INTRODUCTION
The burden of gastrointestinal disease in the United States is substantial. Gastroesophageal reflux disease (GERD) constitutes a considerable portion of the disease burden. Recent data indicate that in 2009, GERD was the most common outpatient gastrointestinal diagnosis with nearly 8.9 million outpatient visits 1. Medical therapy is the primary treatment for acid reflux, and this approach is very effective; however, there are several reasons that patients with GERD would like to avoid chronic PPI use. This includes intolerance to the medication, inability to comply with daily medication, and concern about potential long-term adverse effects. In addition to side effects and tolerance issues, chronic PPI use carries a substantial cost for both the patient and third party payers1.
The main alternative to medical therapy is surgery, with laparoscopic Nissen fundoplication (LNF) being the standard of care. Despite the efficacy of surgery, LNF is invasive and carries procedure morbidity such as dysphagia, gas bloat, and modest long term durability. Given these issues, there has been a great deal of interest in developing an intermediate option as an alternative to chronic prescription drug use, without the morbidity related to surgery. During the last 10 years, a multitude of endoscopic therapies have emerged to try to fill this particular need. These therapies can be mechanistically categorized into four groups: 1) radio-frequency energy delivery to the EGJ, 2) endoluminal suturing of the proximal stomach and/or distal esophagus, 3) injection of non-absorbable inert material into luminal wall in the region of the EGJ and 4) plication techniques attempting to simulate fundoplication (Figure 1). All four methods are intended to bolster the antireflux properties of the EGJ to reduce the occurrence of reflux. This review will focus on the biologic plausibility of these techniques, and also provide an assessment based on current data as to whether these techniques have a role in the current paradigm of GERD management.
Figure 1.
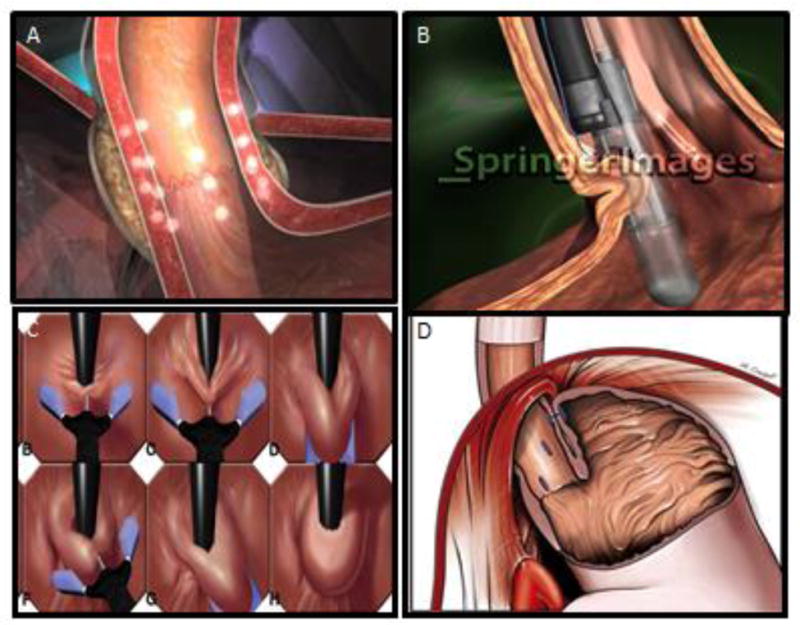
Effect of the various endoscopic procedures on the esophagogastric junction. Panel A) The Stretta effect on the EGJ highlighting the focal thermal injury. Panel B) The Endocinch device illustrating the plication technique. Panel C) The NDO Plicator using a single plication to recreate a flap valve (H). Panel D) The effect of the Esophyx illustrating circumferential plications and the resulting flap valve.
PATHOPHYSIOLOGY OF REFLUX
The fundamental abnormality in GERD is exposure of esophageal or supraesophageal epithelium to gastric juice, resulting in mucosal injury or the elicitation of symptoms. Some degree of gastroesophageal reflux and esophageal acid exposure is considered normal or “physiological” associated with gas venting and belching. GERD results when the balance between what the epithelium is exposed to (related to the frequency of acid reflux, the effectiveness of acid clearance, and the causticity of that reflux) and what that epithelium can tolerate tilts in favor of the aggressive forces. Significant aberrations in one or more potential pathophysiological factors that augment the aggressive forces or deplete the defensive forces can result in shifting from a compensated condition to a decompensated one with the ensuing development of esophagitis or reflux symptoms. Esophagogastric junction (EGJ) competence is the most fundamental defensive factor preventing the complications of GERD, and also the focus of endoscopic therapies for GERD that are the subject of this review. Although esophageal acid clearance, tissue resistance and causticity of the refluxate are also important factors in the pathogenesis of GERD, endoluminal therapies do not primarily target these pathophysiologic mechanisms.
Under normal conditions, reflux of gastric juice into the distal esophagus is prevented as a function of EGJ competence which maintains a closed barrier between the esophagus and the stomach. The EGJ is an anatomically complex zone whose functional integrity as an antireflux barrier has been variably attributed to a number of factors: 1) intrinsic lower esophageal sphincter (LES) pressure, 2) extrinsic compression of the LES by the crural diaphragm, 3) the intra-abdominal location of the LES, 4) integrity of the phrenoesophageal ligament, and 5) maintenance of the acute angle of His promoting a “flap valve” function. Each of these components is operant under specific conditions and the global function of the EGJ as an antireflux barrier is dependent on the sum of the parts. The greater the dysfunction of the individual components that help maintain EGJ competence, the worse the overall antireflux function of the EGJ and by extension, the worse the severity of GERD.
