Abstract
White matter hyperintensities (WMH) of presumed vascular origin as seen on T2-weighted fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI), are known to increase with age and are elevated in Alzheimer’s disease (AD). The cognitive implications of these common markers are not well understood. Previous research has primarily focused on global measures of WMH burden and broad localizations that contain multiple white matter tracts. The aims of this study were to determine the pattern of WMH accumulation with age, risk for AD, and the relationship with cognitive function utilizing a voxel-wise analysis capable of identifying specific white matter regions. Three hundred and forty-nine participants underwent T1-weighted and high-resolution T2FLAIR MRI and neuropsychological testing. Increasing age and lower cognitive speed and flexibility (a component of executive function), were both significantly associated with regional WMH throughout the brain. When age was controlled, lower cognitive speed and flexibility was independently associated with WMH in the superior corona radiata. APOE4 and parental family history of AD were not associated with higher burden of WMH. The results contribute to a larger body of literature suggesting that white matter measures are linked with processing speed, and illustrate the utility of voxel-wise analysis in understanding the effect of lesion location on cognitive function.
Keywords: white matter hyperintensities, aging, processing speed, cognition, MRI
Introduction
White matter hyperintensities (WMH) of presumed vascular origin, as seen on T2-weighted fluid attenuated inversion recovery (FLAIR) MRI, are common features of the aging brain (de Leeuw, de Groot et al. 2001). By the fifth decade of life, approximately 50% of people will have some WMH (Wen, Sachdev et al. 2009), while in healthy adults in their mid-sixties, it’s likely that most will have some degree of WMH as found using T2-weighted imaging (Wen and Sachdev 2004). The underlying cause of these hyperintense regions is thought to be small vessel disease, and accordingly, hypertension and older age are most consistently associated with an increasing burden of WMH (Basile, Pantoni et al. 2006).
Despite the fact that WMH denote localized white matter damage, the associated cognitive changes and risk conferred by WMH for pathological cognitive decline remain incompletely characterized. Several studies suggest a link between WMH and cognitive function even in healthy aging (de Groot, de Leeuw et al. 2000; Gunning-Dixon and Raz 2003; Van Petten, Plante et al. 2004; Soderlund, Nilsson et al. 2006; Frisoni, Galluzzi et al. 2007; Smith, Salat et al. 2011) but other studies have failed to find a link in healthy older adults [for a review see Ferro and Madureira (2002)). While not considered a defining feature of Alzheimer’s disease (AD), WMH are elevated in AD and mild cognitive impairment (MCI) (Yoshita, Fletcher et al. 2006; Cuenco, Green et al. 2008). In AD patients, higher baseline WMH are associated with a greater increase in amyloid-β deposition, potentially due to small vessel disease and subsequently impaired amyloid-β clearance (Grimmer, Faust et al. 2012). WMH also appear to play a role in risk for developing AD; a meta-analysis showed that WMH are a risk factor for AD within population studies (Debette and Markus 2010) and parietal WMH are associated with the risk of incident AD in older adults (Brickman, Provenzano et al. 2012). Whether WMH could be considered a feature of early stage AD, or a result of AD pathological processes is still unknown, and the literature linking WMH to AD risk factors is mixed. Some studies have found elevated WMH in Apolipoprotein E ε4 (APOE4) carriers (de Leeuw, Richard et al. 2004; Lunetta, Erlich et al. 2007), whileBiffi et al. (2010) did not find a relationship between APOE4 status and WHM in the Alzheimer’s Disease NeuroImaging Initiative cohort. The effect of APOE4 may not be specific to AD, as it is also a risk factor for cerebrovascular disease. Parental family history of AD is another well-know risk factor for AD; however,Debette et al. (2009) did not find an effect of parental family history on lesion burden, despite the fact that this risk factor has been linked with white matter alterations as detected with diffusion tensor imaging (DTI) in another study (Bendlin, Ries et al. 2010).
Differences among findings may be due to differences in the population under study, or differences in the way WMH are indexed. WMH in aging and AD have hitherto mainly focused on global lesion volume, e.g. Aggarwal, et al. (2010; Brickman, Siedlecki et al. 2011; Carmichael, Mungas et al. 2012), and broadly defined localization (2010; Guzman, Carmichael et al. 2013).. Given that both aging and AD are associated with regional patterns of white matter change as detected using DTI or volumetric analysis (Good, Johnsrude et al. 2001; Li, Pu et al. 2008; Alves, O'Dwyer et al. 2012), more research may be needed that considers WMH in specific brain locations.
Voxel-wise analysis in which variables of interest can be used to predict WMH throughout the whole brain across a large number of participants can provide regional information with high spatial resolution. Utilizing automated segmentation may also provide a solution to the variability in WMH rating approaches used across laboratories. Largely due to challenges in automated lesion segmentation, voxel-wise approaches to analyzing WMH still rare in the literature. Rostrup, et al. (2012) found differing spatial distribution of WMH with several risk factors for WMH, but did not investigate associations with cognitive symptoms. An elegant study by Smith, et al. (2011) reported a relationship between frontal, posterior, and periventricular white matter lesion burden and executive function. In that same study, frequency of lesions in many of the same posterior and periventricular regions was associated with poorer episodic memory function. The participants in that study were all over the age of 65 and were cognitively normal or diagnosed with MCI or mild dementia. Whether voxel-wise localization of WMH with age is associated with cognitive function or AD risk factors in asymptomatic adults, is relatively unknown.
