million people in the world are infected with HIV and the prevalence of cognitive impairment associated with this disease in the USA is 33–60% of infected individuals [4]. Cognitive impairment in HIV-infected individuals is diagnosed using a battery of neuropsychological and motor function tests, and categorized into three groups using the revised criteria established by the American Academy of Neurology as HIV-associated dementia (HAD), minor neurocognitive disorder (MND) and asymptomatic cognitive impairment (ANI) [1]. Autopsy findings on brain tissue from individuals afflicted with HAD revealed abundant astrocytosis, activated macrophages and microglia, and extensive neuronal apoptosis in the frontal cortex, hippocampus, cerebellum and striatum [5, 6]. With the availability of anti-viral therapy the prevalence of HAD has greatly decreased, but that of MND and ANI has increased [11]. In these latter two types of HIV-associated impairment, synaptodendritic injury, not neuronal loss is the prominent feature [3].
Neurons do not express CD4, the primary receptor required for HIV entry and productive virus replication in cells and hence, the neuronal injury and degeneration seen in HAND occurs predominantly by indirect mechanisms. The release of inflammatory and neurotoxic soluble factors by activated HIV-infected and uninfected macrophages and microglia and the direct interaction of HIV proteins is associated with the development of HAND [9]. In studies to characterize the cell-type specific expression of inflammatory mediators in human brain tissue, we used the well-known anti-NeuN antibody to specifically detect neurons. NeuN is present in the nuclei of most types of neurons throughout the nervous system and is considered a reliable tool to detect post-mitotic neurons. Surprisingly, in contrast to the widely reported nuclear localization of anti-NeuN, we found that in brain sections from HIV-infected cognitively impaired cases compared to controls, staining was visible at a significantly higher frequency throughout the cell body. The protein recognized by anti-NeuN was only recently described as the RNA splicing factor, Rbfox3 [7]. Rbfox3 is a member of the Fox-Family of RNA splicing factors, which recognize a unique motif in introns [15]. While many target genes for Rbfox 1 and Rbfox2 have been identified, very few targets of the brain-specific factor, Rbfox3 are known. This is the first study to show that in the context of a neurological disorder, NeuN/Rbfox3 is not exclusively localized to neuronal nuclei. While the functional significance of Rbfox3 remains to be determined, splicing takes place in the nucleus and therefore, Rbfox3 translocation to the cytoplasm may result in the downregulation of RNA splicing of its target genes, thereby altering the complement of neuronal specific gene expression.
MATERIALS AND METHODS
Patient Samples
Tissue sections were obtained from the National Neuroaids Tissue Consortium (NNTC) after approval for the study was obtained from the Johns Hopkins Institutional Review Board under protocol NA_00030244. The brain tissue was from the occipital lobe, an area of the brain known to have abundant HIV infection and pathology. The exclusion criteria were cytomegalovirus encephalitis, toxoplasmosis (active and healed), aseptic leptomeningitis, bacterial leptomeningitis, lymphoma, contusions, focal infarcts, anoxic/ischemic damage, and tuberculosis. The exclusion criteria for all groups were past and current substance induced major depressive disorder, cannabis, cocaine, opiate, methadone use. Abuse and dependence were also excluded and toxicology reports were examined. Any race was included and medically prescribed drugs were allowed. The revised American Academy of Neurology criteria was used to classify individuals into three groups based on cognitive function: asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND) and HIV-associated dementia (HAD) [1]. It was determined through query of the NNTC database that neurocognitive diagnosis consistent at the last two visits prior to death and by combining the MND and HAD subjects would yield the required number of cases. The three groups based on diagnoses at the last two visits prior to death were: 1) neuropsychological normal (Normal), 2) asymptomatic neurocognitive impairment (ANI) and 3) minor neurocognitive disorder/HIV-associated dementia (MND/HAD). For one case, number 10 (Table 1), although a diagnosis was not available, it was determined by neuropathological examination that the individual had HIV encephalitis (HIVE) and therefore, was included in the MND/HAD group.
