Abstract
Obesity, a pathologic state defined by excess adipose tissue, is a significant public health problem as it affects a large proportion of individuals and is linked with increased risk for numerous chronic diseases. Obesity is the result of fundamental changes associated with modern society including overnutrition and sedentary lifestyles. Proper energy homeostasis is dependent on normal brain function as the master metabolic regulator which integrates peripheral signals, modulates autonomic outflow and controls feeding behavior. Therefore, many human brain diseases are associated with obesity. This review explores the neuropathology of obesity by examining brain diseases which either cause or are influenced by obesity. First, several genetic and acquired brain diseases are discussed as a means to understand the central regulation of peripheral metabolism. These diseases range from monogenetic causes of obesity (leptin deficiency, MC4R deficiency, Bardet-Biedl syndrome and others) to complex neurodevelopmental disorders (Prader-Willi syndrome and Sim1 deficiency) and neurodegenerative conditions (frontotemporal dementia and Gourmand’s syndrome) and serve to highlight the central regulatory mechanisms which have evolved to maintain energy homeostasis. Next, to examine the effect of obesity on the brain, chronic neuropathologic conditions (epilepsy, multiple sclerosis and Alzheimer’s disease) are discussed as examples of obesity leading to maladaptive processes which exacerbate chronic disease. Thus obesity is associated with multiple pathways including abnormal metabolism, altered hormonal signaling and increased inflammation which act in concert to promote downstream neuropathology. Finally, the effect of anti-obesity interventions is discussed in terms of brain structure and function. Together, understanding human diseases and anti-obesity interventions leads to insights into the bidirectional interaction between peripheral metabolism and central brain function, highlighting the need for continued clinicopathologic and mechanistic studies of the neuropathology of obesity.
I. Introduction
Obesity is a pathologic state defined by an excessive accumulation and maintenance of adipose tissue. While direct measures of adiposity are possible such as dual energy X-ray absorptiometry scanning, obesity is often inferred using surrogate markers including body mass index (BMI) because increased body mass is generally associated with excess adipose tissue. Worldwide, obesity rates as measured by BMI have almost doubled since 1980 with ~35% of adults being overweight and ~11% of adults being obese. [190] In the United States, obesity rates are significantly higher at ~35% for adults and ~15% for children. [60,192] Indeed, obesity appears to be linked to societal modernization and, remarkably, 65% of the world’s population live in countries where mortality linked with being overweight or obese is higher than mortality due to being underweight. [190]
In evolutionary terms, humans have only recently been living in environments where sources of cheap, abundant, high calorie food are readily available. Rather, the scarcity of food was a driving force in the development of refined homeostatic mechanisms to protect organisms from starvation. These pathways are now operating under conditions of a sustained positive energy balance, contributing to a variety of chronic diseases including diabetes and vascular disease. Indeed, the trio of central obesity, insulin resistance, dyslipidemia and hypertension are defining hallmarks of “metabolic syndrome.” The neuropathology of obesity which we describe below is linked to alterations in the homeostatic pathways that regulate energy homeostasis, and these changes are associated with increased risk for several neuropathologic conditions.
The goal of this review is to use human diseases associated with obesity to understand both how the brain regulates energy homeostasis and how the brain is influenced by the obesity-related changes. Overall, a general model emerges in which multiple brain circuits cross-regulate each other to affect autonomic neuronal pathways and endocrine organs (thereby directly affecting energy homeostasis), appetite (drive to eat), satiety (sensation of satisfaction or fullness) and food pleasure (palatability and reward derived from food). The hypothalamus and the dorsal medulla act as the two main hubs which receive and integrate peripheral signals which then cross-regulate each other and communicate with higher brain regions such as the anterior forebrain mesolimbic reward system (Figure 1). Furthermore, obesity is associated with fundamental changes in peripheral metabolism resulting in alteration of the hormonal, metabolic and inflammatory milieu – all of which may promote various chronic neurologic diseases. In as much as it is possible, this review strives to discuss the neuropathology of human obesity, although particularly salient references to other components of metabolic syndrome, to animal models of obesity, and to human radiologic findings are also included. We emphasize the pathways linked to obesity, rather than diabetes and cerebrovascular disease which can occur in the absence of obesity. To explore this topic, basic concepts are introduced including those related to energy homeostasis and lipid metabolism, followed by a discussion of the role of the brain in regulating an integrated physiologic network. Second, selected brain diseases which are associated with obesity are described which highlight the central nervous system (CNS) pathways which regulate peripheral metabolism. Third, the deleterious effects of increased adiposity and altered metabolism on the CNS are discussed in terms of how abnormal metabolic, humoral and inflammatory states can affect CNS structure and function. Finally, anti-obesity interventions are discussed in terms of their effects on brain structure and function.
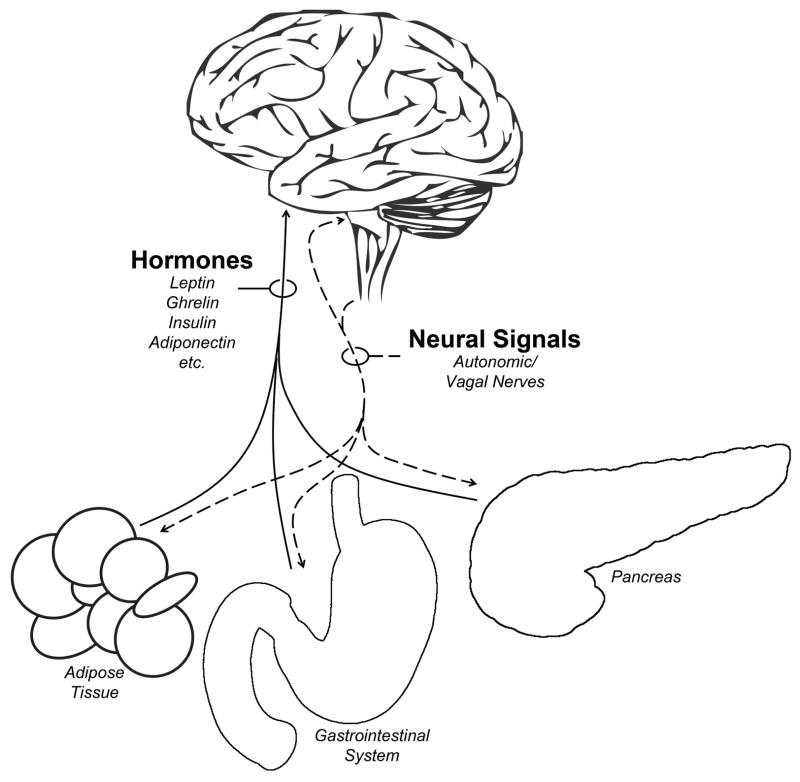
Figure 1. Peripheral-Central Connections.
The brain receives and integrates hormonal and neural signals from peripheral organs including adipose tissue, the gastrointestinal system, the pancreas, skeletal muscle, the liver, and others. We emphasize here the hypothalamic integration of hormonal signals and the hindbrain integration of vagal neural signals. Note that autonomic neural signals are bidirectional such that the brain receives and transmits neural signals with peripheral organs. Not depicted here are peripheral inflammatory or metabolic factors which also affect the CNS.
II. Fundamental Concepts in Obesity
Energy Homeostasis
Obesity results from a chronic disruption in energy homeostasis. Energy homeostasis is the steady-state balance of energy intake versus energy expenditure, and organisms including humans have evolved multiple mechanisms to maintain energy homeostasis. The fundamental biological units of energy are energy-rich molecules such as phosphocreatine, adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+) which are used for nearly all biological functions from maintenance of transmembrane ion gradients, intracellular signaling, neuronal signaling, protein synthesis, etc. We ingest food to supply energy, primarily in the form of carbohydrates, fats and proteins (and alcohol) which are broken down and absorbed by the digestive tract. Within cells, nutrients are taken up and used as fuel in a process whereby glucose, fatty acids and amino acids are hydrolyzed to generate ATP, carbon dioxide, water and heat. This process of obtaining and digesting nutrients accounts for the input side of the equation that defines energy homeostasis. The regulation of feeding behavior, and therefore energy intake, represents one major means whereby the CNS affects energy balance. Feeding behavior can be viewed as the final outcome of appetite, satiety and pleasure sensations, all of which are dependent on the CNS. Dysregulation of feeding behavior contributes to high obesity rates, in part due to the high prevalence of inexpensive palatable foods that promote overfeeding.
On the other side of the equation, multiple processes contribute to energy expenditure including basal metabolic rate, non-shivering thermogenesis, diet-induced thermogenesis, and physical activity. Basal metabolic rate is the energy used in an awake, fasted, rested and supine individual in which there is an energy-neutral temperature environment. Mechanistically, this energy corresponds to the cellular and physiologic processes which are essential to life, ranging from protein synthesis and basic biochemical processes to muscle tone, and cardiovascular and brain function. BMR corresponds roughly to 60–70% of total energy expenditure, and increases with overall body weight as the basal metabolic demand increases with increased body mass. [184,206] Notably, although the brain roughly corresponds to about 2% of total body mass, the brain is responsible for 20% of total oxygen consumption/energy expenditure. [223] In contrast, skeletal muscle uses an equivalent amount of energy, but accounts for greater than 40% of body mass. [71,90] Indeed, the human brain exhibits a particularly high metabolic requirement which may be intimately linked to our dietary choices. Several factors correlate with BMR including adipose tissue mass and age, which together account for ~70% of the inter-subject variance in BMR. [90] Differences in sympathetic tone are another factor which regulates BMR, representing the outflow of neural signaling from the CNS to the periphery. [259,90]
Thermogenesis is another factor which contributes to energy expenditure. Sympathetic regulation of brown adipose tissue is used by some organisms to regulate body temperature in which adipocytes uncouple mitochondrial respiration leading to heat generation. Significant heat is generated in newborn humans due to brown adipose tissue-dependent thermogenesis. Although thermogenesis was thought to be a minor component of adult human energy expenditure, recent evidence suggests that humans do have brown adipose tissue depots which are regulated by ambient temperature. [61,251,256] Energy can also be used in a process called dietary-induced thermogenesis which is the increase in energy expenditure after eating due to digestion, absorption and dispersion of nutrients to target cells.
One final biological process which contributes to energy expenditure is physical activity. Importantly, except in the case of endurance athletes, physical activity is actually responsible for a relatively small proportion of total energy expenditure on the order of 20–30%. [90] However, physical activity is also the most malleable of these three biological processes in that it is difficult to consciously alter BMR or thermogenesis. Indeed, the increase in obesity rates in the United States is at least in part due to sedentary lifestyles.
Integrated Physiology and Energetics
Organisms are able to regulate energy balance with exquisite control. Adipose tissue is by far the main depot of stored energy. A normal, lean 70 kg male has ~12 kg of adipose tissue at ~9.5 kcal/gram (corresponding to ~110,000 kcal or enough fuel to last nearly two months). In contrast, less than 500 grams of carbohydrate is stored in the human body in the form of liver and muscle glycogen which at ~4 kcal/gram only yields 2000 kcal, corresponding to one day of energy. [201] However, because glycogen stores are tapped before fat stores, individuals who eat regular meals may not utilize fat stores. We have evolved multiple mechanisms which protect and maintain adequate adipose tissue mass, and only under chronic exposure to over-nutrition and sedentary lifestyles does obesity ensue. As adipose tissue represents the main energy store for organisms, the maintenance of adipose tissue is a long-term process. In contrast, mechanisms also exist that regulate short-term processes including satiety mechanisms which limit meal size. As the CNS does not use lipids as an energy source, relying almost exclusively on glucose, mechanisms have evolved to maintain carbohydrate levels. Alternatively, under conditions of starvation or in particular diet configurations (high fat, adequate protein, low carbohydrate ketogenic diets), the liver can use acetyl-CoA, a product of lipolysis, to generate the ketone bodies β-hydroxybutyrate, acetoacetate and acetone which can be used by the brain as an alternative fuel source. Ketogenic diets are prescribed for certain types of epilepsy, and ketosis appears neuroprotective through multiple through various mechanisms. [151] Indeed, intermittent energy restriction and exercise have been proposed as a means to promote brain health. [163]
How does the periphery influence the brain and, conversely, how does the brain regulate peripheral metabolism? Peripheral organs send signals to the CNS via three routes: humoral, metabolic and neural. Humoral factors include hormones secreted by peripheral organs including the pancreas, adipose tissue and the gastrointestinal tract (Figure 1). These hormones are found in the peripheral circulation, and in some instances are specifically transported to neuronal populations expressing target receptors. Metabolic factors include carbohydrates, lipids, ketones, alcohols, amino acids and other metabolites which are used for energy and as building blocks of cell structures (membranes, cytoskeleton, extracellular matrix etc.). Finally, the autonomic nervous system transmits signals from peripheral organs to the CNS. No other organ is capable of the remarkable integration of these humoral, metabolic and neuronal signals. After integrating these diverse signals, the brain can alter sympathetic and parasympathetic tone in order to regulate peripheral metabolism via autonomic neuronal pathways, directly altering target organ function. In addition to the autonomic nervous system, the CNS also regulates appetite, satiety, motivation, feeding behavior, and exercise behavior. Thus the brain can be considered the master regulator of energy homeostasis, monitoring short-term energy intake and long-term energy stores in order to modulate both energy intake and energy expenditure.
The Obesogenic Brain
Understanding the CNS in context of whole body energy homeostasis and an integrated physiologic network leads to the possibility that the evolution of the human brain drives our innate desire for high calorie, high fat foods. A comparison of primates including humans shows a tight relationship between total body mass and BMR. [143] However, the human brain represents 20 to 25% of BMR. In contrast, non-human primate brains are responsible for 8 to 10% of BMR, and this drops to 5% or less for non-primate mammals. Indeed, a study of brain weight and BMR across 57 species demonstrates that humans represent an obvious outlier with a very high brain weight to BMR ratio. [143] Stated another way, for a given BMR, non-human primates have brain weights three times larger than non-primate mammals, and similarly human brains are three times heavier than non-human primates. [143] This large allocation of BMR to the CNS raises the question of whether human nutrition has evolved to support the large energetic demands of the brain. Hominin brains have tripled in size over the last 4 million years, with the greatest increases in brain size occurring within the last ~2 million years with the emergence of the Homo genus. This encephalization coincided with a dietary change to foods including animal sources that are denser in terms of both energy and fat, the latter providing essential long-chain polyunsaturated fatty acids (docosahexaenoic acid and arachidonic acid) that are required for brain development. Increased brain mass coincided with changes in diet, the use of tools, the cultivation of stable food sources, and the development of methods for efficient calorie extraction such as cooking. This suggests that the evolution of the human brain is linked with our innate human drive for consumption of high calorie, high fat foods. [143] Thus, perhaps the human drive for high calorie foods is in part due to the high energetic demands of our brains. That is, the evolution of the human brain was linked to our drive for energy dense foods such that humans are particularly susceptible to obesity.
