Abstract
Background
Cystatin C predicts mortality more strongly than does serum creatinine. It is unknown whether this advantage extends to other outcomes, such as kidney failure, or whether other novel renal filtration markers share this advantage in predicting outcomes.
Study Design
Observational cohort study.
Setting &Participants
9,988 participants in the Atherosclerosis Risk in Communities (ARIC) Study, a population-based study in 4 US communities, followed for approximately 10 years.
Predictors
Serum creatinine-based estimated glomerular filtration rate (eGFRcr), cystatin C, β-trace protein (BTP) and β2-microglobulin (B2M).
Outcomes
Mortality, coronary heart disease, heart failure and kidney failure.
Results
Higher concentrations of cystatin C and B2M were more strongly associated with mortality (n=1425) than was BTP, and all were more strongly associated than eGFRcr (adjusted hazard ratio [95% confidence interval] for the upper 6.7 percentile compared to the lowest quintile: 1.6 [1.3-1.9] for eGFRcr; 2.9 [2.3-3.6] for cystatin C; 1.9 [1.5-2.4] for BTP; 3.0 [2.4-3.8] for B2M). Similar patterns were observed for coronary heart disease (n=1279), heart failure (n=803) and kidney failure (n=130). The addition of cystatin C, BTP and B2M to models including eGFRcr and all covariates, including urinary albumin:creatinine ratio, significantly improved risk prediction for all outcomes (p<0.001).
Limitations
No direct measurement of GFR.
Conclusions
B2M, and to a lesser extent, BTP, share cystatin C′s advantage over eGFRcr in predicting outcomes, including kidney failure. These additional markers may be helpful in improving estimation of risk associated with reduced kidney function beyond current estimates based on eGFRcr.
Individuals with decreased kidney function are at substantially higher risk of mortality, cardiovascular disease and kidney failure than the general population.(1-3) Cystatin C, a novel marker of kidney function, is more strongly associated with mortality and cardiovascular disease than is the traditionally-used serum creatinine.(4;5) However, it is unknown if this is a specific property of cystatin C or whether strong associations with these adverse outcomes are shared by other novel kidney function markers, suggesting a greater potential for early detection and prevention. It also is unclear whether the advantage of cystatin C, and potentially other novel markers, extends to prediction of kidney failure.(6;7)
Creatinine is a byproduct of muscle breakdown and, therefore, serum concentrations are affected by an individual's muscle mass.(5) Equations accounting for age, race and sex improve the estimation of glomerular filtration rate (GFR) by accounting for average differences in muscle mass across these factors.(8) Estimating equations, however, cannot account for individual differences in muscle mass for a given age, sex and race. Muscle wasting due to chronic illness is associated with lower creatinine generation, leading to a bias towards higher estimated GFR in such individuals. As these same individuals are at an elevated risk of mortality, this systematic bias results in an underestimate of the association between decreased GFR and mortality risk.(5) In addition, renal tubular secretion of creatinine masks mildly decreased filtration, precluding early detection of disease. Cystatin C is less affected by muscle mass than serum creatinine, is not subject to tubular secretion, and is thought to be a better marker of early kidney dysfunction.(9) Some reports, however, have shown that cystatin C concentrations are affected by non-renal factors, including smoking, obesity and inflammation, which may be associated with increased risk themselves.(9;10) Additional analytes, including the low molecular weight proteins β-trace protein (BTP) and β2 microglobulin (B2M), have recently been examined as alternative markers of kidney function. Several studies have reported correlations of BTP and B2M with directly measured GFR that are better or similar to those observed with creatinine.(11-16) However, data on the putative associations between serum BTP or B2M concentrations with outcomes are limited.(17;18)
To our knowledge, no studies have compared the associations of serum creatinine, cystatin C, BTP, and B2M with the risk of mortality, coronary heart disease, heart failure, and kidney failure. We hypothesized that BTP and B2M will be stronger risk factors in the general population than creatinine-based estimated GFR and show associations of similar strength to cystatin C.
Methods
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective observational cohort of 15,792 black and white individuals between the ages of 45 and 64, recruited from a probability sample of four U.S. communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD). Details of the ARIC Study have been published elsewhere.(19) A total of 9,988 of the 11,656 participants in the fourth ARIC examination (1990-1992) had complete data for all kidney function markers and covariates and are included in these analyses.
Assessment of Covariates
Demographic information was obtained by a trained interviewer during an in-home interview prior to the baseline examination. Medication use was determined by self-report and examination of medication bottles. Smoking status was determined by self-reported cigarette smoking. Diabetes mellitus was defined as fasting glucose of ≥126 mg/dL, non-fasting glucose of ≥ 200 mg/dL, or use of oral hypoglycemic medication or insulin. Two seated systolic blood pressure measurements, following five minutes rest, were taken by certified technicians using a random-zero sphygmomanometer, and the average was recorded. Prevalent coronary heart disease was defined as a reported history of a physician-diagnosed heart attack, evidence of a prior myocardial infarction by electrocardiogram (presence of a major Q-wave abnormality or a minor Q-wave abnormality with T-wave or S-T segment abnormality), or a self-reported prior coronary reperfusion procedure. Body mass index (BMI) was calculated by body weight (in kilograms) divided by height (in meters) squared.
Plasma cholesterol, triglycerides, and HDL cholesterol were determined using enzymatic methods, and LDL cholesterol was calculated using the Friedewald equation.(20) CRP was measured in 2008 on plasma frozen at -70°C from Visit 4 by the immunoturbidimetric assay using the BNII analyzer (Siemens Healthcare Diagnostics, www.medical.siemens.com). The reliability coefficient for blinded quality control replicates of CRP assayed on different days was 0.99 (421 blinded replicates). Spot urine samples were collected and stored at −70°C. Urinary albumin concentrations were measured by nephelometry and urinary creatinine concentrations were measured using the Jaffé method. The urinary albumin:creatinine ratio (ACR) was calculated and, due to its skewed distribution, was logarithmically transformed for analysis.
