Abstract
In adult mice, repeated cocaine administration induces behavioral sensitization measured as increased horizontal locomotor activity. Cocaine-induced locomotor sensitization has been well characterized in adult mice. In adult animals, the D1 dopamine receptor is important for mediating effects of cocaine. The effect of cocaine on D1 receptor expression and function in preadolescent animals is less understood. The recently described drd1-Enhanced Green Fluorescent Protein (drd1-EGFP) reporter mouse is a useful model for performing such mechanistic studies; however, preadolescent drd1-EGFP mice have not been characterized previously. Here we studied cocaine-induced locomotor sensitization in preadolescent drd1-EGFP reporter mice. We administered 15 mg/kg cocaine three times daily at one hour intervals for seven consecutive days beginning on postnatal day 23 to drd1-EGFP reporter mice and the commonly used C57BL/6 mice. Under this regimen, preadolescent mice of both strains exhibited cocaine-induced locomotor sensitization; however, by day 7 the cocaine-induced locomotor activity in the drd1-EGFP mice was maintained for a longer duration compared to the C57BL/6 mice. The preadolescent drd1-EGFP mice also exhibited elevated basal locomotor activity in a novel environment and had higher D1 and D2 dopamine receptor mRNA levels in the caudate nucleus compared to the C57BL/6 mice. The cocaine-induced locomotor sensitization was not retained when the drd1-EGFP mice were maintained cocaine-free for two weeks suggesting that in preadolescent drd1-EGFP mice the cocaine-induced changes do not persist.
Keywords: binge cocaine, preadolescent, behavioral sensitization, withdrawal, locomotor activity, dopamine receptors
1. Introduction
Drugs that affect the dopaminergic system elicit age dependent effects on behavioral and neurochemical responses [16, 35]. In preadolescent mice, the cortico-striatal-thalamo-cortical pathway, which is the primary circuit involved in decision making and motivation, undergoes post natal developmental maturation [7, 13, 24, 25]. In adult mice this pathway is modulated by the dopaminergic system, which undergoes drugs of abuse-induced long-term neurochemical adaptations [1, 23]. While numerous studies have investigated mechanisms underlying cocaine addiction in adult animals and in utero, few studies have investigated the effects of cocaine on preadolescent mice [23, reviewed in 27]. It is of particular interest, given the post natal developmental maturation that occurs in the brain during the preadolescent period [6, 7, 17]. Cocaine-induced behavior sensitization is a progressive augmentation of horizontal locomotor activity in response to repeated cocaine administration. Behavior sensitization has been postulated to underlie the neural basis of drug addiction [20, 26].
Dopamine receptors are divided into two classes, the D1-like (D1 and D5) and the D2-like (D2, D3 and D4), based on their stimulatory and inhibitory effects on adenylate cyclase, respectively [3]. Using pharmacological and genetic tools it has been demonstrated that the D1 receptor is necessary for cocaine-mediated neural and behavioral responses [5, 9, 11, 32]. The dopaminergic system also undergoes postnatal developmental maturation; in particular, the D1 receptor protein expression levels are low at birth and peak at postnatal day 21 [10, 28]. The molecular mechanisms underlying D1 receptor expression and function at the post-weanling, preadolescent developmental stage and the effect of cocaine on these mechanisms are not known. Such studies would be facilitated by the drd1-EGFP reporter mouse model characterized in this paper. The drd1-EGFP transgenic mice are a relatively new transgenic mouse line developed by the Gene Expression Nervous System Atlas project. This reporter mouse model expresses the enhanced green fluorescent protein (EGFP) in cells that endogenously express the D1 receptor which facilitates the identification and characterization of D1 receptor expression and function in vivo. These mice have been used recently to study D1 receptor function in adult animals [12, 15, 30]. Given the role of D1 receptor in mediating the effects of cocaine, the drd1-EGFP mouse strain is a valuable in vivo model for studying the underlying molecular mechanisms [21]. In this study our primary goal was to determine the effects of cocaine on preadolescent drd1-EGFP mice.
