Abstract
Methamphetamine (METH) abuse results in long-term damage to the dopaminergic system, manifesting as decreases in dopamine (DA) tissue content, DA transporter (DAT) binding, as well as tyrosine hydroxylase (TH) and vesicular monoamine transporter (VMAT) immunostaining. However, the exact cascade of events that ultimately results in this damage has not been clearly elucidated. One factor that has been heavily implicated in METH-induced DA terminal degeneration is the production of nitric oxide (NO). Unfortunately, many of the studies attempting to clarify the role of NO in METH-induced neurotoxicity have been confounded by issues such as the disruption of METH-induced hyperthermia, preventing the formation of strong conclusions. As a result, there is a body of work suggesting that NO is sufficient for METH-induced neurotoxicity, while other studies suggest that NO does not play a role in METH-induced degeneration of DA nerve terminals. This review summarizes the existing studies investigating the role of NO in METH-induced neurotoxicity, and argues that while NO may be necessary for METH-induced neurotoxicity, it is not sufficient. Finally, important areas of future investigation are highlighted and discussed.
Keywords: Dopamine, Methamphetamine, Neurotoxicity Nitric Oxide, Nitric Oxide Synthase
Methamphetamine (METH) abuse continues to be a significant public health concern in the United States. According to the National Survey on Drug Use and Health, approximately 12 million Americans report using METH at least once in their lifetime (SAMHSA/OSM, 2011). Additionally, new evidence indicates that individuals with a history of METH abuse have an increased risk for developing Parkinson’s Disease, compared to both healthy controls and individuals with a history of cocaine use (Callaghan et al., 2010; Callaghan et al., 2012). Thus, abuse of amphetamines, and METH in particular, continues to be a significant public health concern and will remain a significant burden to society for years to come. Importantly, exposure to high doses or repeated administration of METH results in long-lasting damage to the dopamine (DA) system. This damage includes reduced levels of DA (Kogan et al., 1976; Wagner et al., 1980), tyrosine hydroxylase (TH; (Kogan et al., 1976), dopamine transporter (DAT; (Volkow et al., 2001a; Volkow et al., 2001b; McCann et al., 2008), and vesicular monoamine transporter levels (VMAT; (Guilarte et al., 2003) in human METH abusers and animal models. Given the high rates of abuse, as well as the significant damage to the DA system that occurs with METH exposure, studies elucidating factors associated with METH-induced neurotoxicity are essential for prevention of the toxicity and for the development of therapeutic treatments for individuals with a history of METH abuse. One factor that has been heavily implicated in METH-induced neurotoxicity is the production of nitric oxide (NO). This review summarizes the work examining the role of NO in METH-induced neurotoxicity and suggests areas of future study.
Nitric Oxide and Nitric Oxide Synthase
Nitric oxide is a gaseous neuromodualator implicated in various physiological processes, including neuroplasticity (Wang et al., 2005; Serulle et al., 2007), neurovascular coupling (Faraci and Breese, 1993), and neuronal excitability (Centonze et al., 2001; West and Grace, 2004). However, NO has also been implicated in neuronal damage (Louin et al., 2006; Mohammadi et al., 2012) and various central nervous system (CNS) diseases including Alzheimer’s disease (Sultana et al., 2006), Parkinson’s disease (Hunot et al., 1996), and Multiple Sclerosis (Bo et al., 1994), suggesting that in addition to playing a role in normal CNS function, NO may also play a role in neurodegeneration.
Nitric oxide is synthesized by a family of proteins termed nitric oxide synathase (NOS), of which there are three distinct isoforms. The two constitutively expressed, Ca2+-dependent isoforms are neuronal nitric oxide synthase (nNOS or NOS-I) and endothelial nitric oxide synthase (eNOS or NOS-III; (Bredt and Snyder, 1990; Forstermann et al., 1991). These two isoforms are basally expressed in their respective cell types under normal conditions. In the brain, nNOS is predominately expressed by neurons (Bredt et al., 1990; Bredt and Snyder, 1990), although some data suggest possible astrocytic expression of nNOS, as well (Arbones et al., 1996). Importantly, in striatum, nNOS is expressed by a subpopulation of interneurons that co-express GABA, somatostatin (SST), and neuropeptide Y (Kawaguchi et al., 1995; Figueredo-Cardenas et al., 1996). Endothelial nitric oxide synthase is expressed predominantly by endothelial cells (Seidel et al., 1997; Stanarius et al., 1997), although neuronal expression in hippocampus (Dinerman et al., 1994; O'Dell et al., 1994) and astrocytic expression (Lin et al., 2007) have also been described. The third isoform, inducible nitric oxide synthase (iNOS or NOS-II), is not basally expressed under normal conditions, but rather is transcriptionally induced and activated in a Ca2+-independent manner (Yui et al., 1991) during inflammatory reactions via a cytokine-mediated cascade (Lowenstein et al., 1993; Xie et al., 1993; Lin and Murphy, 1997; Park et al., 1997). Inducible nitric oxide synthase is expressed mainly by astrocytes, microglia, and macrophages throughout the brain (Endoh et al., 1994; Liu et al., 1996). Therefore, although all three isoforms convert the precursor l-arginine to NO and l-citrulline, each isoform arises from a different gene product (Janssens et al., 1992; Geller et al., 1993; Hall et al., 1994) with unique expression patterns.