III. Neuropathology of Obesity-related Conditions
There are multiple CNS-based humoral and neural mechanisms that regulate energy homeostasis. In this section, various neuropathologic conditions associated with obesity will be described which highlight different types of mechanisms used by the human brain to regulate peripheral metabolism. Instead of providing an exhaustive list of CNS causes of obesity, the purpose of this section is to highlight particular diseases or manipulations which highlight how the CNS regulates energy homeostasis. Although there is significant overlap and cross-talk between these various mechanisms, these conditions are broadly categorized into peripheral to central hormonal signaling, peripheral to central neural signaling, and central signaling networks. Thus human diseases will be used to provide insights into how the human brain regulates energy homeostasis. A simplified model consists of two main signaling hubs, the hypothalamus which receives and integrates peripheral hormonal signals in order to affect appetite and the dorsal medulla which receives and integrates vagal signals in order to affect satiety (Fig 2B–D). These hubs cross-regulate each other and higher brain regions, such as the mesolimbic reward system which regulates feelings of reward and pleasure associated with food. Thus a complex system has evolved in which diverse signals and neural circuits act in concert to regulate energy homeostasis.
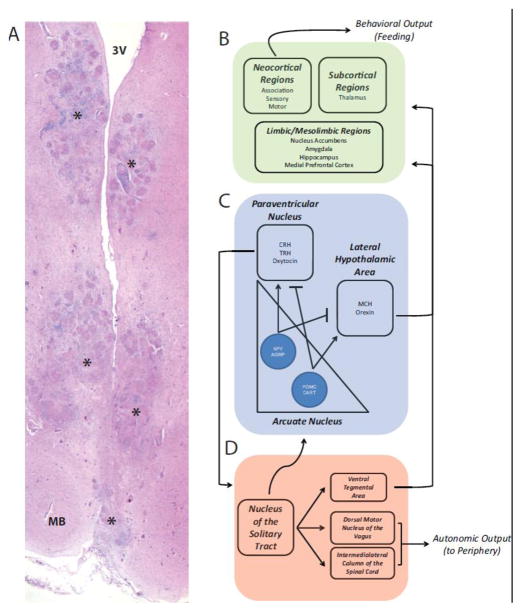
Figure 2. Neuroanatomic Regulation of Energy Homeostasis.
A) A case of hypothalamic obesity secondary to sarcoidosis involving the hypothalamus demonstrates florid granulomatous inflammation involving multiple areas without regard for neuroanatomic boundaries. Shown here is involvement of the posterior hypothalamus (other sections demonstrated destruction throughout the hypothalamus). Areas with granulomatous inflammation are marked with an asterisk. MB, mamillary body, 3V, third ventricle. (B) Multiple forebrain regions receive input from the hypothalamus and brainstem including neocortex, deep grey structures and limbic/mesolimbic brain regions. These areas give rise to sensations of appetite, satiety and pleasure, all of which regulate feeding behavior. (C) Hypothalamic circuits which are modulated by peripheral hormonal cues are shown, including distinct neuronal populations within the arcuate nucleus expressing neuropeptide Y (NPY), agouti-related protein (AGRP), pro-opiomelanocortin (POMC), cocaine and amphetamine-related transcript (CART). These neurons reciprocally regulate other hypothalamic areas including the paraventricular nucleus and the lateral hypothalamic area with diverse neuronal subtypes expressing corticotrophin-release hormone (CRH), thyrotropin-releasing hormone (TRH), oxytocin, melanin concentrating hormone (MCH) and orexin. (D) In the hindbrain, the nucleus of the solitary tract integrates neural signals via the vagus nerve from the periphery, and regulates autonomic output to the periphery via projections to the dorsal motor nucleus of the vagus and the intermediolateral column of the spinal cord. The hindbrain also projects to the hypothalamus and the forebrain, for example via the ventral tegmental area. Notably, there are cross-connections between the forebrain, hypothalamus and hindbrain such that peripheral hormonal and neural signals are integrated across multiple brain circuits resulting in a coordinated CNS response.
Classical Neuropathology of Obesity: The Neuroanatomic Basis of Obesity
The neuropathologic basis of obesity was firmly established in the classic descriptions of various hypophyseal or hypothalamic syndromes. Probably the earliest description of a pituitary tumor in association with obesity was published in 1840 by Mohr, although a cause-effect relationship between hypophyseal tumors and obesity was not surmised until a pair of publications by Babinski in 1900 and Fröhlich in 1901. [20,36,39,171] These case reports described the clinical and pathologic features of what has been variably termed adiposogenital dystrophy, Fröhlich syndrome, Babinski-Fröhlich syndrome or hypothalamic infantilism-obesity. In Fröhlich’s case, histology of a sellar tumor showed “an abnormality of the pituitary in a precancerous stage” while Babinski described “an epithelioma developed from the epithelium of the pituitary gland … of Malpigian type”. [20,36] Clinically, these cases of hypothalamic obesity were complex disorders characterized by headaches, changes or loss of vision, obesity and “infantilism” (i.e. hypogonadism) in the absence of acromegaly (the latter which was gaining recognition as a manifestation of pituitary tumors through the works of Pierre Marie and Harvey Cushing).
Hypogonadism in these cases is now known to be due to hypopituitarism, namely the disruption of the gonadotropin-releasing hormone-gonadotropin axis. In contrast, obesity is more attributed to damage to hypothalamic brain regions which regulate appetite and energy homeostasis. We now know that any pathology which causes structural damage to the hypothalamus can lead to obesity including neoplasms (craniopharyngiomas, macroadenomas of the pituitary, meningiomas, gliomas, germ cell tumors, metastatic tumors), vascular malformations, radiation-induced damage, sarcoidosis and other inflammatory conditions. [200] The incidence of hypothalamic obesity is rare, given that the most common cause of hypothalamic structural damage leading to obesity are craniopharyngiomas which occurs at a rate of 1.3 cases per one million person years. [43] These diverse lesions typically do not demonstrate specificity with regards to a particular anatomic region, limiting our ability to draw detailed mechanistic knowledge from these cases in terms of the neuronal circuits or signaling pathways which regulate appetite and energy expenditure (see Figure 2A). Despite this variability, hypothalamic obesity is generally associated with damage to the medial hypothalamus which is linked to variable levels of hyperphagia, autonomic dysfunction, decreased energy expenditure, increased somnolence and hormonal abnormalities. [200] The relative contribution of various hypothalamic (or pituitary) regions to each of the phenotypes cannot easily be discerned. However, there is clearly an anatomic means for regulating peripheral metabolism.
Classic lesion studies on rats showed that damage to the medial hypothalamus, more specifically the ventromedial hypothalamus (VMH), resulted in hyperphagia and obesity. Similarly, lesions at the same level in the lateral hypothalamic area (LHA) resulted in aphagia. [12] These two experimental findings led to the dual-center hypothesis in which the VMH contains a satiety center which is reciprocally connected to the LHA which contains a feeding center. These findings were challenged later by apparently more precise lesion experiments and neuroanatomic studies, [95,248,235,148] and undoubtedly represent an oversimplification of the hypothalamic regulation of feeding and energy expenditure. [127] As will be discussed below, there is now substantial evidence that many neurochemically distinct populations of hypothalamic neurons including those within the VMH, LHA and arcuate nucleus lie at the junction between peripheral signal detection and central neuronal circuits that influence energy homeostasis.
Another classic CNS cause of obesity was described by Harvey Cushing in 1932 in which basophil adenomas of the pituitary gland were associated with clinical features including truncal obesity of what is now known as Cushing’s syndrome. [37,58] The mechanism of obesity due to Cushing’s disease is fundamentally different than that of hypothalamic obesity. In Cushing’s disease, ACTH secreting tumors in the anterior pituitary result in hypercortisolemia, promoting protein catabolism (accounting for peripheral muscle wasting) which is used by the liver for gluconeogenesis, leading to hyperglycemia and hyperinsulinemia which results in increased adipose tissue (accounting for truncal obesity). Although this review will not further explore the neuroendocrine control of metabolism via the pituitary gland, it should be recognized that the CNS directly regulates pituitary hormones and neuroendocrine diseases can lead to peripheral obesity through a variety of peripheral mechanisms.
Hormonal Signals from the Periphery: Leptin
The detection and transmission of peripheral signals of metabolic status is one key function whereby the CNS regulates body weight. While the increase in energy-dense foods and sedentary lifestyles undoubtedly contribute to obesity, there is a genetic component which confers risk to developing obesity because adiposity is highly heritable with an estimated genetic contribution of ~60–85%. [81] While most forms of human obesity are polygenic and multifactorial, obesity can be associated with single genetic defects. These monogenic forms of obesity have led to several mechanistic insights, and remarkably most of the monogenic forms of obesity demonstrate that the CNS is the master regulator of energy homeostasis. The first monogenic cause of human obesity to be discovered is leptin deficiency. [172] Multiple reports have demonstrated that homozygous frameshift or missense mutations in the Ob gene which encodes for leptin is associated with severe, early onset obesity, voracious hyperphagia, hyperinsulinemia and accelerated bone aging. [80,172,191,237] Immunologic abnormalities and hypogonadotrophic hypogonadism were also observed in some individuals. [80,191] This clinical phenotype is remarkably similar to that observed in mice with congenital leptin deficiency. [81] Mutation of the human leptin receptor also leads to obesity and pituitary dysfunction. [51]
Leptin is a hormone secreted by adipose tissue, and in normal individuals, circulating leptin levels correlates with adipose tissue mass. [137] Leptin receptor is a cytokine receptor family member which is alternatively spliced into multiple isoforms. The longest isoform, LRb, is the only isoform containing a signaling-competent intracellular domain, and is expressed in distinct neuronal subsets within the brain, in particular neurons of the arcuate nucleus of the hypothalamus, and also other hypothalamic, brainstem and cerebrocortical neurons. [137] Leptin has pleiotropic effects and regulates energy expenditure, feeding behavior, locomotor activity, bone mass, growth, thermogenesis, fertility, life span, adrenal function and thyroid function. Overall, these effects are most consistent with the absence of leptin acting as a signal of starvation. [6] Thus leptin-deficient humans (and rodents) essentially develop a complex phenotype which includes severe obesity and hyperphagia because leptin-responsive neurons respond to the absence of leptin by modulating CNS pathways meant to protect organisms from starvation. [6] Indeed, treating leptin-deficient individuals with leptin leads to a remarkable reversal of obesity, hyperphagia and diabetes consistent with leptin treatment acting as a satiety factor that signals to the CNS that adipose stores are adequate. [79,80,145]
The original cloning of leptin was met with hopes that this hormone would lead to a treatment for polygenic obesity. [24] However, polygenic obesity is associated with hyperleptinemia, [152] resulting in a state of relative leptin resistance such that physiologic responses to exogenous leptin are blunted and ineffective at reducing adiposity. [137] A further complication of obesity is that weight reduction from an obese state is associated with a drop in leptin levels which is then perceived as a state of relative starvation, promoting weight gain. [89,207,218] Stated another way, the brain is relatively insensitive to rising levels of leptin but is exquisitely sensitive to reduced leptin levels. This can be considered an evolutionarily advantageous system as it allows for excess energy storage when resources are transiently available but drives feeding behavior under more limiting conditions. However, leptin signaling becomes maladaptive under modern circumstances when the availability of excess calories is constant and not transient.
Leptin may also affect the structure of CNS neuronal circuits. Leptin deficiency has pleiotropic effects on neuronal morphology and connectivity during development. Mice normally exhibit a large postnatal surge in circulating leptin independent of any metabolic effect which was suggested to be involved in postnatal brain development. [4,5] Indeed, the brains of leptin deficient (ob/ob) mice are smaller and have synaptic protein alterations, both of which are partially reversed by exogenous leptin treatment. [3] At this point in development, hypothalamic circuits are functionally and structurally immature. Leptin may regulate hypothalamic circuit development via neurotrophic signaling during this critical developmental period, and impaired leptin signaling results in long-term alterations in hypothalamic structure and function. [34,35,273] In considering the development of hypothalamic circuits in humans, the mouse brain is considerably less mature than the human brain at birth and the leptin-sensitive developmental period in humans is likely the last trimester of pregnancy. [50,130] Leptin is indeed detectable in fetal cord blood as early as 18 weeks of gestation with dramatic increases in leptin levels after 34 weeks gestation, although a “surge” in leptin has not been documented. [120] However, human congenital leptin deficiency is associated with neurocognitive deficits and radiologic structural abnormalities in multiple brain regions and changes in mesolimbic reward system activation, each of which can be reversed upon exogenous leptin treatment. [160,194,21,78]
Integration of Hormonal Signals: Hypothalamic Circuits
Although leptin receptors are widely expressed in neurons throughout the brain, leptin action on neurons within the arcuate nucleus of the hypothalamus is best understood (see Figure 2B). Two distinct populations of neurons are found within the arcuate. When leptin levels are low due to fasting, neurons expressing the orexigenic neuropeptides agouti-related protein (AGRP) and neuropeptide Y (NPY) are activated, with a concomitant inhibition of neurons coexpressing anorexic neuropeptides cocaine and amphetamine-related transcript (CART) and pro-opiomelanocortin (POMC). Arcuate neurons form synapses with multiple second-order neurons, including strong projections to several hypothalamic nuclei including the lateral hypothalamic area (LHA) and the paraventricular nucleus (PVN). LHA neurons express orexigenic neuropeptides (melanin concentrationg hormone and orexins) while PVN neurons express anorexic neuropeptides (corticotrophin-release hormone, thyrotropin-releasing hormone and oxytocin). Indeed, oxytocin PVN neurons that project to the hindbrain and spinal cord are particularly critical for controlling acute feeding behavior in mice. [18] Leptin’s effects on these hypothalamic circuits are neuromodulatory, in essence stimulating or repressing various neuronal circuits which regulate appetite and feeding behavior. For example, arcuate neurons convert POMC into alpha-melanocyte-stimulating hormone (αMSH) which binds to and activates melanocortin receptors. In contrast, AGRP is a potent antagonist of melanocortin receptors. Melanocortin receptors (MC3R and MC4R) are expressed on PVN neurons and stimulation of melanocortin receptors decreases appetite and feeding behavior. Thus the brain has evolved a mechanism whereby the relative balance of αMSH versus AGRP secretion on PVN neurons regulates appetite and feeding behavior.