Measurement of Kidney Function Markers
A modified kinetic Jaffe method was used to measure serum creatinine (reliability coefficient = 0.95 in 439 blinded replicates) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) equation was used to estimate glomerular filtration rate (eGFRCKD-Epi).(8) The creatinine concentration was indirectly calibrated to the IDMS-traceable measurements at the Cleveland Clinic.(21)
Cystatin C, BTP and B2M were measured by a particle-enhanced immunonephelometric assay with a BNII nephelometer (Siemens Healthcare Diagnostics, Deerfield, IL). The reliability coefficients after removing outliers (>3 sd difference) for blinded replicates were 0.94 in 411 replicates (10 outliers) for Cystatin C, 0.96 in 381 replicates (7 outliers) for BTP and 0.98 in 390 replicates (9 outliers) for B2M.
Outcomes Assessment
Active surveillance for all events before January 1, 2008, included annual telephone calls and screening all known hospitalizations as well as local obituaries. A CHD event was defined as a definite or probable myocardial infarction, definite CHD death, or coronary revascularization procedure as adjudicated by the ARIC Morbidity and Mortality Classification Committee.(22) Incident heart failure was defined as the first heart failure hospitalization (ICD-9 code 428) or death with heart failure as an underlying condition (ICD-9 code 428 or ICD-10 code I50). Study participants with evidence of prevalent heart failure at baseline were excluded from analyses.(23) Incident end-stage renal disease (ESRD) was defined by ICD-9 codes specified for kidney transplant (V42.0), dialysis (V45.1) or a procedural code indicating dialysis (hemodialysis -39.95 or peritoneal dialysis - 54.98) or death with kidney failure (584.5-587) including codes for acute renal failure (ARF; codes: 586, 584, 788.9) as an underlying cause of death if they had an earlier diagnosis of CKD (as defined by an eGFR<60 mL/min/1.73 m2 and/or hospitalization code). However, individuals that had a transplant or dialysis code and an ARF code without previous CKD and those with an ARF code of 958.5 (traumatic anuria) were not included. A total of 52 participants reached ESRD prior to Visit 4 and were excluded from analyses of this outcome.
Statistical Analysis
Categories of eGFRCKD-Epi and concentrations of kidney function markers (cystatin C, BTP, B2M) were created by dividing the study population into quintiles and subdividing the highest quintile into tertiles, comparable to seminal work on cystatin C.(4) Pairwise correlation coefficients were determined using the reciprocal of each kidney function markers, since the reciprocals reflect kidney function more closely and are more normally distributed. Associations of eGFRCKD-Epi and marker concentrations with outcomes were assessed with Cox proportional hazards regression models, using the seven categories of each marker. Multivariable models adjusted for age, sex, race, field center, diabetes, prevalent CHD, current smoking, BMI, systolic blood pressure, antihypertensive medication use, LDL cholesterol, HDL cholesterol, log(triglycerides), log(hsCRP), log(ACR). Adjusted hazard ratios were calculated for each category of eGFRCKD-Epi and marker concentration, using the lowest quintile of each marker concentration (highest quintile of eGFRCKD-Epi) as the reference category. Strengths of associations were compared between fully adjusted models using seemingly unrelated regression methods.(25) Analyses were repeated in models limited to participants with baseline eGFRCKD-Epi ≥ 60 mL/min/1.73 m2. Due to the relatively small number of ESRD events, quintiles 1-4 were used as the reference category for these analyses of ESRD.
Continuous net reclassification improvement (NRI) at 10 years of follow-up (approximate median follow-up) was calculated from fully adjusted Poisson models and defined as the sum of those classified upward to higher risk in those with an event plus those classified downward to lower risk in those without an event, less the sum of those classified downward to lower risk in those with an event plus those classified to higher risk in those without an event. We also report NRI separately for participants with an event (eventNRI) and without an event (non-eventNRI). (26) Confidence intervals were calculated by bootstrapping (1,000 repetitions). Analyses were performed using Stata software (StataCorp, www.stata.com). The ARIC Study was reviewed and approved by the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health
Role of the funding source
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute. The study sponsors had no other role in the study design, the collection, analysis, and interpretation of data, or the writing of the report or the decision to submit the paper for publication.
Results
Lower eGFRCKD-Epi categories were associated with higher age, greater prevalence of black race, greater prevalence of CHD, higher systolic blood pressure, greater prevalence of antihypertensive medication use, higher LDL cholesterol, higher hsCRP concentrations and higher urinary ACR values, and with a lower prevalence of smoking (Table 1). The highest eGFRCKD-Epi quintile (≥96.7 mL/min/1.73 m2), however, often had worse rather than better risk factors levels, including a greater prevalence of black race and diabetes and higher hsCRP concentrations.
