Abstract
Objective
To evaluate the association of community health indicators with outcomes for kidney transplant recipients.
Design
Retrospective observational cohort study using multivariable Cox proportional hazards models.
Setting
Transplant recipients in the United States from the Scientific Registry of Transplant Recipients merged with health indicators compiled from several national databases and the Centers for Disease Control and Prevention, including the National Center for Health Statistics, the Behavioral Risk Factor Surveillance System, and the National Center for Chronic Disease Prevention and Health Promotion.
Patients
A total of 100 164 living and deceased donor adult (aged ≥18 years) kidney transplant recipients who underwent a transplant between January 1, 2004, and December 31, 2010.
Main Outcome Measures
Risk-adjusted time to post-transplant mortality and graft loss.
Results
Multiple health indicators from recipients’ residence were independently associated with outcomes, in cluding low birth weight, preventable hospitalizations, inactivity rate, and smoking and obesity prevalence. Recipients in the highest-risk counties were more likely to be African American (adjusted odds ratio, 1.59, 95% CI, 1.51-1.68), to be younger (aged 18-39 years; 1.46; 1.32-1.60), to have lower educational attainment (<high school; 1.84; 1.62-2.08), and to have public insurance (1.46; 1.38-1.54). Proportions of recipients from higher-risk counties varied dramatically by center and region. There was an independent graded effect between health indicators and posttransplant mortality, including notable hazard associated with the highest-risk counties (adjusted hazard ratio, 1.26; 95% CI, 1.13-1.40).
Conclusions
In a national cohort of patients undergoing complex medical procedures, health indicators from patients’ communities are strong independent predictors of all-cause mortality. Findings highlight the importance of community conditions for risk stratification of patients and development of individualized treatment protocols. Findings also demonstrate that standard risk adjustment does not capture important factors that may affect unbiased performance evaluations of transplant centers.
Numerous factors contribute to an individual's mortality risk, including biological characteristics, environmental conditions, behavior, and psychosocial conditions in addition to access to and quality of health care. Although research studies often focus on clinical factors associated with a specific intervention, the contribution of underlying health conditions, continuity of care, and cumulative exposure to risks in patients’ communities indicative of environmental and social conditions may be significant and perhaps unappreciated predictors of outcomes. In the general US population, leading biological causes of death are heart disease, cancer, stroke, and chronic lower respiratory diseases.1 Studies have also highlighted the prevalence and significant contribution of “actual causes of death,” generally described as behavioral and modifiable factors that are not genetic.2 Based on this definition, leading actual causes of death are tobacco, poor diet, a reduced level of physical activity, alcohol consumption, exposure to toxic agents, and motor vehicle crashes.3 In addition, social factors, such as low educational level, racial segregation, low social support, and poverty, have effects on mortality that are comparable to biological or behavioral causes.4,5
For kidney transplant recipients, well-documented clinical factors and biological conditions (eg, recipient and donor demographic characteristics, cause of end-stage renal disease, HLA antigen mismatching, and body mass index) are associated with recipient outcomes. There is also evidence that outcomes are affected by certain behavioral factors, such as smoking and alcohol use,6-9 diet, and exercise level.10,11 Documented disparities in access to healthcare between transplant recipients are associated with processes of care as well as outcomes.12-14 In addition, socioeconomic status is associated with posttransplant survival.15-17
Cumulatively, substantial evidence suggests that factors beyond clinical and physiological conditions are strongly associated with mortality in the general population, and these factors also may pertain to transplant recipients.3-5 Distinguishing sources of risks for patients is important to optimize care and to individualize treatment and interventions. Moreover, identifying whether risks and the extent to which those risks vary by region of the country and individual providers may have important implications related to quality assessment and pay-for-performance initiatives. In particular, providers that treat large proportions of patients with risk factors that are not codified or that reflect conditions not directly associated with quality of care may be disadvantaged with regard to performance evaluations. Documentation of underlying risks and identification of potential systematic biases related to hospital performance assessments may improve prospective evaluations of quality, as well as mitigate disincentives to treat patients with unmeasured risks.