2. Experimental procedures
2.1. Animals
All experiments described in this paper were performed with male mice. Two breeding pairs of drd1-EGFP mice (Tg(Drd1-a-EGFP)X60Gsat/Mmmh MMRRC:000297) were obtained from the Mutant Mouse Regional Resource Center at University of Missouri, Columbia, Missouri, USA and a local breeding colony established at Rutgers-New Jersey Medical School. The drd1-EGFP transgenic mice have a mixed Swiss Webster/FVB genetic background. Male C57BL/6 mice were purchased from Charles River Laboratories (Kingston, NY). The mice were weaned at P21 and used for experiments on P23 or P30. Animals were housed in individual cages on a 12 hour light/dark cycle (lights on at 0700), and provided food and water ad lib. All procedures were approved by the IACUC committee at Rutgers-New Jersey Medical School.
2.2. Cocaine administration
Beginning on P23, mice received three daily intraperitoneal (i.p.) injections of saline or 15mg/kg of cocaine HCl (Medisca, Plattasburgh, NY), one hour apart, for seven consecutive days in the locomotor arena. The injection volume was 0.2 mL. The dose of cocaine and the binge administration protocol have been previously described [21, 22, 33].
2.3. Activity measurement
Horizontal locomotor activity was recorded on each of the 7 treatment days using the open field photobeam activity system (PAS; SD Instruments, San Diego, CA). The PAS recording software was programmed to collect data over 4 phases with 12 intervals per phase. Each interval was 300 seconds long. The animals were placed in the open field for half an hour prior to injections for habituation. Photobeam breaks were collected in 5 min bins for half an hour prior to injections and one hour after each of three injections for a total recording time of 3.5 hours. In some experiments the locomotor activity of naïve non-injected P30 mice were recorded for 30 minutes. Photobeam breaks were converted to total distance traveled in cm using the PAS reporter software (version 2). The resting time parameter in the software was set at 4 seconds.
2.4. Brain tissue harvest
Brains were harvested for mRNA analysis from naïve non-injected mice on P30. Whole brain was isolated and immersed in ice-cold saline. Brain sections (300µm thick) were obtained using a refrigerated Vibratome® 1500 sectioning system (Vibratome, St. Louis, MO) maintained at 3°C. The nucleus accumbens and caudate brain regions were micro-punched (2mm) from 300µm coronal sections obtained from following coordinates- interaural 5.4mm/bregma 1.94mm to interaural 3.70mm/bregma -1.10mm. The micro-punches for RNA isolation were stored in RNAlater® (Ambion) and stored at − 80°C.
2.5. Real-time reverse transcriptase PCR
RNA isolation and RT-PCR was performed as described previously [18]. D1, D2 and D3 dopamine receptor cDNA levels were measured using TaqMan® gene expression assays Mm0135211, Mm00438545 and Mm00432887, respectively. The internal control GAPDH cDNA was detected using Mm99999915 TaqMan® gene expression assay. Appropriate negative and positive controls were included in the RT-PCR experiments [18, 28].