Thus, the aims of this study were to: (1.) determine the pattern of regional WMH found with increasing age; (2.) determine the extent to which regional WMH are associated with cognitive function; and (3.) assess the impact of parental family history and APOE4 genotype on regional distribution of WMH. In addition to regional analyses, secondary analyses also examined total WMH in order to compare with existing studies. We hypothesized that older age would be associated with higher regional WMH, especially in frontal brain regions, that regional burden would be linked to cognitive dysfunction, and that AD risk would be associated with higher frequency of WMH, especially in AD specific regions, including parietal and temporal lobes.
Methods
Participants
Three hundred and fifty-nine participants from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) underwent brain imaging as part of studies on memory, aging, and risk for AD. WRAP is a longitudinally followed cohort comprising participants who either have a family history of late onset AD or no family history of AD (Sager, Hermann et al. 2005). The majority of the WRAP participants are adult children of persons with AD who were evaluated at the Memory Assessment Clinic at the University of Wisconsin-Madison or satellite memory assessment clinics affiliated with the Wisconsin Alzheimer's Institute, and other participants who learned about the study from educational presentations, health fairs, newsletters, or word of mouth. A positive family history was defined as having one or both parents with autopsy-confirmed or probable AD as defined by NINCDS-ADRDA research criteria (McKhann, Drachman et al. 1984) and reviewed by a multidisciplinary diagnostic consensus panel. Absence of family history of AD required that the father survive to at least age 70 and the mother to age 75 without incurring a formal diagnosis of dementia or exhibiting cognitive deterioration. The inclusion criteria for this imaging study consisted of: normal cognitive function determined by neuropsychological evaluation (MMSE ≥ 25), no contraindications for magnetic resonance imaging (MRI) and a subsequent normal MRI scan, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., myocardial infarction, or recent history of cancer), and no history of head trauma, stroke or transient ischemic attack. All participants underwent MRI and neuropsychological testing. Brain images were reviewed by a neuroradiologist to exclude infarcts and other abnormalities. Ten participants were excluded because of abnormal radiological findings from the reviewing radiologist, leaving 349 participants. Demographic information for this sample is presented in Table 1. The University of Wisconsin Institutional Review Board approved all study procedures and each participant provided signed informed consent before participation.
Table 1.
Demographics (N = 349)
| n (%) | ||
|---|---|---|
| female | 238(68%) | |
| parental fam hist of AD | 260(74%) | |
| ApoE4-carriers | 131 (38%) | |
| diabetics | 7 (2.2%) | |
| current smokers | 17 (5%) | |
| history of hypertension | 178 (51%) | |
| M ± SD | range | |
| Age | 59.7 ± 6.4 | [42 – 73] |
| Edu | 16.2 ± 2.4 | [12 – 20] |
| systolic BP | 124 ±16 | [85 – 176] |
| diastolic BP | 73 ± 9.5 | [48 – 104] |
| WMH volume (mm^3) | 11.7 ± 6.7 | [2.3 – 45.4] |
| WMHr (% of ICV) | 0.85 ± 0.49 | [0.2 – 3.3] |
Cognitive testing
As part of their participation in WRAP, participants received at least one comprehensive neuropsychological assessment (Sager, Hermann et al. 2005). For participants with multiple assessments, factor scores were used from the testing date in closest proximity to the MR scan. On average, neuropsychological testing occurred within nine months of the MRI scan (SD = 5.3 months). We analyzed four cognitive factor scores that were determined from a factor analytic study of the WRAP neuropsychological battery and adapted from work published in Dowling et al.(2010). Factor scores represented cognitive domains known to change with age: Immediate Memory, Verbal Learning & Memory, Working Memory, and Speed & Flexibility. The individual tests which loaded onto the factors were as follows: Immediate Memory - Rey Auditory Verbal Learning Test (RAVLT) Trials 1 and 2 (Spreen and Strauss 1998); Verbal Learning & Memory - RAVLT Trials 3–5 and Delayed Recall Trial; Working Memory - Wechsler Adult Intelligence Scale – 3rd edition Digit Span, Arithmetic, and Letter-Numbering Sequencing subtests (Wechsler 1997); Speed & Flexibility - Stroop Test interference trial (Trenerry 1989), and Trail Making Test A and B(Reitan and Wolfson 1993). Factor scores from all waves were standardized around WRAP baseline data.
The Speed and Flexibility factor score was unavailable for eight participants (five were colorblind and unable to perform the Stoop test, and Trail Making Tests were unavailable for three participants due to tester error). These eight participants were excluded for all analyses involving the Speed and Flexibility factor score.
Brain Imaging Acquisition
MR scanning was performed on a General Electric 3.0 Tesla Discovery MR750 (Waukesha, WI) MRI system with an 8-channel head coil and parallel imaging (ASSET).
A T1-weighted volume was acquired in the axial plane with a 3D fast spoiled gradient-echo (3D FSPGR) sequence using the following parameters: TI = 450 ms; TR = 8.1 ms; TE = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 mm, FOV = 256 mm; slice thickness = 1.0 mm.
A 3D T2-weighted fluid attenuated inversion recovery (FLAIR) sequence was acquired in the sagittal plane using the following parameters: TI = 1868 ms; TR = 6000 ms; TE = 123 ms; flip angle = 90°; acquisition matrix = 256×256, FOV = 256 mm; slice thickness = 2.0 mm, no gap, yielding a voxel resolution of 1 mm × 1 mm × 2 mm.
