Abstract
Purpose
Sugarcane juice (ScJ) is a natural drink popular in most tropical Asian regions. However, research on its effect in enhancing sports performance is limited. The present investigation was to study the effect of sugarcane juice on exercise metabolism and sport performance of athletes in comparison to a commercially available sports drinks.
Methods
Fifteen male athletes (18-25 yrs) were asked to cycle until volitional exhaustion at 70% VO2 max on three different trials viz. plain water (PW), sports drink (SpD) and ScJ. In each trial 3ml/kg/BW of 6 % of carbohydrate (CHO) fluid was given at every 20 min interval of exercise and a blood sample was taken to measure the hematological parameters. During recovery 200 ml of 9% CHO fluid was given and blood sample was drawn at 5, 10, 15 min of recovery.
Results
Ingestion of sugarcane juice showed significant increase (P<0.05) in blood glucose levels during and after exercise compared to SpD and PW. However, no significant difference was found between PW, SpD and ScJ for total exercise time, heart rate, blood lactate and plasma volume.
Conclusion
ScJ may be equally effective as SpD and PW during exercise in a comfortable environment (<30°C) and a more effective rehydration drink than SpD and PW in post exercise as it enhances muscle glycogen resynthesis.
Keywords: Sugarcane Juice, Exercise Performance, Fluids, Natural Drink
INTRODUCTION
Fluid intake during prolonged exercise is effective in improving exercise performance and delaying the onset of fatigue [1]. To sustain high rate of work output or exercise performance in heat requires availability of carbohydrate (CHO) as fuel for the working muscle. Ingestion of CHO from 20 to 15 min prior to the exercise results in an increase in plasma glucose [2–4], time of performance [5], CHO oxidation during exercise [6] and reduces endogenous glucose production [1, 7]. CHO solutions containing 6-8% will increase exercise performance [4, 8–10] more than 8-10% solutions [11, 12] in hot and humid conditions.
Ingestion of plain water post exercise results in rapid fall in plasma sodium and plasma osmolality and with subsequent diuresis [13, 14]. CHO ingestion during short term recovery from prolonged exercise has been shown to increase the capacity for subsequent exercise in a warm environment [15]. The rate of glycogen resynthesis is dependent on the amount of CHO ingested [16, 17]. In recovery, a beverage containing 5-10% CHO with 30-40 mEq sodium should be ingested to achieve euhydration. A minimum of 50g/ hour of CHO should be ingested in the first 2 hours to maximize glycogen depletion? [18].
Sports beverages containing CHOs and electrolytes may be consumed before, during, and after exercise to help maintain blood glucose concentration, provide fuel for muscles, and decrease risk of dehydration and hyponatremia. Natural fruit drinks such as young coconut water [19, 20], honey [21] and milk [22, 23] have been shown to be effective hydration drinks.
Sugarcane juice (ScJ) is a common indigenous drink, economical and widely consumed by Indian athletes. It is rich in CHOs and a few electrolytes, comparable with planned sports drinks. Though it is consumed regularly, the efficacy of ScJ in sports performance hasn't been reported. Hence, the present investigation was envisaged to study the effect of sugarcane juice on exercise metabolism and sport performance of athletes in comparison to a commercially available sports drinks and plain water.
METHODS AND SUBJECTS
Subjects
Fifteen healthy male Indian athletes in the age range of 18-25 years were participated in the present study. The physical characteristics were (Mean ± Standard Deviation) age: 19.4±0.58 yrs, Height 172.1±1.52 cms, body weight 65.2 ±1.21 kg, body fat 15.1±0.74%, lean body mass 55.0 ±1.13 kg and maximal oxygen uptake (VO2max) 51.0± 2.10 ml.kg-1.min-1. The study was approved by Sports Authority of India Research committee. The volunteers were briefed on the purpose of the study, experimental protocol and their written consent was obtained.
Experimental Procedure
The study had a randomized crossover counterbalance design. The subjects participated in three experimental trials, at least one week apart.
Pre Exercise Phase
Maximal oxygen uptake (VO2 max) was determined prior to the experimental trials [24]. The volunteers were restricted from performing routine exercise a day before each trial and were required to maintain similar training volume throughout the study. The volunteers were instructed to report to the laboratory after 10-12 hours of fast. On reporting to the laboratory, a standardized breakfast was given which comprised of two slices of bread and 500 ml of cold plain water. One hour later, the participants emptied their bladder and body weight with minimal clothing was measured. The heart rate was taken through a monitor attached to an elastic belt positioned below the pectoral muscle. A blood sample was taken to measure the hematological parameters such as blood glucose, hemoglobin, hematocrit and plasma volume.