Table 1.
Patient Sample Demographics
| PATIENT ID | AGE | SEX | RACE | HIV STATUS | DIAGNOSIS | SECTION TYPE |
|---|---|---|---|---|---|---|
| Case 1 | 47 | F | Black | Negative | Normal | Frozen |
| Case 2 | 44 | F | White | Negative | Normal | Frozen |
| Case 3 | 51 | M | White/Hispanic | Negative | Normal | Frozen |
| Case 4 | 49 | M | White | Negative | Normal | Frozen |
| Case 5 | 46 | M | White | Negative | Normal | Frozen |
| Case 6 | 39 | M | Hispanic | Positive | ANI | Frozen |
| Case 7 | 43 | M | White | Positive | ANI | Frozen |
| Case 8 | 39 | M | White | Positive | MND | Frozen |
| Case 9 | 33 | M | Hispanic | Positive | MND | Frozen |
| Case 10 | 35 | M | White | Positive | MND/HAD* (HIVE) | Frozen |
| Case 11 | 37 | M | White | Positive | MND | Frozen |
| Case 12 | 31 | M | White | Positive | MND | Frozen |
| Case 13 | 57 | M | Asian | Positive | HAD | Frozen |
| Case 14 | 30 | F | Hispanic | Negative | Normal | Paraffin |
| Case 15 | 48 | F | Hispanic | Negative | Normal | Paraffin |
| Case 16 | 50 | M | Hispanic | Negative | Normal | Paraffin |
| Case 17 | 56 | F | White | Negative | Normal | Paraffin |
| Case 18 | 57 | M | Black | Positive | ANI | Paraffin |
| Case 19 | 34 | M | White | Positive | ANI | Paraffin |
| Case 20 | 46 | M | White | Positive | ANI | Paraffin |
| Case 21 | 45 | M | Hispanic | Positive | MND/HAD | Paraffin |
| Case 22 | 31 | F | Hispanic | Positive | MND/HAD | Paraffin |
Abbreviations: ANI, asymptomatic neurocognitive impairment; MND, minor neurocognitive disorder; HAD, HIV-associated dementia; HIVE, HIV encephalitis;
diagnosis not available and placed in MND/HAD group
Immunohistochemical staining
Following deparaffinization (not needed for frozen sections), slides were rinsed in distilled water and placed in 1X TBS buffer (20 MM Tris, 13.8 mM NaCl, pH 7.4). Sections were incubated for 20 min at 37°C with proteinase K solution (IHC World) in a humidified chamber then immersed in antigen retrieval buffer (10 mM Tris pH 9.0, 1 mM EDTA, 0.05% Tween-20) and placed in a steamer for 45 min. Frozen sections were fixed in acetone before blocking. Slides were incubated for 1 hr in 10% goat serum/TBS followed by incubation with 1:100 dilution of rabbit anti-NeuN antibody (Millipore, #ABN78) at 4°C overnight. The slides were rinsed thoroughly in 1X TBS followed by incubation in 1:500 dilution of goat anti-rabbit-alkaline phosphatase antibody (Cell Signaling, #7054) at room temperature for 1 hr. Slides were rinsed in 1X TBS and developed with Permanent Fast Red Quanto as directed by the manufacturer (Thermo Scientific) for 10–15 min at room temperature. The slides were rinsed in 1XTBS followed by counterstaining with hematoxylin QS (Vector Labs) for 1–2 min. Slides were dehydrated and mounted in Cytoseal 60 (Thermo Scientific). Images were taken at 63x/1.4 oil plan-apochromat on a Zeiss AxioObserver A1 inverted microscope. Image processing to adjust contrast and sharpen was performed with Adobe Photoshop 5.5.