Under normal conditions, NO plays a role in normal physiological processes; however, over-production of NO may result in CNS damage. For example, under normal conditions, NO is known to bind soluble guanylate cyclase (sGC) resulting in activation of the enzyme, cyclic guanosine monophosphate (cGMP) production, and activation of cellular events downstream of cGMP (Stone and Marletta, 1996). However, NO has also been heavily implicated in several CNS injuries and neurodegenerative diseases. In particular, NO can interact with superoxide (02) to form peroxynitrite (ONOO−; (Beckman et al., 1990), a potent oxidant (Radi et al., 1991). Peroxynitrite, in turn, can interact with various cellular targets, resulting in protein nitration, lipid peroxidation (Rubbo et al., 1994), and DNA damage (Salgo et al., 1995; Yermilov et al., 1995; Yermilov et al., 1996). More specifically, peroxynitrite can interrupt cellular respiration by inhibiting components of the mitochondrial electron transport chain, including complexes I and III (Radi et al., 1994; Clementi et al., 1998; Riobo et al., 2001). NO can also directly nitrate proteins, resulting in protein/enzyme malfunction (Konorev et al., 1998; Blanchard-Fillion et al., 2001). Thus, while it is clear that NO is an important mediator of normal physiological processes, NO can also be detrimental to cellular function, and overproduction of NO can thus contribute to cellular injury.
To date, several studies have described increased NO production following METH exposure. For example, detection of nitrated proteins, an indirect measure of peroxynitrite formation, is increased in striatum following METH exposure (Imam et al., 1999; Imam et al., 2000; Anderson and Itzhak, 2006; Wang et al., 2008; Friend et al., 2013). Our lab has also shown an increase in NADPH diaphorase histochemical staining (Friend et al., 2013), a measure of NOS activity (Dawson et al., 1991; Hope et al., 1991), following exposure to a neurotoxic regimen of METH. Furthermore, the co-administration of peroxynitrite decomposition catalysts or NOS inhibitors is reported to result in decreased NO production following METH exposure (Imam et al., 1999; Imam et al., 2000). Thus, it is apparent that METH results in NO production.
Source of Nitric Oxide Following Methamphetamine Exposure
Given the increase in NO production following METH exposure and data suggesting roles for NO and peroxynitrite in neurodegeneration, several studies have attempted to identify which isoform of NOS contributes to the METH-induced NO production. One study examined nNOS and iNOS expression in striatum and found that nNOS protein, as well as the number of cells positive for NADPH-diaphorase histochemical staining, were increased following METH exposure (Deng and Cadet, 1999). However, since this study was published, our lab and others have examined nNOS expression via immunohistochemistry (Wang and Angulo, 2011) or in situ hybridization (Friend et al., 2013) and have failed to see any change in the amount of nNOS expression at either the mRNA or protein level. Additionally, we also examined the number of cells with histochemical staining for NADPH diaphorase—a stain produced by the enzymatic activity of NOS (Hope et al., 1991)—and again, we did not observe a METH-induced change in the number of cells positively stained. It is possible that the discrepancy between the studies reflect a mouse vs. rat difference, as differences in nNOS expression have been observed between species and strains of animals within a species (Blackshaw et al., 2003). Furthermore, it is generally accepted that nNOS is constitutively expressed and that NO production via nNOS arises as a consequence of Ca2+-calmodulin and Ca2+ influx through NMDA receptors (Bredt and Snyder, 1990; Sattler et al., 1999). In fact, although we have not observed changes in the numbers of cells expressing nNOS mRNA or the number of cells positive for NADPH diaphorase histochemical staining, we did observe an increase in total NADPH diaphorase histochemical staining (i.e. percent of the total imaged field with signal; (Friend et al., 2013). These data suggest that METH increases NO production via activation of constitutively expressed nNOS rather than a change in its expression.