The aims of our study were to capture information that describes the environmental risks, prevalence of comorbidities, and psychosocial and behavioral attributes of the populations from communities where transplant recipients reside using data aggregated from several national registries. We then sought to evaluate the independent association of these community health indicators with transplant outcomes across the United States. We planned to evaluate characteristics of recipients who reside in regions with a higher prevalence of community risk factors and to describe the distribution by state and transplant center. Finally, we discuss the implications of community health indicators on hospital performance assessments. This study was approved by the Cleveland Clinic Institutional Review Board.
METHODS
DATA COLLECTED
The primary data source for the study was the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States; these data are submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration of the US Department of Health and Human Services provides oversight to the activities of the Organ Procurement and Transplantation Network and SRTR contractors.
We used zip codes of recipients in the SRTR database to merge data with health indicators collected at the county level. The county-level health indicators were derived from publicly available data that have been compiled through a Robert Wood Johnson Foundation project and the University of Wisconsin Population Health Institute.18 These data have been aggregated from several national registries and surveys and the Centers for Disease Control and Prevention. We merged these data with the SRTR so that county indicators associated with each transplant recipient's permanent residence were available for analyses. From the available list of community risk factors, we selected 12 that we considered could be directly related to transplant outcomes or serve as reasonable proxies for community health, environmental and behavioral risks, social condition, or access to care.
We considered the following community health indicators (listed with the primary source) for analysis as predictor variables: years of potential life lost and proportion of low birth weights (National Center for Health Statistics); poor or fair reported health, poor reported physical health days, poor reported mental health days, and proportion of individuals reporting tobacco use (Behavioral Risk Factor Surveillance System); adult obesity and physical inactivity proportions (National Center for Chronic Disease Prevention and Health Promotion); preventable hospital stays and screening for diabetes mellitus (Medicare/Dartmouth Institute); illiteracy rates (National Assessment of Adult Literacy); and median annual household income (US Census).
CUMULATIVE COMMUNITY HEALTH RISK SCORE
We generated a cumulative community risk score for each recipient's county on the basis of the combined presence of health indicators. For each of the 12 indicators, we categorized prevalence into quintiles. We evaluated the association of each factor with recipient mortality after adjustment for standard risk factors. Two of the indicators (the percentage of diabetic individuals who underwent hemoglobin A1c screening and the illiteracy rate) were not associated with outcomes or did not demonstrate a graded response and were not incorporated into the analysis. A cumulative score for each county was based on the remaining 10 indicators as the sum of each quintile above the lowest risk level. For instance, a county that was in the second quintile for each risk factor received 1 risk score for each of the 10 factors, resulting in a community risk score of 10. The range of scores possible was from 0 (if a county was in the top quintile [and had the least risk] for each factor) to 40 (if the county was in the bottom quin-tile [and had the most risk] for each factor). We selected this approach to measure the aggregate risk for each county and assumed an equal weight for each of the included factors. For the purpose of displaying results, we also generated county risk groups categorized on approximately the 10th, 20th, 50th, 80th, and 90th percentiles. To validate the risk score, we used a resampling (bootstrap) technique in which we extracted 1000 samples (with replacement) using the equivalent sample size as our original study population. For the primary analysis, we then replicated the Cox proportional hazards model for time to patient death for each sample. From the output of the models, we extracted the concordance index and the parameter estimate of the primary explanatory variable of interest (the cumulative risk score). Finally, we reported the empirical 95% CI of each of these statistics to represent the variability of the estimates and evaluate the likelihood of an overfitted model.19
The study population consisted of adult (aged ≥ 18 years) kidney transplant recipients who underwent transplant between January 1, 2004, and December 31, 2010, and had available zip codes from their permanent residence. These years were selected because the county-level risk factors were also derived from this period. We evaluated recipient characteristics associated with the presence of county indicators and used the Cochran-Armitage trend test to evaluate linear associations of characteristics with increasing scores. We generated a multivariable logistic model to assess the association of recipient factors with residence in the highest-risk communities. We defined highest-risk counties as those with 36 or more cumulative risk indicators, which constituted approximately the top decile of the study population. Multivariable Cox proportional hazards models were used to assess the adjusted hazard of time to patient death and overall graft loss (defined as graft failure or death) associated with community risk factors. Each of the multivariable models was adjusted for recipient and donor demographic characteristics, recipients’ primary diagnosis, whether the operation was for a primary transplant vs a retransplant, recipients’ body mass index, recipients’ primary insurance, recipients’ educational attainment, HLA antigen mismatches, panel reactive antibody percentage, and deceased or living donor source. A 2-sided type I error probability of .05 was selected as the threshold for statistical significance. All analyses were conducted with SAS statistical software (version 9.2; SAS Institute, Inc).