2.6 Statistics
One-way, two-way, two-way repeated measure analysis of variance (ANOVA), post-hoc multiple comparison tests and two-tailed Student’s t-test were performed with the SigmaPlot® 11 (SPSS Inc.). For the two-way ANOVA tests, the main factors were treatment and time. Data were considered statistically significant when the probability value (P) was less than 0.05. The number of animals used in each experiment is indicated in the figure legends.
3. Results
3.1. Preadolescent drd1-EGFP and C57BL/6 mice exhibits cocaine-induced locomotor sensitization
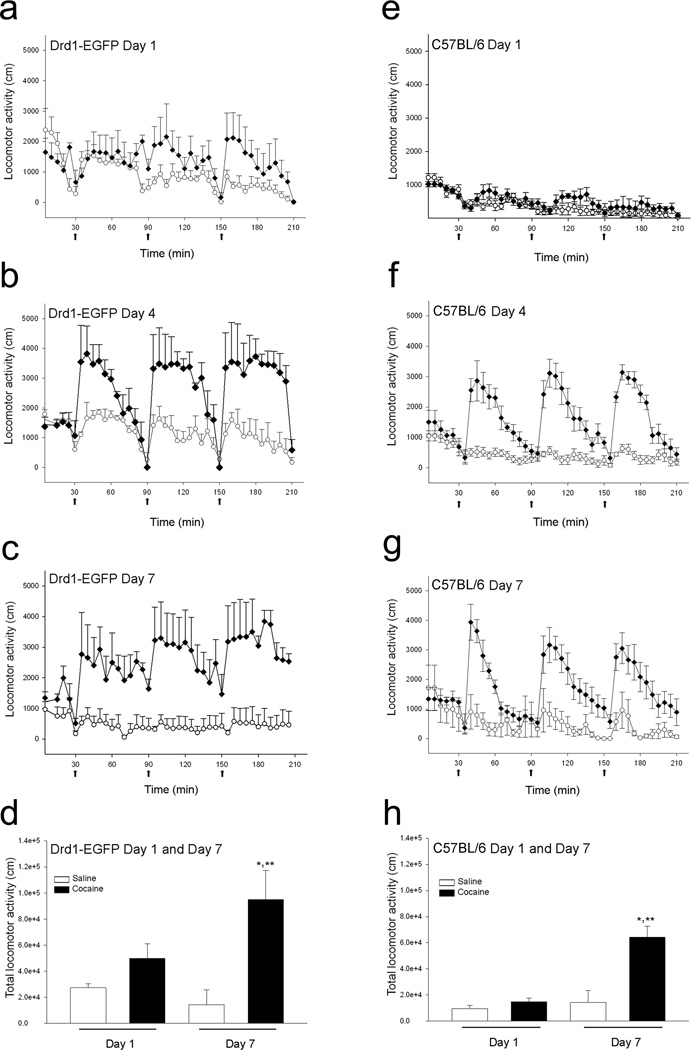
To determine if preadolescent mice exhibit cocaine-induced locomotor sensitization, we treated male drd1-EGFP and C57BL/6 mice beginning at P23 with saline or cocaine as described in the Experimental procedure section. Statistical analysis of the results in Figure 1 using two-way repeated measure ANOVA with time and treatment (saline and cocaine) as main factors, suggests that the preadolescent drd1-EGFP and C57BL/6 mice exhibits significant cocaine-induced locomotor sensitization commencing day 4 (Fig. 1b and 1f; F1,123 = 2.918, p<0.001 and F1, 205 = 10.782, p<0.001, respectively). Two-way ANOVA analysis with days (day 1 and day 7) and treatment (saline and cocaine) groups as main factors, followed by Holm-Sidak post-hoc multiple comparison test revealed that the total cocaine-induced locomotor activity of drd1-EGFP and C57BL/6 mice is significantly higher on day 7 than on day 1 (Fig. 1d and 1h; F1,12 = 13.93, p=0.003 and F1,20 =18.003, p<0.001). Initially, in both strains, peak cocaineinduced locomotor activity is observed 5 to 10 minutes after each cocaine injection with the locomotor activity returning to baseline in 45 to 60 minutes; however, by day 7 the cocaine-induced locomotor activity of the C57BL/6 mice (Fig. 1g) returned to base line faster (τ=0.168 ± 0.04) than the drd1-EGFP mice (τ= 0.0339 ± 0.01; Fig. 1c). The decay constant (τ) for cocaine-induced locomotor activity in C57BL/6 mice was significantly different compared to drd1-EGFP mice (P=0.008, Student’s t-test).
Figure 1.
Effect of binge cocaine administration on the horizontal locomotor activity in drd1-EGFP and C57BL/6 mice. Locomotor activity on days 1 (a, e), 4 (b, f), and 7 (c, g) in drd1-EGFP (a–d) and C57BL/6 (e–h) mice. Mice were administered saline (circle) or 15 mg/kg cocaine (diamonds) intraperitoneally at 9:30, 10:30, and 11:30 A.M. daily (black arrows). Photo beam break counts were collected in 5 minute bins for half an hour before and 3 hours after the injections commenced. The total horizontal distance traveled (cm) for three hours in response to saline (white bars) and cocaine (black bars) in drd1-EGFP (d) and C57BL/6 (h) mice were significantly different between the cocaine treated groups on day 1 and day 7 (*, p<0.05, n=4–6, two-way ANOVA) and between the saline and cocaine treated groups on day 7 (**, p<0.05, n=4–6, two-way ANOVA). Error bars represent ± SEM.