Inducible nitric oxide synthase expression has also been examined following a neurotoxic regimen of METH, and no induction of iNOS protein was observed (Deng and Cadet, 1999). However, Deng and Cadet examined iNOS expression at 1hr, 24hr, and 1 week following exposure to a neurotoxic regimen of METH—time points at which glial cells, the cell types in which induction of iNOS mRNA expression typically occurs (Gibson et al., 2005), may not be fully reactive (LaVoie et al., 2004). Therefore, we examined iNOS mRNA expression in animals 1 hr and also 48 hr following a neurotoxic regimen of METH, as glial reactivity is maximal at 48 hr after exposure to a neurotoxic regimen of METH (LaVoie et al., 2004). Consistent with the data from Deng and Cadet (Deng and Cadet, 1999), we also failed to see any induction of iNOS mRNA (Friend et al., 2013). Thus, because iNOS must be transcriptionally induced in order to produce NO (Lowenstein et al., 1993; Xie et al., 1993), these data suggest that NO is not produced via iNOS after exposure to a neurotoxic regimen of METH.
Finally, our lab is the first to have examined eNOS mRNA expression following a neurotoxic regimen of METH. As was the case for iNOS, we did not observe any change in eNOS expression in animals sacrificed 1 hr or 48 hr after exposure to the neurotoxic regimen of METH (Friend et al., 2013). However, given that eNOS is also constitutively expressed, there remains the possibility that eNOS may contribute, at least in part to METH-induced NO production. In this regard, our data show that when we limit our analysis of NOS activity to the nNOS expressing interneurons in striatum by excluding blood vessels from the analysis of the NADPH diaphorase histochemical staining, we still observe an increase in NOS activity, suggesting that eNOS expressing endothelial cells are not contributing to METH-induced increases in NOS activation. Taken together, these data suggest that nNOS, rather than eNOS, is the source of NO production in response to METH exposure. However, a better general understanding of how the constitutively expressed NOS isoforms are regulated will lead to a more definitive answer regarding the particular isoforms responsible for METH-induced NO production. For instance, studies examining NO in the context of long-term potentiation (LTP) in the hippocampus have demonstrated compensatory interactions between nNOS and eNOS. These data show that LTP is disrupted only if both nNOS and eNOS are eliminated (Son et al., 1996), suggesting that in the absence of one isoform of NOS the other may suffice in generating the NO necessary for LTP to occur. If a similar scenario exists in the context of METH-induced neurotoxicity, then it is conceivable that either isoform may contribute to METH-induced increases in NO.
Nitric Oxide in Methamphetamine-Induced Neurotoxicity
Several attempts have been made to elucidate the role of NO in METH-induce DA nerve terminal degeneration by using either pharmacological or genetic manipulations. Unfortunately, these studies have been inconclusive. For example, the co-administration of peroxnitrite decomposition catalysts with METH protects against METH-induced DA depletions (Imam et al., 1999). Furthermore, studies using genetic manipulations have shown that METH-induced DA depletions are blocked in mice with deletion of nNOS (Itzhak et al., 1998; Itzhak et al., 2000b) and partially attenuated in mice with deletion of iNOS (Itzhak et al., 1999; Itzhak et al., 2000b), suggesting a role for NO and its downstream mediator, peroxynitrite, in METH-induced neurotoxicity. However, although the use of peroxynitrite decomposition catalysts or the use of nNOS and iNOS knockout mice afforded protection against the neurotoxic effects of METH, these manipulations also mitigated the METH-induced hyperthermia (Itzhak et al., 1998; Imam et al., 1999; Itzhak et al., 1999) known to be tightly associated METH-induced monoamine toxicity (Ali et al., 1994; Bowyer et al., 1994). In fact, simply cooling animals during METH exposure protects animals against METH-induced toxicity (Ali et al., 1994). Therefore it is difficult to determine whether the attenuation of neurotoxicity is a result of the manipulations of the NOS system or whether it arose from the mitigation of METH-induced hyperthermia. Finally, while some studies suggest protection against METH-induced DA depletions when NOS inhibitors are co-administered with METH (Di Monte et al., 1996; Itzhak and Ali, 1996; Ali and Itzhak, 1998; Itzhak et al., 2000a), others suggest that the neuroprotective effects of NOS inhibitors simply result from mitigation of METH-induced hyperthermia (Taraska and Finnegan, 1997; Callahan and Ricaurte, 1998). Therefore, results from the work using pharmacological inhibition of NOS in the context of METH-induced neurotoxicity remain inconclusive. Conducting these studies while carefully controlling for METH-induced hyperthermia (i.e. placing knockout animals in an environment with increased ambient temperature to maintain METH-induced hyperthermia) should lead to more conclusive results in this regard. Additionally, studies using knockdown approaches, particularly in specific cell types, (e.g. shRNA driven by cell type specific promoters such as SST) should more clearly elucidate not only the NOS isoform contributing to increased NO during exposure to METH, but also the particular cell population involved.