With the above brief overview of the pathogenesis of GERD, one can assess the biologic plausibility of endoluminal therapies. All of these methods are intended to alter the mechanical properties of the EGJ to reduce the occurrence of reflux and, perhaps, the volume of the refluxate. Altered compliance may result from scarring in the case of radiofrequency technique, plication in the case of the various suturing techniques, or by thickening the luminal wall in the case of injection therapies. The net result of these approaches will be to raise the threshold for opening the EGJ and reduce the diameter of the EGJ during reflux events. None of the techniques can adequately address the anatomical issues associated with severe reflux and thus, these techniques will have a limited role in patients with hiatus hernia as evidenced by the use of this as exclusion criteria. Theoretically, the trans-oral fundoplication devices could reduce small hernias and recreate a flap valve similar to the standard laparoscopic fundoplication; however, it still does not address the crural defect and is not appropriate in patients with a large hiatus hernia.
Thus, there is biological plausibility that these devices could reduce reflux burden; however, they are obviously limited by their inability to restore normal anatomy and physiologic function of the antireflux barrier. Conceptually, these devices would likely target patients with mild anatomical defects and thus, they should not be viewed as an alternative to surgery for patients with significant anatomical abnormalities.
EVIDENCE FOR CLINICAL EFFICACY
Whether the biologic plausibility of the endoluminal therapies for GERD translates into clinical outcomes can only be addressed by well-designed clinical trials that use relevant clinical endpoints. Most studies assessing endoluminal antireflux procedures have focused on outcome measures related to symptom response, medication utilization, objective measures of reflux burden during ambulatory testing and manometric measures focused on the LES. Although these variables are reasonable targets, they are far from perfect and thus, they should be evaluated in the context of their limitations (Table 1).
Table 1.
Outcome measures utilized in research trials assessing endoscopic therapy for GERD
| Outcome | Pros | Cons | Bottom Line |
|---|---|---|---|
| Clinical Symptoms |
|
|
Clinical symptoms should be used in well-designed studies, however, they are less helpful in the absence of a sham arm. |
| PPI utilization |
|
|
When PPI utilization is used as an outcome, this should be correlated with objective data to document whether the PPI is used for ongoing abnormal acid reflux, or for dyspeptic symptoms. |
| Reflux Testing |
|
|
Sham arm is important. Unclear if pH testing alone, or combined pH-impedance testing is the optimal strategy. In addition, a correlation with symptoms and PPI use will ultimately be needed. |
| Manometry |
|
|
Is an important marker of device success, however, the clinical relevance is unclear. May be a surrogate marker, but should not be the primary endpoint. |
ENDOLUMINAL THERAPIES
The summary data using level I evidence for both short- and long-term are presented in Tables 2 and 3. Each individual device will be discussed separately.
Table 2.
Randomized Sham controlled trials [Short outcome]
| Device | %AET | Clinical symptoms | PPI withdrawal | LES tone |
|---|---|---|---|---|
| Stretta | ||||
| Corley 2003 | ||||
| Rx | 9.9 | 161 | 58% | 16.2 |
| Sham | 10.7 | 21 | 57% | 18 |
| Arts 2012 | ||||
| Rx | 15 | 8.32 | Na | 13.3 |
| Sham | 9 | 15.6 | na | 16.3 |
| Summary | 0 (ns) | ++ | 0 | 0 |
| Enteryx | ||||
| Deviere 2005 | ||||
| Rx | 11.2 | 14.51 | 67% | NA |
| Sham | 12.7 | 19.9 | 42% | NA |
| Summary | 0 | ++ | + | NA |
| Endocinch | ||||
| Schwartz 2007 | ||||
| Rx | No diff | 153 | 65% | No diff |
| Sham | 8.5 | 25% | ||
| Montgomery 2006 | ||||
| Rx | 6.6 | 94 | 50% | 11.2 |
| Sham | 7.2 | 12 | 25% | 14 |
| Summary | 0 | ++ | ++ | 0 |
| NDO Plicator | ||||
| Rothstein 2006 | ||||
| Rx | 7 | 12.52 | 57% | NA |
| Sham | 10 | 20.1 | 25% | NA |
| Summary | ++ | ++ | ++ | NA |
NA- no data available, 0- no effect, + 20% above sham, ++ 21–50% above sham, +++ >50% above sham
AET, total 24 hour percentage acid exposure time
HRQL
composite score (tack et al 2008 digestion)
frequency x severity heartburn (bais lancet 2000)
GSRS
Table 3.
Randomized Sham controlled trials [≥ 12 month outcomes outcome]
| Device | %AET | Clinical symptoms | PPI withdrawal | LES tone |
|---|---|---|---|---|
| Stretta | ||||
| Aziz 2010 | ||||
| Rx | 6.7 | 14.41 | 16% | 16.2 |
| Sham | 8.2 | 24.8 | 0% | 15.9 |
| Summary | 0 | + | + | 0 |
| Endocinch | ||||
| Montgomery 2006 | ||||
| Rx | 4.7 | 114 | 45% | 9.9 |
| Sham | 7.4 | 12 | 21% | 14 |
| Summary | 0 (NS) | 0 (NS) | 0 (NS) | 0 (NS) |
| Lotus trial | ||||
| PPI | 1.9 | 0 | - | |
| Fundoplication | 0.7 | 82% | - | |
NA- no data available, 0- no effect, + 20% above sham, ++ 21–50% above sham, +++ >50% above sham
%AET, total 24 hour percentage acid exposure time
HRQL
composite score (tack et al 2008 digestion)
frequency x severity heartburn (bais lancet 2000)
GSRS
Stretta
Technical Aspects
The Stretta procedure was first FDA approved in 2000, and was one of the earliest endoscopic devices conceived to treat reflux. The ultimate goal of the procedure was to augment the tone and integrity of the lower esophageal sphincter (LES). Conceptually, however, this procedure would do very little to improve the intrinsic LES function as the mechanism of action is focused on inducing fibrosis in the submucosa and muscle. Hence, this would lead to a less compliant distal esophagus and not impact the muscular function of the LES. The device consists of an ablation catheter and an electrical generator unit. The catheter is 20Fr and has a soft tip bougie configuration. The tip of the bougie contains a balloon that is encased by a basket. The basket has needle electrodes which are used to deliver the radiofrequency energy deep into the submucosa. The device is rotated to allow for circumferential therapy and the catheter continuously irrigates the esophageal lumen to minimize mucosal thermal injury. In animal models, the Stretta device was able to increase gastric yield pressure, and reduce transient lower esophageal sphincter relaxation (tLESR) 2.