3.2. Drd1-EGFP mice have higher basal locomotor activity than C57BL/6 mice
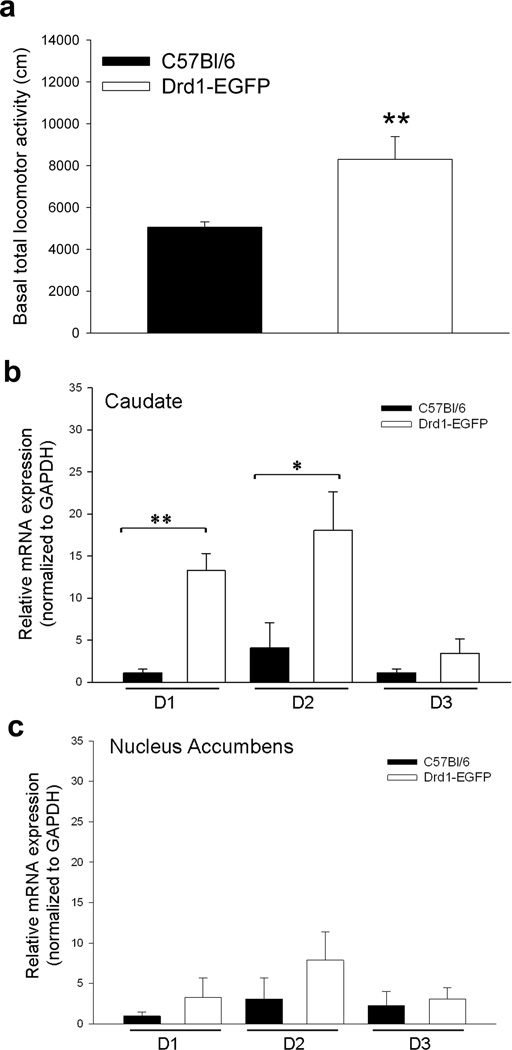
While both preadolescent C57BL/6 and drd1-EGFP mice exhibited robust cocaine-induced locomotor sensitization, the locomotor data gathered on P23, during the initial 30 minute habituation period in the open field arena, suggested that the drd1-EGFP mice have higher basal locomotor activity than the C57BL/6 mice (Fig. 1). To investigate this further, we measured basal locomotor activity in naïve non-injected drd1-EGFP and C57BL/6 mice on P30. The results in Figure 2a show that basal locomotor activity in a novel environment is significantly higher in the drd1-EGFP mice.
Figure 2.
Differences in basal locomotor activity and dopamine receptor expression between C57BL/6 and drd1-EGFP mice strains. (a) Total distance traveled (cm) during the initial 30 min after preadolescent (P30) C57BL/6 (black bar, n=12) and drd1-EGFP (white bar, n=8) mice are placed in the locomotor arena. The drd1-EGFP exhibited significantly greater locomotor activity (**, p<0.0.5, Student’s t-test). Error bars represent ± SEM. Quantitative real-time RT-PCR results of basal D1, D2, and D3 dopamine receptor mRNA levels normalized to GAPDH mRNA levels in caudate putamen (b) and nucleus accumbens (c) of C57BL/6 (black bars, n=4) and drd1-EGFP (white bars, n=4) preadolescent (P30) mice. Error bars represent ± SEM. The drd1-EGFP mice express significantly higher levels of D1 (**p< 0.001) and D2 (*p<0.05) receptor mRNA than the C57BL/6 mice in the caudate putamen (Student’s t-test).
Previous studies have shown that activation of both D1 and D2 receptors induce locomotor activity whereas activation of D3 receptor inhibits locomotor activity [3, 19]. To determine if the difference in basal locomotor activity between C57BL/6 and drd1-EGFP mice strains was due to a difference in the expression of D1, D2 and D3 dopamine receptors, we used RT-PCR to measure the mRNA level of these dopamine receptor subtypes in the caudate and nucleus accumbens isolated from naïve non-injected P30 mice after the locomotor activity measurement. The results show that the drd1-EGFP mice express significantly higher level of D1 and D2 receptors in the caudate compared to the C57BL/6 mice (Fig. 2b). The expression level of D1 and D2 receptors was not significantly different in the nucleus accumbens (Fig. 2c) and the level of D3 receptor mRNA was similar in both strains.