ICV calculation
Intracranial volume (ICV) was calculated to scale for differences in head size in the WMH analyses using a “reverse brain masking” method (Keihaninejad, Heckemann et al. 2010). First, summing the gray, white and CSF ICBM probability maps created an ICV probability map. Then, the inverse deformation field resulting from unified segmentation on each participant image was applied to the ICV probability map, in order to produce an ICV mask in native space. A threshold of 90% was applied to this participant specific ICV probability map and the total volume was extracted. Total and regional analyses were adjusted for ICV in order to control for the variability in brain size.
WMH segmentation
Total volume of WMH was calculated using the Lesion Segmentation Tool (LST) version 1.2.2 in SPM8 (Schmidt, Gaser et al. 2012). Utilizing automated segmentation provides the advantage of high reliability. The toolbox is open source and utilizes T1-weighted and T2FLAIR images for lesion segmentation. Lesions are seeded based on spatial and intensity probabilities from T1 images and hyperintense outliers on T2FLAIR images. The initial threshold was set at 0.30 and is used to create the binary conservative lesion belief map from the gray and white matter lesion belief maps. Next, a growth algorithm grows these seeds from the conservative lesion belief map toward a probabilistic liberal lesion belief map from gray matter, white matter, and cerebral spinal fluid belief maps. Lastly, we used a threshold of 1.00 on the resulting lesion belief map to remove any voxels that have a lower probability of being a lesion. Schmidt et al. (2012) demonstrated high agreement (R2 = 0.94) between LST and manual tracing in a sample of patients with multiple sclerosis and controls using a version of the software that utilized only the gray matter belief map to seed the algorithm. We found that using the gray and white matter belief maps to seed the algorithm produced more accurate segmentation of lesions in our sample. The resulting total volume of WMH was divided by ICV and multiplied by 100 to give a ratio (WMHr) in units of percent of ICV, which was used for analysis using total lesion volume. In accordance with recently published consensus standards for research into small vessel disease in aging (Wardlaw, Smith et al. 2013), all final segmentation maps were visually inspected.
For voxel-wise WMH analysis, probability lesion belief maps were normalized to Montreal Neurological Institute (MNI) space and smoothed with a 12mm Gaussian kernel. Since the results were in MNI space, we utilized the ICBM DTI-81 white matter atlas (http://www.loni.ucla.edu/Atlases) to identify specific regions of association.
Finally, a constant of one was added and a log transformation was applied to WMHr values and lesion probability maps in order to normalize the distribution of WMH.
Statistical Analyses
Statistical tests were considered significant at p < 0.05. Voxel-wise statistics in SPM8 were corrected for multiple comparisons with a familywise error rate correction (FWE). Voxel-wise analyses were restricted to white matter using an explicit mask made by thresholding the ICBM Tissue Probabilistic Atlases white matter map at 0.30. Results were cluster thresholded at >20 voxels to exclude very small clusters and increase anatomical plausibility.
1. Association between age and total and regional WMH
To test the hypothesis that global WMHr increased with age, linear multiple regression was used in IBM SPSS version 20.0 (Chicago, IL), assessing the significance of age (independent variable), controlling for sex on WMHr (dependent variable). The multiple regression model was used in SPM8 to determine regional correlations between age and lesion probability on a voxel-wise basis. Age was the predictor variable and the voxel values of the smoothed lesion probability belief maps were the dependent variable. Sex and ICV were entered as covariates.
2. Association between cognition and total and regional WMH
To test the hypothesis that cognitive functioning decreases with increasing WMHr, linear multiple regression analysis was used. Cognitive factor scores (dependent variable) and total WMHr (independent variable) were entered into the analysis, adjusting for the effects of age, sex, and years of education. Multiple regression in SPM8 was used to test linear regional correlations between cognitive factor scores and WMH probability across the whole brain (voxel-wise), controlling for age, sex, years of education, and ICV as covariates. Age was used as a covariate because of the strong association between age and both cognitive function and WMH and the possibility of spurious correlations. Since WMH are believed to underlie declines in age related cognitive function, a voxel-wise model was performed without controlling for age.
3. Associations between WMH and Alzheimer’s risk (APOE4 and parental family history of AD)
To test the hypothesis that APOE4 carriage and parental family history of AD incurs higher total WMHr, ANOVA was used with APOE4 and parental family history as the independent variables and WMHr as the dependent variable, controlling for the effects of age, sex, and years of education. The two-sample t-test design was used in SPM8 to test for regional group differences between APOE4 carriers and non carriers and between those with a parental family history of AD and those without. Age, sex, years of education and ICV were entered as covariates.
Results
1. Association between age and total and regional WMH
In a multiple regression model controlling for sex, age was a significant predictor of global WMHr, β = 0.39, t(346) = 7.8, p < 0.001 (Figure 1). Based on the possibility of a non-linear pattern of white matter change with age, we also fit a non-linear slope; the quadratic function had an r(346) = 0.39 and did not explain more variance than the linear fit.
Figure 1.