Level II Data
The first human study assessing the Stretta procedure was an open label trial involving 20 consecutive patients with GERD. These patients all had objective evidence of GERD with either reflux esophagitis on endoscopy, or abnormal acid exposure on ambulatory pH monitoring. Manometry was performed in all patients. Patients with Barrett’s esophagus, hiatal hernia >2cm, LES pressure <10mmHg or a primary motility disorder were excluded. After undergoing the procedure, patients were given standard quality of life and GERD questionnaires. Complications, patient satisfaction and GERD medication therapy were also monitored as endpoints. In 3 month follow up, patients who underwent the Stretta procedure reported improved quality of life, decreased GERD symptoms and decreased proton pump inhibitor (PPI) use3. This early, open label investigation demonstrated feasibility and relative safety with Stretta and was followed up with a larger multicenter prospective open label study. This study included 118 patients and the duration of the study was extended to 12 months. In addition to GERD and quality of life surveys, this study performed manometry and pH testing at 6 months and 12 months. In regards to the subjective measures, there was significant improvement in GERD-HRQL surveys, SF-36, and patient satisfaction in the 6 and 12 month time point. Objective measures such as %time pH<4 also improved from 10.2 at baseline to 6.4 at 6 month follow up. Interestingly, LES pressure decreased from 15 to 12.6 mmHg. At 12 month follow up, 40% of patients did not require any antireflux medication and 60% needed only as needed antacids4. These findings were corroborated in other similarly designed open label studies5–7.
Level I Studies
There have been three randomized controlled trials of Stretta for the treatment of reflux. All are sham controlled and prospective. The first study was a multicenter prospective double-blind sham controlled trial of Stretta for the treatment of GERD8. Inclusion criteria included both symptoms and pathologic esophageal acid exposure and the primary endpoint was symptom improvement. A priori secondary endpoints included medication use, LES pressure and % acid exposure time (AET). At 6 months, the treatment arm had significant improvement in heartburn score, HRQL and SF-36 compared to the sham arm. Unlike the open label studies, there was no difference in % AET, LES pressure or PPI use in the sham arm compared to the control arm. A subgroup analysis did reveal that patients with symptomatic improvement were more likely to have decreased % AET than non-responders in the treatment arm 8.
The second study was a three-arm sham controlled prospective investigation. The first arm was a “sham” arm where patients underwent sedated endoscopy and had the stretta catheter placed, but not deployed. The second arm received a single session of Stretta, and the third arm included patients who received a single dose of Stretta, but did not attain 75% improvement in the GERD-HRQL survey, and as such, underwent a second session of Stretta [not included in the Table 2]. The primary endpoint was improvement in GERD-HRQL from baseline. The study revealed that GERD-HRQL improved in the sham and treatment arm when comparing pre and post endoscopy scores. The extent of GERD-HRQL improvement was greater in the treatment group compared to the sham group, but this was not significant. Interestingly, the %AET improved in both the sham and treatment arm (8.2 vs 6.7), though it was only statistically significant in the treatment arm. Mean LES pressure was also increased in the treatment arm, but this was not much different from the sham arm (15.9 vs 16.2)9.
The third RCT was a prospective double blind sham controlled study. This was a more rigorous investigation which blinded both patient and investigator. Inclusion criteria were abnormal % AET along with the presence of reflux symptoms. The study was designed as a crossover with all patients acting as their own personal control. Patients were initially randomized to receive either sham or Stretta. At the three month point, patients who received sham initially then received Stretta, and patients who received Stretta initially, received sham. Motility studies and ambulatory pH was reported at baseline, 3 months and 6 months after the initial procedure. Symptom scores were significantly better in the treatment arm at three months when comparing to the sham arm (15.6 vs 8.3 P<0.05). At the 6 month point, all patients received treatment. In patients who initially received sham, the symptom scores improved after crossing over to Stretta. LES pressure was unchanged after Stretta treatment in both arms. In addition, there was no change in %AET or need for PPI in either arm after Stretta10
Complications
Post procedure events were common, but often self-limited. The most commonly reported post procedure event was chest pain, which was almost universal after Stretta. This is relevant, especially when taken into the context of a sham study, where study patients may have known that they received treatment. Transient fever and esophageal ulcer were also reported. Serious post procedure events include necrotizing pancreatitis and prolonged gastroparesis. When reviewing the MAUDE database (a manufacturer and user self-reported database of adverse events), there were several reports of gastroparesis. The cause for gastroparesis is presumed to be related to inadvertent vagal nerve injury. In addition, unlike the published reports, there were several reports of suspected esophageal perforation.
Summary
The Stretta device revealed promising results with early open label trials. However, the randomized sham controlled trials did not support the findings of the open label trials. Thus, high quality evidence suggests that the Stretta procedure only provides a mild subjective improvement in symptoms but no objective improvement in reflux burden, EGJ function or reduction in PPI utilization. The mechanism of the symptom improvement has been postulated to be related to alteration in esophageal visceral afferent fibers resulting from thermal injury. The lack of improvement in objective parameters, along with complications noted that are not much less frequent or severe compared to fundoplication make this approach less attractive. Further studies are unlikely to change this recommendation and thus, we would not recommend utilization of Stretta for the treatment of GERD.
