Abstract
Non-verbal referential communication is impaired in children with Autism Spectrum Disorders (ASD). However, the development of difficulties with referential communication in the younger siblings of children with ASD (High-Risk Siblings)—and the degree to which early referential communication predicts later autism symptomatology—is not clear. We modeled the early developmental trajectories of three types of referential communication: responding to joint attention (RJA), initiating joint attention (IJA), and initiating behavioral requests (IBR) across 8, 10, 12, 15, and18 months of age in High-Risk Siblings (n = 40) and the infant siblings of children without ASD (Low-Risk Siblings; n = 21). Hierarchical Linear Modeling indicated that High-Risk Siblings exhibited lower levels of baseline RJA and IJA and a lower rate of linear change in IBR than Low-Risk Siblings. When the 10 High-Risk Siblings who received an ASD diagnosis were excluded from analyses, group differences in the development of referential communication remained significant only for RJA. Baseline levels of IJA were associated with later ASD symptomatology among High-Risk Siblings, suggesting that individual differences in referential communication development at 8 months may index early manifestations of ASD.
Keywords: Autism Spectrum Disorders, at-risk infants, referential communication, developmental trajectories, symptom severity
Prior to the development of verbal communication, infants communicate with social partners about objects and events through the use of eye contact and/or gestures: non-verbal referential communication. These abilities emerge and develop between 8 and 18 months of age. Responding to joint attention (RJA), initiating joint attention (IJA), and initiating behavioral requests (IBR) are three classes of behaviors central to early, non-verbal referential communication. In RJA, infants respond to and follow the joint attention behavior (e.g., pointing) of a social partner. This ability emerges around 6 months of age in typically developing infants, with improving accuracy in identifying the intended target through 18 months of age (e.g., Bakeman & Adamson, 1984; Sullivan et al., 2007). In IJA, infants convey interest about an object or event to a social partner (Jones & Carr, 2004; Messinger & Fogel, 1998, Mundy & Burnette, 2005). In typically developing infants, IJA is demonstrated by 9 months of age and becomes more complex (e.g., coordinating eye contact with gestures) through the second year of life (e.g., Bakeman & Adamson, 1984). In IBR, infants convey requests for help or a specific object (Mundy, Sigman, Ungerer, and Sherman, 1986). The development of IBR is less frequently discussed, but appears to emerge within the second half of the first year of life (e.g., Bates, Camaioni, & Volterra, 1975) during typical development. Mundy and colleagues (2007) documented the developmental course of these non-verbal referential communication abilities in typically developing infants, providing a basis from which to investigate the trajectories of these abilities in atypical populations during the first two years of life.
While these behaviors are keystones in the typical development of social communication, impairments in non-verbal referential communication are characteristic deficits in children with an Autism Spectrum Disorder (ASD). However, the early developmental trajectories of these important social communication abilities are not well-documented in children with ASD. Yoder, Stone, Walden, & Malesa (2009) documented the development of weighted triadic communication, a composite of verbal and non-verbal referential communication, and RJA in the infant siblings of children with an ASD (High-Risk Siblings) beginning in the second year of life. The current study seeks to build upon this work by examining the early developmental trajectories of IJA, RJA, and IBR prior to 12 months of age, in order to understand the earliest development of these crucial social communication abilities in High-Risk Siblings. In addition, we ask whether the early developmental trajectories of RJA, IJA, and IBR predict later severity of ASD symptomatology in infants at risk for ASD.
Referential Communication in ASD
Joint attention deficits appear to be integrally related to impairments in social cognition in children with ASD such as theory of mind (Mundy, Sullivan, & Mastergeorge, 2009; Mundy & Newell, 2007). Decreased levels of IJA in young children with ASD distinguish them both from typically developing children and from children with other disabilities (Baranek, 1999; Dawson et al., 2004; Jones & Carr, 2004, Mundy et al., 1986). Although behavioral requesting impairments are not as pronounced as joint attention deficits in older children with an ASD (Mundy, Sigman, & Kasari, 1990), requesting is a key referential communication behavior that mediates early infant-parent interaction (Messinger & Fogel, 1998) and is a component of gold standard autism assessments like the Autism Diagnostic Observation Schedule (Lord et al., 1999).
The infant siblings of children with an ASD (High-Risk Siblings) are at heightened risk (18.7%) of themselves developing an ASD (Ozonoff et al., 2011). In addition, as many as 40% of High-Risk Siblings exhibit sub-clinical deficits in social and communicative functioning, such as atypical eye contact and difficulty relating to others, which are indicative of a broader autism phenotype (Constantino et al., 2006; Dawson et al., 2002; Landa & Garrett Mayer, 2006; Losh, Sullivan, Trembath, & Piven, 2008; Zwaigenbaum et al., 2005). Due to their increased genetic vulnerability and the opportunity they present for studying early development prospectively, High-Risk Siblings have been the focus of many recent studies characterizing the early development of ASD symptomatology, including possible deficits in referential communication.
It is not clear whether High-Risk Siblings engage in lower levels of referential communication than the infant siblings of children without an ASD (Low-Risk Siblings) in the first two years of life. While some studies find that High-Risk Siblings engage in lower levels of RJA than Low-Risk Siblings (Cassel et al., 2007; Goldberg et al., 2005; Presmanes, Walden, Stone, & Yoder, 2007), not all groups have found such differences (Toth, Dawson, Meltzoff, Greenson, & Fein, 2007; Yirmiya et al., 2006).
Similarly, some investigations indicate that High-Risk Siblings initiate joint attention and behavioral requests less frequently than Low-Risk Siblings (Cassel et al., 2007; Goldberg et al., 2005; Stone, McMahon, Yoder, & Walden, 2007), while others have not identified such differences (Toth et al., 2007; Yirmiya et al., 2006). Stone et al. (2007) and Goldberg et al. (2005) reported less frequent IJA in High-Risk Siblings at an average age of 16 and17 months, respectively. Other investigations have not detected differences in IJA between High- and Low-Risk Siblings at 14 and 20 months (Toth et al., 2007; Yirmiya et al., 2006). High-Risk Siblings have demonstrated lower rates of IBR than Low-Risk Siblings at 12, 14, and 17 months (Cassel et al., 2007; Goldberg et al., 2005; Yirmiya et al., 2006).