Adding further debate to the role of NO in METH-induced neurotoxicity are studies that use other manipulations in attempts to clarify its role in METH-induced neurotoxicity. For example, ablation of nNOS-expressing interneurons in striatum does not protect against METH-induced TH or DAT depletions (Zhu et al., 2006; Fricks-Gleason and Keefe, 2013); however, there was incomplete mitigation of METH-induced NO production in such preparations (Fricks-Gleason and Keefe, 2013) raising questions as to whether the NO detected could be produced by constitutively expressed eNOS or result from diffusion away from residual nNOS-containing interneurons.
An alternative conclusion for the results of these studies is that NO is not sufficient for METH-induced neurotoxicity. To address this possibility, our lab utilized animals resistant to the acute neurotoxic consequences of METH exposure. In this model, animals were initially treated with METH or saline on PND60 and then allowed to recover for 30 days. At PND90, the rats were treated again with either METH or saline, resulting in four treatment groups based on PND60:PND90 treatment (Saline:Saline, Saline:METH, METH:Saline, and METH:METH). Under this paradigm, we and others have found that animals with partial DA loss induced by a neurotoxic regimen of METH fail to exhibit further decreases in striatal DA when re-exposed to METH at PND90 (Thomas and Kuhn, 2005; Hanson et al., 2009). Using this paradigm we were able to compare changes in NOS enzyme activity and protein nitration in animals experiencing acute toxicity when exposed to METH at PND90 (i.e. the saline:METH group) compared to animals not experiencing acute toxicity when exposed to METH at PND90 (i.e. the METH:METH group; (Friend et al., 2013). We found that both protein nitration and NOS activity were increased in both groups exposed to METH at PND90 (i.e. Saline:METH and METH:METH). Thus, NO was produced regardless of whether an animal was experiencing acute toxicity or not. These data, combined with data showing that METH exposure results in DA terminal damage in several brain regions (i.e. amygdala, hippocampus, and cortex) that do not exhibit changes in protein nitration (Anderson and Itzhak, 2006), indicate a significant dissociation between indices of NO production and acute DA neuron toxicity, suggesting that generation of NO is not sufficient for METH-induced DA toxicity.
Although NO does not appear to be sufficient for METH-induced DA nerve terminal degeneration, it may be necessary when toxicity does occur, as NO may act together with other factors under those conditions to contribute to the toxicity. Also, it is important to note that while this review has focused on the role, or lack thereof, of METH-induced NO production in DA nerve terminal degeneration, there remains the possibility that METH-induced NO production may contribute to METH-induced apoptosis of cells postsynaptic to DA nerve terminals. For example, ablation of nNOS-expressing interneurons in striatum prior to a bolus regimen of METH (1 injection of 30 mg/kg) protected against METH-induced cell death in striatum, but not DA depletions (Zhu et al., 2006; Wang et al., 2008; Zhu et al., 2009). Further, manipulations that decrease METH-induced protein nitriation also mitigate METH-induced striatal cell death (Zhang et al., 2013). Therefore, while METH-induced NO production may not be sufficient for METH-induced DA terminal degeneration, it may contribute to METH-induced striatal neuron apoptosis. Together, these data may suggest that mechanisms underlying METH-induced DA depletions differ from those underlying METH-induced striatal cell death, and thus studies examining the distinct mechanisms mediating METH-induced DA terminal degeneration versus METH-induced apoptosis are needed.