3.3. Preadolescent drd1-EGFP mice do not maintain cocaine-induced sensitization following a two-week abstinence period
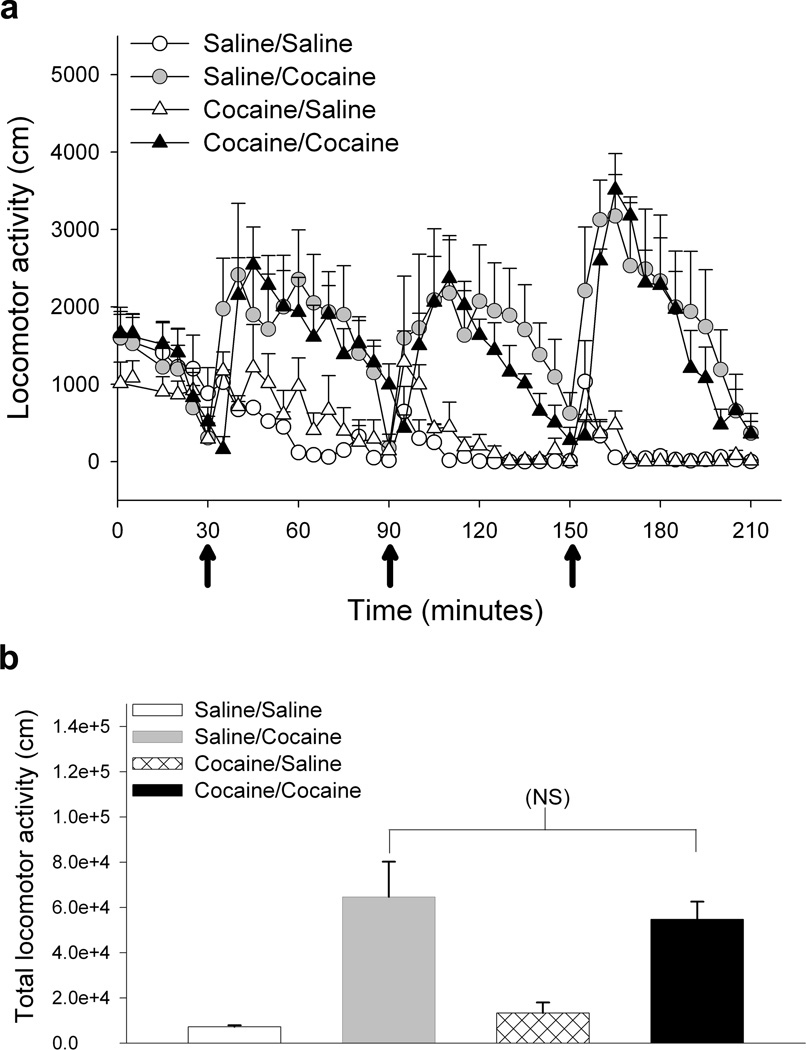
Studies in adult animals have shown that cocaine-induced sensitization persists even after an extended cocaine abstinence period [8, 14]. To determine if preadolescent mice maintained the cocaine-induced sensitization, drd1-EGFP mice at P23 were administered saline or cocaine for seven days as described above. Locomotor activity was monitored and the mice exhibited cocaine-induced locomotor sensitization as shown in Figure 1. The mice were then maintained in home cages without cocaine for two weeks and then challenged with saline or the same dose (15 mg/kg) of cocaine on P44. Statistical analysis of the results in Figure 3 using two-way repeated measure ANOVA with time and treatment as main factors, suggested no overall significant effect of treatment (F1,246 = 3.001, p=0.117); however there was a significant interaction between time and treatment (F1,246 = 2.697, p<0.001) with the Holm-Sidak post-hoc test revealing significant difference in the time period following the third binge cocaine injection between groups that received saline and cocaine at P44 (Fig. 3a). There was no significant difference in the locomotor response elicited by the challenge dose of cocaine in the P44 animals that were previously administered saline or cocaine during the preadolescent period (P23 to P29) (Fig. 3b). This suggests that the preadolescent drd1-EGFP mice do not retain the cocaine-induced sensitization after a two week abstinence period. To determine if the increased cocaine-induced locomotor activity observed in the P44 mice that were saline treated during the preadolescent period was due to the developmental maturation of the underlying pathways, we compared its cocaine-induced locomotor response to that of naïve P44 drd1-EGFP mice that were never administered saline or cocaine previously. The results in Supplementary Fig. 1 show that the cocaine-induced locomotor activity in the naïve P44 drd1-EGFP mice is similar to the cocaine-induced locomotor activity in P44 drd1-EGFP mice that were administered saline during the preadolescent period.
Figure 3.