Exercise Phase (During Exercise)
After warm up for 5 min at 50 % VO2 max, the subjects cycled at 70% VO2max until volitional exhaustion. In each trial subjects consumed 3ml / kg body weight of refrigerated fluid (<15°C) at every 20 min interval [25]. The subjects were fully aware of the drinks consumed during trials. The heart rate was recorded every 5 min. At every 20 min and at exhaustion, a blood sample was taken in sitting position on bike in order to measure the hematological parameters. Perceived rate of exertion (PRE) was measured for every 10 min and fluid sensory scale was administered every 20 min to measure the gastro intestinal tolerance (GIT). All the trials were conducted at 28-30° C and at a relative humidity of 65 -70%.
Post Exercise Phase
Participant's body weight with minimal clothing was measured after the trial. During recovery 9% of CHO fluid; 3ml / kg body weight was given for rehydration. A blood sample was drawn at 5, 10, 15 min to measure the hematological parameters.
Liquid Supplements
In the present study trials were done with plain water (PW), sports drink (SpD), and Sugarcane juice (ScJ). The nutrient composition of SpD and ScJ is given in Table 1. Bottled mineral water (Bisleri) was used as control (PW). Glucogen Sport of Applied Nutritional Sciences, Mumbai was used as sports drink. ScJ used for the experiment was grown exclusively for research purpose from organic sources and free from pesticides. The juice was pasteurized to kill the microorganisms and to avoid bacterial contamination. To prepare a 6 % CHO solution of SpD, a 25gm sachet was dissolved in 385 ml of water, which was given during exercise and to prepare a 9% solution it was dissolved in 255 ml of water, which was given after exercise. To prepare a 6% solution, 67 ml of ScJ was mixed with 33 ml of water, which was given during exercise and the 9% solution, in the form of pure sugarcane juice was given after exercise. Fluids were refrigerated at <15 °C.
Table 1.
Nutrient composition of sports drink and sugarcane juice
| Nutrients | Sports drink(25mg) | Sugarcane Juice (100g) |
|---|---|---|
| Energy (k.cal) | 91 | 39 |
| Carbohydrate (g) | 25 | 9.1 |
| Vitamin A (IU) | 500 | 188 |
| Vitamin B1 (mcg) | - | 12 |
| Vitamin B12 * (mcg) | 2 | - |
| Vitamin –C* (mg) | 60 | - |
| Vitamin –D3* (IU) | 400 | - |
| Vitamin –E* (mg) | 10 | - |
| Calcium Pancothenate* (mg) | 10 | - |
| Potassium (mg) | 100 | 360 |
| Sodium (mg) | 100 | 20 |
| Calcium (mg) | 200 | 74 |
| Phosphorus (mg) | 150 | 22 |
| Iron (mg) | - | 0.1 |
| Zinc* (mg) | 1.25 | - |
| Magnesium* (mg) | 75 | - |
| Manganese* (mcg) | 100 | - |
| Selenium* (mcg) | 20 | - |
Not measured in ScJ
Biochemical Estimation
A Venous blood sample was drawn to estimate the bio chemical parameters. Blood glucose was analyzed using glucometer (Boehringer Mannheim Germany accu check sensor). Blood lactate was analyzed using lactate analyzer (1500, YSI, USA). Cyanmethaemoglobin method was used for the estimation of hemoglobin [26]. Micro hematocrit capillary tube coated with anticoagulant was used for the estimation of hematocrit. Plasma volume was calculated indirectly from hemoglobin and hematocrit [27].
Physiological Transients
Exercise test was performed on an electronically operated computerized bicycle ergometer (ER 900: Erich Jaeger, Germany). V02max measurement was carried out using a portable metabolic analyzer, Cosmed K4 (Cosmed srl, Italy) using a test protocol that consisted of graded ergometry). The heart rate was recorded through a heart rate sensor (Polar HR sensor, USA). Total exercise time till exhaustion was recorded to determine the maximal endurance performance.
Measurement of PRE, GIT and Sweat loss
The Borg's scale was administered to assess PRE [42] and fluid sensory scale was administered to measure GIT [45]. Sweat loss was calculated using the formulae:
Sweat loss = (body weight before exercise- body weight after exercise) + amount of fluid intake
Statistical Analysis
Statistical Package for Social Sciences (SPSS) version 16.0 was used for the analysis. Mean and standard error was applied for all the variables. Repeated measures analysis of variance (ANOVA) was applied to study the difference between duration and trials. Significant differences were determined by the Duncan Multiple Range Test (Post hoc). Friedman ANOVA was applied to study the significant difference between duration of exercise on PRE and GIT. Kruskal Wallis test was applied to study the significant difference in the ranks obtained for trials with PW, SpD and ScJ. Differences were considered significant at P<0.05.