The importance of the melanocortin pathway is highlighted by the fact that heterozygous mutations of MC4R are a surprisingly common cause of monogenic obesity with an estimated prevalence of ~1 in 1100. [81,11,249,271] The involvement of impaired “melaonocortin-tone” in the development of human obesity is further demonstrated by multiple reports of mutations in POMC associated with hyperphagia and obesity. [131,132,47] The hypothalamic circuitry which regulates appetite and feeding behavior is obviously more complex than presented here. Important extra-hypothalamic projections, which are discussed later in this article, include connections to more caudal brain areas such as the dorsal vagal complex within the medulla and to higher brain regions such as the mesolimbic reward system hippocampus and prefrontal cortex.
Abnormal Signal Detection: Bardet-Biedl Syndrome
Bardet-Biedl syndrome (BBS) is another example of a monogenic cause of obesity which is linked to the abnormal detection of peripheral signals. BBS is clinically heterogeneous but is associated with six core features: obesity, retinal dystrophy, renal abnormalities, polydactyly, learning disability and urogenital tract deficits. [98] BBS is a rare, generally autosomal-recessive disorder with a prevalence of ~1 in 160,000 in European populations which can increase to 1in 13,500 in certain populations with high consanguinity such as isolated areas of Canada and the Middle East. [84,103] Obesity affects 72–92% of BBS patients. [84] Even when comparing BBS patients to control subjects with a similar BMI, BBS patients exhibit higher fat mass and increased visceral fat. [82,97] Furthermore, heterozygous BBS carriers exhibit increased rates of obesity. [56] Thus far, mutations in 16 genes account for ~80% of BBS cases. BBS is the prototypic ciliopathy; all of the BBS proteins analyzed thus far localize to the basal body or the ciliary axoneme and BBS genes are restricted evolutionarily to ciliated species. [103] Cilia are thin projections found on virtually all mammalian cell types and play a critical role sensing of extracellular signals and transmitting these signals intracellularly to affect various cellular processes including gene transcription, cell division and cell differentiation. [224] Although reports of postmortem neuropathologic examination of BBS are scarce, [166] MRI imaging of BBS individuals has revealed various CNS abnormalities including empty sellae, cerebellar vermis hypoplasia, hippocampal dysgenesis, cortical dysplasia and cerebral and/or cerebellar atrophy. [8,23,26,29,123,210,225]
The dominant theory explaining obesity in BBS is abnormal leptin receptor signaling due to defective cilia. BBS patients are hyperleptinemic which is not surprising given that obesity leads to hyperleptinemia. [82] BBS mutant mice are also hyperleptinemic and obese, but also exhibit increased leptin levels even at an early age when body weights were equivalent to normal control mice, suggestive of a primary leptin signaling defect. [203] Furthermore, leptin resistance was observed in BBS mutant mice even after caloric restriction. [219] BBS mice also demonstrated hyperphagia, decreased locomotor activity, and a blunted response to exogenous leptin, all consistent with leptin resistance contributing to maintenance of obesity. [203] On a molecular level, BBS1 protein may interact directly with leptin receptor and regulate leptin receptor trafficking. [219] Interestingly, not all leptin-responsive pathways are equally affected by BBS mutations. For example, activation of the anorexigenic POMC gene is blunted while the expression of orexigenic AgRP and NPY genes are normal. [203,219] This is consistent with another study which showed that disruption of intraflagellar transport in POMC neurons leads to hyperphagia and obesity. [64] Others have argued that obesity seen in ciliopathies may not be primarily due to leptin receptor signaling defects per se, but rather more complex mechanisms, perhaps associated with other signaling pathways, neurodevelopmental defects or neurodegenerative processes. [30] Regardless, given the role of the primary cilium in detecting and integrating extracellular signals, BBS demonstrates that a molecular defect linked to signal detection in key neurons which regulate energy homeostasis can lead to obesity.
While we focused here on leptin-related signaling pathways, multiple hormonal signals including insulin, adiponectin and ghrelin are believed to directly modulate CNS neurons. [138] In particular, the hormone ghrelin is secreted by fundic cells in the stomach and is highest before meals and during periods of fasting, falling postprandially. Ghrelin binds to the growth hormone secretagogue receptor, a G protein-coupled receptor expressed by several neuronal populations including vagal afferents, the hypothalamic arcuate neurons, and neurons within the hypothalamic ventromedial nucleus. [22,102] Ghrelin thus serves as an orexigenic signal increasing appetite and feeding behavior, in many ways counter to the effects of leptin. [138]
Neural Signaling from the Periphery
Bariatric surgery (gastric bypass and gastric banding surgery) is a highly effective treatment for morbid obesity. The effectiveness of bariatric surgery is linked to effects on curbing hunger (i.e. promoting satiety), changes in metabolism and alterations in food preferences, many of which are dependent on the CNS. [135,136,204,31] Understanding the neural connections between the gastrointestinal system and the brain highlights the role of neural signaling from the periphery to the CNS in the development and treatment of obesity. While the primary role of the gastrointestinal tract is to digest and absorb nutrients, it also plays a role in energy homeostasis via mechanoreceptors and chemosensors which detect the amount and quality of food intake. Gastric distension leads to vagal stimulation due to the secretion of serotonin from gastric enterochromaffin cells or due to direct stimulation via stretch receptors. [100,138] The small intestine also responds to nutrients by secreting various satiety signals including cholecystokinin (CCK), peptide YY, serotonin, glutamate, enterostatin and glucagon like peptide-1. For example, CCK is a satiety hormone, but unlike leptin CCK does not act directly on the brain but rather has paracrine activity, binding to receptors on local vagal sensory afferent terminals. [100] Indeed, numerous gastrointestinal signals are integrated by vagal afferents and transmitted to the hindbrain, namely the medullary dorsal vagal complex and in particular the nucleus of the solitary tract (NTS, see Figure 2C). [100] Numerous projections from the NTS regulate peripheral metabolism and are related to obesity, including projections to the hypothalamus, mesolimbic reward areas and higher brain regions. One relatively simple circuit is a projection from the NTS to the visceral sensory thalamus which integrates gut signals and sends projections to the visceral sensory neocortex, resulting in the conscious feeling of fullness and satiety. [100,138]
With the sole exception of ghrelin, the net effect of gut-to-brain signaling is to inhibit short term food intake and limit meal size. [138] Notably, experimental models suggest that gut-to-brain signals are most important in the regulation of short term energy consumption. For example, CCK regulates short term feeding behavior in mice, but the absence of CCK signaling has no effect on long-term energy homeostasis. [129] Although the regulation of short-term energy intake via gut-brain signaling is considerably different from the long-term adipostatic pathways (the latter exemplified by leptin signaling), there is considerable cross-talk between forebrain and hindbrain pathways such that obesity likely involves dysregulation of both short-term and long-term homeostatic pathways. Indeed, human studies indicate that the inability to accurately estimate caloric intake by overweight individuals is due to large meal size. [258,147] Bariatric surgery is effective in part due to gut-brain signaling which promotes the perception of satiety, limiting meal size and calorie intake. [135,136] Consistent with this hypothesis is the fact that some types of bariatric surgery are associated with alterations in gut-brain hormones including markedly suppressed ghrelin levels, supporting the view that gut-brain signaling is at least in part responsible for the anti-obesity effects of bariatric surgery. [57,122,204] Of course, neurologic complications of bariatric surgery are well documented, often linked to nutritional deficiencies leading to Wernicke’s encephalopathy, polyneuropathies or other manifestations of nutritional deficiency. [1] There is no clear consensus as to which gut-brain signaling pathways, neural or humoral, are responsible for the efficacy of bariatric surgery. Rather, multiple pathways are probably acting in concert to improve energy homeostasis, alter food preferences and improve metabolic status.
Central Neuronal Circuits: Development and Degeneration
There are several developmental disorders linked with obesity including Prader-Willi syndrome (PWS). [46] PWS is a complex multisystem disorder characterized by several clinical features including excessive eating and morbid obesity unless feeding is restricted. Other clinical features include severe hypotonia in early infancy, motor and language developmental delay, behavioral problems, hypogonadism, short stature and mild to moderate intellectual disability. [46] PWS affects ~1 to 3 per 30,000 individuals and is linked to the loss of expression of paternal genes in chromosome 15q11.2-q13. [46] Several genes in this critical region are imprinted such that only the paternal gene is active, and disease is caused either by deletion of this region from the paternal chromosome (~65–75% of cases), maternal uniparental disomy of chromosome 15 (~20–30% of cases) or imprinting defects (i.e. abnormalities in the epigenetic imprinting process, which occurs in <3% of cases). [46] The clinical phenotype associated with obesity is due to insatiability linked to hypothalamic dysfunction. Although multiple mechanisms have been proposed for PWS eating behavior such as abnormalities in gut-brain signaling (in particular ghrelin signaling), [46,165] neuropathologic analysis of PWS brains identified several hypothalamic abnormalities which correlate well with many of the clinical phenotypes observed. [240,241] In particular, PWS patients have significantly fewer oxytocin-expressing neurons within the PVN. As mentioned already, AGRP neurons within the arcuate nucleus which are key for integration of peripheral hormonal signals project to oxytocin-expressing neurons in the PVN. In turn, these neurons project rostrally to the medulla and spinal cord, and central oxytocin potently inhibits feeding behavior. [32,242,13] The reduction in these oxytocin neurons in PWS was postulated to be the anatomic cause of overeating in PWS, [240,241] a hypothesis which is bolstered nearly two decades later by advanced optogenetic and pharmacogenetic approaches in mice which demonstrate the key role of oxytocin-expressing PVN neurons in the regulation of acute feeding behavior. [18]
A similar mechanism may account for cases of PWS-like hyperphagia and early-onset obesity which have been linked to mutations, deletions or translocations affecting the SIM1 gene. [117,238,257,7,255,93,76] Single-minded homolog 1 (Sim1) is a basic helix-loop-helix-PAS domain transcription factor that regulates gene expression in midline cells. [169,170] Mice lacking Sim1 die shortly after birth with hypocellular PVN and supraoptic nuclei including the loss of oxytocin-expressing neurons. [170] Mice with only one functional copy of Sim1 exhibit hypocellular PVNs, hyperphagia and obesity apparently in large part due to oxytocin deficiency. [169,133] Postnatal Sim1 haploinsufficiency also leads to hyperphagic obesity in part linked to decreased oxytocin expression despite an otherwise structurally normal PVN. [247] Thus, data from human neuropathology, human genetics and experimental mouse studies demonstrate that abnormal neurodevelopment of key neuronal circuits leads to obesity, highlighting the delicate control mechanisms whereby the brain regulates energy homeostasis.
On the other end of the spectrum of neuropathology, neurodegenerative diseases are also associated with obesity. For example, frontotemporal dementia (FTD) is associated with weight gain. FTD is the second most common dementia in individuals under 65 years of age and is characterized by executive or language dysfunction and progressive neurodegeneration preferentially affecting the frontal and temporal lobes. Many individuals with FTD exhibit hyperphagia with episodes of binge eating and may continue eating despite feeling full. [265] This suggests that overeating in FTD is not linked to dysfunction of satiety pathways per se, but rather due to dysfunctional reward circuits. Neuroanatomic analysis of these patients demonstrates that atrophy of the right orbitofrontal-insular-striatal circuit is closely associated with abnormal feeding behavior. [265] The peripheral signals discussed above (hormonal or vagal) are largely homeostatic signals that regulate short-term (acute feeding behavior) or long-term (adiposity) energy balance. For example, satiety is often linked to feelings of satisfaction and fullness. In contrast, hedonic responses to food are essentially nonhomeostatic driven by pleasure and palatability. Food reward is encoded in part by the mesolimbic reward system in which the ventral tegmental area of the midbrain sends dopaminergic projections to the limbic system via nucleus accumbens (ventral striatum), and involves multiple limbic and cortical regions such as the amygdala, hippocampus, medial prefrontal cortex and orbitofrontal cortex (see Figure 2D). In addition to FTD, these brain regions are implicated in multiple human diseases with feeding abnormalities including bulimia and obsessive-compulsive disorder. Another interesting disease is Gourmand syndrome which is caused by focal lesion such as trauma, stroke or tumor in the same brain regions that are linked to overeating in FTD, namely right anterior cortical, basal ganglia and limbic regions. [208] Post-injury, individuals with Gourmand syndrome exhibit a pathological preoccupation with food and fine dining. [208] Thus diverse developmental abnormalities (leptin deficiency, Prader-Willi, Sim1 deficiency) and degenerative diseases (FTD, Gourmand syndrome) affect appetite, satiety and food reward, highlighting central neuronal circuits which regulate energy intake. Disruption of these circuits leads to obesity due to insatiable appetite and constant overnutrition. More common forms of obesity are likely linked to similar dysfunction of appetite and food reward pathways due to the high prevalence of readily available and palatable foods. Indeed, neurosurgical intervention targeting the nucleus accumbens for deep brain stimulation has been suggested as a possible treatment for obesity. [107]
Central Intracellular Signaling Pathways: BDNF
In addition to central neuronal circuits, intracerebral signaling pathways are also linked to obesity. Brain-derived neurotrophic factor (BDNF) is produced and released from excitatory neurons throughout the brain in an activity-dependent manner. Best known for its critical roles in neuronal survival, synaptic plasticity, and learning and memory, BDNF also regulates energy intake and metabolism. [54,211] BDNF acts on hypothalamic PVN and VMH neurons to suppress appetite; BDNF may mediate the anorexigenic effects of MSH acting on the MCH-4 receptor. [267] BDNF may also act on neurons in the brainstem to regulate peripheral energy metabolism; as evidence, central infusion of BDNF increases peripheral insulin sensitivity, [179] and BDNF signaling in the brainstem enhances parasympathetic tone. [99] Some human inherited disorders that involve obesity are associated with deficits in BDNF levels or signaling. For example, patients with PWS exhibit reduced levels of plasma BDNF compared to control subjects. [109] Mutation of trkB, the high-affinity BDNF receptor, results in obesity in humans. [270] Patients with BDNF haploinsufficiency due to truncation of the region of chromosome 11 that contains the Bdnf gene are obese. [108] Reduced BDNF signaling may also contribute to the epidemic of obesity in industrialized countries where many people are sedentary and consume large amounts of energy-dense foods. Obese and diabetic mice exhibit reduced BDNF levels in the hippocampus and other bran regions, and associated deficits in learning and memory, synaptic plasticity and neurogenesis. [233,234] Exercise and intermittent fasting, which protect against obesity, increase BDNF levels and signaling in multiple brain regions. [163] Mice with a genetic BDNF haploinsufficiency are obese and insulin resistant, and exhibit impaired adaptive responses to exercise and intermittent fasting, including reduced neurogenesis. [140,70] Thus, BDNF plays critical roles in the regulation of body weight and reduced BDNF signaling may be involved in obesity resulting from both genetic and environmental factors.