Table 1. Baseline characteristics (sd), by category of estimated glomerular filtration rate.
| Estimated GFRCKD-Epi (mL/min/1.73 m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5a | Quintile 5b | Quintile 5c | ||
| ≥96.7 | 89.3-96.6 | 81.6-89.2 | 71.8-89.1 | 66.7-71.7 | 60.0-66.6 | <60.0 | p-trend | |
| N | 2029 | 1977 | 1995 | 2014 | 661 | 672 | 640 | - |
| Age, years | 59.3 (4.4) | 61.8 (5.6) | 64.5 (4.6) | 63.6 (5.7) | 64.2 (6.1) | 65.7 (5.1) | 66.6 (5.2) | <0.001 |
| Male, % | 63.3 | 52.5 | 56.2 | 53.8 | 64.0 | 54.9 | 56.4 | 0.03 |
| Black, % | 42.4 | 14.3 | 15.3 | 15.4 | 18.9 | 15.8 | 23.2 | <0.001 |
| Diabetes, % | 20.8 | 13.8 | 13.9 | 13.5 | 14.4 | 13.7 | 26.4 | 0.9 |
| Prevalent CHD, % | 6.3 | 7.1 | 8.0 | 7.5 | 7.9 | 10.3 | 17.3 | <0.001 |
| Current smoking, % | 21.4 | 16.2 | 13.3 | 10.9 | 11.2 | 9.1 | 11.1 | <0.001 |
| Body mass index, kg/m2 | 29.5 (6.3) | 28.3 (5.4) | 28.5 (5.5) | 28.5 (5.1) | 28.7 (5.0) | 28.6 (5.0) | 29.4 (5.5) | 0.2 |
| Systolic blood pressure, mmHg | 127.0 (18.7) | 126.1 (18.2) | 127.7 (18.4) | 126.4 (18.2) | 127.5 (20.2) | 129.0 (21.2) | 132.4 (21.5) | <0.001 |
| Antihypertensive medication use, % | 35.5 | 28.9 | 32.5 | 32.9 | 40.1 | 42.7 | 64.5 | <0.001 |
| LDL cholesterol, mg/dL | 119.0 (33.6) | 122.3 (33.1) | 122.4 (32.1) | 123.7 (33.1) | 124.6 (31.2) | 125.7 (35.9) | 126.9 (37.0) | <0.001 |
| HDL cholesterol, mg/dL | 51.9 (16.6) | 50.8 (16.7) | 51.0 (16.5) | 49.6 (16.2) | 49.4 (16.0) | 48.3 (15.5) | 47.2 (16.2) | <0.001 |
| Triglycerides,mg/dL | 128.3 (66.3) | 136.6 (67.9) | 135.4 (66.2) | 137.5 (65.8) | 140.9 (67.2) | 148.3 (71.6) | 157.8 (71.5) | <0.001 |
| hsCRP, mg/dL* | 2.9 (1.2-6.5) | 2.2 (0.9-5.1) | 2.3 (1.0-5.0) | 2.2 (1.0-4.9) | 2.5 (1.1-5.4) | 2.4 (1.1-5.5) | 3.5 (1.4-7.4) | 0.07 |
| Urinary albumin:creatinine, mg/mg* | 0.05 (0.01-0.17) | 0.06 (0.02-0.17) | 0.06 (0.02-0.17) | 0.05 (0.02-0.15) | 0.05 (0.02-0.15) | 0.06 (0.02-0.16) | 0.09 (0.02-0.43) | <0.001 |
| Standardized creatinine, mg/dL | 0.7 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.9 (0.1) | 1.0 (0.1) | 1.1 (0.1) | 1.4 (1.0) | <0.001 |
|
| ||||||||
| eGFRCKD-Epi, mL/min/1.73 m2 | 103.5 (6.3) | 92.4 (2.1) | 85.7 (2.2) | 76.7 (2.9) | 69.4 (1.4) | 63.6 (2.0) | 49.8 (10.1) | <0.001 |
| Cystatin C, mg/L | 0.83 (0.20) | 0.90 (0.22) | 0.95 (0.26) | 0.98 (0.23) | 1.03 (0.24) | 1.09 (0.25) | 1.49 (0.77) | <0.001 |
| BTP, mg/dL | 0.59 (0.12) | 0.66 (0.37) | 0.69 (0.16) | 0.71 (0.14) | 0.75 (0.16) | 0.80 (0.30) | 1.11 (0.85) | <0.001 |
| B2M, mg/dL | 0.18 (0.04) | 0.19 (0.04) | 0.20 (0.04) | 0.21 (0.04) | 0.23 (0.06) | 0.24 (0.07) | 0.36 (0.30) | <0.001 |
eGFRCKD-Epi: Estimated glomerular filtration rate based on serum creatinine, using Chronic Kidney Disease Epidemiology Collaboration equation; BTP: β-trace protein; B2M: β2 microglobulin; sd: standard deviation.
Median (inter-quartile range)
Each of the kidney function markers was significantly correlated with the other markers (p<0.001 for all pairwise correlations). The strongest correlation was between 1/cystatin C and 1/B2M (r=0.74). The correlations of 1/cystatin C with 1/BPT (r=0.59) and eGFRCKD-Epi with 1/cystatin C (r=0.57), 1/BTP (r=0.52) and 1/B2M (r=0.55) were of similar magnitude.