RESULTS
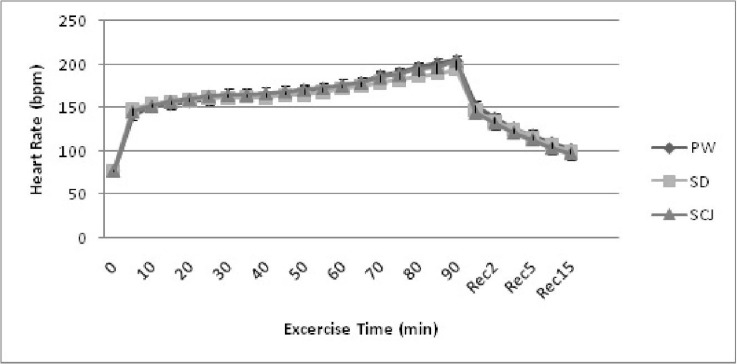
The mean pre exercise heart rate (PHR) of the subjects showed no significant difference between the trials. During exercise, heart rate showed significant increase (P<0.05) with increasing exercise time and in post exercise, significant decrease (P<0.05) with increasing recovery time. There was no significant difference between the trials with PW, SpD and ScJ trials during exercise and recovery (Fig. 1). The mean total exercise time showed no significant difference between the three trials (Table 2). Most of the athletes could not exercise more than 60 min and a few athletes could exercise for about 90 min.
Fig. 1.
Mean heart rate responses of the subjects before, during and after exercise with select liquid drinks PW: plain water; SD: Sports Drink; ScJ: Sugarcane juice
Table 2.
Total exercise time and peak blood lactate concentration of athletes with select liquid drinks
| Parameter | Liquid Supplements | Total Exercise Time (min) (Mean ± Standard Error) | ANOVA value |
|---|---|---|---|
| Total exercise time | Plain Water | 62.0 (5.29) | F= 0.07NS |
| Sports Drink | 64.3 (5.63) | ||
| Sugarcane Juice | 63.4 (5.54) | ||
| Peak Blood Lactate Concentration | Water | 12.0 (0.66) | F= 0.01NS |
| Sports Drink | 12.0 (0.57) | ||
| Sugarcane Juice | 12.1 (0.68) |
NS: Not significant
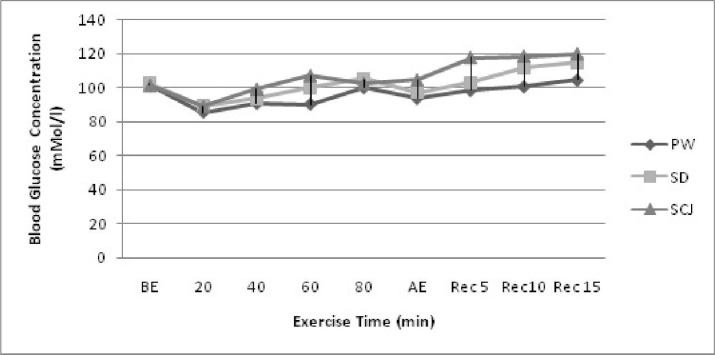
The subjects were in similar pre exercise blood glucose level with PW, SpD and ScJ trials. During exercise the blood glucose levels showed significant increase (P<0.05) with increase in exercise time (P<0.05). However, at each time (20, 40, 60 min) point no significant difference was observed for the blood glucose levels between the trials (Fig. 2). The post exercise blood glucose levels of the subjects in all the three trials increased significantly with the recovery time (P<0.05) and showed a significant difference (P<0.05) between the three trials. The trials with SpD and ScJ recorded significantly higher (P<0.05) blood glucose levels when compared with water. Sugarcane juice recorded significantly higher blood glucose levels than the sports drink trials. The mean peak blood lactate had no significant difference between the three trials (Table 2).
Fig. 2.
