Abstract
Recombinant adeno-associated viruses are important vectors for retinal gene delivery. Currently utilized vectors have relatively slow onset and for efficient transduction it is necessary to deliver treatment subretinally, with the potential for damage to the retina. Amino-acid substitutions in the viral capsid improve efficiency in rodent eyes by evading host responses. As dogs are important large animal models for human retinitis pigmentosa, we evaluated the speed and efficiency of retinal transduction using capsid-mutant vectors injected both subretinally and intravitreally. We evaluated AAV serotypes 2 and 8 with amino-acid substitutions of surface exposed capsid tyrosine residues. The chicken beta-actin promoter was used to drive green fluorescent protein expression. Twelve normal adult beagles were injected, 4 dogs received intravitreal injections, 8 dogs received subretinal injections. Capsid-mutant viruses tested included AAV2(quad Y-F) (intravitreal and subretinal), and self-complementary scAAV8(Y733F) (subretinal only). Contralateral control eyes received injections of scAAV5 (subretinal) or scAAV2 (intravitreal). Subretinally delivered vectors had a faster expression onset than intravitreally delivered vectors. Subretinally delivered scAAV8(Y733F) had a faster onset of expression than scAAV5. All subretinally injected vector types transduced the outer retina with high efficiency, and the inner retina with moderate efficiency. Intravitreally delivered AAV2(quad Y-F) had a marginally higher efficiency of transduction of both outer retinal and inner retinal cells than scAAV2. Because of their rapid expression onset and efficient transduction, subretinally delivered capsid-mutant AAV8 vectors may increase the efficacy of gene therapy treatment for rapid photoreceptor degenerative diseases. With further refinement, capsid-mutant AAV2 vectors show promise for retinal gene delivery from an intravitreal approach.
Keywords: (3–6): gene therapy, rAAV, canine, retina, capsid-mutant
Introduction
Significant recent developments have occurred in the field of retinal gene therapy. Phase I/II human clinical trials aimed at treating Leber congenital amaurosis (LCA) type 2 caused by RPE65 mutations have demonstrated the safety and efficacy of recombinant adeno-associated viral (AAV) vector-mediated gene augmentation therapy when delivered by subretinal injection. 1–6 Pre-clinical proof-of-concept and safety studies using the Rpe65-deficient canine model provided a critical step in the approval process for the human trials. 7–9
Retinal dystrophies are reported to cause visual dysfunction in up to 1 in 3500 humans, 10 with causative mutations being identified in almost 200 genes (https://sph.uth.edu/retnet/). As treatments for additional retinal dystrophies are attempted, increasingly sophisticated therapeutic approaches will be sought. These are likely to include the need to achieve faster onset of expression to combat rapid degenerative disease, accomplishing efficient retinal cellular transduction from a more straightforward intravitreal injection of vector and more efficient targeting of inner retinal cells to introduce novel proteins into this location. By refining the methods and approaches to gene therapy in the canine retina, important advances can be made in the prospective treatment of early-onset and rapidly progressive retinal dystrophy in people. Ongoing studies in additional canine models are paving the way for human gene therapy trials aiming to treat diseases such as achromatopsia, 11 autosomal recessive retinitis pigmentosa 12, 13 and X-linked retinitis pigmentosa. 14
Although conventional AAV-mediated gene therapy can effectively treat diseases such as RPE65 deficiency that cause slow photoreceptor degeneration, the treatment of very rapid degenerations will require early intervention with rapid-onset treatment. Human patients with LCA type 4 caused by mutations in the AIPL1 gene suffer early onset and severe LCA characterized by severely reduced vision from birth, extinguished ERGs within the first year of life 15 and profound retinal thinning detectable within the second decade of life. 16, 17 We reported a spontaneously occurring frameshift mutation in Pde6a causing retinal dystrophy in the Cardigan Welsh Corgi dog, in which peak photoreceptor apoptosis occurs by about 4 weeks of age. 18, 19 Gene augmentation therapy in dogs with this mutation has proven challenging (Petersen-Jones, unpublished data), at least in part due to the time lag from initial vector delivery to onset of transgene expression, reported as approximately 6 weeks using conventional AAV2 vectors. 20 We have shown that self-complementary AAV vector constructs have a faster onset of transgene expression than conventional single stranded AAV vectors in dogs. 21 However, these self-complementary AAV vectors have significant restrictions on the size of gene that can be introduced, 22 limiting their potential for replacement of all but the smaller genes.
Subretinal injection is used as standard for delivery of gene therapy to the outer retina and retinal pigment epithelium (RPE) and results in the creation of a temporary retinal detachment. Although this usually resolves within a few days of its creation, detachment of the retina can have immediate deleterious effects on the retinal cells due to the separation of the retina from the RPE and choroid. 23–26 Damage to the retina in the context of active disease or degeneration may be even more detrimental. A subset of gene therapy treated LCA2 patients suffered retinal complications that may, in part, be related to the subretinal method of vector delivery. Complications included the development of a macular hole, 6 and two cases of foveal thinning that persisted in one case, causing negative effects on visual acuity. 1 Intravitreal injection can safely provide therapeutic levels of drugs to the retina, including anti-inflammatories, antibiotics and anti-angiogenic treatments, and is a commonly adopted technique in the treatment of many retinal diseases. Intravitreal injections are performed in people under local analgesia and a recent large study reported a complication rate of less than 0.03% per injection. 27 If gene therapy vectors delivered by intravitreal injection could efficiently transduce the outer retina and/or the retinal pigment epithelium, many of the technical challenges and potential complications of subretinal injection could be circumvented.
Recombinant adeno-associated viruses (AAVs) are widely used as gene therapy vectors in the eye due to their low immunogenicity, the ability to transduce a variety of nondividing cells in the retina, and their ability to facilitate sustained expression of replacement genes. 28 Pseudotyped AAV vectors using the AAV serotype 2 inverted terminal repeats, and alternative AAV serotype capsids have been widely studied in the retina. 29, 30 Subretinal injection of vectors containing the genome from AAV serotype 2 and capsids from serotypes 2 (AAV2), 5 (AAV5) and 8 (AAV8) have been used in gene therapy trials in rodents and dogs. 31 Advances in the understanding of molecular trafficking of AAV through the host cell have identified capsid tyrosine phosphorylation as a signal for AAV vector ubiquitination and subsequent targeting for degradation by cellular proteasomes. 32 Mutations introduced to substitute exposed tyrosine for phenylalanine residues result in reduced virus degradation, enhanced nuclear transport and increased transgene expression. 33 Using single point-mutations in the genes encoding viral capsid tyrosine residues, delivery in rodents showed superior transduction characteristics in multiple retinal cells compared with constructs without the capsid mutations. 34 Using vitreal delivery, efficient transduction of inner retinal cells was achieved using AAV2, and using subretinal delivery, rapid-onset efficient photoreceptor expression was achieved with AAV2 and AAV8. Additional substitutions (triple mutation of Y444, 500, 730F) in AAV2 capsid further enhanced transduction efficiency. 35 Following these discoveries, capsid-mutant AAV vectors have been investigated in proof-of-concept therapy studies for retinal dystrophies in rodent models and have been shown to provide long-term rescue of a variety of photoreceptor dystrophies in mice 36–39 and rats. 40
These next-generation vectors therefore promise improvements in the treatment of early onset dystrophies and show potential for efficient gene delivery to the retina and RPE from an intravitreal injection. Our studies in the normal canine retina provide essential information to allow further refinement of gene therapy approaches in an important animal model species with the ultimate goal of translation into human patients.