The majority of these studies, however, assessed the presence or absence of group differences on RJA, IJA, and IBR at a single age (Yirmiya et al., 2006) that occasionally reflected an average over an age range as large as 5 months (Goldberg et al., 2005; Stone et al., 2007). Longitudinal investigations may offer a more stable portrait of developmental differences. A longitudinal study of typically developing infants and infants with non-ASD developmental delays indicated that IJA increased between 9 and 12 months, decreased between 12 and 15 months, and exhibited renewed growth through 18 months (Mundy et al., 2007). These researchers found that IBR rose between 9 and 12 months, and then remained stable through 18 months of age. By contrast, there was consistent growth in RJA from 9 to 18 months of age. Examining the developmental trajectories of these behaviors in High-Risk Siblings will allow a better understanding of potential developmental differences in nonverbal referential communication within the first two years of life in the context of ASD risk.
In older children with ASD, referential communication is associated with ASD symptomatology. IJA has been associated with severity of symptomatology in preschool-age children with ASD (Charman, 2004, Mundy, Gwaltney, & Henderson, 2010). Yoder, Stone, Walden, & Malesa (2009) examined RJA and weighted triadic communication, which indexed the use of gestural, vocal, gaze, and/or symbolic communication directed at a social partner and assigned greater weight to more sophisticated communications (e.g., multi-word utterance) in High-Risk Siblings with an ASD diagnosis. Baseline levels of RJA and growth in weighed triadic attention from 15 to 30 months predicted later ASD symptomatology. Their results highlight the importance of studying the developmental trajectories of referential communication in High-Risk Siblings to better understand the implications of developmental atypicalities in the emergence of ASD.
A number of recent studies examine the social behavior and referential communication of infants eventually diagnosed with an ASD. These studies find evidence of behavioral deficits beginning at 12 months among infants who go on to an ASD diagnosis, but find no evidence of deficits at 6 months (Ozonoff et al., 2010; Rogers, 2009; Rozga et al., 2011). The current study is the first, to our knowledge, to ask whether earlier trajectories of referential communication predict later ASD symptomatology in High-Risk Siblings.
In the current study, we examined trajectories of referential communication among High-Risk Siblings beginning at 8 months and continuing through 10, 12, 15, and 18 months. In line with the range of outcomes that affect High-Risk Siblings, we conceptualize ASD symptomatology at 30 months as a continuum of severity. In order to understand the impact of referential communication on ASD outcomes (see Yoder et al., 2009), we employed trajectories of referential communication to predict a continuous ASD severity score using a recently developed continuous algorithm (Gotham, Pickles, & Lord, 2009). In supplementary analyses, we used referential communication trajectories to predict ASD diagnostic classification. We also re-ran analyses examining group differences in developmental trajectories and the prediction of continuous ASD symptomatology while removing those High Risk Siblings who received an ASD diagnosis.
Method
Participants
Informed consent was obtained from parents prior to participation in the research procedures. Participants were enrolled in a longitudinal study investigating the early social and emotional development of High-Risk Siblings. High-Risk Siblings (n = 40; males = 27) had at least one older sibling with a community diagnosis of an ASD that was confirmed via administration of the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999) and a DSM-IV-TR (APA, 2000) -based clinical diagnosis from a licensed psychologist experienced in ASD. Low-Risk Siblings (n = 21; males = 9) had no reported history of ASD in first degree relatives, and all of their older siblings received a cut-off score lower than 9, a conservative score indicating no evidence of ASD, on the Social Communication Questionnaire (SCQ; Berument, Rutter, Lord, Pickles, & Bailey, 1999).
Children were excluded from both groups if they had a gestational age below 37 weeks, or major birth complications. There was no gestational age difference between High-Risk Siblings (M = 38.8, SD = 1.4) and Low-Risk Siblings (M = 39.1, SD = 1.3). There was no overall ethnicity difference between High-Risk Siblings (White/Caucasian = 28.9%, Hispanic = 47.4%, and Other = 23.7%) and Low-Risk Siblings (White/Caucasian = 40.0%, Hispanic = 25.0%, and Other = 35.0%). There was no difference in the education of the parent who accompanied the infant to assessments (all but one were mothers) between High-Risk Siblings (65.8% had at least four years of college) and Low-Risk Siblings (80.0% had at least four years of college). Forty-five percent of the participants in the current sample were included in an early report of group differences in referential communication (citation removed for blind review). The previous study did not examine the developmental trajectories of RJA, IJA, and IBR and their associations with later ASD symptomatology.
Measures
Referential communication
The Early Social Communication Scales (ESCS; Mundy et al., 2003; Mundy et al., 2007) was used to measure RJA, IJA, and IBR at 8, 10, 12, 15, and 18 months. The ESCS is a 15–25 minute procedure in which an examiner engages the child in semi-structured interaction with a standardized toy set. Tasks are designed to elicit referential communication with the examiner through the use of high-interest objects such as wind-up toys. The ESCS was coded from video by coders who were blind to the risk status of the participants. Twenty percent of ESCSs were double coded to assess reliability. Mean absolute intra-class correlations indicated that reliability was high across all ages for RJA (M = .87; SD = .07), IJA (M = .91; SD = .06), and IBR (M = .85; SD = .07).
Responding to joint attention (RJA) referred to the child’s ability to follow the joint attention behavior of the examiner. RJA was coded when infants followed the examiner’s point combined with a vocalization (i.e. the child’s name) to a distal stimulus. RJA was indicated as the proportion of trials correctly followed (out of 8 trials; see Table 1).
Table 1.