Enteryx
Technical Aspects
Enteryx is a biodegradeable ethylene-vinyl-alcohol copolymer that is used as a sphincter bulking agent. It is directly injected into the lower esophageal sphincter using a sclerotherapy needle. Initial animal models revealed relative tolerability of Enteryx injection in the lower esophageal sphincter, however manometric characteristics were not sufficiently different after injection.
Level Il Data
The initial multicenter open label study included 85 patients with GERD symptoms and abnormal pH studies. The primary outcome was PPI usage 6 months post procedure. Secondary outcomes included GERD symptoms, quality of life and % AET at 6 months. In regards to the primary endpoint, 74% of patients were not taking any PPI at 6 months post procedure, and 10% had a 50% reduction in PPI usage11. In the 12 month follow up of this cohort 70% of patients were able to discontinue PPI entirely12. Symptom scores and quality of life 6 months post procedure was markedly improved from scores taken pre procedure off medication, and were comparable to scores pre procedure while taking PPI. %AET 6 months after Enteryx injection revealed improvement in % AET from 9.5% to 7%, with a majority of the improvement in supine % AET. LES pressure was unchanged. At 12 month follow up, the reduction in % AET continued to be significant compared to baseline, but the difference was numerically small. Similar improvement in PPI usage was noted in a European cohort study13, however, pH testing did not reveal any significant decrease in % acid exposure 12 months post treatment compared to baseline. Furthermore, there were no differences in manometric characteristics of the LES 12 months after Enteryx injection13
Level I Evidence
The same European group performed a single blind prospective sham controlled investigation of Enteryx. This group randomized 32 patients to Enteryx and 32 to sham (endoscopy without intervention). At the 3 month time point, 81% of Enteryx treated patients achieved the primary endpoint of a 50% reduction in PPI use compared to 53% of sham treated patients. 68% of Enteryx treated had discontinued all PPI use compared to 41% of sham treated patients.14
Complications
Common published adverse events include chest pain and dysphagia. Occasional self-limited fever have also been reported. More concerning were the increasing reports of serious mediastinal injury related to Enteryx injection. Case reports have revealed mediastinal abscess and mediastinitis. Review of the MAUDE database indicated numerous cases of mediastinal injury, abscess and perforation. In addition, there were reported cases of inadvertent injection into the aorta as well as embolization of the enteryx polymer into distal organs. These events led to the voluntary withdrawal of Enteryx from the market.
Summary
The Enteryx procedure was relatively simple and did not require advance endoscopic training, which made it an attractive option for most general gastroenterologists. The single Level I study did reveal a modest effect in terms of symptom control and PPI utilization. However, Enteryx was taken off the market voluntarily due to multiple serious adverse events 15–17 after the FDA issued a warning on October 14, 2005 related to these complications.
Endocinch
Technical Aspects
The Endocinch was manufactured by BARD and was first introduced by Swain and collegues as an internal plication device to mimic the effect of surgical plication to enhance the antireflux barrier. The plication achieved with Endocinch is mucosal, and unlike the devices described later, is not full thickness. An initial multicenter prospective study investigated 64 patients who underwent Endocinch. The authors note marked improvement in heartburn severity, frequency and regurgitation in 3 and 6 months post procedure. Medication usage had also decreased at 3 and 6 months. There was a modest, though statistically significant, improvement in % AET and upright, but not supine reflux events. The authors did note that accurate placement of the plicator stitch was challenging18.
Level II Data
A follow up investigation with one year follow up evaluating 21 patients was then reported. All patients had GERD symptoms and abnormal % AET. At 12 month follow up, GERD symptom score and regurgitation score was significantly improved from baseline. The DeMeester score was mildly decreased, but total % AET was no different pre and post procedure. PPI use had decreased from 100% to 36% at 12 month. Three of twenty-one patients had significant adverse events19. Three subsequent follow up studies showed lack of durability of response to Endocinch, despite short term favorable outcomes20–22. This lack of durable response was felt to mirror the lack of durability of suture retention as many of the patients with recurrent symptoms did not have visible suture23.
Level I Data
Two randomized, sham controlled trials have been published. The first study had three arms, treatment, sham and control. The primary outcomes were PPI usage and symptoms scores, with % AET as a secondary outcome. Three months post treatment, PPI usage dropped significantly in the Endocinch group compared to sham. Interestingly, PPI usage was also decreased in the sham group compared to the observation group. At one year, roughly 50% of the Endocinch were considered treatment failures and only 29% were able to discontinue PPI altogether. There was no difference in % AET in the treatment group compared to sham. This study concluded that despite early symptom improvement, Endocinch failed to yield sustained symptom improvement and control of intraesophageal acid24. The findings were confirmed in a second sham controlled RCT. In this study 46 patients were randomized to either Endocinch or sham procedure. Manometry and pH testing were performed at 3 and 12 months. There were no differences in LES pressure or % AET at either 3 or 12 months in the Endocinch group compared to sham. PPI use was slightly decreased in the treatment group at 3 months, but this was not sustained at 12 months. Regarding symptoms, gastrointestinal symptom rating scale (GSRS) scores were significantly better at 3 months in the treatment arm, but by 12 months, there was no difference between treatment and sham25.
Complications
Most complications associated with Endocinch were mild and self-limited. The most common being sore throat, which is likely related to the overtube required for this device. Serious complications noted in the open label studies as well as the MAUDE database were bleeding events requiring intervention or transfusion. This is likely from tissue ulceration or inadvertent damage to a submucosal blood vessel during suture deployment.