Mortality
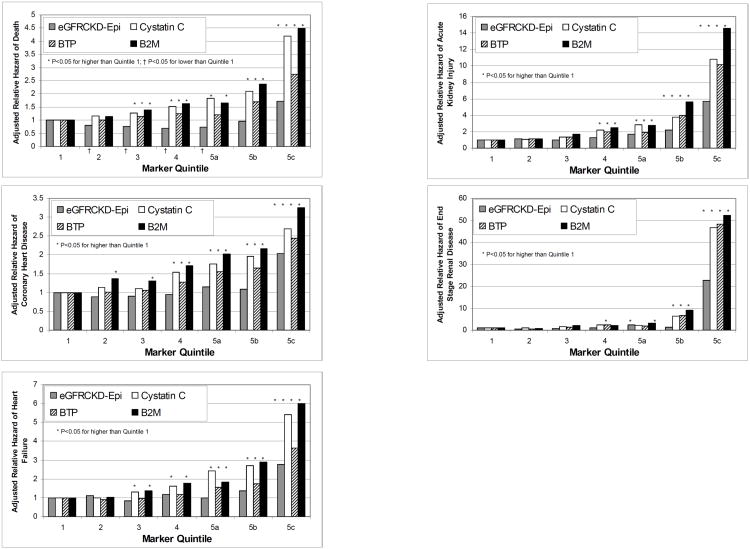
A total of 1425 deaths occurred during a median follow-up of 10.2 years. Concentrations of cystatin C and B2M were more strongly associated with mortality than was eGFRCKD-Epi in models adjusted for age, race, sex, and field center (Figure 1.A.). For eGFRCKD-Epi, only the lowest category (subquintile 5c; eGFRCKD-Epi <60.0 mL/min/1.73 m2) was significantly associated with a greater incidence of mortality (hazard ratio [HR]=1.57; 95% confidence interval [CI]: 1.27, 1.93), whereas quintiles 3-5c were significantly (p<0.003 with HRs of 2.89, 1.90 and 3.01 for subquintile 5c of cystatin C, BTP and B2M) associated with a greater risk of mortality for the other markers. The association between higher BTP category and mortality was stronger than eGFRCKD-Epi, (p<0.001) but weaker than cystatin C and B2M (both p<0.001). Additional adjustment for diabetes, prior coronary heart disease, smoking, systolic blood pressure, antihypertensive medication use, LDL and HDL cholesterol, triglycerides, hsCRP and urinary ACR attenuated all associations, but the patterns remained similar and statistically signficant, with cystatin C and B2M having the strongest associations, and eGFRCKD-Epi having the weakest associations with mortality overall (Table 2). The addition of cystatin C, BTP and B2M categories to a fully-adjusted model with eGFRCKD-EPI categories resulted in improved risk classification for mortality (NRI=27.41; 10.49 for those with an event and 16.92 for those without an event; Table 4).
Figure 1.
Adjusted relative hazard by marker quantile, adjusted for age, sex, race and field center. (A) death (1425 events in 9988 participants), (B) coronary heart disease (1279 events in 9988 participants), (C) heart failure (803 events in 9414 participants), (D) end-stage renal disease (130 events in 9936 participants). * p<0.05 for higher than Quantile 1. † p<0.05 for lower than Quantile 1.
Table 2. Adjusted* hazard ratio (95% CI), by quintile of kidney function marker.
| Quintile | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | 5b | 5c | p-trend | |
| Mortality (1425 events/9988 participants) | ||||||||
| eGFRCKDEPI | Reference | 0.90 (0.75, 1.09) | 0.87 (0.72, 1.05) | 0.87 (0.72, 1.05) | 0.90 (0.69, 1.16) | 1.12 (0.89, 1.41) | 1.57‡ (1.27, 1.93) | <0.001 |
| Cystatin C | Reference | 1.21 (0.98, 1.50) | 1.29† (1.04, 1.59) | 1.46† (1.19, 1.80) | 1.67‡ (1.31, 2.14) | 1.77‡ (1.39, 2.25) | 2.89‡ (2.32, 3.61) | <0.001 |
| BTP | Reference | 1.06 (0.87,1.29) | 1.22 (1.00, 1.48) | 1.29† (1.07, 1.56) | 1.19 (0.92, 1.53) | 1.55‡ (1.23, 1.95) | 1.90‡ (1.54, 2.36) | <0.001 |
| B2M | Reference | 1.08 (0.87, 1.34) | 1.36‡ (1.10, 1.67) | 1.49‡ (1.22, 1.83) | 1.44‡ (1.12, 1.85) | 1.87‡ (1.47, 2.38) | 3.01‡ (2.41, 3.75) | <0.001 |
| Coronary Heart Disease (1279 events/9988 participants) | ||||||||
| eGFRCKDEPI | Reference | 0.97 (0.80, 1.18) | 0.97 (0.80, 1.19) | 1.05 (0.86, 1.27) | 1.15 (0.89, 1.48) | 1.07 (0.83, 1.38) | 1.46‡ (1.16, 1.84) | 0.001 |
| Cystatin C | Reference | 1.09 (0.89, 1.35) | 0.97 (0.78, 1.20) | 1.29† (1.05, 1.57) | 1.32† (1.03, 1.70) | 1.35† (1.05, 1.72) | 1.53‡ (1.20, 1.95) | <0.001 |
| BTP | Reference | 1.05 (0.86, 1.30) | 1.05 (0.86, 1.29) | 1.25† (1.02, 1.52) | 1.42† (1.11, 1.82) | 1.41† (1.10, 1.80) | 1.50‡ (1.19, 1.89) | <0.001 |
| B2M | Reference | 1.28† (1.05, 1.58) | 1.14 (0.93, 1.41) | 1.37† (1.11, 1.68) | 1.45† (1.13, 1.87) | 1.37† (1.06, 1.78) | 1.78‡ (1.39, 2.28) | <0.001 |
| Heart Failure (803 events/9414 participants) | ||||||||
| eGFRCKDEPI | Reference | 1.27 (0.98, 1.65) | 0.95 (0.73, 1.24) | 1.43† (1.11, 1.84) | 1.14 (0.80, 1.60) | 1.42† (1.04, 1.94) | 2.14‡ (1.61, 2.83) | <0.001 |
| Cystatin C | Reference | 1.01 (0.76, 1.35) | 1.24 (0.94, 1.63) | 1.44† (1.10, 1.88) | 1.91‡ (1.40, 2.62) | 1.95‡ (1.44, 2.66) | 3.18‡ (2.39, 4.24) | <0.001 |
| BTP | Reference | 0.94 (0.73, 1.22) | 1.00 (0.77, 1.29) | 1.13 (0.88, 1.45) | 1.41† (1.04, 1.92) | 1.43† (1.05, 1.94) | 2.02‡ (1.54, 2.65) | <0.001 |
| B2M | Reference | 0.97 (0.73, 1.29) | 1.22 (0.93, 1.60) | 1.48† (1.14, 1.93) | 1.36† (0.98, 1.90) | 1.95‡ (1.42, 2.66) | 3.25‡ (2.43, 4.33) | <0.001 |
| End-stage renal disease (130 events/9936 participants) | ||||||||
| eGFRCKDEPI | Reference | 0.74 (0.28, 1.93) | 1.02 (0.45, 2.32) | 1.15 (0.52, 2.55) | 2.77† (1.18, 6.47) | 1.50 (0.53, 4.24) | 11.75‡ (6.29, 21.95) | <0.001 |