Obviously, the brain is exquisitely tuned to monitor and, in turn, influence energy homeostasis. CNS diseases reveal some of the pathways which have evolved to regulate short term energy intake and long term energy stores (see Table I) including non-specific hypothalamic damage (tumors, infections, etc), monogenic causes of obesity (deficiencies of leptin, leptin receptor, MC4R, POMC, trkB, BDNF, BBS, SIM1), neurodevelopmental genetic syndromes associated with obesity (PWS) and neurodegenerative diseases (FTD, Gourmand syndrome). Furthermore, manipulation of peripheral to central neural signaling is a proven means to treat morbid obesity (bariatric surgery). These diverse disease processes reveal the brain as an integrator of peripheral signals through two main hubs, namely the hypothalamus in the forebrain and the dorsal medulla in the hindbrain. Together with anterior cerebral structures such as the mesolimbic reward system, the brain regulates peripheral metabolism (sympathetic tone), drives feeding behavior (appetite), regulates acute food intake (satiety) and dictates food choices (hedonism). Although not discussed extensively here, autonomic neural signaling is another way the CNS regulates peripheral metabolism. Thus the central regulation of energy homeostasis is characterized by integration, cross-communication and execution across numerous CNS circuits. In essence, understanding natural human diseases associated with obesity leads to an understanding of which process among many are critical in terms of maintaining energy homeostasis, ranging from specific cellular signaling defects to damage of particular brain regions.
IV. Effects of Obesity on the CNS
Emerging findings have revealed adverse effects of obesity on CNS structure and function outside of those brain regions which regulate energy homeostasis. While cerebrovascular disease can lead to observable neuropathologic changes within the CNS, neuropathologic changes due to obesity on a macropscopic or microscopic scale are subtle to absent. Perhaps a difficulty in defining these changes is that the effects of obesity on the brain are maladaptive (i.e. not evolutionary) and thus more difficult to dissect. Alternatively, changes due to obesity may be unperceptively progressive over many years or may not manifest until decades after the onset of obesity in susceptible individuals, making it difficult to link neuropathologic features with the obesity phenotype. However, the brain is a highly metabolic organ and so the expectation is that changes in metabolism due to obesity adversely affect the brain. The latter possibility is supported by recent brain imaging studies which have shown that obesity, insulin resistance and diabetes are associated with reduced volumes of hippocampus, prefrontal cortex and precuneus. [119,40,263] In addition to metabolic changes, hormonal changes are likely to alter CNS structure and function. Furthermore, obesity is associated with low-grade chronic inflammation in multiple organs, and so chronic activation of inflammatory pathways may also potentially affect the CNS. Although actual human neuropathologic data are scarce, this section will explore a select set of major neuropathologic conditions which may be influenced by obesity in terms of potential hormonal, metabolic and inflammatory pathways that may affect the human brain. The goal is to provide a framework for understanding how obesity may affect the human CNS such that future neuropathology studies can begin to address whether particular changes can be seen in brains of the obese.
Epilepsy: Metabolites and Hormones
Numerous studies have shown increased obesity rates in patients with seizure disorders, and many of these associations were assumed to be secondary to medication-related changes in metabolism or disability-associated decreases in physical activity. [33,118,213] Indeed, many effective anti-epilepsy drugs (AEDs) are known to alter metabolic pathways resulting in increased or decreased body weight. [33,118,213] Up to 10% of all individuals will have a seizure sometime during their lifetime, and millions are affected by chronic seizure disorders. [52,112,215,216] Epilepsy is a clinical syndrome characterized by recurrent spontaneous seizures in the context of hyperexcitable and hypersynchronous neuronal activity. Although epilepsy is often a pediatric disorder, seizures and epilepsy are increasingly affecting the elderly such that epilepsy incidence is now higher in the elderly relative to pediatric populations, concordant with the rise of chronic diseases such as obesity, diabetes and cerebrovascular disease. [52,112,215,216]
There is a well-known interaction between diet and epilepsy as ketogenic diets (high fat, low carbohydrate, adequate protein) have been used for refractory epilepsy for nearly a century. Various ketogenic diets have been proven clinically effective by randomized or blinded trials. [86,182,183] Ketogenic diets essentially shift metabolism towards the use of lipids (acetyl-CoA) to generate ketoacids and ketones which can be used by the CNS as an alternative to glucose. Under normal conditions, glucose is converted into energy via glycolysis to generate pyruvate which is shunted into the tricarboxylic acid (TCA) cycle. Ketone bodies, in contrast, bypass the glycolytic pathway and are shunted into the TCA cycle. The diet was formulated in the 1920s to mimic fasting which had been used to treat epilepsy since at least the time of Hippocrates ca. 400 BC. [260] During fasting, liver glycogen can be converted into glucose but is depleted within 12 to 14 hours, after which lipids are used to generate ketone bodies. [85] Thus, ketogenic diets mimic prolonged fasting due to the switch in fuel usage from glucose to ketone bodies but differ because caloric and protein intake is maintained. While the efficacy of ketogenic diets is likely linked to metabolic changes, there is no consensus as to the mechanism of action be it increased ketone bodies, decreased glucose or calorie availability, increased energy stores, altered mitochondrial function, increased glutathione, increased polyunsaturated fatty acids or other metabolic alteration. [186,85] Moreover, given the clinical heterogeneity and numerous molecular causes of epilepsy, the fact that the ketogenic diet is effective for a wide range of epilepsy syndromes suggests the ketogenic diet works through multiple complementary mechanisms. [110]
While many metabolic changes may occur as a consequence of epilepsy or AED usage, [33,118,213] one study has shown that the rates of obesity are higher in children at time of presentation before the use of AEDs. [63] Although causality is not established by such studies, the association between obesity and epilepsy suggests that obesity may prime the CNS for seizures. Consistent with the latter possibility, obese leptin receptor mutant mice and adiponectin-deficient mice on a high fat diet exhibit increased vulnerability of hippocampal neurons to seizure-induced degeneration. [139,231] Conversely, intermittent fasting can protect against seizure-induced memory impairment and neuronal degeneration in rats. [38] The mechanisms by which obesity endangers, while dietary energy restriction protects, neurons in epilepsy may involve opposite effects on adaptive cellular stress response pathways. Obesity and diabetes are associated with reduced expression of BDNF, and elevated levels of oxidative stress and pro-inflammatory processes in brain cells. [234,66] In contrast, intermittent fasting up-regulates neurotrophic (BDNF and FGF2), protein chaperones (HSP-70 and GRP-78) and antioxidant (HO-1) proteins, while suppressing production of pro-inflammatory cytokines (TNF, IL-1, IL-6). [14,68]
In addition to metabolic pathways, hormonal alterations may affect seizure threshold. Indeed, both leptin and ghrelin inhibit seizures and seizure-related neuropathology in mice, although under certain conditions leptin also appears to increase neural activity thereby decreasing the threshold for seizure. [17,19,72,104,150,189,220,268,141,187,188] The adipose hormone adiponectin also inhibits seizures and seizure-related neuropathology. [121,139] Supporting the potential modulatory effect of adiponectin is that PPARγ agonists which increase adiponectin expression protect against seizure or seizure-related damage. [2,164,239,272] In addition, the AED valproic acid alters PPARγ signaling, adiponectin expression and adiponectin receptor expression. [134,202,205] Taken together, these experimental studies suggest that seizure threshold, epilepsy and/or seizure-related damage may be modulated by peripheral hormones such as leptin, ghrelin and adiponectin, all of which are altered in the obese state.
Multiple Sclerosis: Inflammatory Pathways
Obesity is associated with more than a twofold increase in risk for multiple sclerosis (MS) in longitudinally followed cohorts. [175,174] However, only ~50% of MS patients are overweight or obese in cross-sectional studies which is similar to the general population. [156,155,124] This discrepancy highlights an important facet to obesity’s effect on the brain. Only obesity during late childhood and adolescence confers risk for MS as an adult, while birth weight or adult weight is not associated with increased risk. [175,174] Thus, obesity appears to be deleterious during a critical period during which susceptibility for disease is developing. Although the exact mechanism linking obesity and MS is not known, modulation of inflammation appears to account for some of this risk. MS is an idiopathic inflammatory disease characterized by adaptive autoimmunity resulting in targeting and destruction of myelin and neurodegeneration. Obesity is associated with chronic inflammation characterized predominantly by activation of the innate immune system within multiple organ systems including adipose tissue, blood vessels, the liver, the pancreas and muscle. [158,149] Activation of hypothalamic inflammatory pathways has also been observed to be both a cause and a consequence of obesity in experimental models, [42,128,144,173,275,246] and is associated with subtle neuroimaging changes within the hypothalamus of obese humans (mildly increased T2 signal) which raises the possibility of low-grade inflammation or gliosis. [246] Functional neuroimaging studies also have found dysfunctional activation of hypothalamic areas in obese humans, and these changes are partially corrected upon weight loss after bariatric surgery coincident with a more anti-inflammatory (increased interleukin-10 and interleukin-6) CSF profile. [250] Amazingly, inhibiting innate immunity pathways within the mouse hypothalamus results in reduced aging phenotypes and increased longevity, possibly through a modulation of gonadotropin-releasing hormone levels. [274] While obesity is generally associated with increased innate immunity (non-specific immunity via phagocytes, macrophages, neutrophils, dendritic cells, basophils, mast cells, eosinophils, natural killer cells), MS is an autoimmune disease with a directed immune response linked to abnormal activation of the adaptive immune system. However, these two arms of immunity are not entirely separable and there is considerable evidence of cross-regulation consistent with obesity causing changes in both innate and adaptive immunity. [92,149,158]
What mechanisms may account for the association between obesity and MS? Vitamin D intake and serum 25-hydroxyvitamin D (25(OH)D) levels are protective against MS in humans, hypovitaminosis D is a risk factor for MS in humans, and increased serum 25-(OH)D protects against experimental models of MS. [177,178,176,199,226,142,45] Obesity is associated with reduced vitamin D and body fat is inversely correlated to 25(OH)D. [28,146,266,53,115,209,10,15] These observations are cogent given that vitamin D has immunomodulatory functions and that the protective effects of vitamin D in experimental MS models have been related to immunologic changes. [113,180,181,195,227–229] Leptin has also been postulated to play a modulatory role in MS as leptin is known to act on multiple immune cell types including CD4+, CD8+, and regulatory T-cells which express the long signaling-competent form of leptin receptor. [65] Humans with congenital leptin deficiency exhibit several immune deficiencies including impaired cellular and cytokine immune responses which are reversed by exogenous leptin. [80] Furthermore, leptin deficient ob/ob mice are resistant to experimental auto-immune encephalomyelitis (EAE) but become susceptible upon leptin treatment due to enhancement of autoimmune T-cell responses. [159] MS patients have increased serum and CSF leptin levels which correlate with interferon-γ production and decreased numbers of regulatory T-cells, [157] Moreover, leptin induces inflammatory cytokine release from peripheral blood mononuclear cells from relapsing MS patients but not from stable patients or normal controls, [87] and leptin receptor expression and signaling is increased in CD8+ T-cells and monocytes from relapsing MS patients compared to stable patients or normal controls. [88] Together with other inflammatory cytokines, obesity may increase the risk for MS through modulation of immune function leading to increased autoimmune susceptibility.
