Abstract
A method for direct, auxiliary-assisted alkoxylation and phenoxylation of β-sp2 C−H bonds of benzoic acid derivatives and γ-sp2 C−H bonds of amine derivatives is reported. The reaction employs (CuOH)2CO3 catalyst, air as an oxidant, phenol or alcohol coupling partner, DMF, pyridine, or DMPU solvent, and K2CO3, tetramethylguanidine, or K3PO4 base at 90–130 °C.
Carbon-hydrogen bond functionalization methodology is perhaps the most direct way to introduce functionality into organic molecules. In the last decade, significant advances in C-H activation and functionalization have been realized.1 However, relatively scarce second-and third row transition metals are most commonly used for these transformations. It would be beneficial if abundant first-row transition metals could replace their heavier analogues as catalysts for C-H bond functionalization.2 Most examples of direct hydroxylation or etherification of sp2 C-H bonds have been performed under palladium or ruthenium catalysis.3 While copper-promoted oxygenation of sp2 C-H bonds has been studied in context of evaluating mechanisms of oxidations by enzymes, catalytic applications of such reactions in synthetically relevant systems have been rare.4 An early report by Reinaud showed that copper(II) mediates aromatic hydroxylation by trimethylamine N-oxide.4a More recently, Yu has shown that a number of nucleophiles, including water, can be coupled with 2-phenylpyridine by employing Cu(II) salts and oxygen oxidant.4b Subsequently, other groups have reported related copper-catalyzed sp2 C-H bond functionalization reactions.4 Martin has shown that copper-catalyzed hydroxylation of 2-phenylbenzoic acid is possible.5a This report is especially interesting since carboxylate functionality remains intact.5b We disclose here a general method for aminoquinoline and picolinamide-directed benzoic acid and amine derivative ortho-etherification by employing air as an oxidant.
In 2005, we introduced picolinic acid and 8-aminoquinoline auxiliaries for palladium-catalyzed C-H bond functionalization.6 Subsequently, these auxiliaries have been used by a number of groups for palladium-, nickel-, iron-, and ruthenium-catalyzed sp2 and sp3 C-H bond functionalization.7 Recently, we have developed aminoquinoline- and picolinamide-directed, copper-catalyzed sulfenylation, amination, and fluorination of arene and heteroarene C-H bonds.8 The common feature of these reactions is the coupling of a nucleophile with a C-H bond. We speculated that copper-catalyzed etherification of sp2 C-H bonds should be possible if aminoquinoline or picolinic acid directing groups are employed. Additionally, in an insightful mechanistic study of Cu(II)-mediated sp2 C-H bond oxidation, Stahl has reported the methoxylation of N-(8-quinolinyl)benzamide by employing methanol solvent and 2 equiv of Cu(OAc)2.9a
Reaction of 8-aminoquinoline 3-trifluoromethyl-benzamide 1 was investigated with respect to Cu catalyst, oxidant, and base (Table 1). The conditions developed for arene amination8b resulted in low yield of coupling product (entry 1). Replacing Ag2CO3 with K2CO3 was beneficial (entry 2), and the reaction could be run under an atmosphere of oxygen for additional increase of yield (entry 3). Interestingly, omission of NMO oxidant and running the reaction under air in an open flask was successful (entry 4). Furthermore, yield could be increased if reaction time was decreased from 12 to 6 hours (entry 5). Thus, the optimal reaction conditions involve 1 equiv of phenol, K2CO3 base, 11 % (22% based on Cu) of (CuOH)2CO3 catalyst, DMF solvent, 110 °C, and air as oxidant.
Table 1.