Determination of NeuN localization and statistical analyses
For our experimental design we had determined, based on preliminary studies, that six samples per group would be needed to have a power of 80% to detect a statistically significant result. Tissue sections were divided into four quadrants and ten random images captured. A total of 200–300 cells in an equal area for each patient sample were analyzed and scored for NeuN localization as: nuclear only, cytoplasmic only, or nuclear and cytoplasmic. Cytoplasmic exclusive staining was defined as filling the cell body while the nucleus remained only hematoxylin positive. Neuronal nuclei that costained for NeuN and hematoxylin had a deep color that was readily distinguishable from cytoplasmic only staining. NeuN nuclear exclusive staining was defined by complete overlap of NeuN and hematoxylin staining within the boundary of the nucleus. Data was analyzed for significance using a one-tailed t test (P>0.05) with GraphPad software.
RESULTS
In studies to characterize the cell-type specific expression of inflammatory mediators in human brain tissue, we used the well-known anti-NeuN antibody to specifically detect neurons. In use for more than 15 years, NeuN reactivity is found largely to be restricted to neuronal nuclei. Interestingly, for a subset of neurons in a paraffin brain section from an HIV-infected individual with ANI compared to a normal control, NeuN staining was visible throughout the cell body and axon (Fig. 1b, arrows). Abundant nuclear NeuN reactivity was also seen in the HIV+ ANI case (Fig. 1b, stars). In contrast, NeuN reactivity was largely nuclear in the uninfected normal control sample (Fig. 1a, star).
Figure 1. Cytoplasmic localization of anti-NeuN staining in a patient with HIV-associated asymptomatic cognitive impairment (ANI) (Paraffin sections).
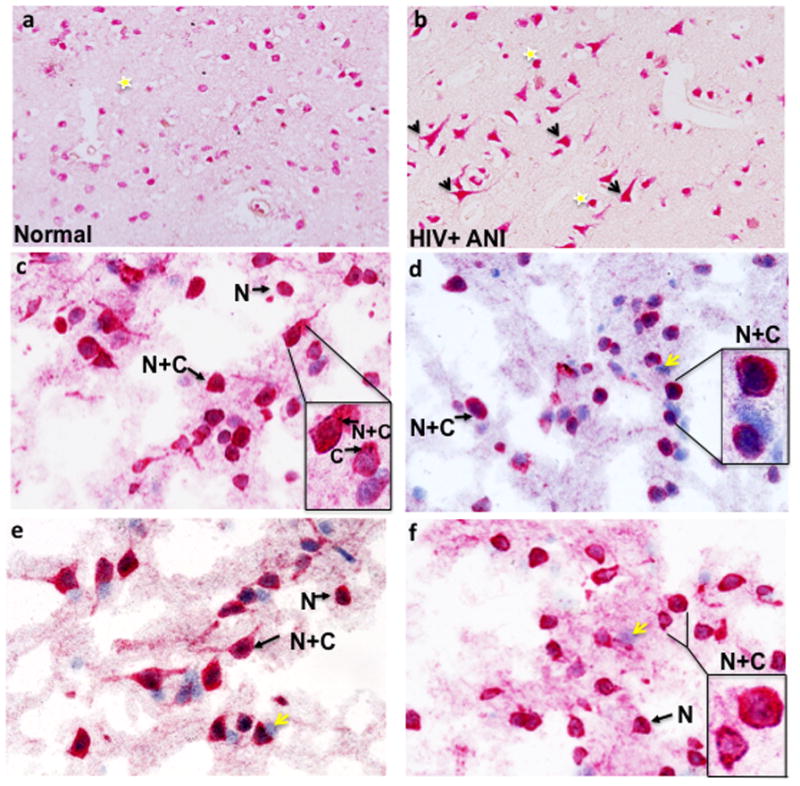
Yellow stars indicate nuclear localization and arrows highlight neurons with NeuN positivity in the cell body and axon. (a) Case number 14 with normal cognition and (b) case number 20, HIV+ ANI (see Table 1). NeuN immunoreactivity in four different normal controls (Frozen sections). Arrows highlight neurons with either nuclear exclusive staining (N) or nuclear and cytoplasmic reactivity (N+C). In the insets (Fig. 1c–f), cells are magnified to illustrate NeuN localization, including cytoplasmic exclusive staining. Yellow arrows indicate hematoxylin positive cells that do not react with anti-NeuN antisera. (a) case number 4, (b) case number 5, (c) case number 2 and (d) case number 1 (see Table 1).