| Cystatin C | Reference | 1.26 (0.46, 3.51) | 1.47 (0.57, 3.78) | 2.18 (0.90, 5.32) | 1.53 (0.45, 5.19) | 3.61† (1.43, 9.12) | 16.62‡ (7.45, 37.00) | <0.001 |
| BTP | Reference | 0.48 (0.15, 1.50) | 1.18 (0.47, 2.97) | 1.75 (0.78, 3.92) | 1.07 (0.29, 3.88) | 3.40† (1.42, 8.15) | 12.44‡ (6.18, 25.06) | <0.001 |
| B2M | Reference | 0.87 (0.30, 2.52) | 1.71 (0.68, 4.34) | 1.87 (0.73, 4.78) | 1.70 (0.57, 5.10) | 5.11‡ (2.06, 12, 66) | 16.99‡ (7.48, 38.58) | <0.001 |
Adjusted for age, sex, race, field center, diabetes, prevalent CHD, current smoking, systolic blood pressure, antihypertensive medication use, LDL cholesterol, HDL cholesterol, log(triglycerides), log(hsCRP), and log(ACR). eGFRCKDEPI (GFR estimated using CKD-EPI equation for serum creatinine); BTP: β-trace protein; B2M: β2 microglobulin.
p<0.05 versus Quintile 1
p<0.001 versus Quintile 1.
Table 4.
Net reclassification indices (NRI) comparing fully adjusted* models including quantiles of estimated glomerular filtration rate (eGFRCKDEPI), cystatin C, β-trace protein and β2-microglobulin to fully adjusted models including quantiles of eGFRCKDEPI only, by subgroup.
| All participants | |||
|---|---|---|---|
| EventNRI (95% CI) | Non-eventNRI (95% CI) | NRI (95% CI) | |
| Mortality | 10.49 (5.03, 15.95) | 16.92 (14.81, 19.03) | 27.41 (21.51, 33.32) |
| Coronary Heart Disease | 12.79 (6.98, 18.60) | 5.14 (3.04, 7.24) | 17.93 (11.62, 24.25) |
| Heart Failure | 9.50 (2.25, 16.74) | 19.52 (17.52, 21.50) | 29.01 (21.41, 36.61) |
| End-stage renal disease | 34.79 (17.84, 51.74) | 11.76 (9.88, 13.64) | 46.53 (29.41, 63.68) |
| Participants with eGFRCKDEPI ≥ 60 mL/min/1.73 m2 | |||
| Mortality | 22.33 (15.42, 29.24) | 5.27 (-1.15, 11.70) | 17.06 (14.93, 19.19) |
| Coronary Heart Disease | 8.54 (2.53, 14.55) | 8.11 (5.94, 10.27) | 16.64 (10.27, 23.02) |
| Heart Failure | 2.72 (-5.19, 10.63) | 23.40 (21.29, 25.51) | 26.12 (17.91, 34.34) |
| End-stage renal disease† | 11.46 (-17.27, 40.19) | 9.63 (7.63, 11.62) | 32.77 (3.35, 62.19) |
Adjusted for age, sex, race, field center, diabetes, prevalent CHD, current smoking, systolic blood pressure, antihypertensive medication use, LDL cholesterol, HDL cholesterol, log(triglycerides), log(hsCRP), and log(ACR). eGFRCKDEPI (GFR estimated using CKD-EPI equation for serum creatinine.
Four categories of each marker.
Coronary Heart Disease
A total of 1279 CHD incident events occurred. The associations with CHD events were weaker than the associations with mortality for each kidney function marker examined. Similar to the findings for mortality, however, cystatin C and B2M concentrations were more strongly associated with CHD events than was eGFRCKD-Epi (both p<0.001; Figure 1.B.). The magnitude of the association between BTP category and CHD events was again between that of cystatin C and B2M and that of eGFRCKD-Epi. In fully adjusted models, there was little difference between the strength of the associations for cystatin C, BTP and B2M, whereas the association was relatively weak for eGFRCKD-Epi in all but the lowest category (Table 2). The improvement in risk classification with the addition of cystatin C, BTP and B2M categories to a fully-adjusted model with eGFRCKD-EPI categories was slightly lower for CHD than for mortality, due to a lower NRI for those without an event (NRI=17.93; 12.79 for those with an event and 5.14 for those without an event; Table 4).
Heart Failure
A total of 803 events occurred among 9414 participants without prevalent heart failure. Associations between higher marker quintiles and heart failure were of greater magnitude than the associations with mortality. Again, B2M and cystatin C were most strongly associated with risk, with BTP having a slightly stronger association than eGFRCKD-Epi (Figure 1.C. and Table 2). The improvement in risk classification was similar to that for mortality (Table 4).
End Stage Renal Disease
A total of 130 of 9936 eligible participants reached ESRD during follow-up. The associations between each of the markers and incidence of ESRD were much stronger at the lowest levels of kidney function than for the other outcomes (Figure 1.E.). The associations were much stronger for cystatin C, BTP and B2M than for eGFRCKD-Epi (Table 2) The improvement in risk classification for ESRD was substantially greater than for the other outcomes, primarily due to a higher NRI (34.79; 95% CI: 17.84, 51.74) among individuals with an ESRD event (Table).