Results
Onset of transgene expression
Fluorescent fundus images were taken serially in all 12 dogs following subretinal and intravitreal viral vector injection until 30 days post-injection. Images were examined closely by 2 masked observers to detect the onset of green fluorescent protein (GFP) expression. Images taken immediately following injection clearly delineated the site of subretinal injection (Figure 1). In all eyes in which subretinal injections were performed, complete retinal reattachment was observed by 3 days post-injection. All eyes that received subretinal injections had detectable GFP expression by 6 days post-injection. Intensity of GFP expression continued to increase throughout the study period, and by termination at 30 days post-injection, robust GFP expression was present within the boundary of the subretinal bleb for all vector types evaluated (Figure 1). GFP expression was detected later in eyes receiving intravitreal injections, with fluorescence noted in all eyes by 9 days post-injection. Fluorescence intensity continued to increase throughout the study period. By 14 days post-injection in all intravitreally injected eyes, fluorescence of the optic nerve head was observed likely indicating GFP expression within ganglion cell axons (Figure 2). There was a significant correlation (p < 0.0001 Spearman correlation, R2 = 0.61) between the findings of two masked observers evaluating onset of fluorescence in post-injection fundus images (Figure 3A). There was a significantly faster onset of expression using subretinally delivered AAV2(quad Y-F) than intravitreally delivered AAV2(quad Y-F) (p < 0.01 Figure 3B) or scAAV8(Y733F) (one-way ANOVA with Bonferroni post-test; p < 0.0001 Figure 3B).
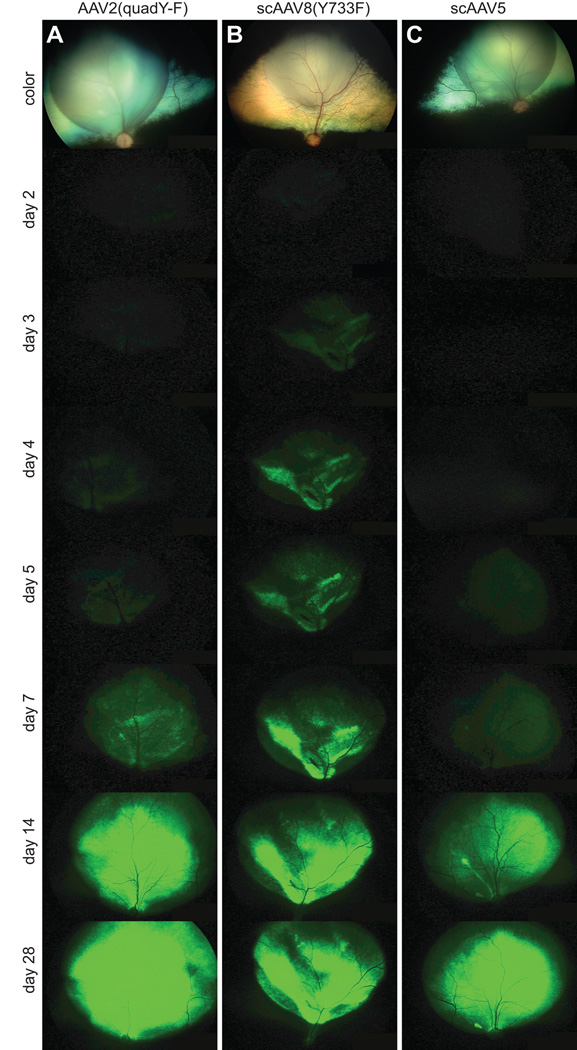
Figure 1.
In vivo images showing the onset of GFP expression following subretinal injection. Representative digitally enhanced images from time points post-injection are shown for subretinal injection of AAV2(quad Y-F) (A), scAAV8(Y733F) (B) and the control vector scAAV5 (C). The top color image in each case is taken immediately after injection showing the site of injection, subsequent pictures are of GFP fluorescence. scAAV8(Y733F) appeared to have a faster onset than the other two vector-types. The final extent of expression at the end of the study period (28 days) appeared similar between the 3 vector-types.
Figure 2.
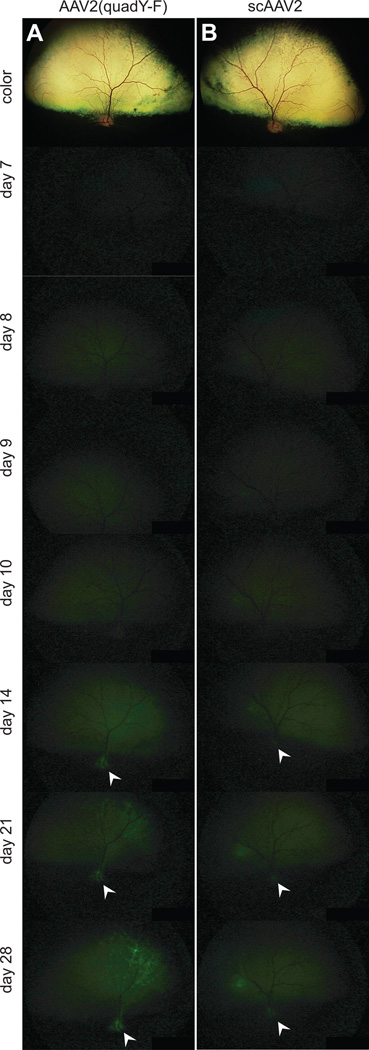
In vivo images showing the onset of GFP expression following intravitreal injection. Representative digitally enhanced images from time points post-injection are shown for intravitreal injection of AAV2(quad Y-F) (A) and scAAV2 (B). The top color image in each case is taken immediately after injection, subsequent pictures are of GFP fluorescence. Presence of GFP in nerve fibers and within the optic nerve head (white arrowheads) was detectable with both vector-types by 14 days post-injection, confirming that fluorescence was derived from vector expression within the retinal neurons, including ganglion cells.
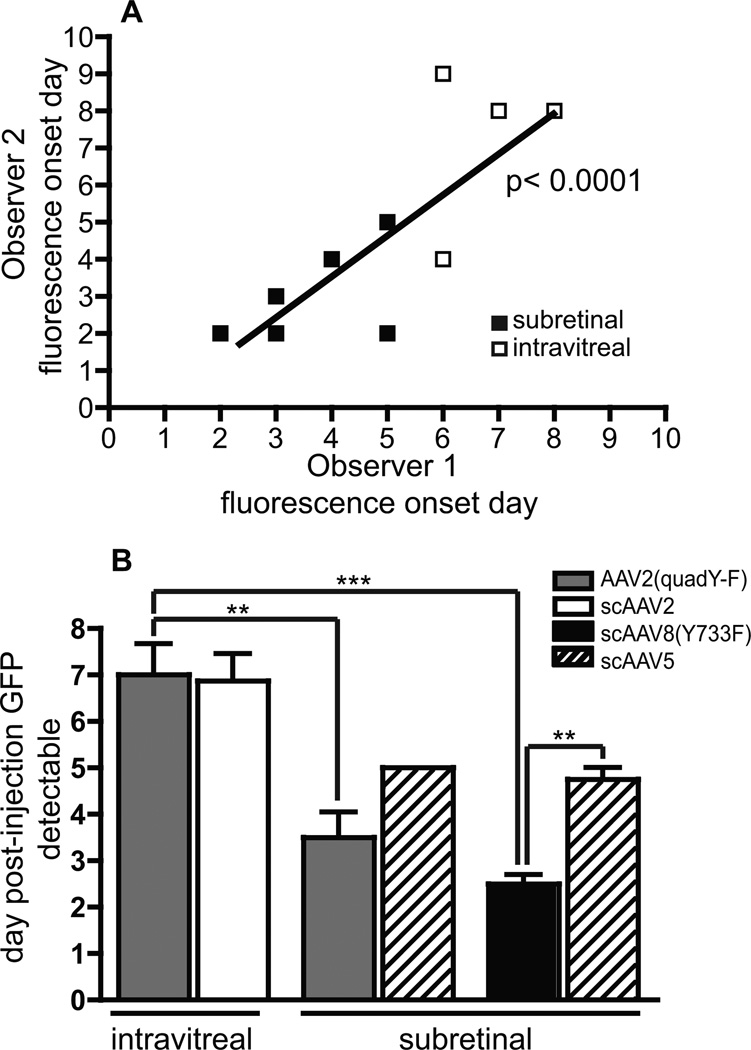
Figure 3.