To determine whether this observation was related to HIV infection, and/or the presence of cognitive impairment or might be explained by complex genetic differences between individuals, we characterized more precisely NeuN subcellular localization in brain tissue sections obtained from the NNTC. This prospective longitudinal cohort has existed since 1998 and collects brain tissue at four different sites in the United States [12]. Individuals consenting to upon death have their brain tissues preserved are followed clinically in a longitudinal fashion and subjected to a battery of neuropsychological tests to ascertain the level of cognitive function. In order to obtain a sufficient number of cases, in this study, three groups were analyzed based on neuropsychological diagnoses at the last two visits prior to death: 1) neuropsychological normal (normal), 2) asymptomatic cognitive impairment (ANI) and 3) minor neurocognitive disorder/HIV-associated dementia (MND/HAD). Sections were immunostained with anti-NeuN antisera and to unambiguously identify nuclei, counterstained with the nucleic acid binding dye hematoxylin. The clinical characteristics of the patient samples are given in Table 1 and the exclusion/inclusion criteria are detailed in the methods. The cohort was predominantly composed of males (73%) and the average age of the cases upon death was 43.1 +/− 8.37 years. Fifty percent of the cohort was classified as White, 31.8% Hispanic, 0.09% Black and .04% as Asian or mixed race.
In frozen brain tissue from uninfected controls, NeuN staining was predominantly nuclear (N) with complete colocalization of NeuN and hematoxylin staining (Fig. 1c–f, N arrows). In addition, neurons having NeuN reactivity in the cell body and nucleus (N+C) were also present (Fig. 1c–f, N+C arrows). Cells stained only by hematoxylin were also visible, confirming the specificity of the anti-NeuN antibody for neurons (Fig. 1c–f, yellow arrows). In two HIV+ cases with ANI, a similar pattern of NeuN reactivity was seen, although neurons with both cytoplasmic and nuclear NeuN staining were more abundant (Fig. 2a–b). In contrast, in frozen sections of the HIV+ MND/HAD group, neurons with exclusively cytoplasmic or cytoplasmic and nuclear staining were very abundant (Fig. 2c–f, four different cases shown).