Subgroup Analyses
Each of the non-creatinine markers remained strongly positively associated with mortality among participants with eGFRCKD-Epi ≥ 60 mL/min/1.73 m2, while eGFRCKD-Epi showed a flatter or “U”-shaped association with risk (Table 3). As in the overall population, the associations of cystatin C and B2M with mortality were stronger than the association of BTP (both p<0.001). There were only 54 ESRD events among 9277 participants with eGFRCKD-Epi ≥60 mL/min/1.73 m2. Nonetheless, each of the novel markers was significantly associated with incidence of ESRD in this subgroup. Compared to a fully adjusted model with eGFRCKD-Epi categories alone, a fully-adjusted model with all four markers improved risk classification among participants with eGFRCKD-Epi ≥ 60 mL/min/1.73 m2 for mortality (NRI=22.33; p<0.001), CHD (NRI=16.64; p<0.001), CHF (NRI=26.12; p<0.001) and ESRD (NRI=32.77; p=0.03).
Table 3. Adjusted hazard ratio (95% CI) among participants with eGFRCKD-Epi ≥ 60 mL/min/1.73 m2, by quantile of kidney function marker.
| Quintile | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | 5b | 5c | p-trend | |
| Mortality (1201 events/9320 participants) | ||||||||
| eGFRCKDEPI | Reference | 0.88 (0.73, 1.07) | 0.84 (0.69, 1.02) | 0.84 (0.69, 1.02) | 0.87 (0.67, 1.13) | 1.09 (0.86, 1.38) | - | 0.82 |
| Cystatin C | Reference | 1.20 (0.97, 1.49) | 1.26† (1.02, 1.56) | 1.44† (1.17, 1.77) | 1.70‡ (1.32, 2.20) | 1.69‡ (1.31, 2.18) | 2.56‡ (1.96, 3.34) | <0.001 |
| BTP | Reference | 1.07 (0.87, 1.30) | 1.23† (1.01, 1.50) | 1.27† (1.04, 1.55) | 1.21 (0.93, 1.58) | 1.45† (1.13, 1.86) | 1.49† (1.14, 1.95) | <0.001 |
| B2M | Reference | 1.07 (0.87, 1.33) | 1.35† (1.10, 1.67) | 1.51‡ (1.23, 1.86) | 1.45† (1.12, 1.88) | 1.83‡ (1.42, 2.37) | 2.62‡ (2.00, 3.42) | <0.001 |
| Coronary Heart Disease (1115 events/9320 participants) | ||||||||
| eGFRCKDEPI | Reference | 0.95 (0.78, 1.16) | 0.95 (0.78, 1.17) | 1.02 (0.84, 1.25) | 1.13 (0.87, 1.46) | 1.08 (0.83, 1.39) | - | 0.2 |
| Cystatin C | Reference | 1.07 (0.87, 1.33) | 0.93 (0.75, 1.15) | 1.24† (1.01, 1.52) | 1.31† (1.01, 1.70) | 1.14 (0.87, 1.49) | 1.26 (0.91, 1.73) | 0.02 |
| BTP | Reference | 1.05 (0.85, 1.29) | 1.02 (0.82, 1.26) | 1.19 (0.97, 1.46) | 1.35† (1.04, 1.76) | 1.33† (1.03, 1.73) | 1.22 (0.91, 1.62) | 0.006 |
| B2M | Reference | 1.26† (1.02, 1.54) | 1.10 (0.89, 1.37) | 1.34† (1.08, 1.65) | 1.26 (0.96, 1.65) | 1.30 (0.98, 1.71) | 1.55† (1.13, 2.14) | 0.01 |
| Heart Failure (672 events/8840 participants) | ||||||||
| eGFRCKDEPI | Reference | 1.24 (0.96, 1.62) | 0.92 (0.70, 1.21) | 1.38† (1.07, 1.79) | 1.08 (0.76, 1.53) | 1.36 (0.99, 1.87) | - | 0.05 |
| Cystatin C | Reference | 0.99 (0.74, 1.32) | 1.21 (0.91, 1.60) | 1.38† (1.05, 1.82) | 1.94‡ (1.40, 2.69) | 1.91‡ (1.38, 2.65) | 2.94‡ (2.08, 4.17) | <0.001 |
| BTP | Reference | 0.93 (0.72, 1.20) | 1.00 (0.77, 1.29) | 1.08 (0.83, 1.39) | 1.41† (1.02, 1.94) | 1.26 (0.90, 1.76) | 1.60† (1.14, 2.25) | 0.001 |
| B2M | Reference | 0.94 (0.70, 1.26) | 1.21 (0.92, 1.60) | 1.49† (1.14, 1.95) | 1.29 (0.91, 1.84) | 1.96‡ (1.41, 2.74) | 2.97‡ (2.10, 4.21) | <0.001 |
| End-stage renal disease (54 events/9277 participants) | ||||||||
| eGFRCKDEPI | Reference | 3.05 (1.45, 6.43) | 1.00 (0.30, 3.31) | - | 0.2 | |||
| Cystatin C | Reference | 0.76 (0.18, 3.24) | 2.22 (0.96, 5.13) | 5.73‡ (2.55, 12.87) | <0.001 | |||
| BTP | Reference | 0.50 (0.07, 3.71) | 3.04† (1.25, 7.36) | 4.36‡ (2.04, 9.31) | <0.001 | |||
| B2M | Reference | 0.76 (0.18, 3.24) | 2.22 (0.96, 5.13) | 5.73‡ (2.55, 12.87) | <0.001 | |||
Adjusted for age, sex, race, field center, diabetes, prevalent CHD, current smoking, systolic blood pressure, antihypertensive medication use, LDL cholesterol, HDL cholesterol, log(triglycerides), log(hsCRP), and log(ACR). eGFRCKDEPI (GFR estimated using CKD-EPI equation for serum creatinine); BTP: β-trace protein; B2M: β2 microglobulin
p<0.05 versus Quintile 1;
p<0.001 versus Quintile 1.