Analysis of onset of GFP expression. Two masked observers graded fluorescent fundus images to evaluate the onset of GFP expression following vector injection. The results from the two observers were positively correlated (R2 = 0.61; A). There was no statistically significant difference in onset between capsid-mutant vectors and control vectors from an intravitreal injection or between AAV2(quad Y-F) and control scAAV5 from a subretinal injection (B; paired t-test). scAAV8(Y733F) had a significantly faster onset than the control scAAV5 following subretinal injection (B; paired t-test). Subretinal injection of the capsid-mutant vectors resulted in a faster onset of expression than intravitreal injection (B; one-way ANOVA with Bonferroni post-test). Two individual bars are shown for the control vector scAAV5 to reflect the data from the paired eye for the AAV2(quad Y-F) and the scAAV8(Y733F) groups separately. ** p <0.01, *** p <0.001
There was no significant difference in time to detection of initial GFP expression between AAV2(quad Y-F) and scAAV5 vectors delivered by subretinal injection (AAV2(quad Y-F) mean onset 3.5 days ± 0.5 days, scAAV5 mean onset 5.0 ± 0.0 days, p = 0.07 paired t-test; Figure 3B). Subretinal delivery of scAAV8(Y733F) resulted in significantly faster time to detection of initial GFP expression compared to scAAV5 (scAAV8(Y733F) mean onset 2.5 days ± 0.2 days, range 2–3 days, scAAV5 mean onset 4.8 ± 0.3 days, range 4–5 days, p = 0.006 paired t-test; Figure 3B). There was no significant difference in time to detection of initial GFP expression between AAV2 vectors delivered by intravitreal injection (AAV2(quad Y-F) mean onset 7.0 ± 0.7 days, scAAV2 mean onset 6.9 ± 0.6 days, p = 0.81, paired t-test; Figure 3B). There was no significant difference in time to detection of initial GFP expression between AAV2(quad Y-F) vector and scAAV8(Y733F) delivered by subretinal injection (one-way ANOVA with Bonferroni post-test).
Retinal cell tropism of subretinally injected vectors
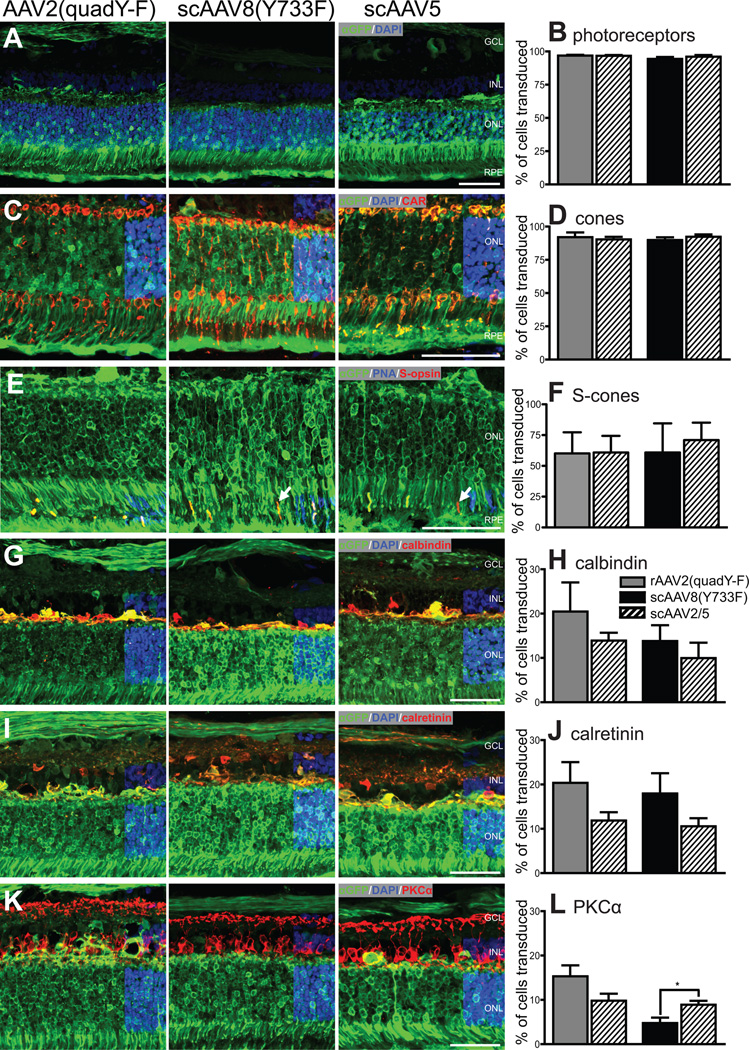
Immunohistochemistry labeling on sections through injected areas of all 16 subretinally injected eyes was quantified to assess retinal cell tropism and transduction efficiency. Photoreceptor transduction efficiency was greater than 90% for all three vectors (AAV2(quad Y-F), scAAV8(Y733F) and scAAV5) delivered by subretinal injection, and there was no significant difference between vector types (range 91.8%-98%; p = 0.83 scAAV5 vs. AAV2(quad Y-F), p = 0.41 scAAV5 vs. scAAV8(Y733F); Figure 4A, B). Cone photoreceptors were efficiently transduced by all vector types, there was no difference in efficiency between vector types (range 81.6%–97.2%; p = 0.57 scAAV5 vs. AAV2(quad Y-F), p = 0.84 scAAV5 vs. scAAV8(Y733F); Figure 4C, D). Although there was no difference in S-cone transduction efficiency between different vector types, S-cone transduction efficiency was variable between different animals irrespective of vector-type (range 22.8%–100%; p = 0.12 scAAV5 vs. AAV2(quad Y-F), p = 0.67 scAAV5 vs. scAAV8(Y733F); Figure 4E, F). Inner retinal cell-transduction was achieved using all tested vector types from a subretinal injection, although at a lower efficiency than outer nuclear layer transduction efficiency (Figure 4G–L). There were no differences between vector types in the efficiency of transduction of calbindin positive cells (range 2.1–38.8%, p = 0.32 scAAV5 vs. AAV2(quad Y-F), p = 0.98 scAAV5 vs. scAAV8(Y733F); Figure 4G, H) or calretinin positive cells (range 7.4–31.1%, p = 0.17 scAAV5 vs. AAV2(quad Y-F), p = 0.36 scAAV5 vs. scAAV8(Y733F);Figure 4 I, J). There was no difference in transduction efficiency of protein kinase-C alpha positive cells between subretinally delivered AAV2(quad Y-F) and scAAV5 (p = 0.16) but scAAV8(Y733F) transduced significantly fewer rod bipolar cells than the contralateral scAAV5 injected eye (4.8 ± 1.2% scAAV8(Y733F) vs. 8.9 ±0.8% scAAV5, p = 0.03; Figure 4 K, L). The numbers of transduced ganglion cells were not quantified. However, visible nerve fiber layer GFP expression was observed in all eyes, indicating ganglion cell transduction (Figure 4G–L).
Figure 4.