Alzheimer’s disease: The Rise and Fall of Weight
The relationship between body weight and Alzheimer’s disease (AD) is complex in that there are age-dependent changes in body weight in individuals with dementia. [238] AD is a progressive neurodegenerative disease and the most common cause of dementia responsible for tremendous physical, psychological and financial burden. The neuropathology of AD is characterized by neuron loss, gliosis, amyloid plaques and neurofibrillary tangles. AD is associated with decreased body weight often presumed to be due to malnutrition leading to a negative energy balance. [137] However, the loss of body weight may be linked to disease pathogenesis as reductions in body weight in the elderly appears to precede onset of dementia, and increases the subsequent risk for dementia. [25,41,185,232] Low BMI is associated with reduced CSF levels of amyloid-β peptide, increased CSF levels of tau protein, and increased numbers of neurofibrillary tangles and amyloid plaques. [75,254] Caution is warranted because BMI may not be an accurate measure of adiposity in elderly populations, and the weight loss in AD may be due to other processes such as sarcopenia and not necessarily linked to reductions in fat mass. [44] The exact mechanisms linking weight loss and AD are not known and multiple processes are likely involved. Lower body weight appears to be primarily associated with decreased dietary intake and altered feeding behavior as opposed to increased energy expenditure. [94] These changes are related to physiologic and behavioral changes as the disease progresses and with factors related to caregiver burden. Given that limbic brain regions regulate feeding behavior, it is not surprising that medial temporal cortex atrophy is associated with low body weight. [101] Since pathologic changes in AD initiate within the medial temporal cortex, limbic dysfunction in the presymptomatic phase of AD may be one factor leading to weight loss prior to clinical dementia. Other factors which may affect energy balance in AD include changes in NPY secretion, elevated inflammatory cytokines such as TNFα and the pharmacologic effects of anticholinesterase inhibitors. [94]
In contrast, mid-life obesity is associated with increased risk for late-life AD by multiple epidemiologic studies (hazards or odds ratios ~1.4 to 3.6). [238] In as much as such factors are separable, the risk due to obesity is reported to be independent of the risk due to diabetes or vascular disease. Given the protracted nature of these studies, groups were defined based on clinical diagnosis and no clinicopathologic studies have been performed correlating AD neuropathology with mid-life obesity. The importance of clinicopathologic studies is reflected in the literature for diabetes and AD neuropathology. Although diabetes is a known risk factor for Alzheimer’s-type dementia, the mechanisms responsible for this risk are unknown and may not be specific to AD. The Religious Orders Study of 233 elderly Catholic clergy found that a clinical history of diabetes was not associated with AD neuropathology as determined by multiple histopathologic metrics for plaques and tangles, but rather was associated with increased cerebrovascular disease. [16] Multiple studies have found diabetes was associated with no differences in AD-specific pathologies or with reduced AD-specific pathology. [9,27,114] Several autopsy studies found an association between diabetes and AD neuropathology (plaques and tangles) amongst APOE E4 carriers but not in the general cohort. [153,161,197]. These clinicopathologic series demonstrate multiple difficulties in understanding the interaction between metabolism and neurologic diseases. Thus an important question which remains unanswered is whether obesity increases the risk for AD neuropathology (amyloid plaques and neurofibrillary tangles) versus other pathology such as vascular disease as this can help guide further mechanistic studies. The possibility exists that obesity acts in the same pathways which lead to amyloid plaques and neurofibrillary tangles, or act on largely coincident pathways such as cerebrovascular-mediated damage, or may act synergistically with AD pathways such that the CNS is especially vulnerable to AD processes.
AD and Obesity: Genetic Associations
Large scale genome-wide association studies have now demonstrated multiple polymorphisms linked to both obesity and AD. For obesity, multiple rounds of genome-wide association studies and meta-analyses have been performed including several large-scale studies from the GIANT consortium (Genetic Investigation of Anthropometric Traits) and deCODE as reviewed elsewhere. [77] Numerous single nucleotide polymorphisms (SNPs) have been found associated with obesity or related traits. Overall, no obvious biological pathway or mechanism has emerged from these data, although many of the genes are highly expressed within the brain consistent with the central role of the CNS in regulating energy homeostasis including genes known to be hypothalamic regulators of energy homeostasis including MC4R, POMC, SH2B1 and BDNF. [261,77,230] Overall, the 32 confirmed loci linked to BMI account for only 1.45% of inter-individual variation. [230] Thus most of the heritability of obesity is yet unaccounted for and awaits additional studies which evaluate gene x environment interactions, copy number variations or other genetic changes, epigenetic modifications, or large effects due to low frequency or rare SNPs which may not be represented in current genome-wide association studies. The SNP associated with the greatest effect on BMI is an intronic SNP within the FTO gene, accounting for ~0.34% of BMI variance. [230] The exact function of the protein is not known, but FTO is expressed widely throughout the brain including the hypothalamus. [91,167] Loss of Fto in mice leads to post-natal growth retardation, reduced adipose tissue and reduced lean mass, while overexpression leads to increased body and fat mass. [48,49,83] Interestingly, the FTO SNP is associated with globally reduced brain volume in both adolescent and elderly humans suggesting that FTO is associated with neurodevelopmental changes. [116,168] Whether these structural MRI changes are associated with increased risk for dementia or AD is not known.
Genetic risk for AD has also been assessed with large scale genome-wide association studies. [217] These studies have confirmed that APOE polymorphism is a major risk for AD as first described using more traditional linkage analyses in 1991. [198,236] This gene encodes apolipoprotein E (ApoE) which is a multifunctional protein best known for its role in lipid metabolism and transport. Subsequently, genome-wide association studies have identified 11 SNPs associated with AD risk including at least four that are related to lipid metabolism including APOE, CLU (clusterin, also known as apolipoprotein J), SORL1 (sortilin-related receptor) and ABCA7 (ABC transporter member 7). [217] An additional three SNPs are associated with genes involved in innate immunity including CR1 (complement receptor type 1), CD33 (cluster of differentiation 33 which is expressed by myeloid cells and monocytes), and the MS4A4A/MS4A4E/MS4A6E locus (part of a cluster of 15 MS4A genes with homology to the B-lymphocyte surface marker CD20 but expressed on myeloid cells and monocytes). [217] Accepting that innate immunity is intimately linked to obesity, the vast majority of SNPs associated with AD are at least conceptually connected to obesity or metabolism.
AD and obesity: Lipids
The regulation of central lipids is highly complex as lipids play important biological roles ranging from cellular structure to intracellular signaling. Indeed, the concentration of lipids within the CNS is second only to adipose tissue. There are three common variants of ApoE, ε2, ε3, and ε4, of which the ε4 allele is associated with increased AD risk, the ε3 allele is neutral and the ε2 allele is associated with reduced AD risk. [217] ApoE is one component of chylomicron and intermediate-density lipoproteins and is the main CNS cholesterol transport protein. Thus, altered cholesterol transport has been suggested as one mechanism linked to ApoE ε4 risk, particularly given the key role of cholesterol in cellular membrane maintenance including synaptic structure. We have found that atherosclerosis of the circle of Willis as a marker of chronic dyslipidemia correlates with neurodegenerative disease pathology, a result seen in many but not all autopsy series. [269] More specifically, a higher proportion of AD subjects had grossly apparent atherosclerosis compared to normal or other neurodegenerative disease subjects, and atherosclerosis ratings correlated with amyloid plaque and tau pathology. [269] While it is tempting to associate dyslipidemia with AD pathogenesis, alternative mechanisms are entirely possible including differential cerebrovascular perfusion of the CNS. Similarly, epidemiologic and experimental studies in general support the hypothesis that high cholesterol exacerbates AD pathogenesis. However, statins have a variable to absent clinical effect in human trials, although issues surrounding clinical trial design and blood-brain-barrier penetration may have confounded some trials. [222,221]
Two other hypotheses have garnered favor regarding ApoE and AD pathogenesis, both related to the Aβ peptide. First, ApoE has been proposed to act as a chaperone to the hydrophobic Aβ peptide with the ε4 variant promoting Aβ fibrillogenesis. [125] Second, ApoE was thought to bind to the Aβ peptide inhibiting its clearance from the extracellular space, recent data suggests that under more physiologic conditions, ApoE and Aβ compete for the low-density lipoprotein receptor-related protein (LRP1) which is a cell surface receptor expressed on astrocytes and is involved in clearance and degradation of soluble Aβ peptide. [214,253] Regardless of whether ApoE directly or indirectly affects Aβ clearance from the extracellular space, most human brain analyses have shown that APOE ε4 carriers have increased amyloid burden, biomarker studies have shown that CSF Aβ is reduced in APOE ε4 carriers, and neuroimaging with fibrillar Aβ agents show increased Aβ deposition in APOE ε4, all supporting the hypothesis that APOE mediated risk is linked to Aβ accumulation or clearance. [125]
Finally, the amino acid sequences among the three APOE isoforms differ only in residues 112 and 158, with APOE ε2 having cysteines at both positions, APOE ε3 having a cysteine at position 112 and an arginine at position 158, and APOE ε4 having arginines at both positions. The lipid peroxidation product 4-hydroxynonenal covalently modifies cysteine residues and is implicated in the pathogenesis of Aβ- and Tau-associated neuropathology in AD. [105,154] APOE ε2 is an effective scavenger of 4-hydroxynonenal, whereas APOE ε3 is less effective and APOE ε4 is ineffective, suggesting that the increased risk for AD in APOE ε4 carriers may result from reduced detoxification of 4-hydroxynonenal. [196]
While the mechanisms linking the other lipid-related genes to AD are obscure, SorLA is a lipoprotein receptor that binds ApoE while clusterin is another lipoprotein found both peripherally and centrally. [217] The function of ABCA7 is not clear; it may be involved in phagocytosis or regulate phospholipid or cholesterol efflux, and loss of Abca7 in mice promotes amyloid plaque accumulation. [217,126] Clearly, lipid homeostasis plays a role in the pathogenesis of AD and it remains to be determined how obesity alters these pathways. Although cholesterol has been mentioned specifically thus far, a variety of additional lipid species have been suggested to play a role in both obesity and AD including phospholipids, isoprostanes, endocannabinoids, lipid aldehydes such as 4-hydroxynonenal and acrolein, sphingomyelins, ceramides and essential long-chain polyunsaturated fatty acids such as docosahexaenoic acid. [59,162] The potential links between obesity and AD are not limited to changes in lipid homeostasis. As is common from chronic multifactorial diseases, both obesity and AD are associated with changes in many domains. Thus inflammation (in particular activation of innate immunity), dysfunctional hormonal signaling (insulin, leptin), impaired neurogenesis, altered epigenetic programming and altered neuronal excitability are all processes which potentially link obesity and AD. While each of these pathways cannot be explored in detail here, there are many commonalities between obesity and AD such that many of these changes are likely to act in concert to adversely affect CNS structure and function. While not all pathways may lead to increased amyloid plaques or tangles, there is strong epidemiologic evidence supporting the maladaptive effects of obesity on the aging brain.
In summary, several neurologic diseases appear to be modulated by obesity (see Table I), with a few prototype examples presented here (epilepsy, MS and AD). While other neurologic diseases are closely associated with obesity (such as idiopathic intracranial hypertension), obesity may affect the pathogenesis of many neurologic diseases in yet undiscovered ways. As shown for both MS and AD, identifying these correlations in human populations can be confounded by age-dependent critical periods and significant time-delays in terms of measurable effects of obesity on disease. Furthermore, the mechanisms linking obesity to these chronic diseases are not entirely understood. However, the above discussion on epilepsy, MS and AD broadly demonstrates that obesity is associated with distinct changes in the metabolic, hormonal and inflammatory milleu which together negatively impact the human brain. Clearly, additional experimental, clinicopathologic and intervention studies are needed to better understand which pathways are particularly relevant to either the pathogenesis or cure of these human neurologic diseases.
V. Impact of Anti-Obesity Interventions on Brain Stucture and Function
In most cases, obesity can be prevented and reversed by adherence to a program of regular exercise and reduced energy intake. This fact provides the opportunity to determine, by evaluating brain structure and function in longitudinal studies of the same individuals, how the brain is affected by obesity and interventions that prevent/reverse obesity. While the emphasis here is on human studies, we will briefly summarize salient finding from animal studies that have elucidated cellular and molecular mechanisms by which exercise and energy intake affect brain structure and function. An in-depth review of this topic was recently published. [163] Running wheel exercise can increase synapse density, stimulate neurogenesis, and suppress inflammation in the hippocampus of rats and mice. [193,234,252] Cognitive function in several domains is improved by exercise, including spatial pattern separation, a process fundamental to most if not all aspects of cognition. [55] Caloric restriction (CR; a reduction in calories without a reduction in meal frequency) and intermittent fasting (IF; a reduction in meal frequency without an increase of meal size) each reduce markers of oxidative stress and inflammation in multiple brain regions. [14] IF can also increase the survival of neurons arising from stem cells in the hippocampus, [140] and can preserve function of neurons in animal models of AD, Parkinson’s disease and Huntington’s diseases. [67,69,106] The mechanisms by which IF promotes neuronal plasticity and resistance to injury and disease involves stimulation of the production of FGF2 and BDNF, protein chaperones and antioxidant enzymes. [14] Collectively, the results of animals demonstrate that two interventions that prevent and reverse obesity enhance neuroplasticity and can protect the brain against injury and age-related neurodegenerative disorders.
Studies of humans have shown that exercise can improve cognitive and motor function, by mechanisms involving changes in brain structure and neuronal network activity. In a cross-sectional study of elderly subjects it was found that higher levels of aerobic fitness are associated with greater hippocampal volumes and with better spatial memory. [73] Another study found that older adults with higher levels of fitness exhibit preserved hippocampal volume and fewer episodes of forgetting compared to age-matched subjects who are less fit. [244] A 6 month exercise intervention resulted in improved episodic memory and increased gray matter volumes in the prefrontal cortex and cingulate of elderly subjects. [212] In a randomized interventional study of older adults, regular aerobic exercise significantly increased the size of the anterior hippocampus, improved spatial memory ability, and increased levels of BDNF in the serum. [74] However, another study found no effect of exercise on serum BDNF [243], and there have as yet been no studies in which the effect of exercise on brain/CSF BDNF levels were measured. Magnetic resonance spectroscopy analysis revealed higher concentrations of N-acetyl-aspartate and choline in the brains of middle-aged endurance athletes compared to less fit control subjects, indicating that aerobic exercise enhances metabolic efficiency and neurotrophic signaling. [96]
Interventional studies of the effects of energy intake on brain structure and function, and vulnerability to neurodegenerative disorders, in humans are very limited. Performance on tests of verbal memory improved significantly in elderly human subjects that had been maintained for 3 months on a CR diet (30% reduction in daily calorie intake). [264] Improvements in memory capacity were associated with reductions in plasma levels of insulin and C-reactive protein (a marker of inflammation/oxidative stress). In a comparison of rhesus monkeys that had been maintained on either an ad libitum diet or a 30% CR diet for more than 10 years it was found that CR results in enhanced motor function, greater amounts of gray matter in the parietal and frontal cortices, and greater hippocampal volumes. [262] In another study of non-human primates, lemurs maintained for 18 months on a 30% CR diet exhibited superior performance in a spontaneous alternation test of learning and memory. [62] More than 2000 years ago Romans discovered that fasting can inhibit epileptic seizures; of course at that time it was not recognized that epilepsy was a disease, and instead it was believed that affected individuals were possessed by ‘demons’. [245] Today, fasting can be as effective as or even more effective than drugs in suppressing seizures. [111] The mechanism underlying this therapeutic action of fasting involves the generation of ketones, which can suppress seizures [151], and the production of neurotrophic factors. [68] Future studies of human subjects will clarify the cellular and molecular mechanisms by which obesity, exercise and energy intake influence brain energy metabolism and function in the contexts of aging and disease.