Blood glucose concentration of the subjects before, during and after exercise with select liquid drinks PW: plain water; SD: Sports Drink; ScJ: Sugarcane juice
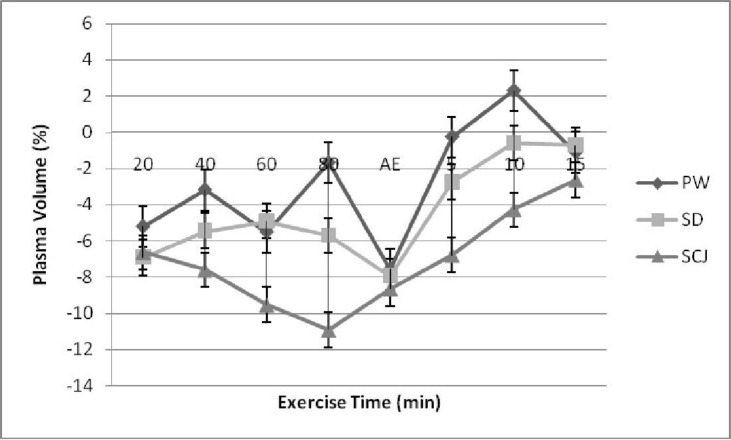
The mean hemoglobin and hematocrit level of the subjects before and during and after exercise was not significantly different between the trials. During exercise, the mean hemoglobin and hematocrit level was increased with increase in exercise time (P<0.05) and decreased during recovery (P<0.05). During exercise with increasing exercise time the mean plasma volume decreased for all the trials. However, the difference between the trials was not significant. In post exercise, the mean plasma volume significantly increased (P<0.05) in all the three trials with increase in recovery time. However, no significant difference was found (Fig. 3). The sweat loss showed no significant difference between the three trials.
Fig. 3.
Plasma volume level of the subjects before, during and after exercise with select liquid drinks PW: plain water; SD: Sports Drink; ScJ: Sugarcane juice
In the present study the percent change in body weight with PW, SpD and ScJ trials shows marginal dehydration with test fluids. The total amount of fluid ingested and sweat loss was almost similar in all the trials, indicating similar level of hydration with the test fluids (Table 3). The PRE significantly increased with the time of exercise in all the three trials. However, there was no significant difference between the trials during exercise for PRE (Table 4). During exercise, at different time points and during recovery no significant difference was observed for thirst, stomach upsets and nausea in PW, SpD and ScJ trials. However, significant difference (P<0.05) was observed for fullness and sweetness (Table 5). Feeling of fullness and sweetness was more in ScJ trial than SpD trial.
Table 3.
Pre and post exercise changes in body weight and sweat loss of subjects in the trials with select liquid drinks
| Hydration Indicators | Mean ± Standard Error | ANOVA Value | ||
|---|---|---|---|---|
| Plain Water | Sports drink | Sugarcane juice | ||
| Pre exercise body weight (kg) | 63.7(1.52) | 64.0(1.48) | 63.7(1.52) | F = 0.01NS |
| Post exercise body weight (kg) | 63.5(1.56 | 63.7(1.49) | 63.5(1.54) | F= 0.00NS |
| Change in body weight (%) | 0.4(0.07) | 0.5(0.08) | 0.4(0.07) | F= 0.15NS |
| Sweat loss (ml) | 797.6(58.83) | 799.6(100.82) | 759.6(102.77) | F = 0.04NS |
NS: Not significant
Table 4.
Kruskals wallis test results for perceived rate of exertion of the subjects during exercise with select liquid drinks
| Trial | Exercise Time (min) (Mean Ranks) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | |
| Plain Water | 24.9 | 24.4 | 23.1 | 19.5 | 15.2 | 16.1 | 10.1 | 8.0 | 3.0 |
| Sport Drink | 21.7 | 21.5 | 20.6 | 16.7 | 15.6 | 13.6 | 8.9 | 6.0 | 3.0 |
| Sugarcane juice | 22.3 | 23.0 | 22.1 | 17.6 | 15.6 | 11.6 | 10.7 | 5.5 | 3.0 |
| Chi- square | 0.57NS | 0.41 NS | 0.3 NS | 0.48 NS | 0.01 NS | 1.55 NS | 0.39NS | 1.23 NS | 0.00 NS |
* Significant at P<0.05; NS: Not significant
Table 5.