Mean and Standard Deviations of Referential Communication Measures
| Assessment | High-Risk Siblings |
Low-Risk Siblings |
|||
|---|---|---|---|---|---|
| n | M(SD) | n | M(SD) | Cohen’s d | |
| 8 month | |||||
| IJA Total (rpm) | 24 | .88 (.44) | 15 | 1.35 (.68) | −.86 |
| IBR Total (rpm) | 24 | .45 (.34) | 15 | .46 (.24) | −.03 |
| RJA % Correct | 24 | 08 (19) | 15 | 19(17) | −.60 |
| 10 month | |||||
| IJA Total (rpm) | 28 | 1.36 (.70) | 17 | 1.83 (.64) | −.69 |
| IBR Total (rpm) | 28 | .52 (.45) | 17 | .68 (.59) | −.32 |
| RJA % Correct | 28 | 08 (17) | 17 | 22 (27) | −.65 |
| 12 month | |||||
| IJA Total (rpm) | 38 | 1.14 (.63) | 15 | 1.40 (.45) | −.44 |
| IBR Total (rpm) | 38 | .90 (.76) | 15 | 1.19 (.65) | −.40 |
| RJA % Correct | 38 | 15(15) | 15 | 32(31) | −.82 |
| 15 month | |||||
| IJA Total (rpm) | 28 | 1.00 (.71) | 16 | 1.03 (.42) | −.05 |
| IBR Total (rpm) | 28 | 1.07 (.84) | 16 | 1.66(1.04) | −.64 |
| RJA % Correct | 28 | 26 (23) | 16 | 52(31) | −.99 |
| 18 month | |||||
| IJA Total (rpm) | 26 | 1.00 (.61) | 13 | 1.35 (.84) | −.51 |
| IBR Total (rpm) | 26 | 1.54 (.84) | 13 | 1.78 (.42) | −.33 |
| RJA % Correct | 26 | 28 (21) | 13 | 51 (30) | −.96 |
Note. IJA Total (rpm) = Initiating Joint Attention Total (rate per minute); IBR Total (rpm) = Initiating Behavioral Requesting Total (rate per minute); RJA % correct = Responding to Joint Attention percentage of trials correctly followed. For each of the variables at each age, Cohen’s d provides a measure of the effect size of the difference between the groups.
Initiating joint attention (IJA) referred to sharing interest in an object or event with the examiner. IJA included eye contact directed at the examiner with or without the simultaneous use of gestures (e.g., pointing or showing), and pointing without eye contact that was proto-declarative in intent. At each age, IJA was coded across the entire ESCS administration and the total number of instances of IJA was indexed as a rate per minute.
Initiating behavioral requests (IBR) referred to requesting help from the examiner. IBR behaviors include making eye contact to request a toy, or reaching toward, proto-imperative pointing to, or giving the examiner a desired toy with or without eye contact. At each age, IBR was coded across the entire ESCS administration and the total number of acts was indexed as a rate per minute (see Table 1).
Autism outcome
The Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999) consists of a series of behavioral presses that provide opportunities to observe behavior in the areas of social interaction, communication, and play. Infants were administered the ADOS at 30 months (M = 30.13, SD = 1.20, Range = 26–36 months), which was scored by a research reliable clinician. The ADOS calibrated severity score functioned as a continuous measure of ASD symptomatology. The calibrated severity score is the sum of the algorithm items within the Social Affect and Restricted and Repetitive Behavior domains adjusted for age, module, and verbal ability (Gotham et al., 2009). High-Risk Siblings (M = 3.05; SD = 1.88) had significantly higher ASD severity scores than Low-Risk Siblings (M = 1.57, SD = .81), t(57.46)= −4.27, p < .01, Cohen’s d = 1.02; the same pattern of significance was observed when High-Risk Siblings who received an ASD diagnosis were removed from the analysis, p = .02, Cohen’s d = .66 Diagnostic outcome was determined via administration of the ADOS and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), and a DSM-IV-based clinical diagnosis from a licensed psychologist experienced in ASD. The ADI-R, a semi-structured parent interview, was administered when children were 36 months of age. At 36 months of age, 10 High-Risk infants received an ASD diagnosis. None of the Low-Risk infants received an ASD diagnosis.
Cognitive characteristics at outcome
Participants were administered the Mullen Scales of Early Learning at 36 months of age by a trained administrator. The Mullen scales measure nonverbal problem solving (visual reception), gross and fine motor abilities, and expressive and receptive language abilities in children 1–70 months of age. The four domain scores of Visual Reception, Fine Motor, Expressive Language, and Receptive Language comprise an overall Early Learning Composite (ELC) t-score. Six participants (High-Risk siblings = 5) were missing cognitive outcome. High-Risk siblings (M = 90.67; SD = 16.01) had significantly lower ELC scores than Low-Risk Siblings (M = 106.53; SD = 14.85), t(53) = 3.58, p < .01, Cohen’s d = −1.02; the same pattern of significance difference was observe when High-Risk Siblings diagnosed with ASD were removed from the analysis, p < .01, Cohen’s d = −.83. Specifically, High-Risk Siblings (M = 43.70; SD = 10.51) were significantly lower on receptive language than Low-Risk Siblings (M = 50.58; SD = 8.54), t(53) = 2.46, p = .02, Cohen’s d = −.72; this difference was no longer significant when High-Risk Siblings diagnosed with ASD were removed from the analysis, p = .06, Cohen’s d = −.46. There were no significant differences between High-Risk Siblings (M = 48.97; SD = 8.80) and Low-Risk Siblings (M = 53.05; SD = 10.28) on expressive language, t(53) = 1.54, p = .13, Cohen’s d = −.43.
Results
Hierarchical Linear Modeling overview
Hierarchical Linear Modeling (HLM) was used to determine whether risk group was associated with the developmental trajectories of RJA, IJA, and IBR. HLM parsimoniously models developmental trajectories and adjusts for missing data in longitudinal designs. The current sample of 61 infants at 5 assessment ages was adequate for HLM (Kreft & De Leeuw, 1998; Maas & Hox, 2005; Snijders & Bosker, 1999). Full Maximum Likelihood was used in the estimation of parameters. The homogeneity of variance assumption was met in the HLM models. Age parameters were centered so that eight months was the intercept, representing baseline levels of RJA, IJA, or IBR.