VI. Conclusions
Human diseases can provide insights into basic biological processes. In this review, we presented greater than a dozen human diseases which either promote obesity or are modulated by obesity. From these diseases, one can build an understanding of the CNS as the master regulator of energy homeostasis in which two signaling hubs integrate and transmit signals either directly to peripheral organs to regulate metabolism or to higher brain regions that regulate feeding behavior. Abnormalities ranging from cellular signaling defects to destruction of neuronal circuits lead to a chronic imbalance in energy homeostasis and obesity. Furthermore, treatments based on these specific pathways have been shown effective for both rare and common forms of human obesity. The net effect of obesity is difficult to measure as many factors and pathways are altered. However, obesity appears to be associated with increased risk for several chronic neurologic diseases. Although the mechanisms underlying this risk are not entirely elucidated, it appears that global alterations in metabolites, hormones or inflammatory mediators can negatively impact the CNS and promote disease. What remains to be learned is which of these pathways are particularly harmful, or whether interventions based on these pathways will prove beneficial. This review attempts to show that the description of human disease can help guide our understanding of disease mechanisms. However, the final tome on the neuropathology of obesity has yet to be written and will require new observations and insights based on the integration of experimental studies with human physiology, genetics and neuropathology.
Figure 3. Energetic challenges (exercise and energy restriction) counteract adverse effects of obesity on brain structure and function.
Obesity most commonly results from chronic excessive energy intake and a sedentary lifestyle, a situation in which the brain (and body) do not experience energetic challenges. As a result, signaling pathways involved in neuroplasticity and stress resistance are not engaged, resulting in low levels of synaptic plasticity and neurogenesis, and increased vulnerability to degeneration. Exercise and dietary energy restriction (caloric restriction and intermittent fasting) stimulate the production of neurotrophic factors such as BDNF and FGF2, and enhance neuronal bioenergetics so as to promote neuronal plasticity and protect neurons against injury and disease. The inset at the lower left shows three dentate granule neurons with their dendrites receiving synapses from the entorhinal cortex. Neurons and stem cells in this region of the hippocampus play important roles in learning and memory, are highly responsive to exercise and energy intake, and are adversely affected by obesity. In contrast, obesity is associated with alterations in hormones, metabolites and inflammatory mediators which adversely affect brain structure and function. Adapted from Stranahan and Mattson (2012).
Table 1.
Obesity-associated human neuropathologic conditions
| Disease | Description |
|---|---|
| Hypothalamic obesity | Structural damage to medial hypothalamus region due to tumor, infection or other pathology. |
| Cushing’s disease | ACTH secreting pituitary adenoma leading to hormonal alterations and truncal obesity. |
| Leptin deficiency | Congenital absence of leptin leading to severe obesity and other neuroendocrine abnormalities |
| Leptin receptor deficiency | Mutation of leptin receptor leading to severe obesity and other neuroendocrine abnormalities |
| MC4R deficiency | Mutation of MC4R decresing melanocortin signaling leading to obesity. |
| POMC deficiency | Mutation of POMC altering melanocortin signaling leading to obesity. |
| Bardet-Biedl Syndrome | Mutations in genes related to the primary cilium altering cellular signaling leading to obesity. |
| Prader-Willi syndrome | Loss of expression in 15p11.2-q13 due to genetic mutaiton or imprinting defects leading to abnormal hypothalamic development including oxyt.cin neurons within the PVN leading to insatiability/hyperphagic obesity. |
| SIM1 deficiency | Mutation of SIM1 causing abnormal hypothalamic development including oxytocin neurons within the PVN leading to hyperphagic obesity. |
| Frontotemporal degeneration | Progressive degeneration of frontal and temporal lobes including mesolimbic reward circuits leading to hyperphagia and weight gain. |
| Gourmand’s syndrome | Focal lesions such as stroke or trauma involving the mesolimbic reward system leading to pathologic obsession with food and fine dining. |
| BDNF deficiency | Mutation of BDNF receptor, trkB, or BDNF haploinsufficiency associated with obesity. |
| Epilepsy | Chronic seizure disorder associated with obesity in children at time of presentation. Ketogenic diets are effective against epilepsy. |
| Multiple sclerosis | Obesity during late childhood or early adolescence associated with increased risk for multiple sclerosis as an adult. |
| Alzheimer’s disease | Mid-life obesity associated with incresaed risk for dementia including Alzheimer’s disease. Elderly weight loss associated with subsequent Alzheimer’s disease. |
| Idiopathic intracranial hypertension | Strongly associated with obesity, particularly in young women |
| Cerebrovascular disease | Associated with obesity and dyslipidemia. |
Acknowledgments
EBL is funded in part by grants from the National Institutes of Health (AG039510, DK19525, AG010124) and MPM is funded in part by the Intramural Research Program of the National Institute on Aging. EBL thanks Dr. Rexford Ahima for useful discussions in developing this review.
Bibliography
- 1.Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248 (5):714–720. doi: 10.1097/SLA.0b013e3181884308. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah DM. Anticonvulsant potential of the peroxisome proliferator-activated receptor gamma agonist pioglitazone in pentylenetetrazole-induced acute seizures and kindling in mice. Brain Res. 2010;1351:246–253. doi: 10.1016/j.brainres.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140 (6):2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept. 2000;92 (1–3):1–7. doi: 10.1016/s0167-0115(00)00142-7. [DOI] [PubMed] [Google Scholar]
- 5.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101 (5):1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382 (6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 7.Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Martin J, Hebert S, Doelle H, Ersoy B, Kryukov G, Schmidt S, Yosef N, Ruppin E, Sharan R, Vaisse C, Sunyaev S, Dent R, Cohen J, McPherson R, Pennacchio LA. Medical sequencing at the extremes of human body mass. Am J Hum Genet. 2007;80 (4):779–791. doi: 10.1086/513471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksoy A, Karaguzel G, Akbulut U, Turk A. Two sisters with Bardet-Biedl syndrome: brain abnormalities and unusual facial findings. Turk J Pediatr. 2011;53 (4):460–463. [PubMed] [Google Scholar]
- 9.Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H. Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol. 2009;35 (1):60–68. doi: 10.1111/j.1365-2990.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 10.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57 (2):183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Alharbi KK, Spanakis E, Tan K, Smith MJ, Aldahmesh MA, O’Dell SD, Sayer AA, Lawlor DA, Ebrahim S, Davey Smith G, O’Rahilly S, Farooqi S, Cooper C, Phillips DI, Day IN. Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Hum Mutat. 2007;28 (3):294–302. doi: 10.1002/humu.20404. [DOI] [PubMed] [Google Scholar]
- 12.Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77 (2):323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 13.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48 (6):825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 14.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Annals of neurology. 2010;67 (1):41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88 (1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 16.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67 (11):1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 17.Aslan A, Yildirim M, Ayyildiz M, Guven A, Agar E. Interaction of leptin and nitric oxide pathway on penicillin-induced epileptiform activity in rats. Brain Res. 1321:117–124. doi: 10.1016/j.brainres.2010.01.054. S0006-8993(10)00208-8 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488 (7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayyildiz M, Yildirim M, Agar E, Baltaci AK. The effect of leptin on penicillin-induced epileptiform activity in rats. Brain Res Bull. 2006;68 (5):374–378. doi: 10.1016/j.brainresbull.2005.09.012. S0361-9230(05)00412-0 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Babinski JP. Tumor of the body of the pituitary without acromegaly and with arrest of development of the genital organs. 1900. Obes Res. 1993;1 (4):332–333. doi: 10.1002/j.1550-8528.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 21.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104 (46):18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey AR, von Engelhardt N, Leng G, Smith RG, Dickson SL. Growth hormone secretagogue activation of the arcuate nucleus and brainstem occurs via a non-noradrenergic pathway. J Neuroendocrinol. 2000;12 (3):191–197. doi: 10.1046/j.1365-2826.2000.00398.x. [DOI] [PubMed] [Google Scholar]
- 23.Baker K, Northam GB, Chong WK, Banks T, Beales P, Baldeweg T. Neocortical and hippocampal volume loss in a human ciliopathy: A quantitative MRI study in Bardet-Biedl syndrome. Am J Med Genet A. 2011;155A (1):1–8. doi: 10.1002/ajmg.a.33773. [DOI] [PubMed] [Google Scholar]
- 24.Barinaga M. “Obese” protein slims mice. Science. 1995;269 (5223):475–476. doi: 10.1126/science.7624769. [DOI] [PubMed] [Google Scholar]
- 25.Barrett-Connor E. The menopause, hormone replacement, and cardiovascular disease: the epidemiologic evidence. Maturitas. 1996;23 (2):227–234. doi: 10.1016/0378-5122(95)00975-2. [DOI] [PubMed] [Google Scholar]
- 26.Baskin E, Kayiran SM, Oto S, Alehan F, Agildere AM, Saatci U. Cerebellar vermis hypoplasia in a patient with Bardet-Biedl syndrome. J Child Neurol. 2002;17 (5):385–387. doi: 10.1177/088307380201700514. [DOI] [PubMed] [Google Scholar]
- 27.Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC, Haroutunian V. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60 (4):471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76 (1):370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennouna-Greene V, Kremer S, Stoetzel C, Christmann D, Schuster C, Durand M, Verloes A, Sigaudy S, Holder-Espinasse M, Godet J, Brandt C, Marion V, Danion A, Dietemann JL, Dollfus H. Hippocampal dysgenesis and variable neuropsychiatric phenotypes in patients with Bardet-Biedl syndrome underline complex CNS impact of primary cilia. Clin Genet. 2011;80 (6):523–531. doi: 10.1111/j.1399-0004.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- 30.Berbari NF, Pasek RC, Malarkey EB, Yazdi SM, McNair AD, Lewis WR, Nagy TR, Kesterson RA, Yoder BK. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1210192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav. 2011;105 (1):106–119. doi: 10.1016/j.physbeh.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520 (1):6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology. 2001;56 (2):172–177. doi: 10.1212/wnl.56.2.172. [DOI] [PubMed] [Google Scholar]
- 34.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304 (5667):108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 35.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7 (2):179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bray GA. Commentary on classics of obesity. 4. Hypothalamic obesity. Obes Res. 1993;1 (4):325–328. doi: 10.1002/j.1550-8528.1993.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA. Harvey Cushing and the neuroendocrinology of obesity. Obes Res. 1994;2 (5):482–485. doi: 10.1002/j.1550-8528.1994.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Annals of neurology. 1999;45 (1):8–15. [PubMed] [Google Scholar]
- 39.Bruch H. The Frohlich syndrome: report of the original case. 1939. Obes Res. 1993;1 (4):329–331. doi: 10.1002/j.1550-8528.1993.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 40.Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–194. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65 (6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 42.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2013;521 (6):1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89 (4):547–551. doi: 10.3171/jns.1998.89.4.0547. [DOI] [PubMed] [Google Scholar]
- 44.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67 (4):428–433. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93 (15):7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14 (1):10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 47.Challis BG, Pritchard LE, Creemers JW, Delplanque J, Keogh JM, Luan J, Wareham NJ, Yeo GS, Bhattacharyya S, Froguel P, White A, Farooqi IS, O’Rahilly S. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11 (17):1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- 48.Church C, Lee S, Bagg EA, McTaggart JS, Deacon R, Gerken T, Lee A, Moir L, Mecinovic J, Quwailid MM, Schofield CJ, Ashcroft FM, Cox RD. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet. 2009;5 (8):e1000599. doi: 10.1371/journal.pgen.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Bruning JC, Nolan PM, Ashcroft FM, Cox RD. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42 (12):1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105 (1):7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 51.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392 (6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 52.Cockerell OC, Eckle I, Goodridge DM, Sander JW, Shorvon SD. Epilepsy in a population of 6000 re-examined: secular trends in first attendance rates, prevalence, and prognosis. J Neurol Neurosurg Psychiatry. 1995;58 (5):570–576. doi: 10.1136/jnnp.58.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34 (11):2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 54.Cordeira J, Rios M. Weighing in the role of BDNF in the central control of eating behavior. Mol Neurobiol. 2011;44 (3):441–448. doi: 10.1007/s12035-011-8212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107 (5):2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Croft JB, Morrell D, Chase CL, Swift M. Obesity in heterozygous carriers of the gene for the Bardet-Biedl syndrome. Am J Med Genet. 1995;55 (1):12–15. doi: 10.1002/ajmg.1320550105. [DOI] [PubMed] [Google Scholar]
- 57.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346 (21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 58.Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). 1932. Obes Res. 1994;2 (5):486–508. doi: 10.1002/j.1550-8528.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 59.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101 (7):2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogden Cynthia L, Carroll Margaret D, Kit Brian K, Flegal Katherine M. Prevalence of Obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–7. [PubMed] [Google Scholar]
- 61.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360 (15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dal-Pan A, Pifferi F, Marchal J, Picq JL, Aujard F. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6 (1):e16581. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniels ZS, Nick TG, Liu C, Cassedy A, Glauser TA. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology. 2009;73 (9):658–664. doi: 10.1212/WNL.0b013e3181ab2b11. WNL.0b013e3181ab2b11 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17 (18):1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26 (2):241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011;25 (6):1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100 (5):2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76 (2):619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 69.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57 (2):195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 70.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elia M, Folmer P, Schlatmann A, Goren A, Austin S. Carbohydrate, fat, and protein metabolism in muscle and in the whole body after mixed meal ingestion. Metabolism. 1988;37 (6):542–551. doi: 10.1016/0026-0495(88)90169-2. [DOI] [PubMed] [Google Scholar]
- 72.Erbayat-Altay E, Yamada KA, Wong M, Thio LL. Increased severity of pentylenetetrazol induced seizures in leptin deficient ob/ob mice. Neurosci Lett. 2008;433 (2):82–86. doi: 10.1016/j.neulet.2007.12.051. S0304-3940(07)01327-4 [pii] [DOI] [PubMed] [Google Scholar]
