Abstract
A (Mg2+ + Ca2+)ATPase (ATP phosphohydrolase, EC 3.6.1.3) has been purified from sarcoplasmic reticulum using a single step centrifugation procedure. The preparation is >95% pure by weight and contains only 25-30% of the lipid associated with the enzyme in native sarcoplasmic reticulum. The purified enzyme is unable to accumulate Ca2+. Using a sedimentation-substitution technique, >98% of the lipid associated with the purified enzyme can be replaced by dioleoyl lecithin without grossly affecting the ATPase activity of the enzyme. The Ca2+ pump can be restored to this dioleoyl lecithin-substituted enzyme by addition of excess sarcoplasmic reticulum lipids in the presence of cholate. Removal of the cholate by dialysis generates a system which accumulates Ca2+ at a rate and to a level comparable to native sarcoplasmic reticulum. Significant reconstitution of the Ca2+ pump can also be achieved using excess dioleoyl lecithin, but since the full expression of the capacity to accumulate Ca2+ requires the presence of oxalate, these vesicles would appear to be more leaky than those reconstituted with an excess of sarcoplasmic reticulum lipids. Of about 90 lipid molecules which are associated with one molecule of ATPase in native sarcoplasmic reticulum, an average of less than one lipid molecule remains in these reconstituted systems. We have therefore achieved a fully functional Ca2+ pump containing essentially a single protein and exogenous lipid.
Keywords: (Mg2+ + Ca2+)ATPase, sarcoplasmic reticulum, active transport, membrane protein purification, lipid substitution
Full text
PDF

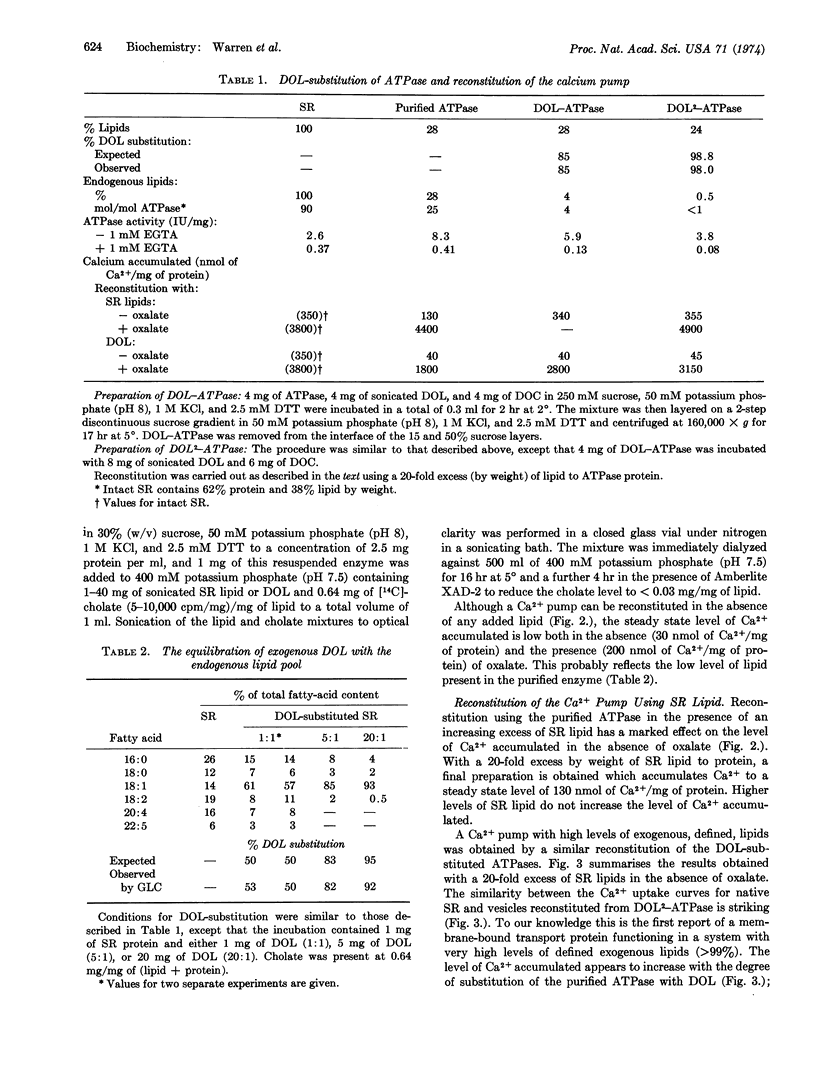
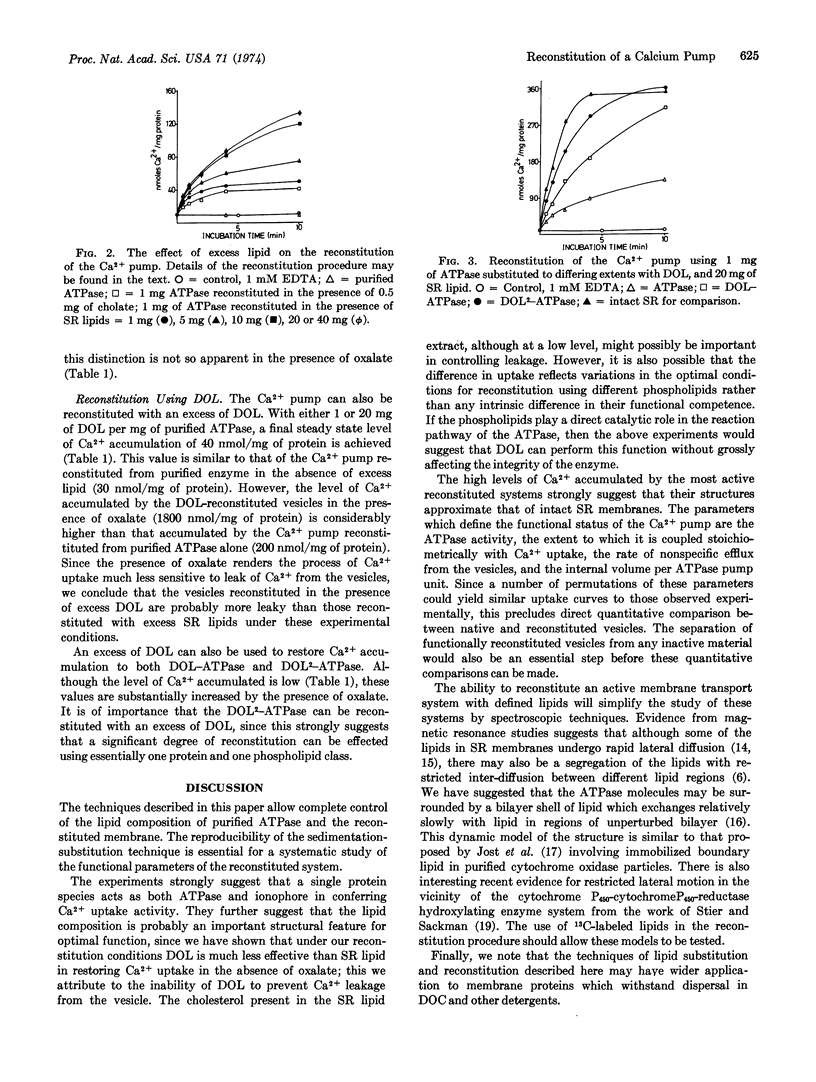

