Abstract
Objective
Contingency management (CM) is an empirically validated treatment for substance use disorders, but its effects on psychiatric symptoms have not been systematically examined. The purpose of this study was to examine the impact of CM on psychiatric symptoms of cocaine dependent patients receiving CM with standard care versus standard care alone.
Methods
Psychiatric symptoms were evaluated in 393 patients participating in one of three randomized trials of CM at treatment initiation and 1, 3 (post-treatment), 6, and 9 months later.
Results
Patients randomized to CM evidenced significant reductions in psychiatric symptoms over time throughout the 9 month follow-up. In contrast, psychiatric symptoms remained stable relative to baseline in patients randomized to standard care. A significant time by treatment condition effect was noted (p < .05) for overall psychiatric distress as well as for specific indices of depression, hostility, interpersonal sensitivity, phobic anxiety and psychoticism symptoms. Reductions in drug use mediated the effects of CM on psychiatric symptoms.
Conclusions
These data demonstrate that the effects of CM extend beyond their impact on drug use behaviors and the period in which reinforcers are in effect.
Keywords: cocaine, contingency management, substance abuse treatment, psychiatric symptoms
Substance abusers suffer from high rates of psychiatric problems. Nationally representative data reveal that 60% of substance abusers also have a mood disorder and 43% an anxiety disorder (Grant et al., 2004). Although many substance abuse treatment programs do not formally diagnose, and even fewer treat, non-substance related disorders, psychiatric symptoms are common in patients initiating drug abuse treatment. Cocaine use disorders, in particular, are linked to depression, anxiety and psychotic symptoms (Conway et al., 2006; Falck, Wang, Carlson, Eddy, & Siegal, 2002; Tang, Kranzler, Gelernter, Farrer, & Cubells, 2007).
Because drug use can exacerbate psychiatric symptoms (Curran, Kirchner, Worley, Rookey, & Booth, 2002), effective treatments for drug use may reduce psychiatric distress. Polcin, Korcha, Bond, and Galloway (2010) found psychiatric symptoms significantly decreased while patients resided in sobriety houses for 18 months. However, durations of stay in outpatient clinics are typically shorter, and changes in psychiatric symptoms during the time patients remain engaged in outpatient settings, or following discharge, are not well characterized.
Contingency management (CM) interventions that reinforce submission of drug-negative samples are highly efficacious in reducing drug use (Lussier, Heil, Mongeon, Badger, & Higgins, 2006), and they may also improve other areas of functioning. In terms of psychiatric symptoms, benefits of CM were noted on Addiction Severity Index (ASI; McLellan, Luborsky, Cacciola, & Griffin, 1985) psychiatric composite scores in one of Higgins and colleagues’ CM studies (2003), but not others (Higgins, Wong, Badger, Haug Ogden, & Dantona, 2000; Higgins et al., 2007). The ASI may not be a sensitive indicator of change as only a few dimensions of distress are measured primarily on binary indices, and/or the lack of systematic findings may relate to power. Although CM studies find moderate to large effect sizes for reducing drug use (Lussier et al., 2006), individual studies may be underpowered for uncovering effects in other areas, which are likely to be less robust as psychiatric symptoms are not directly targeted by the intervention.
The purpose of this study was to examine changes in psychiatric symptoms over time and in response to CM versus usual care in cocaine treatment patients using a sensitive index of psychiatric distress. To enhance power, data from three independent, yet similar, trials of CM were combined (Petry, Alessi, Tedford, Austin, & Tardif, 2005; Petry et al., 2004, 2006). Hypotheses were that psychiatric symptoms would decrease as patients initiate drug abuse treatment, and CM treatments would be especially efficacious in reducing psychiatric distress. Reductions in cocaine use were expected to mediate the beneficial effects of CM.