Screening of Reaction Conditionsa
 | ||||
|---|---|---|---|---|
| entry | catalyst | base | oxidant | yield, % |
| 1b | Cu(OAc)2 | Ag2CO3 | NMO | 21 |
| 2c | Cu(OAc)2 | K2CO3 | NMO | 64 |
| 3c,d | Cu(OAc)2 | K2CO3 | NMO/O2 | 67 |
| 4e | (CuOH)2CO3 | K2CO3 | air | 68 |
| 5e,f | (CuOH)2CO3 | K2CO3 | air | 88 |
Amide (0.1 mmol), phenol (0.1 mmol), catalyst (0.02 mmol), base (0.2 mmol). Yields determined by NMR of crude reaction mixtures.
NMO (N-methylmorpholine oxide) 0.2 mmol, NMP solvent.
NMO 0.2 mmol, DMF solvent.
Under 1 atm of O2.
Reaction open to air, 11 mol % catalyst (22% Cu).
Isolated yield, reaction time − 6 h.
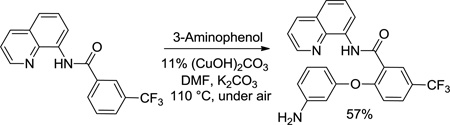
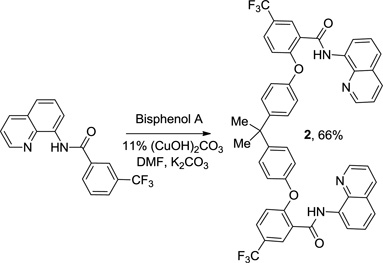
The reaction scope with respect to phenols is presented in Table 2 and Equation 1. Reactions were run on a 0.5 mmol scale. Electron-rich (entries 1, 8, 9) and electronpoor (entries 3–6) phenols are reactive. The reactions tolerate most functional groups, such as thioether (entry 2), bromide (entry 4), chloride (entries 7 and 8), amino (entry 9) and even iodide (entry 5). Ester functionality is also tolerated (entry 6). ortho-Substituted phenols are reactive (entry 8). Interestingly, bisphenol A can be used and double arylation product 2 was isolated in 66% yield (eq 1). The yields range from good to excellent, and reaction times are 2–8 hours. A 3.2 mmol scale reaction of 1 with 4-t-butylphenol afforded 73% isolated yield of product (entry 1).
Table 2.
Reaction Scope with Respect to Phenolsa
 | |||
|---|---|---|---|
| entry | phenol | product | yield, % |
| 1 | 4-tBu-phenol | 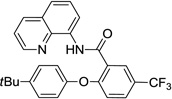 |
88 73b |
| 2 | 4-SMe-phenol | 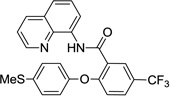 |
75 |
| 3 | 3-CF3-phenol | 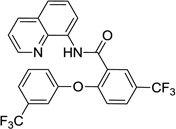 |
55 |
| 4 | 4-Br-phenol | 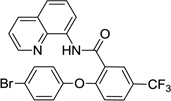 |
78 |
| 5 | 3-I-phenol | 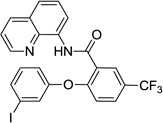 |
56 |
| 6 | 3-CO2Me-phenol | 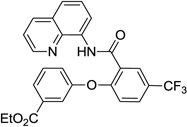 |
84 |
| 7 | 3-Cl-4-Me-phenol | 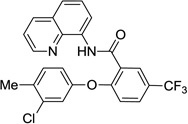 |
77 |
| 8 | 2-Cl-4-MeO-phenol | 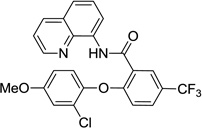 |
78 |
| 9c | 3-amino-phenol | 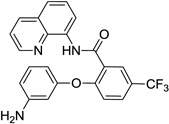 |
57 |
Scale: 0.5 mmol; time: 2–8 h; 1/1 amide/phenol ratio, 110 °C, 11% catalyst = 22 mol % Cu. Please see Supporting Information for details.
Scale: 3.2 mmol, 5.5 mol % (CuOH)2CO3.
Amide/phenol ratio: 1/2.