Figure 2. NeuN immunoreactivity in two different HIV+ ANI cases (Frozen sections).
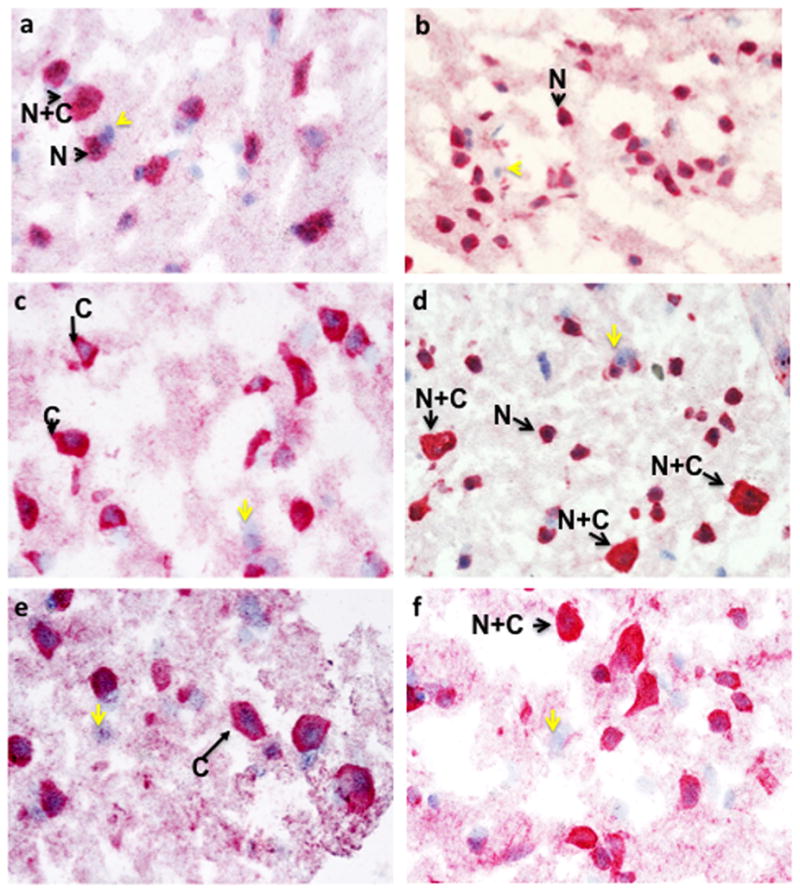
Arrows highlight neurons with either nuclear exclusive staining (N) or nuclear and cytoplasmic reactivity (N+C). Yellow arrows indicate hematoxylin positive cells that did not react with anti-NeuN antisera. (a) case number 6 and (b) case number 7 (see Table 1). NeuN immunoreactivity in four different HIV+ MND/HAD cases (Frozen sections). Arrows highlight neurons with either nuclear exclusive staining (N) or nuclear and cytoplasmic reactivity (N+C) or exclusively cytoplasmic staining (C). Yellow arrows indicate hematoxylin positive cells that did not react with anti-NeuN antisera. In a and c, hematoxylin stained nuclei are surrounded by NeuN reactivity in the cytoplasm. (a) case number 9, (b) case number 13, (c) case number 8, and (d) case number 11 (see Table 1).
A similar pattern of NeuN reactivity was also observed for paraffin-embedded sections. Nuclear exclusive staining was abundant in four different paraffin sections from normal controls (Fig. 3a–d). In three HIV+ ANI cases, NeuN staining was pronounced in nuclei, but neurons with both nuclear and cytoplasmic reactivity were also readily detected (Fig. 3e–f, only two shown). In two cases with HIV infection and a diagnosis of MND/HAD, nuclear exclusive as well as nuclear and cytoplasmic NeuN reactivity was detected (Fig. 4).
Figure 3. NeuN immunoreactivity in four different normal paraffin-embedded cases.
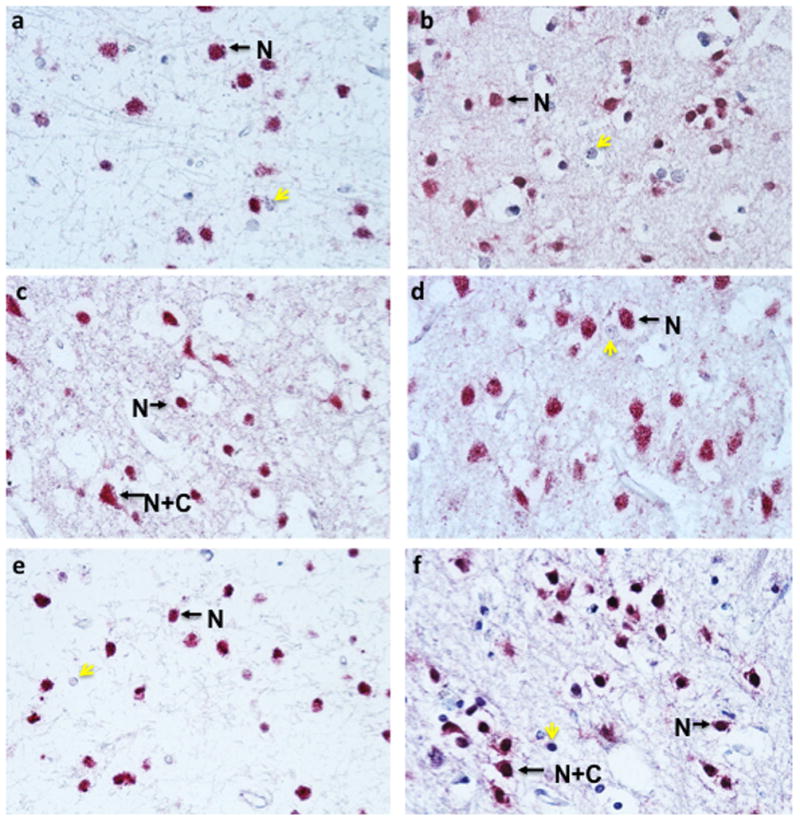
Arrows highlight neurons with either nuclear exclusive staining (N) or nuclear and cytoplasmic reactivity (N+C). Yellow arrows indicate hematoxylin positive cells that did not react with anti-NeuN antisera. (a) case number 16, (b) case number 17, (c) case number 15, and (d) case number 14 (see Table 1). NeuN immunoreactivity in two different HIV+ ANI paraffin-embedded cases. Arrows highlight neurons with either nuclear exclusive staining (N) or nuclear and cytoplasmic reactivity (N+C). Yellow arrows indicate hematoxylin positive cells that did not react with anti-NeuN antisera. (a) case number 19, (b) case number 18 (see Table 1).