RESULTS
From the target population, 13 029 recipients did not have available zip code information in the SRTR or in the county database. There were no marked differences in characteristics between the patients with and without zip code information available. The final study population (N=100 164) consisted of 63% deceased-donor transplant recipients, 61% men, 26% African Americans, and 24% individuals with diabetes mellitus as a primary diagnosis. The mean (SD) age of the recipients was 50.3 (13.6) years and of the donors was 39.6 (14.9) years.
COMMUNITY RISK FACTORS
The mean (SD) cumulative community risk score for the recipient population was 19.9 (10.2). Approximately 10% of recipients resided in counties with a cumulative risk score of less than 6, and 8% of recipients resided in counties with a cumulative risk score of more than 35.
Recipients in higher-risk counties were less likely to receive a living-donor transplant, undergo a preemptive transplant, have private insurance, or be older than 65 years. In contrast, recipients in higher-risk counties were more likely to be African American and have a higher body mass index (Table 1). Recipient factors independently associated with residence in the highest-risk counties included younger age (aged 18-39 years; adjusted odds ratio, 1.46; 95% CI, 1.32-1.60), hypertension as a primary diagnosis (1.46; 1.36-1.56), deceased-donor recipients (1.42; 1.33-1.50), lower educational attainment (1.84; 1.62-2.08), African American race (1.59; 1.51-1.68), and Medicare as the primary insurance (1.46; 1.38-1.54; eTable 1 [http://www.archsurg.com]).
Table 1.
Demographic Characteristics of Study Population by Community Risk Factors
| Cumulative Community Risk Factors, No. (%)a |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | 0-5 | 6-10 | 11-20 | 21-30 | 31-35 | 36-40 | P Valueb |
| Living donor | 4319 (45) | 4783 (43) | 11364 (39) | 11067 (37) | 3702 (31) | 2116 (27) | <.001 |
| Primary transplant | 8407 (87) | 9607 (87) | 26 063 (88) | 26771 (88) | 10490 (89) | 7088 (89) | <.001 |
| Female recipient | 3679 (38) | 4306 (39) | 11571 (39) | 11919 (39) | 4709 (40) | 3088 (39) | .08 |
| Preemptive transplant | 2681 (28) | 3045 (28) | 7320 (25) | 6977 (23) | 2620 (22) | 1791 (23) | <.001 |
| PRA percentage of 0% | 4617 (48) | 5521 (50) | 14182 (48) | 14321 (47) | 5612 (47) | 3838 (48) | .03 |
| HLA-Ag MM of 0 | 1141 (12) | 1353 (12) | 3281 (11) | 3315 (11) | 1148 (10) | 773 (10) | <.001 |
| Private insurance | 4747 (49) | 5153 (47) | 12212 (41) | 11319 (37) | 4032 (34) | 2325 (29) | <.001 |
| BMI ≥30c | 2315 (26) | 2858 (28) | 8147 (29) | 9229 (33) | 3809 (35) | 2577 (35) | <.001 |
| African American recipient | 1300 (13) | 1573 (14) | 6979 (24) | 8465 (28) | 3982 (34) | 3590 (45) | <.001 |
| Recipient with primary diagnosis of diabetes mellitus | 2227 (23) | 2600 (24) | 7195 (24) | 7508 (25) | 3031 (26) | 1889 (24) | <.001 |
| Donor age ≥60y | 820 (9) | 874 (8) | 2497 (8) | 2400 (8) | 747 (6) | 563 (7) | <.001 |
| Recipient age ≥65y | 1604 (17) | 1809 (16) | 4638 (16) | 4537 (15) | 1752 (15) | 1095 (14) | <.001 |
| Total (N = 100 164) | 9650 (10) | 11012(11) | 29483 (29) | 30 266 (30) | 11831 (12) | 7922 (8) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HLA-Ag MM, HLA antigen mismatching; PRA, panel reactive antibody.