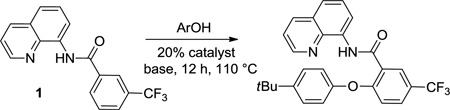
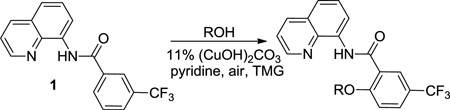
Reaction scope with respect to aliphatic alcohols is presented in Table 3. Optimal base for alkoxylation is tetramethylguanidine (TMG), and the best results were obtained by employing 5 equiv of alcohol in pyridine solvent. Air was used as an oxidant. Simple primary alkyl alcohols such as cyclopropylmethanol afford good yields of the product (entry 1). Trifluoromethanol (entry 2) and allyl alcohol (entry 3) are reactive and coupling products were isolated in 73 and 71% yields. More complex structures such as carbitol (entry 4), cinchonine (entry 5), and ethyl lactate (entry 6) gave products in fair to excellent yields. The latter examples show that aliphatic esters, amines, esters, and polyethers are tolerated in the reaction. Reaction with trifluoroethanol was carried out in closed vessel pressurized with O2 due to volatility of the alcohol.
 |
(1) |
Table 3.
Reaction Scope with Respect to Aliphatic Alcoholsa
 | |||
|---|---|---|---|
| entry | alcohol | product | yield, % |
| 1 | cyclopropyl-methanol | 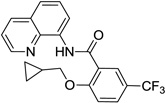 |
75 |
| 2b | HOCH2CF3 | 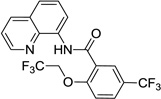 |
73 |
| 3 | allyl alcohol | 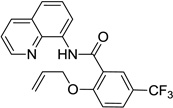 |
71 |
| 4 | carbitol | 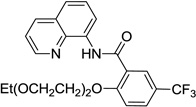 |
72 |
| 5 | cinchonine | 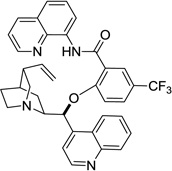 |
85 |
| 6 | ethyl lactate | 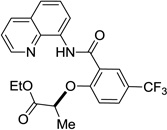 |
39 |
Scale: 0.5 mmol; time: 2–8 h; 1/5 amide/alcohol ratio, 110 °C, 11% catalyst = 22 mol % Cu. Please see Supporting Information for details.
Closed vessel pressurized with O2.
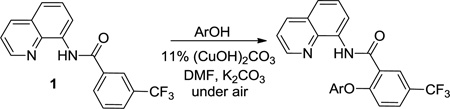
The reaction scope with respect to aminoquinoline benzamides is presented in Table 4. Cyano- (entry 1), trifluoromethyl- (entry 2), nitro- (entry 3), and methoxysubstituted (entries 4 and 5) amides are reactive and monosubstitution products are obtained in moderate to good yields. Heterocyclic amides are compatible with the reaction conditions (entires 7 and 8). In contrast with aminoquinoline benzamide amination where diamination products were not observed,8b bis-phenoxylation is possible by employing higher phenol/amide rations and DMPU solvent (entry 8). The disubstitution product was obtained in 47% yield.
Table 4 .