Kruskals wallis test results for gastro intestinal tolerance of the subjects during exercise and recovery with select liquid drinks
| Gastrointestinal tolerance | Trial | Exercise Time (min) (Mean Ranks) | Recovery | |||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | |||
| Thirst | Plain Water | 16.5 | 16.0 | 11.5 | 7.5 | 22.0 |
| Sport Drink | 27.0 | 16.0 | 14.5 | 6.0 | 20.5 | |
| Sugarcane juice | 25.5 | 22.3 | 16.5 | 6.0 | 26.5 | |
| Chi- square Value | 9.09* | 9.57NS | 4.10 NS | 2.00 NS | 2.63 NS | |
| Sweetness | Plain Water | 9.0 | 9.0 | 5.5 | 3.0 | 8.2 |
| Sport Drink | 26.9 | 21.5 | 17.8 | 9.0 | 29.3 | |
| Sugarcane juice | 33.1 | 27.5 | 19.2 | 10.0 | 31.4 | |
| Chi- square Value | 31.9* | 24.9* | 21.9* | 11.0* | 30.9* | |
| Stomach Upset | Plain Water | 23.0 | 18.0 | 13.5 | 5.0 | 22.5 |
| Sport Drink | 23.0 | 18.0 | 15.0 | 6.5 | 24.0 | |
| Sugarcane juice | 23.0 | 18.0 | 13.5 | 8.0 | 22.5 | |
| Chi- square Value | 0.00NS | 0.00 NS | 2.00 NS | 2.44 NS | 2.00 NS | |
| Fullness | Plain Water | 19.5 | 15.5 | 10.5 | 3.0 | 18.5 |
| Sport Drink | 22.5 | 18.9 | 15.7 | 7.5 | 21.4 | |
| Sugarcane juice | 27.0 | 22.0 | 17.5 | 9.0 | 29.0 | |
| Chi- square Value | 6.28* | 3.98NS | 6.32* | 8.17* | 10.72* | |
| Nausea | Plain Water | 23.0 | 18.5 | 15.5 | 6.50 | 23.0 |
| Sport Drink | 23.0 | 18.5 | 15.5 | 6.50 | 23.0 | |
| Sugarcane juice | 23.0 | 18.5 | 15.5 | 6.50 | 23.0 | |
| Chi- square Value | 0.00 NS | 0.00 NS | 0.00 NS | 0.00 NS | 0.00 NS | |
Significant at P<0.05; NS: Not significant
DISCUSSION
In the present context, HR responses were not significantly different during and after exercise with ingestion of plain water, sports drink and sugarcane juice. Owen et al[28] reported that no significant difference in heart rate was noticed with the ingestion of either plain water or CHO beverages during prolonged exercise in the hot environment.
The time to exhaustion showed no significant difference between three trials viz; SpD, ScJ and PW, but the mean values showed that the subjects performed exercise for a longer time with SpD and ScJ than PW. The primary reason for the difference in cycling time was due to presence of CHO and electrolyte sources in SpD & ScJ which were able to maintain blood glucose at a higher level for oxidation by muscle thus sparing the muscle glycogen stores [29–31]. In a competitive sport the margin between victory and defeat is small. This small difference might sway the result. Athletes may improve their exercise performance with consumption of CHO fluids during exercise for the winning edge.
Ingestion of CHO during endurance exercise results in maintenance of blood glucose levels and a concomitant increase in the rate of CHO oxidation during the later stages of the exercise and spares muscle glycogen [8, 32–35]. In the present context at different points of exercise time (20, 40, 60, 80 minutes) the glucose values significantly increased in SpD, ScJ and PW trials and no significant difference was found between the trials. The exogenous CHO source might have resulted in the increases observed in blood glucose levels in both SpD and ScJ trials. The subjects performing with water might have depended for glucose on the internal mobilization of muscle glycogen, glycogenolysis, lactate conversion and free fatty acids.
This increase in glucose concentrations with CHO beverages in this study was also similar to the responses found in studies conducted by Owen et al [28] and Ivy et al [36]. Both studies showed increased performance endurance with the increase of blood glucose concentration. In the present context a trend of increased exercise performance with SpD and ScJ trials compared to water was evident, however the differences were not significant.
Muscle glycogen resynthesis is enhanced if CHO beverages are ingested immediately following an exercise bout [15, 16, 37]. The rate of resynthesis is dependent on the amount of CHO ingested [38] and the addition of sodium to CHO solutions results in increased replenishment of muscle glycogen. In recovery, significantly increased blood glucose concentration was observed in the SpD, ScJ compared to PW trials. The increase in blood glucose concentration with SpD and ScJ indicates that perhaps this increase in blood glucose was because of exogenous CHO and in PW trial free fatty acids (FFA) may have contributed. Seidman et al [39] haveobserved similar results with glucose polymer and water which shows increased blood glucose with glucose polymer and increased FFA with water.
The lactate levels showed no significant difference between the three trials. This shows that blood lactate concentration was not affected either by ingestion of water or by sports drink or sugarcane juice. Fritzsche et al [40] found similar results with ingestion of water and a CHO drink.
The precent change in body weight with PW, SpD and ScJ trials shows marginal dehydration with test fluids. The total amount of fluid ingested and sweat loss was almost similar in all the trials, indicating similar level of hydration was observed with the test fluids. Owen et al [28] have reported that sweat rates remain the same with different CHO beverages during exercise in the heat. Sweating rates were reported to be similar with a 10% glucose polymer, 10% glucose or water.