Defined as the sum of the number of quintiles beyond the first for each of 10 community-level risk factors (range, 0-40).
Cochran-Armitage trend test for linear association of transplant characteristics with levels of community risk score.
Missing values excluded for calculation of percentages.
POSTTRANSPLANT OUTCOMES AND COMMUNITY HEALTH INDICATORS
The distribution of individual community health indicators is given in eTable 2. Relative to patients from counties with the lowest presence of risk (lowest 20th percentile), each factor demonstrated a significant association with mortality after adjustment for clinical risk factors (Table 2). Factors generally demonstrated a dose-dependent association and had at least 10% increased hazard with the highest-risk quintile.
Table 2.
Adjusted Hazard Ratio for Death Associated With County Health Indicators
| Patient Death by Percentile, AHR (95% CI)a |
||||
|---|---|---|---|---|
| Community Health Indicator | 21st-40th | 41st-60th | 61st-80th | 81st-100th |
| Potential life lost, y | 1.09 (1.02-1.18) | 1.07 (1.00-1.16) | 1.07 (1.00-1.15) | 1.14 (1.06-1.22) |
| Poor physical health, d | 1.00 (0.92-1.08) | 1.07 (0.99-1.15) | 1.16 (1.08-1.24) | 1.10 (1.02-1.18) |
| Low birth weight, % | 1.09 (1.02-1.18) | 1.09 (1.02-1.18) | 1.12 (1.04-1.20) | 1.13 (1.05-1.22) |
| Poor mental health, d | 1.01 (0.93-1.09) | 1.06 (0.98-1.13) | 1.07 (0.99-1.15) | 1.12 (1.04-1.21) |
| Fair or poor health, % | 1.04 (0.97-1.12) | 1.04 (0.97-1.12) | 1.10 (1.03-1.19) | 1.17 (1.09-1.25) |
| Annual household median income, in $1000sb | 1.10 (1.02-1.18) | 1.08 (1.00-1.16) | 1.13 (1.05-1.21) | 1.17 (1.09-1.26) |
| Preventable hospital stayrate | 1.07 (0.99-1.16) | 1.12 (1.04-1.20) | 1.11 (1.04-1.20) | 1.20 (1.13-1.29) |
| Smokers, % | 1.03 (0.96-1.11) | 1.06 (0.98-1.14) | 1.14 (1.06-1.22) | 1.17 (1.09-1.25) |
| Obesity prevalence, % | 1.02 (0.95-1.10) | 1.04 (0.97-1.12) | 1.06 (0.98-1.14) | 1.13 (1.05-1.21) |
| Inactivity rate, % | 1.03 (0.95-1.11) | 1.16 (1.08-1.25) | 1.17 (1.09-1.26) | 1.13 (1.05-1.22) |
Abbreviations: AHR, adjusted hazard ratio; CI, confidence interval.
All models are adjusted for recipient and donor age, race, and sex; recipient primary diagnosis; HLA antigen mismatching; panel reactive antibody level; waiting time while receiving dialysis; primary vs re-transplant; recipient body mass index; recipient primary insurance; and deceased or living donor type. The reference group is 0 to the 20th percentile.
The reference level for this variable is the highest income quintile; subsequent quintiles represent descending income levels.