Methods
Participants were 393 patients randomized in three studies of CM (Petry et al., 2004,2005,2006). The trials were conducted at four outpatient community-based drug abuse treatment clinics, which provided similar content and types of treatment to patients of comparable ages, education and drug use histories. The trials also employed similar treatment intensities and duration (3 months), and used the same assessments. For each, inclusion criteria were initiating treatment, age >18 years, and Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) criteria for cocaine abuse or dependence. Exclusion criteria were significant uncontrolled psychopathology (e.g., suicide attempt in past 30 days, active psychosis) or in recovery for pathological gambling (because of potential similarity of gambling and prize CM; but see Petry & Alessi, 2010). Only one patient was excluded for severe psychopathology. All patients provided written informed consent, approved by Institutional Review Boards.
Procedures
Patients completed demographic questionnaires, drug use diagnoses modules derived from the Structured Clinical Interview for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996), and the Brief Symptom Inventory (BSI; Derogatis, 1993) to assesses severity of past week psychiatric symptoms. It uses a 5-point scale (0 to 4), with higher scores reflecting greater severity. The Global Severity Index (GSI) averages overall severity of symptoms, with scores ranging from 0–4; the mean score in healthy controls is 0.30 (standard deviation (SD) = 0.31) (Derogatis, 1993). Subscales assess nine dimensions: somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychotocism. The BSI is well validated, including in drug abusing samples (Wang et al., 2010).
The BSI was administered at baseline, and at 1, 3 (post-treatment), 6 and 9 month follow-ups, for which patients were compensated $15-$35. Follow-up rates were 84.5%, 81.2%, 73.8%, and 69.0% at the respective time points. No differences were noted on baseline characteristics or treatment assignments in follow-up completers versus non-completers (ps > .19).
Treatments
A computer program randomized patients to treatment conditions. Each study (Petry et al., 2004, 2005, 2006) included three conditions, one of which was standard care, and the other two were CM. Treatments are detailed in the original publications.
Standard care (SC) patients received usual care at the clinic. Intensive outpatient therapy, consisting of relapse prevention, coping and life skills training, AIDS education, and 12-step therapy, was provided for 2–4 weeks; it did not include interventions for psychiatric conditions other than drug use disorders. Intensity gradually reduced to one session per week. Treatment was provided in group format. Patients were also expected to submit 21 breath and urine samples over the 12-week studies. Alcosensor IV Altometers (Intoximeters, St Louis, MO, USA) tested for alcohol, and Ontrak TesTstiks (Roche, Somersville, NJ, USA) tested for cocaine and opioids.
CM patients received SC and sample testing as above, plus reinforcers contingent upon abstinence and/or completion of treatment-related activities. Each trial (Petry et al., 2004, 2005, 2006) applied two different CM conditions, and four of the six CM conditions reinforced both abstinence and activities. Abstinence CM conditions applied reinforcers when samples tested negative for cocaine, opioids and alcohol concurrently, and activity CM conditions reinforced patients for completing steps related to goals, such as attending medical or legal appointments; objective verification was required for reinforcement. Schedules were independent such that failure to complete an activity did not affect abstinence reinforcement or vice versa. Five of the six CM conditions used chances to win prizes as reinforcers, and one used vouchers; maximal expected reinforcement was $80-$882. Because six CM conditions were included across studies, sample sizes were too small (and confounded by study) to divide treatments based on reinforcement targets, magnitudes or types. Nevertheless, in each study, CM significantly improved drug abuse outcomes, with no differences between CM conditions within studies, providing rationale for combining CM conditions for these analyses.
Data Analysis
All patients assigned to a CM condition were classified into the CM group, and patients not randomized to CM into the SC group. We compared the two treatment groups in terms of demographics and baseline drug use diagnoses using Chi-square or F tests.
Hierarchial linear models (HLM) evaluated changes in GSI scores from baseline to month 9. These analyses accommodate actual assessment intervals, and within-participants (Level-1) and between-participants (Level-2) equations were specified, with partial regression coefficients estimated for intercept and slopes at each level. Models below were used to predict outcome variable Y (GSI scores) from the Level-1 predictor Time and Level-2 predictor Group:
To examine group differences over time, group was coded 0 for CM patients, so significant effects of Intercept (γ10) indicate that the slope of CM patients differed significantly from 0, and significant effects of slope (γ11) reveal that slopes of the two groups differed. Models were rerun with SC coded 0 such that significant effects of γ10 indicate changes over time differed significantly from 0 in SC. Predictor variables were treated as fixed and entered uncentered. Final estimation of fixed effects (with robust standard errors) is presented.