The major target of fluid replacement during exercise and recovery is to maintain plasma volume, so that circulation and sweating can progress at optimum level [27]. Progressive, uncompensated reduction in plasma volume result in increased heart rate and cardiac stroke volume and eventually in the hearts ability to maintain its output [41].
In this study, the mean values showed increased haemoglobin and hematocrit and decreased plasma volume at different time points of exercise with PW, SpD and ScJ trials. This shows an insufficient amount of fluid ingestion with sweat loss. This also is evident from change in body weight. The post exercise mean values showed decreased haemoglobin and hematocrit level and increased plasma volume than the pre exercise levels with PW, SpD and ScJ. This could be due to fluid replacement post exercise.
Exercise intensity is interpreted via oxygen uptake, relative values such as heart rate and in terms of ratings of subjective intensity as perceived by the subject [42]. The perception of exertion may be looked upon as a kind of gestalt, or configuration of sensations: strain, aches and fatigue from the peripheral muscles and the pulmonary system, and some other sensory cues [43]. The increased rate of PRE in the present study indicates that ingestion of CHO drink can make the task of exercise easier. Carter et al [44] also noticed that the PRE was significantly lower during the CHO ingestion trials compared to the plain water trials.
Gastric emptying rates and intestinal absorption may have impact on actual performance. There was a significant difference in sweetness and fullness with ScJ when compared with SpD. This may be due to the composition of sugarcane juice. Cool water, at a temperature of 5 to 15°C empties quicker than warmer solutions [46]. In this study beverages were served were at a temperature of 13-15°C.
Limitations
The researchers could assess the rehydration status only up to 15 min. We could not assess the serum electrolyte status during and after exercise, blood lactate level during exercise, total body water and plasma free fatty acids.
CONCLUSION
ScJ may be equally effective as SpD and PW during exercise in a comfortable environment (<30°C) and as effective a rehydration drink as SpD and PW. Reduction of the feeling of fullness with ScJ and maintenance of plasma volume may be achieved with modification through addition of NaCl.
ACKNOWLEDGMENTS
This work was supported by Sports Authority of India, New Delhi.
Conflict of interests: None
REFERENCES
- 1.Below PR, Mora-Rodriguez R, Gonzalez–Alonso J, et al. Fluid and carbohydrate ingestion independently improve performance during one hour of intense exercise. Med Sci Sports Exer. 1995;17:456–61. [PubMed] [Google Scholar]
- 2.Snyder AC, Moorhead K, Luedtke J, et al. Carbohydrate consumption prior to repeated bouts of high-intensity exercise. Eu J Appl Physio. 1993;66:141–5. doi: 10.1007/BF01427055. [DOI] [PubMed] [Google Scholar]
- 3.Criswell D, Renschler K, Powers S, et al. Fluid replacement beverages and maintenance of plasma volume during exercise: role of aldosterone and vasopressin. Eur J Appl Physio. 1992;65:445–51. doi: 10.1007/BF00243512. [DOI] [PubMed] [Google Scholar]
- 4.Angus JD, Hargreaves M, Dancey J, et al. Effect of carbohydrate or carbohydrate plus medium –chain triglyceride ingestion on cycling time trial performance. J Appl Physio. 2000;88:113–9. doi: 10.1152/jappl.2000.88.1.113. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki BL, Engell DB, Maller O. Perception of water temperature and effects for humans after exercise. Physio Behaviour. 1987;32:851–5. doi: 10.1016/0031-9384(84)90205-1. [DOI] [PubMed] [Google Scholar]
- 6.Arkinstall JM, Bruce CR, Nikolopoulos V, et al. Effect of carbohydrate ingestion on metabolism during running and cycling. J Appl Physio. 2001;91:2125–34. doi: 10.1152/jappl.2001.91.5.2125. [DOI] [PubMed] [Google Scholar]