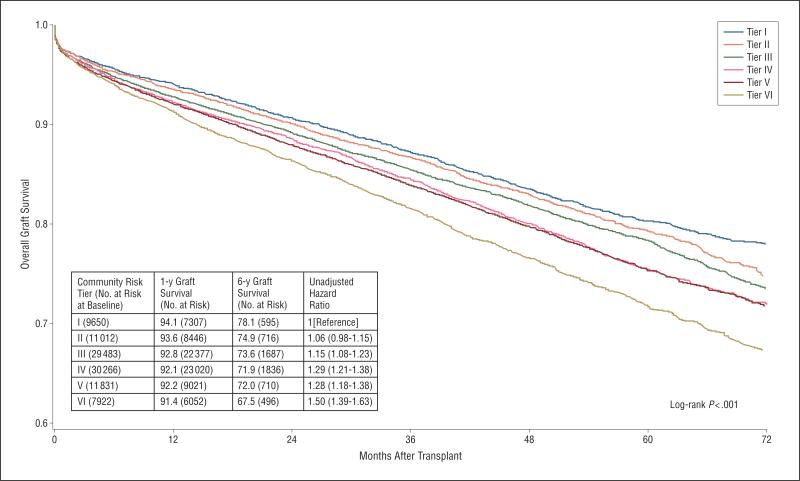
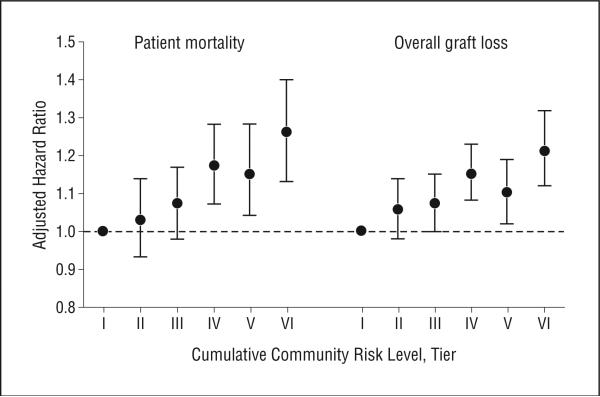
One-year overall graft survival rates varied from 91.4% to 94.1%, and 6-year survival rates ranged between 67.5% in the highest-risk group to 78.1% in the lowest-risk group (Figure 1). Patients from the highest-risk counties were associated with significant unadjusted hazard for overall graft loss (hazard ratio [HR], 1.50; 95% CI, 1.39-1.63) and posttransplant mortality (1.45; 1.31-1.61). For mortality and overall graft loss, we also found a statistically significant dose-response–adjusted association with the cumulative risk score, including a 26% hazard for mortality (adjusted HR [AHR], 1.26; 95% CI, 1.13-1.40) with the highest-risk counties (Figure 2). The association of highest-risk counties with overall graft loss (AHR, 1.21; 95% CI, 1.12-1.32) and death-censored graft loss (1.18; 1.06-1.32) were also significant. The association of the cumulative community risk score with mortality was relatively consistent by adjusting for the center-level proportion of patients from higher-risk counties. The association of the highest-risk counties with mortality was stronger among counties with larger populations. Among counties with populations of more than 90 000 (the approximate median level), the AHR of patients from the highest-risk counties was 1.39 (95% CI, 1.19-1.62).
Figure 1.
Kaplan-Meier plots of time to overall graft loss by county-level risk.
Figure 2.
Adjusted hazard ratio for patient death and overall graft loss by cumulative community risk level. Tier I indicates a cumulative risk of 0-5; tier II, 6-10; tier III, 11-20; tier IV, 21-30; tier V, 31-35; and tier VI, 36-40.
The overall contribution of the cumulative risk score to the explained variation of the posttransplant mortality Cox proportional hazards model was moderate. The type III Wald χ2 statistics contributing to the log likelihood of the Cox model indicated that the cumulative risk score explained more variation in the model than did retransplantation vs primary transplantation , dialysis waiting time , HLA antigen mismatching , panel reactive antibody percentage level , or donor race . However, the explained variation was significantly less than those for recipient age , donor age , primary diagnosis , or donor source . The concordance index of the Cox model for time to patient death was 0.71. Based on the bootstrapped 1000 resamples, the point estimate (empirical 95% CI) for this estimate was 0.709 (0.703-0.715). The AHR of the primary explanatory variable of interest (ie, the cumulative community risk score) when considered as a continuous variable was 1.03 per 5 units of the risk score. The point estimate (95% CI) based on the bootstrapped samples was 1.031 (1.022-1.041). In addition, when we weighted the effects of the community risk score based on the estimated effect of each component (rather than weighting each risk factor equally), the association of the community risk score increased slightly (AHR, 1.032; 95% CI, 1.020-1.043).