Among the 528 participants with eGFRCKD-Epi 45-59 mL/min/1.73 m2 and no albuminuria (ACR <30 mg/g), cystatin C remained significantly associated (p≤0.05) with mortality and CHD, BTP remained significantly associated with heart failure and ESRD, and B2M remained significantly associated only with heart failure. Improvements in risk classification remained significant (p<0.001) for all outcomes in this subgroup.
In models adjusted for 7 categories (Quantiles 1-5a, 5b, 5c) of cystatin C, B2M remained significantly associated with all outcomes (p-trend<0.001) except CHD (p=0.10), and BTP remained significantly associated with CHD (p-trend=0.02) and ESRD (p-trend<0.001). Using Cystatin C, BTP and B2M significantly improved risk classification for all outcomes (all p<0.001) compared to cystatin C categories alone.
Discussion
Several studies have demonstrated that cystatin C concentrations, or GFR estimates based on cystatin C, predict mortality and cardiovascular disease better than GFR estimates based on serum creatinine.(4;5) In this community-based cohort study, we found that two filtration markers which have not been compared prospectively, B2M, and to a lesser extent, BTP, share the advantage of cystatin C over creatinine-based estimates of GFR in predicting these outcomes. We further found that cystatin C, B2M and BTP also predict kidney failure more strongly than does serum creatinine-based eGFR. These data provide the first evidence that the advantages of cystatin C over serum creatinine in risk prediction are shared by other novel filtration markers, and show that several filtration markers together improve risk prediction beyond serum creatinine for a number of clinical outcomes. These improvements in prediction associated with cystatin C, BTP and B2M are beyond that provided by other relevant predictors of outcomes, including urinary ACR.
Serum creatinine provides useful estimates of GFR which are strong risk factors for a wide range of outcomes.(27) However, it is unclear to what extent associations with creatinine under-estimate the full importance of decreased GFR. Creatinine production varies with muscle mass and catabolism and tubular secretion add to its renal elimination beyond filtration.(9) As a result, it provides a useful but imprecise estimate of GFR whose errors are negatively correlated with muscle mass. This is especially true at near-normal levels of GFR. Low molecular weight proteins, including cystatin C, BTP and B2M, provide independent measures of kidney function which are less likely to be confounded by muscle mass.(12;14;15) Each is produced at a relatively constant rate, is freely filtered by the glomeruli and is nearly completely reabsorbed and metabolized by the proximal tubules. Non-filtration influences on these markers may exist but are unlikely to be dominated by the same pathways as for serum creatinine. It is unknown whether the non-filtration factors found in some studies to be associated with circulating levels of cystatin C, including smoking, obesity, and inflammation, also affect BTP and B2M. The stronger associations with outcomes remained after adjustment for these factors, however.
Beta-trace protein acts as a prostaglandin D synthase, promoting the conversion of prostaglandin H2 to prostaglandin D2.(28) The major site of the brain form measured here is in the epithelial cells of the choroid plexus in the central nervous system.(16) Several studies have found that serum levels of BTP are higher at lower levels of kidney function, and that BTP is comparable to creatinine for estimation of GFR.(14, 29) In a study of 146 patients, serum BTP-based eGFR equations had lower bias and greater precision in children (n=54) and adults (n=92) than did eGFR based on serum creatinine.(29) Evidence of risk associated with elevated BTP is very limited. In a recent study of 227 patients with CKD (median GFR = 63 L/min/1.73 m2; inter-quartile range = 38-96 mL/min/1.73 m2), higher concentrations of BTP were strongly associated with incident ESRD or doubling of serum creatinine (median follow-up of 53 months), as were higher creatinine and cystatin C concentrations.(18) It is uncertain what non-filtration factors may influence BTP.
Beta 2-microglobulin is a small subunit of the major histocompatibility (MHC) class I molecule present on all nucleated cells.(15) B2M concentrations are increased in lymphoproliferative disorders and myeloma. Most studies analyzing the relationship between B2M concentration and GFR report relatively strong correlations, including one study of 44 participants with varying etiologies of kidney disease, in which the reciprocal of B2M was more strongly correlated with GFR by inulin than was serum creatinine.(15, 30) B2M is a predictor of survival in patients with lymphoproliferative disorders,(31) and the association between higher B2M concentrations and mortality is well known for patients on maintenance dialysis.(32) Few studies have examined the association between serum B2M concentrations and morbidity and mortality in the general population. The Tokyo Metropolitan Institute of Gerontology Longitudinal Interdisciplinary Study on Aging followed 1,034 individuals age 65 years and older for 8 years.(17) The middle tertile and upper tertile of B2M concentrations were associated with significantly higher risk of mortality (HR = 2.02 [95%CI: 1.35-3.04] and 2.84 [95% CI, 1.92-4.20], respectively) than the lowest tertile.