Models above were repeated with Y defined as BSI subscale scores. Analyses are protected against multiple comparisons when a change in an overall score comprised of those same domains is significant (Rosenthal & Rosnow, 1991), as was the case herein.
To evaluate whether reductions in cocaine use mediated the effects, four steps (Baron & Kenny, 1986) were evaluated. Step 1 (outlined above) evaluated if CM improved GSI scores over time relative to SC. Step 2 examined if CM increased proportion of negative urine samples provided, using a t-test. Step 3 evaluated partial correlations between proportion of negative samples and month 9 GSI scores, controlling for baseline GSI scores. Step 4 assessed if inclusion of proportion of negative samples as a between-participants predictor rendered the relationship in Step 1 between group and GSI scores non-significant. The Level-1 Model was identical to that described earlier, and the Level-2 Model was:
If all four steps follow these patterns, changes in GSI scores can be attributed to abstinence engendered by CM.
Results
No between group differences were noted on baseline characteristics (Table 1). Baseline BSI scores did not differ by treatment condition, or across studies or sites (data not shown).
Table 1.
Demographic and Baseline Characteristics
| Standard treatment | Contingency Management | Significance test | p-value | |
|---|---|---|---|---|
| N | 115 | 278 | ||
| Study, % (n) | χ2 (2) = 0.68 | .71 | ||
| Petry et al. (2004) | 32.2 (37) | 29.9 (83) | ||
| Petry et al. (2005) | 34.8 (40) | 32.7 (91) | ||
| Petry et al. (2006) | 33.0 (38) | 37.4 (104) | ||
| Men, n (%) | 63 (54.8) | 134 (48.2) | χ2 (1) = 1.41 | .24 |
| Ethnicity % (n) | χ2 (3) = 3.30 | .35 | ||
| African American | 51.3 (59) | 52.9 (147) | ||
| European American | 37.4 (43) | 33.5 (93) | ||
| Hispanic/Latino | 11.3 (13) | 11.2 (31) | ||
| Other | 0.0 (0) | 2.5 (7) | ||
| Years of educationa | 11.4 (1.7) | 11.7 (1.6) | F (1, 391) = 3.57 | .06 |
| Ever married, % (n) | 25.2 (29) | 24.1 (67) | χ2 (1) = 0.06 | .82 |
| Employed full time, % (n) | 42.6 (49) | 46.4 (130) | χ2 (1) = 0.45 | .57 |
| Annual earned income ($)b | 3,500 (13,200) | 3,224 (12,528) | U (df = 1) = 15366.5 | .54 |
| Cocaine dependent, % (n) | 84.3 (97) | 86.0 (239) | χ2 (1) = 0.17 | .68 |
| Alcohol dependent, % (n) | 53.0 (61) | 52.9 (147) | χ2 (1) = 0.001 | .98 |
| Opiate dependent, % (n) | 18.3 (21) | 20.1 (56) | χ2 (1) = 0.09 | .77 |
| Positive urine/breath sample at treatment initiation, n (%) | 17.4 (20) | 19.1 (53) | χ2 (1) = 0.16 | .69 |
| Ever received psychiatric treatment, n (%) | 53.9 (62) | 49.6 (138) | χ2 (1) = 0.59 | .44 |
| Currently prescribed psychiatric medication, n (%) | 33.3 (38) | 27.7 (77) | χ2 (1) = 1.12 | .29 |
Note. Values are percentages (n’s in parentheses) unless noted.
Mean ± standard deviation.
Median (interquartile range).