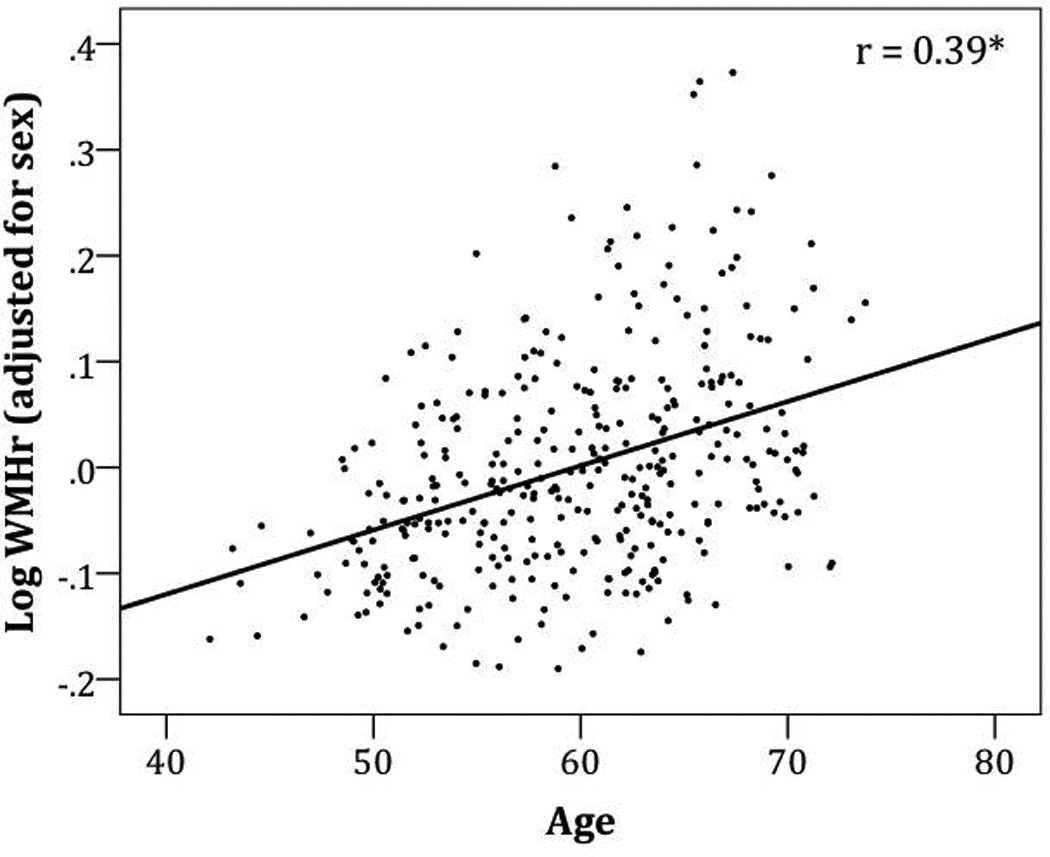
Residuals of white matter hyperintensity ratio [WMHr (% of intracranial volume)] after adjusting for sex as a function of age. r(346) = 0.39, p <0.001.
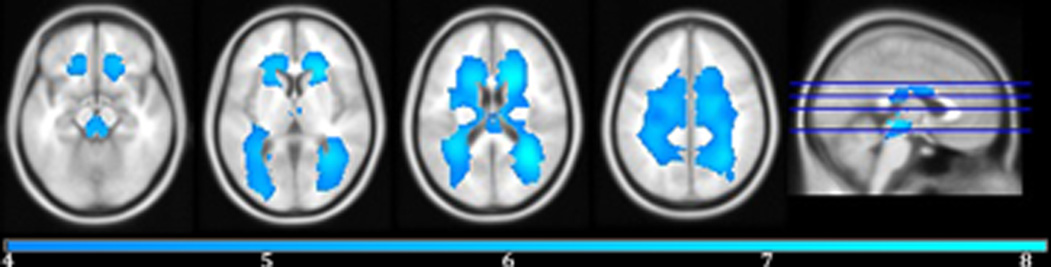
Controlling for sex and ICV, a voxel-wise analysis revealed a linear relationship between increasing age and increasing lesion probability in large areas of bilateral WM including all portions of the corpus callosum, the fornix, the superior cerebellar peduncles, the cerebral peduncles, all portions of the internal capsule, the anterior, posterior, and superior corona radiata, the posterior thalamic radiation, the external capsule, the cingulum, the superior longitudinal fasciculus, and the superior and inferior fronto-occipital fasciculus. (Figure 2 & Table 2).
Figure 2.
Areas of positive correlation with age on smoothed WMH probability belief maps controlling for intracranial volume and sex are displayed in cool colors (blue). The color bar indicates the t-value. FWE-correction, p < 0.05. Peak voxel coordinates, t-value, p-value, and cluster size are listed in Table 2.
Table 2.
For each significant cluster, peak voxel MNI coordinates (x, y, z), t-statistic and p-value, and cluster size(k) are listed separately for each voxel-wise analysis.