REGIONAL VARIATION IN PRESENCE OF RECIPIENTS FROM HIGH-RISK COUNTIES
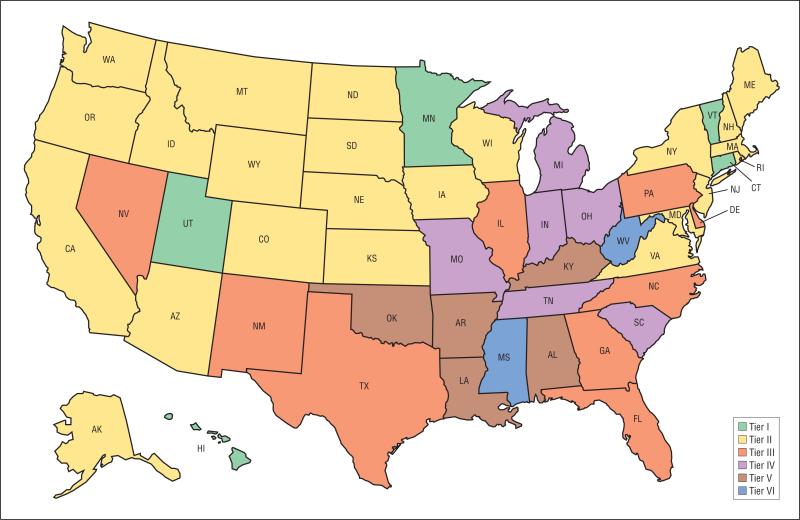
The mean cumulative community risk score by transplant center was 20 (eFigure 1). The proportion of recipients in counties with a risk score of at least 20 ranged from 0% to 97.5% between centers. Ten percent of centers had recipients with a mean risk score of 10 or less, whereas 10% of centers had a recipient population with a mean risk score of 31 or higher. As displayed in Figure 3, this variability was also highly significant at the state level. Recipients from 2 states (West Virginia and Mississippi) were in counties with mean cumulative risk scores higher than 35. In contrast, 5 states (Connecticut, Vermont, Utah, Minnesota, and Hawaii) had a recipient population residing in counties with mean risk scores of less than 10. Individual county cumulative risk scores are listed in eTable 3.
Figure 3.
State-level variation of mean county-level risk factors for transplant recipients. Tier I indicates a cumulative risk of 0-5; tier II, 6-10; tier III, 11-20; tier IV, 21-30; tier V, 31-35; and tier VI, 36-40.
COMMENT
There are several principal findings of our study. First, there is wide variation in health indicators from the communities of kidney transplant recipients in the United States. Second, recipients who are younger, are African American, have lower educational attainment, and have public insurance are more likely to reside in communities with poor health indicators. Third, community health indicators are significantly associated with posttransplant mortality independent of traditional risk factors and explain more variation in outcomes than many factors considered clinically relevant. Finally, the prevalence of community health indicators varies markedly by region and individual transplant center.
Cumulatively, our findings indicate that underlying factors in the community are important for risk stratification of transplant recipients. The substantial regional variation of risks and the failure to account for them in quality assessments indicate sources of potential bias for center performance evaluations. Inclusion of community risk factors in models evaluating provider performance may alleviate disincentives to treat patients from high-risk communities. Further understanding of the mechanisms by which community health indicators are associated with diminished outcomes and the development of interventions addressing these risks may improve long-term survival for kidney transplant recipients.
There are a number of potential explanations for the association of community health indicators and transplant outcomes. Health indicators evaluated in this study included characteristics describing comorbid conditions, socioeconomic status, access to care, and behavior. Potential explanations for our findings are that these aggregate community measures are proxies for individual patient factors and that the association with poorer outcomes reflects these risks, which are not traditionally codified. Access to primary and specialist care is likely clustered within communities and can be reflected in outcomes.20,21 The behavioral and socioeconomic conditions of the communities in which patients reside may also reflect risks that patients are exposed to in their environment before and after undergoing transplant but that are not consistently documented. However, it is also important for interpretation of our study findings that ascribing broad area risks to each individual within that area is an ecological fallacy. Thus, although there appear to be significant risks associated with certain communities, it is inappropriate to directly assign risks to individuals within that community.