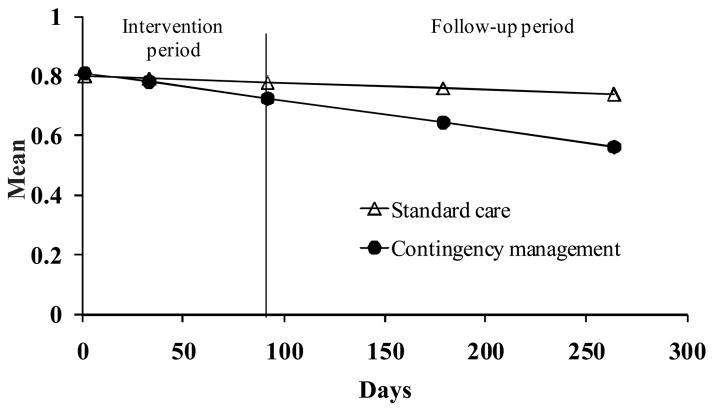
Table 2 shows means and standard deviations of BSI scores at each assessment point and results from HLM analyses. GSI scores decreased over time in CM (p < .001), but not SC (p = .39). Changes over time differed by group, T ratio (391) = 2.19, p = .03 (Figure 1).
Table 2.
Raw Means and Standard Errors of Brief Symptom Inventory Scores and Hierarchial Linear Model Results with Within-Group Slope Estimates Representing Main Effects of Time.
| Outcome variable Group | Raw scores | β1 Coefficient | Standard error | T ratio (df = 391) | p < | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 1 | Month 3 | Month 6 | Month 9 | |||||
| Global Symptom Index | |||||||||
| Standard care | .87 ± .69 | .71 ± .72 | .73 ± .81 | .71 ± .76 | .75 ± .80 | −0.000230* | 0.000268 | −0.86 | .39 |
| CM | .90 ± .71 | .64 ± .63 | .72 ± .75 | .65 ± .66 | .57 ± .65 | −0.000940 | 0.000184 | 5.12 | .001 |
| Somatization | |||||||||
| Standard care | .58 ± .69 | .56 ± .72 | .57 ± .75 | .53 ± .67 | .56 ± .64 | −0.000097 | 0.000269 | −0.36 | .72 |
| CM | .60 ± .68 | .47 ± .66 | .55 ± .29 | .51 ± .66 | .49 ± .68 | −0.000249 | 0.000190 | −1.32 | .19 |
| Obsessive-compulsive | |||||||||
| Standard care | 1.11 ± .91 | .94 ± .88 | .87 ± .94 | .84 ± .94 | .95 ± .98 | −0.000477 | 0.000321 | −1.49 | .14 |
| CM | 1.13 ± .95 | .80 ± .82 | .91 ± .94 | .78 ± .84 | .71 ± .81 | −0.001156 | 0.000226 | −5.12 | .001 |
| Interpersonal sensitivity | |||||||||
| Standard care | .99 ± .92 | .74 ± .93 | .81 ± 1.02 | .76 ± .96 | .81 ± 1.02 | −0.000310* | 0.000357 | −0.87 | .39 |
| CM | 1.04 ± .99 | .69 ± .86 | .77 ± 1.09 | .69 ± .83 | .61 ± .84 | −0.001242 | 0.000225 | −5.53 | .001 |
| Depression | |||||||||
| Standard care | 1.01 ± .94 | .77 ±.93 | .82 ± .99 | .81 ± 1.01 | .85 ± 1.05 | −0.000137* | 0.000358 | −0.38 | .70 |
| CM | 1.05 ± .89 | .70 ± .80 | .79 ± .93 | .72 ± .87 | .62 ± .79 | −0.001152 | 0.000240 | −4.80 | .001 |
| Anxiety | |||||||||
| Standard care | .84 ± .80 | .70 ± .84 | .71 ± .93 | .69 ± .86 | .69 ± .80 | −0.000372 | 0.000301 | −1.236 | .22 |
| CM | .92 ± .89 | .67 ± .85 | .70 ± .83 | .63 ± .77 | .55 ± .79 | −0.001040 | 0.000218 | −4.76 | .001 |
| Hostility | |||||||||
| Standard care | .63 ± .76 | .64 ± .86 | .68 ± .87 | .63 ± .82 | .73 ± .91 | 0.000294* | 0.000352 | 0.84 | .40 |
| CM | .78 ± .79 | .60 ± .72 | .68 ± .81 | .59 ± .70 | .55 ± .72 | −0.000648 | 0.000201 | −3.23 | .002 |
| Phobic anxiety | |||||||||
| Standard care | .62 ± .72 | .52 ± .69 | .50 ± .72 | .52 ± .73 | .60 ± .85 | 0.000054* | 0.000274 | 0.20 | .84 |
| CM | .64 ± .79 | .44 ± .65 | .49 ± .69 | .44 ± .66 | .36 ± .62 | −0.000832 | 0.000186 | −4.47 | .008 |