Figure 4. NeuN immunoreactivity in two different HIV+ MND/HAD paraffin-embedded cases.
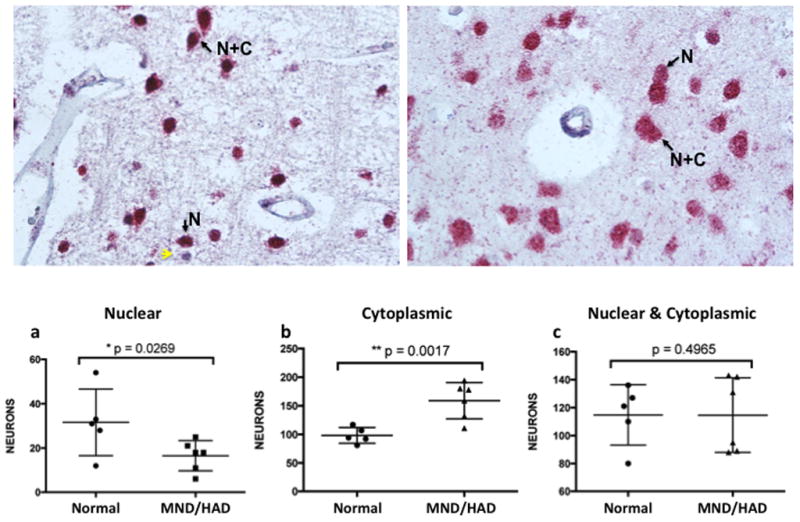
Arrows highlight neurons with either nuclear exclusive staining (N) or nuclear and cytoplasmic reactivity (N+C). Yellow arrows indicate hematoxylin positive cells that did not react with anti-NeuN antisera. (a) case number 22 and (b) case number 21 (see Table 1). NeuN staining is significantly decreased in nuclei and increased in the cytoplasm of neurons found in the brains of HIV-infected individuals with MND/HAD. Approximately 200–300 neurons from frozen sections for each case was scored (mean +/− s.e.m.; one-tailed t-test; *p<.05, **p<.001). (a) The number of neurons with exclusively nuclear localized NeuN in controls (N=5; 31.6 +/− 6.71) and HIV+ MND/HAD (N=6; 16.5 +/− 2.81 was significantly decreased, p=0.0269. (b) The number of neurons with exclusively cytoplasmic localized NeuN in controls (N=5; 98.2 +/− 19.3 (mean +/− s.e.m.) and HIV+ MND/HAD (N=6; 159 +/− 12.99) was significantly increased, p=0.0017. (c) The number of neurons with both nuclear and cytoplasmic localized NeuN in controls (N=5; 115 +/− 9.67) and HIV+ MND/HAD (N=6; 114.7 +/−10.9) did not differ significantly p=0.4965.