- 73.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19 (10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108 (7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ewers M, Schmitz S, Hansson O, Walsh C, Fitzpatrick A, Bennett D, Minthon L, Trojanowski JQ, Shaw LM, Faluyi YO, Vellas B, Dubois B, Blennow K, Buerger K, Teipel SJ, Weiner M, Hampel H. Body mass index is associated with biological CSF markers of core brain pathology of Alzheimer’s disease. Neurobiol Aging. 2012;33 (8):1599–1608. doi: 10.1016/j.neurobiolaging.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faivre L, Cormier-Daire V, Lapierre JM, Colleaux L, Jacquemont S, Genevieve D, Saunier P, Munnich A, Turleau C, Romana S, Prieur M, De Blois MC, Vekemans M. Deletion of the SIM1 gene (6q16.2) in a patient with a Prader-Willi-like phenotype. J Med Genet. 2002;39 (8):594–596. doi: 10.1136/jmg.39.8.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 78.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317 (5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341 (12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 80.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110 (8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farooqi IS, O’Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- 82.Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E, Zheng YC, Caruso RC, Brooks BP, Johnston JJ, Yanovski JA, Biesecker LG. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J Clin Endocrinol Metab. 2011;96 (3):E528–535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458 (7240):894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 84.Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21 (1):8–13. doi: 10.1038/ejhg.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv Pediatr. 2010;57 (1):315–329. doi: 10.1016/j.yapd.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Freeman JM, Vining EP, Kossoff EH, Pyzik PL, Ye X, Goodman SN. A blinded, crossover study of the efficacy of the ketogenic diet. Epilepsia. 2009;50 (2):322–325. doi: 10.1111/j.1528-1167.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- 87.Frisullo G, Angelucci F, Mirabella M, Caggiula M, Patanella K, Nociti V, Tonali PA, Batocchi AP. Leptin enhances the release of cytokines by peripheral blood mononuclear cells from relapsing multiple sclerosis patients. J Clin Immunol. 2004;24 (3):287–293. doi: 10.1023/B:JOCI.0000025450.48267.a5. [DOI] [PubMed] [Google Scholar]
- 88.Frisullo G, Mirabella M, Angelucci F, Caggiula M, Morosetti R, Sancricca C, Patanella AK, Nociti V, Iorio R, Bianco A, Tomassini V, Pozzilli C, Tonali PA, Matarese G, Batocchi AP. The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;192 (1–2):174–183. doi: 10.1016/j.jneuroim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57 (1):35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 90.Galgani J, Ravussin E. Principles of Human Energy Metabolism. In: Ahima RS, editor. Metabolic Basis of Obesity. Springer; New York: 2011. pp. 1–23. [Google Scholar]
- 91.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, Rorsman P, Robins P, Prieur X, Coll AP, Ma M, Jovanovic Z, Farooqi IS, Sedgwick B, Barroso I, Lindahl T, Ponting CP, Ashcroft FM, O’Rahilly S, Schofield CJ. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318 (5855):1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Getz GS. Thematic review series: the immune system and atherogenesis. Bridging the innate and adaptive immune systems. J Lipid Res. 2005;46 (4):619–622. doi: 10.1194/jlr.E500002-JLR200. [DOI] [PubMed] [Google Scholar]
- 93.Gilhuis HJ, van Ravenswaaij CM, Hamel BJ, Gabreels FJ. Interstitial 6q deletion with a Prader-Willi-like phenotype: a new case and review of the literature. Eur J Paediatr Neurol. 2000;4 (1):39–43. doi: 10.1053/ejpn.1999.0259. [DOI] [PubMed] [Google Scholar]
- 94.Gillette Guyonnet S, Abellan Van Kan G, Alix E, Andrieu S, Belmin J, Berrut G, Bonnefoy M, Brocker P, Constans T, Ferry M, Ghisolfi-Marque A, Girard L, Gonthier R, Guerin O, Hervy MP, Jouanny P, Laurain MC, Lechowski L, Nourhashemi F, Raynaud-Simon A, Ritz P, Roche J, Rolland Y, Salva T, Vellas B. IANA (International Academy on Nutrition and Aging) Expert Group: weight loss and Alzheimer’s disease. J Nutr Health Aging. 2007;11 (1):38–48. [PubMed] [Google Scholar]
- 95.Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182 (4111):488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 96.Gonzales MM, Tarumi T, Kaur S, Nualnim N, Fallow BA, Pyron M, Tanaka H, Haley AP. Aerobic fitness and the brain: increased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr. 2013;26 (1):126–134. doi: 10.1007/s10548-012-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grace C, Beales P, Summerbell C, Jebb SA, Wright A, Parker D, Kopelman P. Energy metabolism in Bardet-Biedl syndrome. Int J Obes Relat Metab Disord. 2003;27 (11):1319–1324. doi: 10.1038/sj.ijo.0802420. [DOI] [PubMed] [Google Scholar]
- 98.Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, Johnson G, Heath O, McManamon PJ, O’Leary E, Pryse-Phillips W. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321 (15):1002–1009. doi: 10.1056/NEJM198910123211503. [DOI] [PubMed] [Google Scholar]
- 99.Griffioen KJ, Wan R, Brown TR, Okun E, Camandola S, Mughal MR, Phillips TM, Mattson MP. Aberrant heart rate and brainstem brain-derived neurotrophic factor (BDNF) signaling in a mouse model of Huntington’s disease. Neurobiol Aging. 2012;33 (7):1481, e1481–1485. doi: 10.1016/j.neurobiolaging.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16 (3):296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grundman M, Corey-Bloom J, Jernigan T, Archibald S, Thal LJ. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46 (6):1585–1591. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 102.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48 (1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 103.Guo DF, Rahmouni K. Molecular basis of the obesity associated with Bardet-Biedl syndrome. Trends Endocrinol Metab. 2011;22 (7):286–293. doi: 10.1016/j.tem.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283 (3):1754–1763. doi: 10.1074/jbc.M703753200. M703753200 [pii] [DOI] [PubMed] [Google Scholar]
- 105.Gwon AR, Park JS, Arumugam TV, Kwon YK, Chan SL, Kim SH, Baik SH, Yang S, Yun YK, Choi Y, Kim S, Tang SC, Hyun DH, Cheng A, Dann CE, 3rd, Bernier M, Lee J, Markesbery WR, Mattson MP, Jo DG. Oxidative lipid modification of nicastrin enhances amyloidogenic gamma-secretase activity in Alzheimer’s disease. Aging Cell. 2012;11 (4):559–568. doi: 10.1111/j.1474-9726.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26 (1):212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 107.Halpern CH, Wolf JA, Bale TL, Stunkard AJ, Danish SF, Grossman M, Jaggi JL, Grady MS, Baltuch GH. Deep brain stimulation in the treatment of obesity. J Neurosurg. 2008;109 (4):625–634. doi: 10.3171/JNS/2008/109/10/0625. [DOI] [PubMed] [Google Scholar]
- 108.Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359 (9):918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han JC, Muehlbauer MJ, Cui HN, Newgard CB, Haqq AM. Lower brain-derived neurotrophic factor in patients with prader-willi syndrome compared to obese and lean control subjects. J Clin Endocrinol Metab. 2010;95 (7):3532–3536. doi: 10.1210/jc.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hartman AL. Does the effectiveness of the ketogenic diet in different epilepsies yield insights into its mechanisms? Epilepsia. 2008;49(Suppl 8):53–56. doi: 10.1111/j.1528-1167.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hartman AL, Rubenstein JE, Kossoff EH. Intermittent fasting: a “new” historical strategy for controlling seizures? Epilepsy Res. 2013;104 (3):275–279. doi: 10.1016/j.eplepsyres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34 (3):453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 113.Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol. 2003;49 (2):277–300. [PubMed] [Google Scholar]
- 114.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49 (5):1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 115.Hey H, Stokholm KH, Lund B, Sorensen OH. Vitamin D deficiency in obese patients and changes in circulating vitamin D metabolites following jejunoileal bypass. Int J Obes. 1982;6 (5):473–479. [PubMed] [Google Scholar]
- 116.Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, DeCarli CS, DeChairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Raji CA, Lopez OL, Becker JT, Carmichael OT, Thompson PM. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A. 2010;107 (18):8404–8409. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9 (1):101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 118.Isojarvi JI, Laatikainen TJ, Knip M, Pakarinen AJ, Juntunen KT, Myllyla VV. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol. 1996;39 (5):579–584. doi: 10.1002/ana.410390506. [DOI] [PubMed] [Google Scholar]
- 119.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62 (10):1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 120.Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83 (4):1243–1246. doi: 10.1210/jcem.83.4.4731. [DOI] [PubMed] [Google Scholar]
- 121.Jeon BT, Shin HJ, Kim JB, Kim YK, Lee DH, Kim KH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Res Rev. 2009;61 (2):81–88. doi: 10.1016/j.brainresrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 122.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247 (3):401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 123.Keppler-Noreuil KM, Blumhorst C, Sapp JC, Brinckman D, Johnston J, Nopoulos PC, Biesecker LG. Brain tissue- and region-specific abnormalities on volumetric MRI scans in 21 patients with Bardet-Biedl syndrome (BBS) BMC Med Genet. 2011;12:101. doi: 10.1186/1471-2350-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khurana SR, Bamer AM, Turner AP, Wadhwani RV, Bowen JD, Leipertz SL, Haselkorn JK. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88 (2):83–91. doi: 10.1097/PHM.0b013e318194f8b5. [DOI] [PubMed] [Google Scholar]
- 125.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63 (3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim WS, Li H, Ruberu K, Chan S, Elliott DA, Low JK, Cheng D, Karl T, Garner B. Deletion of Abca7 increases cerebral amyloid-beta accumulation in the J20 mouse model of Alzheimer’s disease. J Neurosci. 2013;33 (10):4387–4394. doi: 10.1523/JNEUROSCI.4165-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87 (2):221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10 (4):249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest. 1999;103 (3):383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koutcherov Y, Mai JK, Paxinos G. Hypothalamus of the human fetus. J Chem Neuroanat. 2003;26 (4):253–270. doi: 10.1016/j.jchemneu.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 131.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19 (2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 132.Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Gruters A. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88 (10):4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- 133.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22 (7):1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lan MJ, Yuan P, Chen G, Manji HK. Neuronal peroxisome proliferator-activated receptor gamma signaling: regulation by mood-stabilizer valproate. J Mol Neurosci. 2008;35 (2):225–234. doi: 10.1007/s12031-008-9056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lonroth H, Fandriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes. 2012;36 (3):348–355. doi: 10.1038/ijo.2011.217. [DOI] [PubMed] [Google Scholar]
- 136.Laurenius A, Larsson I, Melanson KJ, Lindroos AK, Lonroth H, Bosaeus I, Olbers T. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur J Clin Nutr. 2013;67 (2):168–173. doi: 10.1038/ejcn.2012.208. [DOI] [PubMed] [Google Scholar]
- 137.Lee EB. Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci. 2011;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee EB, Ahima RS. Central Regulation of Appetite and Satiety Behavior. In: Preedy VR, Marin CR, Watson RR, editors. Handbook of Behavior, Food and Nutrition. Springer; New York: 2011. pp. 1023–1034. [Google Scholar]
- 139.Lee EB, Warmann G, Dhir R, Ahima RS. Metabolic dysfunction associated with adiponectin deficiency enhances kainic acid-induced seizure severity. J Neurosci. 2011;31 (40):14361–14366. doi: 10.1523/JNEUROSCI.3171-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82 (6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 141.Lee J, Lim E, Kim Y, Li E, Park S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J Endocrinol. 205(3):263–270. doi: 10.1677/JOE-10-0040. JOE-10-0040 [pii] [DOI] [PubMed] [Google Scholar]
- 142.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87 (3):1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leonard WR, Snodgrass JJ, Robertson ML. Effects of brain evolution on human nutrition and metabolism. Annu Rev Nutr. 2007;27:311–327. doi: 10.1146/annurev.nutr.27.061406.093659. [DOI] [PubMed] [Google Scholar]
- 144.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14 (10):999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101 (13):4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43 (4):199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 147.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 148.Luiten PG, ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28 (1):1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 149.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121 (6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lynch JJ, 3rd, Shek EW, Castagne V, Mittelstadt SW. The proconvulsant effects of leptin on glutamate receptor-mediated seizures in mice. Brain Res Bull. 82(1–2):99–103. doi: 10.1016/j.brainresbull.2010.02.003. S0361-9230(10)00036-5 [pii] [DOI] [PubMed] [Google Scholar]
- 151.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59 (2):293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1 (11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 153.Malek-Ahmadi M, Beach T, Obradov A, Sue L, Belden C, Davis K, Walker DG, Lue L, Adem A, Sabbagh MN. Type 2 Diabetes Is Associated With Increased Alzheimer’s Disease Neuropathology in ApoE epsilon4 Carriers. Curr Alzheimer Res. 2013 doi: 10.2174/15672050113109990006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17 (3):1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15 (1):105–113. doi: 10.1177/1352458508096680. [DOI] [PubMed] [Google Scholar]
- 156.Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol. 2010;9 (8):820–828. doi: 10.1016/S1474-4422(10)70135-6. [DOI] [PubMed] [Google Scholar]
- 157.Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2005;102 (14):5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Matarese G, Carrieri PB, Montella S, De Rosa V, La Cava A. Leptin as a metabolic link to multiple sclerosis. Nat Rev Neurol. 2010;6 (8):455–461. doi: 10.1038/nrneurol.2010.89. [DOI] [PubMed] [Google Scholar]
- 159.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166 (10):5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 160.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90 (5):2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 161.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75 (9):764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 162.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44 (10):625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16 (6):706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maurois P, Rocchi S, Pages N, Bac P, Stables JP, Gressens P, Vamecq J. The PPARgamma agonist FMOC-L-leucine protects both mature and immature brain. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2008;62 (4):259–263. doi: 10.1016/j.biopha.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 165.McAllister CJ, Whittington JE, Holland AJ. Development of the eating behaviour in Prader-Willi Syndrome: advances in our understanding. Int J Obes. 2011;35 (2):188–197. doi: 10.1038/ijo.2010.139. [DOI] [PubMed] [Google Scholar]
- 166.McLoughlin TG, Shanklin DR. Pathology of Laurence-Moon-Bardet-Biedl syndrome. J Pathol Bacteriol. 1967;93 (1):65–79. doi: 10.1002/path.1700930106. [DOI] [PubMed] [Google Scholar]