| Paranoid ideation | |||||||||
| Standard care | 1.05 ± .84 | .81 ± .86 | .81 ± .95 | .80 ± .90 | .79 ± .92 | −0.000634 | 0.000327 | −1.94 | .05 |
| CM | 1.01 ± .83 | .76 ± .79 | .84 ± .94 | .77 ± .84 | .67 ± .84 | −0.000969 | 0.000227 | −4.27 | .001 |
| Psychoticism | |||||||||
| Standard care | .89 ± .82 | .69 ± .88 | .71 ± .93 | .70 ± .90 | .76 ± .95 | −0.000201* | 0.000325 | −062 | .54 |
| CM | .93 ± .87 | .63 ± .79 | .72 ± .92 | .62 ± .76 | .50 ± .73 | −0.001253 | 0.000218 | −5.75 | .001 |
Note. CM = Contingency management. β1 coefficient = γ10 for the CM condition when coded 0 (and standard care coded 1); β1 coefficient = γ10 for the standard care condition when coded 0 (and CM coded 1); β1coefficients indicate the rate of change in scores per day from baseline through Month 9.
= slopes are significantly different between treatment conditions, p < .05.
Figure 1.
Brief Symptom Inventory Global Symptom Index scores over time by treatment condition. Data are derived from hierarchical linear models analyses as described in the text, and represent group means. Data from participants assigned to the standard treatment condition are shown in filled triangles, and data from participants assigned to a contingency management condition are shown in open circles. The treatment condition by time interaction effect is significant.
Scores on all subscales except somatization significantly decreased over time in CM patients (Table 2). In contrast, reductions over time trended toward significance on only one subscale (paranoid ideation) in the SC group (p = .05). For interpersonal sensitivity, depression, hostility, phobic anxiety, and psychoticism subscales, group by time interactions were significant (T ratio (391) = 2.21, 2.35, 2.32, 2.67, and 2.69, respectively, all ps < .05).
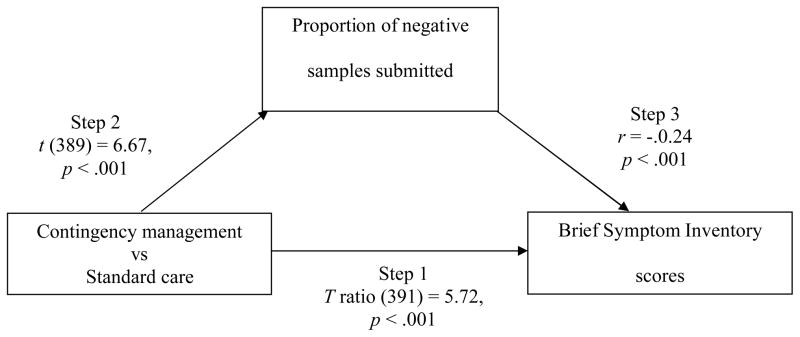
To evaluate if changes in cocaine use mediated these effects (Figure 2), between group differences in proportions of negative sample were examined in Step 2. In CM patients, 85.1% (SD = 19.6) of samples tested negative vs 68.2% (SD = 29.3) in SC patients, t (389) = 6.67, p < .001. Step 3 was also significant; after controlling for baseline GSI scores, correlations between proportions of negative samples submitted and GSI scores at month 9 were −0.24 (p < .001). The final condition for mediation was also met. When proportion of negative samples submitted was included in the HLM model, treatment condition was no longer significant, T ratio (391) = −1.18, p = .24, but proportion negative samples was, T ratio (391) = −2.27, p = .02. Figure 3 shows GSI scores over time based on treatment condition and proportion of negative samples submitted.
Figure 2.