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cubero Robles E., van den Berg D. Synthesis of lecithins by acylation of O-(sn-glycero-3-phosphoryl) choline with fatty acid anhydrides. Biochim Biophys Acta. 1969 Dec 17;187(4):520–526. doi: 10.1016/0005-2760(69)90049-6. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C. Measurement of fast lateral diffusion of lipids in vesicles and in biological membranes by 1 H nuclear magnetic resonance. Biochemistry. 1973 Apr 10;12(8):1650–1659. doi: 10.1021/bi00732a029. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Wong P. T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- Racker E. Reconstitution of a calcium pump with phospholipids and a purified Ca ++ - adenosine triphosphatase from sacroplasmic reticulum. J Biol Chem. 1972 Dec 25;247(24):8198–8200. [PubMed] [Google Scholar]
- Robinson J. D., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C and 1 H nuclear magnetic resonance relaxation measurements of the lipids of sarcoplasmic reticulum membranes. Biochemistry. 1972 Jul 18;11(15):2903–2909. doi: 10.1021/bi00765a025. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Devaux P., McConnell H. M. Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2056–2060. doi: 10.1073/pnas.69.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier A., Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta. 1973 Jul 6;311(3):400–408. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- Stoffel W., LeKim D., Tschung T. S. A simple chemical method for labelling phosphatidylcholine and sphingomyelin in the choline moiety. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1058–1064. doi: 10.1515/bchm2.1971.352.2.1058. [DOI] [PubMed] [Google Scholar]
- Walter H., Hasselbach W. Properties of the calcium-independent ATPase of the membranes of the sarcoplasmic reticulum delipidated by the nonionic detergent Triton X-100. Eur J Biochem. 1973 Jul 2;36(1):110–119. doi: 10.1111/j.1432-1033.1973.tb02890.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]