The three types of NeuN reactivity were quantified and analyzed for significant differences using the five frozen normal cases and six MND/HAD sections. A significant decrease in the total number of neurons with exclusively nuclear localized NeuN between the non-infected controls (N=5; 31.6 +/− 6.71 (mean +/−s.e.m.) and HIV+ MND/HAD group (N=6; 16.5 +/− 2.81) was detected (Fig. 8p=0.0269). A significant increase in the number of neurons with exclusively cytoplasmic NeuN was found for HIV+ MND/HAD group (N=6; 159 +/−12.99) compared to normal controls (N=5; 98.2 +/− 19.3) (Fig. 8p=0.0017), while no significant differences were found between the groups in the number of neurons displaying both nuclear and cytoplasmic staining (Fig. 8, Normal, N=5; 115 +/− 9.67) and HIV+ MND/HAD (N=6; 114.7 +/− 10.9113) p=0.4965). There were an insufficient number of paraffin cases in the MND/HAD group to perform a similar analysis, but the results for the normal and HIV+ ANI groups showed a similar trend. These results suggest that an increase in the number of neurons with cytoplasmic or cytoplasmic and nuclear localized NeuN is more prevalent in brain tissue from cognitively impaired HIV-infected individuals.
DISCUSSION
Despite its extensive use in neuroscience research, the identity of the protein recognized by anti-NeuN antibody was only recently reported as Rbfox3, a member of the Fox family of RNA splicing factors [7]. While Rbfox2 is more widely expressed, Rbfox1 is found in the brain and striated tissues while, Rbfox3 is restricted to neurons [7]. The tissue-specific Rbfox RNA splicing factors are unique in recognizing a conserved UGCAUG motif within introns [15]. While over a 100 genes, including those involved in many neuromuscular disorders, have been identified as targets of Rbfox1 and Rbfox2 [10] those regulated by Rbfox3 remain largely unknown. Recently non-muscle myosin heavy chain (NMHC) was reported to be a substrate of Rbfox3 and the polypyrimidine tract binding protein associated splicing factor (PSF) was found to interact with Rbfox3 in the splicing of NMHC [8]. In addition, Rbfox2 has been suggested to be a substrate of Rbfox3 that generates a dominant negative form of Rbfox2 leading to nonsense-mediated decay of its mRNA [2]. Moreover, this study suggested that four alternatively spliced isoforms of Rbfox3 are made and that one of these preferentially localizes to the cytoplasm. However, the use of epitope tags in the latter report may have confounded interpretation of the subcellular localization studies [2]. Nevertheless, cytoplasmic localization of NeuN positive Dogiel type II neurons abundant in the calcium binding protein calbindin, where found in the gastrointestinal tract of the pig [17].
Interestingly, of all the tissues in the body, the brain exhibits the highest levels of splicing, likely reflecting the importance for rapid and responsive changes in gene expression to maintain brain activity and homeostasis and perhaps could explain why Rbfox3 is dedicated exclusively to the brain. The sorting of RNA splicing factors to the cytoplasm as a mechanism for downregulating its function has been previously suggested [16, 19]. The spliceosome, a multicomponent ribonucleoprotein complex is utilized for basal splicing. Mutations in any of the components in the spliceosome or in the intron/exon target sequences can cause disease. Of relevance are the tauopathies that result from a change in the ratio of protein isoforms due to defects in splicing and frontotemporal lobar degeneration (FTLD) caused by the loss of the splicing factor TDP43 [14]. Interestingly, in ALS and FTLD, TDP43 is ubiqutinated and mislocalized to the cytoplasm, while in normal neurons it is located in the nucleus [13]. In addition to expanding these findings in a larger cohort, future studies are aimed at identifying the genes regulated by Rbfox3, which may be important in neuronal survival and homeostasis.
HIGHLIGHTS.
Anti-NeuN recognizes the brain-specific RNA splicing factor, Rbfox3.
NeuN/Rbfox3 localization is altered in the context of a neurological disorder.