Mediational model. Treatment group differences predicted changes in Brief Symptom Inventory scores in Step 1 (see Figure 1). Contingency management treatment increased abstinence in Step 2. Increases in abstinence, in turn, accounted for differential changes in Brief Symptom Inventory scores in Step 3. The strengths of each of these associations are shown by the statistics and p values included. The final step (4) of the medication model tested whether group differences remained significant after abstinence was added to the analysis; because group differences were no longer significant, T ratio (391) = −1.18, p = .24, but proportion of negative samples was, T ratio (391) = −2.27, p = .02, abstinence fulfilled criteria for a mediator.
Figure 3.
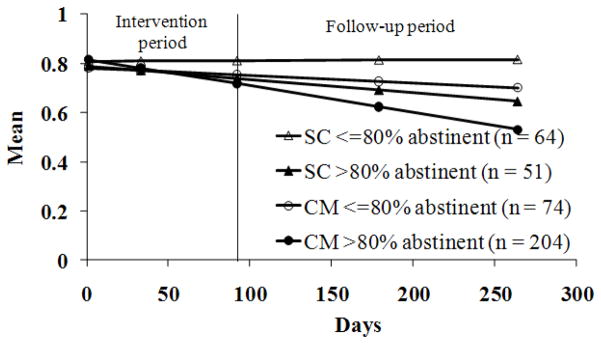
Brief Symptom Inventory Global Symptom Index scores over time by treatment condition and proportion of negative samples submitted. Data are derived from hierarchical linear models analyses as described in the text, and represent group means. Data from patients assigned to the standard care (SC) condition are shown in triangles, and data from patients assigned to a contingency management (CM) condition are shown in circles. Groups are further subdivided based upon proportion of negative samples submitted; patients who submitted less than 80% negative samples are shown in open symbols, and patients who submitted more than 80% negative samples are shown in filled symbols.
Discussion
CM significantly reduced psychiatric symptoms compared to SC. Group by time interaction effects were noted for a global measure of psychiatric distress and for five of nine specific types of symptoms assessed. Given the high rates of psychiatric comorbidity and distress in substance abusers (Conway et al., 2006; Curran et al., 2002; Grant et al., 2004), these data demonstrate important benefits of CM. Further, this study found that the effects of CM on reducing psychiatric symptoms are mediated by reductions in drug use.
The baseline level of psychiatric distress in these patients is similar to that reported in other clinical samples. Using this same index, GSI scores range from 0.83 to 1.52 in other substance abusing populations (Johnson et al., 2007; Polcin et al., 2010). Additionally, these levels of psychiatric distress are two to three standard deviations above the means of controls and indicative of clinical distress (Derogatis, 1993).
Psychiatric symptoms did not change significantly over time among patients randomized to SC, but they decreased in patients randomized to CM. The magnitude of reduction in GSI scores in CM-treated patients was similar to that reported with depressed patients receiving depression treatment (Beulter et al., 1991), suggestive of clinically significant reductions in psychiatric symptoms with CM. Given high rates of psychiatric distress and comorbidity in substance abusers, these benefits of CM for improving psychiatric symptoms are noteworthy. Research is ongoing advocating for the inclusion of psychiatric specific treatment alongside drug abuse treatment (Kelly, Daley, & Douaihy, 2012). Although additional psychiatric services are clearly beneficial for some subsets of substance abusing patients, these data suggest that provision of CM confers benefits in reducing psychiatric distress via increases in drug abstinence, without requiring pharmacology or extensive training and oversight in administration of specific psychiatric treatment protocols.
Importantly, the benefits of CM on reducing psychiatric distress extended beyond the duration of the treatment period. Decreases in psychiatric symptoms were seen throughout the 9-month follow-up period. These data suggest that CM can evidence long-term changes.
However, limitations to this study exist. Although this study examined the impact of CM on psychiatric symptoms for 9 months, longer-term evaluations would be useful. Additionally, this study utilized the BSI. This instrument is widely used and has well established psychometric properties (Derogatis, 1993), but it is not a diagnostic instrument. Whether CM engenders remission in actual psychiatric diagnoses requires further investigation.
A potential concern about this study design relates to including data from several studies and CM interventions, which differed in behaviors targeted for reinforcement and magnitudes and types of reinforcers provided. However, the presence of a significant effect across studies and CM conditions speaks to the generalization of CM’s effect in reducing psychiatric symptoms.