- 7.Mc Conell GK, Canny BJ, Daddo MC, et al. Effect of carbohydrate ingestion on glucose kinetics and muscle metabolism. J Str Cond Res. 2000;18:189–93. doi: 10.1152/jappl.2000.89.5.1690. [DOI] [PubMed] [Google Scholar]
- 8.Millard SM, Sparling PB, Rosskopf LB, et al. Should carbohydrate concentration of a sports drink be less than 8 % during exercise in the heat. Int J Sports Nut Exer metab. 2005;15:117–30. doi: 10.1123/ijsnem.15.2.117. [DOI] [PubMed] [Google Scholar]
- 9.Wong SH, Williams C, Simpson M, Ogaki T. Influence of fluid intake pattern on short-term recovery from prolonged, submaximal running and subsequent exercise capacity. J Sports Sci. 1998;16:143–52. doi: 10.1080/026404198366858. [DOI] [PubMed] [Google Scholar]
- 10.Murray R. The effects of consuming carbohydrate –electrolyte beverage on gastric emptying and fluid absorption during and following exercise. Sports Med. 1987;21:275–82. doi: 10.2165/00007256-198704050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kingwell B, McKenna MJ, Sandstrom ER, Hargreaves M. Effect of glucose polymer ingestion on energy and fluid balance during exercise. J Sports Sci. 1989;7:3–8. doi: 10.1080/02640418908729817. [DOI] [PubMed] [Google Scholar]
- 12.Murray R, Paul GL, Seifert JG, et al. Carbohydrate feeding and exercise: effect of beverage carbohydrate content. Eur J Appl Physiol. 1989;59:152–8. doi: 10.1007/BF02396594. [DOI] [PubMed] [Google Scholar]
- 13.Nose H, Mack GW, Shi XR, et al. Involvement of sodium retention hormones during re hydration in humans. J Appl Physio. 1988;65:332–6. doi: 10.1152/jappl.1988.65.1.332. [DOI] [PubMed] [Google Scholar]
- 14.Reilly J, Ekblom B. The use of recovery method post exercise. J Sports Sci. 2005;23:619–27. doi: 10.1080/02640410400021302. [DOI] [PubMed] [Google Scholar]
- 15.Bilzon JL, Murply JL, Allsopp AJ, et al. Influence of glucose ingestion by humans during recovery from exercise on substrate utilization during subsequent exercise in a warm environment. Eur J Appl Physio. 2002;87:318–26. doi: 10.1007/s00421-002-0614-4. [DOI] [PubMed] [Google Scholar]
- 16.Burke LM, Collier GR, Hargreves M. Muscle glycogen storage after prolonged exercise: Effect of the glycemic index of carbohydrate feeding. J Appl Physiol. 1993;75:1019–23. doi: 10.1152/jappl.1993.75.2.1019. [DOI] [PubMed] [Google Scholar]
- 17.Lambert CP, Costill DL, McConnell GR, et al. Fluid replacement after dehydration: influence of beverage carbonation and carbohydrate content. Int J Sports Med. 1992;13:285–92. doi: 10.1055/s-2007-1021268. [DOI] [PubMed] [Google Scholar]
- 18.Gisolfi CV, Duchman M. Guidelines for optimal replacement beverages for different athletic events. Med Sci Sports Exer. 1992;24:679–87. [PubMed] [Google Scholar]
- 19.Saat M, Singh R, Sirisingle RG, et al. Rehydration after exercise with fresh young coconut water, carbohydrate and electrolyte beverage and plain water. J Phy Anthro Appl Human Sci. 2002;21:93–104. doi: 10.2114/jpa.21.93. [DOI] [PubMed] [Google Scholar]
- 20.Ismail I, Singh R, Siri Singh RG. Rehydration with sodium enriched coconut water after exercise induced dehydration. South East Asian J Tropical Med Public Health. 2007;38:764–85. [PubMed] [Google Scholar]
- 21.Earnest CP, Lancastu SL, Rasmussen RJ, et al. Low versus high glycemic index meals carbohydrate gel ingestion during stimulated 64 km cycling time performance. J Str Cond Res. 2004;18:466–72. doi: 10.1519/R-xxxxx.1. [DOI] [PubMed] [Google Scholar]
- 22.Watson P, Love JD, Maugham RJ, et al. A comparison of the effects of milk and a carbohydrate electrolyte drink on the restoration of fluid balance and exercise capacity in a hot humid environment. Eu J Appl Physio. 2008;104:633–42. doi: 10.1007/s00421-008-0809-4. [DOI] [PubMed] [Google Scholar]
- 23.Shirreffs SM, Aragon vargas LF, Keil M, et al. Rehydration after exercise in the heat: a comparison of 4 commonly used drinks. Int J Sports Nut Exer Metab. 2007;17:244–58. doi: 10.1123/ijsnem.17.3.244. [DOI] [PubMed] [Google Scholar]
- 24.Astrand PO, Rodhal K. Text book of work physiology. New York: Mc Graw Hills; 1970. [Google Scholar]