Reaction Scope with Respect to Amides
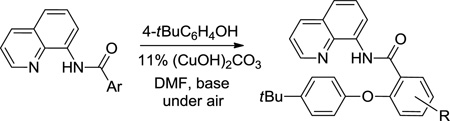 | |||
|---|---|---|---|
| entry | Ar | product | yield, % |
| 1 | 4-CNC6H4 | 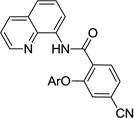 |
55 |
| 2 | 4-CF3C6H4 | 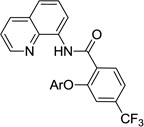 |
74 |
| 3 | 4-NO2C6H4 | 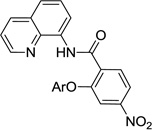 |
59 |
| 4 | 3,4-(OMe)2C6H4 | 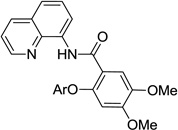 |
57 |
| 5 | 3-MeOC6H4 | 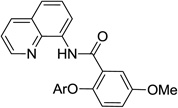 |
54 |
| 6 | 3-MeC6H4 | 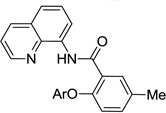 |
60 |
| 7 | 4-C5H4N | 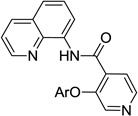 |
51 |
| 8b | 4-C5H4N | 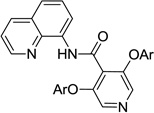 |
47 |
Scale: 0.5 mmol; time: 2–8 h; 1/1 amide/alcohol ratio, 90–110 °C, 11% catalyst = 22 mol % Cu. Please see Supporting Information for details.
Amide/alcohol ratio: 1/3, DMPU solvent.
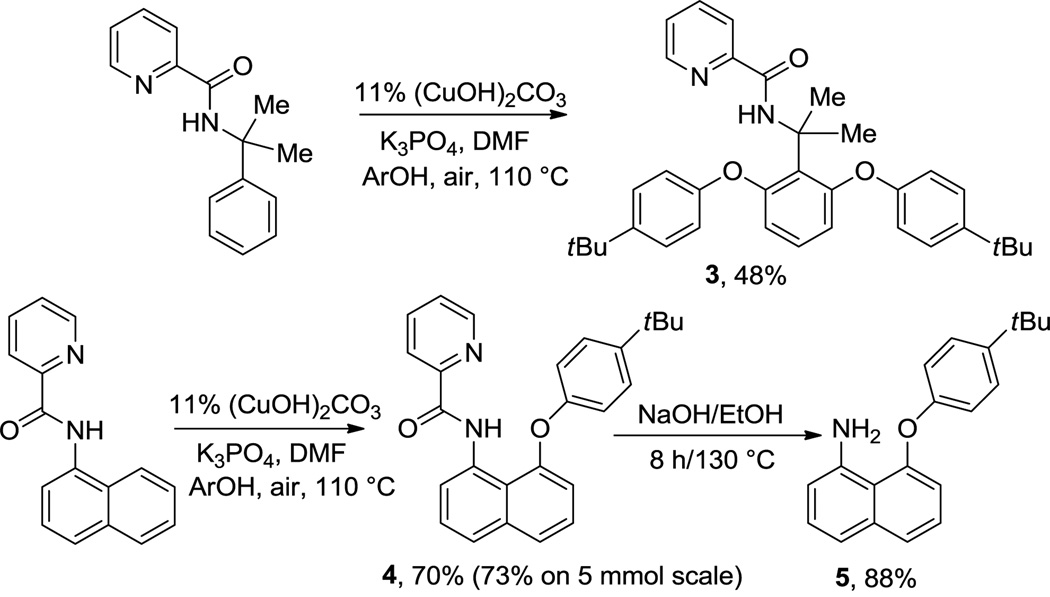
Picolinamide can also serve as a directing group (Scheme 1). Thus, α,α-dimethylbenzylamine picolinamide can be phenoxylated in moderate yield at 110 °C by employing (CuOH)2CO3 catalyst, K3PO4 base, and DMF solvent. 1-Naphthylamine picolinamide phenoxylation occurs at 8-position and the product was isolated in 70% (0.5 mmol scale reaction) or 73% yield (5 mmol scale reaction). The reactions can be scaled up at least tenfold without loss of yield. The directing group can be removed by base hydrolysis affording 8-aryloxynaphthylamine 5 in high yield. Aminoquinoline auxiliary can be removed as well (eq 2).
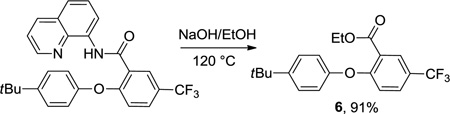 |
(2) |
Scheme 1.