This study shows that while serum creatinine-based filtration estimates are associated with increased risk, this risk is lower than that observed for any of the other three markers examined. All three novel markers were strongly associated with outcomes among participants with a baseline eGFRCKD-Epi ≥ 60 mL/min/1.73 m2, where associations with eGFRCKD-Epi are much flatter and sometimes inverse, as well as among patients with eGFRCKD-Epi 45-59 mL/min/1.73 m2 and no albuminuria, where markers distinguishing lower from higher risk are particularly needed. These two novel filtration markers, in addition to cystatin C, show promise in distinguishing risk in the range of kidney function where serum creatinine-based GFR estimates have a weaker relationship to risk. The reason for this difference could be that only serum creatinine suffers from strong negative confounding by lower muscle mass. The other markers could be more tightly correlated with GFR. Alternatively, they may be associated with other factors that both increase the concentration of these factors and increase the risk of mortality. The findings were similar for each of the outcomes studied, however, reducing the likelihood that a common third factor can explain these patterns.
The limitations of the study include no direct measure of GFR and a single baseline measurement of each marker. The observed associations should be replicated in similar populations and extended to more diverse settings, assays should be standardized, and the optimal set or markers for specific settings should be determined before making specific recommendations for use in clinical practice. The finding that cystatin C shares its predictive advantage over creatinine-based GFR estimates with two other novel filtration markers supports its use and interpretation in risk prediction and indicates the potential for improved risk prediction from using markers beyond creatinine. Defining the optimal combination of markers, however, will require further study across a range of settings and populations.
In summary, we found that B2M shares the advantages of cystatin C over serum creatinine in terms of predicting outcomes mortality, CHD, heart failure, and ESRD in the general population. Serum concentrations of BTP also predicted these outcomes more strongly than did estimated creatinine-based eGFR, but not as strongly as cystatin C and B2M. These markers strongly predicted events among individuals with normal creatinine-based eGFR, extending the range over which risk is related to markers of kidney function. Overall, multiple markers of kidney function improve risk prediction beyond serum creatinine alone, suggesting that they have promise as adjuncts to serum creatinine in evaluating kidney function and assessing prognosis in the general population.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions. Drs. Astor and Coresh are supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1 R01 DK076770-01). Siemens Healthcare Diagnostics provided the reagents and loan of a BNII instrument to conduct the high sensitivity CRP, beta-2 microglobulin and beta-trace protein assays.
Footnotes
The authors have no conflicts of interest.
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55(1):31–41. doi: 10.1053/j.ajkd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 5.Astor BC, Levey AS, Stevens LA, Van LF, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20(10):2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22(1):147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kottgen A, Selvin E, Stevens LA, Levey AS, Van LF, Coresh J. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51(3):385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Giessing M. Beta-trace protein as indicator of glomerular filtration rate. Urology. 1999;54(5):940–941. [PubMed] [Google Scholar]
- 12.Priem F, Althaus H, Birnbaum M, Sinha P, Conradt HS, Jung K. Beta-trace protein in serum: a new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem. 1999;45(4):567–568. [PubMed] [Google Scholar]
- 13.Poge U, Gerhardt TM, Stoffel-Wagner B, et al. beta-Trace protein is an alternative marker for glomerular filtration rate in renal transplantation patients. Clin Chem. 2005;51(8):1531–1533. doi: 10.1373/clinchem.2005.048959. [DOI] [PubMed] [Google Scholar]
- 14.Filler G, Priem F, Lepage N, et al. Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem. 2002;48(5):729–736. [PubMed] [Google Scholar]
- 15.Woitas RP, Stoffel-Wagner B, Poege U, Schiedermaier P, Spengler U, Sauerbruch T. Low-molecular weight proteins as markers for glomerular filtration rate. Clin Chem. 2001;47(12):2179–2180. [PubMed] [Google Scholar]
- 16.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. 1997;7(4):499–506. doi: 10.1093/glycob/7.4.499. [DOI] [PubMed] [Google Scholar]
- 17.Shinkai S, Chaves PH, Fujiwara Y, et al. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch Intern Med. 2008;168(2):200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 18.Spanaus KS, Kollerits B, Ritz E, Hersberger M, Kronenberg F, von EA. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem. 2010;56(5):740–749. doi: 10.1373/clinchem.2009.138826. [DOI] [PubMed] [Google Scholar]
- 19.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators 162. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 20.Operations Manual 7: Blood Collection and Processing. Bethesda: National Heart,Lung and Blood Institute. National Heart Lung and Blood Institute Atherosclerosis Risk in Communities (ARIC) Study; 1987. Ref Type: Report. [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 22.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 24.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21(10):1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zellner A. An efficient method of estimating seemingly unrelated regressions and rests for aggregation bias. J Am Stat Assoc. 1962;57:348–368. [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, de Jong PE, Coresh J, et al. The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 28.Melegos DN, Grass L, Pierratos A, Diamandis EP. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53(1):32–37. doi: 10.1016/s0090-4295(98)00453-1. [DOI] [PubMed] [Google Scholar]
- 29.White CA, Akbari A, Doucette S, et al. A novel equation to estimate glomerular filtration rate using beta-trace protein. Clin Chem. 2007;53(11):1965–1968. doi: 10.1373/clinchem.2007.090126. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi C, Donadio C, Tramonti G, Consani C, Lorusso P, Rossi G. Reappraisal of serum beta2-microglobulin as marker of GFR. Ren Fail. 2001;23(3-4):419–429. doi: 10.1081/jdi-100104725. [DOI] [PubMed] [Google Scholar]
- 31.Delgado J, Pratt G, Phillips N, et al. Beta2-microglobulin is a better predictor of treatment-free survival in patients with chronic lymphocytic leukaemia if adjusted according to glomerular filtration rate. Br J Haematol. 2009;145(6):801–805. doi: 10.1111/j.1365-2141.2009.07699.x. [DOI] [PubMed] [Google Scholar]
- 32.Canaud B, Assounga A, Flavier JL, et al. Beta-2 microglobulin serum levels in maintenance dialysis. What does it mean? ASAIO Trans. 1988;34(4):923–929. [PubMed] [Google Scholar]