Summary
The pooled data for Endocinch suggest that there may be a possible modest benefit in clinical symptoms and medication use at 3 months, but this benefit is not durable and still associated with mild complications. The lack of durability may be related to the inability of Endocinch to provide a full thickness plication. Based on high quality data, there is strong evidence to support that the Endocinch device is not clinically useful in the long-term management of GERD.
Endoscopic Plication System
Technical Aspects
The Endoscopic plication system (EPS NDO surgical) is similar to the Endocinch in that the goal is to form a gastric plication to tighten the EGJ. Unlike Endocinch, it is a full thickness plication with serosa to serosa healing. The device contains a channel where a pediatric gastroscope fits to view the plication under retroflexion. The goal is plication of the stomach to the distal esophagogastric junction. The first pilot investigation enrolled 7 patients with reflux. Six of the seven patients were able to undergo the procedure. GERD symptoms improved at 3 and 6 months and no major complications were reported26.
Level II Data
Several prospective open label investigations have occurred since the initial safety/feasibility study. A multicenter prospective study included 64 patients with abnormal % AET and GERD symptoms27. Only 57 were available for follow up. At 12 months post procedure, GERD-HRQL and SF-36 scores improved significantly, and 63% of patients were able to discontinue PPI. In addition, % AET decreased at 6 months, though only 30% of patients achieved normalization.
Level I Data
A sham controlled RCT was performed with the EPS. In total 78 patients were randomized to full thickness plication and 81 underwent sham (defined as undergoing placement of the endoscopic device without deploying the plication suture.) At three month follow up, GERD symptom scores were significantly better in the treatment group compared to sham, and comparable to baseline scores on PPI. In an intention to treat analysis, complete PPI cessation was seen in 50% of the treatment group compared to 24% of the sham group. % AET was significantly improved with 23% achieving normalization of % AET in the treatment arm compared to the sham arm with 15% achieving normalization. Significantly more patients in the treatment arm reported adverse events, though these were mild and self-limited28.
While the RCT revealed promising symptom scores, the improvement in objective measures was only modest. As such, there has been a follow up investigation using multiple plications in each patient in an attempt to improve reflux parameters. In a multicenter investigation, 41 patients with two or more plications were evaluated with 6 month follow up data. While clinical symptoms improved, median %AET only decreased from 11 to 9, with only 31% achieving complete normalization of % AET29.
Complication
Similar to the Endocinch, sore throat was the most common self-limited complaint, and is related to the use of a large overtube. Interestingly, there were several reports of shoulder pain related to the Plicator. Some were mild and self-limited, and others were persistent for several months. This suggests a probable inadvertent diaphragm injury during deployment of the transmural plication. Indeed there have been published reports of pneumothorax and pneumoperitoneum suggesting diaphragm and pleura injury. Review of the MAUDE database reveals several accounts of diaphragmatic injury, shoulder pain and pneumoperitoneum.
Summary
The data from the RCTs was encouraging and the finding of better results with multiple plications without an increase in adverse events supported that this device could have clinical utility. However, the company ceased operations in 2008 and the device is no longer clinically available.
Esophyx
Technical Aspects
Similar to the EPS plicator, transoral incisionless fundoplication with Esophyx creates serosa to serosa plications. The main difference is the ability to perform circumferential, transmural plications with Esophyx. The device consists of a flexible catheter that contains a tissue retractor and fasteners. The endoscope fits within this catheter. It is placed orally, and with the endoscope retroflexed in the stomach, the tissue retractor facilitates apposition of the gastric cardia to the distal esophagus.
Level II Data
The initial multicenter prospective study of Esophyx enrolled 86 patients with GERD symptoms requiring daily PPI use with pathologic % AET. The follow up was 12 months. This study reported 3 major adverse events, two esophageal perforations and one major luminal hemorrhage. One month post procedure, there were few reported adverse events. Regarding clinical outcomes, GERD-HRQL were significantly improved at 6 months. This improvement was sustained at 12 months. Regurgitation scores were also improved. 68% of patients were able to discontinue PPI use at 6 months, which was sustained at 12 months. % AET was decreased compared to baseline, but only normalized in 37% of patients30. In one of the centers, clinical outcomes of improved GERD symptoms persisted in 2 year follow up, and PPI use was eliminated in 71% of patients31. Three year follow up from the multicenter cohort revealed sustained subjective improvement, with PPI cessation in 61%. A second group evaluated 48 patients for up to 24 months post TIF. Symptom scores were improved consistently at 6, 12 and 24 months. Twenty-four months post procedure, 42% of patients had discontinued their PPI, however, there were no changes in Demeester score or reflux events (acidic, weakly acidic or total) at 24 months compared to pre procedure32. In a small group of patients who underwent impedence testing 6 months post procedure, it appeared that total and acidic reflux events were slightly reduced33. In a community setting, TIF was studied in 100 consecutively enrolled patients. Of these, 51 had laryngopharyngeal reflux (LPR). At 6 month follow up, 42 of the 51 LPR patients were off PPI and noted subjective improvement in their LPR symptoms. In total 80 out of the initial 100 patients were off PPI at 6 month follow up. There was improvement in % acid exposure, but normalization in only 15 of the 28 patients available for follow up 34
Level I Data
At present, there are no published placebo/sham randomized trials evaluating TIF. However, there is an ongoing prospective investigation that is currently enrolling patients. It is a randomized controlled trial comparing TIF to sham (RESPECT study). The primary endpoint is a reduction in GERD symptoms at 6 months, with secondary outcomes being normalization of esophageal AET and healing of esophagitis.
Complications
Similar to the Plicator, published studies reveal that shoulder pain is a relatively common early complaint after TIF with Esophyx. In addition, abdominal pain and self-limited bleeding was commonly reported. Published reports also noted severe hemorrhage and one case of cervical esophageal perforation. Review of the MAUDE database revealed several accounts of life threatening episodes of hemorrhage. In addition, there were several accounts of pleural effusion and perforation. These complications are likely related to inadvertent vascular or adjacent organ damage related to blind placement of the TIF fasteners. Further data will be needed to see if image guided deployment (ie fluoroscopy or endosonography) can prevent such complications.