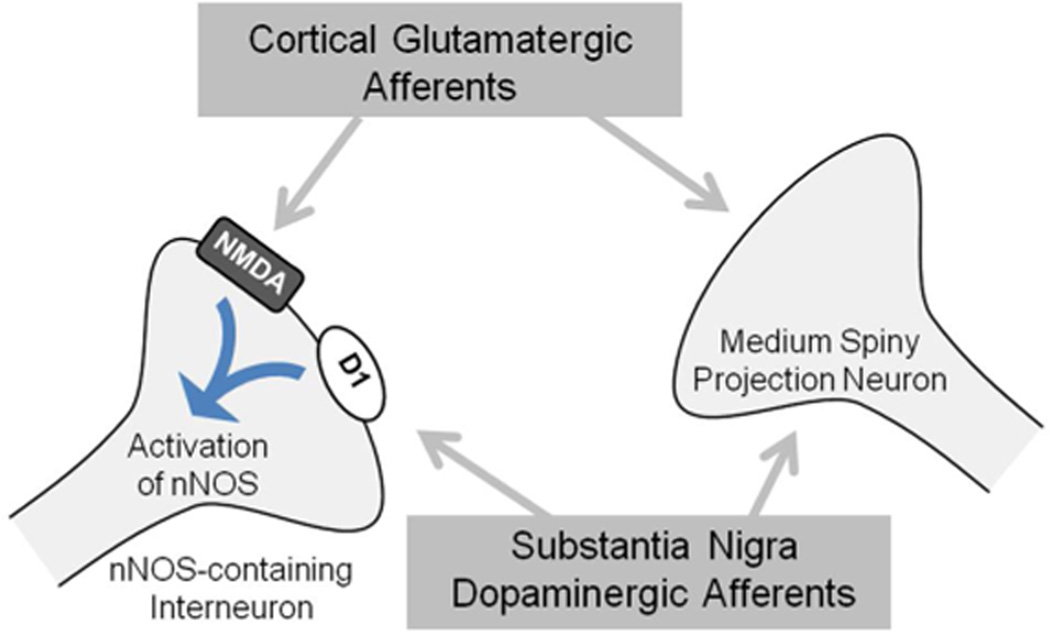
Finally, given the significant glutamatergic and dopaminergic inputs to striatum, increased NO production during and following METH exposure may simply result from activity of these inputs. In fact, several studies have demonstrated how the projections to striatum influence the production of NO via nNOS. Both DA and glutamate (GLU) can regulate NO production via nNOS-expressing interneurons in striatum (Figure 1). Furthermore, the striatum receives extensive glutamatergic inputs from the cortex and the thalamus (Kemp and Powell, 1971; Kitai et al., 1976; Gerfen, 1989; Bellomo et al., 1998). The nNOS-expressing interneurons in striatum express NMDA-type GLU receptors (Gracy and Pickel, 1997), and intra-striatal infusion of NMDA receptor agonists (Iravani et al., 1998; Rossetti and Crespi, 2004) or application in vitro (Garthwaite et al., 1988; Bredt and Snyder, 1989) increases NO production. Furthermore, stimulation of corticostriatal afferents, both in vitro and in vivo, increases the production of NO via an nNOS-dependent mechanism (Kawaguchi, 1993; Sammut et al., 2007). In addition to GLU influencing NO production from nNOS-expressing interneurons, nNOS production can also be influenced by DA. For instance, the striatum also receives extensive dopaminergic inputs from the substantia nigra (Gerfen et al., 1987; Kubota et al., 1988; Vuillet et al., 1989), and nNOS-expressing neurons are known to express D1-type (D1 and D5) DA receptors (Le Moine et al., 1991; Rivera et al., 2002; Centonze et al., 2003). D1-type DA receptor or substantia nigra (Sammut et al., 2006) stimulation induces the production of NO (Le Moine et al., 1991; Sammut et al., 2006) and increases NADPH diaphorase staining in striatum (Morris et al., 1997; Hoque et al., 2010). Finally, NMDA and D1 DA receptor activation work together to increase NO production in striatum (Park and West, 2009). Therefore, these data, combined with studies demonstrating significant increases in both GLU (Nash and Yamamoto, 1992; Mark et al., 2004) and DA (O'Dell et al., 1991; Nash and Yamamoto, 1992; O'Dell et al., 1993) during and following exposure to a neurotoxic regimen of METH, suggest that NO produced by nNOS during METH exposure may simply be a readout of NMDA and DA receptor stimulation rather than a contributor to the neurotoxic process. Studies using specific manipulations of NMDA or DA receptors and then examining NO production during METH exposure would more directly address this possibility.
Figure 1.
Model of striatal nNOS regulation (adapted from West, 2010). nNOS-containing interneurons receive input from corticostriatal and nigralstriatal projections. nNOS-containing interneurons express both NMDA and D1 receptors. Stimulation of NMDA receptors activates nNOS to produce NO. In addition to corticostriatal activation of NMDA receptors, nigrostriatal DA inputs activate D1 receptors, increasing nNOS activity.
In conclusion, while a significant amount of data suggest that NO may play an important role in METH-induced DA terminal degeneration a growing amount of data also suggest that this NO production may not be sufficient for such neurotoxicity. Future work carefully manipulating the nitric oxide syanthases while controlling for METH-induced neurotoxicity will more clearly answer this question.
Contributor Information
Danielle M. Friend, Email: da.friend@utah.edu.
Ashley N. Fricks-Gleason, Email: a.fricks@utah.edu.
Kristen A. Keefe, Email: k.keefe@utah.edu.