Rbfox3 mislocalization could be a mechanism that downregulates its activity.
Acknowledgments
This work could not have been done without the resources provided by The US National NeuroAIDS Tissue Consortium through the following grants: Manhattan HIV Brain Bank: U01MH083501, R24MH59724, Texas NeuroAIDS Research Center U01MH083507, R24 NS45491, National Neurological AIDS Bank 5U01MH083500, NS 38841, California NeuroAIDS Tissue Network U01MH083506, R24MH59745, Statistics and Data Coordinating Center U01MH083545, N01MH32002. This study was also made possible through funding from the US National Institutes of Mental Health grant R21 MH095646 awarded to A.B. We thank Himadri Patel for her interest in the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Calixto-Hope Lucas, Email: clucas7@jhu.edu, Johns Hopkins University School of Medicine, Department of Neurology, 600 North Wolfe Street, Meyer 6-181, Baltimore, MD 21287.
Mathilde Calvez, Email: Mathilde.calvez@ens-lyon.fr, Johns Hopkins University School of Medicine, Department of Neurology, 600 North Wolfe Street, Meyer 6-181, Baltimore, MD 21287.
Roshni Babu, Email: robabu@gmail.com, Johns Hopkins University School of Medicine, Department of Neurology, 600 North Wolfe Street, Meyer 6-181, Baltimore, MD 21287.
Amanda Brown, Email: abrown76@jhmi.edu, Johns Hopkins University School of Medicine, Department of Neurology, 600 North Wolfe Street, Meyer 6-181, Baltimore, MD 21287.
References
- 1.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dredge BK, Jensen KB. NeuN/Rbfox3 nuclear and cytoplasmic isoforms differentially regulate alternative splicing and nonsense-mediated decay of Rbfox2. PLoS One. 2011;6:e21585. doi: 10.1371/journal.pone.0021585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorder before and during the era of combination antiretroviral therapy: differences in rates, nature and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012 doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaul M. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KK, Kim YC, Adelstein RS, Kawamoto S. Fox-3 and PSF interact to activate neural cell-specific alternative splicing. Nucleic Acids Res. 2011;39:3064–3078. doi: 10.1093/nar/gkq1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft-Terry SA, SD, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroyanagi H. Fox-1 family of RNA-binding proteins. Cell Mol Life Sci. 2009;66:3895–3907. doi: 10.1007/s00018-009-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArthur J, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 12.Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol and Applied Neurobiol. 2001;27:326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 13.Polymenidou M, Lagier-Tourenne C, Hutt KR, Bennett CF, Cleveland DW, Yeo G. Misregulated RNA processing in amyotrophic lateral sclerosis. Brain Res. 2012;1462:3–15. doi: 10.1016/j.brainres.2012.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Underwood JG, Boutz PL, Dougherty JD, Stoilov P, Black DL. Homologues of the Caenorhabditis elegans Fox-1 Protein Are Neuronal Splicing Regulators in Mammals. Mol Cell Biol. 2005;25:10005–10016. doi: 10.1128/MCB.25.22.10005-10016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Houven van Oordt W, Diaz-Meco MT, Lozano J, Krainer AR, Moscat J, Caceres JF. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J Cell Biol. 2000;149:307–316. doi: 10.1083/jcb.149.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Nassauw L, Wu M, De Jonge F, Adriaensen D, Timmermans JP. Cytoplasmic, but not nuclear, expression of the neuronal nuclei (NeuN) antibody is an exclusive feature of Dogiel type II neurons in the guinea-pig gastrointestinal tract. Histochem Cell Biol. 2005;124:369–377. doi: 10.1007/s00418-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 18.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 19.Xie J, Lee JA, Kress TL, Mowry KL, Black DL. Protein kinase A phosphorylation modulates transport of the polypyrimidine tract-binding protein. Proc Natl Acad Sci USA. 2003:8776–8781. doi: 10.1073/pnas.1432696100. [DOI] [PMC free article] [PubMed] [Google Scholar]