Summary
To date, there have not been any sham controlled, or even randomized/blinded studies evaluating the efficacy of TIF. As with other devices, it appears that Esophyx is able to improve subjective parameters, but does not adequately improve objective parameters such as % AET or reflux events on impedance monitoring. Among the previously discussed therapies, TIF most closely mimics the effect of LNF with the exception of the crural repair. Long term data reveal no new adverse events up to 3 years, however there are several reports of early complications such as bleeding and perforation. The plication appears durable during long term follow up; however, in one prospective study, a majority of patients required either ongoing PPI use or were referred for LNF due to persistent symptoms 35 Studies suggest that in patients with persistent symptoms after TIF, LNF is associated with increased complication such as gastric perforation36. A multicenter placebo/sham randomized trial comparing TIF to PPI in patients with GERD is ongoing. Thus, there is low to moderate data to support a weak recommendation that Esophyx could be a viable treatment for GERD. However, we would recommend that this device be used in the context of research trials (including strict post-marketing surveillance) to establish efficacy and safety.
Comparison of Risks
The risks associated with laparoscopic fundoplication are well established in both high level clinical trials and long-term experience from prospective and retrospective evaluation of large cohorts. The mortality related to laparascopic fundoplication is low (<1%). Complications related to LNF can be categorized as immediate post procedural, delayed post procedure, and treatment failures. Early complications include perforation (0–4%), bleeding (<1%) and pneumothorax (0–10%)37. The most common delayed complication is gas-bloat, which occurs to some degree, in almost all patients. Roughly 25% of patients can experience dysphagia persistent after 3 months post fundoplication; however, most patients do not require significant intervention38.
The need for proton pump inhibitors after LNF is surprisingly common, as shown by Spechler et al, with roughly 50% of patient needing daily PPI 10 years after LNF39. Follow up studies indicate that only a fraction of patients taking PPI after fundoplication have abnormal acid reflux, with the majority taking PPI for nonspecific dyspeptic symptoms 40. Perhaps the most concerning late complication is the need for revisional surgery. The indication for revisional fundoplication is typically due to persistent reflux symptoms, dysphagia or herniation. Symptoms usually present within two years of the initial fundoplication and herniation may be an early or late complication. The redo-fundoplication is considerably more complicated, and associated with higher perioperative risk38. As such, it should only be undertaken by experienced foregut surgeons.
Future Directions
Given the waning enthusiasm for endoscopic procedures, a shift toward creating alternatives for fundoplication that are less invasive and associated with less dysphagia and gas-bloat are currently being investigated. Recently, there has been interest in the LINX device. Though not an endoscopic device, the LINX is a ring of magnetic beads placed around the esophagus to bolster the EGJ during laparoscopy. Prospectively collected data suggest relative safety and efficacy with 3 year follow up and objective parameters improved at 12 months41. This data, however, is neither randomized nor blinded. Furthermore, it is unclear from the current data whether this benefit was the result of concomitant surgical crural repair or from the device alone. As such, this data should be interpreted with caution and the device is currently limited to centers of excellence for continued post-approval assessment.
Another non-endoscopic device that is also being investigated is the implantable EndoStim LES stimulator which has been shown to improve LES pressure without altering deglutitive relaxation42. This device was assessed in an open label trial in 24 patients and shown to improve both symptom score and objective parameters of reflux burden 43. Although the device has received the CE Mark approval, it is currently not available in the United States.
Conclusions
The current data support that the risk/benefit of endoluminal therapies do not favor utilization of these techniques in our current management paradigm for GERD. The effectiveness of these devices is mild to modest compared to sham procedures in high quality studies and the risks have either been too great or not studied to the degree that we can confidently state that these approaches are safer than fundoplication. This is in line with the current recommendations of both the AGA and ACG regarding utilization of endoscopic therapies in the management of GERD38, 44 In fact, two devices are no longer available and currently there is only one device (Esophyx) that has not been fully vetted in a randomized controlled trial.
Although the current state of endoscopic antireflux procedures would seem discouraging, we have learned a great deal regarding GERD pathophysiology from these trials and we have also learned that our current treatment modalities are very good (Table 3). The data from the Lotus trial suggest that both PPI therapy and Fundoplication are safe and effective and thus, the bar was set quite high in terms of our expectations for these devices. While potential long-term risks of PPI therapy still requires further evaluation, the risk/benefit ratio favors treatment in patients with documented GERD. Similarly, Fundoplication remains an effective treatment for patients who are intolerant to medical treatment, unwilling to take PPIs and refractory to PPI therapy if there is objective evidence that ongoing reflux is the cause of the refractory symptoms. Thus, there is no urgency in developing new treatments for GERD, and we have the luxury of time so that new devices, such as the Esophyx, EndoStim and Linx, can be adequately assessed before they are considered ready for widespread use.
Table 4.