Final models were built using theory and deviance statistics to indicate which level-1 and level-2 predictors improved the fit of the model when entered. Linear, quadratic, and cubic representations of age (in months) were examined as level-1 predictors within infants. Linear, quadratic, and cubic representations of age were modeled as random effects when they exhibited significant variance between infants. When significant variance between infants was not present, indicating there was no variance to be explained by level-2 predictors, these parameters were modeled as fixed effects (Singer & Willet, 2003). Risk group and gender were examined as level-2 predictors of the intercepts and rates of growth that were modeled as random effects. Gender was not a significant predictor of intercepts or rates of change and was not retained in any of the final models.
Modeling RJA development
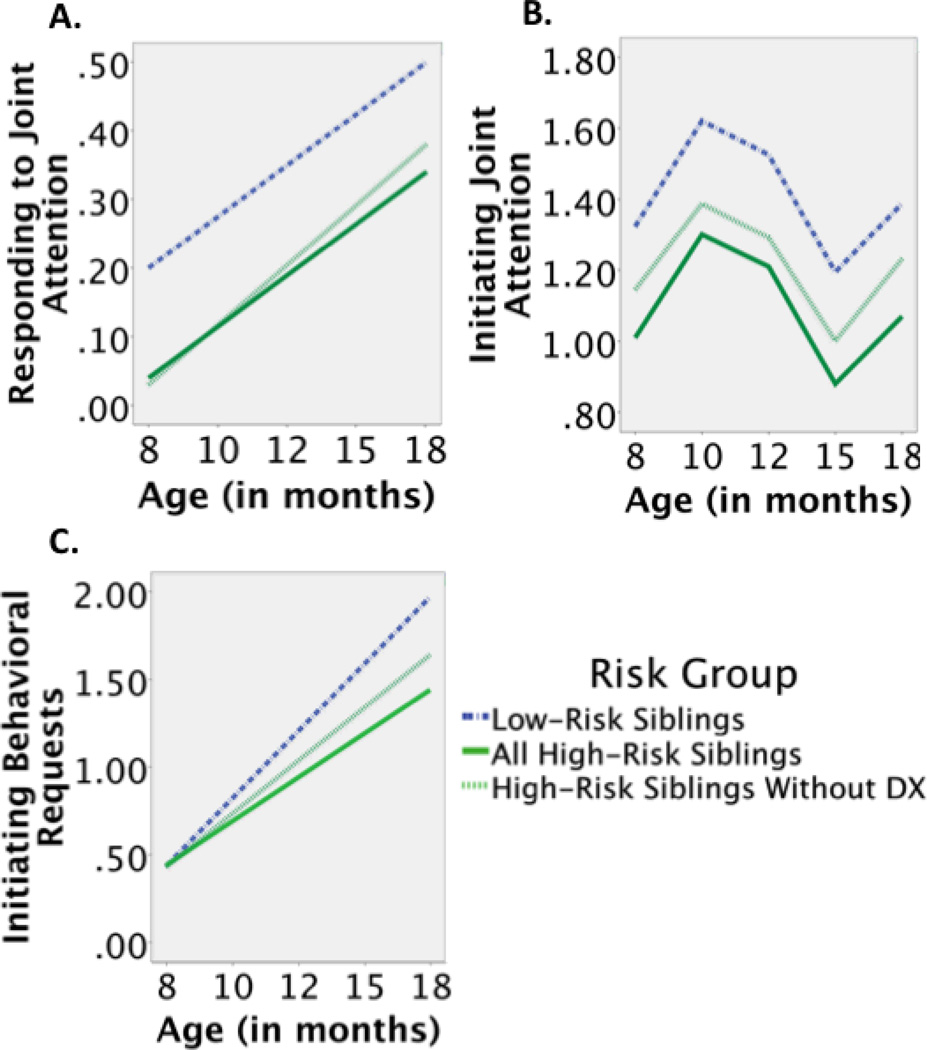
The level-1 growth model contained significant random intercept and random linear rate of change terms. At level-2, risk group significantly predicted the intercept (i.e. baseline, 8 month RJA), but not the linear term. High-Risk Siblings exhibited lower baseline levels of RJA than Low-Risk Siblings. The linear term indicated that all infants, regardless of risk group, exhibited similar rates of growth between 8 and 18 months of age (see Table 2 and Figure 1a). In sum, the model revealed a lower intercept of RJA in High-Risk Siblings than Low-Risk Siblings with similar subsequent overall trajectories for the two groups.
Table 2.
HLM Continuous Final Models for IJA, IBR, and RJA
| Models | ||||
|---|---|---|---|---|
| Estimated parameters | RJA Final Model |
IJA Final Model |
IBR Final Model |
|
| Fixed Effects Initial Status |
||||
| Intercept, β00 (s.e.) | .20 (.03)** | 1.32 (.12)** | .44 (.06)** | |
| Risk Group, β01(s.e.) | −.16 (.04)** | −.32 (.13)* | − | |
| Rate of Change |
||||
| Age (Linear), β10(s.e.) | .03 (.01)** | .29 (.07)** | .15 (.02)** | |
| Risk Group, β11(s.e.) | − | − | −.05 (.02)* | |
| Age (Quadratic), β20(s.e.) | − | −.08 (.02)** | − | |
| Age (Cubic),β30(s.e.) | − | .01 (.001)** | − | |
| Model Fit | ||||
| Deviance | −86.59 | 371.72 | 419.71 | |
| # parameters | 7 | 7 | 7 | |
Note. (Coded as Low-Risk Siblings = 0 and High-Risk Siblings = 1) Unstandardized beta coefficients and (standard errors).
p <.05,
p <.01
Figure 1.