Picolinic Acid Directing Group
In conclusion, we have developed a method for direct, auxiliary-assisted alkoxylation and phenoxylation of β-sp2 C−H bonds of benzoic acid derivatives and γ-sp2 C−H bonds of amine derivatives. The reaction employs Cu2(OH)2CO3 catalyst, air as an oxidant, phenol or alcohol coupling partner, DMF, pyridine, or DMPU solvent, and K2CO3, tetramethylguanidine, or K3PO4 base at 90–130 °C. The method is advantageous compared to the existing sp2 C-H bond etherification procedures due to utilization of inexpensive copper basic carbonate (malachite) catalyst and a removable directing group. The method shows high generality and functional group tolerance, with ester, amine, nitro, nitrile, and halogen functionalities compatible with the reaction conditions.
Supplementary Material
Acknowledgment
We thank the Welch Foundation (Grant No. E-1571), NIGMS (Grant No.R01GM077635), and Camille and Henry Dreyfus Foundation for supporting this research.
Footnotes
Supporting Information Available Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org..
References
- 1.Reviews: Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. Ackermann L. Chem. Rev. 2011;111:1315. doi: 10.1021/cr100412j. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Nakamura E, Yoshikai N. J. Org. Chem. 2010;75:6061. doi: 10.1021/jo100693m. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. Sun C-L, Li B-J, Shi Z-J. Chem. Commun. 2010:677. doi: 10.1039/b908581e. Li C-J. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n.
- 2.(a) Kulkarni AA, Daugulis O. Synthesis. 2009:4087. [Google Scholar]; (b) Wendlandt AE, Suess AM, Stahl SS. Angew. Chem. Int. Ed. 2011;50:11062. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]; (c) Allen SE, Walvoord RR, Padilla-Salinas R, Kozlowski MC. Chem. Rev. 2013;113:6234. doi: 10.1021/cr300527g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hickman AJ, Sanford MS. Nature. 2012;484:177. doi: 10.1038/nature11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Liu W, Ackermann L. Org. Lett. 2013;15:3484. doi: 10.1021/ol401535k. [DOI] [PubMed] [Google Scholar]; (b) Shan G, Yang X, Ma L, Rao Y. Angew. Chem. Int. Ed. 2012;51:13070. doi: 10.1002/anie.201207458. [DOI] [PubMed] [Google Scholar]; (c) Gulevich AV, Melkonyan FS, Sarkar D, Gevorgyan V. J. Am. Chem. Soc. 2012;134:5528. doi: 10.1021/ja3010545. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Desai LV, Stowers KJ, Sanford MS. J. Am. Chem. Soc. 2008;130:13285. doi: 10.1021/ja8045519. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Thirunavukkarasu VS, Ackermann L. Org. Lett. 2012;14:6206. doi: 10.1021/ol302956s. [DOI] [PubMed] [Google Scholar]; (f) Zhang YH, Yu J-Q. J. Am. Chem. Soc. 2009;131:14654. doi: 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Reinaud O, Capdevielle P, Maumy M. J. Chem. Soc. Chem. Commun. 1990:566. [Google Scholar]; (b) Chen X, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:6790. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; (c) Hong S, Huber SM, Gagliardi L, Cramer CC, Tolman WB. J. Am. Chem. Soc. 2007;129:14190. doi: 10.1021/ja0760426. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang W, Luo F, Zhang S, Cheng J. J. Org. Chem. 2010;75:2415. doi: 10.1021/jo1000719. [DOI] [PubMed] [Google Scholar]; (e) Liu Q, Wu P, Yang Y, Zeng Z, Liu J, Yi H, Lei A. Angew. Chem. Int. Ed. 2012;51:4666. doi: 10.1002/anie.201200750. [DOI] [PubMed] [Google Scholar]; (f) Xu R, Wan J-P, Mao H, Pan Y. J. Am. Chem. Soc. 2010;132:15531. doi: 10.1021/ja107758d. [DOI] [PubMed] [Google Scholar]