References
- Ali SF, Itzhak Y. Effects of 7-nitroindazole, an NOS inhibitor on methamphetamine-induced dopaminergic and serotonergic neurotoxicity in mice. Ann N Y Acad Sci. 1998;844:122–130. [PubMed] [Google Scholar]
- Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Itzhak Y. Methamphetamine-induced selective dopaminergic neurotoxicity is accompanied by an increase in striatal nitrate in the mouse. Ann N Y Acad Sci. 2006;1074:225–233. doi: 10.1196/annals.1369.021. [DOI] [PubMed] [Google Scholar]
- Arbones ML, Ribera J, Agullo L, Baltrons MA, Casanovas A, Riveros-Moreno V, Garcia A. Characteristics of nitric oxide synthase type I of rat cerebellar astrocytes. Glia. 1996;18:224–232. doi: 10.1002/(SICI)1098-1136(199611)18:3<224::AID-GLIA6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo M, Giuffrida R, Palmeri A, Sapienza S. Excitatory amino acids as neurotransmitters of corticostriatal projections: immunocytochemical evidence in the rat. Arch Ital Biol. 1998;136:215–223. [PubMed] [Google Scholar]
- Blackshaw S, Eliasson MJ, Sawa A, Watkins CC, Krug D, Gupta A, Arai T, Ferrante RJ, Snyder SH. Species, strain and developmental variations in hippocampal neuronal and endothelial nitric oxide synthase clarify discrepancies in nitric oxide-dependent synaptic plasticity. Neuroscience. 2003;119:979–990. doi: 10.1016/s0306-4522(03)00217-3. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fillion B, Souza JM, Friel T, Jiang GC, Vrana K, Sharov V, Barron L, Schoneich C, Quijano C, Alvarez B, Radi R, Przedborski S, Fernando GS, Horwitz J, Ischiropoulos H. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J Biol Chem. 2001;276:46017–46023. doi: 10.1074/jbc.M105564200. [DOI] [PubMed] [Google Scholar]
- Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Callahan BT, Ricaurte GA. Effect of 7-nitroindazole on body temperature and methamphetamine-induced dopamine toxicity. Neuroreport. 1998;9:2691–2695. doi: 10.1097/00001756-199808240-00001. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Pisani A, Bonsi P, Giacomini P, Bernardi G, Calabresi P. Stimulation of nitric oxide-cGMP pathway excites striatal cholinergic interneurons via protein kinase G activation. J Neurosci. 2001;21:1393–1400. doi: 10.1523/JNEUROSCI.21-04-01393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Cadet JL. Methamphetamine administration causes overexpression of nNOS in the mouse striatum. Brain Res. 1999;851:254–257. doi: 10.1016/s0006-8993(99)02087-9. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, Royland JE, Jakowec MW, Langston JW. Role of nitric oxide in methamphetamine neurotoxicity: protection by 7-nitroindazole, an inhibitor of neuronal nitric oxide synthase. J Neurochem. 1996;67:2443–2450. doi: 10.1046/j.1471-4159.1996.67062443.x. [DOI] [PubMed] [Google Scholar]
- Dinerman JL, Dawson TM, Schell MJ, Snowman A, Snyder SH. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci U S A. 1994;91:4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994;651:92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- Figueredo-Cardenas G, Morello M, Sancesario G, Bernardi G, Reiner A. Colocalization of somatostatin, neuropeptide Y, neuronal nitric oxide synthase and NADPH-diaphorase in striatal interneurons in rats. Brain Res. 1996;735:317–324. doi: 10.1016/0006-8993(96)00801-3. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Pollock JS, Schmidt HH, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Keefe KA. Evaluating the Role of Neuronal Nitric Oxide Synthase-Containing Striatal Interneurons in Methamphetamine-Induced Dopamine Neurotoxicity. Neurotox Res. 2013 doi: 10.1007/s12640-013-9391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Son JH, Keefe KA, Fricks-Gleason AN. Expression and activity of nitric oxide synthase isoforms in methamphetamine-induced striatal dopamine toxicity. J Pharmacol Exp Ther. 2013;344:511–521. doi: 10.1124/jpet.112.199745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Geller DA, Lowenstein CJ, Shapiro RA, Nussler AK, Di Silvio M, Wang SC, Nakayama DK, Simmons RL, Snyder SH, Billiar TR. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993;90:3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Coughlan TC, Murphy SP. Glial nitric oxide and ischemia. Glia. 2005;50:417–426. doi: 10.1002/glia.20143. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Ultrastructural localization and comparative distribution of nitric oxide synthase and N-methyl-D-aspartate receptors in the shell of the rat nucleus accumbens. Brain Res. 1997;747:259–272. doi: 10.1016/s0006-8993(96)01249-8. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA. Structural organization of the human neuronal nitric oxide synthase gene (NOS1) J Biol Chem. 1994;269:33082–33090. [PubMed] [Google Scholar]