The developmental trajectories of RJA, IJA, and IBR between 8 and 18 months of age. The ordinary least squares estimates of RJA, IJA, and IBR are shown. RJA is proportion of trials correctly followed and IJA and IBR are rate per minute scores.
Modeling IJA development
IJA was modeled with a polynomial model (Singer and Willet, 2003) in light of previous findings (Mundy et al., 2007). The model included linear rate of change, quadratic rate of change, and cubic rate of change. The linear term indexed linear change in IJA from 8 to 18 months, the quadratic term indexed the rate of the curvature in IJA growth, and the cubic term indexed the rate of reversal of the curvature in IJA growth.
The level-1 growth model contained a significant random intercept term; linear, quadratic, and cubic rates of change were modeled as fixed effects. At level-2, risk group significantly predicted 8 month (i.e., baseline) IJA. High-Risk Siblings exhibited lower baseline levels of IJA than Low-Risk siblings. Risk group explained approximately 11% of the inter-individual variance of IJA at baseline. No predictors were examined for the linear, quadratic, and cubic rates of change, as they did not vary between infants. The linear, quadratic, and cubic terms indicated that all infants, regardless of risk status, exhibited similar patterns of growth between 8 and 18 months of age (see Table 2 and Figure 1b). In sum, the model revealed lower IJA baseline in High-Risk Siblings than Low-Risk Siblings with similar subsequent overall trajectories for the two groups.
Modeling IBR development
The level-1 growth model contained a random intercept and random linear rate of change. At level-2, risk group did not predict the intercept, but significantly predicted the linear term (i.e. growth). High-Risk Siblings exhibited a lower rate of growth than Low-Risk Siblings (see Table 2 and Figure 1c). Risk group explained approximately 6% of the inter-individual variance of the growth of IBR. In sum, the model revealed that while both groups demonstrated similar levels of IBR at baseline, High-Risk Siblings’ rate of growth in IBR between 8–18 months of age was lower than that of Low-Risk Siblings.
Predicting ASD Severity
Three HLM parameters—RJA intercept (RJA), IJA intercept (IJA), and IBR linear rate of change (IBR)—varied between infants (level-2) and were different between groups. Ordinary least squares estimates of these parameters were examined as univariate predictors of ASD severity in each risk group. Among High-Risk Siblings, IJA, r(38) = −.48, p < .01, and IBR , r(33) = −.36, p < .05, were each correlated with ASD severity. In contrast, among Low-Risk Siblings, there were no significant correlations between IJA, r(19) = --.09, p = .70, or IBR, r(18) = .15, p = .54, and ASD severity. There were no significant correlations between RJA and later ASD severity among High-Risk, r(33) = .23, p = .19, or Low-Risk Siblings, r(18) = − .13, p = .58.
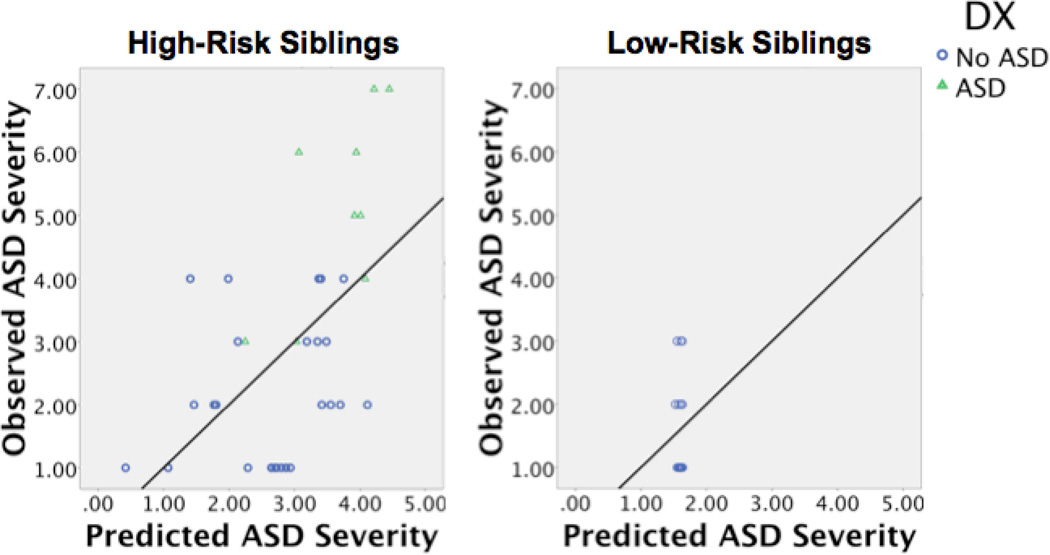
Multiple regression was used to determine whether the IJA intercept (i.e. baseline) and IBR linear term (i.e. growth) uniquely predicted ASD severity, and whether these associations were moderated by risk group. The severity of ASD symptomatology was regressed on risk group, IJA, IBR, and the interactions of these variables with risk group. This initial regression model significantly predicted severity of ASD symptomatology, R2 = .43, F(5, 54) = 7.45, p ≤ .01 (see Table 3). Risk group significantly predicted severity of ASD symptomatology, β = .48, t = 3.15, partial r2 = .17, p ≤ .03. There were no main effects of IJA, IBR, or the interaction between IBR and risk group. However, the interaction between IJA and risk group, β = −.42, t = −2.20, partial r2 = .09, p = .03, significantly predicted severity of ASD symptomatology. IBR and its interaction with risk group were then removed as predictors in the model due to non-significance. There was no significant change between the initial model and the more parsimonious model, R2 change, .06, p = .09. The final model, which only included risk group, IJA and the interaction between risk group and IJA, significantly predicted severity of ASD symptomatology, R2 = .37, F(3, 54) = 10.10, p ≤ .01 (see Table 3 and Figure 2). As in the initial model, the interaction between IJA and risk group, β = −.46, t = −2.33, partial r2 = .10, p = .02, significantly predicted severity of ASD symptomatology (see Figure 2). IJA intercept was a more efficient predictor of ASD symptomatology among High-Risk than Low-Risk Siblings.