Complications associated with endoscopic antireflux procedures.
| Device | Self-limited complications (published) | Serious complications (published) | Unpublished complications (MAUDE database) |
|---|---|---|---|
| Stretta | Chest pain +++ Fever ++ Esophagitis/Ulcer + Nausea |
Gastroparesis + Necrotizing pancreatitis |
Esophageal perforation + Gastroparesis |
| Enteryx | Chest pain +++ Dysphagia +++ Fever +++ Ulcer |
Esophageal Abscess Pneumomediastinum Mediastinal injury |
Mediastinal Injury +++ Vascular penetration + Pneumonitis ++ Embolization |
| Endocinch | Sore throat +++ Nausea ++ Dysphagia ++ Hemorrhage ++ |
Hemorrhage | Bleeding Esophageal ulcer |
| Plicator | Sore throat +++ Chest pain +++ Shoulder pain ++ Hemorrhage+ |
Pneumoperitoneum Pneumothorax |
Diaphragm injury Pneumoperitoneum Pneumothorax Perforation |
| Esophyx | Abdominal pain + Shoulder pain++ Hemorrhage ++ Dysphagia |
Hemorrhage Shoulder pain Cervical esophageal perforation |
Perforation + Pleural effusion + Hemorrhage |
| Fundoplication |
Chest Pain Dysphagia Bloating |
Perforation Repeat surgery Gastroparesis |
NA |
| PPI therapy |
Headache Diarrhea |
Hypomagnesemia C. Difficile colitis Pneumonia Hip fracture |
NA |
Relative frequency of reporting:
( ) Rare- <5 cases
(+) 5–10 cases
(++) 11–20 cases
(+++) >20 cases
Acknowledgments
This work was supported by R01 DK079902 (JEP) from the Public Health Service
Abbreviations
- EGJ
esophagogastric junction
- LES
lower esophageal sphincter
Footnotes
Conflicts of Interest: JEP: Astra Zeneca [Speaker]
KK: none
References
- 1.Dominitz JA, Dire CA, Billingsley KG, et al. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol. 2006;4:299–305. doi: 10.1016/j.cgh.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Utley DS, Kim M, Vierra MA, et al. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the gastroesophageal junction: a porcine model. Gastrointest Endosc. 2000;52:81–6. doi: 10.1067/mge.2000.105981. [DOI] [PubMed] [Google Scholar]
- 3.Richards WO, Scholz S, Khaitan L, et al. Initial experience with the stretta procedure for the treatment of gastroesophageal reflux disease. J Laparoendosc Adv Surg Tech A. 2001;11:267–73. doi: 10.1089/109264201317054546. [DOI] [PubMed] [Google Scholar]
- 4.Triadafilopoulos G, DiBaise JK, Nostrant TT, et al. The Stretta procedure for the treatment of GERD: 6 and 12 month follow-up of the U.S. open label trial. Gastrointest Endosc. 2002;55:149–56. doi: 10.1067/mge.2002.121227. [DOI] [PubMed] [Google Scholar]
- 5.Go MR, Dundon JM, Karlowicz DJ, et al. Delivery of radiofrequency energy to the lower esophageal sphincter improves symptoms of gastroesophageal reflux. Surgery. 2004;136:786–94. doi: 10.1016/j.surg.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Cipolletta L, Rotondano G, Dughera L, et al. Delivery of radiofrequency energy to the gastroesophageal junction (Stretta procedure) for the treatment of gastroesophageal reflux disease. Surg Endosc. 2005;19:849–53. doi: 10.1007/s00464-004-2169-4. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi K, Fujiwara Y, Okazaki H, et al. Feasibility, safety, and efficacy of the Stretta procedure in Japanese patients with gastroesophageal reflux disease: first report from Asia. J Gastroenterol. 2007;42:205–10. doi: 10.1007/s00535-006-1944-5. [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Katz P, Wo JM, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology. 2003;125:668–76. doi: 10.1016/s0016-5085(03)01052-7. [DOI] [PubMed] [Google Scholar]
- 9.Aziz AM, El-Khayat HR, Sadek A, et al. A prospective randomized trial of sham, single-dose Stretta, and double-dose Stretta for the treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:818–25. doi: 10.1007/s00464-009-0671-4. [DOI] [PubMed] [Google Scholar]
- 10.Arts J, Bisschops R, Blondeau K, et al. A double-blind sham-controlled study of the effect of radiofrequency energy on symptoms and distensibility of the gastro-esophageal junction in GERD. Am J Gastroenterol. 2012;107:222–30. doi: 10.1038/ajg.2011.395. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DA, Ganz R, Aisenberg J, et al. Endoscopic, deep mural implantation of Enteryx for the treatment of GERD: 6-month follow-up of a multicenter trial. Am J Gastroenterol. 2003;98:250–8. doi: 10.1111/j.1572-0241.2003.07291.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DA, Ganz R, Aisenberg J, et al. Endoscopic implantation of enteryx for treatment of GERD: 12-month results of a prospective, multicenter trial. Am J Gastroenterol. 2003;98:1921–30. doi: 10.1111/j.1572-0241.2003.08109.x. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher B, Neuhaus H, Ortner M, et al. Reduced medication dependency and improved symptoms and quality of life 12 months after enteryx implantation for gastroesophageal reflux. J Clin Gastroenterol. 2005;39:212–9. doi: 10.1097/01.mcg.0000152751.10268.fa. [DOI] [PubMed] [Google Scholar]
- 14.Deviere J, Costamagna G, Neuhaus H, et al. Nonresorbable copolymer implantation for gastroesophageal reflux disease: a randomized sham-controlled multicenter trial. Gastroenterology. 2005;128:532–40. doi: 10.1053/j.gastro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Tintillier M, Chaput A, Kirch L, et al. Esophageal abscess complicating endoscopic treatment of refractory gastroesophageal reflux disease by Enteryx injection: a first case report. Am J Gastroenterol. 2004;99:1856–8. doi: 10.1111/j.1572-0241.2004.40554.x. [DOI] [PubMed] [Google Scholar]
- 16.Noh KW, Loeb DS, Stockland A, et al. Pneumomediastinum following Enteryx injection for the treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:723–6. doi: 10.1111/j.1572-0241.2005.41293.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong RF, Davis TV, Peterson KA. Complications involving the mediastinum after injection of Enteryx for GERD. Gastrointest Endosc. 2005;61:753–6. doi: 10.1016/s0016-5107(04)02645-8. [DOI] [PubMed] [Google Scholar]