- 5.(a) Gallardo-Donaire J, Martin R. J. Am. Chem. Soc. 2013;135:9350. doi: 10.1021/ja4047894. [DOI] [PubMed] [Google Scholar]; (b) Bhadra S, Dzik WI, Gooßen LJ. Angew. Chem. Int. Ed. 2013;52:2959. doi: 10.1002/anie.201208755. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nadres ET, Daugulis O. J. Am. Chem. Soc. 2012;134:7. doi: 10.1021/ja210959p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) He G, Lu C, Zhao Y, Nack WA, Chen G. Org. Lett. 2012;14:2944. doi: 10.1021/ol301352v. [DOI] [PubMed] [Google Scholar]; (b) He G, Zhao Y, Zhang S, Lu C, Chen G. J. Am. Chem. Soc. 2012;134:3. doi: 10.1021/ja210660g. [DOI] [PubMed] [Google Scholar]; (c) Zhang S-Y, He G, Zhao Y, Wright K, Nack WA, Chen G. J. Am. Chem. Soc. 2012;134:7313. doi: 10.1021/ja3023972. [DOI] [PubMed] [Google Scholar]; (d) Gou F-R, Wang X-C, Huo P-F, Bi H-P, Guan Z-H, Liang Y-M. Org. Lett. 2009;11:5726. doi: 10.1021/ol902497k. [DOI] [PubMed] [Google Scholar]; (e) Gutekunst WR, Gianatassio R, Baran PS. Angew. Chem. Int. Ed. 2012;51:7507. doi: 10.1002/anie.201203897. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ano Y, Tobisu M, Chatani N. J. Am. Chem. Soc. 2011;133:12984. doi: 10.1021/ja206002m. [DOI] [PubMed] [Google Scholar]; (g) Gutekunst WR, Baran PS. J. Am. Chem. Soc. 2011;133:19076. doi: 10.1021/ja209205x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]; (i) Aihara Y, Chatani N. J. Am. Chem. Soc. 2013;135:5308. doi: 10.1021/ja401344e. [DOI] [PubMed] [Google Scholar]; (j) Nishino M, Hirano K, Satoh T, Miura M. Angew. Chem. Int. Ed. 2013;52:4457. doi: 10.1002/anie.201300587. [DOI] [PubMed] [Google Scholar]; (k) Shang R, Ilies L, Matsumoto A, Nakamura E. J. Am. Chem. Soc. 2013;135:6030. doi: 10.1021/ja402806f. [DOI] [PubMed] [Google Scholar]
- 8.(a) Tran LD, Popov I, Daugulis O. J. Am. Chem. Soc. 2012;134:18237. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tran LD, Roane J, Daugulis O. Angew. Chem. Int. Ed. 2013;52:6043. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Truong T, Klimovica K, Daugulis O. J. Am. Chem. Soc. 2013;135:9342. doi: 10.1021/ja4047125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Suess AM, Ertem MZ, Cramer CJ, Stahl SS. J. Am. Chem. Soc. 2013;135:9797. doi: 10.1021/ja4026424. [DOI] [PubMed] [Google Scholar]; (b) Ribas X, Jackson DA, Donnadieu B, Mahía J, Parella T, Xifra R, Hedman B, Hodgson KO, Llobet A, Stack TDP. Angew. Chem. Int. Ed. 2002;41:2991. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (c) Huffman LM, Stahl SS. J. Am. Chem. Soc. 2008;130:9196. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]; (d) King AE, Huffman LM, Casitas A, Costas M, Ribas X, Stahl SS. J. Am. Chem. Soc. 2010;132:12068. doi: 10.1021/ja1045378. [DOI] [PubMed] [Google Scholar]; (e) Campbell AN, Stahl SS. Acc. Chem. Res. 2012;45:851. doi: 10.1021/ar2002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.