Table 3.
Hierarchical Regression Predicting Severity of ASD Symptomatology
| Variables | R2 | B | SE B | β | F | |
|---|---|---|---|---|---|---|
| Initial Model | .43 | 7.45** | ||||
| Group Status | 1.63 | .52 | 48** | |||
| IJA Intercept | −.04 | .64 | −.01 | |||
| IBR Linear Change | .55 | 1.38 | .06 | |||
| IJA Intercept X Group Status | −1.71 | .78 | −.42* | |||
| IBR Linear Change X Group Status | −3.84 | 2.03 | −.31 | |||
| Final Model | .37 | 10.10** | ||||
| Group Status | 1.14 | .40 | .34** | |||
| IJA Intercept | −.07 | .66 | −.02 | |||
| IJA Intercept X Group Status | −1.86 | .80 | −.46* |
Note. (coded as Low Risk Siblings = 0 and High Risk Siblings = 1),
p≤ .05,
p ≤ .01
Figure 2.
Predicted ASD severity versus observed ASD severity. ASD severity was predicted by risk group, the interaction between risk group and IJA intercept, and the interaction between risk group and the IBR growth parameter, R2 = .37
The Role of ASD Diagnosis
We next re-examined the final HLM models (see Figure 1), correlations, and the multiple regression after removing the 10 High-Risk Siblings who went on to receive an ASD diagnosis from the analyses. In the HLM examining RJA, risk group remained a significant predictor of the intercept, β01 = −.16, s.e.= .04, p < .01. In the HLM examining IJA, risk group was no longer a significant predictor of the intercept, β01 = −.20, s.e.= .13, p = .14. In the HLM examining IBR, risk group was no longer a significant predictor of linear change, β11 = −.03, s.e.= .02, p = .19. IJA, r(28) = −.39, p = .03, but not IBR r(24) = −.09, p = .68, was still significantly correlated with ASD severity among High-Risk Siblings. In the multiple regression examining predictors of ASD severity, the final model no longer significantly predicted severity of ASD symptomatology, R2 = .11, F(3, 45) = 1.68, p = .19. There were no main effects of risk group, intercept of IJA, or the interactions between these variables. This finding indicates that referential communication parameters predicted ASD severity in the High-Risk Siblings, but only when the High-Risk Siblings who went on to receive an ASD diagnosis were included in the model. We next examined whether the referential communication parameters could predict categorical ASD diagnosis.
Binary logistic regression1 was used to examine whether the intercept of IJA and the linear rate of change of IBR predicted diagnostic outcome (ASD vs. no ASD) in High-Risk Siblings, χ2 = 10.31, p < .01, Cox and Snell R2 = .25. The intercept of IJA significantly predicted diagnostic outcome, b = −3.03, s.e. = 1.41, Wald = 4.04, p= .03; linear rate of change of IBR was not a significant predictor. This model correctly classified 67% of the children who received an ASD diagnosis (6/9) and 96% (25/26) of the children who did not receive an ASD diagnosis.
Discussion
This study examined the early developmental trajectories of referential communication in High- and Low-Risk Siblings and the extent to which these trajectories were associated with later severity of ASD symptomatology and diagnostic outcome. High-Risk Siblings exhibited lower levels of baseline RJA and IJA, and a lower rate of linear change in IBR, than Low-Risk Siblings. Baseline levels of IJA as well as growth in IBR predicted severity of ASD symptomatology among High-Risk Siblings. The results are based on repeated observations of key communicative parameters, suggesting they are relatively stable indices of the nonverbal referential communication constructs of interest (Fogel, 2011).
The Developmental Trajectory of RJA
High-Risk Siblings were less responsive to RJA bids than Low-Risk Siblings at 8 months and remained less responsive through 18 months of age (see Figure 1a). Findings of lower levels of RJA in High-Risk Siblings have been inconsistent in the second year of life, with some studies (Presmanes et al., 2007), but not others (Goldberg et al., 2005) reporting differences. This is the first prospective study to model RJA in High-Risk Siblings from eight months of age. Along with a difference in baseline levels, the longitudinal assessment of RJA indicated the groups had comparable rates of growth, which suggests a stable difference in responding to the referential cues of others. Finally, differences in RJA trajectories were present even after the High-Risk Siblings who received ASD diagnosis were removed from HLM analyses, suggesting widespread vulnerabilities in the development of RJA in High-Risk Siblings.
The Developmental Trajectory of IJA
Both High- and Low-Risk Siblings had IJA trajectories that were characterized by early growth, followed by a decline, and then a subsequent rebound, which was indexed by significant linear, quadratic, and cubic change (see Figure 1b). These results mirror Mundy et al.’s (2007) description of IJA development in typically-developing infants between 9 and 18 months of age. This perturbation in IJA growth may be due to the acquisition of motor and language competencies (Mundy et al., 2007; Parlade & Iverson, 2011). During these acquisition periods, infants may rely less on their nonverbal abilities as their communicative repertoires expand to accommodate their rapidly developing verbal communication.
Group differences in the intercept indicated that High-Risk Siblings had lower baseline levels of IJA at 8 months than Low-Risk Siblings. These findings were dependent on the presence of High-Risk siblings who received an ASD diagnosis, suggesting that these infants exhibit specific deficits in sharing attention with a partner (Ozonoff et al., 2010; Rozga et al., 2011). In a departure from previous studies that have examined a specific age or the average over an age range (Goldberg et al., 2005; Stone et al., 2007; Toth et al. 2007; Yirmiya et al., 2006), the current study examined IJA at 5 distinct ages, yielding a model of change with stable growth parameters. When considered along with the difference in baseline levels, the comparable rates of growth between the groups suggest that the development of IJA in children at risk for ASD is largely characterized by differences that appear early—as IJA comes on line—and persist through at least 18 months of age. IJA reflects an infant’s awareness of their social partner’s potential interest in an object or event. It provides potentially rewarding experiences of integrating an interest in objects with the attention of others. Consequently, lower rates of IJA in High-Risk Siblings may deny infants the reinforcing experiences of engaging the attention and intention of others (Charman, 2003; Mundy et al., 2009; Tomasello, 1995).