- 25.Mc Ardle WD, Katch FL, Katch VI. Philadelphia: Lea and Febiger; 1991. Exercise physiology: Energy, Nutrition and Human Performance. Chap.9. [Google Scholar]
- 26.Crosby WH, Munn JC, Furtt FW. Standardizing a method for clinical hemoglobinometry, US. Armed Force Med Jl. 1965:693–696. [PubMed] [Google Scholar]
- 27.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma and red cells in dehydration. J Appl Physio. 1974;37:247–8. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 28.Owen MD, Kregel KC, Wall PT, et al. Effects of ingesting carbohydrate beverages during exercise in the heat. Med Sci Sports Exer. 1986;18:568. [PubMed] [Google Scholar]
- 29.Hargreaves M, Costill DL, Coggan A, et al. Effect of Carbohydrate feedings on muscle glycogen utilization and exercise performance. Med Sci Sports Exer. 1985;16:219–22. [PubMed] [Google Scholar]
- 30.Davies JM, Jackson DA, Broadwell MS, et al. Carbohydrate drinks delay fatigue during intermittent high intensity cycling in active women. Int J Sports Med. 1997;7:261–73. doi: 10.1123/ijsn.7.4.261. [DOI] [PubMed] [Google Scholar]
- 31.Yaspelkis BB, Ivy JL. Effect of carbohydrate supplements and water on exercise metabolism in the heat. J Appl Physio. 1991;71:680. doi: 10.1152/jappl.1991.71.2.680. [DOI] [PubMed] [Google Scholar]
- 32.Wright DA, Sherman WM, Dernbach AR. Carbohydrate feedings before, during or in combination improve cycling performance. J Appl Physio. 1991;171:598. doi: 10.1152/jappl.1991.71.3.1082. [DOI] [PubMed] [Google Scholar]
- 33.Murray R, Paul GL, Seifert JG, et al. Responses to varying rates of CHO ingestion during exercise. Med Sci Sports Exer. 1997;23:713. [PubMed] [Google Scholar]
- 34.Rehrer RJ. Fluid and electrolyte balance in ultra endurance sport. Sports Med. 2001;31:701–15. doi: 10.2165/00007256-200131100-00001. [DOI] [PubMed] [Google Scholar]
- 35.Schramm J, Pradel HG. Volume and electrolyte disturbances in endurance sport. Internist. 2006;47:1145–50. doi: 10.1007/s00108-006-1724-6. [DOI] [PubMed] [Google Scholar]
- 36.Ivy TL, Miller W, Dover V, et al. Endurance improved by ingestion of a glucose polymer supplement. Med Sci Sports Exer. 1983;52:466–71. [PubMed] [Google Scholar]
- 37.Maugham RJ, Leiper JB, Shirreffs SM. Factors influencing the restoration of fluid and electrolyte balance after exercise in the heat. Br J Sports Med. 1997;31:175–82. doi: 10.1136/bjsm.31.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blom PCS, Hostmark AJ, Vaege O, et al. Effect of different post sugar diets on the rate of muscle glycogen synthesis. Med Sci Sports Exer. 1987;19:491–6. [PubMed] [Google Scholar]
- 39.Seidman DS, Ashkenazi I, Arnon R, et al. The effects of glucose polymer beverage ingestion during prolonged outdoor exercise in the heat. Med Sci Sports Exer. 1991;23:S152. [PubMed] [Google Scholar]
- 40.Fritzsche RG, Switzer TW, Hodgkinson BJ, et al. Water and carbohydrate ingestion during prolonged exercise increase maximal neuro muscular power. J Appl Physio. 2000;88:730–7. doi: 10.1152/jappl.2000.88.2.730. [DOI] [PubMed] [Google Scholar]
- 41.Murray R. The role of salt and glucose replacement drinks in the marathon. Sports Med. 2007;37:358–60. doi: 10.2165/00007256-200737040-00021. [DOI] [PubMed] [Google Scholar]
- 42.Borg G. Perceived exertion and pain scales. Champaign. Illinios: Human Kinetics Publishers; 1982. [Google Scholar]
- 43.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exer. 1982;4:377–81. [PubMed] [Google Scholar]
- 44.Carter J, Jeukendrup AE, Mundel T, et al. Carbohydrate supplementation improves moderate and high intensity exercise in the heat. Eur J Physiol. 2003;446:211–9. doi: 10.1007/s00424-003-1020-4. [DOI] [PubMed] [Google Scholar]
- 45.Peryam DR, Pilgrim FJ. Hedonic scale method of measuring food preference. Food Tech. 1957;11:9–14. [Google Scholar]
- 46.Caspary WF. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. 1992;55:299S–305S. doi: 10.1093/ajcn/55.1.299s. [DOI] [PubMed] [Google Scholar]