An important consideration for the study findings in this and other health care contexts is the association of community health indicators and performance indicators. In transplantation, the recent Conditions of Participation by the Centers for Medicare and Medicaid Services22 explicitly aligns transplant center performance with program credentialing. In addition, for many other health care contexts, report cards and quality performance assessments have increasing ramifications on hospital reimbursement. The profound regional variability of community health indicators implies that the degree of systematic bias between providers may be substantial.23,24 In general, health care providers that more commonly treat patients from higher-risk communities may be more likely to lose reimbursement and credentialing of their programs.25-27 In fact, there is evidence that transplant centers use more conservative criteria as a consequence of receiving poor performance evaluations and may exclude patients from care.28 Therefore, in health care contexts in which there are mechanisms to select patients for treatment, failure to account for community risk factors could lead to diminished access to care for patients who may already be experiencing disparities in access to care. Alternatively, incorporating community risks into performance evaluations may alleviate conscious or subconscious barriers to care for patients from higher-risk communities.
There are several limitations for interpretation of our findings. First, there is clear heterogeneity of risks within counties. By applying a county-level health indicator to individuals within counties, a clear degree of misspecification is evident. However, this limitation would tend to dilute effects, and the fact that county-level health indicators were associated with outcomes despite this lack of granularity suggests that effects may be stronger if measured at a more specific level.29,30 Second, the study was not designed to identify specific mechanisms for associations but rather suggests that community-level factors are sources of unexplained variation in outcomes. Third, it could be argued that adjustment for community risks may implicitly provide an “excuse” for poorer outcomes among certain patient groups rather than charge providers with attaining equivalent outcomes despite patients’ individual needs. Finally, it is possible that community-level measures used for this analysis included transplant recipients even though transplant recipients likely represent only a few people in a county.
In summary, the principal findings of our study are that community health indicators are significantly associated with transplant recipient outcomes. These indicators are independent of traditional clinical risk factors and are highly variable across the country. The impact of these risks for providing unbiased measurements of transplant center quality is important for future study, as well as for proper interpretation of program evaluations by regulators, caregivers, and patients. The potential for improving access to care to vulnerable populations by accounting for these risks is an important consideration for future study and for policymakers. Further understanding of the mechanisms for these associations and potential interventions are needed to improve patient outcomes, minimize disparities, and elucidate the most effective individualized treatment planning.
Supplementary Material
Acknowledgments
Disclaimer: The data reported herein have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US government.
Footnotes
Financial Disclosure: None reported.
Online-Only Material: The 3 eTables and the eFigure are available at http://www.archsurg.com.
REFERENCES
- 1.Miniño AM, Xu J, Kochanek KD, Tejada-Vera B. Death in the United States, 2007. NCHS Data Brief. 2009;(26):1–8. [PubMed] [Google Scholar]
- 2.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270(18):2207–2212. [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.Galea S, Tracy M, Hoggatt KJ, Dimaggio C, Karpati A. Estimated deaths attributable to social factors in the United States. Am J Public Health. 2011;101(8):1456–1465. doi: 10.2105/AJPH.2010.300086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher MP, Kelly PJ, Jardine M, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21(5):852–858. doi: 10.1681/ASN.2009101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gueye AS, Chelamcharla M, Baird BC, et al. The association between recipient alcohol dependency and long-term graft and recipient survival. Nephrol Dial Transplant. 2007;22(3):891–898. doi: 10.1093/ndt/gfl689. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Klinger D. Cigarette smoking in renal transplant recipients. J Am Soc Nephrol. 2000;11(4):753–759. doi: 10.1681/ASN.V114753. [DOI] [PubMed] [Google Scholar]