The Developmental Trajectory of IBR
While High-Risk Siblings demonstrated similar levels of IBR as Low-Risk Siblings at baseline, they exhibited slower growth than Low-Risk Siblings and “lost ground” between 8 and 18 months of age (see Figure 1c). This may explain why previous studies have found lower rates of IBR in High-Risk than Low-Risk Siblings at 12, 14, and 17 months (Cassel et al., 2007; Goldberg et al., 2005; Yirmiya et al., 2006). As with IJA, the current findings were dependent on the inclusion of the High-Risk Siblings who received an ASD diagnosis. Comparably, Rozga et al. (2011) found that High-Risk Siblings who receive an ASD diagnosis demonstrated fewer IBR bids than non-diagnosed High-Risk Siblings and Low-Risk Siblings at 12 months (the only assessment age reported). The slowed growth of behavioral requesting in High-Risk Siblings suggests that their motivation to communicate a desire for objects to others emerges at a slower pace than in Low-Risk Siblings.
Predicting ASD severity and diagnostic outcome
Individual differences in the developmental trajectories of referential communication were associated with differences in ASD symptomatology and diagnostic outcome among High-Risk Siblings; associations between referential communication and ASD severity were not expected or detected for Low-Risk Siblings due to the limited variability in symptomatology exhibited by this group. In the current study, individual differences in baseline levels of RJA were not related to later ASD severity. This finding indicates that RJA in the ESCS is a robust marker of overall vulnerability to difficulties with referential communication in High-Risk Siblings, but is not a powerful predictor of later ASD symptomatology. Low levels of RJA were common in the ESCS among High-Risk Siblings but did not sufficiently distinguish among High-Risk Siblings with varying levels of later ASD symptomatology. RJA is thought to be a more fundamental and less volitional type of referential communication than IJA, and both are depressed in the early development of High-Risk Siblings. In preschool age children with ASD, however, RJA improves while IJA remains a unique indicator of ASD impairment (Mundy et al., 2009). Therefore, RJA may be more mutable than IJA throughout development, which in the current study may have led to its lack of developmental association with later ASD symptomatology.
Previous studies, however, have found a link between early RJA and later ASD diagnosis (Sullivan et al., 2007; Yoder et al., 2009). Specifically, Yoder et al. (2009) found that RJA at 15 months was associated with ASD diagnosis, and Sullivan et al. (2007) found that low levels of RJA (<50% correct) at 14 months were associated with diagnosis. while the current study used the conventional ESCS task, which employs a set of redundant gestural and vocal cues, to measure RJA, Yoder and Sullivan used an RJA task that included different attention-eliciting prompts that included different combinations of physical and verbal cues. These varying levels of redundancy in RJA cues may yield a more sensitive measure of individual differences in responding to others that is especially predictive of ASD outcome.
Among High-Risk Siblings, baseline eight month IJA predicted ASD symptomatology. Previous studies have indicated that IJA predicts ASD symptomatology among older children already diagnosed with an ASD. Among children with an ASD, Charman (2003) found that IJA at 20 months predicted severity of ASD symptomatology. Yoder and colleagues (2009) found that linear growth in weighted triadic communication—referential communications weighted by sophistication, particularly the use of vocalizations—between 15 and 30 months predicted later social functioning in children with an ASD. By contrast, the current study utilized a nonverbal measure of IJA, which indexed the fundamental skill of coordinating attention between an object and a social partner. The current results and previous studies highlight the capacity of early nonverbal measures of IJA to predict ASD symptomatology as well as diagnostic outcome. Infants who engaged in fewer IJA bids had fewer opportunities to share their experience of objects with social partners. These limitations in experience may lead to an impoverished understanding of the social meaning of objects and events (Mundy et al., 2009). Consequently, lower initial levels of IJA in infants may index emerging difficulties in the ability to communicate about objects with social partners, a characteristic ASD deficit.
We found that growth in behavioral requesting between 8 and 18 months of age was associated with later ASD severity, indicating the importance of this slowed developmental growth rate. Rozga et al. (2011) found that High-Risk Siblings who received an ASD diagnosis engaged in fewer IBR bids than High-Risk Siblings who did not receive a diagnosis at 12 months of age. However, IBR did not uniquely predict ASD symptomatology and categorical ASD diagnosis. Relatively little significance has been accorded to behavioral requesting in the development of autism symptomatology, in part because requesting is believed to be less socially motivated than initiating joint attention. Both types of referential communication, however, involve communicating with social partners about objects. Infants who engage with others to request items demonstrate an understanding that social partners can meet their needs.
Limitations
The current study had limitations in sample characteristics similar to other investigations of the development of High-Risk infants (Gamliel et al., 2007; Landa & Garrett Mayer, 2006; Zwaigenbaum et al., 2005). Of 61 infants, 40 were in the high-risk group, of whom 10 received an ASD diagnosis. The small group size of the children diagnosed with ASD indicates that the current predictive findings should be interpreted cautiously and further examination is needed. In that context, the findings related to the severity of autism symptomatology are likely more robust than findings related to the prediction of ASD diagnosis. In addition, the size of the High- and Low-Risk groups was not matched, and gender was not matched within groups. However, modern statistical methods such as HLM are robust to non-matched group characteristics, and gender was not a significant predictor of referential communication.