| Brain Region | x | y | z | k | t | p |
|---|---|---|---|---|---|---|
| WMH increase with age (Figure 2) | ||||||
| R posterior thalamic radiation | 33 | −54 | 19 | 71551 | 8.13 | <0.0001 |
| WMH increase with lower Speed and Flexibility factor score (Figure 4a) | ||||||
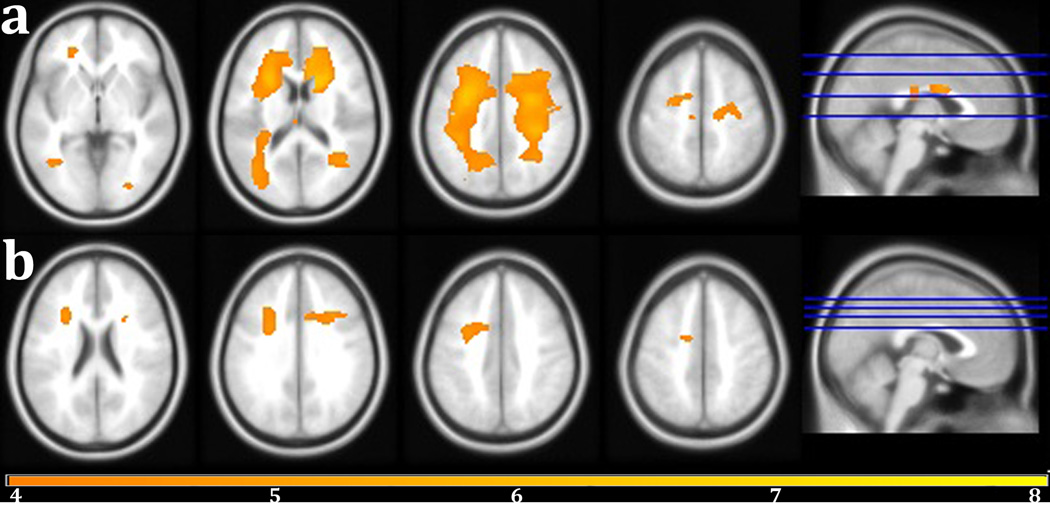
| R superior corona radiata | 26 | 14 | 25 | 43,869 | 6.67 | <0.0001 |
| R middle occipital WM | 27 | −84 | 4 | 325 | 4.57 | <0.0001 |
| Body of the corpus callosum | −6 | −22 | 22 | 296 | 4.38 | <0.0001 |
| L medial frontal WM | −12 | −21 | 58 | 90 | 4.35 | <0.0001 |
| WMH increase with lower Speed and Flexibility factor score independent of age (Figure 4b) | ||||||
| L superior corona radiata | −27 | 5 | 34 | 1,838 | 4.97 | <0.0001 |
| R superior corona radiata | 28 | 17 | 28 | 726 | 4.87 | <0.0001 |
2. Association between cognition and total and regional WMH
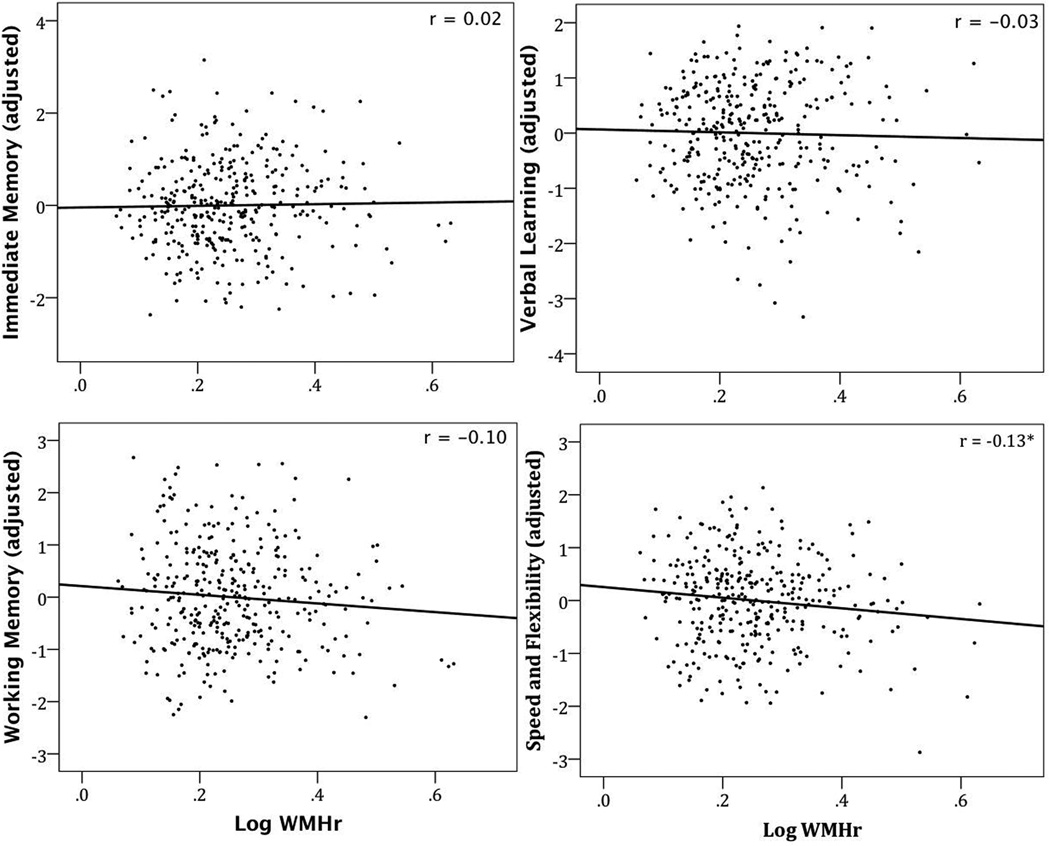
Speed and Flexibility was the only cognitive measure that was associated with global WMHr controlling for age, sex, and years of education, β = −0.13, t(336) = −2.5, p = 0.01 (Figure 3). Controlling for sex and years of education, age significantly predicted the Speed and Flexibility factor score, β = −0.41, t(337) = −8.3, p < 0.001.
Figure 3.