Inner and outer retinal transduction efficiency following subretinal injection. 30 days following subretinal injection, highly efficient transduction of retinal photoreceptors was evident (A); no difference was detected in the efficiency of photoreceptor transduction between vector types (B). Similarly, cones (identified by positive cone arrestin (CAR) expression) (C) were transduced with high efficiency, but no differences were detected between vector types (D). S-cones were transduced with relatively high efficiency (E,F) white arrows in E delineate S-opsin cones with no GFP expression. There was no difference between vector types in the transduction efficiency of inner retinal cells labeled with calbindin (G, H) or calretinin (I, J). PKC-alpha positive rod bipolar cells were not transduced with great efficiency with any vector-type (K), and scAAV8(Y733F) transduced significantly fewer rod bipolar cells than the control vector scAAV5 (L). Two individual bars are shown for the control vector scAAV5 to reflect the data from the paired eye for the AAV2(quad Y-F) and the scAAV8(Y733F) groups separately. Original magnification x40 for all images. GCL ganglion cell layer; INL inner nuclear layer; ONL outer nuclear layer; RPE retinal pigment epithelium. Scale bars = 50 µm
Retinal cell tropism of intravitreally injected vectors
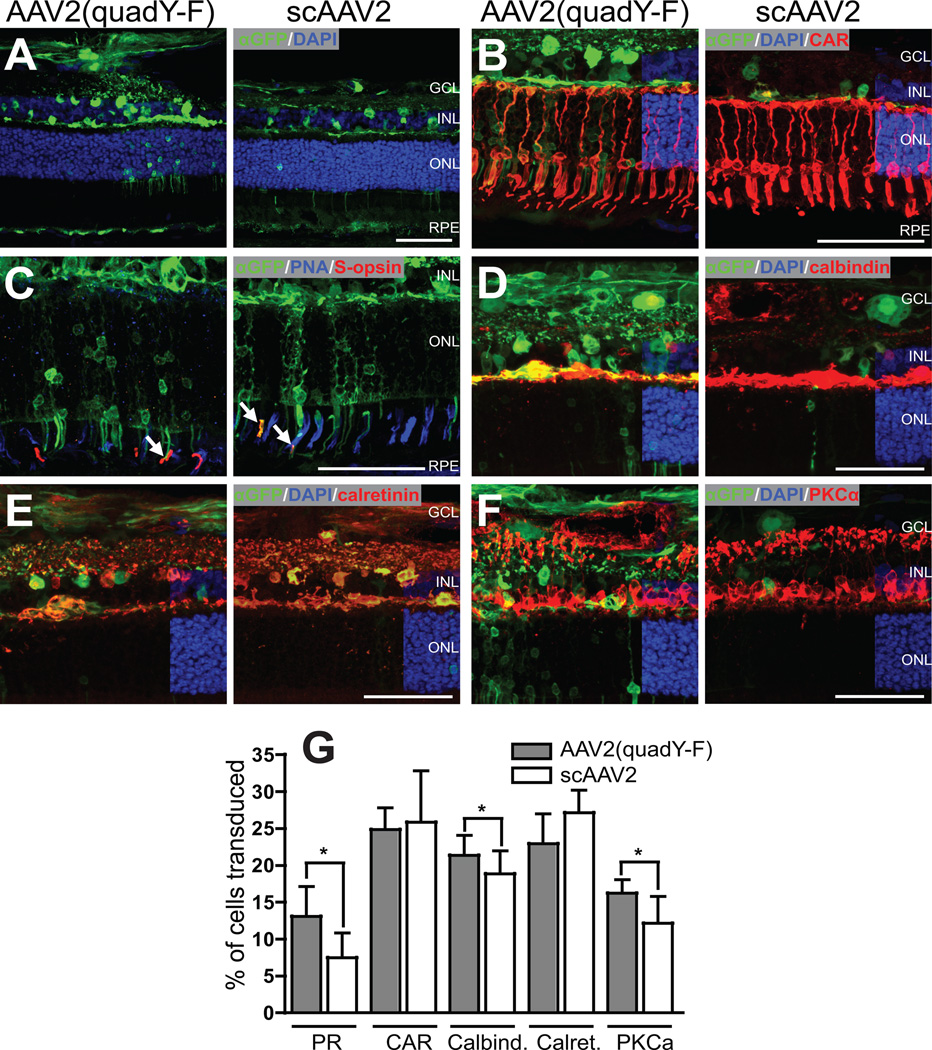
Immunohistochemistry labeling on sections through the central retina of all 8 intravitreally-injected eyes was quantified to assess retinal cell tropism and transduction efficiency. The efficiency of cell transduction was lower than from a subretinal approach although detectable GFP expression was present throughout the retina following injection with either AAV2(quad Y-F) or scAAV2 (Figure 5A). Using all vector types delivered intravitreally, the highest GFP expression was present around large retinal blood vessels (data not shown for scAAV2, see figure 6 for AAV2(quadY-F)). Quantification of transduction efficiency was performed in these areas of maximal transduction. Photoreceptors were transduced with relatively low efficiency using either vector type, but AAV2(quad Y-F) vector transduced photoreceptors to a greater extent than the scAAV2 (13.0 ± 4.1% AAV2(quad Y-F) vs. 7.4 ± 3.3% scAAV2; p = 0.02; Figure 5B, G). This difference likely reflected increased transduction of rod photoreceptors, as there was no difference in cone transduction efficiency between different vector types (range 17.3%–46.5%; p = 0.87; Figure 5B, G). Very low numbers of transduced S-cones could be detected in intravitreally-injected eyes using either vector type (Figure 5C). The low numbers of transduced cells precluded statistical analysis. Inner retinal cells were also transduced with low efficiency from the intravitreal approach but we detected GFP positive calbindin positive cells (range 10.4%–25.5%; Figure 5D), calretinin positive cells (range 15.8%–36.1%, Figure 5E) and PKC-alpha positive cells (range 5.5%–20.2%, Figure 5F) in retinas injected with both vector types. AAV2(quad Y-F) transduced more calbindin positive cells (p = 0.04) and PKC-a positive cells (p = 0.04) but not calretinin positive cells (p = 0.53) compared with scAAV2 (Figure 5G).
Figure 5.
Inner and outer retinal transduction efficiency following intravitreal injection. 30 days following intravitreal injection, limited transduction of retinal photoreceptors was evident in the regions of maximal transduction around larger superficial retinal vessels (A). Similarly, a relatively low proportion of cones (identified by positive cone arrestin (CAR) expression) were transduced by both vector types (B). Occasional S-cones positive for GFP were identified in both treatment groups (GFP positive S-cone identified with white arrow in C). Vectors transduced inner retinal cells labeled with calbindin (D), calretinin (E) and PKC-alpha (F). AAV2(quad Y-F) vector transduced significantly more photoreceptors, calbindin positive inner retinal cells and PKC-alpha positive rod bipolar cells (G). GCL ganglion cell layer; INL inner nuclear layer; ONL outer nuclear layer; RPE retinal pigment epithelium. Scale bars = 50 µm
Figure 6.