- Hanson JE, Birdsall E, Seferian KS, Crosby MA, Keefe KA, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced dopaminergic deficits and refractoriness to subsequent treatment. Eur J Pharmacol. 2009;607:68–73. doi: 10.1016/j.ejphar.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque KE, Indorkar RP, Sammut S, West AR. Impact of dopamine-glutamate interactions on striatal neuronal nitric oxide synthase activity. Psychopharmacology (Berl) 2010;207:571–581. doi: 10.1007/s00213-009-1687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, Hirsch EC. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Crow JP, Newport GD, Islam F, Slikker W, Jr, Ali SF. Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837:15–21. doi: 10.1016/s0006-8993(99)01663-7. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Islam F, Itzhak Y, Slikker W, Jr, Ali SF. Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci. 2000;914:157–171. doi: 10.1111/j.1749-6632.2000.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Millar J, Kruk ZL. Differential release of dopamine by nitric oxide in subregions of rat caudate putamen slices. J Neurochem. 1998;71:1969–1977. doi: 10.1046/j.1471-4159.1998.71051969.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Gandia C, Huang PL, Ali SF. Resistance of neuronal nitric oxide synthase-deficient mice to methamphetamine-induced dopaminergic neurotoxicity. J Pharmacol Exp Ther. 1998;284:1040–1047. [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ail SF. nNOS inhibitors attenuate methamphetamine-induced dopaminergic neurotoxicity but not hyperthermia in mice. Neuroreport. 2000a;11:2943–2946. doi: 10.1097/00001756-200009110-00022. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. Methamphetamine- and 1-methyl-4-phenyl- 1,2,3, 6-tetrahydropyridine-induced dopaminergic neurotoxicity in inducible nitric oxide synthase-deficient mice. Synapse. 1999;34:305–312. doi: 10.1002/(SICI)1098-2396(19991215)34:4<305::AID-SYN6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. Comparison between the role of the neuronal and inducible nitric oxide synthase in methamphetamine-induced neurotoxicity and sensitization. Ann N Y Acad Sci. 2000b;914:104–111. doi: 10.1111/j.1749-6632.2000.tb05188.x. [DOI] [PubMed] [Google Scholar]
- Janssens SP, Shimouchi A, Quertermous T, Bloch DB, Bloch KD. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992;267:14519–14522. [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971;262:429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Kocsis JD, Wood J. Origin and characteristics of the cortico-caudate afferents: an anatomical and electrophysiological study. Brain Res. 1976;118:137–141. doi: 10.1016/0006-8993(76)90848-9. [DOI] [PubMed] [Google Scholar]
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur J Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- Konorev EA, Hogg N, Kalyanaraman B. Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett. 1998;427:171–174. doi: 10.1016/s0014-5793(98)00413-x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Inagaki S, Kito S, Shimada S, Okayama T, Hatanaka H, Pelletier G, Takagi H, Tohyama M. Neuropeptide Y-immunoreactive neurons receive synaptic inputs from dopaminergic axon terminals in the rat neostriatum. Brain Res. 1988;458:389–393. doi: 10.1016/0006-8993(88)90484-2. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Murphy S. Regulation of astrocyte nitric oxide synthase type II expression by ATP and glutamate involves loss of transcription factor binding to DNA. J Neurochem. 1997;69:612–616. doi: 10.1046/j.1471-4159.1997.69020612.x. [DOI] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili O, Talman WT. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res. 2007;1171:42–51. doi: 10.1016/j.brainres.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao ML, Brosnan CF, Lee SC. Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1beta and IL-1 receptor antagonist. J Immunol. 1996;157:3569–3576. [PubMed] [Google Scholar]
- Louin G, Marchand-Verrecchia C, Palmier B, Plotkine M, Jafarian-Tehrani M. Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury. Neuropharmacology. 2006;50:182–190. doi: 10.1016/j.neuropharm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Mohammadi MT, Shid-Moosavi SM, Dehghani GA. Contribution of nitric oxide synthase (NOS) in blood-brain barrier disruption during acute focal cerebral ischemia in normal rat. Pathophysiology. 2012;19:13–20. doi: 10.1016/j.pathophys.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Simpson CS, Mundell S, Maceachern K, Johnston HM, Nolan AM. Dynamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacology. 1997;36:1589–1599. doi: 10.1016/s0028-3908(97)00159-7. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J Neurochem. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Park DJ, West AR. Regulation of striatal nitric oxide synthesis by local dopamine and glutamate interactions. J Neurochem. 2009;111:1457–1465. doi: 10.1111/j.1471-4159.2009.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Lin HL, Murphy S. Nitric oxide regulates nitric oxide synthase-2 gene expression by inhibiting NF-kappaB binding to DNA. Biochem J. 1997;322(2):609–613. doi: 10.1042/bj3220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, Poderoso JJ. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J. 2001;359:139–145. doi: 10.1042/0264-6021:3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvaez JA, de la Calle A, Moratalla R. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Crespi F. Inhibition of nitric oxide release in vivo by ethanol. Alcohol Clin Exp Res. 2004;28:1746–1751. doi: 10.1097/01.alc.0000145755.72834.f1. [DOI] [PubMed] [Google Scholar]