Residuals of WMHr after adjusting for age, sex, and years of education graphed as a function of cognitive factor scores. * significant p < 0.05
The regional voxel-wise analysis revealed that lower Speed and Flexibility factor score was associated with higher lesion probability in many of the same regions observed with age, controlling for sex, years of education, and ICV (Figure 4a & Table 2). Qualitatively, the relationship between age and WMH probability extended over a greater portion of WM compared to the Speed and Flexibility result map, which lacked significant associations in the splenium and tapetum of the corpus callosum, the fornix, the cerebellar and cerebral peduncles, the posterior limb of the internal capsule, and the inferior fronto-occipital fasciculus. Considering that WMH and Speed and Flexibility are both strongly related to age, this result not controlling for age most likely contains effect from confounds also associated with age. When age was controlled, Speed and Flexibility was correlated with WMH in the body of the corpus callosum, bilateral areas of the anterior and superior corona radiata, and the right cingulum (Figure 4b & Table 2). Since it is believed that WMH may be a neurological substrate responsible for some portion of age related cognitive decline, controlling for age most likely removes some of the variance shared by age and Speed and Flexibility in this result.
Figure 4.
(a)Areas of negative correlation with Speed and Flexibility factor score controlling for sex, intracranial volume, and years of education are shown in warm colors (yellow-orange). (b) Areas of negative correlation with Speed and Flexibility factor score controlling for age, sex, years of education, and ICV. FWE-correction, p < 0.05. The color bar indicates the t-value. Peak voxel coordinates, t-value, p-value, and cluster size are listed in Table 2.
3. Associations between WMH and Alzheimer’s Risk (APOE4 and parental family history of AD)
Parental family history of AD did not predict WMHr controlling for age, sex, and years of education, F(1, 344) = 0.05, p = 0.83. Controlling for the same variables, APOE4 carrier status did not predict WMHr, F(1, 344) = 0.19, p = 0.66. Using the same models in SPM8, there were no regional associations between WMH probability and APOE4 or parental family history of AD.
Discussion
The goals of this study were to determine the regional localization of WMH with age, to determine the effect of WMH on cognitive function in the context of aging, and assess the effect of Alzheimer’s risk on WMH in a large sample of healthy late middle aged participants. We observed that increasing age and lower cognitive speed and flexibility are strongly associated with WMH throughout white matter. Furthermore, the factor score representing speed and flexibility was independently associated with WMH in an area containing portions of the superior corona radiata bilaterally, the anterior corona radiata bilaterally, and the right cingulum, where higher WMH probability was associated with lower cognitive performance. The Speed and Flexibility factor score reflects processes that are considered under the broad category of executive function. Alzheimer’s risk was not associated with regional lesion burden in this study.
Our results are consistent with and extend the established literature, which suggests that WMH are often associated with decreasing executive functioning and speed of processing. The localization of correlations between cognitive speed and flexibility and WMH in this study is consistent with studies that have shown a relationship between visually rated periventricular WMH and speed of processing and executive function (de Groot, de Leeuw et al. 2000; Soderlund, Nilsson et al. 2006). Using voxel-wise analysis,Smith et al. (2011) observed that frontal and periventricular WMH are associated with lower executive functioning in older participants with AD, MCI, and normal cognition. Using tracing methods in a population with a similar mean age to our sample, Raz et al found a relationship between frontal WMH, including periventricular regions, and executive function (Raz, Rodrigue et al. 2003). Other studies have found impacts on executive function and processing speed when looking across several brain regions; in a study of patients with subcortical ischemic vascular disease and AD, Tullberg et al found that WMH contribute to impairments in executive function regardless of lesion location (Tullberg, Fletcher et al. 2004). Using lobar parcellation of the brain and a region of interest approach, Murray et al. found that higher WMH in all regions except the occipital lobe was associated with lower executive function (Murray, Senjem et al. 2010).
We did not find a relationship between WMH and memory. Several other studies have observed an association between memory measures and WMH (Gunning-Dixon and Raz 2000; Van Petten, Plante et al. 2004; Smith, Salat et al. 2011; Carmichael, Mungas et al. 2012), and indeed, WMH have been found to underlie episodic memory deficits, even in the absence of hippocampal atrophy (Nordahl, Ranganath et al. 2005). The current study focused on a relatively healthy and well-educated population (with approximately 16 years of education), which may explain why effects were found for the Speed and Flexibility factors score, but not other aspects of cognitive function. Factors such as brain and cognitive reserve have been noted to mitigate the impact of pathology on cognition (Brickman, Siedlecki et al. 2011). Other factors that may play a role in differing outcomes include age and disease status, WMH rating methods, and the choice of neuropsychological tests. Carmichael et al. found a relationship between WMH burden and both executive and memory function when examining participants who ranged in cognitive function from healthy to demented (Carmichael, Mungas et al. 2012), however, when controlling for diagnostic status, the relationship with memory was trending, but not significant. Bunce et al. studied a relatively young population of community-dwelling adults aged 44 to 48 years, and found that temporal WMH were associated with memory, however in this case the task was non-verbal (face recognition), and only present in men (Bunce, Anstey et al. 2010). In a primarily male population studied in the SMART-MR study, WMH burden interacted with brain volume measures to produce lower executive function, but did not have an effect on memory function (Muller, Appelman et al. 2011). Likewise, in a study which assessed the contribution of childhood IQ on later-life cognitive function in an older, but non-demented sample, Valdés Hernández et al. found that WMH have an incremental effect on reducing general cognitive ability and processing speed, but not memory(Valdes Hernandez, Booth et al. 2013), and results from the Framingham Heart study also point toward a relatively stronger relationship between WMH and frontal lobe based cognitive tasks compared to medial temporal lobe based memory tests (Au, Massaro et al. 2006). A recent study in a cognitively healthy older sample found that memory function was affected by amyloid burden, while executive function was more closely associated with WMH (Hedden, Mormino et al. 2012). While episodic memory function can be clearly impacted by WMH burden, at least one study suggests that this effect is mediated by executive functioning (Parks, Iosif et al. 2011). Given that the participants in the current study—while younger—showed an effect of WMH on subcomponents of executive function, suggests that effects on memory may be impending as the WRAP sample ages, or as age-related diseases manifest. Longitudinal work in this area indicates that WMH continue to evolve over time with significant consequences for cognitive function (Maillard, Carmichael et al. 2012).