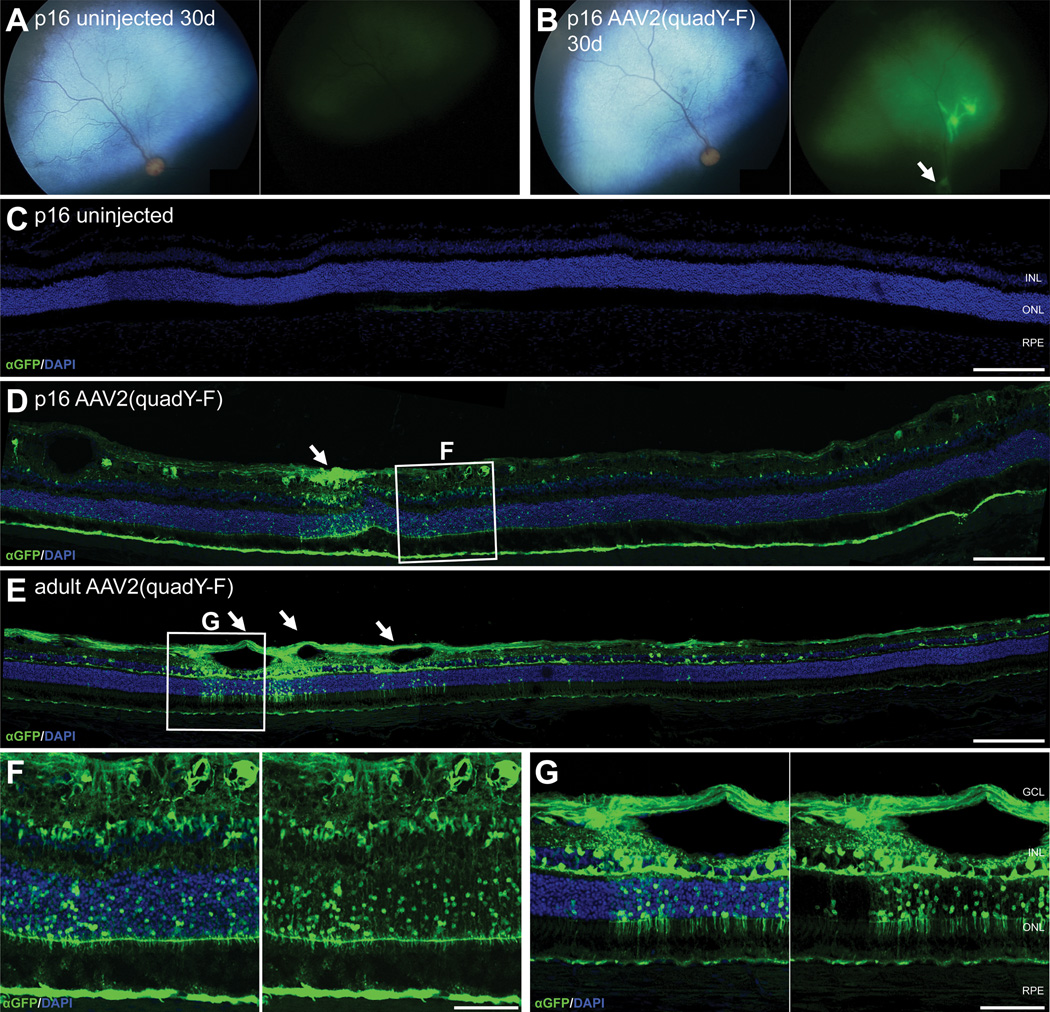
Comparison of cellular transduction characteristics between juvenile and adult dogs injected intravitreally. Two p16 dogs had a unilateral intravitreal injection with the AAV2(quad Y-F) vector. In vivo images taken at 30 days post-injection are shown from uninjected (A) and injected eyes (B; optic nerve expression indicated with arrow), demonstrating GFP expression in the AAV2(quad Y-F) injected eye. 30 days following injection, no GFP expression could be detected in the uninjected eye (C), but significant GFP expression was present throughout the retinal layers in the injected eyes (D), particularly within the retinal pigment epithelium. This was in contrast to the low amount of RPE expression seen in eyes injected as adults (E). Although this was seen to a limited extent following injections in juveniles, retinal transduction in adult retinas appeared to correlate more heavily with regions of superficial retinal vessels (arrows in D and E) High-magnification views of the areas delineated in D and E are shown in F and G respectively. GCL ganglion cell layer; INL inner nuclear layer; ONL outer nuclear layer; RPE retinal pigment epithelium. Scale bars in C–E = 200 µm, in F, G = 50 µm
We compared the inner retinal cell transduction efficiency of capsid-mutant vectors between the subretinal and intravitreal approach (AAV2(quad Y-F) delivered both subretinally and intravitreally and scAAV8(Y733F) delivered subretinally). There was no difference between groups in the transduction efficiency of calbindin positive cells (p = 0.74) or calretinin positive cells (p = 0.48). Using AAV2(quad Y-F), the route of injection did not affect the transduction efficiency of PKC-alpha positive rod bipolar cells. However, AAV2(quad Y-F) transduced significant more rod bipolar cells than scAAV8(Y733F) when delivered either subretinally or intravitreally (p < 0.05 for AAV2(quad Y-F) subretinal vs. scAAV8(Y733F) subretinal, p < 0.01 for AAV2(quad Y-F) intravitreal vs. scAAV8(Y733F) subretinal, one way ANOVA with Bonferroni post-test).
Intravitreal injection in juvenile dogs
Because of the limited transduction efficiency following intravitreal injection in adult dogs, we performed intravitreal injection of AAV2(quad Y-F) in one eye each of two juvenile dogs (postnatal day 16; Figure 6). Onset time was not assessed. Thirty days post-injection, only mild tapetal autoflorescence was detected in the uninjected control eyes (Figure 6A), but robust GFP expression was present in the superior retina in injected eyes, with evidence of nerve fiber expression in the optic nerve (Figure 6B). No expression was noted histologically in uninjected eyes (Figure 6C). Using histological evaluation, AAV2(quad Y-F) appeared to produce more efficient cell transduction following intravitreal injection in juveniles (Figure 6D, F) compared with adult dogs (Figure 6E, G). Although just two dogs were analyzed, a mean of 15.5% of photoreceptors and 15.2% of inner nuclear layer cells expressed GFP in analyzed maximal regions of expression in these juvenile animals. Particularly notable was the extensive GFP expression within the retinal pigment epithelium (RPE) of the juvenile animals. In both juveniles and adults, subjectively the highest transduction efficiency in the photoreceptor and inner nuclear layers appeared to occur in areas where superficial retinal blood vessels were present in the nerve fiber layer. However, in the juvenile retinas, retinal GFP expression was more widespread in regions distant from a retinal blood vessel (Figure 6D, F).
Discussion
We have demonstrated that an AAV8 vector carrying a single amino acid substitution on the capsid surface generates significantly faster onset (2.5 days) of GFP expression compared to a self-complementary AAV5 vector (4.8 days), previously reported to produce the fastest onset of expression following subretinal injection in the dog. 21 Although no difference in the transduction efficiency was detected between the capsid-mutant scAAV8(Y733F) and scAAV5, rod and cone photoreceptor transduction approached 100% using either vector type. The enhanced speed of onset achieved using the capsid-mutant vector may allow therapeutic protein expression at an earlier stage and facilitate rescue of severe, early onset retinal dystrophies. Although an improvement in the speed of onset of 2–3 days may not make significant clinical difference in therapeutic trials, it is likely that by evading host degradation, capsid-mutant vectors reduce presentation of capsid as an antigen by the host immune system. Additionally they will have a higher multiplicity of infection per cell when delivered subretinally. 32–35, 41 It was beyond the scope of this study to examine the level of gene expression following delivery, but recent studies in rodents found increased nuclear transport and increased transduction efficiency when using capsid-mutant vectors compared with non-mutated vectors. 42 A higher multiplicity of infection may therefore help to achieve higher levels of transgene expression and greater and more sustained therapeutic benefit. This is particularly pertinent to RPE65 deficiency, as a recent study suggested higher transgene expression, in combination with other approaches to neuroprotection, might be necessary to effectively treat RPE65 deficiency in people in the long-term. 43 Proof of efficacy studies have been performed in mouse models of rod 36 and cone 38 dystrophies showing that the efficient and stable transduction using capsid-mutant AAV8 can preserve structure and function long-term in mouse models of retinitis pigmentosa. We plan to apply these vectors in the treatment of the rapid rod photoreceptor degeneration in the Pde6a mutant dog retina, in which apoptosis peaks at 25 to 28 days of age. 19 The data presented here indicate that capsid-mutant AAV8, with its faster onset of highly efficient gene expression may be more efficacious in the rescue of this severe, early phenotype, and will have wider application to the treatment of early-onset retinal dystrophies in people.