- 9.Sung RS, Althoen M, Howell TA, Ojo AO, Merion RM. Excess risk of renal allograft loss associated with cigarette smoking. Transplantation. 2001;71(12):1752–1757. doi: 10.1097/00007890-200106270-00009. [DOI] [PubMed] [Google Scholar]
- 10.Gordon EJ, Prohaska T, Siminoff LA, Minich PJ, Sehgal AR. Needed: tailored exercise regimens for kidney transplant recipients. Am J Kidney Dis. 2005;45(4):769–774. doi: 10.1053/j.ajkd.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon EJ, Prohaska TR, Gallant MP, et al. Prevalence and determinants of physical activity and fluid intake in kidney transplant recipients. Clin Transplant. 2010;24(3):E69–E81. doi: 10.1111/j.1399-0012.2009.01154.x. doi:10.1111/j.1399-0012.2009.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280(13):1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs RB, Lobo PI, Nock SL, Hanson JA, Ojo AO, Pruett TL. Racial disparities in access to simultaneous pancreas-kidney transplantation in the United States. Am J Kidney Dis. 2000;36(3):526–533. doi: 10.1053/ajkd.2000.9793. [DOI] [PubMed] [Google Scholar]
- 14.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6(7):1760–1767. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 15.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5(12):2276–2288. doi: 10.2215/CJN.04940610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, et al. Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol. 2006;1(2):313–322. doi: 10.2215/CJN.00630805. [DOI] [PubMed] [Google Scholar]
- 17.Woodward RS, Schnitzler MA, Lowell JA, Spitznagel EL, Brennan DC. Effect of extended coverage of immunosuppressive medications by Medicare on the survival of cadaveric renal transplants. Am J Transplant. 2001;1(1):69–73. doi: 10.1034/j.1600-6143.2001.010113.x. [DOI] [PubMed] [Google Scholar]
- 18.County Health Rankings Web site [June 1, 2011];2011 http://www.countyhealthrankings.org.
- 19.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Hall YN, O'Hare AM, Young BA, Boyko EJ, Chertow GM. Neighborhood poverty and kidney transplantation among US Asians and Pacific Islanders with end-stage renal disease. Am J Transplant. 2008;8(11):2402–2409. doi: 10.1111/j.1600-6143.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 21.Prakash S, Rodriguez RA, Austin PC, et al. Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol. 2010;21(7):1192–1199. doi: 10.1681/ASN.2009101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [September 1, 2011];New Medicare hospital conditions of participation for transplant centers. Centers for Medicare & Medicaid Services Web site. 2007 Mar;27 http://www .cms.hhs.gov/apps/media/press/release.asp?Counter=2093&intNumPerPage=10&checkDate=&checkKey=&srchType=&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. [Google Scholar]
- 23.Kasiske BL, Snyder JJ, Skeans MA, Tuomari AV, Maclean JR, Israni AK. The geography of kidney transplantation in the United States. Am J Transplant. 2008;8(3):647–657. doi: 10.1111/j.1600-6143.2007.02130.x. [DOI] [PubMed] [Google Scholar]
- 24.Schold JD, Harman JS, Chumbler NR, Duncan RP, Meier-Kriesche HU. The pivotal impact of center characteristics on survival of candidates listed for deceased donor kidney transplantation. Med Care. 2009;47(2):146–153. doi: 10.1097/MLR.0b013e31818475c9. [DOI] [PubMed] [Google Scholar]
- 25.Howard RJ, Cornell DL, Schold JD. CMS oversight, OPOs and transplant centers and the law of unintended consequences. Clin Transplant. 2009;23(6):778–783. doi: 10.1111/j.1399-0012.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 26.Schold JD, Srinivas TR, Howard RJ, Jamieson IR, Meier-Kriesche HU. The association of candidate mortality rates with kidney transplant outcomes and center performance evaluations. Transplantation. 2008;85(1):1–6. doi: 10.1097/01.tp.0000297372.51408.c2. [DOI] [PubMed] [Google Scholar]
- 27.Weinhandl ED, Snyder JJ, Israni AK, Kasiske BL. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant. 2009;9(3):506–516. doi: 10.1111/j.1600-6143.2008.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schold JD, Arrington CJ, Levine G. Significant alterations in reported clinical practice associated with increased oversight of organ transplant center performance. Prog Transplant. 2010;20(3):279–287. doi: 10.1177/152692481002000313. [DOI] [PubMed] [Google Scholar]
- 29.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the Public Health Disparities Geo-coding Project (US). J Epidemiol Community Health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.