The mainly periventricular distribution of WMH with increasing age in this study is consistent with the voxel-wise WMH age distribution found inRostrup et al. (2012), which studied an older population. WMH appear to accumulate in periventricular and deep white matter near the precentral gyrus. A voxel-wise study using diffusion tensor imaging (DTI) from our group which included participants from the WRAP cohort showed a relationship between lower fractional anisotropy in the superior corona radiata and poorer performance on complex attention and set shifting as measured by Trails B (Bendlin, Fitzgerald et al. 2010). Additional DTI studies have also indicated that altered measures of white matter microstructure in the superior corona radiata are associated with poorer performance in task switching and processing speed (Sasson, Doniger et al. 2012; Leunissen, Coxon et al. 2013). A tractography study performed by Leunissen et al.(2013) showed that injury to fibers of the superior corona radiata—which connect the basil ganglia and thalamus to superior frontal gyrus and supplementary motor area—contributes to deficits in task switching. They point to an emerging body of evidence that cortico-subcortical damage may cause executive dysfunction through a lack of cognitive control (2013). The results of the current study, which found that WMH probability in the superior corona radiata was associated with speed and flexibility independently of age (Figure 4b), provides further support that portions of the superior corona radiata play a role in cognitive speed and flexibility.
We did not find a relationship between WMH and APOE4 or parental family history of AD. Our results concur with some studies that have also failed to find a link (Debette, Wolf et al. 2009; Biffi, Anderson et al. 2010). In studies that have found a relationship between APOE4 and WMH, the participants were older than the sample in the current study (de Leeuw, Richard et al. 2004; Lunetta, Erlich et al. 2007) suggesting that APOE4 may be associated with WMH but only at an older age. Perhaps providing more information on the link between white matter health and AD risk, several studies have shown that the APOE4 effect on white matter microstructure as measured by DTI often interacts with age, such that a greater APOE4 effect is observed in older age (Nierenberg, Pomara et al. 2005; Persson, Lind et al. 2006; Smith, Chebrolu et al. 2010; Ryan, Walther et al. 2011).
This study has a few limitations that should be noted. This study is cross-sectional and longitudinal studies are needed to understand the temporal relationship between cognitive performance and the development of WMH (Wardlaw, Smith et al. 2013). The present sample was recruited from an established registry for AD research enriched for family history of AD, potentially limiting the generalizability of these results. Further, it’s important to note that while WMH account for some of the effects on cognitive function,(Soderlund, Nyberg et al. 2003; Vannorsdall, Waldstein et al. 2009), other aspects of neural health are important, as are the myriad of lifestyle and genetic factors that affect neural health and cognition. Finally, given the heterogeneous nature of WMH in aging, larger studies, different samples or different methods of assessing WMH could produce different results.
Conclusion
This large study examined the effects of age and AD risk on regional WMH, in addition to assessing the relationship between regional lesion burden and cognitive function in a large sample of healthy middle to older aged adults. The results suggest that white matter alterations partly underlie age-related changes in processing speed. Identifying factors associated with age-related decrease in processing speed is important, given that slowed processing speed has been implicated as the broad underlying deficit for other age-related cognitive declines (Salthouse 1996). The findings underscore the importance of considering lesion location for informing upon age-related cognitive decline.
Acknowledgements
This project was supported by the Alzheimer’s Association, NIRG-09-132626, the National Institute on Aging (R01 AG027161 [MAS], ADRC P50 AG033514 [SA], R01 AG021155 [SCJ], R01 AG037639 [BBB]), and by the Veteran’s Administration Merit Review award I01CX000165. The project was also facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. GRECC MS # 2013-03. The authors gratefully acknowledge Nancy Davenport-Sis, Amy Hawley, Jennifer Bond, Caitlin Cleary, Jennifer Oh, Chuck Illingworth, and the support of researchers and staff at the Waisman Center, University of Wisconsin-Madison, for their assistance in recruitment, data collection, and data analysis. Above all, we wish to thank our dedicated volunteers for their participation in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
None of the authors have actual or potential conflicts of interest.