An alternative approach is to target the photoreceptors and retinal pigment epithelium via intravitreal injection. We have shown in this study that a quadruple tyrosine capsid-mutant AAV2 vector (AAV2(quad Y-F)) more efficiently transduces the photoreceptors from an intravitreal approach than a self-complementary AAV vector (scAAV2). However, despite these improvements in efficiency, the intravitreal approach was not as efficient as the subretinal approach (5.1–24% of photoreceptors intravitreal vs. 91.8–97.7% subretinal). A recent study in normal mice showed that additional substitutions in the viral capsid (four Y to F mutations, and a T to V mutation) had a 3-fold increase in photoreceptor transduction from intravitreal administration compared with AAV2(quad Y-F). 44 These data suggest that further enhancements in vector design can increase the efficiency of photoreceptor transduction from an intravitreal injection. In addition, there are several barriers to retinal penetration from the vitreous that might be overcome to enhance transduction efficiency. An intact inner limiting membrane is a significant barrier for penetration. In comparison with the injection of (AAV2(quad Y-F) intravitreally in the mouse eye, 35 the canine transduction efficiency from a vitreal approach was more limited. We hypothesize that this is related to the barrier to penetration through the inner limiting membrane and nerve fiber layer - which is thicker in the canine (141µm in the peripapillary location, 45 26µm in the superior central retina 46) than the mouse (19µm in the central retina). 47 A similar finding was noted when capsid-mutant vectors were injected into the vitreous cavity of non-human primates, which also have a thicker inner limiting membrane than mice. 48 Receptors for AAV2 are present at the vitreo-retinal junction in rodents, 49 causing accumulation of virus in this location. The barrier appears to be reduced in the context of retinal degeneration related to inner limiting membrane disorganization in the rodent eye. 50 We also report two additional interesting findings in the canine retina. Firstly, more efficient outer (and inner) retinal transduction in adult retinas appears to center predominantly around retinal blood vessels (also found in non-human primates following intravitreal AAV2 delivery)48 and secondly that the juvenile (postnatal day 16) canine retina appears to have fewer barriers for penetration of the retina from the vitreous, resulting in more widespread and efficient transduction of both photoreceptors and retinal pigment epithelium. These findings might suggest that the inner limiting membrane is a barrier to vitreal penetration in the canine retina, as the inner limiting membrane is thinner in immature human eyes, 51 and thickens with age in the non-human primate. 52 The ILM may be thinner over retinal blood vessels due to the reduction in Müller cell end-feet in those locations. 53 To enhance transduction efficiency, future studies could investigate the effect of combined intravitreal capsid-mutant AAV2 delivery and inner limiting membrane disruption such as enzymatic digestion. 49 Investigating the transduction efficiency of intravitreally delivered virus in the context of active retinal degeneration may provide additional evidence to support the application of capsid-mutant vectors in the treatment of retinal degeneration. 50
In addition to targeting retinal photoreceptors, intravitreal injection of capsid-mutant AAV2 showed mildly enhanced transduction of a proportion of inner retinal cells compared to self-complementary AAV2. Inner retinal transduction through either subretinal or intravitreal approaches was less efficient than photoreceptor transduction, but vectors targeted approximately 10–25% of inner retinal cells over a wide area from an intravitreal approach (compared to a similar percentage of cells transduced from a subretinal approach using the same capsid-mutant vector type). Intravitreal AAV2(quad Y-F) had marginally higher transduction of rod bipolar cells and calbindin positive inner retinal cells compared with scAAV2. Further investigation of the intravitreal approach to gene delivery is warranted as this method would be straightforward, could overcome many of the deleterious effects of subretinal injection and is capable of treatment of a wide area of the retina from a single injection. Intravitreal injection is already a reality in the delivery of drugs such as angiostatic agents, to the eye. Sustained protein delivery using viral vector mediated expression would negate the need for repeated injections that carry a cumulative increase in complication risk. 54 These vectors also have promise for the optogenetic approach, which aims to introduce novel light-sensitive protein into cells of the inner retina. Previous studies have demonstrated light-sensitive protein expression in the ganglion cells 55–57 and in some instances horizontal cells 58 from a vitreal approach, and the current study shows that capsid-mutant vector delivery would also facilitate the expression of protein in amacrine/horizontal cells and rod on-bipolar cells from an intravitreal approach. The advantage of expression in this location is that further processing of light-perception is possible by utilizing the normal neural pathways in the retina that process signals derived from photoreceptors. This will help to facilitate restored visual perception, as has recently been shown in the rd10 mutant mouse where capsid-mutant AAV2 was used to target rod ON-bipolar cells, restoring light perception. 59
In conclusion, compared with the previous generation of AAV vectors, capsid mutations in certain pseudotyped AAV vectors injected into the canine eye can result in a faster onset of expression from a subretinal injection. Intravitreal delivery of capsid-mutant AAV2 results in improved transduction of inner and outer retina cells. Future studies will aim to evaluate the outcome following therapeutic gene delivery in canine models of retinal dystrophy.
Materials and methods
Animals
Normal purpose-bred, adult beagle dogs (n = 12 dogs; 6 male, 6 female; mean age at injection 308 days, range 285–345 days) were obtained from a commercial supplier (Covance, Kalamazoo, MI) and acclimated for 1 week in the Michigan State University (MSU) animal facility prior to injection. Additionally, 2 normal juvenile mixed-breed dogs (16 days old) were obtained from a colony maintained at MSU. Animals were housed on a 12-hour light: dark cycle. Animal care was in compliance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research and all procedures were performed with approval by the MSU Institutional Animal Care and Use Committee.
AAV constructs
Recombinant AAV vectors were manufactured and purified by previously described methods. 33–35 This included purification and concentration by column chromatography. Vector titer was determined by real time PCR and final aliquots were resuspended in balanced salt solution (BSS Alcon Laboratories, Forth Worth TX, USA) containing 0.014% Tween 20. Four different AAV vectors were used: self-complementary AAV2/5 construct expressing humanized green fluorescent protein (hGFP) driven by the ubiquitous, truncated chimeric CMV-chicken beta actin promoter (smCBA) (subsequently referred to as scAAV5) was used as a control for subretinal injection. 21 Self-complementary AAV 2/2 (scAAV2) was used as a control for intravitreal injection, as scAAV2 transduces the murine retina following intravitreal delivery. 34 Capsid-mutant vectors included: an AAV2/2 with four surface exposed capsid tyrosine residues replaced by phenylalanine (Y272F, Y444F, Y500F and Y730F) expressing green fluorescent protein driven by smCBA (subsequently referred to as AAV2(quad Y-F), and a self-complementary AAV2/8 construct with a single capsid tyrosine to phenylalanine substitution (Y733F) expressing hGFP driven by smCBA (subsequently referred to as scAAV8(Y733F)).
Subretinal and intravitreal injections
All vectors were diluted to 5×1011 vg/mL using sterile balanced salt solution prior to injection. Adult dogs were randomly allocated into one of three groups of 4 dogs (Table 1). Under general anesthesia 250 µl of vector preparation was delivered by subretinal 21, 60 or intravitreal 61 injection as previously described. Subretinal injections targeted the superior retina delineated by the boundary of the tapetum lucidum; intravitreal injections were administered immediately anterior to the retinal surface of the tapetal fundus. The two juvenile dogs both received a unilateral right eye intravitreal injection of 100 µl AAV2(quad Y-F) preparation. Immediately after subretinal injection, all dogs received a subconjunctival injection of 2mg dexamethasone solution and 8mg gentamicin. Oral prednisone was administered post-operatively at 1mg/kg daily for week 1, 0.5mg/kg for week 2, 0.25mg/kg for week 3 and 0.125mg/kg for week 4.