Preadolescent drd1-EGFP mice do not retain the cocaine-induced locomotor sensitization following a two-week cocaine-free period. (a) Horizontal distance travelled (cm) by drd1-EGFP mice on P44. Mice received saline (circles) or 15 mg/kg cocaine (triangles) during the 7-day binge sensitization period beginning P23. On P44, following a two-week injection-free period, the mice in the two treatment groups were separated into four groups and challenged with either saline (saline/saline, open circles and cocaine/saline, open triangles) or 15 mg/kg cocaine (saline/cocaine, gray circles and cocaine/cocaine, black triangles) in the locomotor arena. The three injections were administered at 9:30, 10:30, and 11:30 A.M (arrows) and data collected in 5 minute bins. Error bars represent ± SEM. (b) The total horizontal distance traveled (cm) in three hours for groups shown in panel a. Error bars represent ± SEM. NS, no statistically significant difference between saline-cocaine and cocaine-cocaine treatment groups. (p>0.05, one way ANOVA, post-hoc Dunnett’s test).
4. Discussion
The primary objective of this study was to determine the effect of cocaine administration on preadolescent drd1-EGFP mice. The results show that both drd1-EGFP and C57BL/6 preadolescent mice develop cocaine-induced behavioral sensitization as a result of repeated cocaine administration. While both strains manifest behavioral sensitization, there are subtle differences in their locomotor responses. Both strains start to display increased cocaine-induced locomotor activity on Day 4 in response to the same dose of cocaine, but the drd1-EGFP strain maintains an elevated locomotor response while the C57BL/6 strain returns to baseline within an hour of each cocaine injection. This difference in response termination became more pronounced by Day 7 (Fig. 1c and 1g). In addition, when comparing the initial locomotor activity of naïve animals during the habituation period on P23 and P30, we observed that the drd1-EGFP strain had a significantly higher locomotor activity than the C57BL/6 strain. The difference in locomotor activity in a novel environment might represent inherent genetic differences between the two strains. Indeed, the higher level of D1 and D2 dopamine receptor mRNA in the caudate of the drd1-EGFP mouse strain and increased locomotor behavior are consistent with the reported role of these two receptor subtypes in the induction of locomotor activity [reviewed in 34]. Differences in ontogeny of dopamine receptor expression between the strains might also contribute to the behavior and expression differences observed in this study.
One of the goals of this study was to determine if cocaine exposure during the preadolescent period induced long-lasting changes in the locomotor activity of the drd1-EGFP mice. Studies in adult animals have shown that cocaine-sensitized animals maintain their behavioral sensitization even after an extended cocaine-free period. For example, in adult rats, cocaine-induced behavioral sensitization persists after a one month cocaine-free period [14, 29]. In contrast to these adult animal studies, we observed that while preadolescent drd1-EGFP mice manifest cocaine-induced behavioral sensitization, this sensitization is not maintained after a two week cocaine- and injection-free period. This result suggests that cocaine-induced neuronal adaptations in the preadolescent drd1-EGFP brain might not persist. A previous study using black Swiss Webster mice, which were sensitized from P15 to P28, demonstrated retention of behavioral sensitization following a cocaine-free period [8]. This suggests that the retention of cocaine-induced sensitization might be different between strains. However, it should be noted that in the study with the black Swiss Webster strain, the mice continued to receive saline injections during the cocaine-free period [8]. These saline injections could confound the results by serving as a conditioned stimulus reinforcing the behavioral response.
The drd1-EGFP mouse model facilitates the identification of D1 receptor mRNA-expressing neurons, making detailed mechanistic studies possible [4]. However before such mechanistic studies are performed using the preadolescent drd1-EGFP model, it is important to characterize this animal model. This is particularly important since previous studies using adult drd1-EGFP mice with different genetic backgrounds have shown contradictory results [2, 12, 15, 30]. Given this, comparison of results obtained in different strains might be difficult to reconcile and, as such, mechanistic investigation of cocaine effects will need to be performed within individual strains. Characterization of cocaine-induced behavioral sensitization in preadolescent drd1-EGFP mice will facilitate future studies that investigate the role of D1 receptor expression and function during post natal brain development. In particular, this mouse model will be useful for investigating the role of post-natal developmental changes that occur in the D1 receptor-expressing cells and how these are affected by drugs of abuse.
Supplementary Material
Highlights.
Drd1-EGFP mice exhibit higher basal locomotor activity than C57BL/6 mice
Drd1-EGFP mice express higher levels of D1 and D2 receptors than C57BL/6 mice
Preadolescent mice of both strains exhibit cocaine-induced behavioral sensitization
Termination of cocaine-induced response is different between the two mice strains
Sensitization does not persist in drd1-EGFP mice after a 2-week cocaine withdrawal
Acknowledgements
This work was supported by NIH grants R03DA026030 and R03DA026030-02S1 and a grant from the F. M. Kirby Foundation to EVK. KET was supported by a PhRMA Foundation pre-doctoral fellowship. We would like to acknowledge the late Dr. Steven S. Zalcman who helped us with design and interpretation of the locomotor assays.