References
- Aggarwal NT, Wilson RS, et al. The association of magnetic resonance imaging measures with cognitive function in a biracial population sample. Archives of neurology. 2010;67(4):475–482. doi: 10.1001/archneurol.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves GS, O'Dwyer L, et al. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One. 2012;7(12):e52859. doi: 10.1371/journal.pone.0052859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R, Massaro JM, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Basile AM, Pantoni L, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovascular diseases. 2006;21(5- 6):315–322. doi: 10.1159/000091536. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, et al. White matter in aging and cognition: a crosssectional study of microstructure in adults aged eighteen to eighty-three. Developmental neuropsychology. 2010;35(3):257–277. doi: 10.1080/87565641003696775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, et al. White matter is altered with parental family history of Alzheimer's disease. Alzheimers Dement. 2010;6(5):394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Anderson CD, et al. Genetic variation and neuroimaging measures in Alzheimer disease. Archives of neurology. 2010;67(6):677–685. doi: 10.1001/archneurol.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Provenzano FA, et al. Regional White Matter Hyperintensity Volume, Not Hippocampal Atrophy, Predicts Incident Alzheimer Disease in the Community. Archives of neurology. 2012:1–7. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Siedlecki KL, et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging. 2011;32(9):1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, et al. Cognitive deficits are associated with frontal and temporal lobe white matter lesions in middle-aged adults living in the community. PloS one. 2010;5(10):e13567. doi: 10.1371/journal.pone.0013567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, et al. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2012;33(1):83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenco KT, Green RC, et al. Magnetic resonance imaging traits in siblings discordant for Alzheimer disease. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2008;18(3):268–275. doi: 10.1111/j.1552-6569.2007.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Annals of neurology. 2000;47(2):145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. Journal of neurology, neurosurgery, and psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, Richard F, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke; a journal of cerebral circulation. 2004;35(5):1057–1060. doi: 10.1161/01.STR.0000125859.71051.83. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and metaanalysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Wolf PA, et al. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73(24):2071–2078. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling NM, Hermann B, et al. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer's disease. Neuropsychology. 2010;24(6):742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro JM, Madureira S. Age-related white matter changes and cognitive impairment. Journal of the neurological sciences. 2002;203–204:221–225. doi: 10.1016/s0022-510x(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Galluzzi S, et al. The effect of white matter lesions on cognition in the elderly--small but detectable. Nat Clin Pract Neurol. 2007;3(11):620–627. doi: 10.1038/ncpneuro0638. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grimmer T, Faust M, et al. White matter hyperintensities predict amyloid increase in Alzheimer's disease. Neurobiology of aging. 2012;33(12):2766–2773. doi: 10.1016/j.neurobiolaging.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Guzman VA, Carmichael OT, et al. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairment. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, et al. Cognitive Profile of Amyloid Burden and White Matter Hyperintensities in Cognitively Normal Older Adults. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(46):16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keihaninejad S, Heckemann RA, et al. A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T) NeuroImage. 2010;50(4):1427–1437. doi: 10.1016/j.neuroimage.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunissen I, Coxon JP, et al. Task switching in traumatic brain injury relates to cortico-subcortical integrity. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Pu F, et al. Regional white matter decreases in Alzheimer's disease using optimized voxel-based morphometry. Acta Radiol. 2008;49(1):84–90. doi: 10.1080/02841850701627181. [DOI] [PubMed] [Google Scholar]
- Lunetta KL, Erlich PM, et al. Heritability of magnetic resonance imaging (MRI) traits in Alzheimer disease cases and their siblings in the MIRAGE study. Alzheimer Disease and Associated Disorders. 2007;21(2):85–91. doi: 10.1097/WAD.0b013e3180653bf7. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, et al. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79(5):442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Muller M, Appelman AP, et al. Brain atrophy and cognition: interaction with cerebrovascular pathology? Neurobiol Aging. 2011;32(5):885–893. doi: 10.1016/j.neurobiolaging.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Murray ME, Senjem ML, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67(11):1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg J, Pomara N, et al. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16(12):1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, et al. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43(11):1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CM, Iosif AM, et al. Executive function mediates effects of white matter hyperintensities on episodic memory. Neuropsychologia. 2011;49(10):2817–2824. doi: 10.1016/j.neuropsychologia.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, et al. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66(7):1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, et al. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press. 1993 [Google Scholar]
- Rostrup E, Gouw AA, et al. The spatial distribution of age-related white matter changes as a function of vascular risk factors--results from the LADIS study. NeuroImage. 2012;60(3):1597–1607. doi: 10.1016/j.neuroimage.2012.01.106. [DOI] [PubMed] [Google Scholar]
- Ryan L, Walther K, et al. Age-related differences in white matter integrity and cognitive function are related to APOE status. NeuroImage. 2011;54(2):1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager MA, Hermann B, et al. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, et al. Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain Struct Funct. 2012;217(2):503–515. doi: 10.1007/s00429-011-0344-7. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Gaser C, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, et al. White matter diffusion alterations in normal women at risk of Alzheimer's disease. Neurobiology of aging. 2010;31(7):1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Salat DH, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund H, Nilsson LG, et al. Cerebral changes on MRI and cognitive function: the CASCADE study. Neurobiology of aging. 2006;27(1):16–23. doi: 10.1016/j.neurobiolaging.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Soderlund H, Nyberg L, et al. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex. 2003;39(4–5):1093–1105. doi: 10.1016/s0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests : administration, norms, and commentary. New York: Oxford University Press; 1998. [Google Scholar]
- Trenerry MR. Stroop Neuropsychological Screening Test Manual. Psychological Assessment Resources; 1989. [Google Scholar]
- Tullberg M, Fletcher E, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes Hernandez MD, Booth T, et al. Brain white matter damage in aging and cognitive ability in youth and older age. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C, Plante E, et al. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42(10):1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Vannorsdall TD, Waldstein SR, et al. White matter abnormalities and cognition in a community sample. Arch Clin Neuropsychol. 2009;24(3):209–217. doi: 10.1093/arclin/acp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. Psychological Corporation; 1997. [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22(1):144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev PS, et al. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp. 2009;30(4):1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]