Table 1.
Details of dogs used for experimental procedures
| Number of dogs | 4 | 4 | 4 |
|---|---|---|---|
| Route | intravitreal | subretinal | subretinal |
| Right eye | AAV2(quad Y-F) | AAV2(quad Y-F) | scAAV8(Y733F) |
| Left eye | scAAV2 | scAAV5 | scAAV5 |
Ophthalmic examination and imaging
Slit-lamp biomicroscopy (SL, Kowa, Japan), binocular indirect ophthalmoscopy (Welch Allyn, Skaneateles Falls, NY, USA), and wide-angle color and fluorescent digital fundus imaging (RetCam II, Clarity Medical Systems, Pleasanton CA) were performed pre-injection, immediately post-injection, daily for 10 days post-injection, then weekly until the end of the study.
Tissue processing/sectioning
Animals were sacrificed 30 days post-injection. Eyes were enucleated following euthanasia by barbiturate overdose (Fatal Plus, Vortech Pharmaceuticals, Dearborn MI). Eyes were prepared for sectioning as previously described 62 and embedded eyecups were stored at −20 °C until sectioning. 14 µm vertical sagittal retinal cryosections were collected using a cryomicrotome (Leica CM3050-S, Leica Microsystems, Buffalo Grove IL) onto poly-L-lysine coated glass slides (Electron Microscopy Sciences, Hatfield MO). Sections were taken from the portion of the retina that included the optic nerve, and approximately 1mm nasal and temporal to this location.
Immunohistochemistry
Reagents utilized and basic immunohistochemistry protocols performed were as previously described. 62 Primary and secondary antibody labeling was performed at the concentrations outlined in Table 2. Primary antibodies were incubated overnight at 4 °C, secondary antibodies for 2 hours at room temperature. Representative images of immunohistochemistry labeling were taken using a confocal microscope (Olympus FluoView 1000 confocal, Center Valley, PA).
Table 2.
Antibodies used for immunohistochemistry
| Antigen target | Antibody details and working dilution |
Manufacturer | Secondary antibody and concentration (all from Invitrogen Corp, Carlsbad CA) |
|---|---|---|---|
| Green-fluorescent protein | rabbit FITC conjugated 1:1000 | Invitrogen Corp, Carlsbad CA | N/A |
| Cone arrestin (LUMif) 63 | rabbit polyclonal 1:5000 | kind gift of Cheryl M. Craft, University of Southern California | AlexaFluor anti-rabbit 546 1:500 |
| S-cone opsin | rabbit polyclonal 1:1000 | Millipore Billerica MA | AlexaFluor anti-rabbit 546 1:500 |
| Lectin labeling cone matrix sheaths | Biotinylated peanut agglutinin lectin (PNA) 1:500 | Vector Laboratories Burlingame CA | AlexaFluor streptavadin 405 1:500 |
| Calbindin | Mouse monoclonal 1:500 | Swant, Marly Switzerland | AlexaFluor anti-mouse 546 1:500 |
| Calretinin | Rabbit polyclonal 1:500 | Swant, Marly Switzerland | AlexaFluor anti-rabbit 546 1:500 |
| Protein-kinase C alpha | Mouse monoclonal 1:500 | BD Biosciences, San Diego CA | AlexaFluor anti-mouse 546 1:500 |
Image analysis
To evaluate the onset speed of GFP expression, fluorescent fundus images were equally digitally enhanced using a commercial program (Adobe Photoshop CS4; San Jose CA), number coded, randomized (www.randomizer.org), and inserted into a slide-show (Powerpoint 2007, Microsoft inc. Redmond WA) for examination. Two masked observers independently scored images for presence or absence of GFP fluorescence at each study time-point.
To evaluate retinal transduction efficiency, images of IHC were captured using a fluorescence microscope (Nikon Eclipse 80i, Nikon Instruments Inc., Melville NY) at x40 magnification. Images were captured from three sections 140µm apart, resulting in triplicate counts generated for each eye. Within each individual dog, the same region in both eyes was selected, to limit the effect of regional variation in cell density. Sections including the optic nerve were imaged in eyes that had received intravitreal injections. All images were cropped to 1200×800 pixels using the rectangular marquee tool (Adobe Photoshop CS4) and randomized. A masked observer counted the total numbers of antigen-positive cells and the number of these cells co-expressing GFP. Average percentages of specific cell-types expressing GFP were calculated.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 5.0a (Graphpad software Inc. La Jolla, CA). When comparing the right eye and left eye within individual animals, a two-tailed paired student's t-test was performed. When results from eyes of different dogs were compared, a one-way ANOVA was performed with Bonferroni's multiple comparison post-test to evaluate the significance of individual differences. To evaluate the agreement between the two observers evaluating the fundus images, a regression line was fit to the scatter plot data. Due to low n-numbers, a Spearman rank correlation was used to compare results. Effects were considered significant if p<0.05. In all figures, graphs represent mean +/− standard error of the mean (SEM).
Acknowledgements
Janice Querubin and Lisa Allen for animal assistance, Joe Hauptman for statistical assistance, Cheryl Craft for hCAR antibody and Vince Chiodo for AAV purification. JTB and FMM acknowledge the following funding sources: Michigan State University College of Veterinary Medicine Endowed Research Fund. SPJ acknowledges the following funding sources: The Glassen Memorial Foundation, Myers-Dunlap Endowment for Canine Health. WWH acknowledges the following funding sources: NIH grant EY021721 and grants from the Macula Vision Research Foundation, Foundation Fighting Blindness, Eldon Family Foundation, Overstreet Fund and Research to Prevent Blindness, Inc.
Footnotes
Conflict of interest
WWH and the University of Florida have a financial interest in the use of AAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. SLB and WWH hold a patent (No. 8,298,818) that covers some aspects of the AAV technology used in this study. The other authors acknowledge no conflict of interest.