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- Salgo MG, Bermudez E, Squadrito GL, Pryor WA. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes [corrected] Arch Biochem Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SAMHSA/OSM. Results from the 2010 National Survey on Drug Use and Health. 2011 [Google Scholar]
- Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR. Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacology. 2006;31:493–505. doi: 10.1038/sj.npp.1300826. [DOI] [PubMed] [Google Scholar]
- Sammut S, Park DJ, West AR. Frontal cortical afferents facilitate striatal nitric oxide transmission in vivo via a NMDA receptor and neuronal NOS-dependent mechanism. J Neurochem. 2007;103:1145–1156. doi: 10.1111/j.1471-4159.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Seidel B, Stanarius A, Wolf G. Differential expression of neuronal and endothelial nitric oxide synthase in blood vessels of the rat brain. Neurosci Lett. 1997;239:109–112. doi: 10.1016/s0304-3940(97)00912-9. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman MC, Kandel ER. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- Stanarius A, Topel I, Schulz S, Noack H, Wolf G. Immunocytochemistry of endothelial nitric oxide synthase in the rat brain: a light and electron microscopical study using the tyramide signal amplification technique. Acta Histochem. 1997;99:411–429. doi: 10.1016/S0065-1281(97)80034-7. [DOI] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Taraska T, Finnegan KT. Nitric oxide and the neurotoxic effects of methamphetamine and 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1997;280:941–947. [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Vuillet J, Kerkerian L, Kachidian P, Bosler O, Nieoullon A. Ultrastructural correlates of functional relationships between nigral dopaminergic or cortical afferent fibers and neuropeptide Y-containing neurons in the rat striatum. Neurosci Lett. 1989;100:99–104. doi: 10.1016/0304-3940(89)90667-8. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wang J, Angulo JA. Synergism between methamphetamine and the neuropeptide substance P on the production of nitric oxide in the striatum of mice. Brain Res. 2011;1369:131–139. doi: 10.1016/j.brainres.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu W, Ali SF, Angulo JA. Connection between the striatal neurokinin-1 receptor and nitric oxide formation during methamphetamine exposure. Ann N Y Acad Sci. 2008;1139:164–171. doi: 10.1196/annals.1432.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR. Nitric Oxide Signaling in Striatum. In: Steiner HaT, KY., editor. Handbook of the Basal Ganglia Structure and Function. Elsevier Inc; 2010. pp. 187–196. [Google Scholar]
- West AR, Grace AA. The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity States and electrophysiological properties of striatal medium spiny neurons recorded in vivo. J Neurosci. 2004;24:1924–1935. doi: 10.1523/JNEUROSCI.4470-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermilov V, Rubio J, Ohshima H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376:207–210. doi: 10.1016/0014-5793(95)01281-6. [DOI] [PubMed] [Google Scholar]
- Yermilov V, Yoshie Y, Rubio J, Ohshima H. Effects of carbon dioxide/bicarbonate on induction of DNA single-strand breaks and formation of 8-nitroguanine, 8-oxoguanine and base-propenal mediated by peroxynitrite. FEBS Lett. 1996;399:67–70. doi: 10.1016/s0014-5793(96)01288-4. [DOI] [PubMed] [Google Scholar]
- Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C. Purification of nitric oxide synthase from rat macrophages. J Biol Chem. 1991;266:12544–12547. [PubMed] [Google Scholar]
- Zhang F, Chen L, Liu C, Qiu P, Wang A, Li L, Wang H. Up-regulation of protein tyrosine nitration in methamphetamine-induced neurotoxicity through DDAH/ADMA/NOS pathway. Neurochem Int. 2013;62:1055–1064. doi: 10.1016/j.neuint.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Zhu J, Xu W, Wang J, Ali SF, Angulo JA. The neurokinin-1 receptor modulates the methamphetamine-induced striatal apoptosis and nitric oxide formation in mice. J Neurochem. 2009;111:656–668. doi: 10.1111/j.1471-4159.2009.06330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. Distinct mechanisms mediating methamphetamine-induced neuronal apoptosis and dopamine terminal damage share the neuropeptide substance p in the striatum of mice. Ann N Y Acad Sci. 2006;1074:135–148. doi: 10.1196/annals.1369.013. [DOI] [PMC free article] [PubMed] [Google Scholar]