Implications for Intervention
The associations in which lower levels of IJA and tapered growth in IBR predict ASD severity in High-Risk Siblings indicate that these are areas where early intervention may be helpful. Some recent intervention studies have successfully targeted and increased joint attention in children with ASD as early as 21 months of age (Landa, Holman, O’Neill, & Stuart, 2010; Kasari, Gulsrud, Wong, Kwon, & Locke, 2010). The current findings suggest that High-Risk Siblings might benefit from similar intervention strategies within the first year of life. Supportive interventions that raise IJA levels at 8 months might lead to improvements among High-Risk Siblings in IJA that would make their IJA trajectories through 18 months more comparable to those of Low-Risk Siblings. Further, while intervention studies frequently focus on improving joint attention abilities, there has been less emphasis on behavioral requesting, as these deficits are not as pronounced in older children with ASD (Stone et al., 1997; Mundy et al., 1986). The current findings suggest that behavioral requesting may also be an important focus of early intervention because impairments are evident by 12 months of age and are linked to later ASD symptomatology.
Conclusion
The current study indicated that developmental trajectories of referential communication distinguished High- and Low-Risk Siblings and predicted ASD symptomatology. Specifically, IJA at 8 months and IBR growth between 8–18 months predicted later ASD outcomes. IJA and IBR are important components of a rich social feedback loop that may impact the development of autism symptomatology. The findings highlight the importance of examining the growth of referential communication during the first year of life to better understand development in typical and at-risk children.
Acknowledgments
We would like to thank the families that have kindly given their time to participate in our study. This research was supported by the National Institutes of Health (R01 HD047417), the Marino Autism Research Institute (MARI), and the National Science Foundation (0808767 & 1052736).
Footnotes
One infant with an ASD diagnosis who had referential communication data only at 12 months—and no growth parameter estimate for IBR—could not be included in this analysis.
References
- Adamson LB, Bakeman R. Affect and attention: Infants observed with mothers and peers. Child Development. 1985;56(3):582–593. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. text revision. [Google Scholar]
- Bakeman R, Adamson LB. Coordinating attention to people and objects in mother-infant and peer-infant interaction. Child Development. 1984;55(4):1278–1289. [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bates E, Camaroni L, Volterra V. The acquisition of performatives prior to speech. Merrill-Palmer Quarterly. 1975;21:206–226. [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175(5):444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism and Developmental Disorders. 2007;37(1):122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society of London. 2003;358(1430):315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2000;15(4):481–498. [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorders. American Journal of Psychiatry. 2006;163(2):294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Fogel A. Theoretical and Applied Dynamic Systems Research in Developmental Science. Child Development Perspectives. 2011;5(4):267–272. [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, Filipek P, Spence M. Brief report: Early social communication behaviors in the younger siblings of children with autism. Journal of Autism and Developmental Disorders. 2005;35(5):657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez LV, Messinger DS, Newell L, Lambert B, Sheskin M. Visual disengagement in the infant siblings of children with an autism spectrum disorder (ASD) Autism. 2008;12(5):473–485. doi: 10.1177/1362361308094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Carr EG. Joint attention in children with autism: Theory and intervention. Focus on Autism and Other Developmental Disabilities. 2004;19(1):13–26. [Google Scholar]
- Kasari C, Gulsrud AC, Wong C, Kwon S, Locke J. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. Journal of Autism and Developmental Disorders. 2010;40(9):1045–1056. doi: 10.1007/s10803-010-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft IGG, Leeuw J. Introducing multilevel modeling. London: Sage Publications Limited; 1998. [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, O'Neill AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(1):13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Manual. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: From phenome to genome. Journal of Neuropathology and Experimental Neurology. 2008;67(9):829–837. doi: 10.1097/NEN.0b013e318184482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas CJM, Hox JJ. The influence of violations of assumptions on multilevel parameter estimates and their standard errors. Computational Statistics and Data Analysis. 2004;46(3):427–440. [Google Scholar]
- Messinger DS, Fogel A. Give and take: The development of conventional infant gestures. Merrill-Palmer Quarterly. 1998;44(4):566–590. [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78(3):938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Burnette C. Joint attention and neurodevelopment. In: Volkmar F, Klin A, Paul R, editors. Handbook of Autism and Pervasive Developmental Disorders. Vol. 3. Hoboken, NJ: John Wiley; 2005. pp. 650–681. [Google Scholar]
- Mundy P, Delgado C, Block J, Venezia M, Hogan A, Seibert J. A manual for the abridged Early Social Communication Scales (ESCS) Sacramento, California: Available through the University of California at Davis M.I.N.D. Institute; 2003. http://www.ucdmc.ucdavis.edu/mindinstitute/_ourteam/faculty_staff/ESCS.pdf. [Google Scholar]
- Mundy P, Gwaltney M, Henderson H. Self-referenced processing, neurodevelopment and joint attention in autism. Autism. 2010;14(5):408–429. doi: 10.1177/1362361310366315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman MD, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in autism. Development and Psychopathology. 1994;6(3):389–401. [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Research. 2009;2(1):2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Losif A-M, Baguio F, Cook IC, Hill MM, Rogers SJ, Sangha S, Steinfeld MB, Young GS, Hutman T, Rozga A, Sigman M. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Rogers SJ, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Dobkins K, Constantino JN, Sigma M, Iverson JM, Landa R, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128(3):488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlade MV, Iverson JM. The interplay between language, gesture, and affect during communicative transition: A dynamic systems approach. Developmental Psychology. 2011;47(3):820–833. doi: 10.1037/a0021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother-infant interaction and non-verbal communication. Journal of Autism and Developmental Disorders. 2011;41(3):287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Methods for Studying Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. London: Sage Publications Limited; 1999. [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social communicative and cognitive development of younger sibling of children with Autism Spectrum Disorders. Archive of Pediatrics and Adolescent Medicine. 2007;161(4):384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Stone WL, Ousley OY, Yoder PJ, Hogan KL, Hepburn SL. Nonverbal communication in two- and three-year-old children with autism. Journal of Autism and Developmental Disorders. 1997;27(6):677–696. doi: 10.1023/a:1025854816091. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders. 2007;37(1):37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Tomasello M. Joint attention as social cognition. In: Dunham PJ, editor. Joint attention: Its origins and role in development. Hillsdale, NJ England: Lawrence Erlbaum Associates, Inc; 1995. pp. 103–130. [Google Scholar]
- Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37(1):145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39(10):1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]