References
- 1.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18(3):643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20(9):999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 6.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 8.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14(4):292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 10.Anasagasti A, Irigoyen C, Barandika O, Lopez de Munain A, Ruiz-Ederra J. Current mutation discovery approaches in Retinitis Pigmentosa. Vision Res. 2012;75:117–129. doi: 10.1016/j.visres.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19(13):2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowat FM, Bartoe JT, Bruewer A, Dinculescu A, Boye SL, Hauswirth WW, et al. Evaluation Of Rod Photoreceptor Function And Preservation Following Retinal Gene Therapy In The PDE6A Mutant Dog. ARVO Meeting Abstracts. 2012;53(6):1928. [Google Scholar]
- 13.Petit L, Lheriteau E, Weber M, Le Meur G, Deschamps JY, Provost N, et al. Restoration of vision in the pde6beta-deficient dog, a large animal model of rod-cone dystrophy. Mol Ther. 2012;20(11):2019–2030. doi: 10.1038/mt.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci U S A. 2012;109(6):2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennesi ME, Stover NB, Stone EM, Chiang PW, Weleber RG. Residual electroretinograms in young Leber congenital amaurosis patients with mutations of AIPL1. Invest Ophthalmol Vis Sci. 2011;52(11):8166–8173. doi: 10.1167/iovs.11-8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Swider M, et al. Human retinal disease from AIPL1 gene mutations: foveal cone loss with minimal macular photoreceptors and rod function remaining. Invest Ophthalmol Vis Sci. 2011;52(1):70–79. doi: 10.1167/iovs.10-6127. [DOI] [PubMed] [Google Scholar]
- 17.Testa F, Surace EM, Rossi S, Marrocco E, Gargiulo A, Di Iorio V, et al. Evaluation of Italian patients with leber congenital amaurosis due to AIPL1 mutations highlights the potential applicability of gene therapy. Invest Ophthalmol Vis Sci. 2011;52(8):5618–5624. doi: 10.1167/iovs.10-6543. [DOI] [PubMed] [Google Scholar]
- 18.Petersen-Jones SM, Entz DD, Sargan DR. cGMP phosphodiesterase-alpha mutation causes progressive retinal atrophy in the Cardigan Welsh corgi dog. Invest Ophthalmol Vis Sci. 1999;40(8):1637–1644. [PubMed] [Google Scholar]
- 19.Tuntivanich N, Pittler SJ, Fischer AJ, Omar G, Kiupel M, Weber A, et al. Characterization of a canine model of autosomal recessive retinitis pigmentosa due to a PDE6A mutation. Invest Ophthalmol Vis Sci. 2009;50(2):801–813. doi: 10.1167/iovs.08-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarra GM, Stephens C, Schlichtenbrede FC, Bainbridge JW, Thrasher AJ, Luthert PJ, et al. Kinetics of transgene expression in mouse retina following sub-retinal injection of recombinant adeno-associated virus. Vision Res. 2002;42(4):541–549. doi: 10.1016/s0042-6989(01)00230-9. [DOI] [PubMed] [Google Scholar]
- 21.Petersen-Jones SM, Bartoe JT, Fischer AJ, Scott M, Boye SL, Chiodo V, et al. AAV retinal transduction in a large animal model species: comparison of a self-complementary AAV2/5 with a single-stranded AAV2/5 vector. Mol Vis. 2009;15:1835–1842. [PMC free article] [PubMed] [Google Scholar]
- 22.Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker SE, Smith AJ, et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther. 2008;15(6):463–467. doi: 10.1038/sj.gt.3303074. [DOI] [PubMed] [Google Scholar]
- 23.Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36(6):990–996. [PubMed] [Google Scholar]
- 24.Fisher SK, Lewis GP. Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003;43(8):887–897. doi: 10.1016/s0042-6989(02)00680-6. [DOI] [PubMed] [Google Scholar]
- 25.Fisher SK, Stone J, Rex TS, Linberg KA, Lewis GP. Experimental retinal detachment: a paradigm for understanding the effects of induced photoreceptor degeneration. Prog Brain Res. 2001;131:679–698. doi: 10.1016/s0079-6123(01)31053-1. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi T, Miyake K, Asakawa N, Miyake N, Shimada T, Takahashi H. Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr Eye Res. 2013;38(5):569–577. doi: 10.3109/02713683.2013.779720. [DOI] [PubMed] [Google Scholar]
- 27.Englander M, Chen TC, Paschalis EI, Miller JW, Kim IK. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br J Ophthalmol. 2013 doi: 10.1136/bjophthalmol-2012-302435. [DOI] [PubMed] [Google Scholar]
- 28.Stieger K, Schroeder J, Provost N, Mendes-Madeira A, Belbellaa B, Le Meur G, et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther. 2009;17(3):516–523. doi: 10.1038/mt.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auricchio A. Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vision Res. 2003;43(8):913–918. doi: 10.1016/s0042-6989(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 30.Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10(4):375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21(3):509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15(7):1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 33.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17(3):463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011;19(2):293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19(2):234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ku CA, Chiodo VA, Boye SL, Goldberg AF, Li T, Hauswirth WW, et al. Gene therapy using self-complementary Y733F capsid mutant AAV2/8 restores vision in a model of early onset Leber congenital amaurosis. Hum Mol Genet. 2011;20(23):4569–4581. doi: 10.1093/hmg/ddr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boye SL, Conlon T, Erger K, Ryals R, Neeley A, Cossette T, et al. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest Ophthalmol Vis Sci. 2011;52(10):7098–7108. doi: 10.1167/iovs.11-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dinculescu A, Estreicher J, Zenteno JC, Aleman TS, Schwartz SB, Huang WC, et al. Gene therapy for retinitis pigmentosa caused by MFRP mutations: human phenotype and preliminary proof of concept. Hum Gene Ther. 2012;23(4):367–376. doi: 10.1089/hum.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng WT, Dinculescu A, Li Q, Boye SL, Li J, Gorbatyuk MS, et al. Tyrosine-mutant AAV8 delivery of human MERTK provides long-term retinal preservation in RCS rats. Invest Ophthalmol Vis Sci. 2012;53(4):1895–1904. doi: 10.1167/iovs.11-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martino AT, Basner-Tschakarjan E, Markusic DM, Finn JD, Hinderer C, Zhou S, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224–2233. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One. 2013;8(3):e59142. doi: 10.1371/journal.pone.0059142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110(6):E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, et al. Targeting Photoreceptors via Intravitreal Delivery Using Novel, Capsid-Mutated AAV Vectors. PLoS One. 2013;8(4):e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Sanchez GA, Gil-Carrasco F, Roman JJ, Brooks DE, Alvarez-Clau A, Hosgood G, et al. Measurement of retinal nerve fiber layer thickness in normal and glaucomatous Cocker Spaniels by scanning laser polarimetry. Vet Ophthalmol. 2007;(10 Suppl 1):78–87. doi: 10.1111/j.1463-5224.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez-Merino E, Kecova H, Jacobson SJ, Hamouche KN, Nzokwe RN, Grozdanic SD. Spectral domain optical coherence tomography (SD-OCT) assessment of the healthy female canine retina and optic nerve. Vet Ophthalmol. 2011;14(6):400–405. doi: 10.1111/j.1463-5224.2011.00887.x. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson LR, Dominguez Ii JM, Balaiya S, Grover S, Chalam KV. Retinal Thickness Normative Data in Wild-Type Mice Using Customized Miniature SD-OCT. PLoS One. 2013;8(6):e67265. doi: 10.1371/journal.pone.0067265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5(189):189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 49.Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17(12):2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, Schaffer DV, et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther. 2010;21(5):571–578. doi: 10.1089/hum.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heegaard S. Structure of the human vitreoretinal border region. Ophthalmologica. 1994;208(2):82–91. doi: 10.1159/000310458. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto B, Blanks JC, Ryan SJ. Topographic variations in the rabbit and primate internal limiting membrane. Invest Ophthalmol Vis Sci. 1984;25(1):71–82. [PubMed] [Google Scholar]
- 53.Wolter JR. Pores in the Internal Limiting Membrane of the Human Retina. Acta Ophthalmol (Copenh) 1964;42:971–974. doi: 10.1111/j.1755-3768.1964.tb03664.x. [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 55.Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50(1):23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S, et al. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci. 2007;48(8):3821–3826. doi: 10.1167/iovs.06-1501. [DOI] [PubMed] [Google Scholar]
- 57.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105(41):16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Ivanova E, Bi A, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29(29):9186–9196. doi: 10.1523/JNEUROSCI.0184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19(7):1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruewer AR, Mowat FM, Bartoe JT, Boye SL, Hauswirth WW, Petersen-Jones SM. Evaluation of Lateral Spread of Transgene Expression following Subretinal AAV-Mediated Gene Delivery in Dogs. PLoS One. 2013;8(4):e60218. doi: 10.1371/journal.pone.0060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gearhart PM, Gearhart C, Thompson DA, Petersen-Jones SM. Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol. 2010;128(11):1442–1448. doi: 10.1001/archophthalmol.2010.210. [DOI] [PubMed] [Google Scholar]
- 62.Mowat FM, Bartoe JT, Bruewer A, Smith AJ, Bainbridge JB, Ali RR, et al. RPE65 Gene Therapy Promotes Survival Of S-cones In The RPE65-deficient Dog. Invest Ophthalmol Vis Sci. 2011;52:1. [Google Scholar]
- 63.Li A, Zhu X, Brown B, Craft CM. Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2003;44(3):996–1007. doi: 10.1167/iovs.02-0434. [DOI] [PubMed] [Google Scholar]