- 167.McTaggart JS, Lee S, Iberl M, Church C, Cox RD, Ashcroft FM. FTO is expressed in neurones throughout the brain and its expression is unaltered by fasting. PLoS One. 2011;6 (11):e27968. doi: 10.1371/journal.pone.0027968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Melka MG, Gillis J, Bernard M, Abrahamowicz M, Chakravarty MM, Leonard GT, Perron M, Richer L, Veillette S, Banaschewski T, Barker GJ, Buchel C, Conrod P, Flor H, Heinz A, Garavan H, Bruhl R, Mann K, Artiges E, Lourdusamy A, Lathrop M, Loth E, Schwartz Y, Frouin V, Rietschel M, Smolka MN, Strohle A, Gallinat J, Struve M, Lattka E, Waldenberger M, Schumann G, Pavlidis P, Gaudet D, Paus T, Pausova Z. FTO, obesity and the adolescent brain. Hum Mol Genet. 2013;22 (5):1050–1058. doi: 10.1093/hmg/dds504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Levy E, Mitchell GA, Himms-Hagen J, Fan CM. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10 (14):1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 170.Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12 (20):3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohr B. Neuropathology communication from Dr. Mohr, privat docent in Wurzburg. 1840. Obes Res. 1993;1 (4):334–335. doi: 10.1002/j.1550-8528.1993.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 172.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387 (6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 173.Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4 (4):e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Munger KL, Bentzen J, Laursen B, Stenager E, Koch-Henriksen N, Sorensen TI, Baker JL. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler. 2013 doi: 10.1177/1352458513483889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73 (19):1543–1550. doi: 10.1212/WNL.0b013e3181c0d6e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Munger KL, Chitnis T, Frazier AL, Giovannucci E, Spiegelman D, Ascherio A. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol. 2011;258 (3):479–485. doi: 10.1007/s00415-010-5783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296 (23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 178.Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62 (1):60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 179.Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49 (3):436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- 180.Nashold FE, Hoag KA, Goverman J, Hayes CE. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119 (1):16–29. doi: 10.1016/s0165-5728(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 181.Nashold FE, Miller DJ, Hayes CE. 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;103 (2):171–179. doi: 10.1016/s0165-5728(99)00247-7. [DOI] [PubMed] [Google Scholar]
- 182.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7 (6):500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 183.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50 (5):1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 184.Norgan NG. Laboratory and field measurements of body composition. Public Health Nutr. 2005;8 (7A):1108–1122. doi: 10.1079/phn2005799. [DOI] [PubMed] [Google Scholar]
- 185.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60 (1):117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 186.Nylen K, Likhodii S, Burnham WM. The ketogenic diet: proposed mechanisms of action. Neurotherapeutics. 2009;6 (2):402–405. doi: 10.1016/j.nurt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Obay BD, Tasdemir E, Tumer C, Bilgin HM, Atmaca M. Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides. 2008;29 (3):448–455. doi: 10.1016/j.peptides.2007.11.020. S0196-9781(07)00474-3 [pii] [DOI] [PubMed] [Google Scholar]
- 188.Obay BD, Tasdemir E, Tumer C, Bilgin HM, Sermet A. Antiepileptic effects of ghrelin on pentylenetetrazole-induced seizures in rats. Peptides. 2007;28 (6):1214–1219. doi: 10.1016/j.peptides.2007.04.003. S0196-9781(07)00122-2 [pii] [DOI] [PubMed] [Google Scholar]
- 189.Obeid M, Frank J, Medina M, Finckbone V, Bliss R, Bista B, Majmudar S, Hurst D, Strahlendorf H, Strahlendorf J. Neuroprotective effects of leptin following kainic acid-induced status epilepticus. Epilepsy Behav. doi: 10.1016/j.yebeh.2010.07.023. S1525–5050(10)00524-X [pii] [DOI] [PubMed] [Google Scholar]
- 190.Organization WH. Fact Sheet. 2013. Obesity and overweight; p. 311. [Google Scholar]
- 191.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84 (10):3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 192.Pan L, Blanck HM, Sherry B, Dalenius K, Grummer-Strawn LM. Trends in the prevalence of extreme obesity among US preschool-aged children living in low-income families, 1998–2010. Jama. 2012;308 (24):2563–2565. doi: 10.1001/jama.2012.108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30 (1):121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Paz-Filho GJ, Babikian T, Asarnow R, Delibasi T, Esposito K, Erol HK, Wong ML, Licinio J. Leptin replacement improves cognitive development. PLoS One. 2008;3 (8):e3098. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85 (11):2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 196.Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74 (4):1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- 197.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51 (4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 198.Pericak-Vance MA, Bebout JL, Gaskell PC, Jr, Yamaoka LH, Hung WY, Alberts MJ, Walker AP, Bartlett RJ, Haynes CA, Welsh KA, et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991;48 (6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- 199.Pierrot-Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain. 2010;133 (Pt 7):1869–1888. doi: 10.1093/brain/awq147. [DOI] [PubMed] [Google Scholar]
- 200.Pinkney J, Wilding J, Williams G, MacFarlane I. Hypothalamic obesity in humans: what do we know and what can be done? Obes Rev. 2002;3 (1):27–34. doi: 10.1046/j.1467-789x.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 201.Principles and Practice of Endocrinology and Metabolism. 3. Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 202.Qiao L, Schaack J, Shao J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology. 2006;147 (2):865–874. doi: 10.1210/en.2005-1030. en.2005-1030 [pii] [DOI] [PubMed] [Google Scholar]
- 203.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118 (4):1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rao RS. Bariatric surgery and the central nervous system. Obes Surg. 2012;22 (6):967–978. doi: 10.1007/s11695-012-0649-5. [DOI] [PubMed] [Google Scholar]
- 205.Rauchenzauner M, Laimer M, Luef G, Kaser S, Engl J, Tatarczyk T, Ciardi C, Tschoner A, Lechleitner M, Patsch J, Ebenbichler CF. Adiponectin receptor R1 is upregulated by valproic acid but not by topiramate in human hepatoma cell line, HepG2. Seizure. 2008;17 (8):723–726. doi: 10.1016/j.seizure.2008.03.002. S1059-1311(08)00065-4 [pii] [DOI] [PubMed] [Google Scholar]
- 206.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78 (6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4 (2):e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Regard M, Landis T. “Gourmand syndrome”: eating passion associated with right anterior lesions. Neurology. 1997;48 (5):1185–1190. doi: 10.1212/wnl.48.5.1185. [DOI] [PubMed] [Google Scholar]
- 209.Reinehr T, de Sousa G, Alexy U, Kersting M, Andler W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur J Endocrinol. 2007;157 (2):225–232. doi: 10.1530/EJE-07-0188. [DOI] [PubMed] [Google Scholar]
- 210.Rooryck C, Pelras S, Chateil JF, Cances C, Arveiler B, Verloes A, Lacombe D, Goizet C. Bardet-biedl syndrome and brain abnormalities. Neuropediatrics. 2007;38 (1):5–9. doi: 10.1055/s-2007-981466. [DOI] [PubMed] [Google Scholar]
- 211.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264 (1):49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32 (7):1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 213.Sachs GS, Guille C. Weight gain associated with use of psychotropic medications. J Clin Psychiatry. 1999;60(Suppl 21):16–19. [PubMed] [Google Scholar]
- 214.Sagare AP, Bell RD, Srivastava A, Sengillo JD, Singh I, Nishida Y, Chow N, Zlokovic BV. A lipoprotein receptor cluster IV mutant preferentially binds amyloid-beta and regulates its clearance from the mouse brain. J Biol Chem. 2013 doi: 10.1074/jbc.M112.439570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet. 1990;336 (8726):1267–1271. doi: 10.1016/0140-6736(90)92959-l. [DOI] [PubMed] [Google Scholar]
- 216.Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry. 1996;61 (5):433–443. doi: 10.1136/jnnp.61.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012;124 (3):305–323. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schwartz A, Doucet E. Relative changes in resting energy expenditure during weight loss: a systematic review. Obes Rev. 2010;11 (7):531–547. doi: 10.1111/j.1467-789X.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 219.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18 (7):1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545(Pt 3):933–944. doi: 10.1113/jphysiol.2002.029488. PHY_029488 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2011;68 (10):1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68 (11):1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27 (8):489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 224.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313 (5787):629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 225.Soliman AT, Rajab A, AlSalmi I, Asfour MG. Empty sellae, impaired testosterone secretion, and defective hypothalamic-pituitary growth and gonadal axes in children with Bardet-Biedl syndrome. Metabolism. 1996;45 (10):1230–1234. doi: 10.1016/s0026-0495(96)90240-1. [DOI] [PubMed] [Google Scholar]
- 226.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175 (6):4119–4126. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 227.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177 (9):6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 228.Spach KM, Pedersen LB, Nashold FE, Kayo T, Yandell BS, Prolla TA, Hayes CE. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18 (2):141–151. doi: 10.1152/physiolgenomics.00003.2004. [DOI] [PubMed] [Google Scholar]
- 229.Spanier JA, Nashold FE, Olson JK, Hayes CE. The Ifng gene is essential for Vdr gene expression and vitamin D(3)-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis, a multiple sclerosis model. J Immunol. 2012;189 (6):3188–3197. doi: 10.4049/jimmunol.1102925. [DOI] [PubMed] [Google Scholar]
- 230.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Magi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segre AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpelainen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proenca C, Chen YD, Chen CM, Chines PS, Clarke R, Coin L, Connell J, Day IN, den Heijer M, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJ, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Grassler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen AL, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jorgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, Konig IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaloy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimaki T, Lettre G, Liu J, Lokki ML, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O’Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Pare G, Parker AN, Perola M, Pichler I, Pietilainen KH, Platou CG, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstrale M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo ML, Tardif JC, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tonjes A, Tuomi T, van Meurs JB, van Ommen GJ, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CI, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kahonen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Gronberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin MR, Kaprio J, Karpe F, Khaw KT, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PE, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann HE, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, McCarthy MI, Hirschhorn JN, Ingelsson E, Loos RJ. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42 (11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sriram K, Benkovic SA, Miller DB, O’Callaghan JP. Obesity exacerbates chemically induced neurodegeneration. Neuroscience. 2002;115 (4):1335–1346. doi: 10.1016/s0306-4522(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 232.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62 (1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 233.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11 (3):309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19 (10):951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stricker EM. Biological bases of hunger and satiety: therapeutic implications. Nutr Rev. 1984;42 (10):333–340. doi: 10.1111/j.1753-4887.1984.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 236.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90 (5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18 (3):213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 238.Stutzmann F, Ghoussaini M, Couturier C, Marchand M, Vatin V, Corset L, Lecoeur C, Balkau B, Horber F, Driscoll DJ, Goldstone AP, Weill J, Michaud JL, Meyre D, Froguel P. Loss-of-function mutations in SIM1 cause a specific form of Prader-Willi-like syndrome. Diabetologia. 2009;52:S104–S104. [Google Scholar]
- 239.Sun H, Huang Y, Yu X, Li Y, Yang J, Li R, Deng Y, Zhao G. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2008;26 (5):505–515. doi: 10.1016/j.ijdevneu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 240.Swaab DF. Prader-Willi syndrome and the hypothalamus. Acta Paediatr Suppl. 1997;423:50–54. doi: 10.1111/j.1651-2227.1997.tb18369.x. [DOI] [PubMed] [Google Scholar]
- 241.Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab. 1995;80 (2):573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- 242.Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 1980;198 (1):190–195. doi: 10.1016/0006-8993(80)90354-6. [DOI] [PubMed] [Google Scholar]
- 243.Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, Church TS. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS One. 2012;7 (8):e42785. doi: 10.1371/journal.pone.0042785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, Wojcicki TR, White SM, Gothe N, Olson EA, Kramer AF. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011;25 (5):545–553. doi: 10.1037/a0022733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Temkin O. The falling sickness: a history of epilepsy from the Greeks to the beginnings of modern neurology. 2. Johns Hopkins Univ. Press; 1945. [Google Scholar]
- 246.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122 (1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30 (10):3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 249.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20 (2):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 250.van de Sande-Lee S, Pereira FR, Cintra DE, Fernandes PT, Cardoso AR, Garlipp CR, Chaim EA, Pareja JC, Geloneze B, Li LM, Cendes F, Velloso LA. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes. 2011;60 (6):1699–1704. doi: 10.2337/db10-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360 (15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 252.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10 (2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 253.Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110 (19):E1807–1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vidoni ED, Townley RA, Honea RA, Burns JM. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77 (21):1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Villa A, Urioste M, Bofarull JM, Martinez-Frias ML. De novo interstitial deletion q16.2q21 on chromosome 6. Am J Med Genet. 1995;55 (3):379–383. doi: 10.1002/ajmg.1320550326. [DOI] [PubMed] [Google Scholar]
- 256.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360 (15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 257.Wang JC, Turner L, Lomax B, Eydoux P. A 5-Mb microdeletion at 6q16.1–q16.3 with SIM gene deletion and obesity. Am J Med Genet A. 2008;146A (22):2975–2978. doi: 10.1002/ajmg.a.32555. [DOI] [PubMed] [Google Scholar]
- 258.Wansink B, Chandon P. Meal size, not body size, explains errors in estimating the calorie content of meals. Ann Intern Med. 2006;145 (5):326–332. doi: 10.7326/0003-4819-145-5-200609050-00005. [DOI] [PubMed] [Google Scholar]
- 259.Webber J. Energy balance in obesity. Proc Nutr Soc. 2003;62 (2):539–543. doi: 10.1079/pns2003256. [DOI] [PubMed] [Google Scholar]
- 260.Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 261.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41 (1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, Sridharan A, Allison DB, Anderson R, Voytko ML, Kemnitz JW, Weindruch RH, Johnson SC. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. 2012;61 (5):1036–1042. doi: 10.2337/db11-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, Hermann BP, La Rue A, Asthana S, Bendlin BB. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36 (2):443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106 (4):1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69 (14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 266.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72 (3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 267.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6 (7):736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118 (1):272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135 (Pt 12):3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7 (11):1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 271.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20 (2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 272.Yu X, Shao XG, Sun H, Li YN, Yang J, Deng YC, Huang YG. Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus. Brain Res. 2008;1200:146–158. doi: 10.1016/j.brainres.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 273.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1 (6):371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 274.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497 (7448):211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135 (1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]