- 18.Filipi CJ, Lehman GA, Rothstein RI, et al. Transoral, flexible endoscopic suturing for treatment of GERD: a multicenter trial. Gastrointest Endosc. 2001;53:416–22. doi: 10.1067/mge.2001.113502. [DOI] [PubMed] [Google Scholar]
- 19.Mahmood Z, McMahon BP, Arfin Q, et al. Endocinch therapy for gastro-oesophageal reflux disease: a one year prospective follow up. Gut. 2003;52:34–9. doi: 10.1136/gut.52.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abou-Rebyeh H, Hoepffner N, Rosch T, et al. Long-term failure of endoscopic suturing in the treatment of gastroesophageal reflux: a prospective follow-up study. Endoscopy. 2005;37:213–6. doi: 10.1055/s-2005-860994. [DOI] [PubMed] [Google Scholar]
- 21.Arts J, Lerut T, Rutgeerts P, et al. A one-year follow-up study of endoluminal gastroplication (Endocinch) in GERD patients refractory to proton pump inhibitor therapy. Dig Dis Sci. 2005;50:351–6. doi: 10.1007/s10620-005-1610-4. [DOI] [PubMed] [Google Scholar]
- 22.Schiefke I, Zabel-Langhennig A, Neumann S, et al. Long term failure of endoscopic gastroplication (EndoCinch) Gut. 2005;54:752–8. doi: 10.1136/gut.2004.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmood Z, Zaheer A, Ang YS, et al. Endocinch treatment for gastro-oesophageal reflux (GORD): retention of plications are essential to control GORD. Gut. 2007;56:1027. doi: 10.1136/gut.2007.122978. author reply 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz MP, Wellink H, Gooszen HG, et al. Endoscopic gastroplication for the treatment of gastro-oesophageal reflux disease: a randomised, sham-controlled trial. Gut. 2007;56:20–8. doi: 10.1136/gut.2006.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery M, Hakanson B, Ljungqvist O, et al. Twelve months’ follow-up after treatment with the EndoCinch endoscopic technique for gastro-oesophageal reflux disease: a randomized, placebo-controlled study. Scand J Gastroenterol. 2006;41:1382–9. doi: 10.1080/00365520600735738. [DOI] [PubMed] [Google Scholar]
- 26.Lubezky N, Goykhman Y, Nakache R, et al. Early and Late Presentations of Graft Arterial Pseudoaneurysm Following Pancreatic Transplantation. World J Surg. 2013 doi: 10.1007/s00268-013-1972-2. [DOI] [PubMed] [Google Scholar]
- 27.Blanc PD, Quinlan PJ, Katz PP, et al. Higher environmental relative moldiness index values measured in homes of adults with asthma, rhinitis, or both conditions. Environ Res. 2013 doi: 10.1016/j.envres.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothstein R, Filipi C, Caca K, et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: A randomized, sham-controlled trial. Gastroenterology. 2006;131:704–12. doi: 10.1053/j.gastro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 29.von Renteln D, Schiefke I, Fuchs KH, et al. Endoscopic full-thickness plication for the treatment of GERD by application of multiple Plicator implants: a multicenter study (with video) Gastrointest Endosc. 2008;68:833–44. doi: 10.1016/j.gie.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Cadiere GB, Buset M, Muls V, et al. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg. 2008;32:1676–88. doi: 10.1007/s00268-008-9594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadiere GB, Van Sante N, Graves JE, et al. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication using EsophyX. Surg Endosc. 2009;23:957–64. doi: 10.1007/s00464-009-0384-8. [DOI] [PubMed] [Google Scholar]
- 32.Testoni PA, Vailati C, Testoni S, et al. Transoral incisionless fundoplication (TIF 2. 0) with EsophyX for gastroesophageal reflux disease: long-term results and findings affecting outcome. Surg Endosc. 2012;26:1425–35. doi: 10.1007/s00464-011-2050-1. [DOI] [PubMed] [Google Scholar]
- 33.Testoni PA, Corsetti M, Di Pietro S, et al. Effect of transoral incisionless fundoplication on symptoms, PPI use, and ph-impedance refluxes of GERD patients. World J Surg. 2010;34:750–7. doi: 10.1007/s00268-010-0394-7. [DOI] [PubMed] [Google Scholar]
- 34.Bell RC, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215:794–809. doi: 10.1016/j.jamcollsurg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Witteman BP, Strijkers R, de Vries E, et al. Transoral incisionless fundoplication for treatment of gastroesophageal reflux disease in clinical practice. Surg Endosc. 2012;26:3307–15. doi: 10.1007/s00464-012-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furnee EJ, Broeders JA, Draaisma WA, et al. Laparoscopic Nissen fundoplication after failed EsophyX fundoplication. Br J Surg. 2010;97:1051–5. doi: 10.1002/bjs.7078. [DOI] [PubMed] [Google Scholar]
- 37.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383–1391. 1391 e1–5. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 39.Khajanchee YS, Ujiki M, Dunst CM, et al. Patient factors predictive of 24-h pH normalization following endoluminal gastroplication for GERD. Surg Endosc. 2009;23:2525–30. doi: 10.1007/s00464-009-0448-9. [DOI] [PubMed] [Google Scholar]
- 40.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392–1413. 1413 e1–5. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 41.Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:719–27. doi: 10.1056/NEJMoa1205544. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez L, Rodriguez P, Neto MG, et al. Short-term electrical stimulation of the lower esophageal sphincter increases sphincter pressure in patients with gastroesophageal reflux disease. Neurogastroenterol Motil. 2012;24:446–50. e213. doi: 10.1111/j.1365-2982.2012.01878.x. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez L, Rodriguez P, Gomez B, et al. Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: final results of open-label prospective trial. Surg Endosc. 2012 doi: 10.1007/s00464-012-2561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2012;108:308–28. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]