Strengths of this study include the large sample size, use of broad inclusion and few exclusion criteria, and integration of CM within the context of usual care in four community-based clinics. These procedures all enhance generalization of the findings. Further, the studies achieved acceptable rates of follow-up, and HLM analyses accounted for missing data, allowing for a true intent-to-treat approach including all randomized patients.
In sum, this study suggests that CM reduces psychiatric symptoms by its effects on decreasing drug use. This result is important because it speaks to an additional and enduring benefit of CM. Although CM is criticized for cost, it consistently yields benefits in reducing drug use (Lussier et al., 2006). These data extend its positive effects to another important dimension. Greater widespread implementation of CM may ultimately save health care costs by reducing the need for ancillary services and improving psychiatric functioning in drug abusing patients.
Acknowledgments
This research and preparation of this report were supported by NIH grants: P30-DA023918, R01-DA027615, R01-DA022739, R01-DA13444, P50-DA09241, P60-AA03510, R01-HD075630, DP3-DK09770, and R21-DA03189.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beutler LE, Engle D, Mohr D, Daldrup RJ, Bergan J, Meredith K, Merry W. Predictors of differential response to cognitive, experiential, and self-directed psychotherapeutic procedures. Journal of Consulting and Clinical Psychology. 1991;59:333–340. doi: 10.1037//0022-006x.59.2.333. [DOI] [PubMed] [Google Scholar]
- Booth BM, Curran G, Han X, Wright P, Frith S, Leukefeld C, Falck R, Carlson RG. Longitudinal relationship between psychological distress and multiple substance use: Results from a three-year multisite natural-history study of rural stimulant users. Journal of Studies on Alcohol and Drugs. 2010;71:258–267. doi: 10.15288/jsad.2010.71.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2006;67:247–57. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Curran GM, Kirchner JE, Worley M, Rookey C, Booth BM. Depressive symptomatology and early attrition from intensive outpatient substance use treatment. Journal of Behavioral Health Services and Research. 2002;29:138–43. doi: 10.1007/BF02287700. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory (BSI): Administration, Scoring and Procedure manual. Minneapolis: National Computer Systems; 1993. [Google Scholar]
- Falck RS, Wang J, Carlson RG, Eddy M, Siegal HA. The prevalence and correlates of depressive symptomatology among a community sample of crack-cocaine smokers. Journal of Psychoactive Drugs. 2002;34:281–288. doi: 10.1080/02791072.2002.10399964. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–81. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry. 2003;60:1043–52. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Brems C, Mills M, Fisher D. Psychiatric symptomatology among individuals in alcohol detoxification treatment. Addictive Behaviors. 2007;32:1745–1752. doi: 10.1016/j.addbeh.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addictive Behaviors. 2012;37:11–24. doi: 10.1016/j.addbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola JS, Griffin JE. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;163:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine abusers with and without recent gambling problems. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, Sierra S. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006;74:592–601. doi: 10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Tedford J, Austin M, Tardif M. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treatment of cocaine abusers: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcin DL, Korcha RA, Bond J, Galloway G. Sober living houses for alcohol and drug dependence: 18-month outcomes. Journal of Substance Abuse and Treatment. 2010;38:356–365. doi: 10.1016/j.jsat.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R. Essentials of Behavioral Research: Methods and Data Analysis (2nd ed.) New York: McGraw Hill; 1991. [Google Scholar]
- Tang YL, Kranzler HR, Gelernter J, Farrer LA, Cubells JF. Comorbid psychiatric diagnoses and their association with cocaine-induced psychosis in cocaine-dependent subjects. American Journal on Addiction. 2007;5:343–351. doi: 10.1080/10550490701525723. [DOI] [PubMed] [Google Scholar]
- Wang J, Kelly BC, Booth BM, Falck RS, Leukefeld C, Carlson RG. Examining factorial structure and measurement invariance of the Brief Symptom Inventory (BSI)-18 among drug users. Addictive Behaviors. 2010;35:23–29. doi: 10.1016/j.addbeh.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]