Abbreviations
- EGFP
Enhanced green fluorescent protein
- PAS
photobeam activity system
- ANOVA
Analysis of variance
- RT-PCR
Reverse transcriptase-polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interests.
References
- 1.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav. Neurosci. 1998;112(5):1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 2.Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampà C, Calabresi P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease. J. Neurosci. 2011;31(35):12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 4.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 2008;28(22):5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J. Neurosci. 2007;27(48):13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32(11):2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- 7.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerriero RM, Hayes MM, Dhaliwal SK, Ren JQ, Kosofsky BE. Preadolescent methylphenidate versus cocaine treatment differ in the expression of cocaine-induced locomotor sensitization during adolescence and adulthood. Biol. Psychiatry. 2006;60(11):1171–1180. doi: 10.1016/j.biopsych.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J. Cell. Physiol. 2002;191(1):17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- 10.Jung AB, Bennett JP., Jr Development of striatal dopaminergic function. I. Pre- and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Brain Res. Dev. Brain Res. 1996;94(2):109–120. doi: 10.1016/0165-3806(96)00033-8. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200(1):117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J. Neurosci. 2011;31(1):126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci. Biobehav. Rev. 1999;23(7):993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 14.Marin MT, Cruz FC, Planeta CS. Cocaine-induced behavioral sensitization in adolescent rats endures until adulthood: lack of association with GluR1 and NR1 glutamate receptor subunits and tyrosine hydroxylase. Pharmacol. Biochem. Behav. 2008;91(1):109–114. doi: 10.1016/j.pbb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Nelson AB, Hang GB, Grueter BA, Pascoli V, Luscher C, Malenka RC, Kreitzer AC. A comparison of striatal-dependent behaviors in wild-type and hemizygous Drd1a and Drd2 BAC transgenic mice. J. Neurosci. 2012;32(27):9119–9123. doi: 10.1523/JNEUROSCI.0224-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol. Biochem. Behav. 2005;82(2):280–288. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell P. Adolescent maturation of cortical dopamine. Neurotox. Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- 18.Pasuit JB, Li Z, Kuzhikandathil EV. Multi-modal regulation of endogenous D1 dopamine receptor expression and function in the CAD catecholaminergic cell line. J. Neurochem. 2004;89(6):1508–1519. doi: 10.1111/j.1471-4159.2004.02450.x. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA, Xu M, Zhang J, Richtand NM. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol. Biochem. Behav. 2007;86(4):718–726. doi: 10.1016/j.pbb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 21.Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of "binge" pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 1998;60(2):593–599. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 22.Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic "binge" cocaine in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 2003;75(1):123–131. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 23.Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacol. (Berl) 2004;173(1–2):41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- 24.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 25.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 26.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol. Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci. Biobehav. Rev. 2003;27(1–2):163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 28.Tobón KE, Chang D, Kuzhikandathil EV. MicroRNA 142-3p mediates post-transcriptional regulation of D1 dopamine receptor expression. PLoS One. 2012;7(11):e49288. doi: 10.1371/journal.pone.0049288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ujike H, Tsuchida K, Akiyama K, Fujiwara Y, Kuroda S. Ontogeny of behavioral sensitization to cocaine. Pharmacol. Biochem. Behav. 1995;50(4):613–617. doi: 10.1016/0091-3057(94)00352-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50(3):443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Westrich L, Kuzhikandathil EV. The tolerance property of human D3 dopamine receptor is determined by specific amino acid residues in the second cytoplasmic loop. Biochim. Biophys. Acta. 2007;1773(12):1747–1758. doi: 10.1016/j.bbamcr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852(1):198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or "binge" cocaine in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 2002;73(3):655–662. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Ouagazzal A-M, Sun B-C, Creese I. In: Regulation of motor behavior by dopamine receptor subtypes. The Dopamine Receptors Chapter 14. Neve KA, Neve RL, editors. New Jersey: Humana Press; 1997. pp. 425–455. [Google Scholar]
- 35.Zombeck JA, Swearingen SP, Rhodes JS. Acute locomotor responses to cocaine in adolescents vs. adults from four divergent inbred mouse strains. Genes Brain Behav. 2010;9(8):892–898. doi: 10.1111/j.1601-183X.2010.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.